Abstract
The gut microbiota play an important role in the growth and intestinal health of broilers. The present study was to investigate the gut microbiota, short-chain fatty acids, and intestinal morphology of broilers at different ages. A total of 320 one-day-old male broilers were raised in 8 replicates and fed the same corn–soybean diets for 42 D. The duodenal, jejunal, and ileal segments and their and cecal microbiota were collected on day 1, 7, 14, 21, and 42, respectively. The villous height (VH), crypt depth (CD), and their ratio of VH:CD in the duodenum, jejunum, and ileum all increased (P < 0.05) with age. Caecal acetate, propionate, butyrate, valerate, and isovalerate increased (P < 0.01), but isobutyrate decreased (P < 0.001) with age. The cecum had the greatest (P < 0.001) alpha diversity of bacterial community in broilers at different ages. Beta diversities showed distinct differences in gut microbial compositions among different ages (R = 0.55, P < 0.002) and different intestinal segments (R = 0.53, P < 0.002). Lactobacillus was the most abundant genus in the duodenum (36∼97%), jejunum (39∼72%), and ileum (24∼96%) at all ages, and in the ileum, it was positively correlated with VH (R = 0.559, P < 0.03), VH:CD (R = 0.55, P < 0.03), and acetate contents (R = 0.541, P < 0.04) but negatively correlated (R = -0.50, P < 0.05) with isobutyrate contents. Escherichia–Shigella and Salmonella dominated in the cecum of newly hatched broilers, and then the Bacteroides dominated in the cecum on day 42. In the cecum, Escherichia–Shigella was positively correlated (R = 0.577∼0.662, P < 0.05) with isobutyrate contents and Salmonella negatively correlated (R = -0.539∼-0.843, P < 0.05) with isovalerate, butyrate, and acetate contents. These aforementioned results indicated that the most abundant Lactobacillus from the small intestine and the most diversity of microflora community and short-chain fatty acids in the cecum might contribute to the development of intestinal structure in the whole growing period of broilers.
Key words: broiler, gut microbiota, intestinal morphology, short-chain fatty acid
Introduction
The intestinal tracts of chickens are inhabited by complex microbial communities (Gong et al., 2007). These communities perform an important role in the growth and gut health of animals by producing short-chain fatty acids (SCFAs) (Dunkley et al., 2007), modulating the morphological structure of the intestinal tract (Shakouri et al., 2009), and consequently influencing nutrient digestion and absorption (Choct, 2009). The indigestible carbohydrates in the gut can be used and converted into SCFAs by the microbial communities (Józefiak et al., 2004). The microbial communities and SCFAs interact in a complex way with the intestinal morphological structure (Besten et al., 2013; Calik and Ergun, 2015). The SCFAs have been shown to be the primary energy sources of the colonic epithelial cells in mice (Donohoe et al., 2011) and are essential for the development in the intestinal villus of broilers (Panda et al., 2009). In turn, the morphological structure of the gastrointestinal tract has significant correlations with the composition of microbial communities, indicating spatial heterogeneity along gastrointestinal tracts in chickens (Gong et al., 2007; Choi et al., 2014). It has also been demonstrated that the diverse microbial communities are distributed along the entire gastrointestinal tract of broilers (Gong et al., 2007) and the interactions between microbial communities or SCFAs and intestinal morphology are primarily in the ileum or cecum in broilers (Sekelja et al., 2012). However, the roles of microbial communities and SCFAs in the development of the duodenum and jejunum are unclear. In addition, the composition of the microbial communities is also influenced by days of age. It has been reported that stable microflora in the small intestine are established within 2 weeks of hatch; however, the cecal flora may take up to 30 D to develop (Amit-Romach et al., 2004). Importantly, the increased populations of Lactobacillus salivarius and clostridia between day 20 and 30 of broilers deconjugated bile acids, thus leading to growth reduction and dysbacteriosis (Ranjitkar et al., 2016). Therefore, an increased understanding of the changing patterns of microbial communities and SCFAs at different ages is essential for the improvement of the intestinal health and growth in the broiler production. It was hypothesized that the microbial communities and SCFAs in the duodenum and jejunum might change with age and these changes might be associated with the development of the healthy gut morphology. Therefore, the specific objectives of the present study were to investigate the changing pattern of the microbial population and the SCFAs in the duodenum, jejunum, ileum, and cecum, and the histomorphology in the duodenum, jejunum, and ileum, as well as their correlations of growing and healthy broilers at different ages.
Materials and methods
Birds, Diets, and Experimental Design
All experimental procedures were approved by the Animal Management Committee (in charge of animal welfare) of the Institute of Animal Science, Chinese Academy of Agricultural Sciences (IAS-CAAS, Beijing, China), and performed in accordance with the guidelines. A completely randomized design was used in this study. A total of 320 one-day-old Arbor Acres commercial male broiler chicks (Huadu Broiler Breeding corp., Beijing, China), with average initial body weight of 47.8 g, were randomly allotted to 1 of 8 replicates of 40 birds per replicate. The birds in each replicate were kept in 2 neighboring cages, with 20 birds per cage. All birds were housed in an electrically heated, thermostatically controlled cages (160 × 90 × 75 cm, length × width × height) equipped with fiberglass feeders, waterers, and stainless steel cages coated with plastics. Environmental temperature was maintained at 35°C for the first 3 D, after which it was gradually reduced by 3°C per week until it reached 24°C. They had free access to feed and tap water. The same corn–soybean meal diets were formulated to meet or exceed the National Research Council (1994) requirements of broilers for all nutrients (Table 1). The experiment lasted for 42 D, and the health status and mortality were visually observed and recorded daily throughout the experiment. At 7, 14, 21, and 42 D of age, the body weight was measured to check whether the broilers were close to the standard weight in accordance with Arbor Acres plus Broiler Performance Objectives, 2014.
Table 1.
Ingredients and nutrient composition of basal diets for broilers (as-fed basis).
| Ingredients, % | Day 1–21 | Day 22–42 | Nutrient composition, % unless noted | Day 1–21 | Day 22–42 |
|---|---|---|---|---|---|
| Maize | 53.85 | 59.54 | ME, kcal/kg | 3,004 | 3,052 |
| Soybean meal | 37.40 | 32.10 | Crude protein3 | 21.55 | 20.44 |
| Soybean oil | 4.68 | 4.95 | Lysine | 1.11 | 1.03 |
| CaHPO4·2H2O1 | 1.94 | 1.64 | Methionine | 0.55 | 0.43 |
| Limestone1 | 1.18 | 1.12 | Methionine + cysteine | 0.90 | 0.76 |
| NaCl1 | 0.30 | 0.30 | Calcium3 | 1.04 | 0.90 |
| DL-Methionine1 | 0.33 | 0.14 | Total P3 | 0.69 | 0.63 |
| Micronutrients2 | 0.32 | 0.21 | Nonphytate P | 0.45 | 0.35 |
Feed grade.
