Abstract
The present study evaluated the effects of natural astaxanthin (ASTA) from Haematococcus pluvialis on the antioxidant capacity, lipid metabolism, and ASTA accumulation in the egg yolk of laying hens. Hy-Line Brown layers (n = 288, 50 wk old) were randomly assigned to 1 of 4 dietary treatment groups. Each group had 6 replicates of 12 hens each. All birds were given a corn-soybean meal–based diet containing 0, 25, 50, or 100 mg/kg ASTA for 6 wk. The results showed that the total antioxidant capacity, superoxide dismutase level, and glutathione peroxidase level in the plasma, livers, and egg yolks were significantly increased in the ASTA groups compared with those of the control group (P < 0.05), whereas the content of malondialdehyde linearly decreased (P < 0.05). The plasma levels of high-density and very-low-density lipoprotein cholesterol in the ASTA groups were significantly higher than those in the control group (P < 0.05). In addition, ASTA supplementation decreased low-density lipoprotein cholesterol and triglyceride plasma levels (P < 0.05). However, there were no significant differences in the other lipid metabolism parameters among the ASTA-supplemented groups relative to the control group except for an increase in high-density lipoprotein cholesterol in the liver. Compared with the control, dietary ASTA supplementation significantly increased the enrichment of ASTA in egg yolks at the end of week 2, 4, and 6 (P < 0.05). The mRNA expression of scavenger receptor class B type 1 (SCARB1) and very-low-density lipoprotein receptor (VLDLR) in the ASTA groups was markedly higher (P < 0.05) than that in the control group in the liver and ovaries, respectively. In conclusion, these results suggest that dietary ASTA enhances the antioxidant capacity and regulates lipid metabolism in laying hens. ASTA enrichment in egg yolks may be closely related to the upregulation of SCARB1 and VLDLR gene expression.
Key words: natural astaxanthin, laying hen, antioxidant enzyme, SCARB1, VLDLR
Introduction
Currently, with the increasing demand for poultry meat and eggs, nutraceuticals are used as dietary supplements to improve feed quality characteristics in poultry. Recently, many studies have reported that dietary supplementation with L-carnitine (Ghoreyshi et al., 2019a, Ghoreyshi et al., 2019b; Ghoreyshi et al., 2019a, Ghoreyshi et al., 2019b), quercetin (Goliomytis et al., 2014), and saponins (Bera et al., 2019) in broiler chickens improves meat quality. In addition, dietary supplementation with spirulina (Omri et al., 2019a, Omri et al., 2019b, Omri et al., 2019c), linseeds (Omri et al., 2019a, Omri et al., 2019b, Omri et al., 2019c), and fenugreek seeds (Omri et al., 2019a, Omri et al., 2019b, Omri et al., 2019c) has successfully improved the egg quality of laying hens. Hen eggs are considered one of the most healthy foods in nature because they contain high-quality proteins and lipids, as well as trace elements and vitamins (Fredriksson et al., 2006; Walker et al., 2012). Carotenoids have been used in the poultry industry for many years to pigment eggs and meat (Gervasi et al., 2018). Lycopene (Panaite et al., 2019) and lutein (Leeson and Caston, 2004) can be deposited in the yolk and significantly deepen the color of egg yolk. Compared with β-carotenoid and canthaxanthin, astaxanthin (ASTA) is deposited more efficiently in laying hens (Lee et al., 2010).
ASTA is a xanthophyll carotenoid found in shrimp, crab shells, and salmon (Ritto et al., 2017) in both synthetic and natural forms. Synthetic ASTA is produced from petrochemicals by chemical companies. Although it has the same chemical formula as ASTA, its physiological function, for example, the ability to scavenge free radicals, is far less than that of ASTA (Capelli et al., 2013). Haematococcus pluvialis (a freshwater unicellular microalga) is one of the most effective organisms for the production of ASTA (Shao et al., 2019). It has been shown that H. pluvialis can produce a large amount of ASTA under stress conditions, such as high temperature, light, and high salinity (Sarada et al., 2002). ASTA has a higher antioxidant activity than various carotenoids, such as lutein, lycopene, α-carotene, and β-carotene (Naguib, 2000) and more than 550 times the scavenging activity of singlet oxygen than vitamin E (Sajad Fakhri and Jorjani, 2018). It can effectively scavenge free radicals, increase antioxidant enzyme activity (Dose et al., 2016; Li et al., 2018), and enhance immune responses (Callie et al., 2018) in rats. However, the effects of high levels of dietary ASTA on the lipid metabolism and antioxidant capacity of laying hens and the enrichment of ASTA in egg yolks remain poorly understood. The United States Food and Drug Administration has approved ASTA as an edible pigment in animal and fish feed, and the European Commission considers natural ASTA a food dye (Singh et al., 2019). Recently, there has been a growing demand for ASTA from many industries, such as pharmaceutical, cosmetic, food, nutraceutical, and feed industries (Gervasi et al., 2019). Therefore, ASTA is an ideal choice as a feed additive.
High-density lipoprotein (HDL) is a large molecular complex of lipoproteins mainly synthesized in the liver and small intestine (Zhang et al., 2018). Scavenger receptor B class 1 (SCARB1), known as the HDL receptor, mediates carotenoid uptake in mammals (Hoekstra and Sorci-Thomas, 2017) and also plays a key role in hepatic HDL metabolism (Trigatti, 2017). In addition, as HDL levels decrease, carotenoid levels in the blood and surrounding tissues also decrease (Dias et al., 2014).
Vitellogenin is a major protein widely found in oviparous animals that can bind and transport lipids, thyroxine, vitamins, carotenoids, and riboflavin to oocytes (Zheng et al., 2012). As a yolk protein precursor, vitellogenin recognizes very-low-density lipoprotein receptors before entering egg cells; it then enters the cells to promote the maturation of oocytes (Barber et al., 1991). Therefore, the VLDLR gene is crucial for the production of laying hens. Whether SCARB1 or VLDLR is involved in the deposition of dietary ASTA into the egg yolk in laying hens remains unclear.
Although numerous reports regarding the effects of dietary carotenoids on lipid metabolism and antioxidant capacity in laying hens have been published (Feng Xue, 2013; Sun et al., 2014), there is limited information available on the effects of dietary ASTA from H. pluvialis on laying hens. Therefore, this study investigated the effects of dietary ASTA on the antioxidant enzyme activity, lipid metabolism, and gene expression related to ASTA enrichment in the egg yolks of laying hens.
