Abstract
Mercuric chloride (HgCl2) is a widely distributed environmental pollutant with multiorgan toxicity including immune organs such as spleen. Selenium (Se) is an essential trace element in animal nutrition and exerts biological activity to antagonize organ toxicity caused by heavy metals. The objective of this study was to explore the underlying mechanism of the protective effects of Se against spleen damage caused by HgCl2 in chicken. Ninety male Hyline brown chicken were randomly divided into 3 groups namely Cont, HgCl2, and HgCl2+Se group. Chicken were provided with the standard diet and nontreated water, standard diet and HgCl2-treated water (250 ppm), and sodium selenite-treated diet (10 ppm) plus HgCl2-treated water (250 ppm), respectively. After being fed for 7 wk, the spleen tissues were collected, and spleen index, the microstructure of the spleen, and the indicators of oxidative stress, inflammation, apoptosis as well as heat shock proteins (HSP) were detected. First, the results of spleen index and pathological examination confirmed that Se exerted an antagonistic effect on the spleen injury induced by HgCl2. Second, Se ameliorated HgCl2-induced oxidative stress by decreasing the level of malondialdehyde and increasing the levels of glutathione, glutathione peroxidase, and total antioxidant capacity. Third, Se attenuated HgCl2-induced inflammation by decreasing the protein expression of nuclear factor kappa-B, inducible nitric oxide synthase, and cyclooxygenase-2, and the gene expression of interleukin (IL)-1β, IL-6, IL-8, IL-12β, IL-18 as well as tumor necrosis factor-α. Fourth, Se inhibited HgCl2-induced apoptosis by downregulating the protein expression of BCL2 antagonist/killer 1 and upregulating the protein expression of B-cell lymphoma-2. Finally, Se reversed HgCl2-triggered activation of HSP 60, 70, and 90. In conclusion, Se antagonized HgCl2-induced spleen damage in chicken, partially through the regulation of oxidative stress, inflammatory, and apoptotic signaling.
Key words: mercuric chloride, selenium, oxidative stress, inflammation, apoptosis
Introduction
Mercury (Hg) is a potentially toxic element and ubiquitous environmental pollutant, which exists in different forms including Hg0, Hg+, Hg2+, and methylmercury (Driscoll et al., 2013). All forms of Hg produce a corresponding degree of organ toxicity in animals through ingestion, inhalation, and dermal contact, ultimately incur heavy threaten to human's health by the means of the enriched food chains. With the enhancement of human activities (e.g., batteries, thermometers, cosmetics, and pharmaceuticals.), the pollution of inorganic Hg, especially mercuric chloride (HgCl2), is becoming more and more serious. It is important to note that in ecosystems, HgCl2 is converted into organic Hg such as methylmercury, a compound that is considered as the most toxic Hg compound (Jonsson et al., 2014). Therefore, the preventions of tissue and organ damage caused by HgCl2 exposure have attracted considerable attention (Frisbie et al., 2015). Unfortunately, there is no effective antagonist that can antagonize the toxicity of HgCl2.
The accumulating evidence indicates that the biological toxicity of HgCl2 is characterized by multiple target organs, especially kidney, liver, brain, and immune organs such as spleen. Previous studies have shown that disorder of mitochondrial dynamics triggered oxidative stress and eventually resulted in apoptosis, which was involved in the nephrotoxicity caused by HgCl2 (Li et al., 2019). It was also reported that HgCl2-induced hepatotoxicity was closely related to oxidative stress and inflammation (Lee et al., 2014). In addition, prior study indicated that oxidative stress and disruption of intracellular calcium homeostasis contributed to HgCl2-mediated neurotoxicity (Gasso et al., 2001). The spleen, as the most important peripheral immune organ, is susceptible to toxicity caused by heavy metals including cadmium (Cd) (Qu et al., 2019), lead (Pb) (Corsetti et al., 2017), and arsenic (As) (Soria et al., 2017). More recently, it has become evident that disruption of the antioxidant system, apoptosis, and autophagy may play an important role in the spleen and splenic lymphocyte toxicity induced by Cd (Chen et al., 2018) and Pb (Zhao and Zhang, 2018) exposure in chicken. The information of mechanism involved in Hg-induced organ toxicity is growing, but knowledge gaps still exist in which spleen injury caused by HgCl2 exposure in chicken.
Selenium (Se) is an essential trace element for humans and animals, with important functions in antioxidant defense (Zhang et al., 2011), detoxification, and enhancement of immunity. Previous studies have shown that Se deficiency caused organ and tissue damage in chicken, and the main mechanisms included oxidative stress (Yang et al., 2017), inflammatory response (Wang et al., 2018a), autophagy (Khoso et al., 2017), and apoptosis (Wan et al., 2019). In view of its unique biological functions, Se has become a candidate for antagonizing the toxicity of heavy metals in recent years. A series of recent studies in chicken have shown that Se regulated oxidative stress, inflammation, apoptosis, and autophagy signaling pathway to antagonize heavy metals–induced damage of kidney (Jin et al., 2017), liver (Cong et al., 2019), and pancreas (Jin et al., 2018). Moreover, in vitro experiments also reported that Se can antagonize spleen lymphocyte toxicity induced by Cd (Liu et al., 2014) and Pb (Zhao and Zhang, 2018). However, the specific mechanism of chicken splenic injury induced by HgCl2 and the availability of Se as an effective antagonist are unknown.
Therefore, we established an in vitro model of Se alleviating spleen injury caused by HgCl2 exposure and revealed its underlying molecular mechanism from the aspects of oxidative stress, inflammation, and apoptosis.
Materials and methods
Experimental Protocol and Sample Collection
All animal experiments were in accordance with the guidelines for the Care and Use of Laboratory Animals in Shandong Agricultural University (NO:SDAUA-2019-057). All chemicals provided were of the highest-grade purity. Mercuric chloride and sodium selenite were purchased from Sigma-Aldrich (Carlsbad, CA). Ninety male Hyline brown chicken (1-day-old, Dongyuezhongqin Co. Ltd., Tai'an, China) were randomly divided into 3 groups (30 chicken per group and 3 replicates per group). After acclimatization for 1 wk, Cont, HgCl2, and HgCl2+Se group chicken were provided with the standard diet and nontreated water, standard diet and HgCl2-treated water (250 ppm), and sodium selenite-treated diet (10 ppm) plus HgCl2-treated water (250 ppm), respectively. After being fed for 7 wk, following euthanasia with sodium pentobarbital and weighed, the chicken were killed, and spleen tissues were quickly removed. The spleen index was calculated according to the formula, spleen index = wt of spleen (g)/body wt (g) × 100. After rinsed with ice-cold sterile deionized water, the partial spleen tissues were ground into homogenates, and another partial spleen tissues were fixed in 4% formaldehyde solution. All the remaining spleen tissues were stored at -80°C until needed.