Supplied per kilogram of diet:day 1–21: vitamin A 12,500 IU, vitamin D3 3,750 IU, vitamin E 20 U, vitamin K3 2.5 mg, vitamin B1 2.5 mg, vitamin B2 8 mg, vitamin B6 2.5 mg, vitamin B12 0.015 mg, pantothenic acid calcium 12.5 mg, niacin 32.5 mg, folic acid 1.25 mg, biotin 0.125 mg, choline 700 mg, Zn (ZnSO4·7H2O) 60 mg, Cu (CuSO4·5H2O) 8 mg, Mn (MnSO4·H2O) 110 mg, Fe (FeSO4·7H2O) 40 mg, I (KI) 0.35 mg, Se (Na2SeO3) 0.15 mg, chlortetracycline 50 mg. day 22–42: vitamin A 10,000 IU, vitamin D3 3,400 IU, vitamin E 12.8 U, vitamin K3 1.6 mg, vitamin B1 0.8 mg, vitamin B2 6.8 mg, vitamin B6 1.6 mg, vitamin B12 0.008 mg, pantothenic acid calcium 8 mg, niacin 26 mg, folic acid 0.8 mg, biotin 0.158 mg, choline 500 mg, Zn (ZnSO4·7H2O) 40 mg, Cu (CuSO4·5H2O) 8 mg, Mn (MnSO4·H2O) 80 mg, Fe (FeSO4·7H2O) 30 mg, I (KI) 0.35 mg, Se (Na2SeO3) 0.15 mg.
Analyzed values and each value based on triplicate determinations.
Sample Collections and Preparations
At 1, 7, 14, 21, and 42 D of age, 4 birds closest to the cage average body weight were selected from each replicate and killed by cervical dislocation (Liao et al., 2017). The contents of the duodenum, jejunum, ileum, and cecum were collected aseptically, snap-frozen in liquid nitrogen, and stored at -80°C, and equal amounts of intestinal contents from 4 birds in each replicate were pooled into 1 sample for DNA extraction and SCFAs analyses. Samples (about 1 cm) of the midpoint of the duodenum, the midpoint between the bile duct entry and Meckel's diverticulum (jejunum), and the midpoint of the Meckel's diverticulum and the ileocecal junction (ileum) were taken from 1 bird in each replicate and washed in PBS (flushed with 0.05 mol/L PBS, pH 7.2), fixed in 10% formalin solution and kept at 4°C for histological examination.
Intestinal Histomorphology
Formalin-fixed intestinal samples were prepared using paraffin embedding techniques. Consecutive sections (5 μm) were stained using hematoxylin and eosin and observed for histomorphology. The villous height (VH) and crypt depth (CD) were measured from 10 randomly selected villi and associated crypts (Shao et al., 2014). The ratio of VH:CD was then calculated from the aforementioned measurements. All examinations and measurements were performed with an Olympus optical microscope using ProgRes CapturePro software, version 2.7 (Jenoptik, Germany).
SCFA Extraction and Analysis
A 0.5 g sample of intestinal contents was suspended in 1.5 mL 2.5% metaphosphoric acid solution. The suspensions were placed in ice water for 30 min immediately, homogenized with a vortex intermittently, and then centrifuged for 10 min at 14,000 g at 4°C. The supernate was used to determine the concentrations of SCFAs (acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, and isovaleric acid) using gas chromatography (Ailgent 7890A, Wilmington, NC) as described previously (García-Villalba et al., 2012).
DNA Extraction
Bacterial cells were lysed and then the chromosomal DNA was extracted from cell lysates using the TIANamp Stool DNA Kit (TIANGEN, Beijing, China) in accordance with the manufacturer's instructions. The concentration of DNA extracts was estimated by measuring its optical density at 260 nm with a spectrophotometer (Q5000; Quawell Technology, San Jose, CA).
Cloning and Sequencing of 16S rRNA Gene
The V3–V4 hypervariable regions of the bacteria 16S rRNA gene were amplified with primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) using a thermocycler PCR system (GeneAmp 9700; ABI, Vernon, CA). The PCR reactions were conducted with a high-fidelity polymerase in the following program: 3 min of denaturation at 95°C, 29 cycles of 30 s at 95°C, 30 s for annealing at 55°C, and 45 s for elongation at 72°C, and a final extension at 72°C for 10 min. After amplification, the PCR products were visualized in a 2% agarose gel and further purified with the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA) and then quantified with QuantiFluor-ST (Promega, WI) in accordance with the manufacturer's protocol. Purified amplicons were pooled in equimolar and paired-end sequenced (2 × 300) on an Illumina MiSeq platform (Illumina, San Diego, CA) in accordance with the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China).
Processing of Sequencing Data
Raw FASTQ files were quality-filtered by Trimmomatic software and merged by FLASH software with the following criteria: (i) the reads were truncated at any site receiving an average quality score < 20 over a 50 bp sliding window; (ii) sequences with overlap longer than 10 bp were merged in accordance with their overlap with mismatch no more than 2 bp; (iii) sequences of each sample were separated in accordance with barcodes (exactly matching) and primers (allowing 2 nucleotide mismatching), and reads containing ambiguous bases were removed. Operational taxonomic units (OTUs) were clustered with 97% similarity cutoff using UPARSE (version 7.1; http://drive5.com/uparse/) with a novel greedy algorithm which performed chimera filtering and OTU clustering simultaneously. The taxonomy of each 16S rRNA gene sequence was analyzed by RDP classifier algorithm (http://rdp.cme.msu.edu/) against the Silva (SSU123) 16S rRNA gene database using a confidence threshold of 70%.
Statistical Analyses
All data were analyzed using SPSS statistical software (version 17.0; Chicago, IL). Data from the same intestinal segments of broilers at different ages or from different intestinal segments of broilers at the same day of age were analyzed using one-way ANOVA, and differences among means were tested by the least significant difference method. Polynomial orthogonal contrasts were used to determine the linear and quadratic responses of dependent variables to ages (Kamely et al., 2016). The replicate of 1 bird used for sample collection was regarded as an experimental unit to determine the small intestinal morphology, and 4 birds used for 1 mixed sample collections were regarded as an experimental unit to determine gut microbiota and SCFAs. Results were presented as means ± SE. The beta diversities of the intestinal microbiota were analyzed by analysis of similarities and permutational multivariate analysis of variance (Ranjitkar et al., 2016). The relationships between luminal bacteria (the top 15% genus level) and the small intestinal morphological structure or SCFAs were analyzed by Spearman correlation test. The small intestinal morphological structure and SCFAs were served as explanatory variables, and the bacterial genera were used as response variables. The P ≤ 0.05 was considered to be statistically significant.
Results
Growth Performance
The broilers grew well during the whole experimental period, and the mortality was 4.1% throughout the experiment. The average body weights on day 7, 14, 21, and 42 were 185 ± 1.6, 450 ± 3.2, 849 ± 3.7, and 2,740 ± 24.4 g, respectively. These weights were close to the standard weight of Arbor Acres broilers in accordance with Arbor Acres plus Broiler Performance Objectives, 2014. The average daily gain, average daily feed intake, and the ratio of feed:gain increased linearly and quadratically (P < 0.0001, Supplementary Table 1) as the age increased.