Materials and methods
Birds and Diets
All experimental protocols were approved by the Animal Care and Use Committee of Beijing University of Agriculture. H. pluvialis was purchased from Jingzhou Natural Astaxanthin Inc (Jingzhou, China), and the ASTA content was 1.3%. Two hundred eighty-eight 50-week-old Hy-Line Brown laying hens with a similar weight and genetic background were randomly assigned to 1 of 4 dietary treatment groups following a completely randomized single factor design. Each group had 6 replicates of 12 birds, and 3 birds were housed in one cage (45 cm × 45 cm × 45 cm). All birds had free access to water and were given a corn-soybean meal–based diet containing a 0, 25, 50, or 100 mg/kg ASTA mixture for 6 wk. The composition and nutrient levels of the corn-soybean meal–based diet are shown in Table 1.
Table 1.
Composition and nutrient content of basal diets of laying hens (air dried basis, %).1
| Ingredient | Content, % | Nutrient level | |
|---|---|---|---|
| Corn | 62.66 | Metabolizable energy [MJ/kg]2 | 11.68 |
| Soybean meal | 25.66 | Crude protein [%]3 | 16.50 |
| Limestone | 8.33 | Calcium [%]3 | 3.50 |
| Soybean oil | 0.60 | Methionine [%]2 | 0.32 |
| (Wheat) bran | 0.06 | Lysine [%]2 | 0.77 |
| Premix1 | 0.54 | Total phosphorus [%]3 | 0.60 |
| Salt | 0.49 | Tryptophan [%]2 | 0.17 |
| Choline | 0.16 | ||
| Dicalcium phosphate | 1.50 | ||
| Total | 100.00 |
The premix provided the following per kilogram of the diet: vitamin A 9,000 IU, vitamin D 2,000 IU, vitamin E 10 IU, vitamin K 0.5 mg, vitamin B2 0.006 mg, vitamin B6 52.5 mg, vitamin B12 0.4 mg, biotin 4.5 mg, folic acid 4.0 mg, D-pantothenic acid 4 mg, nicotinic acid 30 mg, Cu (as copper sulfate) 8 mg, Fe (as ferrous sulfate) 60 mg, Mn (as manganese sulfate) 65 mg, Zn (as zinc sulfate) 90 g, I (as potassium iodide) 0.5 mg, and Se (as sodium selenite) 0.3 mg.
Calculated values.
Measured values.
Sample Collection
At the end of the 6-week feeding trial, one bird from each replicate was randomly selected and sacrificed by cervical dislocation. Immediately after euthanasia, blood samples were collected via exsanguination of the left jugular vein with scalpels and centrifuged at 3,000 × g for 10 min at 4°C to separate the plasma. Then, the plasma samples were frozen at −80°C until analysis. The liver and ovaries were immediately removed and quickly frozen at −80°C for further analysis. Two eggs in each replicate were sampled on day 14, 28, and 42 to separate egg yolks from egg whites. Egg yolks were collected in sterile dishes and mixed. Frozen egg yolks were lyophilized for 48 h, with the vacuum level reaching 133 × 10−3 mbar and a condenser temperature of at least −40°C in a Freeze Dry System (Tian Feng, Shanghai, China).
Antioxidant Enzymes and Lipid Metabolism Assay
The levels of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), total antioxidant capacity (T-AOC), and malondialdehyde (MDA) were measured in the plasma, liver, and egg yolk. In addition, total cholesterol, triglycerides (TG), HDL cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and very-low-density lipoprotein cholesterol (VLDL-C) were measured in the plasma and liver. These assays were performed using commercial chicken ELISA kits (Nanjing JianCheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions. The method and principle to determine antioxidant and lipid parameter indicators using these kits were described elsewhere (Qi et al., 2011).
Determination of ASTA in Egg Yolks
A 2.5-g egg yolk sample was accurately weighed into a 50-mL centrifuge tube. After 5 mL of deionized water was added to the sample, the centrifuge tube was placed in an ultrasonic water bath at 50°C for 30 min. The cooled sample was transferred to a separating funnel, the suspension was extracted with 30 mL of dichloromethane 3 times, and then the liquid was separated. The dichloromethane extract was transferred to a 250-mL round-bottomed flask, and the suspension was extracted with 30 mL of dichloromethane 3 more times. The extracts were mixed in the same round-bottomed flask and evaporated to dryness at 35°C. Then, 5 mL of methanol was added, and the mixture was passed through a 0.45-μm membrane filter. Finally, the sample was analyzed by HPLC (Waters, Milford, MA) according to a previous report (Du et al., 2016). Briefly, ASTA was separated from other components on a Sun Fire C18 column (150-mm long, 4.6 mm i.d., 5 μm particle size; Waters). The elution procedure used methanol as mobile phase A and acetonitrile as mobile phase B. The analysis time was 10 min, followed by a 2-min rebalance time for a total run time of 12 min. According to the standard chromatogram of ASTA, the retention time was 4.5 min. The mobile phase flow rate was 1 mL/min, the injection volume was 10 μL, the column temperature was maintained at 30°C, and ASTA was detected at a wavelength of 474 nm.
Quantitative PCR Analysis
Total RNA was isolated from livers and ovaries using TRIzol reagent (Ambion/Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. RNA purity was estimated by measuring the ratio of the absorbance at 260 and 280 nm (A260/A280) and then visually confirmed by examining the 18S and 28S bands in a 1% agarose gel stained with ethidium bromide. Then, total RNA concentrations were measured using a spectrophotometer at 260 nm. Reverse transcription was performed using a Thermo First cDNA Synthesis Kit (#33-20102; SinoGene, Beijing, China). Quantitative PCR analyses were performed using the Step One Plus Real-time PCR system (Applied Biosystems, Foster City, CA). The primers of the selected genes are listed in Table 2. Quantitative PCR reactions were performed using a programmable thermal cycler (denaturation at 95°C for 3 min followed by 40 cycles of denaturation at 95°C for 15 s and annealing at 62°C for 30 s followed by an additional step at 72°C for 30 s, 95°C for 15 s, 60°C for 30 s, and 95°C for 15 s for dissociation curve analysis). Each sample was measured in triplicate. All primer concentrations were optimized before the experiment. The relative gene expression levels were calculated using the 2−ΔΔCt method (Livak and Schmittgen., 2001), with β-actin as the reference gene.
Table 2.
