Abstract
Probiotics, toxin binders, and plant extracts improve health and immunity of broiler chickens exposed to aflatoxin. The effects of licorice extract (LE), Protexin probiotic, toxin binder (Agrabound), and poultry litter biochar (PLB) in experimental aflatoxicosis were evaluated. In a completely randomized design, 504 broiler chickens were allotted to 7 treatments and 6 replicates with 12 broiler chickens in each. The experimental groups were as follows: T1) basal diet (B) without any feed additive or aflatoxin B1 (AFB1); T2) B + 0.5 mg AFB1/kg; T3) T2 + 3 g LE/kg; T4) T2 + 6 g LE/kg; T5) T2 + 0.5 g Protexin/kg; T6) T2 + 1 g toxin binder/kg, and T7) T2 + 5 g/kg PLB. Broiler chickens fed AFB diet (T2) had lower body weight gain at the end of grower period and higher feed conversion ratio at the end of the finisher period, whereas inclusion of LE, probiotic, toxin binder, or PLB restores body weight of broiler chickens to that of the control group. Aflatoxicosis decreased total protein, TG, albumin, Ca, and P concentrations and greater uric acid concentration in broiler chickens as compared with the control group (P < 0.05). As compared with the T2 group, inclusion of 3 mg LE/kg increased serum total protein; inclusion of 3 mg LE/kg, probiotic, and toxin binder increased TG; inclusion of 3 and 6 mg LE/kg, probiotic, and PLB increased serum albumin; and the whole additive decreased serum uric acid of broiler chickens comparing with the control group. Lymphocyte percentage, avian influenza antibody titer, thymus relative weight, and immune response to phytohemagglutinin were decreased in the T2 group, whereas heterophil percentage and heterophil-to-lymphocyte ratio were increased (P < 0.05). Aflatoxicosis increased breast meat malondialdehyde concentration, liver enzymes activities, and number of fat vacuoles (P < 0.05). As compared with the T2 group, all of the additives lowered alkaline phosphatase, aspartate aminotransferase, and alanine transaminase activities, breast meat malondialdehyde concentration, and liver pathological damages (P < 0.05). It can be concluded that all of the additives are capable to decrease the negative impact of AFB1 on broiler chickens' performance, blood indices, and immunity.
Key words: aflatoxin, broiler, performance, medicinal herb, adsorbents
Introduction
Aflatoxins (AFs) are a group of mycotoxins that are largely produced by the fungi Aspergillus flavus and Aspergillus parasiticus (Zhao et al., 2010). Aflatoxin B1 (AFB1) is known to depress performance, immune response, and induce liver disorders in poultry species (Andretta et al., 2011). Significant changes in serum biochemistry such as serum total protein, albumin, cholesterol, glucose (Zhao et al., 2010), uric acid (Oğuz et al., 2002), calcium, and phosphorus concentrations (Denli et al., 2009) reduction were reported as indicative signs of aflatoxicosis. Immune response suppression by AF including lower antibody production against Newcastle disease (ND), avian influenza (AI) viruses, higher skin response to mitogens (Bagherzadeh Kasmani et al., 2012), and impaired cell-mediated immunity (Hoerr, 2010) has been well documented.
There are 3 ways to control mycotoxins (Khatoon and Abidin, 2018): 1) biological method: use of microorganisms such as probiotics for AF biotransformation (Bagherzadeh Kasmani et al., 2012) and prebiotic such as mannan oligosaccharide (Zaghini et al., 2005); 2) physical method: including use of toxin binders and adsorbing agents such as hardwood charcoal (Yamauchi et al., 2014), biochar, zeolite, bentonite (Prasai et al., 2017), and calcium aluminosilicates (Chen et al., 2014) that metabolize or absorb AFs in gastrointestinal tract (GIT) and prevent their absorption and entrance to the liver; and 3) chemical method: such as using extracts or essential oils of some plants as antioxidant agent.
Biochar is a carbon compound created under relatively low level or absence of oxygen and moderate-pressure pyrolysis of a variety of feedstock such as wood products, agricultural residues, animal litters. Because of its highly porous nature, biochar has high surface area per unit of volume and very good absorptive properties (Spokas et al., 2012). Chemical and physical properties of biochar can vary significantly depending on the type of feedstock used. It is reported that biochar improved growth rates, feed conversion ratio (FCR), and deactivate toxins in the broiler chickens' digestive system (Prasai et al., 2016).
Agrabond toxin binder (Agranco Corp. Co., FL) is a calcium- and sodium-based mycotoxin binder selected to have high affinity for effective adsorption of polar and nonpolar toxins, including AF, fusarium, and zearalenone. It has cation exchange properties and is capable of trapping molecules within its pores. Owing to toxin binder porosity, particle and crystal size, it could be used as adsorbent in broiler feed to be bound to mycotoxins (Boudergue et al., 2009).
It appears that AFB1 is bound to the surface components of probiotic bacteria (Haskard et al., 2001). The polysaccharide part of the lactic acid bacteria (LAB) cell wall is involved in the surface binding to AFs and teichoic acids has dominant role in binding mechanism (Bagherzadeh Kasmani et al., 2012).
Licorice extract (LE) has powerful antioxidants properties and absorb free radicals. Licorice root extract has antifungal spectrum against A. flavus and its essential oils completely inhibited AFB1 production and exhibited antioxidant activity as a free radical scavenger (Saxena, 2005).
We hypothesized that the inclusion of LE and biochar into the diet of broiler chickens exposed to aflatoxicosis may ameliorate adverse effects of aflatoxicosis like probiotic and toxin binders. In addition, to date, no report is available on the effects of poultry litter biochar (PLB) and LE on broiler chickens exposed to AF. The objectives of the present study were as follows: 1) to evaluate the protective effect of Protexin probiotic, toxin binder, PLB, and LE on broiler chickens against AFB1 and 2) comparing PBL, Protexin, and toxin binder (as agents act in GIT) with LE (as antioxidative agent that acts in the liver) to determine which group is better in preventing or reducing aflatoxicosis in broiler chickens by measuring performance, carcass traits, antibody titers, serum biochemical parameters, as well as liver enzymes activities, hepatocyte pathology, and antioxidant status.
Materials and methods
All experimental procedures being used were approved by the Animal Welfare Committee of the Department of Animal Science, University of Ilam.
Animals, Experimental Design, Diet, and Housing
In a completely randomized design, 504 1-day-old Ross-308 broiler chickens (initial weight = 43 g) were assigned to 7 dietary treatments with 6 replicates and 12 broiler chickens in each pen. The experimental groups were as follows: T1) basal diet without any feed additive or AFB1; T2) basal diet + 0.5 mg AFB1/kg; T3) T2 + 3 g LE/kg; T4) T2 + 6 g LE/kg; T5) T2 + 0.5 g Protexin/kg; T6) T2 + 1 g toxin binder/kg, and T7) T2 + 5 g/kg PLB. The probiotic Protexin has 9 bacterial strains including Streptococcus salivarius spp. Thermophilus, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus rhamnosus, Bifidobacterium bifidum, Enterococcus faecium, Candida pintolopesii, and Aspergillus oryzae 2 × 109 CFU/g (Kavyani et al., 2012). No antibiotics were offered to the broiler chickens via either feed or water during the trial. Protexin and toxin binder were used based on catalog recommendations. Poultry litter biochar and LE usage levels were selected based on previous studies (Sedghi et al., 2010; Prasai et al., 2017). Licorice root extract was provided by Giah-Essanse Co., Golestan, Iran. All of the additives were added to the feed at the expense of corn. The broiler chickens were kept in the pens with 1.2 × 1.2 m2 area (10 broiler chickens in 1.44 m2) under conventional conditions for vaccination, temperature, ventilation, and lighting. The broiler diets were formulated based on standardized ileal digestible amino acids (Hoehler et al., 2005), and micronutrient requirements were obtained from Ross catalog recommendations as the starter (1–11 d), grower (12–24 d), and finisher (25–42 d) periods (Aviagen, 2014). The chemical composition of basal diet such as crude protein, crude fiber, Ca, P, Na, and Cl concentration were analyzed by AOAC methods (AOAC, 1990) (Table 1).