Small Intestinal Histomorphology
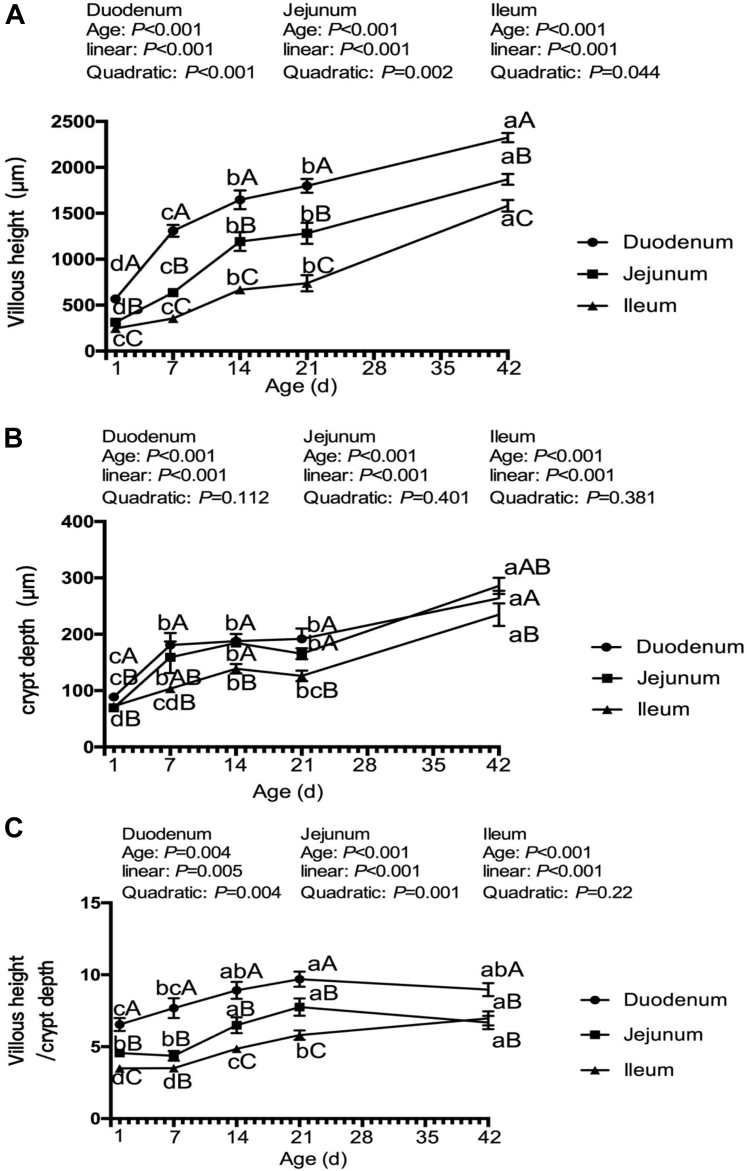
The VH, CD, and VH:CD of the duodenum, jejunum, and ileum increased linearly (P < 0.006, Figure 1) as the age increased. Over the whole growing period, the VH of the duodenum was the highest, followed by jejunum and then ileum (P < 0.001). The CD was higher (P < 0.01) in the duodenum and jejunum than in ileum on day 14 and 21, and there was no difference (P > 0.11) in CD between the duodenum and jejunum from 7 to 42 D of age. The VH:CD in the duodenum was higher (P < 0.005) than in the jejunum and ileum, and the jejunum had a higher (P < 0.005) VH:CD than the ileum over the whole growing period except for day 7 and 42 (P > 0.05).
Figure 1.
The small intestinal morphology structure of broilers at different ages. A, villous height; B, crypt depth; C, villous height:crypt depth. Lacking the same letters in the curves (a, b, c, d) means that the differences at P < 0.05 were observed among different ages for the same small intestinal segments. Lacking the same letters on the same day (A, B, C) means that the differences at P < 0.05 were observed among different small intestinal segments.
Intestinal SCFAs Production
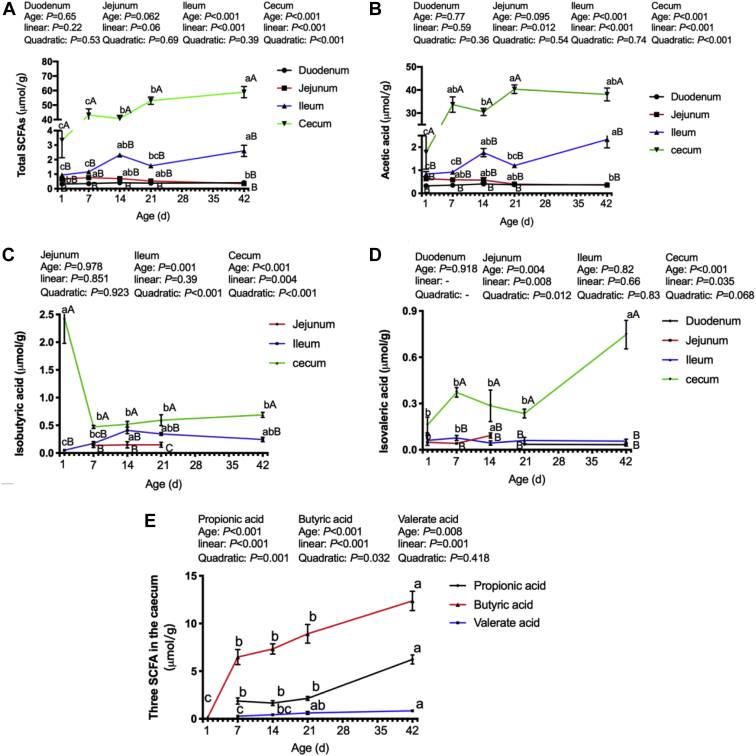
The total SCFAs concentration increased linearly (P < 0.001, Figure 2) in the ileum and cecum over the whole growing period. The acetic acid concentration increased linearly (P < 0.001) in the jejunum, ileum, and cecum over the whole growing period. In addition, the total SCFAs and acetate concentrations in the cecum were higher (P < 0.01) than those in the duodenum, jejunum, or ileum, but no differences (P > 0. 81) were observed among the duodenum, jejunum, and ileum during the whole growing period.
Figure 2.
The concentrations of short-chain fatty acids (SCFAs) in the intestine of broilers. A, total SCFAs; B, acetic acid; C, isobutyric acid; D, isovaleric acid; E, propionic acid, butyric acid and valeric acid in the cecum. Lacking the same letters in the curves (a, b, c) means that the differences at P < 0.05 were observed among different age for the same intestinal segments. Lacking the same letters on the same day (A, B, C) means that the differences at P < 0.05 were observed among different intestinal segments.
Quadratic changes (P < 0.005) were observed in isobutyric acid concentration in the ileum and cecum, and the concentration of isobutyric acid was higher (P < 0.05) in the cecum than that in the ileum during the whole growing period. The lowest (P < 0.05) concentrations of isobutyric acid were detected in the jejunum on day 7, 14, and 21. However, isobutyric acid was not detected in the duodenum over the whole growing period.
There was a linear increase (P < 0.04) in isovaleric acid concentration in the cecum, but no influence (P > 0.80) of age on the concentration of this SCFA in the ileum over the whole growing period. The isovaleric acid in the duodenum and jejunum were detected in the older broilers (day 21 and 42) and in the younger broilers (day 1, 7, and 14), respectively. In addition, the isovaleric acid concentration in the cecum was higher (P < 0.001) than that in the small intestinal tracts of the broilers from 7 to 14 D of age. No differences (P > 0.08) were observed in the isovaleric acid concentration between the duodenum and jejunum on day 1, 7, and 14, and between the duodenum and ileum on day 21 and 42.
There was a linear increase (P < 0.002) in propionic acid, butyric acid, and valeric acid in the cecum, but propionic acid and valeric acid were not detected in the cecum of newly hatched broilers. In addition, the 3 SCFAs were also not detected in the small intestine of broilers during the whole growing period.