Primer sequences used for quantitative real-time PCR.
| Genes | Primer sequence (5′-3′) | Product size | Tm (°C) | Accession number |
|---|---|---|---|---|
| ACTB | F- CGCATAAAACAAGACGAGA R- GACACCTTCACCATTCCA |
91 bp | 62 | NM_205518.1 |
| VLDLR | F- TGAGGATGGGTCTGACGAGAG R- CACACTCCAACTCATCACTACCAT |
275 bp | 62 | NM_205229.1 |
| SCARB1 | F- TCACTTCTACAATGCTGACCCAA R- TGAGCCATCAATGTATCCACTC |
241 bp | 62 | XM_015275627.2 |
Statistical Analysis
All data were expressed as the mean ± SEM. Individual birds were treated as the experimental unit for parameter determination, and all data were analyzed by a one-way ANOVA using SPSS 22.0 (IBM Corp., Armonk, NY). In addition, polynomial regression analysis was used to test the linear and quadratic nature of the response to the additive dosage of ASTA, and Duncan's multiple comparison tests were used to analyze the differences among various treatments. Statistical significance was defined at P < 0.05.
Results and discussion
Antioxidant Capacity and Lipid Peroxidation
Normal aerobic metabolism in organisms generates free radicals, such as hydroxyls, peroxides, and reactive oxygen species (Higuera-Ciapara et al., 2006). These molecules arise from the disturbance of the equilibrium state of prooxidation/antioxidation reactions (Valko et al., 2007). Excess oxidative molecules are associated with various disorders because they induce protein and lipid oxidation and DNA damage. An antioxidant is a molecule that inhibits oxidation, thus ameliorating oxidative damage, which plays an important role in the treatment and prevention of disease (Seifried et al., 2007). In the present study, the effects of dietary ASTA supplementation on plasma, liver, and egg yolk antioxidant parameters are presented in Table 3, Table 4, Table 5. SOD activity in the plasma, liver, and egg yolk of laying hens increased dramatically in the 50- and 100-mg/kg ASTA groups compared with that in the control group (P < 0.05). The activity of GSH-Px in the plasma, liver, and egg yolk was linearly and quadratically affected (P < 0.05) by dietary ASTA. In addition, 50-mg/kg ASTA significantly increased the activity of SOD (P < 0.05). Supplementation with ASTA increased the T-AOC level (P < 0.05) in the plasma and liver relative to the control group. However, no significant differences in T-AOC were observed among ASTA-supplemented groups in the plasma. Numerous studies have demonstrated the antioxidant properties of SOD and GSH-Px (Battin and Brumaghim, 2009; Azarabadi et al., 2017; Olson et al., 2018). Dietary ASTA has been reported to enhance the activities of GSH-Px and SOD and markedly reduce hepatic lipid peroxidation and oxidative stress in the liver of rats (Xu et al., 2017). Similarly, Chen et al. (2015) demonstrated that dietary ASTA increased the activity of GSH-Px and SOD in the liver of Mesocricetus auratus (Chen et al., 2015). In addition, ASTA plays a potential role in repairing damage in UV C-irradiated mice (Nia et al., 2018). Similar to previous reports, we showed that dietary ASTA increased the activities of SOD and GSH-Px and the T-AOC in the plasma, liver, and egg yolk.
Table 3.
Effect of dietary astaxanthin (ASTA) supplementation on antioxidant enzyme activities and lipid peroxidation levels in the plasma of laying hens.
| Items | ASTA levels/(mg/kg) |
SEM |
P value |
||||
|---|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 100 | Linear | Quadratic | ||
| GSH-Px (U/mL) | 417.13b | 438.16a | 440.46a | 438.05a | 2.43 | <0.01 | <0.01 |
| MDA (nmol/mL) | 7.87a | 6.31b | 2.85c | 1.92d | 0.52 | <0.01 | 0.30 |
| SOD (U/mL) | 311.39b | 334.55a,b | 348.33a | 346.47a | 5.37 | 0.011 | 0.20 |
| T-AOC (U/mL) | 0.34b | 0.43a | 0.46a | 0.45a | 0.02 | <0.01 | 0.02 |
a,bMeans within a row with no common superscripts differ significantly (P < 0.05).
Abbreviations: GSH-Px, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase; T-AOC, total antioxidant capacity.
Table 4.
Effect of dietary astaxanthin (ASTA) supplementation on antioxidant enzyme activities and lipid peroxidation levels in the liver of laying hens.
| Items | ASTA levels/(mg/kg) |
SEM |
P value |
||||
|---|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 100 | Linear | Quadratic | ||
| GSH-Px (U/mg prot) | 24.36d | 29.11c | 37.97a | 31.68b | 1.07 | <0.01 | <0.01 |
| MDA (nmol/mg prot) | 0.80a | 0.63a,b | 0.58b | 0.57b | 0.023 | <0.01 | 0.12 |
| SOD (U/mg prot) | 268.97c | 315.02b | 369.55a | 318.76b | 7.77 | <0.01 | <0.01 |
| T-AOC (U/mg prot) | 0.034c | 0.038b | 0.048a | 0.037b,c | 0.0012 | <0.01 | <0.01 |
a-cMeans within a row with no common superscripts differ significantly (P < 0.05).
Abbreviations: GSH-Px, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase; T-AOC, total antioxidant capacity.
Table 5.
Effect of dietary astaxanthin (ASTA) supplementation on antioxidant enzyme activities and lipid peroxidation levels in the egg yolk of laying hens.
| Items | Time/week | ASTA levels/(mg/kg) |
SEM |
P value |
||||
|---|---|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 100 | Linear | Quadratic | |||
| GSH-Px (U/ml) | 2 | 1,174.36b | 1,856.39a | 1,535.90a,b | 1,707.70a,b | 103.12 | 0.12 | 0.15 |
| 4 | 1,271.06c | 2,274.73b | 2,915.75a | 2,714.29a,b | 156.22 | <0.01 | <0.01 | |
| 6 | 1,245.61c | 4,695.91a | 4,725.15a | 4,049.71b | 301.79 | <0.01 | <0.01 | |
| MDA (nmol/ml) | 2 | 688.38a | 558.58b | 562.62b | 535.86b | 16.32 | <0.01 | <0.01 |
| 4 | 630.00a | 553.34b | 525.56b | 523.33b | 18.21 | <0.01 | <0.01 | |
| 6 | 663.89a | 467.59b | 438.88b,c | 394.44c | 32.34 | <0.01 | <0.01 | |
| SOD (U/ml) | 2 | 534.72b | 671.72a | 550.71b | 546.77b | 14.78 | 0.034 | <0.01 |
| 4 | 555.31b | 625.78a | 645.99a | 615.59a | 9.14 | <0.01 | <0.01 | |
| 6 | 537.97b | 657.85a | 675.16a | 656.42a | 13.63 | <0.01 | <0.01 | |
a-cMeans within a row with no common superscripts differ significantly (P < 0.05).