Hematoxylin and Eosin Staining
The spleen tissues taken from 4% formaldehyde solution were embedded in paraffin. Then, the paraffin sections were deparaffinized in xylene and rehydrated in a gradient of high percentage ethanol (from 70 to 100%). After that, sections were stained in hematoxylin and differentiated with 1% HCl in 70% alcohol. After rinsing with tap water, the sections were stained with eosin, followed by dehydration and differentiation similar to that described above. Finally, the sections were cleaned with xylene and mounted with neutral resin. To observe the histopathological changes of the spleen, sections were examined under a light microscope (Nikon Eclipse E600 W, Suzhou, China).
Detection of Redox State in Spleen Tissues
The freshly collected spleen tissues were ground into homogenates to measure the levels of glutathione (GSH), glutathione peroxidase (GSH-Px), total antioxidant capacity (T-AOC), and malondialdehyde (MDA) using commercial assay kits, which were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China), according with the operating instructions, respectively (Jiang et al., 2011).
RNA Isolation and Quantitative Real-Time PCR
The total RNA was extracted from spleen tissues using Trizol reagent (Invitrogen, CA) according to the manufacturer's instructions. The dried RNA pellets were resuspended in 50 μL of diethyl-pyrocarbonate treated water. The concentration and purity of the total RNA were determined spectrophotometrically at 260/280 nm. First-strand complementary DNA (cDNA) was synthesized from 5 μg of total RNA using oligo dT primers and Superscript II reverse transcriptase according to the manufacturer's instructions (Invitrogen). β-actin was used as an internal control, and the primer sequences were detailed in Table 1. The relative expression levels of mRNA were evaluated by the 2−ΔΔCt method (Luan et al., 2016).
Table 1.
The primers used in the present study.
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| IL-1β | TTCATCTTCTACCGCCTGGACAG | GCTTGTAGGTGGCGATGTTGA |
| IL-6 | TTCACCGTGTGCGAGAACAGC | CAGCCGTCCTCCTCCGTCAC |
| IL-8 | GGTATGGCTCAGGCACGTTCAG | CATTCTTGCAGTGAGGTCCGCTAG |
| IL-12β | ACTTTCCTTTGCTGCCCTTCTGG | GCTGGTGTCTCATCGTTCCACTC |
| IL-18 | AGATGATGAGCTGGAATGCGATGC | ATCTGGACGAACCACAAGCAACTG |
| TNF-α | CCCAGTTCAGATGAGTTGCCCTTC | GCCACCACACGACAGCCAAG |
| β-actin | CCAGCCATGTATGTAGCCATCCAG | ACGGCCAGCCAGATCCAGAC |
Western Blotting
Spleen tissue protein extracts were subjected to SDS-polyacrylamide gel electrophoresis under reducing conditions on 15% gels. Separated proteins were then transferred to nitrocellulose membranes using tank transfer for 1 h at 100 mA in tris-glycine buffer containing 20% methanol. The membranes were blocked with 5% skim milk for 16 to 24 h and incubated overnight with diluted primary antibody against nuclear factor kappa-B (NF-κB) (1 : 1000 dilution, Servicebio, Wuhan, China), inducible nitric oxide synthase (iNOS) (1 : 1000 dilution, Servicebio), cyclooxygenase-2 (COX-2) (1 : 1000 dilution, Abclonal, Wuhan, China), BCL2 antagonist/killer 1 (Bak1) (1 : 1000 dilution, Abclonal), B-cell lymphoma-2 (Bcl-2) (1 : 1000 dilution, Abclonal), heat shock protein (HSP) 60 (1 : 1000 dilution, Abclonal), HSP70 (1 : 3000 dilution, Abclonal), and HSP90 (1 : 1000 dilution, Abclonal), followed by incubating with a horseradish peroxidase–conjugated secondary antibody against rabbit IgG (1 : 10,000 dilution, Abclonal). To verify equal sample loading, the membrane was incubated with a monoclonal β-actin antibody (1 : 1000 dilution, Abclonal), followed by incubating with a horseradish peroxidase–conjugated goat anti-mouse IgG (1 : 10,000 dilution, Abclonal). The signal was detected using an enhanced chemiluminescence system (Cheml Scope5300, Clinx Science Instruments, Shanghai, China). The optical density of each band was determined using the Image VCD gel imaging system.
Statistical Analysis
Experimental groups were compared using a two-way analysis of variance followed by Scheffe's test when data were normally distributed and by the Kruskal-Wallis test when data were not normally distributed. All data were expressed as mean ± SEM. The statistical significance was evaluated using SPSS 22.0 (SPSS Inc., Chicago, IL), and P < 0.05 was regarded as statistically significant.