Sequence and OTU Data
A total of 3, 316, 558 reads with the average length of 439 bp per read were obtained after quality-filtering using UPARSE software. At 97% similarity, the filtered sequences were clustered into 324 OTUs.
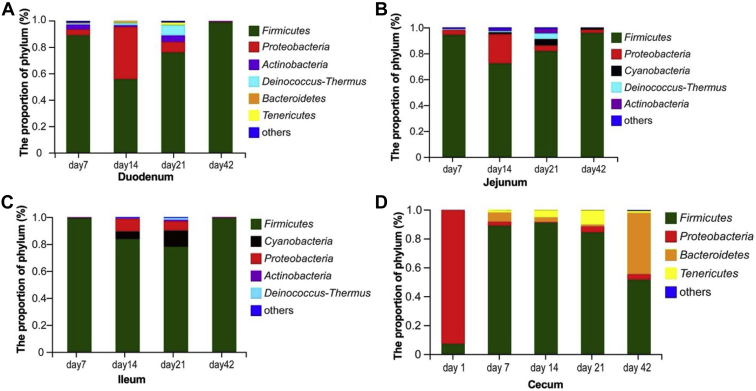
Compositions of Microbial Community at the Phylum Level
At the phylum level (Figure 3, Supplementary Table 2), the Firmicutes was the predominant phylum throughout all the intestinal tracts from 7 to 42 D of age, representing 70∼99% in the duodenum, 74∼95% in the jejunum, 78∼99% in the ileum, and 50∼91% in the cecum. Age affected the relative abundances of Firmicutes in the ileum (P < 0.04) and cecum (P < 0.0001), but it did not affect (P > 0.05) them in the duodenum and jejunum. The lowest (P < 0.05) abundance of Firmicutes in the ileum was observed on day 21. The relative abundances of Firmicutes in the cecum increased from hatching to 14 D of age, and then began to decrease on day 21. In the newly hatched broilers, no bacteria were detected in the duodenum, jejunum, and ileum, but the phylums of Firmicutes (6.8%) and Proteobacteria (93.3%) were observed in the cecum.
Figure 3.
The composition of microbiota at the level of phylum in the intestine of broilers. A, Duodenum; B, jejunum; C ileum; D, cecum.
The second most abundant phylum was the Proteobacteria in the duodenum, jejunum, and cecum, and the Cyanobacteria in the ileum. The Proteobacteria abundance was affected by age (P < 0.0001) in the cecum, but not (P > 0.08) in the duodenum and jejunum. The highest (P < 0.01) abundance of the Proteobacteria in the cecum was found in the newly hatched broilers. In addition, the phylum Bacteroidetes was exclusively found in the duodenum and cecum and was affected by age (P < 0.0001) in the cecum, but not (P > 0.50) in the duodenum. The cecum on day 42 had the highest (P < 0.0001) abundance of Bacteroidetes.
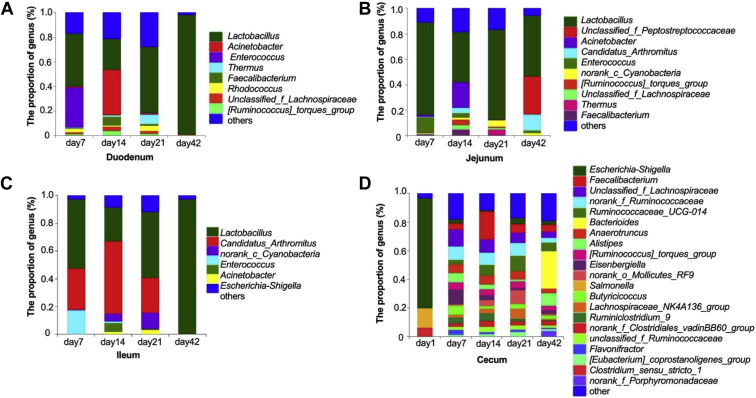
Compositions of the Microbial Community at the Genus Level
Within the Firmicutes, Lactobacillus was the most abundant genus in the duodenum (36∼97%), jejunum (39∼72%), and ileum (24∼96%) at all ages (Figure 4, Supplementary Table 3). In the duodenum, the abundance of Lactobacillus was relatively stable from 7 to 21 D of age, and then sharply increased (P < 0.04) on day 42; the Enterococcus abundance was the highest on day 7, followed by a low level (below 1%) from 14 to 42 D of age (P < 0.05); the Faecalibacterium abundance increased from 7 to 14 D of age, and decreased (P < 0.001) thereafter; the abundance of Ruminococcaceae_UCG-014 increased from 7 to 21 D of age, but disappeared on day 42. In the jejunum, the abundances of Lactobacillus were stable (P > 0.33) throughout the growing period; the highest abundance of the Peptostreptococcaceae family was observed on day 42 (P < 0.03). In the ileum, the abundances of Lactobacillus were stable from 7 to 21 D of age and increased to 96.7% (P < 0.001) on day 42; the Candidatus_Arthromitus remained stable from 7 to 21 D of age, but disappeared on day 42. In the cecum, the abundances of Escherichia–Shigella were high (76.2%) in the newly hatched broilers and then decreased to low levels (0.6∼4.6%) from 7 to 42 D of age; the abundance of Lachnospiraceae family decreased gradually with age from 11.6 to 4.2%; the Ruminococcaceae family showed a high-level abundance (about 9%) from 7 to 21 D of age and then decreased to a low level (3.4%) (P < 0.007) on day 42; the abundance of Ruminococcaceae_UCG-014 increased gradually from 7 to 21 D of age and then decreased (P < 0.003) on day 42; the highest abundance of Eisenbergiella was observed on day 7, followed by a stable low level(P < 0.001). In addition, compared with the small intestinal tracts, the cecum had a greater abundance of Escherichia–Shigella (Firmicutes phylum) in the newly hatched broilers.
Figure 4.
The composition of microbiota at the level of genus in the intestine of broilers. A, Duodenum; B, jejunum; C ileum; D, cecum.
Within the Proteobacteria, the abundance of Acinetobacter in the duodenum and jejunum increased from 7 to 14 D of age and then decreased to a stable low level (P < 0.01, Supplementary Table 3). The cecum of newly hatched broilers had the greatest (28.6%, P < 0.0001) abundance of Salmonella.
Within the Bacteroidetes, the abundances of Alistipes in the cecum were greater (P < 0.04) on day 7 and 42 than on day 21.