Abbreviations: GSH-Px, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase.
MDA is a major oxidation product of peroxidized polyunsaturated fatty acids and an important indicator of lipid peroxidation (Freeman and Crapo, 1981). In this study, MDA concentrations in the plasma, liver, and egg yolk decreased linearly with increasing dietary ASTA levels (P < 0.05), which is similar to previous studies (Kamath et al., 2008; Li et al., 2014). The mechanism by which dietary ASTA reduces lipid peroxidation may be related to 2 factors. First, ASTA is a member of the xanthophyll family of carotenoids, which have a unique molecular structure with both lipophilic and hydrophilic properties. It is located both in and outside of the cell membrane (Ambati et al., 2014). ASTA has a stronger singlet oxygen-quenching ability relative to various carotenoids (Naguib, 2000), reduces the levels of reactive oxygen species, and efficiently inhibits lipid peroxidation. Second, ASTA may reduce MDA concentrations by enhancing antioxidant enzyme activity to scavenge oxygen free radicals.
Lipid Metabolism Parameters
Both HDL and LDL are apolipoproteins and represent a form of lipid transport in the blood. Studies have shown that high levels of plasma HDL can reduce the risk of cardiovascular disease (Zuliani et al., 2010; Kishimoto et al., 2016). The effects of different dietary inclusion levels of ASTA on lipid metabolism in the plasma and liver are shown in Tables 6 and 7. Dietary ASTA supplementation significantly (P < 0.05) increased VLDL-C and HDL-C contents in the plasma. In contrast, we observed a linear decrease in LDL-C levels from 0.40 mmol/L to 0.20 mmol/L (P < 0.05) as dietary ASTA levels increased in the plasma. Meanwhile, the TG level was significantly (P < 0.05) decreased in the 100-mg/kg ASTA group. No significant differences in LDL-C, TG, or total cholesterol levels in the liver were observed among the groups as dietary ASTA levels increased except for an increase in the level of HDL-C. The main function of HDL is to promote the transportation of excess cholesterol from surrounding tissues (including macrophages) back to the liver and their eventual excretion from the body (Arnold Von Eckardstein, 2001). Conversely, LDL is a cholesterol-rich lipoprotein that transports cholesterol from the liver to various tissues of the body (Robert Scott Kiss, 2017). As an important inducer, ASTA plays an essential role in the movement of cholesterol to HDL (Iizuka et al., 2012). Zou et al. (2017) reported that the HDL-C level was significantly higher in the ASTA group than that in the control group in the plasma of mice. A similar result was observed in the present study as dietary ASTA significantly increased HDL-C levels in both the plasma and liver. Although there was no difference in LDL-C levels after dietary supplementation with 25- to 100-mg/kg ASTA in the liver, plasma LDL-C levels were remarkably decreased. For sexually mature laying hens, the serum VLDL-C level is of great significance for the production of laying hens. TGs are synthesized in the liver, transported mainly in the form of VLDL-C, and finally deposited in egg yolks in the form of small-sized yolk-targeted VLDL-C (Yue et al., 2011). In our study, no significant differences in VLDL-C levels were observed among any of the ASTA-added groups in the liver. However, the VLDL-C level in the plasma increased gradually with the addition of increasing ASTA concentrations (from 50–100 mg/kg). Therefore, this result further indicated that ASTA had a positive effect on the regulation of lipid metabolism in laying hens.
Table 6.
Effect of dietary astaxanthin (ASTA) addition on lipid metabolism in the plasma.
| Items | ASTA levels/(mg/kg) |
SEM |
P value |
||||
|---|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 100 | Linear | Quadratic | ||
| HDL-C (mmol/L) | 0.43b | 0.48a,b | 0.53a,b | 0.68a | 0.04 | 0.02 | 0.42 |
| LDL-C (mmol/L) | 0.40a | 0.38a | 0.31b | 0.20c | 0.02 | <0.01 | <0.01 |
| TG (mmol/L) | 4.59a | 4.36a | 4.59a | 3.57b | 0.12 | <0.01 | 0.03 |
| TC (mmol/L) | 3.29 | 3.22 | 3.16 | 2.79 | 0.10 | 0.12 | 0.47 |
| VLDL-C (mmol/L) | 1.78b | 1.88b | 2.26a | 2.33a | 0.08 | <0.01 | 0.88 |
a-cMeans within a row with no common superscripts differ significantly (P < 0.05).
Abbreviations: HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride; VLDL-C, very-low-density lipoprotein cholesterol.
Table 7.
Effect of dietary astaxanthin (ASTA) addition on lipid metabolism in the liver.
| Items | ASTA levels/(mg/kg) |
SEM |
P value |
||||
|---|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 100 | Linear | Quadratic | ||
| HDL-C (mmol/gprot) | 0.024c | 0.032b | 0.039a | 0.033b | 0.0014 | <0.01 | <0.01 |
| LDL-C (mmol/gprot) | 0.0098 | 0.0089 | 0.0087 | 0.0089 | 0.0005 | 0.58 | 0.61 |
| TG (mmol/gprot) | 0.083 | 0.082 | 0.083 | 0.082 | 0.0013 | 0.91 | 0.98 |
| TC (mmol/gprot) | 0.044 | 0.046 | 0.048 | 0.045 | 0.0013 | 0.61 | 0.43 |
| VLDL-C (mmol/gprot) | 0.030 | 0.030 | 0.040 | 0.033 | 0.0036 | 0.57 | 0.66 |
a-cMeans within a row with no common superscripts differ significantly (P < 0.05).
Abbreviations: HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride; VLDL-C, very-low-density lipoprotein cholesterol.