Results
Se Protects Against Spleen Damage Caused by HgCl2 Exposure
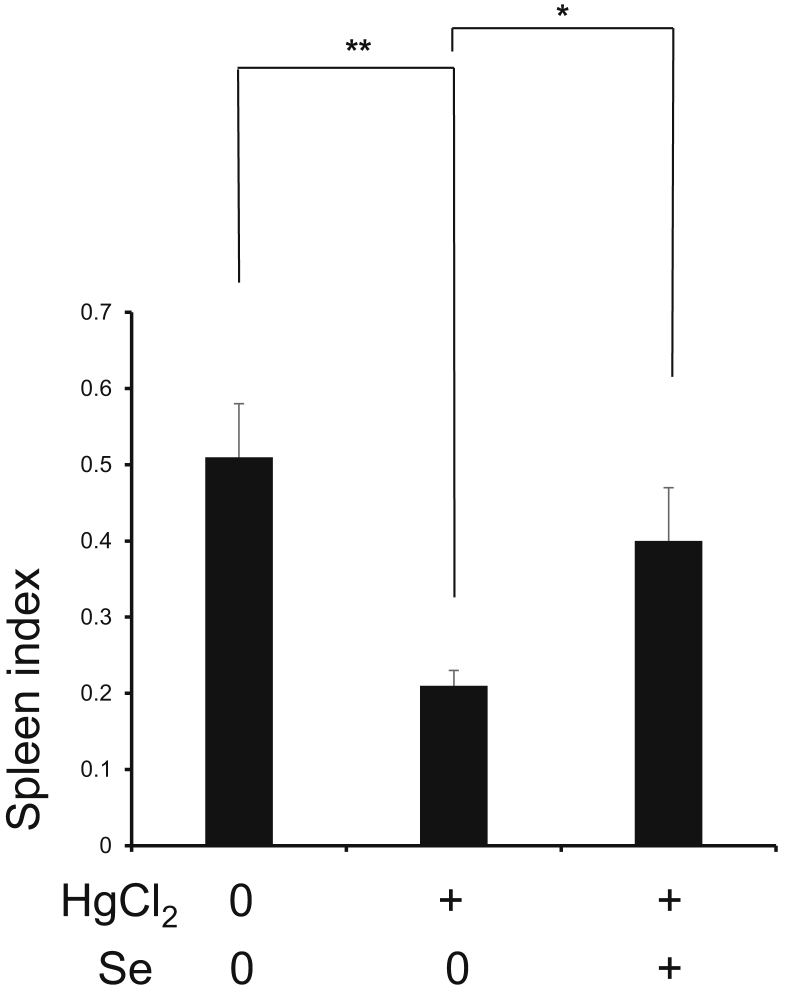
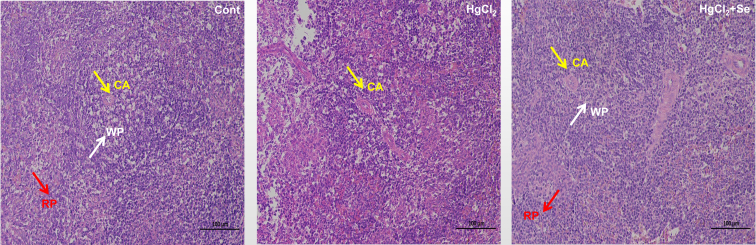
To evaluate the potential protective effects of Se against HgCl2-induced spleen injury, we evaluated the spleen index and the histopathological changes of spleen tissues. We found that spleen index was significantly decreased following HgCl2 exposure compared with Cont group (P < 0.01), and Se significantly reversed this alteration (P < 0.05) (Figure 1). Further analysis of histopathological changes (Figure 2) showed that in Cont group, the lymphocytes were arranged neatly, the boundary between white pith and red pith was clear, and the morphology of central artery was normal. However, HgCl2 exposure resulted in a decrease in the number and disorganization of lymphocytes, a blurring of boundaries between the white pith and red pith, and a thickening of the central artery. At the same time, the above pathological phenomena were improved to some extent in HgCl2+Se group. Therefore, the results confirmed that Se had significant protective effects on HgCl2-induced spleen damage.
Figure 1.
Effect of selenium on the spleen index exposed to mercuric chloride. Data were expressed as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01. Abbreviations: HgCl2, mercuric chloride; Se, selenium.
Figure 2.
Effect of selenium on the microstructure of spleen tissues exposed to mercuric chloride. HE staining and light microscopy were used to observe histomorphological changes of the spleen tissues (magnification: 200 × ). Abbreviations: CA, central artery; HgCl2, mercuric chloride; RP, red pith; Se, selenium; WP, white pith.
Se Ameliorates HgCl2-Induced Oxidative Stress
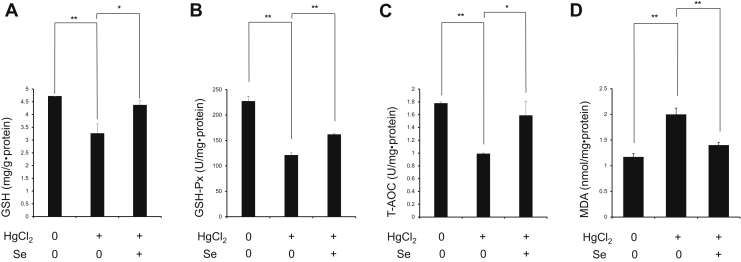
Next, we investigated whether Se can alleviate the spleen damage caused by HgCl2 via relieving oxidative stress (Figure 3). The results showed that HgCl2 exposure significantly decreased the level of GSH, GSH-Px, and T-AOC (P < 0.01); however, it significantly increased the level of MDA (P < 0.01) in spleen tissues compared with Cont group. The results indicated oxidative stress induced by HgCl2 exposure in spleen tissues. Meanwhile, the result also showed that Se significantly increased the level of GSH (P < 0.05), GSH-Px (P < 0.01) as well as T-AOC (P < 0.05) and significantly decreased the level of MDA (P < 0.01) in spleen tissues caused by HgCl2 exposure. Therefore, these results clarified Se ameliorated HgCl2-induced oxidative stress in spleen tissues.
Figure 3.
Effect of selenium on the levels of GSH (A), GSH-PX (B), T-AOC (C), and MDA (D) in spleen tissues exposed to mercuric chloride. Data were expressed as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01. Abbreviations: GSH, glutathione; GSH-Px, glutathion peroxidase; HgCl2, mercuric chloride; MDA, malondialdehyde; Se, selenium; T-AOC, total antioxidant capacity.
Se Attenuates HgCl2-Induced Inflammation
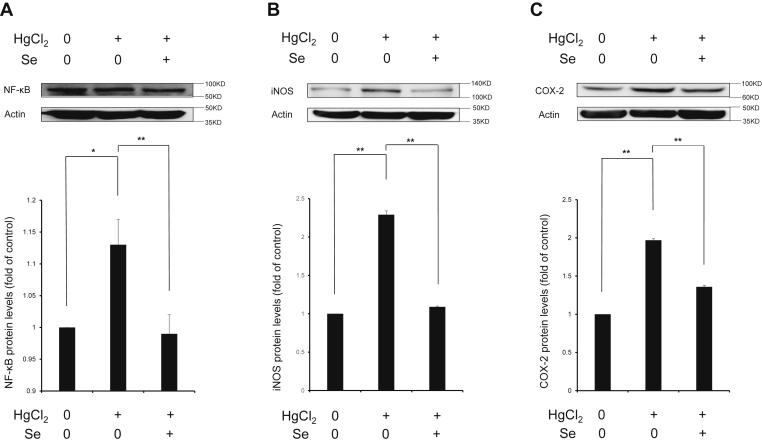
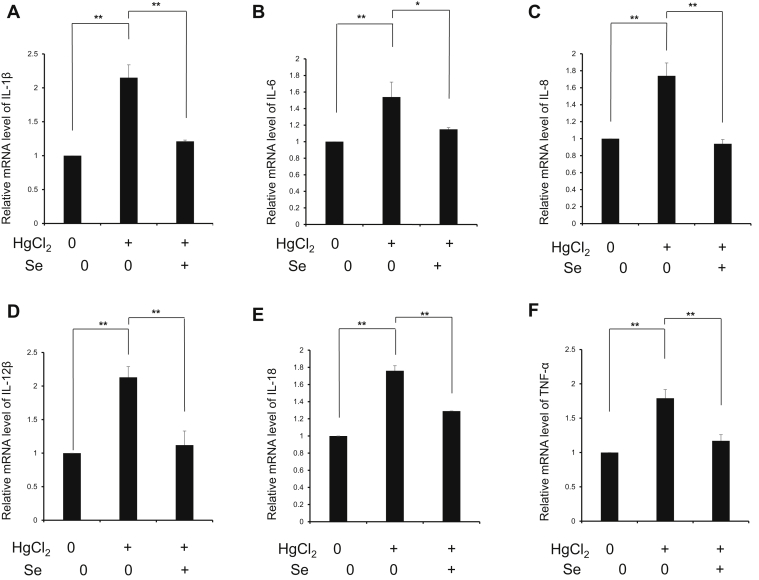
Intermediate oxidative stress mediates inflammatory response, which contributes to further organ damage. Thus, we investigated the effects of Se on spleen injury caused by HgCl2 from the perspective of inflammation. As shown in Figure 4, HgCl2 exposure upregulates the protein expression of NF-κB (P < 0.05), iNOS (P < 0.01), and COX-2 (P < 0.01), which indicates that HgCl2 exposure-induced inflammation though activating NF-κB signaling pathway. Moreover, the results also showed that HgCl2 exposure significantly upregulated the gene expression level of pro-inflammatory cytokines including interleukin (IL)-1β, IL-6, IL-8, IL-12β, IL-18, and tumor necrosis factor (TNF)-α (P < 0.01) (Figure 5). Meanwhile, Se significantly decreased the protein expression of NF-κB (P < 0.01), iNOS (P < 0.01), COX-2 (P < 0.01), and the gene expression levels of IL-1β (P < 0.01), IL-6 (P < 0.05), IL-8 (P < 0.01), IL-12β (P < 0.01), IL-18 (P < 0.01), and TNF-α (P < 0.01). The results indicated that Se attenuated splenic inflammatory response induced by HgCl2 by regulating NF-κB signaling pathway.