Alpha and Beta Diversities of Microbial Community
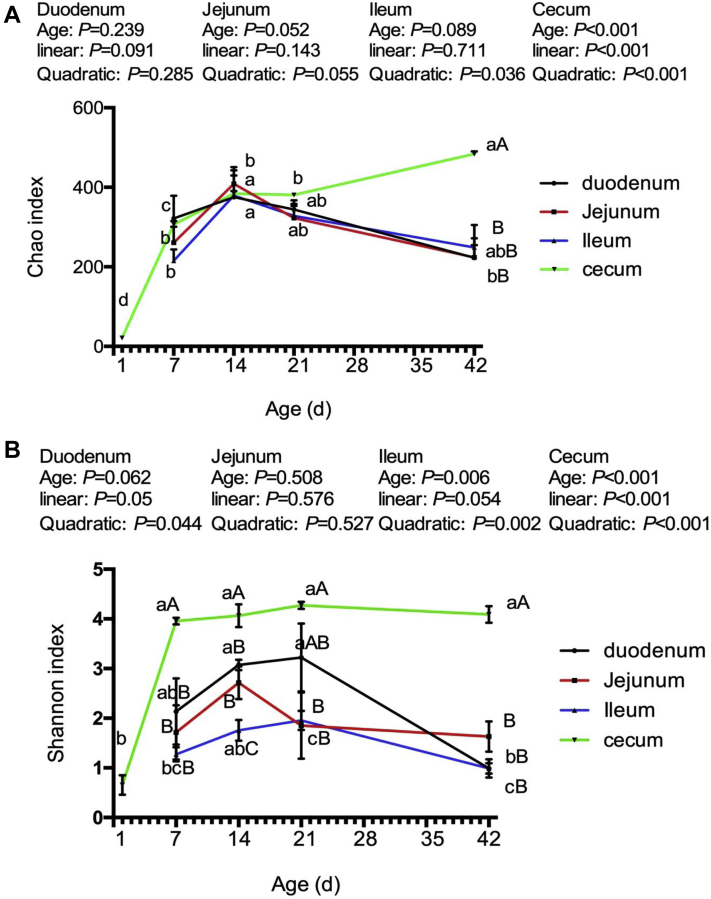
The Chao and Shannon curves were used to evaluate the alpha diversity within a community (Figure 5). The Chao diversity indices of the bacterial community were affected (P < 0.001) by ages in the cecum but not (P > 0.15) in the duodenum, jejunum, and ileum. Both the linear and quadratic responses (P < 0.001) were shown in the Chao diversities in the cecum. In addition, the Shannon diversities of the bacterial community were affected (P < 0.007) by ages in the ileum and cecum but not (P > 0.06) in the duodenum and jejunum. There was a quadratic change (P < 0.003) in the Shannon index in both the ileum and cecum. Among the 4 intestinal segments, the cecum had the greatest (P < 0.01) Shannon index from 7 to 42 D of age, and the Chao index was also the highest (P < 0.01) in the cecum on day 42.
Figure 5.
Alpha diversity of microbiota in the intestine of broilers at different ages. A, Chao index; B, Shannon index. Lacking the same letters in the curves (a, b, c, d) means that the differences at P < 0.05 were observed among different age for the same intestinal segments. Lacking the same letters on the same day (A, B, C) means that the differences at P < 0.05 were observed among different intestinal segments.
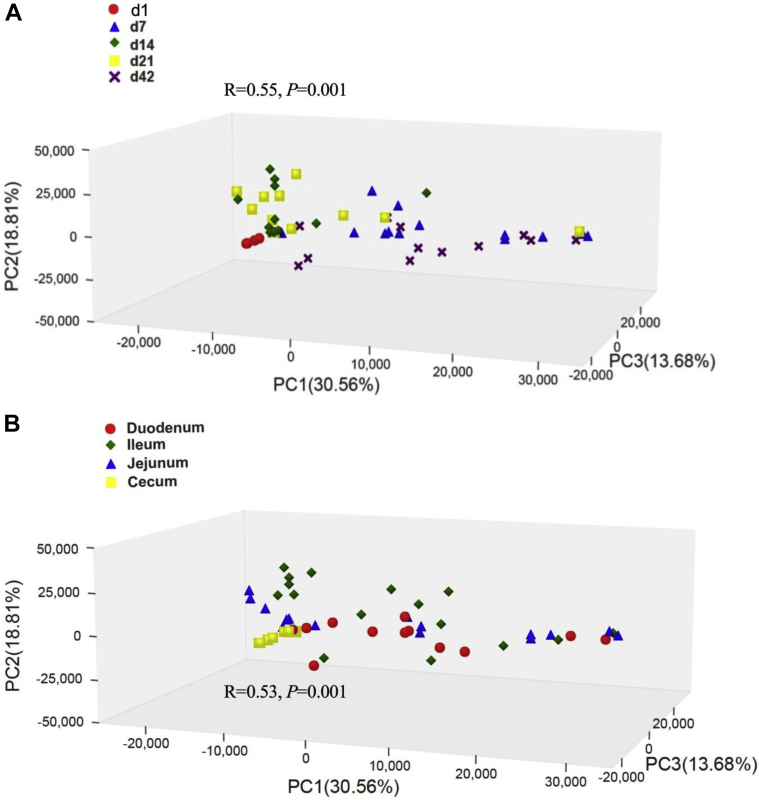
Principal component analysis was used to visualize the beta diversity of the bacterial community from different ages (day 1, 7, 14, 21, and 42, Figure 6A) and different intestinal segments (duodenum, jejunum, ileum, and cecum, Figure 6B) of broilers. Analysis of similarities based on unweighted UniFrac metric showed that significant differences were observed in beta diversity among different ages (R = 0.55, P < 0.002) and different intestinal segments (R = 0.53, P < 0.002).
Figure 6.
Beta diversity of microbiota as shown by principal component analysis. A, Different ages; B, different intestinal segments. ANOSIM R values showed the extent of community variation among different age or different intestinal segments, and the statistical significance was indicated. Axes represent the 3 dimensions explaining the greatest proportion of variances in the communities for each analysis. Abbreviations: ANOSIM, analysis of similarities.
Correlations Among Microbiota, SCFAs, and Morphological Structure in the Intestinal Tracts of Broilers
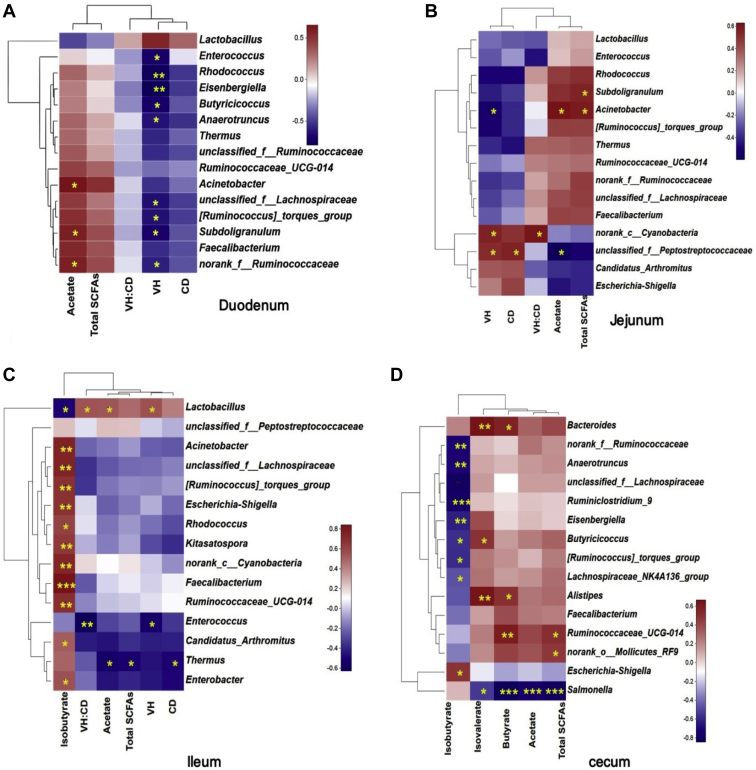
A heatmap showed the Spearman correlations among the bacterial genera, SCFAs, and intestinal morphological structure in the duodenum (Figure 7A), jejunum (Figure 7B), ileum (Figure 7C), and cecum (Figure 7D). In the duodenum, the Acinetobacter and Subdoligranulum were positively correlated with acetate (R = 0.657, P < 0.03; R = 0.594, P < 0.05), and the Enterococcus, Rhodococcus, Eisenbergiella, Butyricicoccus, Anaerotruncus, and Subdoligranulum were all negatively correlated with VH (R = -0.119∼-0.531, P < 0.05). In the jejunum, the Acinetobacter was negatively correlated with VH (R = -0.518, P < 0.05) but positively correlated with acetate (R = 0.629, P < 0.02) and total SCFAs (R = 0.589, P < 0.03). The Subdoligranulum was positively correlated with total SCFAs (R = 0.554, P < 0.04), whereas the norank_c_Cyanobacteria was positively correlated with VH (R = 0.561, P < 0.04) and VH:CD (R = 0.614, P < 0.02). In the ileum, the Lactobacillus was negatively correlated with isobutyrate (R = -0.50, P < 0.05) but positively correlated with acetate (R = 0.541, P < 0.04), VH (R = 0.559, P < 0.03), and VH:CD (R = 0.55, P < 0.03). In addition, the Enterococcus had a negative correlation with VH (R = -0.542, P < 0.04) and VH:CD (R = -0.628, P < 0.01), whereas the Thermus was negatively correlated with acetate (R = -0.55, P < 0.03), total SCFAs (R = -0.518, P < 0.05), and CD (R = -0.503, P < 0.05). And the remaining bacterial genera were all positively correlated with isobutyrate (R = 0.497∼0.841, P < 0.05). In the cecum, the Salmonella was negatively correlated with isovalerate (R = -0.539, P < 0.02), butyrate (R = -0.843, P < 0.001), acetate (R = -0.746, P < 0.001), and total SCFAs (R = -0.793, P < 0.001). Moreover, the Escherichia–Shigella was positively correlated with isobutyrate (R = 0.493, P < 0.04), the Bacteroides, Butyricicoccus, and Alistipes were all positively correlated with isovalerate (R = 0.495∼0.662, P < 0.05), and the Bacteroides, Alistipes, and Ruminococcaceae_UCG-014 were all positively correlated with butyrate (R = 0.506∼0.608, P < 0.05).