ASTA Concentration in Egg Yolks
Carotenoids are transported from the intestinal mucosa through the lymphatic vessels to the blood, then to the liver, and finally transported by very-low-density lipoprotein (VLDL) from the liver to surrounding tissues (Tyssandier et al., 2002). In the present study, dietary ASTA levels linearly and quadratically affected the ASTA concentration in egg yolks (P < 0.05) relative to the control group (Table 8). As expected, the ASTA content gradually increased with increasing ASTA concentrations (from 25–100 mg/kg) in egg yolks (P < 0.05). The highest ASTA content in egg yolks was observed in the 100-mg/kg ASTA group at the end of week 6. It has been shown that hens can convert ASTA esters (ASTA in the algae is mainly in the monoester form.) from algae into free ASTA and deposit the free form in eggs (Holtin et al., 2009). Walker et al. (2012) reported that the egg yolk color reached a maximum on D8. Furthermore, the ASTA content in egg yolks peaked on D10. In addition, other studies have reported that carotenoids can be deposited in various tissues, including the skin, muscle, liver, and egg yolk (Leeson and Caston, 2004; Olson et al., 2008; Lee et al., 2010). The lutein content in egg yolks improved remarkably compared with controls when birds were fed 500 ppm of lutein. However, with an increase in dietary lutein content, the transfer efficiency of lutein into yolk was very low (Leeson and Caston, 2004). Dietary lycopene supplementation (20, 40, or 80 mg/kg) significantly improved the lycopene content in egg yolks (Sun et al., 2014). In the present study, the ASTA content in egg yolks in the ASTA groups was higher than that in the control group, and the highest level of ASTA enrichment was observed in the 100-mg/kg ASTA group at the end of week 6. Different results among studies may be explained by several factors, such as genetics, the concentration of ASTA used, and the experimental conditions.
Table 8.
Effect of dietary astaxanthin (ASTA) on the egg yolk ASTA content.
| Items | Time/week | ASTA levels/(mg/kg) |
SEM |
P value |
||||
|---|---|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 100 | Linear | Quadratic | |||
| ASTA content (mg/kg) | 2 | - | 3.69c | 11.13b | 34.95a | 2.91 | <0.01 | <0.01 |
| 4 | - | 8.07c | 17.56b | 37.47a | 3.06 | <0.01 | <0.01 | |
| 6 | - | 12.87c | 21.06b | 44.20a | 3.46 | <0.01 | <0.01 | |
a-cMeans within a row with no common superscripts differ significantly (P < 0.05).
Gene Expression of SCARB1 and VLDLR
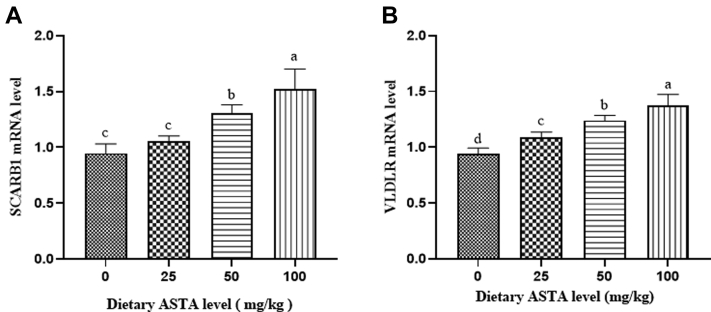
SCARB1 is a member of the class B family of scavenger receptors known to promote the formation of HDL particles and stimulate cholesterol outflow after it is combined with HDL (Acton et al., 1996). In the present study, with increasing dietary ASTA concentrations, SCARB1 mRNA levels in the liver increased gradually compared with those in the control (P < 0.05; Figure 1A). Compared with the control group, SCARB1 mRNA expression increased linearly in the 25 mg/kg to 100 mg/kg ASTA groups (P < 0.05). The SCARB1 mRNA level in the 100-mg/kg ASTA group was higher than that of the other groups (P < 0.05). A previous study showed that lutein is transported by HDL in the plasma (Wang et al., 2007). When the HDL content decreases, carotenoid levels also decrease significantly (Palozza et al., 2012). A severe deficiency in HDL results in low lutein concentrations in the plasma and other tissues in the Wisconsin hypoalpha mutant chicken (Connor et al., 2007).
Figure 1.
The effects of dietary astaxanthin (ASTA) supplementation on the mRNA expression of SCARB1 (A) and VLDLR (B) relative to that of β-actin (ACTB). The values are expressed as means ± SEM of 6 birds per treatment. Means without a common letter differ (P < 0.05).
The distribution of carotenoids in birds varies throughout different growth stages. In the early stages of growth, carotenoids are mainly distributed in the blood, liver, adipose tissue, skin, and feathers. During sexual maturity, carotenoids are gradually transferred to reproductive organs, such as the ovaries (Hansen et al., 2015; Sun et al., 2018). VLDLR, a key receptor in egg yolk deposition, can bind to VLDL and transport the yolk precursor into the oocyte (Nimpf et al., 1989). VLDLR plays an important role in the regulation of egg production, yolk weight, and egg quality (Qian et al., 2012). Triacylglycerols, cholesteryl esters, and free fatty acids are synthesized in the liver and assembled to form egg-yolk precursors, such as VLDL and vitellogenin particles, which are transferred to the developing oocyte as key substances for the growth and development of embryos (Li et al., 2015). In the present study, the increase in dietary ASTA from 0 to 100 mg/kg linearly enhanced the mRNA expression of VLDLR in the ovary (Figure 1B). A significant increase in VLDLR mRNA expression in the 25-, 50-, and 100-mg/kg ASTA groups was observed compared with that in the control group (P < 0.05). The present obtained results indicated that the mRNA expression levels of both SCARB1 and VLDLR were remarkably enhanced by adding ASTA to the diet. In addition, ASTA was enriched in egg yolks. Thus, we speculate that VLDL transports ASTA from the liver to the ovary, where it is bound and taken up by growing chicken oocytes via specific receptor (VLDLR)-mediated endocytosis and finally deposited in the egg yolk.
In summary, dietary ASTA improved the antioxidant capacity and lipid profile in laying hens. The accumulation of ASTA in egg yolks may be partly related to the key receptor for egg yolk deposition-VLDLR. However, the mechanism by which dietary ASTA improves the antioxidant enzyme activity and mRNA expression of SCARB1 and VLDLR needs to be further studied.
Acknowledgments
This study was supported by the Modern Agricultural Industry Technology System-Peking Poultry Innovation Team (BAIC04-2020), Beijing Municipal Education Commission Science and Technology Plan General Project (KM201710020012), and High-level Scientific Research Cultivation Project of BUA. The authors thank Louise Adam, ELS(D), and Melissa Crawford, PhD, from Liwen Bianji, Edanz Editing, China (www.liwenbianji.cn/ac), for editing the English text of a draft of this article.
Conflict of Interest Statement: The authors declare no conflicts of interest.