Figure 4.
Effect of selenium on the protein expressions of NF-κB (A), iNOS (B), and COX-2 (C) in spleen tissues exposed to mercuric chloride. Data were expressed as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01. Abbreviations: COX-2, cyclooxygenase-2; HgCl2, mercuric chloride; iNOS, inducible nitric oxide synthase; NF-κB, nuclear factor kappa-B; Se, selenium.
Figure 5.
Effect of selenium on the gene expressions of IL-1β (A), IL-6 (B), IL-8 (C), IL-12β (D), IL-18 (E), and TNF-α (F) in spleen tissues exposed to mercuric chloride. Data were expressed as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01. Abbreviations: HgCl2, mercuric chloride; IL, interleukin; Se, selenium; TNF-α, tumor necrosis factor-α.
Se Inhibits HgCl2-Induced Apoptosis
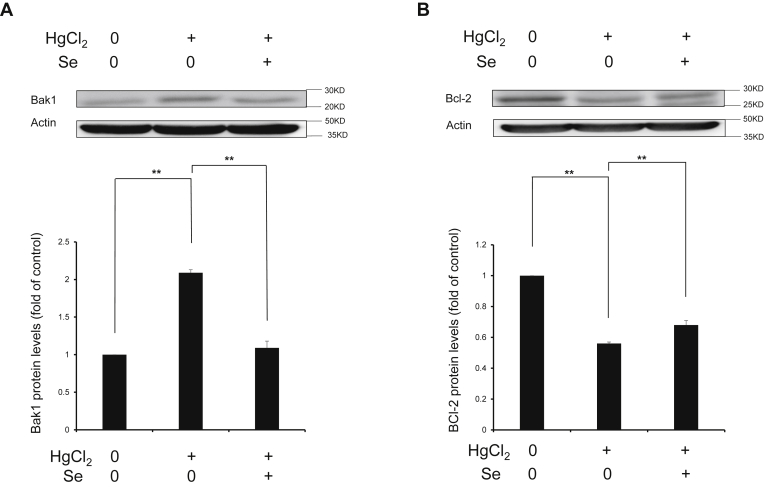
Apoptosis is a common way of death in organ toxicity caused by heavy metals and is closely related to persistent oxidative stress and inflammation. Thus, we examined the expression of apoptotic marker proteins to investigate the antagonistic effects of Se on HgCl2-induced apoptosis in spleen tissues. As shown in Figure 6, HgCl2 significantly elevates Bak1 protein expression (P < 0.01) and significantly declines Bcl-2 protein expression (P < 0.01); however, Se significantly alters these changes (P < 0.01). The results indicated that Se inhibited apoptosis caused by HgCl2 exposure in spleen tissues.
Figure 6.
Effect of selenium on the protein expressions of Bak1 (A) and Bcl-2 (B) in spleen tissues exposed to mercuric chloride. Data were expressed as mean ± SEM. ∗∗P < 0.01. Abbreviations: Bak1, BCL2 antagonist/killer 1; Bcl-2, B-cell lymphoma-2; HgCl2, mercuric chloride; Se, selenium.
Se Reverses HgCl2-Triggered Activation of HSPs
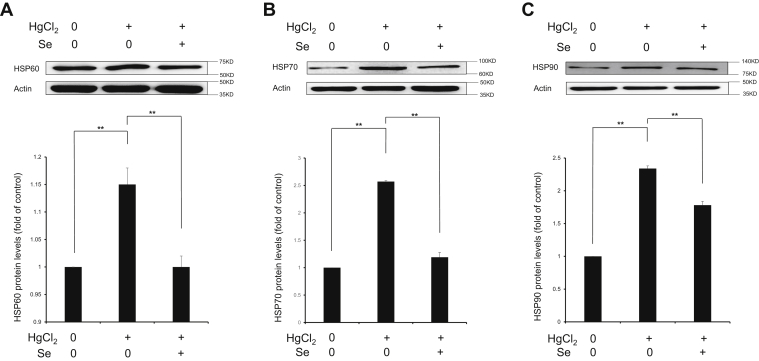
As molecular chaperones, HSPs activation protects cells from adverse stress including oxidative stress, inflammation, and apoptosis. As shown in Figure 7, upregulation of protein expression of HSP60, 70, and 90 (P < 0.01) indicates the splenic toxicity induced by HgCl2 exposure. Meanwhile, Se can significantly inhibit the upregulation of HSP expression induced by HgCl2 exposure (P < 0.01). The results indicated that Se reversed HgCl2-triggered activation of HSPs in spleen tissues.
Figure 7.
Effect of selenium on the protein expressions of HSP60 (A), HSP70 (B), and HSP90 (C) in spleen tissues exposed to mercuric chloride. Data were expressed as mean ± SEM. ∗∗P < 0.01. Abbreviations: HgCl2, mercuric chloride; HSP, heat shock protein; Se, selenium.