Figure 7.
A heatmap showing correlations between predominant genera in intestine and intestinal morphology structure or SCFAs in duodenum (A), jejunum (B), ileum (C) and cecum (D). Red indicates a positive correlation; blue indicates a negative correlation. Abbreviations: SCFAs, short-chain fatty acids; VH, villous height; CD, crypt depth; VH:CD, villous height:crypt depth. ∗ 0.01 < P ≤ 0.05, ∗∗ 0.001 < P ≤ 0.01, ∗∗∗P ≤ 0.001.
Discussion
Our hypothesis that the microbial communities and SCFAs in duodenum and jejunum might change with age and these changes might be associated with the development of healthy gut morphology has been supported by the results from the present study. This would strengthen our understanding on the roles of intestinal microbial communities and their metabolites in the development of the intestinal tract of broilers, and also provide scientific experimental bases for the regulation of intestinal development and health in broilers.
The microbial community plays an important role in digesting complex nutrients and protecting poultry from pathogen colonization via a process of competitive exclusion (Lan et al., 2005; Waite and Taylor, 2014). Chao index and Shannon index were used to estimate the richness and diversity of the microbial community, respectively. The cecum had the most complex microbial community among the different intestinal tracts as shown by the Shannon index in broilers. In addition, the beta diversity of the microbial community showed that the bacterial composition was different among the different age, indicating that the bacterial communities were developing with the increased age. Previous studies have demonstrated that the highest microbial diversity was found in the cecum of broilers (Gong et al., 2002; Sergeant et al., 2014), and it also increased with ages (Danzeisen et al., 2011).
No bacteria were observed in the small intestinal tracts (duodenum, jejunum, and ileum) of newly hatched broilers, and Amit-Romach et al. (2004) reported that the alimentary tract was generally sterile in the newly hatched chicks. However, the bacterial genera Salmonella, Escherichia–Shigella, and Enterobacter were the dominant bacterial community in the cecum of newly hatched chicks. The aforementioned findings suggested that a cecum of newly hatched chicks might be the main site of pathogen colonization because it has not yet established the stable intestinal microflora. Barrow demonstrated that the absence of normal microflora in the cecum was a major factor in the susceptibility of chicks to bacterial infection (Barrow, 1992). Nevertheless, the dominant bacteria to colonize the 3 small intestinal tracts were the genus Lactobacillus and Enterococcus. The Lactobacillus existed in the small intestinal tracts throughout the whole growing period of the broilers, which has also been observed in previous studies (Barnes, 1979; Ranjitkar et al., 2016). The Enterococcus was primarily observed in the small intestine on day 7 and then decreased. These findings indicate that the microbial colonization of the gastrointestinal tract gradually changes until a mature and complex microbial community is established (Babot et al., 2014). Several other studies have demonstrated that Lactobacillus could be potential to prevent the colonization of pathogens, promote intestinal health, and improve growth (Jones and Versalovic, 2009; Zeng et al., 2017). The Spearman analyses further showed that the Lactobacillus was positively correlated with the VH and VH:CD in the ileum of the growing broilers. Increased VH and VH:CD are widely thought to provide a larger surface area and higher ability of nutrient absorption (Olukosi and Dono, 2014).
The microbial community may also influence the structure and function of the intestine through their metabolites, the SCFAs, which have been demonstrated to be essential for appropriate intestinal physiology and the health of gut (Clausen and Mortensen, 1995). Acetic acid suppresses gastric apoptosis and promotes mucin production (Liu et al., 2017). As a source of energy, butyrate plays a vital role in promoting intestinal development and maintaining the integrity of the intestinal epithelial cells (Józefiak et al., 2004; Sun and O'Riordan, 2013). In our study, Lactobacillus was positively correlated with acetate in the ileum, indicating Lactobacillus could improve intestinal health by production of some SCFAs (He et al., 2019; Zhai et al., 2019). Besides, Salmonella was negatively correlated with SCFAs including acetate, butyrate, and isovalerate, indicating that these SCFAs in the cecum might inhibit Salmonella growth and invasion (Lawhon et al., 2002). In addition, the phylum Firmicutes including the genus Faecalibacterium and Ruminococcaceae and the family Lachnospiraceae were the dominant bacterial communities in the cecum from the grower phase of broilers, whereas the phylum Bacteroidetes was predominant in the finisher phase of broilers. It has been reported that Ruminococcaceae is the predominant intestinal SCFAs-producing bacteria (Ohira et al., 2017; Li et al., 2018), and we found that the genus Ruminococcaceae was positively correlated with butyrate production. The Lachnospiraceae may benefit gut development and health by degrading plant fiber and producing SCFAs (Biddle et al., 2013). The Bacteroidetes can degrade complex carbohydrates by fermenting the glucose to synthesize butyrate as energy for epithelial cells (Macy, 1979), and the Spearman analyses in the present study demonstrated that in the cecum, the abundances of Bacteroides were positively correlated with the butyrate concentrations.