Table 1.
Ingredients and nutrient composition of the basal diet (as fed).
| Item | Starter (1–11 d) | Grower (12–24 d) | Finisher (25–42 d) |
|---|---|---|---|
| Ingredient (g/kg, as fed basis) | |||
| Corn yellow (80 g/kg CP) | 535.9 | 630.0 | 665.7 |
| Soybean meal (440 g/kg CP) | 330.0 | 282.3 | 278.0 |
| Corn gluten meal (600 g/kg CP) | 74.0 | 39.2 | 16.0 |
| Soybean oil | 16.0 | 10.0 | 10.0 |
| DL-Methionine (990 g/kg methionine)1 | 2.3 | 1.6 | 1.3 |
| L-Lysine HCl (760 g/kg Lysin)1 | 3.1 | 1.7 | 1.0 |
| L-Threonine (980 g/kg threonine)1 | 0.5 | 0.0 | 0.0 |
| Dicalcium phosphate (180 g/kg P, 220 g/kg Ca) | 15.3 | 16.3 | 9.0 |
| Limestone flour (388 g/kg Ca) | 13.3 | 10.3 | 10.5 |
| Sodium chloride | 2.8 | 2.6 | 3.0 |
| Sodium bicarbonate | 1.8 | 1.0 | 0.5 |
| Mineral and vitamin premix2 | 5.0 | 5.0 | 5.0 |
| Total | 1,000.0 | 1,000.0 | 1,000.0 |
| Chemical composition (g/kg) | |||
| Metabolizable energy (kcal/kg) | 3,000 | 3,050 | 3,100 |
| Crude protein (analyzed) | 231.20 | 205.20 | 183.70 |
| Calcium (analyzed) | 9.93 | 8.81 | 7.85 |
| Total phosphorus (analyzed) | 8.11 | 7.27 | 6.92 |
| Available phosphorus | 4.80 | 4.37 | 4.10 |
| Sodium (analyzed) | 1.70 | 1.80 | 1.85 |
| Chloride (analyzed) | 2.25 | 2.28 | 2.26 |
| DCAB (mEq/kg)3 | 228 | 219 | 209 |
| Linoleic acid | 12.50 | 15.00 | 15.00 |
| Crude fiber (analyzed) | 49.20 | 46.40 | 43.80 |
| Aflatoxin B1 (μg/kg) | 5.40 | 7.50 | 8.30 |
| Digestible amino acids | |||
| Lysine | 12.30 | 9.70 | 9.00 |
| Methionine | 5.70 | 4.30 | 4.00 |
| Cysteine | 3.20 | 3.00 | 2.60 |
| Methionine + cysteine | 8.90 | 7.30 | 6.60 |
| Threonine | 7.70 | 6.10 | 6.10 |
| Tryptophan | 2.20 | 2.10 | 1.90 |
| Arginine | 13.30 | 10.80 | 11.30 |
Degussa Corporation, Kennesaw, GA.
Each kilogram of vitamin and trace mineral premix provided: vitamin A, 13,500 IU; vitamin D3, 2,000 IU; vitamin E 30 IU; vitamin K3, 2 mg; vitamin B1, 1 mg; vitamin B2, 6 mg; niacin, 30 mg; pantothenic acid, 12 mg; vitamin B6, 3 mg; vitamin B12, 10 μg; biotin, 0.1 mg; choline chloride, 500 mg; Fe, 50 mg as ferrous sulfate; Cu, 8 mg as copper sulfate; Mn, 80 mg as magnesium oxide; Zn, 60 mg as zinc oxide; I, 0.5 mg as potassium iodate; Co, 0.1 mg as cobalt carbonate; Se, 0.15 mg as selenium premix.
DCAB = dietary cation–anion balance.
Aflatoxin and Biochar Production
During the gasification process, poultry litter is subjected to temperatures greater than 400°C in an oxygen-controlled environment (in the absence of oxygen or at a highly reduced concentration of oxygen) producing PLB (Zubelena et al., 1990).
Aflatoxin was produced from an A. parasiticus PTCC-5286 culture (obtained from the Iranian Research Organization for Science and Technology) by fermentation of rice grains, and its AFB1 content was determined (Shotwell et al., 1966). A total of 30 kg rice (mesh size 2.00 mm) was placed into 2 100-L containers, 10.0 L distilled water was added to each, and the mixture was autoclaved. The media in both the containers were inoculated with 500 mL A. flavus (1 × 108 spores/mL) and incubated at 28°C for 7 d. The incubated rice was autoclaved to kill the A. flavus, dried, and ground (mesh size 0.425 mm) for the animal feeding experiment. The AFB1 concentrations in the moldy rice from the 2 containers were measured as 6.9 mg/kg and modulated to 0.5 mg of AFB1/kg in the feed at the expense of the corn in the formulation. The AF concentration of the control and AFB diets were detected (Shotwell et al., 1966). The AF concentrations for control diet were 5.40, 7.50, and 8.30 (μg/kg) and for AFB diet were 0.48, 0.485, and 0.4.9 mg/kg at starter, grower, and finisher diets, respectively.
Performance, Serum Biochemistry, and Visceral Organs Weight
Body weight (BW) and feed intake were recorded during starter, grower, and whole of the experiment (1–42 d of age), then the FCR was calculated. Two broiler chickens with BW close to mean BW of each replicate were randomly selected. In nonheparinized collecting tubes, blood samples were collected from the wing vein. Then, serum glucose, total protein, triglyceride, high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol, albumin, uric acid, Ca, and P concentrations were analyzed using diagnostic kits (Pars Azmun, Tehran, Iran) and enzymatic methods. In addition, heterophil and lymphocyte percentage were determined by automated cell counter (Exigo model 25102) and consequently heterophil-to-lymphocyte ratio was calculated.
At the end of the experiment (42 d), 2 broiler chickens of each replicate were randomly selected and then slaughtered by Islamic method. Carcass, liver, abdominal fat, spleen, bursa of Fabricius and thymus weights were measured and relative weight to total BW of broiler chickens were determined. Breast and thigh relative weights were determined as percentage of carcass weight.
Humoral and Cell-Mediated Immunity and Blood Hematology
All the broiler chickens were vaccinated at 7 d of age intramuscularly through the breast muscle with killed ND and influenza vaccines which were provided from Ceva Santé Animale company. The ND LaSota vaccine (Ceva Santé Animale Co.) was used in the drinking water for vaccination at 14 d of age. Two broiler chickens with BW near the mean of BW of each replicate were randomly selected at 24 d of age, and then individual blood samples were collected. Avian influenza and ND virus antibody titers (OIE, 2008) were detected. Toe-web swelling reaction to phytohemagglutinin-P (Sigma L 9017, Sigma Aldrich) was measured in 2 broiler chickens from each replicate at 30 d of age at 4, 24, and 48 h after injection (Corrier and DeLoach, 1990).
Serum and Breast Meat Malondialdehyde and Liver Enzymes Activity
Two broiler chickens with BW near the mean of BW of each replicate were randomly selected. Blood samples were collected and serum malondialdehyde (MDA) was measured (Placer et al., 1966). Serum alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine transaminase (ALT) activities were determined by the colorimetric method. Finally, 2 broiler chickens with BW close to the mean of each replicate were selected, and after killing, the breast samples were collected and breast meat MDA was determined (Placer et al., 1966).
Liver Histopathological Analysis
For histopathological examination, the liver tissues of slaughtered broiler chickens were fixed in 10% of neutral buffered formalin, routinely embedded in paraffin, cut into 5-μm thick sections, and processed for hematoxylin and eosin staining (Kiernan, 2015). Liver sections of the broiler chickens were microscopically examined.