References
- Acton S., Rigotti A., Landschulz K.T., Xu S., Hobbs H.H., Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- Ambati R., Phang S., Ravi S., Aswathanarayana R. Astaxanthin: Sources, extraction, stability, Biological activities and its commercial applications—a review. Mar. Drugs. 2014;12:128–152. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold Von Eckardstein J.N.G.A. High density lipoproteins and Arteriosclerosis : role of cholesterol efflux and Reverse. Arteriosclerosis, Thromb. Vasc. Biol. 2001;21:13–27. doi: 10.1161/01.atv.21.1.13. [DOI] [PubMed] [Google Scholar]
- Azarabadi S., Abdollahi H., Torabi M., Salehi Z., Nasiri J. ROS generation, oxidative burst and dynamic expression profiles of ROS-scavenging enzymes of superoxide dismutase ( SOD ), catalase ( CAT ) and ascorbate peroxidase ( APX ) in response to Erwinia amylovora in pear ( Pyrus communis L) Eur. J. Plant Pathol. 2017;147:279–294. [Google Scholar]
- Barber D.L., Sanders E.J., Aebersold R., Schneider W.J. The receptor for yolk lipoprotein deposition in the chicken oocyte. J. Biol. Chem. 1991;266:18761–18770. [PubMed] [Google Scholar]
- Battin E.E., Brumaghim J.L. Antioxidant activity of Sulfur and Selenium: a review of reactive oxygen species scavenging, glutathione peroxidase, and Metal-binding antioxidant mechanisms. Cell Biochem Biophys. 2009;55:1–23. doi: 10.1007/s12013-009-9054-7. [DOI] [PubMed] [Google Scholar]
- Bera I., Tyagi P.K., Mir N.A., Tyagi P.K., Dev K., Sharma D., Mandal A.B. Dietary supplementation of saponins to improve the quality and oxidative stability of broiler chicken meat. J. Food Sci. Technology. 2019;56:2063–2072. doi: 10.1007/s13197-019-03683-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callie F., Mi-Bo K., Minkyung B., Yoojin L., Tho X P., Yue Y., Myung J.H., Young-Ki P., Ji-Young L. Astaxanthin exerts anti-inflammatory and antioxidant effects in macrophages in NRF2-dependent and independent manners. J. Nutr. Biochem. 2018;62:202–209. doi: 10.1016/j.jnutbio.2018.09.005. [DOI] [PubMed] [Google Scholar]
- Capelli B., Bagchi D., Cysewski G.R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods. 2013;12:145–152. [Google Scholar]
- Chen Y., Lee P., Wu Y., Liu L. In vivo effects of free form astaxanthin powder on anti-oxidation and lipid metabolism with high-cholesterol diet. PLoS One. 2015;10:e134733. doi: 10.1371/journal.pone.0134733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor W.E., Duell P.B., Kean R., Wang Y. The prime role of HDL to transport lutein into the retina: evidence from HDL-deficient WHAM chicks having a mutant ABCA1 transporter. Invest Ophth Vis. Sci. 2007;48:4226. doi: 10.1167/iovs.06-1275. [DOI] [PubMed] [Google Scholar]
- Dias I.H.K., Polidori M.C., Li L., Weber D., Stahl W., Nelles G., Grune T., Griffiths H.R. Plasma levels of HDL and carotenoids are lower in Dementia Patients with Vascular Comorbidities. J. Alzheimer's Dis. 2014;40:399–408. doi: 10.3233/JAD-131964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dose J., Matsugo S., Yokokawa H., Koshida Y., Okazaki S., Seidel U., Eggersdorfer M., Rimbach G., Esatbeyoglu T. Free radical scavenging and Cellular antioxidant properties of astaxanthin. Int. J. Mol. Sci. 2016;17:103. doi: 10.3390/ijms17010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du P., Jin M., Yang L., Chen G., Zhang C., Jin F., Shao H., Yang M., Yang X., She Y., Wang S., Zheng L., Wang J. Determination of astaxanthin in feeds using high performance liquid chromatography and an efficient extraction method. J. Liq Chromatogr. R. T. 2016;39:35–43. [Google Scholar]
- Feng Xue A.C.L.A. In vivo antioxidant activity of carotenoid powder from tomato byproduct and its use as a source of carotenoids for egg-laying hens. Food Funct. 2013:610–617. doi: 10.1039/c3fo30277f. [DOI] [PubMed] [Google Scholar]
- Fredriksson S., Elwinger K., Pickova J. Fatty acid and carotenoid composition of egg yolk as an effect of microalgae addition to feed formula for laying hens. Food Chem. 2006;99:530–537. [Google Scholar]
- Freeman B.A., Crapo J.D. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. Biol.Chem. 1981;256:10986. [PubMed] [Google Scholar]
- Gervasi T., Pellizzeri V., Benameur Q., Gervasi C., Santini A., Cicero N., Dugo G. Valorization of raw materials from agricultural industry for astaxanthin and beta-carotene production by Xanthophyllomyces dendrorhous. Nat. Prod. Res. 2018;32(13):1554–1561. doi: 10.1080/14786419.2017.1385024. [DOI] [PubMed] [Google Scholar]
- Gervasi T., Santini A., Daliu P., Salem A.Z.M., Gervasi C., Pellizzeri V., Barrega L., De Pasquale P., Dugo G., Cicero N. Astaxanthin production by Xanthophyllomyces dendrorhous growing on a low cost substrate. Agroforest Syst. 2019;94:1229–1234. [Google Scholar]
- Ghoreyshi S., Omri B., Chalghoumi R., Bouyeh M., Seidavi A., Dadashbeiki M., Lucarini M., Durazzo A., van den Hoven R., Santini A. Effects of dietary supplementation of L-carnitine and excess Lysine-Methionine on growth performance, Carcass characteristics, and Immunity Markers of broiler chicken. Animals. 2019;9:608. doi: 10.3390/ani9060362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreyshi S., Omri B., Chalghoumi R., Bouyeh M., Seidavi A., Dadashbeiki M., Lucarini M., Durazzo A., van den Hoven R., Santini A. Effects of dietary supplementation of L-carnitine and excess Lysine-Methionine on growth performance, Carcass characteristics, and Immunity Markers of broiler chicken. Animals. 2019;9:362. doi: 10.3390/ani9060362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goliomytis M., Tsoureki D., Simitzis P.E., Charismiadou M.A., Hager-Theodorides A.L., Deligeorgis S.G. The effects of quercetin dietary supplementation on broiler growth performance, meat quality, and oxidative stability. Poult. Sci. 2014;93:1957–1962. doi: 10.3382/ps.2013-03585. [DOI] [PubMed] [Google Scholar]