A previous study in piglets also demonstrated that cecum infusion of butyrate stimulated cell proliferation in the jejunum and ileum (Kien et al., 2007). We found that the butyrate content in the cecum increased with age, which is consistent with the results of VH, CD, and VH:CD of the duodenum, jejunum, and ileum in this study, indicating that the butyrate from the cecum might be moved to the small intestines through antiperistalsis and then promote small intestinal development. Furthermore, all of the SCFAs were found in the cecum in our study, although only acetic acid, isobutryric acid, and isovaleric acid were detected in the duodenum, jejunum, and ileum. The difference of SCFAs between the small intestinal tracts and cecum may primarily depend on intestinal transit, pH, and microbial composition (Macfarlane and Macfarlane, 2012). It is well known that the SCFAs are generated by the gut microbiota through the fermentation of undigested carbohydrates (Besten et al., 2013; Li et al., 2018), especially in the cecum which could digest some carbohydrates including cellulose, starch, and polysaccharides that could not digested in the small intestines (Clench and Mathias, 1995). Awad et al. (2016) found that the changes of fermentation of end products are likely to be associated with the density of the resident bacteria, and the cecum has greatest taxonomic diversity and abundance in the present study, which were in agreement with the previous studies (Gong et al., 2007; Choi et al., 2014). In addition, the diet composition and rearing system are also the important influences of bacteria in the gut, and thus, SCFAs will change as diet component or rearing system varies (Heinritz et al., 2016; Hou et al., 2020). Therefore, the effects of other types of diets or rearing system on gut microbiota, SCFAs, and intestinal morphology of broilers need to be further studied.
In conclusion, the results from the present study indicated that the Lactobacillus was the most abundant genus in the duodenum, jejunum, and ileum at all ages, and the cecum had the most diversity of microbial community and SCFAs among different intestinal tracts of broilers fed a corn–soybean meal diet. These bacteria and metabolites might contribute to the development of intestinal structure in the whole growing period of broilers, which provide scientific experimental basis for the improvement of the intestinal health in the broiler production.
Acknowledgments
The present study was supported by the National Key Research and Development Program of China (project no. 2017YFD0500501; Beijing, P. R. China), the Special Funds of CAAS for Distinguished Scientists (Beijing, P. R. China), the China Agriculture Research System (project no. CARS-41; Beijing, P. R. China), and the Agricultural Science and Technology Innovation Program (ASTIP-IAS09; Beijing, P. R. China). The authors would highly appreciate Dr. David Masters in the University of Western Australia for assisting with the English editing of this article.
Conflict of Interest Statement: All authors declare that there is no conflict of interest.
Availability of Data and Materials: The raw sequence data were submitted to the NCBI SRA database (NCBI BioProject PRJNA490233). The individual reads of samples were available under accession numbers SAMN10031194–SAMN10031256.
Footnotes
Supplementary data associated with this article can be found in the online version at http://doi.org/10.1016/j.psj.2020.08.033.
Contributor Information
Xugang Luo, Email: wlysz@263.net.
Lin Lu, Email: lulin1225@163.com.
Supplementary Data
References
- Amit-Romach E., Sklan D., Uni Z. Microflora ecology of the chicken intestine using 16S ribosomal DNA primers. Poult. Sci. 2004;83:1093–1098. doi: 10.1093/ps/83.7.1093. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Dublecz F., Hess C., Dublecz K., Khayal B., Aschenbach J.R., Hess M. Campylobacter jejuni colonization promotes the translocation of Escherichia coli to extra-intestinal organs and disturbs the short-chain fatty acids profiles in the chicken gut. Poult. Sci. 2016;95:2259–2265. doi: 10.3382/ps/pew151. [DOI] [PubMed] [Google Scholar]
- Babot J.D., Arganaraz-Martinez E., Saavedra L., Apella M M.C., Chaia A.P. Selection of indigenous lactic acid bacteria to reinforce the intestinal microbiota of newly hatched chicken–relevance of in vitro and ex vivo methods for strains characterization. Res. Vet. Sci. 2014;97:8–17. doi: 10.1016/j.rvsc.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Barnes E.M. The intestinal microflora of poultry and game birds during life and after storage. J. Appl. Bacteriol. 1979;46:407–419. doi: 10.1111/j.1365-2672.1979.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Barrow P. Probiotic for chickens. In: Fuller R., editor. Probiotics: The Scientific Basis. Chapman and Hall; London, UK: 1992. pp. 225–257. [Google Scholar]
- Besten D.G., Eunen K.V., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddle A., Stewart L., Blanchard J., Leschine S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity. 2013;5:627–640. [Google Scholar]
- Calik A., Ergun A. Effect of lactulose supplementation on growth performance, intestinal histomorphology, cecal microbial population, and short-chain fatty acid composition of broiler chickens. Poult. Sci. 2015;94:2173–2182. doi: 10.3382/ps/pev182. [DOI] [PubMed] [Google Scholar]
- Choct M. Managing gut health through nutrition. Br. Poult. Sci. 2009;50:9–15. doi: 10.1080/00071660802538632. [DOI] [PubMed] [Google Scholar]
- Choi J.H., Kim G.B., Cha C.J. Spatial heterogeneity and stability of bacterial community in the gastrointestinal tracts of broiler chickens. Poult. Sci. 2014;93:1942–1950. doi: 10.3382/ps.2014-03974. [DOI] [PubMed] [Google Scholar]
- Clausen M.R., Mortensen P.B. Kinetic studies on colonocyte metabolism of short chain fatty acids and glucose in ulcerative colitis. Gut. 1995;37:684–689. doi: 10.1136/gut.37.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clench M., Mathias J. The avian cecum: a review. Wilson Bull. 1995;107:93–121. [Google Scholar]
- Danzeisen J.L., Kim H.B., Isaacson R.E., Tu Z.J., Johnson T.J., Parkinson J. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS One. 2011;6:e27949. doi: 10.1371/journal.pone.0027949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe D.R., Garge N., Zhang X., Sun W., O’Connell T.M., Bunger M.K., Bultman S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley K.D., Dunkley C.S., Njongmeta N.L., Callaway T.R., Hume M.E., Kubena L.F., Nisbet D.J., Ricke S.C. Comparison of in vitro fermentation and molecular microbial profiles of high-fiber feed substrates incubated with chicken cecal inocula. Poult. Sci. 2007;86:801–810. doi: 10.1093/ps/86.5.801. [DOI] [PubMed] [Google Scholar]
- García-Villalba R., Giménez-Bastida J.A., García-Conesa M.T., Tomás-Barberán F.A., Carlos Espín J., Larrosa M. Alternative method for gas chromatography-mass spectrometry analysis of short-chain fatty acids in faecal samples. J. Sep. Sci. 2012;35:1906–1913. doi: 10.1002/jssc.201101121. [DOI] [PubMed] [Google Scholar]
- Gong J., Forster R.J., Yu H., Chambers J.R., Wheatcroft R., Sabour P.M., Chen S. Molecular analysis of bacterial populations in the ileum of broiler chickens and comparison with bacteria in the cecum. FEMS Microbiol. Ecol. 2002;41:171–179. doi: 10.1111/j.1574-6941.2002.tb00978.x. [DOI] [PubMed] [Google Scholar]