Statistical Analysis
All data were analyzed in accordance with a completely randomized design arrangement using GLM procedure (SAS, 2004). Tukey's multiple range tests were used to compare treatments' means (P < 0.05). A repeated measurements analysis was used to compare broiler groups for their cutaneous basophil hypersensitivity test over the time (h). Liver histopathological data were analyzed using chi-square procedure (SAS, 2004). Orthogonal contrasts analysis was performed to compare means of positive control group vs. groups fed AFB1.
Results
Performance and Serum Biochemistry
Effects of dietary treatments on broiler chickens' growth performance are shown in Table 2. Broiler chickens' feed intake during all of the periods and broiler chicken BWs at the end of starter (1–11 d) and finisher periods (25–42 d) and FCR at the end of starter (1–11 d) and total experiment (1–42 d) periods were not affected by dietary treatments (P > 0.05). As compared with the control group, AFB1-contaminated diet decreased broiler chickens' BW at the end of grower (12–24 d) and total experimental periods (P < 0.05), whereas increased FCR of broiler chickens at the end of grower (12–24 d) and finisher periods (25–42 d). Treatment of broiler chickens with LE3 and PLB restored broiler chickens' BW at the end of the grower period, which was comparable with that of broiler chickens fed control diet (P < 0.05). Treatment of broiler chickens with LE6 and probiotic restored broiler chickens' FCR at the end of the finisher period, which was comparable with that of broiler chickens fed control diet (P < 0.05).
Table 2.
Effect of treatments on average daily feed intake (ADFI), average daily body weight gain (ADBWG) and FCR of broiler chickens exposed to aflatoxin B1.
| Items | Treatments1 |
SEM |
P-value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | AFB | LE3 | LE6 | PRO | TXB | PLB | Treatment | Control vs. AFB | ||
| ADFI (g) | ||||||||||
| Starter2 | 23.55 | 23.58 | 23.55 | 23.75 | 23.96 | 23.67 | 23.44 | 0.265 | 0.848 | 0.267 |
| Grower | 103.80 | 101.82 | 103.98 | 102.74 | 103.34 | 103.55 | 103.92 | 0.695 | 0.303 | 0.314 |
| Finisher | 150.24 | 147.16 | 146.47 | 143.00 | 139.91 | 150.24 | 148.93 | 5.344 | 0.739 | 0.142 |
| Total | 102.69 | 100.76 | 101.12 | 99.30 | 98.22 | 102.64 | 102.13 | 2.279 | 0.668 | 0.386 |
| ADBWG (g) | ||||||||||
| Starter | 18.64 | 18.96 | 20.09 | 19.15 | 18.65 | 19.47 | 19.64 | 0.555 | 0.491 | 0.631 |
| Grower | 63.15a | 53.48b | 62.02a | 58.08a,b | 58.11a,b | 57.77a,b | 61.68a | 2.031 | 0.032 | 0.002 |
| Finisher | 83.07 | 74.04 | 76.64 | 76.09 | 77.58 | 78.31 | 71.71 | 1.917 | 0.357 | 0.424 |
| Total | 60.03a | 53.25b | 57.30a | 55.60a,b | 56.12a,b | 56.54a,b | 54.97a,b | 1.160 | 0.012 | 0.003 |
| FCR | ||||||||||
| Starter | 1.26 | 1.24 | 1.17 | 1.25 | 1.28 | 1.21 | 1.20 | 0.232 | 0.512 | 0.486 |
| Grower | 1.65b | 1.91a | 1.68b | 1.77a,b | 1.78a,b | 1.82a,b | 1.68b | 0.051 | 0.043 | 0.004 |
| Finisher | 1.81b | 1.98a | 1.91a | 1.78b | 1.80b | 1.92a | 2.07a | 0.061 | 0.017 | 0.001 |
| Total | 1.71 | 1.89 | 1.77 | 1.78 | 1.75 | 1.81 | 1.85 | 0.047 | 0.048 | 0.237 |
a–c Means within the same column with different letters are statistically significant (P < 0.05).
The values are least square means of the 6 replicates in each treatment.
Control = basal diet, AFB = basal diet + 0.5 mg/kg aflatoxin B1, LE3 = basal diet + AFB1 + 3 g/kg licorice extract, LE6 = basal diet + AFB1 + 6 g/kg licorice extract, PRO = basal diet + AFB1 + 0.5 g/kg Protexin probiotic, TXB = basal diet + AFB1 + 1 g/kg toxin binder, and PLB = basal diet + AFB1 + 5 g/kg poultry litter biochar.
Starter period was 1 to 11 d, grower period was 12 to 24 d, finisher period was 25 to 42 d and total experimental period was 1 to 42 d.
No significant differences were observed in serum glucose, cholesterol, and HDL concentrations (P > 0.05; Table 3). As compared with broiler chickens fed AFB1-contaminated diet, treatment of broiler chickens with PLB and Protexin increased serum albumin concentration (P > 0.05). Broiler chickens fed AFB1-contaminated diet had lower serum total protein, triglyceride, and Ca concentrations and higher uric acid concentration as compared with the group fed uncontaminated diet (P < 0.05). Treatment of broiler chickens with 3 g/kg LE or Protexin increased serum total protein and triglyceride, respectively, and restored them to the values similar to the control group. On the other hand, inclusion of 3 and 6 g/kg LE, Protexin, or toxin binder decreased broiler chickens' serum LDL concentration.
Table 3.
Effect of treatments on blood indices (mg/dL except mentioned) of broiler chickens exposed to aflatoxin B1.2
| Item | Glu | TP (g/dL) | TG | Chol | LDL | HDL | ALB (g/dL) | UA | Ca | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments1 | ||||||||||
| Control | 151.7 | 4.9a | 120.4a,b | 127.8 | 61.9a,b | 35.2 | 1.9a,b | 5.7b | 7.1a | 4.8c,d |
| AFB | 164.5 | 2.8d | 61.6c | 102.3 | 72.7a | 70.7 | 1.6b,c | 7.1a | 4.9c | 2.9d |
| LE3 | 148.3 | 4.7a,b | 78.2b,c | 117.5 | 32.6b,c | 69.3 | 1.9a,b | 6.3b | 4.9c | 3.3d |
| LE6 | 151.3 | 4.0b,c | 107.2a,b,c | 99.6 | 33.1b,c | 45.1 | 1.7a,b,c | 5.7c | 5.9b | 3.4d |
| PRO | 156.0 | 3.9c | 138.7a | 107.1 | 24.4c | 54.9 | 1.9a | 6.3b | 6.1b | 6.1b,c |
| TXB | 153.5 | 3.7c | 94.2ab,c | 105.8 | 25.8c | 61.2 | 1.6c | 5.8b,c | 5.8b,c | 8.3a |
| PLB | 147.5 | 3.7c | 89.9b,c | 124.6 | 56.4a,b,c | 50.2 | 2.0a | 5.8b,c | 5.8b,c | 7.9a,b |
| SEM | 7.12 | 0.26 | 10.14 | 7.02 | 11.13 | 9.53 | 0.09 | 0.18 | 0.29 | 0.65 |
| P-value | 0.163 | 0.004 | 0.005 | 0.053 | 0.011 | 0.134 | 0.015 | 0.001 | 0.001 | 0.001 |
| Contrasts | P-value | |||||||||
| Control vs. AFB | 0.346 | 0.005 | 0.019 | 0.049 | 0.006 | 0.066 | 0.024 | 0.003 | 0.003 | 0.033 |
a–c Means within the same column with different letters are statistically significant (P < 0.05).
The values are least square means of the 6 replicates in each treatment.
Control = basal diet, AFB = basal diet + 0.5 mg/kg aflatoxin B1, LE3 = basal diet + AFB1 + 3 g/kg licorice extract, LE6 = basal diet + AFB1 + 6 g/kg licorice extract, PRO = basal diet + AFB1 + 0.5 g/kg Protexin probiotic, TXB = basal diet + AFB1 + 1 g/kg toxin binder, and PLB = basal diet + AFB1 + 5 g/kg poultry litter biochar.