- Hansen H., Wang T., Dolde D., Xin H. Tocopherol and annatto tocotrienols distribution in laying-hen body. Poult. Sci. 2015;94:2421–2433. doi: 10.3382/ps/pev228. [DOI] [PubMed] [Google Scholar]
- Higuera-Ciapara I., Felix-Valenzuela L., Goycoolea F.M. Astaxanthin: a review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006;46:185–196. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- Hoekstra M., Sorci-Thomas M. Rediscovering scavenger receptor type BI. Curr. Opin. Lipidol. 2017;28:255–260. doi: 10.1097/MOL.0000000000000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtin K., Kuehnle M., Rehbein J., Schuler P., Nicholson G., Albert K. Determination of astaxanthin and astaxanthin esters in the microalgae Haematococcus pluvialis by LC-(APCI)MS and characterization of predominant carotenoid isomers by NMR spectroscopy. Anal Bioanal. Chem. 2009;395:1613–1622. doi: 10.1007/s00216-009-2837-2. [DOI] [PubMed] [Google Scholar]
- Iizuka M., Ayaori M., Uto-Kondo H., Yakushiji E., Takiguchi S., Nakaya K., Hisada T., Sasaki M., Komatsu T., Yogo M., Kishimoto Y., Kondo K., Ikewaki K. Astaxanthin enhances ATP-binding cassette transporter A1/G1 expressions and cholesterol efflux from macrophages. J. Nutr. Sci. Vitaminol (Tokyo) 2012;58:96–104. doi: 10.3177/jnsv.58.96. [DOI] [PubMed] [Google Scholar]
- Kamath B.S., Srikanta B.M., Dharmesh S.M., Sarada R., Ravishankar G.A. Ulcer preventive and antioxidative properties of astaxanthin from Haematococcus pluvialis. Eur. J. Pharmacol. 2008;590:387–395. doi: 10.1016/j.ejphar.2008.06.042. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y., Yoshida H., Kondo K. Potential anti-Atherosclerotic properties of astaxanthin. Mar. Drugs. 2016;14:35. doi: 10.3390/md14020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.Y., Lee B.D., Na J.C., An G. Carotenoid accumulation and their antioxidant activity in spent laying hens as affected by Polarity and feeding Period. Asian-Australas. J. Anim. Sci. 2010;23:799–805. [Google Scholar]
- Leeson S., Caston L. Enrichment of eggs with lutein. Poult. Sci. 2004;83:1709–1712. doi: 10.1093/ps/83.10.1709. [DOI] [PubMed] [Google Scholar]
- Li F., Huang S., Lu X., Wang J., Lin M., An Y., Wu S., Cai M. Effects of dietary supplementation with algal astaxanthin on growth, pigmentation, and antioxidant capacity of the blood parrot ( Cichlasoma citrinellum × Cichlasoma synspilum ) J. Oceanology Limnology. 2018;36:1851–1859. [Google Scholar]
- Li H., Wang T., Xu C., Wang D., Ren J., Li Y., Tian Y., Wang Y., Jiao Y., Kang X., Liu X. Transcriptome profile of liver at different physiological stages reveals potential mode for lipid metabolism in laying hens. Bmc Genomics. 2015;16:763. doi: 10.1186/s12864-015-1943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Wu W., Zhou P., Xie F., Zhou Q., Mai K. Comparison effect of dietary astaxanthin and Haematococcus pluvialis on growth performance, antioxidant status and immune response of large yellow croaker Pseudosciaena crocea. Aquaculture. 2014;434:227–232. [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Naguib Y.M.A. Antioxidant activities of astaxanthin and related carotenoids. J. Agr Food Chem. 2000;48:1150–1154. doi: 10.1021/jf991106k. [DOI] [PubMed] [Google Scholar]
- Nia Y., Lingyan M., Lianxin W., Tianqi Y., Jinlu J., Zeming W., Zhengwei Fua A.Y.J. Astaxanthin has a potential role in Antioxidation and oxidative damage Repair in UVC irradiated Mice1. Anim. Human Physiol. 2018;6:580–588. [Google Scholar]
- Nimpf J., Radosavljevic M.J., Schneider W.J. Oocytes from the restricted ovulator hen lack receptor for very low density lipoprotein. J. Biol. Chem. 1989;264:1393–1398. [PubMed] [Google Scholar]
- Olson K.R., Gao Y., Arif F., Arora K., Patel S., DeLeon E.R., Sutton T.R., Feelisch M., Cortese-Krott M.M., Straub K.D. Metabolism of hydrogen sulfide (H 2 S) and production of reactive Sulfur species (RSS) by superoxide dismutase. Redox Biol. 2018;15:74–85. doi: 10.1016/j.redox.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J.B., Ward N.E., Koutsos E.A. Lycopene Incorporation into egg yolk and effects on laying hen immune Function1. Poult. Sci. 2008;87:2573–2580. doi: 10.3382/ps.2008-00072. [DOI] [PubMed] [Google Scholar]
- Omri B., Larbi Manel B., Jihed Z., Durazzo A., Lucarini M., Romano R., Santini A., Abdouli H. Effect of a Combination of fenugreek seeds, linseeds, Garlic and copper sulfate on laying hens Performances, egg Physical and chemical Qualities. Foods. 2019;8:311. doi: 10.3390/foods8080311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omri B., Amraoui M., Tarek A., Lucarini M., Durazzo A., Cicero N., Santini A., Kamoun M. Arthrospira Platensis (spirulina) supplementation on laying hens' performance: eggs Physical, chemical, and Sensorial Qualities. Foods. 2019;8:386. doi: 10.3390/foods8090386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omri B., Alloui N., Durazzo A., Lucarini M., Aiello A., Romano R., Santini A., Abdouli H. Egg yolk antioxidants profiles: effect of diet supplementation with linseeds and tomato-Red Pepper mixture before and after Storage. Foods. 2019;8:320. doi: 10.3390/foods8080320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palozza P., Catalano A., Simone R.E., Mele M.C., Cittadini A. Effect of lycopene and tomato products on cholesterol metabolism. Ann. Nutr. Metab. 2012;61:126–134. doi: 10.1159/000342077. [DOI] [PubMed] [Google Scholar]