Discussion
Human activities have led to widespread contamination of HgCl2, which is ultimately a major threat to human health. It is urgent to find effective antagonistic substances against HgCl2 toxicity. Emerging evidence has shown that Se can antagonize organ damage (e.g., nephrotoxicity, hepatotoxicity, and neurotoxicity) in chicken caused by heavy metals such as Cd and Pb. Hence, one can hypothesize that it can relieve the spleen damage caused by HgCl2. In the present study, the results of spleen index and spleen histopathological confirmed that Se had antagonistic effect on the spleen injury induced by HgCl2, and then, we revealed the specific molecular mechanism from 3 aspects of oxidative stress, inflammation, and apoptosis.
Oxidative stress has always been regarded as the key event of organ toxicity caused by heavy metals (Wang et al., 2019a; Wang et al., 2020b). Prior study indicated that oxidative stress-mediated autophagy inhibition and apoptosis involved in the spleen injury induced by Cd in rat (Qu et al., 2019). Ma et al. demonstrated that disruption of the Nrf2-Keap1 signaling pathway, which is closely related to oxidative stress, is the main cause of ovarian dysfunction induced by HgCl2 (Ma et al., 2018b). Antioxidant properties, the most fundamental property of Se, are also the main reason why it can be used as a toxic antagonist to heavy metals. In recent years, there have been numerous reports on Se mitigated oxidative stress to relieve marine and freshwater fish toxicity caused by Hg exposure (Khadra et al., 2019; McCormack et al., 2020; Melgar et al., 2019). Therefore, in this study, we firstly focused on the redox state in spleen tissues treatment with HgCl2 and/or Se to investigate the mechanism of Se antagonizing the spleen damage caused by HgCl2 exposure. As expected, HgCl2 treatment significantly increased the level of MDA and decreased the level of GSH, GSHPX, and T-AOC, which indicated that HgCl2-induced oxidative stress in spleen tissues. The results are similar to the previous study in which oxidative stress trigged by HgCl2 exposure in chicken liver and kidney (Ma et al., 2018a). Meanwhile, Se antagonized the oxidative damage caused by HgCl2 by reversing the changes of relevant indicators of oxidative stress in spleen tissues.
Inflammatory response is an important mechanism of Hg toxicity (Gardner et al., 2009; Kempuraj et al., 2010; Pollard et al., 2019) and even carcinogenesis (Zefferino et al., 2017). In addition, specific substances with antioxidant activity can effectively alleviate the inflammatory response to antagonize the organ toxicity caused by HgCl2 (Elblehi et al., 2019; Liu et al., 2018; Toomey et al., 2014). It is important to note that certain signaling pathways (Almeer et al., 2019) such as the NF-κB pathway (Jiang et al., 2018) play a key role in the process of antioxidants against inflammation. As an important transcription factor, NF-κB serves as an important bridge between oxidative stress and inflammation (Hu et al., 2019). After responding to oxidative stress, NF-κB promotes the expression of iNOS and the subsequent production of NO, which further triggers the NF-κB signaling pathway, leading to the release of specific inflammatory factors. In the present study, we intended to investigate whether exposure to HgCl2 induces spleen inflammation and the inhibitory mechanism of Se on this process from aspect of NF-κB signaling pathway. In agreement with other investigators, the data presented here showed that HgCl2 activated NF-κB signaling pathway and promoted the release of proinflammatory cytokines including IL-1β, IL-6, IL-8, IL-12β, IL-18, and TNF-α. The antioxidant activity of Se indicates that it also has potential anti-inflammatory effects, which actually has been confirmed by some previous studies. Tang et al. demonstrated that Se alleviated Cd-induced inflammation in chicken breast muscles (Tang et al., 2019). It was also reported that Se can effectively mitigate the reproductive dysfunction caused by As though antagonizing inflammation in male rat (Adedara et al., 2019). Notably, Se exerts its anti-inflammatory activity by regulating NF-κB signaling pathway (Bi et al., 2016; Zhu et al., 2016; Zhu et al., 2017) and release of proinflammatory cytokines (Zeng et al., 2009). Similar to these findings, our data showed that Se inhibited NF-κB signaling pathway and thus ameliorated the splenic inflammatory response induced by HgCl2.
Apoptosis is the outcome of heavy metal toxicity (Chu et al., 2018; Wang et al., 2017; Zhao et al., 2019). More importantly, oxidative stress and NF-κB signaling pathway are common causes of apoptosis (Wang et al., 2019b; Wang et al., 2018b). This study has confirmed that oxidative stress and inflammatory responses in spleen were caused by HgCl2 exposure, which promoted us to investigate whether HgCl2 results in apoptosis in spleen tissues. Therefore, expression of 2 apoptotic marker proteins was detected in this study. The results showed exposure to HgCl2 significantly upregulated Bak1 protein expression and downregulated Bcl-2 protein expression, which indicated that apoptosis in spleen tissues caused by HgCl2 exposure. In addition, prior study also indicated that Se inhibited apoptosis to protect pancreas (Jin et al., 2018), ovary (Wan et al., 2018), and kidney (Jin et al., 2017) from heavy metal toxicity in chicken. Similar to these findings, the data in this study indicated that Se inhibited apoptosis induced by HgCl2. Cell exposed to specific stresses such as heavy metals, oxidative stress, inflammation, and apoptosis can lead to the expression of an induced HSPs, which act as a molecular chaperone or protease. Resultantly, the activation of HSPs is often considered a marker of an adverse stimulation of the cell (An et al., 2019). In particular, recent studies in chicken have shown that heavy metals can activate HSPs in specific organs including skeletal muscle (Zhao et al., 2018), rectum (Wang et al., 2018c; Yang et al., 2020), and testis (Shao et al., 2018). Our results showed that exposure to HgCl2 activated the protein expression of HSP60, 70, and 90. The results were consistent with previous findings that oxidative stress, inflammation, and apoptosis induced by HgCl2 in the spleen tissues. Also, the results indicated that Se can effectively interfere with the activation of HSPs induced by HgCl2. These findings are compatible with the result that Se can significantly inhibit the activation of HSPs induced by heavy metals in the liver (Wang et al., 2020a) and neutrophils (Xing et al., 2018) of chickens.
In conclusion, we proposed an intriguing mechanism of HgCl2-induced spleen damage. Most importantly, our study revealed that the protective effect of Se is mediated by oxidative stress, inflammation, and apoptosis. Taken together, our findings provided new insight and pharmacological evidence for the application of Se as an effective antagonist for HgCl2-induced organ damage in chicken.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NO. 31902330, NO. 31873030); Natural Science Foundation of Shandong Province (NO. ZR2019MC068); Doctoral Foundation of Shandong Province (NO. ZR2018BC048); and Funds of Shandong ‘‘Double Tops’’ Program.
Conflicts of Interest Statement: The authors declare that they have no conflicts of interest statement.