Glu = glucose, TP = total protein, TG = triglyceride, Chol = cholesterol, HDL = high-density lipoprotein, LDL = low-density lipoprotein, ALB = albumin, UA = uric acid, Ca = calcium, and P = phosphorus.
Blood Hematology and Immune Response
Mean leukocyte percentages are shown in Table 4. Inclusion of LE, Protexin, toxin binder, or PLB to broiler chickens' diet restored heterophil and lymphocyte percentage and heterophil-to-lymphocyte ratio to their original levels which are comparable with the control group. There was no significant difference among the treatments in spleen and bursa of Fabricius relative weight and antibody titer against ND virus (P > 0.05; Table 4). As compared with the control group, feeding AFB1-contaminated diet decreased broiler chickens' thymus relative weight and AI titer (P < 0.05). However, treatment of broiler chickens with Protexin, toxin binder, or 3 mg LE/kg diet did not compensate the adverse effect of AFB1 on AI titer (P > 0.05).
Table 4.
Effect of treatments on blood cell count, relative weights of lymphoid organs and humoral immunity of broilers exposed to aflatoxin B1.2
| Item | Blood cells |
Lymphoid organs (% of live weight) |
Antibody titer (IU) |
|||||
|---|---|---|---|---|---|---|---|---|
| H (%) | L (%) | H:L | Bursa | Thymus | Spleen | ND | AI | |
| Treatments1 | ||||||||
| Control | 34.4b | 63.8a | 0.54c,d | 0.21 | 0.19a | 0.13 | 6.33 | 5.66a |
| AFB | 42.2a | 56.6c | 0.75a | 0.22 | 0.14b | 0.12 | 4.66 | 2.33b |
| LE3 | 35.4b | 63.0a | 0.56c,d | 0.23 | 0.14b | 0.12 | 6.66 | 3.33b |
| LE6 | 34.2b | 63.8a | 0.54c,d | 0.26 | 0.18a,b | 0.14 | 5.00 | 5.33a |
| PRO | 34.0b | 64.0a | 0.53d | 0.28 | 0.21a | 0.13 | 6.33 | 3.00b |
| TXB | 34.0b | 64.4a | 0.52d | 0.29 | 0.18a,b | 0.14 | 4.66 | 3.00b |
| PLB | 38.2a,b | 60.2b | 0.63b,c | 0.25 | 0.17a,b | 0.12 | 6.33 | 5.33a |
| SEM | 1.33 | 1.06 | 0.03 | 0.03 | 0.01 | 0.02 | 0.81 | 0.80 |
| P-value | 0.002 | 0.001 | 0.001 | 0.602 | 0.012 | 0.977 | 0.058 | 0.001 |
| Contrasts | P-value | |||||||
| Control vs. AFB | 0.001 | 0.001 | 0.001 | 0.588 | 0.002 | 0.746 | 0.165 | 0.014 |
a–c Means within the same column with different letters are statistically significant (P < 0.05).
The values are least square means of the 6 replicates in each treatment.
Control = basal diet, AFB = basal diet + 0.5 mg/kg aflatoxin B1, LE3 = basal diet + AFB1 + 3 g/kg licorice extract, LE6 = basal diet + AFB1 + 6 g/kg licorice extract, PRO = basal diet + AFB1 + 0.5 g/kg Protexin probiotic, TXB = basal diet + AFB1 + 1 g/kg toxin binder, and PLB = basal diet + AFB1 + 5 g/kg poultry litter biochar.
H = heterophil, L = lymphocyte, H:L = heterophil-to-lymphocyte ratio, ND = Newcastle disease, and AI = avian influenza.
Feeding AFB1-contaminated diet resulted in significant suppression in toe-web thickness index of broiler chickens (P > 0.05; Table 5). Treatment of broiler chickens with all of the additives resulted in higher toe-web thickness index as compared with the group fed AFB1-contaminated diet, and it was much more pronounced in the group fed Protexin-containing diet.
Table 5.
Effect of dietary treatments on toe web thickness index against PHA-P injection of broiler chickens exposed to aflatoxin B1 at 30 d of age.
| Item | Index |
|---|---|
| Treatments1 | |
| Control | 0.793a |
| AFB | 0.457e |
| LE3 | 0.703c |
| LE6 | 0.706c |
| PRO | 0.805a |
| TXB | 0.743b |
| PLB | 0.615d |
| SEM | 0.023 |
| Time (h) | |
| 4 | 0.803a |
| 24 | 0.669b |
| 48 | 0.595c |
| SEM | 0.017 |
| P-value | |
| Treatment | 0.001 |
| Time | 0.001 |
| Treatment × time | 0.164 |
| Contrasts | P-value |
| Control vs. AFB | 0.001 |
a–c Means within the same column with different letters are statistically significant (P < 0.05).
The values are least square means of the 6 replicates in each treatment.
Abbreviations: PHA-P, phytohemagglutinin-P.
Control = basal diet, AFB = basal diet + 0.5 mg/kg aflatoxin B1, LE3 = basal diet + AFB1 + 3 g/kg licorice extract, LE6 = basal diet + AFB1 + 6 g/kg licorice extract, PRO = basal diet + AFB1 + 0.5 g/kg Protexin probiotic, TXB = basal diet + AFB1 + 1 g/kg toxin binder, and PLB = basal diet + AFB1 + 5 g/kg poultry litter biochar.
Carcass Characteristics
The data of Table 6 illustrate that dietary treatments had no significant effect on the carcass, liver, and thigh relative weights (P > 0.05), whereas breast meat relative weight was decreased by feeding AFB1-contaminated diet (P < 0.05). Inclusion of LE, Protexin, or PLB to AFB1-contaminated diet significantly increased breast meat percentage, and it was more pronounced in the group fed Protexin-containing diet.
Table 6.
Effect of treatments on carcass traits of broiler chickens exposed to aflatoxin B1 (%).
| Item | Carcass2 | Thigh | Breast | Liver | Abdominal fat |
|---|---|---|---|---|---|
| Treatments1 | |||||
| Control | 60.74 | 26.95 | 35.91a | 2.32 | 0.92a,b |
| AFB | 59.38 | 27.06 | 32.28c | 2.54 | 1.15a |
| LE3 | 59.84 | 28.87 | 35.30a,b | 2.31 | 0.92a,b |
| LE6 | 59.96 | 27.01 | 35.16a,b | 2.07 | 0.56b |
| PRO | 60.15 | 27.17 | 35.87a | 2.23 | 0.80a,b |
| TXB | 58.95 | 27.78 | 33.33b,c | 2.29 | 0.90a,b |
| PLB | 59.75 | 27.09 | 35.07a,b | 2.28 | 0.80a,b |
| SEM | 0.67 | 0.73 | 0.75 | 0.15 | 0.11 |
| P-Value | 0.626 | 0.502 | 0.014 | 0.356 | 0.042 |
| Contrasts | P-value | ||||
| Control vs. AFB | 0.233 | 0.941 | 0.033 | 0.313 | 0.041 |
a–c Means within the same column with different letters are statistically significant (P < 0.05).
The values are least square means of the 6 replicates in each treatment.
Control = basal diet, AFB = basal diet + 0.5 mg/kg aflatoxin B1, LE3 = basal diet + AFB1 + 3 g/kg licorice extract, LE6 = basal diet + AFB1 + 6 g/kg licorice extract, PRO = basal diet + AFB1 + 0.5 g/kg Protexin probiotic, TXB = basal diet + AFB1 + 1 g/kg toxin binder, and PLB = basal diet + AFB1 + 5 g/kg poultry litter biochar.
Carcass, liver, and abdominal fat presented as percentage of live body weight and breast and thigh presented as percentage of carcass weight.