- Panaite T.D., Nour V., Vlaicu P.A., Ropota M., Corbu A.R., Saracila M. Flaxseed and dried tomato waste used together in laying hens diet. Arch. Anim. Nutr. 2019;73:222–238. doi: 10.1080/1745039X.2019.1586500. [DOI] [PubMed] [Google Scholar]
- Qian S., Lei Q.X., Cao D.G., Wang C.F. A review on effects of chicken yolk precursor and oocyte Vitellogenesis receptor on egg quality. Food Sci. 2012;33:260–263. [Google Scholar]
- Ritto D., Tanasawet S., Singkhorn S., Klaypradit W., Hutamekalin P., Tipmanee V., Sukketsiri W. Astaxanthin induces migration in human skin keratinocytes via Rac1 activation and RhoA inhibition. Nutr. Res. Pract. 2017;11:275–280. doi: 10.4162/nrp.2017.11.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert Scott Kiss A.S. Shunts, channels and lipoprotein endosomal traffic: a new model of cholesterol homeostasis in the hepatocyte. J. Biomed. Res. 2017;2:95–107. doi: 10.7555/JBR.31.20160139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajad Fakhri F.A.L.D., Jorjani M. Astaxanthin: a Mechanistic review on its Biological activities and Health benefits. Pharmacol. Res. 2018;136:1–20. doi: 10.1016/j.phrs.2018.08.012. [DOI] [PubMed] [Google Scholar]
- Sarada R., Tripathi U., Ravishankar G.A. Influence of stress on astaxanthin production in Haematococcus pluvialis grown under different culture conditions. Process. Biochem. 2002;37:623–627. [Google Scholar]
- Seifried H.E., Anderson D.E., Fisher E.I., Milner J.A. A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 2007;18:567–579. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Shao Y., Gu W., Jiang L., Zhu Y., Gong A. Study on the Visualization of pigment in Haematococcus pluvialis by Raman spectroscopy Technique. Sci. Rep-uk. 2019;9:1–9. doi: 10.1038/s41598-019-47208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K.N., Patil S., Barkate H. Protective effects of astaxanthin on skin: Recent scientific evidence, possible mechanisms, and potential indications. J. Cosmet. Dermatol-us. 2019;19:22–27. doi: 10.1111/jocd.13019. [DOI] [PubMed] [Google Scholar]
- Sun B., Ma J., Zhang J., Su L., Xie Q., Bi Y. Lycopene regulates production performance, antioxidant capacity, and biochemical parameters in breeding hens. Czech J. Anim. Sci. 2014;59:471–479. [Google Scholar]
- Sun T., Yin R., Magnuson A.D., Tolba S.A., Liu G., Lei X.G. Dose-Dependent enrichments and improved redox status in tissues of broiler chicks under Heat stress by dietary supplemental microalgal astaxanthin. J. Agr Food Chem. 2018;66:5521–5530. doi: 10.1021/acs.jafc.8b00860. [DOI] [PubMed] [Google Scholar]
- Trigatti B.L. SR-B1 and PDZK1. Curr. Opin. Lipidol. 2017;28:201–208. doi: 10.1097/MOL.0000000000000396. [DOI] [PubMed] [Google Scholar]
- Tyssandier V., Choubert G., Grolier P., Borel P. Carotenoids, mostly the xanthophylls, exchange between plasma lipoproteins. International journal for vitamin and nutrition research. Internationale Zeitschrift Fur Vitamin- und Ernahrungsforschung. J. Int. de vitaminologie de Nutr. 2002;72:300. doi: 10.1024/0300-9831.72.5.300. [DOI] [PubMed] [Google Scholar]
- Valko M., Leibfritz D., Moncol J., Cronin M.T.D., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Walker L.A., Wang T., Xin H., Dolde D. Supplementation of laying-hen feed with Palm Tocos and algae astaxanthin for egg yolk nutrient enrichment. J. Agr Food Chem. 2012;60:1989–1999. doi: 10.1021/jf204763f. [DOI] [PubMed] [Google Scholar]
- Wang W., Connor S.L., Johnson E.J., Klein M.L., Hughes S., Connor W.E. Effect of dietary lutein and zeaxanthin on plasma carotenoids and their transport in lipoproteins in age-related macular degeneration. Am. J. Clin. Nutr. 2007;85:762–769. doi: 10.1093/ajcn/85.3.762. [DOI] [PubMed] [Google Scholar]
- Qi X.L., Wu S.G., Zhang H.J., Yue H.Y., Xu S.H., Ji F., Qi G.H. Effects of dietary conjugated linoleic acids on lipid metabolism and antioxidant capacity in laying hens. Arch. Anim. Nutr. 2011;65:354–365. doi: 10.1080/1745039x.2011.617546. [DOI] [PubMed] [Google Scholar]
- Xu J., Rong S., Gao H., Chen C., Yang W., Deng Q., Huang Q., Xiao L., Huang F. A Combination of Flaxseed oil and astaxanthin improves hepatic lipid accumulation and reduces oxidative stress in high fat-diet fed rats. Nutrients. 2017;9:271. doi: 10.3390/nu9030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue H.Y., Wang J., Qi X.L., Ji F., Liu M.F., Wu S.G., Zhang H.J., Qi G.H. Effects of dietary oxidized oil on laying performance, lipid metabolism, and apolipoprotein gene expression in laying hens. Poult. Sci. 2011;90:1728–1736. doi: 10.3382/ps.2011-01354. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yang X., Zhang X., Xu Q., Liu Y. Effects of medium-chain fatty acids on high-density-lipoprotein in rats fed with high fat diet. J. Hygiene Research. 2018;47:123–127. [PubMed] [Google Scholar]
- Zheng H., Zhang Q., Liu H., Liu W., Sun Z., Li S., Zhang T. Cloning and expression of vitellogenin (Vg) gene and its correlations with total carotenoids content and total antioxidant capacity in noble scallop Chlamys nobilis (Bivalve: Pectinidae) Aquaculture. 2012;366-367:46–53. [Google Scholar]
- Zou T.B., Zhu S.S., Luo F., Li W.Q., Sun X.R., Wu H.F. Effects of astaxanthin on reverse cholesterol transport and atherosclerosis in mice. Biomed. Res. Int. 2017:4625932. doi: 10.1155/2017/4625932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuliani G., Cavalieri M., Galvani M., Volpato S., Cherubini A., Bandinelli S., Corsi A.M., Lauretani F., Guralnik J.M., Fellin R., Ferrucci L. Relationship between low levels of high-density lipoprotein cholesterol and Dementia in the Elderly. The InChianti study. Journals Gerontol. Ser. A: Biol. Sci. Med. Sci. 2010;65A:559–564. doi: 10.1093/gerona/glq026. [DOI] [PMC free article] [PubMed] [Google Scholar]