References
- Adedara I.A., Adebowale A.A., Atanda O.E., Fabunmi A.T., Ayenitaju A.C., Rocha J.B.T., Farombi E.O. Selenium abates reproductive dysfunction via attenuation of biometal accumulation, oxido-inflammatory stress and caspase-3 activation in male rats exposed to arsenic. Environ. Pollut. 2019;254:113079. doi: 10.1016/j.envpol.2019.113079. [DOI] [PubMed] [Google Scholar]
- Almeer R.S., Albasher G., Alotibi F., Alarifi S., Ali D., Alkahtani S. Ziziphus spina-christi Leaf extract Suppressed mercury chloride-induced nephrotoxicity via Nrf2-antioxidant pathway activation and inhibition of inflammatory and apoptotic signaling. Oxid Med. Cell Longev. 2019;2019:5634685. doi: 10.1155/2019/5634685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An Y., Xing H., Zhang Y., Jia P., Gu X., Teng X. The evaluation of potential immunotoxicity induced by environmental pollutant ammonia in broilers. Poult. Sci. 2019;98:3165–3175. doi: 10.3382/ps/pez135. [DOI] [PubMed] [Google Scholar]
- Bi C.L., Wang H., Wang Y.J., Sun J., Dong J.S., Meng X., Li J.J. Selenium inhibits Staphylococcus aureus-induced inflammation by suppressing the activation of the NF-kappaB and MAPK signalling pathways in RAW264.7 macrophages. Eur. J. Pharmacol. 2016;780:159–165. doi: 10.1016/j.ejphar.2016.03.044. [DOI] [PubMed] [Google Scholar]
- Chen M., Li X., Fan R., Yang J., Jin X., Hamid S., Xu S. Cadmium induces BNIP3-dependent autophagy in chicken spleen by modulating miR-33-AMPK axis. Chemosphere. 2018;194:396–402. doi: 10.1016/j.chemosphere.2017.12.026. [DOI] [PubMed] [Google Scholar]
- Chu B.X., Fan R.F., Lin S.Q., Yang D.B., Wang Z.Y., Wang L. Interplay between autophagy and apoptosis in lead(II)-induced cytotoxicity of primary rat proximal tubular cells. J. Inorg. Biochem. 2018;182:184–193. doi: 10.1016/j.jinorgbio.2018.02.015. [DOI] [PubMed] [Google Scholar]
- Cong Y., Chi Q., Teng X., Li S. The protection of selenium against cadmium-induced mitochondrial damage via the Cytochrome P450 in the livers of chicken. Biol. Trace Elem. Res. 2019;190:484–492. doi: 10.1007/s12011-018-1557-x. [DOI] [PubMed] [Google Scholar]
- Corsetti G., Romano C., Stacchiotti A., Pasini E., Dioguardi F.S. Endoplasmic Reticulum stress and apoptosis triggered by Sub-Chronic lead exposure in mice spleen: a histopathological study. Biol. Trace Elem. Res. 2017;178:86–97. doi: 10.1007/s12011-016-0912-z. [DOI] [PubMed] [Google Scholar]
- Driscoll C.T., Mason R.P., Chan H.M., Jacob D.J., Pirrone N. Mercury as a global pollutant: sources, pathways, and effects. Environ. Sci. Technol. 2013;47:4967–4983. doi: 10.1021/es305071v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elblehi S.S., Hafez M.H., El-Sayed Y.S. L-alpha-Phosphatidylcholine attenuates mercury-induced hepato-renal damage through suppressing oxidative stress and inflammation. Environ. Sci. Pollut. Res. Int. 2019;26:9333–9342. doi: 10.1007/s11356-019-04395-9. [DOI] [PubMed] [Google Scholar]
- Frisbie S.H., Mitchell E.J., Sarkar B. Urgent need to reevaluate the latest World Health Organization guidelines for toxic inorganic substances in drinking water. Environ. Health. 2015;14:63. doi: 10.1186/s12940-015-0050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner R.M., Nyland J.F., Evans S.L., Wang S.B., Doyle K.M., Crainiceanu C.M., Silbergeld E.K. Mercury induces an unopposed inflammatory response in human peripheral blood mononuclear cells in vitro. Environ. Health Perspect. 2009;117:1932–1938. doi: 10.1289/ehp.0900855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasso S., Cristofol R.M., Selema G., Rosa R., Rodriguez-Farre E., Sanfeliu C. Antioxidant compounds and Ca(2+) pathway blockers differentially protect against methylmercury and mercuric chloride neurotoxicity. J. Neurosci. Res. 2001;66:135–145. doi: 10.1002/jnr.1205. [DOI] [PubMed] [Google Scholar]
- Hu X., Chi Q., Liu Q., Wang D., Zhang Y., Li S. Atmospheric H2S triggers immune damage by activating the TLR-7/MyD88/NF-kappaB pathway and NLRP3 inflammasome in broiler thymus. Chemosphere. 2019;237:124427. doi: 10.1016/j.chemosphere.2019.124427. [DOI] [PubMed] [Google Scholar]
- Jiang S.Z., Yang Z.B., Yang W.R., Gao J., Liu F.X., Broomhead J., Chi F. Effects of purified zearalenone on growth performance, organ size, serum metabolites, and oxidative stress in postweaning gilts. J. Anim. Sci. 2011;89:3008–3015. doi: 10.2527/jas.2010-3658. [DOI] [PubMed] [Google Scholar]
- Jiang X., Gu S., Liu D., Zhao L., Xia S., He X., Chen H., Ge J. Lactobacillus brevis 23017 relieves mercury toxicity in the Colon by Modulation of oxidative stress and inflammation through the Interplay of MAPK and NF-kappaB signaling Cascades. Front Microbiol. 2018;9:2425. doi: 10.3389/fmicb.2018.02425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Jia T., Liu R., Xu S. The antagonistic effect of selenium on cadmium-induced apoptosis via PPAR-gamma/PI3K/Akt pathway in chicken pancreas. J. Hazard Mater. 2018;357:355–362. doi: 10.1016/j.jhazmat.2018.06.003. [DOI] [PubMed] [Google Scholar]
- Jin X., Xu Z., Zhao X., Chen M., Xu S. The antagonistic effect of selenium on lead-induced apoptosis via mitochondrial dynamics pathway in the chicken kidney. Chemosphere. 2017;180:259–266. doi: 10.1016/j.chemosphere.2017.03.130. [DOI] [PubMed] [Google Scholar]
- Jonsson S., Skyllberg U., Nilsson M.B., Lundberg E., Andersson A., Bjorn E. Differentiated availability of geochemical mercury pools controls methylmercury levels in estuarine sediment and biota. Nat. Commun. 2014;5:4624. doi: 10.1038/ncomms5624. [DOI] [PubMed] [Google Scholar]