Serum and Meat Malondialdehyde, Enzyme Activity, and Liver Histopathology
As noted in Table 7, results showed that AFB1 increased breast meat MDA concentration and ALP, ALT, and AST activities (P < 0.05). Treatment of broiler chickens with LE, Protexin, or PLB decreased breast meat MDA concentration and ALT and AST activities as low as or lower than that of the control group (P < 0.05).
Table 7.
Effect of treatments on serum and meat MDA and liver enzymes activities (IU/mL) of broilers exposed to aflatoxin B1.2
| Item | Meat MDA (nmol/g) | Serum MDA (nmol/L) | AST | ALT | ALP |
|---|---|---|---|---|---|
| Treatments1 | |||||
| Control | 332.71c | 455.59 | 216.67b,c | 3.66b,c | 6.66b |
| AFB | 498.54a | 498.23 | 301.00a | 5.00a | 8.33a |
| LE3 | 309.45c,d | 512.77 | 241.33b | 4.00a,b | 5.00d |
| LE6 | 250.28d | 467.71 | 209.33c | 3.66b,c | 5.00d |
| PRO | 238.09d | 474.48 | 191.00c,d | 2.66c | 7.66a |
| TXB | 426.09a,b | 491.9 | 161.33e,d | 3.00b,c | 8.00a |
| PLB | 354.44b,c | 477.89 | 147.33e | 1.33d | 6.00b |
| SEM | 24.86 | 24.06 | 14.42 | 0.50 | 0.43 |
| P-Value | 0.013 | 0.906 | 0.001 | 0.001 | 0.002 |
| Contrasts | P-value | ||||
| Control vs. AFB | 0.006 | 0.074 | 0.001 | 0.003 | 0.001 |
a–c Means within the same column with different letters are statistically significant (P < 0.05).
The values are least square means of the 6 replicates in each treatment.
Control = basal diet, AFB = basal diet + 0.5 mg/kg aflatoxin B1, LE3 = basal diet + AFB1 + 3 g/kg licorice extract, LE6 = basal diet + AFB1 + 6 g/kg licorice extract, PRO = basal diet + AFB1 + 0.5 g/kg Protexin probiotic, TXB = basal diet + AFB1 + 1 g/kg toxin binder, and PLB = basal diet + AFB1 + 5 g/kg poultry litter biochar.
MDA = malondialdehyde, ALP = alkaline phosphatase, AST = aspartate aminotransferase, and ALT = alanine transaminase (glutamate-pyruvate transaminase).
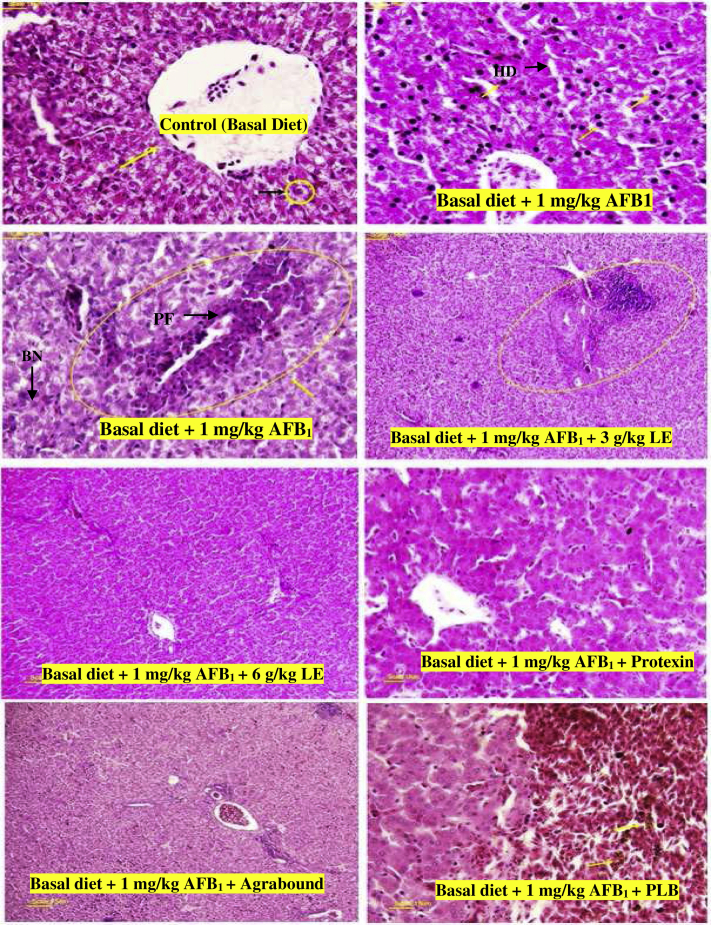
The results of liver histopathology of broiler chickens are shown in Figure 1. These findings revealed a significant damage in the liver tissue of broiler chickens fed AFB1 diet (Figure 1), and as compared with that of the control group, the liver tissue had periportal fibrosis, hydropic degeneration/fatty changes, bile duct hyperplasia, and large number of lymphocyte, heterophil, and eosinophil. Heterochromatin increase in the hepatocytes (black nucleus) indicated that AFB1 increased the hepatocyte activity.
Figure 1.
Hepatic histomorphology and histopathology from various groups of experimental broilers. The liver sections were stained with hematoxylin and eosin (100× magnification). 2- Control = basal diet, AFB = basal diet + 0.5 mg/kg aflatoxin B1, LE3 = basal diet + AFB1 + 3 g/kg licorice extract, LE6 = basal diet + AFB1 + 6 g/kg licorice extract, PRO = basal diet + AFB1 + 0.5 g/kg Protexin probiotic, TXB = basal diet + AFB1 + 1 g/kg toxin binder, and PLB = basal diet + AFB1 + 5 g/kg poultry litter biochar. The values are least square means of the 6 replicates in each treatment. Abbreviations: BN, black nodules; HD, high-density nodes; PF, peripheral fibrosis.
Histopathological analysis showed that hepatocytes of broiler chickens treated with 3 mg LE/kg still had more eosinophil and mononuclear cells than those of the control group but had lower cytoplasmic lipid vacuoles than those of the group fed AFB1 diet. Treatment of broiler chickens with 6 mg LE/kg led to considerable reduction in mononuclear and eosinophil cells around the bob portal and lower lipid vacuole compared with the AFB1-fed group.
Reduction of mononuclear and eosinophil cells were observed in liver of broiler chickens treated with Protexin but sinusoids expansion and lipid vacuole still exist. The microscopic examination of the liver sections of the toxin binder–treated broiler chickens showed normal hepatocytes and central vein similar to the control group, whereas in the PLB-treated group, there was a little sinusoids expansion and some abnormal pigments around the central vein.
Discussion
Effect of AFB1
In this study, the concentration of AFB1 in diets was set to 0.5 mg/kg and decreased BW gain and increased FCR of broiler chickens from 1 to 42 d of age, but it did not cause a mortality difference (data not shown), indicating that the birds had subclinical aflatoxicosis.
Recently it was reported that 0.95 mg of AFB1/kg was the threshold concentration to induce growth depression in broiler chickens (Andretta et al., 2011). The differences in response to induced aflatoxicosis suggested that AFB1 sensitivity depends on the strain, species, and age of broiler chickens.
Almost similar to our results, the addition of 1 mg AFB1/kg to Hubbard male broiler chickens from 1 to 42 d (Modirsanei et al., 2008), 2 mg/kg of diet to broiler chickens (Shannon et al., 2017), 1 mg/kg of diet to Ross male chickens from 1 to 21 d (Gowda et al., 2008), 40 μg/kg AFB1 to Arbor Acres broiler chickens (Liu et al., 2018), and 2.5 mg AFB1/kg to Japanese quails (Bagherzadeh Kasmani et al., 2012) decreased BW, feed intake, FCR, and productive efficiency index. Aflatoxin B1 side effects on growth performance have been related with a decrease in the protein and energy utilization (Verma et al., 2004), probably as a consequence of a deterioration of the digestive and metabolic efficiency of the broiler chickens. The effects of AF on feed efficiency are not always consistent because of different diet composition, particularly different protein sources and levels (Coffey et al., 1989) or different tryptophan concentration in diet (Khanipour et al., 2019), which were reported to alter protein utilization and animal response to AF in poultry or increase AF biotransformation.