- Gong J., Si W., Forster R.J., Huang R., Hai Y., Yulong Y., Yang C., Han Y. 16S rRNA gene-based analysis of mucosaassociated bacterial community and phylogeny in the chicken gastrointestinal tracts: from crops to ceca. FEMS Microbiol. Ecol. 2007;59:147–157. doi: 10.1111/j.1574-6941.2006.00193.x. [DOI] [PubMed] [Google Scholar]
- He T., Zhu Y.H., Yu J., Xia B., Liu X., Yang G.Y., Su J.H., Guo L., Wang M.L., Wang J.F. Lactobacillus johnsonii L531 reduces pathogen load and helps maintain short-chain fatty acid levels in the intestines of pigs challenged with Salmonella enterica infantis. Vet. Microbiol. 2019;230:187–194. doi: 10.1016/j.vetmic.2019.02.003. [DOI] [PubMed] [Google Scholar]
- Heinritz S.N., Weiss E., Eklund M., Aumiller T., Louis S., Rings A., Messner S., Camarinha-Silva A., Seifert J., Bischoff S.C., Mosenthin R. Intestinal microbiota and microbial metabolites are changed in a pig model fed a high-fat/low-fiber or a low-fat/high-fiber diet. Plos One. 2016;11:e0154329. doi: 10.1371/journal.pone.0154329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L.Y., Sun B.S., Yang Y. Effects of added dietary fiber and rearing system on the gut microbial diversity and gut health of chickens. Animals-Basel. 2020;10:107. doi: 10.3390/ani10010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Józefiak D., Rutkowski A., Martin S.A., S Carbohydrate fermentation in the avian ceca: a review. Anim. Feed Sci. Tech. 2004;113:1–15. [Google Scholar]
- Jones S.E., Versalovic J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 2009;9:35. doi: 10.1186/1471-2180-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamely M., Torshizi M.A.K., Rahimi S. Blood biochemistry, thyroid hormones, and performance in broilers with ascites caused by caffeine. Poult. Sci. 2016;95:2673–2678. doi: 10.3382/ps/pew227. [DOI] [PubMed] [Google Scholar]
- Kien C.L., Blauwiekel R., Bunn J.Y., Jetton T.L., Frankel W.L., Holst J.J. Cecal infusion of butyrate increases intestinal cell proliferation in piglets. J. Nutr. 2007;137:916–922. doi: 10.1093/jn/137.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y., Verstegen M.W.A., Tamminga M.S., Williams B.A. The role of the commensal gut microbial community in broiler chickens. World’s Poult. Sci. J. 2005;61:95–104. [Google Scholar]
- Lawhon S.D., Russell M., Mitsu S., Craig A. Intestinal short-chain fatty acids alter Salmonella typhimurium invasion gene expression and virulence through BarA/SirA. Mol. Microbiol. 2002;46:1451–1464. doi: 10.1046/j.1365-2958.2002.03268.x. [DOI] [PubMed] [Google Scholar]
- Li Y., Liu T., Yan C., Xie R., Guo Z., Wang S., Zhang Y., Li Z., Wang B. Diammonium glycyrrhizinate protects against nonalcoholic fatty liver disease in mice through modulation of gut microbiota and restoration of intestinal barrier. Mol. Pharm. 2018;15:3860–3870. doi: 10.1021/acs.molpharmaceut.8b00347. [DOI] [PubMed] [Google Scholar]
- Liao X., Suo H., Lu L., Hu Y., Zhang L., Luo X. Effects of sodium, 1,25-dihydroxyvitamin D3 and parathyroid hormone fragment on inorganic P absorption and Type IIb sodium-phosphate cotransporter expression in ligated duodenal loops of broilers. Poult. Sci. 2017;96:2344–2350. doi: 10.3382/ps/pex033. [DOI] [PubMed] [Google Scholar]
- Liu J., Wang J., Shi Y., Su W., Chen J., Zhang Z., Wang G., Wang F. Short chain fatty acid acetate protects against ethanol-induced acute gastric mucosal lesion in mice. Biol. Pharm. Bull. 2017;40:1439–1446. doi: 10.1248/bpb.b17-00240. [DOI] [PubMed] [Google Scholar]
- Macfarlane G.T., Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012;95:50–60. doi: 10.5740/jaoacint.sge_macfarlane. [DOI] [PubMed] [Google Scholar]
- Macy T.M. The biology of gastrointestinal bacteroides. Annu. Rev. Microbiol. 1979;33:561–594. doi: 10.1146/annurev.mi.33.100179.003021. [DOI] [PubMed] [Google Scholar]
- National Research Council. 9th ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Ohira H., Tsutsui W., Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J. Atheroscler. Thromb. 2017;24:660–672. doi: 10.5551/jat.RV17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olukosi O.A., Dono N.D. Modification of digesta pH and intestinal morphology with the use of benzoic acid or phytobiotics and the effects on broiler chicken growth performance and energy and nutrient utilization. J. Anim. Sci. 2014;92:3945–3953. doi: 10.2527/jas.2013-6368. [DOI] [PubMed] [Google Scholar]
- Panda A.K., Rama R.S.V., Raju M.V.L.N., Shyam S.G. Effect of butyric acid on performance, gastrointestinal tract health and carcass characteristics in broiler chickens. Asian-australian J. Anim. Sci. 2009;22:1026–1031. [Google Scholar]
- Ranjitkar S., Lawley B., Tannock G., Engberg R.M. Bacterial succession in the broiler gastrointestinal tract. Appl. Environ. Microbiol. 2016;82:2399–2410. doi: 10.1128/AEM.02549-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekelja M., Rud I., Knutsen S.H., Denstadli V., Westereng B., Naes T., Rudi K. Abrupt temporal fluctuations in the chicken fecal microbiota are explained by its gastrointestinal origin. Appl. Environ. Microbiol. 2012;78:2941–2948. doi: 10.1128/AEM.05391-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant M.J., Constantinidou C., Cogan T.A., Bedford M.R., Penn C.W., Pallen M.J. Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS One. 2014;9:e91941. doi: 10.1371/journal.pone.0091941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakouri M.D., Iji P.A., Mikkelsen L.L., Cowieson A.J. Intestinal function and gut microflora of broiler chickens as influenced by cereal grains and microbial enzyme supplementation. J. Anim. Physiol. Anim. Nutr. 2009;93:647–658. doi: 10.1111/j.1439-0396.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- Shao Y., Lei Z., Yuan J., Yang Y., Guo Y., Zhang B. Effect of zinc on growth performance, gut morphometry, and cecal microbial community in broilers challenged with Salmonella enterica Serovar Typhimurium. J. Microbiol. 2014;52:1002–1011. doi: 10.1007/s12275-014-4347-y. [DOI] [PubMed] [Google Scholar]
- Sun Y., O’Riordan M.X.D. Regulation of bacterial pathogenesis by intestinal short-chain fatty acids. Adv. Appl. Microbiol. 2013;85:93–118. doi: 10.1016/B978-0-12-407672-3.00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite D.W., Taylor M.W. Characterizing the avian gut microbiota: membership, driving influences, and potential function. Front. Microbiol. 2014;5:1–12. doi: 10.3389/fmicb.2014.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Zeng D., Zhang Y., Ni X.Q., Wang J., Jian P., Zhou Y., Zhou Y., Li Y., Yin Z.Q., Pan K.C., Jing B. Lactobacillus plantarum BS22 promotes gut microbial homeostasis in broiler chickens exposed to aflatoxin B. J. Anim. Physiol. Anim. Nutr. 2017;102:e449–e459. doi: 10.1111/jpn.12766. [DOI] [PubMed] [Google Scholar]
- Zhai Q.X., Zhang Q.S., Tian F.W., Zhao J.X., Zhang H., Chen W. The synergistic effect of Lactobacillus plantarum CCFM242 and zinc on ulcerative colitis through modulating intestinal homeostasis. Food Funct. 2019;10:6147–6156. doi: 10.1039/c9fo00926d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.