- Kempuraj D., Asadi S., Zhang B., Manola A., Hogan J., Peterson E., Theoharides T.C. Mercury induces inflammatory mediator release from human mast cells. J. Neuroinflammation. 2010;7:20. doi: 10.1186/1742-2094-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadra M., Planas D., Brodeur P., Amyot M. Mercury and selenium distribution in key tissues and early life stages of Yellow Perch (Perca flavescens) Environ. Pollut. 2019;254:112963. doi: 10.1016/j.envpol.2019.112963. [DOI] [PubMed] [Google Scholar]
- Khoso P.A., Pan T., Wan N., Yang Z., Liu C., Li S. Selenium deficiency induces autophagy in immune organs of chickens. Biol. Trace Elem. Res. 2017;177:159–168. doi: 10.1007/s12011-016-0860-7. [DOI] [PubMed] [Google Scholar]
- Lee J., Lee S.J., Lim K.T. Preventive effects of ZPDC glycoprotein (24 kDa) on hepatotoxicity induced by mercury chloride in vitro and in vivo. Cell Biochem Funct. 2014;32:520–529. doi: 10.1002/cbf.3046. [DOI] [PubMed] [Google Scholar]
- Li S., Baiyun R., Lv Z., Li J., Han D., Zhao W., Yu L., Deng N., Liu Z., Zhang Z. Exploring the kidney hazard of exposure to mercuric chloride in mice:Disorder of mitochondrial dynamics induces oxidative stress and results in apoptosis. Chemosphere. 2019;234:822–829. doi: 10.1016/j.chemosphere.2019.06.096. [DOI] [PubMed] [Google Scholar]
- Liu B., Yu H., Baiyun R., Lu J., Li S., Bing Q., Zhang X., Zhang Z. Protective effects of dietary luteolin against mercuric chloride-induced lung injury in mice: Involvement of AKT/Nrf2 and NF-kappaB pathways. Food Chem. Toxicol. 2018;113:296–302. doi: 10.1016/j.fct.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Liu S., Xu F.P., Yang Z.J., Li M., Min Y.H., Li S. Cadmium-induced injury and the ameliorative effects of selenium on chicken splenic lymphocytes: mechanisms of oxidative stress and apoptosis. Biol. Trace Elem. Res. 2014;160:340–351. doi: 10.1007/s12011-014-0070-0. [DOI] [PubMed] [Google Scholar]
- Luan H., Wang Y., Li Y., Cui Z., Chang S., Zhao P. Development of a real-time quantitative RT-PCR to detect REV contamination in live vaccine. Poult. Sci. 2016;95:2023–2029. doi: 10.3382/ps/pew147. [DOI] [PubMed] [Google Scholar]
- Ma Y., Shi Y., Li L., Xie C., Zou X. Toxicological effects of mercury chloride on Laying performance, Egg quality, serum Biochemistry, and Histopathology of liver and kidney in Laying Hens. Biol. Trace Elem. Res. 2018;185:465–474. doi: 10.1007/s12011-018-1263-8. [DOI] [PubMed] [Google Scholar]
- Ma Y., Zhu M., Miao L., Zhang X., Dong X., Zou X. Mercuric chloride induced ovarian oxidative stress by suppressing Nrf2-Keap1 signal pathway and its Downstream genes in Laying Hens. Biol. Trace Elem. Res. 2018;185:185–196. doi: 10.1007/s12011-018-1244-y. [DOI] [PubMed] [Google Scholar]
- McCormack M.A., Fielding R., Kiszka J.J., Paz V., Jackson B.P., Bergfelt D.R., Dutton J. Mercury and selenium concentrations, and selenium:mercury molar ratios in small cetaceans taken off St. Vincent, West Indies. Environ. Res. 2020;181:108908. doi: 10.1016/j.envres.2019.108908. [DOI] [PubMed] [Google Scholar]
- Melgar M.J., Nunez R., Garcia M.A. Selenium intake from tuna in Galicia (Spain): health risk assessment and protective role against exposure to mercury and inorganic arsenic. Sci. Total Environ. 2019;694:133716. doi: 10.1016/j.scitotenv.2019.133716. [DOI] [PubMed] [Google Scholar]
- Pollard K.M., Cauvi D.M., Toomey C.B., Hultman P., Kono D.H. Mercury-induced inflammation and autoimmunity. Biochim. Biophys. Acta Gen. Subj. 2019;1863:129299. doi: 10.1016/j.bbagen.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu K.C., Wang Z.Y., Tang K.K., Zhu Y.S., Fan R.F. Trehalose suppresses cadmium-activated Nrf2 signaling pathway to protect against spleen injury. Ecotoxicol Environ. Saf. 2019;181:224–230. doi: 10.1016/j.ecoenv.2019.06.007. [DOI] [PubMed] [Google Scholar]
- Shao Y., Zhao H., Wang Y., Liu J., Li J., Chai H., Xing M. Arsenic and/or copper caused inflammatory response via activation of inducible nitric oxide synthase pathway and triggered heat shock protein responses in testis tissues of chicken. Environ. Sci. Pollut. Res. Int. 2018;25:7719–7729. doi: 10.1007/s11356-017-1042-7. [DOI] [PubMed] [Google Scholar]
- Soria E.A., Perez R.D., Queralt I., Perez C.A., Bongiovanni G.A. Immunotoxicological effects of arsenic bioaccumulation on spatial metallomics and cellular enzyme response in the spleen of male Wistar rats after oral intake. Toxicol. Lett. 2017;266:65–73. doi: 10.1016/j.toxlet.2016.12.014. [DOI] [PubMed] [Google Scholar]
- Tang K.K., Li H.Q., Qu K.C., Fan R.F. Selenium alleviates cadmium-induced inflammation and meat quality degradation via antioxidant and anti-inflammation in chicken breast muscles. Environ. Sci. Pollut. Res. Int. 2019;26:23453–23459. doi: 10.1007/s11356-019-05675-0. [DOI] [PubMed] [Google Scholar]
- Toomey C.B., Cauvi D.M., Hamel J.C., Ramirez A.E., Pollard K.M. Cathepsin B regulates the appearance and severity of mercury-induced inflammation and autoimmunity. Toxicol. Sci. 2014;142:339–349. doi: 10.1093/toxsci/kfu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan N., Xu Z., Chi Q., Hu X., Pan T., Liu T., Li S. microRNA-33-3p involved in selenium deficiency-induced apoptosis via targeting ADAM10 in the chicken kidney. J. Cell Physiol. 2019;234:13693–13704. doi: 10.1002/jcp.28050. [DOI] [PubMed] [Google Scholar]
- Wan N., Xu Z., Liu T., Min Y., Li S. Ameliorative effects of selenium on cadmium-induced injury in the chicken ovary: mechanisms of oxidative stress and Endoplasmic Reticulum stress in cadmium-induced apoptosis. Biol. Trace Elem. Res. 2018;184:463–473. doi: 10.1007/s12011-017-1193-x. [DOI] [PubMed] [Google Scholar]