The serum biochemistry panel results were consistent with the performance of broiler chickens. The negative effect of AFB1 on broilers was further shown by lowered albumin and total protein, triglyceride, and Ca concentrations and raised LDL and uric acid concentrations in this study. The results showed that chronic mycotoxicosis could be diagnosed by changes in serum biochemistry before major symptoms could be observed. When no additives were supplemented, serum albumin and total protein were decreased at 0.5 mg of AFB1/kg, indicating that although low levels of AFB1 did not influence performance, there was impaired protein synthesis.
Serum albumin binds small molecules, such as water, cations, fatty acids, hormones, bilirubin, and pharmaceuticals, to regulate the oncotic pressure of blood. Based on the increase in serum urea N levels, it is likely that impaired protein synthesis was due to lower utilization of amino acids. The other causes of an increase in urea nitrogen are renal failure, hypovolemia, gastrointestinal hemorrhage, and increased catabolism. Impaired protein synthesis and lower utilization of amino acids resulted in higher serum urea nitrogen (Denli et al., 2009). In addition, AFB1 is hepatotoxic and hepatocarcinogenic and can be transported as an AFB1–albumin adduct around the body (Redzwan et al., 2014). It is possible that the circulating toxicant could damage visceral organs and their functions and consequently change the levels of their main metabolites (Kumar and Balachandran, 2016).
Serum total protein, albumin, urea, and glucose concentrations have also been described as valuable parameters of hepatic injury and function (Shannon et al., 2017). Similar to our results, recent documents indicated that AFB1 negatively affected liver physiology and caused injury which resulted in impaired protein synthesis and increased serum urea nitrogen concentration (Zhao et al., 2010).
Similarly, increased liver disfunction was observed in the AFB1 diet in this study, based on the liver function biomarkers, including ALP, ALT, and AST enzymes which was consistent with the reports describing induction of aflatoxicosis by inclusion of 1 mg AFB1 to broiler chickens' diet (Gowda et al., 2008; Denli et al., 2009; Ali Rajput et al., 2017), 0.5 to 2 mg of AFB1 to broiler chickens' diet (Chen et al., 2014), and 1.5 mg AFB1/kg to broiler chickens' diet reduced serum total protein and uric acid concentrations and increased cholesterol and triglyceride concentrations and ALP, ALT, and AST enzymes activities.
The liver is responsible for the production of most of the circulating proteins. Aflatoxin B1 affects the liver function by degenerating hepatocytes. When hepatocyte permeability increased or hepatocytes were damaged, transaminase may have been released from hepatocytes into the blood and increased serum transaminases activities. Elevation in serum AST and ALP indicates cellular (hepatocyte) damage which result necrosis or altering the cell membrane permeability and muscle damage due to impaired cell membrane by AFB1 induced lipid peroxidation (Ali Rajput et al., 2017).
The liver is the target organ for bioconverting of AFB1 to AFB1 epoxide, which can bind with DNA, RNA, and proteins. This binding not only increases liver relative weight (Shannon et al., 2017) but also accumulates peroxides because of antioxidant enzymes inactivation (Shannon et al., 2017). In agreement to our results, it is reported that AFB1 elevated relative weight of liver and numerical increases in liver weight due to lipid accumulation in the liver, which results in hepatomegaly (Ali Rajput et al., 2017).
Aflatoxicosis is characterized mainly by hepatic injury, impaired productivity, and decreased immune response in broiler chickens (Oğuz et al., 2002). In the present study, in line with biochemical parameters, significant changes were observed in histological outcome of the liver of broiler chickens fed AFB1-containing diet, although no changes were observed in growth performance. Similar to the current results, researchers reported that AFs induced hepatic architecture enlargement, bile-duct hyperplasia, periportal fibrosis, hepatocytic vacuolation, and necrosis through microscopic investigation (Ma et al., 2012).
Changes in relative weight of immune organs is one of the major alterations associated with aflatoxicosis. Differences between studies remain unclear, but it is important to note that in the present study, AFB1 was used, whereas previous studies were conducted using an A. parasiticus–contaminated material, which contains all types of AFs (AFB1, aflatoxin B2, aflatoxin G1, and aflatoxin G2).
Although in the present study, clinical signs of aflatoxicosis were not observed, it is important to note that poor humoral immunity in broiler chickens caused by inclusion of 0.5 mg AFB1/kg (Tables 4 and 5). In addition, in this study, the AFB1 challenge increased the heterophil count, indicating that birds suffered from aflatoxicosis which induced leucocyte proliferation.
This is supported by reports that (relative weight of thymus), aflatoxicosis led to increase in relative weight of spleen in broiler chickens (Monson et al., 2015). However, in present study, the relative weight of spleen and bursa of Fabricius were not affected. Similar to our results, intake of AFs from naturally or artificially contaminated feed at 200 ppb to 2.5 mg/kg of diet led to suppressive effects of AFs on cell-mediated immunity and lower specific antibody production in response to sheep red blood cells (Verma et al., 2004), infectious bronchitis and Bursal disease virus and ND virus (Bagherzadeh Kasmani et al., 2012). The immune-suppressive effect of AFs has been related to its direct inhibition of protein synthesis such as immunoglobulins IgG and IgA (Ali Rajput et al., 2017), reduction of the hemolytic activity of complement (Chen et al., 2014), and reduction in the number of lymphocytes through its toxic effect on the bursa of Fabricius.
Effect of Additives
The LE, Protexin, toxin binder, and PLB used in the current experiment were able to numerically restore the BW gain and FCR of broiler chickens fed 0.5 mg of AFB1/kg and the improvement was significant. The results showed protective effects of additives on performance and the serum biochemical parameters in the study. These effects were evident because PLB and Agrabound as an adsorbing agent and LE as a biotransforming agent can effectively bind AFB1 to lower or avoid these harms. In addition, LAB as probiotics were also shown to be capable of enhancing growth and health. Studies have shown that Lactobacillus activates protective immune responses and increases antibody production in chickens.
Lactic acid bacteria are known to inhibit mold growth and bind AFB1 in different matrices. It seems that binding is reversible and that bound AFB1 is released later (Haskard et al., 2001). Therefore, it is necessary to find novel biological methods. Adsorbents such as clays, activated charcoal, bentonites, and synthetic aluminosilicates, feed additives such as prebiotics, probiotics, and plant extracts are capable to bind AFs in GIT or liver preventing or reducing their detrimental effects on animals (Abdel-Wahhab et al., 1998). The basic mechanism seems to involve chemisorption of AFs in the GIT and reduction their bioavailability (Chen et al., 2014). In addition, addition of diatomaceous (Modirsanei et al., 2008), LAB, and smectite (Liu et al., 2018) and 74 and 222 mg total curcumnoids/kg of diet (Zhang et al., 2016) improved broiler chickens performance as it was comparable with the control group.
Protexin is the probiotic product which most of its bacteria are Lactobacillus serotypes and like PLB and LE, reduced AFB1 side effects on broiler chicken performance. It is likely that multiple mechanisms are involved in AFB1 binding: AFB1 is bound to the surface components of probiotic bacteria (Haskard et al., 2001) and thick peptidoglycan layer of the gram-positive bacterial cell wall could interact with AFB1 (Monson et al., 2015) and enhance its excretion.
Poultry litter biochar and toxin binder have cation exchange properties and they are capable to trap molecules within their pores. The effective cation exchange capacity of our PLB was 107.84 Cmol+/kg, whereas effective cation exchange capacity of biochar that other researchers produced was 29.7 Cmol+/kg (Prasai et al., 2016). This porous property of PLB is capable to attract the AFB1 in GIT and eliminate its negative effect on broiler chicken's performance, immunity, serum biochemical parameters, and liver histopathology. Similar to our results, it is reported that inclusion of 2% PLB to fungal contaminated diet in laying hens improved egg production and egg weight (Prasai et al., 2017).
Plant extracts have phenolic compound and currently used in broiler chickens' diet to detoxify the AFB1 because of their antioxidative properties. Glycyrrhizin is a triterpenoid saponin that is present within a range of 2 to 14% in different species of Glycyrrhiza glabra and isoflavones like glycyrrhizin, glabridin, and hispaglabridin-A present in Glycyrrhiza have a very potential antioxidant activity (Saxena, 2005).
It was reported that G. glabra extract has antifungal properties (Fatima et al., 2009), but there is little information about its effect on mycotoxicosis. Because lipid peroxidation plays a major role in the toxicity of AFs, a protective effect of antioxidants is possible. Oxidative stress plays a key role in toxicity mechanism of AFB1 and antioxidants protect animals against AFB1-induced toxicity (Zhang et al., 2016).
Aflatoxicosis induces reactive oxygen species formation and changes in liver enzyme activity could reduce the AFB1-induced cytotoxicity. Cytochrome P450s and glutathione-S-transferase enzymes are believed to be responsible for metabolic activation of AFB1. Glycyrrhizic acid prevents the oxidative and hepatic damage caused by AFs by increasing cytochrome P450-A1 and glutathione-S-transferase activities (Saxena, 2005) and its glutathione peroxidase–like activity seems to play an important role in its antioxidative effects. In addition, glycyrrhizic acid may inhibit the biotransformation of AFB1 to aflatoxicol in the liver (Lee et al., 2001) and detoxify the AFs in broiler chickens' diet.
Conclusion
In conclusion, treatment of broiler chicken with LE, Protexin, toxin binder, and PLB ameliorated the negative effects of aflatoxicosis which resulted in increased serum albumin and total protein concentrations and decreased ALP, AST, ALT activities, LDL, and meat MDA concentrations. Our results showed that some of the additives ameliorated liver pathological damages and immune function of broiler chickens, which they were pronounced in the toxin binder–treated and PLB-treated groups. The probable reason for our result could be due to 1) action as an absorbent or biotransforming action of AFB1 either by LE providing antioxidant protection properties or adsorbing AFB1 in GIT by Protexin, toxin binder, and PLB and 2) increasing antioxidation capacity by liver due to improved liver histopathology or serum antioxidant enzyme activity.
Acknowledgments
The authors thank Kian-Farmino Company (Iran) for providing toxin binder. The biochar production and its FTIR characterization was provided by Dr. Fatemeh Valizadeh Kakhki. This research did not receive any specific funding.
All the applied experimental procedures were approved by the Animal Welfare Committee of the Department of Animal Science, Ilam University. It is stated that all persons gave their informed consent before their inclusion in the study.
Authors declare that no data or models of this research are deposited in an official repository.
Conflict of Interest Statement: The authors have no conflict of interest to declare.
References
- Abdel-Wahhab M.A., Nada S.A., Farag I.M., Abbas N.F., Amra H.A. Potential protective effect of HSCAS and bentonite against dietary aflatoxicosis in rat: with special reference to chromosomal aberrations. Nat. Toxins. 1998;6:211–218. doi: 10.1002/(sici)1522-7189(199809/10)6:5<211::aid-nt31>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Ali Rajput S., Sun L., Zhang N., Mohamed Khalil M., Gao X., Ling Z., Zhu L., Khan F.A., Zhang J., Qi D. Ameliorative effects of grape seed proanthocyanidin extract on growth performance, immune function, antioxidant capacity, biochemical constituents, liver histopathology and aflatoxin residues in broilers exposed to aflatoxin B1. Toxins. 2017;9:371. doi: 10.3390/toxins9110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andretta I., Kipper M., Lehnen C., Hauschild L., Vale M., Lovatto P. Meta-analytical study of productive and nutritional interactions of mycotoxins in broilers. Poult. Sci. 2011;90:1934–1940. doi: 10.3382/ps.2011-01470. [DOI] [PubMed] [Google Scholar]
- AOAC. Vol 2. Association of Official Analytical Chemists Inc; Rockville, MD: 1990. (Official Methods of Analysis of the AOAC). [Google Scholar]
- Avaigen, Scotland, UK; 2014. Aviagen. Ross 308 Broiler Nutrition Specification. [Google Scholar]
- Bagherzadeh Kasmani F., Karimi Torshizi M., Allameh A., Shariatmadari F. A novel aflatoxin-binding Bacillus probiotic: performance, serum biochemistry, and immunological parameters in Japanese quail. Poult. Sci. 2012;91:1846–1853. doi: 10.3382/ps.2011-01830. [DOI] [PubMed] [Google Scholar]
- Boudergue C., Burel C., Dragacci S., FAVROT M.C., FREMY J.M., Massimi C., PRIGENT P., Debongnie P., Pussemier L., Boudra H. EFSA Supporting Publications; 2009. Review of Mycotoxin-Detoxifying Agents Used as Feed Additives: Mode of Action, Efficacy and Feed/Food Safety. [Google Scholar]
- Chen X., Horn N., Applegate T. Efficiency of hydrated sodium calcium aluminosilicate to ameliorate the adverse effects of graded levels of aflatoxin B1 in broiler chicks. Poult. Sci. 2014;93:2037–2047. doi: 10.3382/ps.2014-03984. [DOI] [PubMed] [Google Scholar]
- Coffey M., Hagler W., Jr., Cullen J. Influence of dietary protein, fat or amino acids on the response of weanling swine to aflatoxin B1. J. Anim. Sci. 1989;67:465–472. doi: 10.2527/jas1989.672465x. [DOI] [PubMed] [Google Scholar]
- Corrier D., DeLoach J. Evaluation of cell-mediated, cutaneous basophil hypersensitivity in young chickens by an interdigital skin test. Poult. Sci. 1990;69:403–408. doi: 10.3382/ps.0690403. [DOI] [PubMed] [Google Scholar]
- Denli M., Blandon J., Guynot M., Salado S., Perez J. Effects of dietary AflaDetox on performance, serum biochemistry, histopathological changes, and aflatoxin residues in broilers exposed to aflatoxin B1. Poult. Sci. 2009;88:1444–1451. doi: 10.3382/ps.2008-00341. [DOI] [PubMed] [Google Scholar]
- Fatima A., Gupta V.K., Luqman S., Negi A.S., Kumar J., Shanker K., Saikia D., Srivastava S., Darokar M., Khanuja S.P. Antifungal activity of Glycyrrhiza glabra extracts and its active constituent glabridin. Phytother. Res. 2009;23:1190–1193. doi: 10.1002/ptr.2726. [DOI] [PubMed] [Google Scholar]
- Gowda N., Ledoux D., Rottinghaus G., Bermudez A., Chen Y. Efficacy of turmeric (Curcuma longa), containing a known level of curcumin, and a hydrated sodium calcium aluminosilicate to ameliorate the adverse effects of aflatoxin in broiler chicks. Poult. Sci. 2008;87:1125–1130. doi: 10.3382/ps.2007-00313. [DOI] [PubMed] [Google Scholar]
- Haskard C.A., El-Nezami H.S., Kankaanpää P.E., Salminen S., Ahokas J.T. Surface binding of aflatoxin B1 by lactic acid bacteria. Appl. Environ. Microbiol. 2001;67:3086–3091. doi: 10.1128/AEM.67.7.3086-3091.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehler D., A. Lemme, V. Ravindran, W. Bryden, H. Rostagno. Feed Formulation in Broiler Chickens Based on standardized Ileal Amino Acid Digestibility. Pages 78–91 in Proc. 3rd Mid-Atlantic Mid-Atlantic Nutrition Conference; 15–17 Noviembre, Monterrey, Nuevo León, México: Universidad Autónoma de Nuevo León.