- Wang J., Liu Z., He X., Lian S., Liang J., Yu D., Sun D., Wu R. Selenium deficiency induces duodenal villi cell apoptosis via an oxidative stress-induced mitochondrial apoptosis pathway and an inflammatory signaling-induced death receptor pathway. Metallomics. 2018;10:1390–1400. doi: 10.1039/c8mt00142a. [DOI] [PubMed] [Google Scholar]
- Wang L.Y., Fan R.F., Yang D.B., Zhang D., Wang L. Puerarin reverses cadmium-induced lysosomal dysfunction in primary rat proximal tubular cells via inhibiting Nrf2 pathway. Biochem. Pharmacol. 2019;162:132–141. doi: 10.1016/j.bcp.2018.10.016. [DOI] [PubMed] [Google Scholar]
- Wang S., Chi Q., Hu X., Cong Y., Li S. Hydrogen sulfide-induced oxidative stress leads to excessive mitochondrial fission to activate apoptosis in broiler myocardia. Ecotoxicol Environ. Saf. 2019;183:109578. doi: 10.1016/j.ecoenv.2019.109578. [DOI] [PubMed] [Google Scholar]
- Wang X.Y., Yang H., Wang M.G., Yang D.B., Wang Z.Y., Wang L. Trehalose protects against cadmium-induced cytotoxicity in primary rat proximal tubular cells via inhibiting apoptosis and restoring autophagic flux. Cell Death Dis. 2017;8:e3099. doi: 10.1038/cddis.2017.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Liu J., Chen R., Qi M., Tao D., Xu S. The antagonistic effects of selenium Yeast (SeY) on cadmium-induced inflammatory factors and the heat shock protein expression levels in chicken livers. Biol. Trace Elem. Res. 2020 doi: 10.1007/s12011-020-02039-5. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhao H., Guo M., Fei D., Zhang L., Xing M. Targeting the miR-122/PKM2 autophagy axis relieves arsenic stress. J. Hazard Mater. 2020;383:121217. doi: 10.1016/j.jhazmat.2019.121217. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhao H., Guo M., Shao Y., Liu J., Jiang G., Xing M. Arsenite renal apoptotic effects in chickens co-aggravated by oxidative stress and inflammatory response. Metallomics. 2018;10:1805–1813. doi: 10.1039/c8mt00234g. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhao H., Liu J., Shao Y., Li J., Luo L., Xing M. Copper and arsenic-induced oxidative stress and immune imbalance are associated with activation of heat shock proteins in chicken intestines. Int. Immunopharmacol. 2018;60:64–75. doi: 10.1016/j.intimp.2018.04.038. [DOI] [PubMed] [Google Scholar]
- Xing M., Jin X., Wang J., Shi Q., Cai J., Xu S. The antagonistic effect of selenium on lead-induced immune dysfunction via Recovery of cytokine and heat shock protein expression in chicken neutrophils. Biol. Trace Elem. Res. 2018;185:162–169. doi: 10.1007/s12011-017-1200-2. [DOI] [PubMed] [Google Scholar]
- Yang J., Hamid S., Cai J., Liu Q., Xu S., Zhang Z. Selenium deficiency-induced thioredoxin suppression and thioredoxin knock down disbalanced insulin responsiveness in chicken cardiomyocytes through PI3K/Akt pathway inhibition. Cell Signal. 2017;38:192–200. doi: 10.1016/j.cellsig.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Yang X., Zhao H., Wang Y., Liu J., Guo M., Fei D., Mu M., Xing M. The activation of heat-shock protein after copper(II) and/or arsenic(III)-Induced imbalance of homeostasis, inflammatory response in chicken rectum. Biol. Trace Elem. Res. 2020;195:613–623. doi: 10.1007/s12011-019-01871-8. [DOI] [PubMed] [Google Scholar]
- Zefferino R., Piccoli C., Ricciardi N., Scrima R., Capitanio N. Possible mechanisms of mercury toxicity and Cancer promotion: Involvement of gap Junction Intercellular Communications and inflammatory cytokines. Oxid Med. Cell Longev. 2017;2017:7028583. doi: 10.1155/2017/7028583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J., Zhou J., Huang K. Effect of selenium on pancreatic proinflammatory cytokines in streptozotocin-induced diabetic mice. J. Nutr. Biochem. 2009;20:530–536. doi: 10.1016/j.jnutbio.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Zhang Y.Y., Zhu S.Z., Wang X.P., Wang C.Y., Li F.C. The effect of dietary selenium levels on growth performance, antioxidant capacity and glutathione peroxidase 1 (GSHPx1) mRNA expression in growing meat rabbits. Anim. Feed Sci. Tech. 2011;169:259–264. [Google Scholar]
- Zhao D., Zhang X. Selenium antagonizes the lead-induced apoptosis of chicken splenic lymphocytes in vitro by activating the PI3K/Akt pathway. Biol. Trace Elem. Res. 2018;182:119–129. doi: 10.1007/s12011-017-1088-x. [DOI] [PubMed] [Google Scholar]
- Zhao H., Wang Y., Liu J., Guo M., Fei D., Yu H., Xing M. The cardiotoxicity of the common carp (Cyprinus carpio) exposed to environmentally relevant concentrations of arsenic and subsequently relieved by zinc supplementation. Environ. Pollut. 2019;253:741–748. doi: 10.1016/j.envpol.2019.07.065. [DOI] [PubMed] [Google Scholar]
- Zhao H., Wang Y., Shao Y., Liu J., Wang S., Xing M. Oxidative stress-induced skeletal muscle injury involves in NF-kappaB/p53-activated immunosuppression and apoptosis response in copper (II) or/and arsenite-exposed chicken. Chemosphere. 2018;210:76–84. doi: 10.1016/j.chemosphere.2018.06.165. [DOI] [PubMed] [Google Scholar]
- Zhu C., Ling Q., Cai Z., Wang Y., Zhang Y., Hoffmann P.R., Zheng W., Zhou T., Huang Z. Selenium-containing Phycocyanin from Se-enriched Spirulina platensis Reduces inflammation in Dextran Sulfate sodium-induced colitis by inhibiting NF-kappaB activation. J. Agric. Food Chem. 2016;64:5060–5070. doi: 10.1021/acs.jafc.6b01308. [DOI] [PubMed] [Google Scholar]
- Zhu C., Zhang S., Song C., Zhang Y., Ling Q., Hoffmann P.R., Li J., Chen T., Zheng W., Huang Z. Selenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-kappaB mediated hyper inflammation. J. Nanobiotechnology. 2017;15:20. doi: 10.1186/s12951-017-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]