- Hoerr F.J. Clinical aspects of immunosuppression in poultry. Avian Dis. 2010;54:2–15. doi: 10.1637/8909-043009-Review.1. [DOI] [PubMed] [Google Scholar]
- Kavyani A., Farivar F., Mokhtari Karchegani S., Landy N. IACSIT Press; Singapore: 2012. Efficiency of a Multi-Strain Probiotic (Protexin) on Performance and Carcass Traits in Broiler Chicks. [Google Scholar]
- Khanipour S., Mehri M., Bagherzadeh-Kasmani F., Maghsoudi A., Assadi Soumeh E. Excess dietary tryptophan mitigates aflatoxicosis in growing quails. J. Anim. Physiol. Anim. Nutr. 2019;103:1462–1473. doi: 10.1111/jpn.13167. [DOI] [PubMed] [Google Scholar]
- Khatoon A., Abidin Z.u. Mycotoxicosis–diagnosis, prevention and control: past practices and future perspectives. Toxin Rev. 2018;39:99–114. [Google Scholar]
- Kiernan J. 2015. Histological and Histochemical Methods. Bloxham: Scion Publishing Ltd. [Google Scholar]
- Kumar C., Balachandran C. Effect of citrinin and aflatoxin on haemato-biochemical parameters of broiler chicken. Indian J. Vet. Pathol. 2016;40:51–54. [Google Scholar]
- Lee S.-E., Campbell B.C., Molyneux R.J., Hasegawa S., Lee H.-S. Inhibitory effects of naturally occurring compounds on aflatoxin B1 biotransformation. J. Agric. Food Chem. 2001;49:5171–5177. doi: 10.1021/jf010454v. [DOI] [PubMed] [Google Scholar]
- Liu N., Ding K., Wang J., Deng Q., Gu K., Wang J. Effects of lactic acid bacteria and smectite after aflatoxin B1 challenge on the growth performance, nutrient digestibility and blood parameters of broilers. J. Anim. Physiol. Anim. Nutr. 2018;102:953–961. doi: 10.1111/jpn.12901. [DOI] [PubMed] [Google Scholar]
- Ma Q., Gao X., Zhou T., Zhao L., Fan Y., Li X., Lei Y., Ji C., Zhang J. Protective effect of Bacillus subtilis ANSB060 on egg quality, biochemical and histopathological changes in layers exposed to aflatoxin B1. Poult. Sci. 2012;91:2852–2857. doi: 10.3382/ps.2012-02474. [DOI] [PubMed] [Google Scholar]
- Modirsanei M., Mansoori B., Khosravi A.R., Kiaei M.M., Khazraeinia P., Farkhoy M., Masoumi Z. Effect of diatomaceous earth on the performance and blood variables of broiler chicks during experimental aflatoxicosis. J. Sci. Food Agric. 2008;88:626–632. [Google Scholar]
- Monson M.S., Coulombe R.A., Reed K.M. Aflatoxicosis: lessons from toxicity and responses to aflatoxin B1 in poultry. Agriculture. 2015;5:742–777. [Google Scholar]
- Oğuz H., Kurtoğlu F., Kurtoğlu V., Bırdane Y. Evaluation of biochemical characters of broiler chickens during dietary aflatoxin (50 and 100 ppb) and clinoptilolite exposure. Res. Vet. Sci. 2002;73:101–103. doi: 10.1016/s0034-5288(02)00040-1. [DOI] [PubMed] [Google Scholar]
- OIE A. Office International des Epizooties; Paris, France: 2008. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; pp. 1092–1106. [Google Scholar]
- Placer Z.A., Cushman L.L., Johnson B.C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 1966;16:359–364. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- Prasai T.P., Walsh K.B., Bhattarai S.P., Midmore D.J., Van T.T., Moore R.J., Stanley D. Biochar, bentonite and zeolite supplemented feeding of layer chickens alters intestinal microbiota and reduces campylobacter load. PLoS One. 2016;11:e0154061. doi: 10.1371/journal.pone.0154061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasai T., Walsh K., Midmore D., Bhattarai S. Effect of biochar, zeolite and bentonite feed supplements on egg yield and excreta attributes. Anim. Prod. Sci. 2017;58:1632–1641. [Google Scholar]
- Redzwan S.M., Rosita J., Sokhini A.M., Nurul 'Aqilah A., Wang J.-S., Kang M.-S., Zuraini A. Detection of serum AFB1-lysine adduct in Malaysia and its association with liver and kidney functions. Int. J. Hyg. Environ. Health. 2014;217:443–451. doi: 10.1016/j.ijheh.2013.08.007. [DOI] [PubMed] [Google Scholar]
- SAS, I. SAS Institute, Inc.; Cary NC: 2004. SAS/WATTM User’s Guide. [Google Scholar]
- Saxena S. Glycyrrhiza Glabra: Medicine over the Millennium. IJNPR. 2005;4:358–367. [Google Scholar]
- Sedghi M., Golian A., Kermanshahi H., Ahmadi H. Effect of dietary supplementation of licorice extract and a prebiotic on performance and blood metabolites of broilers. S. Afr. J. Anim. Sci. 2010;40 [Google Scholar]
- Shannon T., Ledoux D., Rottinghaus G., Shaw D., Daković A., Marković M. The efficacy of raw and concentrated bentonite clay in reducing the toxic effects of aflatoxin in broiler chicks. Poult. Sci. 2017;96:1651–1658. doi: 10.3382/ps/pew408. [DOI] [PubMed] [Google Scholar]
- Shotwell O.L., Hesseltine C., Stubblefield R., Sorenson W. Production of aflatoxin on rice. Appl. Microbiol. 1966;14:425–428. doi: 10.1128/am.14.3.425-428.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spokas K.A., Cantrell K.B., Novak J.M., Archer D.W., Ippolito J.A., Collins H.P., Boateng A.A., Lima I.M., Lamb M.C., McAloon A.J. Biochar: a synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2012;41:973–989. doi: 10.2134/jeq2011.0069. [DOI] [PubMed] [Google Scholar]
- Verma J., Johri T., Swain B., Ameena S. Effect of graded levels of aflatoxin, ochratoxin and their combinations on the performance and immune response of broilers. Br. Poult. Sci. 2004;45:512–518. doi: 10.1080/00071660412331286226. [DOI] [PubMed] [Google Scholar]
- Yamauchi K., Manabe N., Matsumoto Y., Takenoyama S.i., Yamauchi K.e. Increased collagen III in culled chicken meat after feeding dietary wood charcoal and vinegar contributes to palatability and tenderness. Anim. Sci. J. 2014;85:468–480. doi: 10.1111/asj.12160. [DOI] [PubMed] [Google Scholar]
- Zaghini A., Martelli G., Roncada P., Simioli M., Rizzi L. Mannanoligosaccharides and aflatoxin B1 in feed for laying hens: effects on egg quality, aflatoxins B1 and M1 residues in eggs, and aflatoxin B1 levels in liver. Poult. Sci. 2005;84:825–832. doi: 10.1093/ps/84.6.825. [DOI] [PubMed] [Google Scholar]
- Zhang N.-Y., Qi M., Zhao L., Zhu M.-K., Guo J., Liu J., Gu C.-Q., Rajput S.A., Krumm C.S., Qi D.-S. Curcumin prevents aflatoxin B1 hepatoxicity by inhibition of cytochrome p450 isozymes in chick liver. Toxins. 2016;8:327. doi: 10.3390/toxins8110327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Shirley R., Dibner J., Uraizee F., Officer M., Kitchell M., Vazquez-Anon M., Knight C. Comparison of hydrated sodium calcium aluminosilicate and yeast cell wall on counteracting aflatoxicosis in broiler chicks. Poult. Sci. 2010;89:2147–2156. doi: 10.3382/ps.2009-00608. [DOI] [PubMed] [Google Scholar]
- Zublena J.P., Barker J.C., Carter T.A. 1990. Poultry manure as a fertilizer source. AG-North Carolina Agricultural Extension Service, North Carolina State University (USA) [Google Scholar]