Abstract
Dietary starch is the major energy source for broiler chickens; therefore, relevant information on its intestinal utilization is important. The present study was designed to evaluate intestinal starch and energy digestibility of broiler chickens fed diets supplemented with α-amylase. A total of 240 day-0 male broiler chicks were randomly assigned to 3 nutritionally adequate corn-soybean–based experimental diets comprising 3 levels of α-amylase supplementation (0, 80, or 160 KNU/kg diet). Each treatment comprised 8 replicate cages of 10 birds each. At day 21 after hatching, digesta was collected from 4 intestinal sites: the anterior jejunum (AJ), posterior jejunum (PJ), anterior ileum (AI), and posterior ileum. Increasing α-amylase supplementation linearly improved (P < 0.01) overall BW gain and feed efficiency of the birds. There were linear and quadratic (P < 0.01) responses of increasing α-amylase supplementation on starch and energy digestibility at the PJ and AI. The total tract digestibility of starch increased (P < 0.05) with increasing α-amylase supplementation. Starch disappearance and digestible energy (kcal/kg) linearly increased (P < 0.01) with digesta flow from the AJ to PJ as dietary α-amylase supplementation increased. There were linear (P < 0.01) and quadratic (P < 0.05) effects of increasing α-amylase supplementation on the villus height in the jejunum. The viscosity of the jejunal digesta decreased (P < 0.05) with increasing dietary α-amylase supplementation. The results from this study showed the efficacy of exogenous amylase in improving growth performance and starch and energy digestibility in broiler chickens. Furthermore, the digestibility of starch and energy and the impact of the exogenous amylase were higher at the PJ than other intestinal sites.
Key words: broiler chicken, digestibility, energy, enzyme, starch
Introduction
Among the nutrients in poultry feed ingredients and diets, starch is quantitatively the most important source of energy. However, starch degradability is affected by the proportion of amylose (Moran, 1982) and its variability in corn, and other cereal grains can significantly influence the AMEn content of feedstuffs to livestock (Wiseman et al., 2000; Tester et al., 2004). In addition, there are physical barriers in the cell walls of feed ingredients that restrict enzyme access to substrates (Ravindran, 2013). Therefore, the use of exogenous carbohydrases such as xylanases, amylases, and glucanases as feed additives have been reported to improve energy utilization and the performance of nonruminant animals (Gracia et al., 2003; Kocher et al., 2003; Olukosi and Adeola, 2008). However, some reports have not found effects in response to these enzyme combinations (Hong et al., 2002; Olukosi et al., 2007). Factors not directly related to starch itself may also affect its digestibility, and the dynamics of starch digestion relative to bird intestinal efficiency may have considerable nutritional consequences. Previously, Weurding et al. (2001) showed that site, rate, and extent of starch digestion in the small intestine of broiler chickens may differ considerably between a wide range of feedstuffs and concluded that rapid starch digestion may lead to the same extent of starch digestion as gradual starch digestion, but the amount of starch digested at specific sites of the intestine would differ. The differences that exist in the site of starch digestion may therefore have metabolic consequences that affect feed utilization in broiler chickens. There are few reports on the impact of exogenous amylase on starch and energy utilization in specific intestinal sections. Therefore, the hypothesis of the present study was that starch and energy digestibility would vary between intestinal sections and would be affected by exogenous amylase administration. The objective of the present study was to evaluate the influence of dietary α-amylase supplementation on the digestibility of starch and energy in the different intestinal sites in broiler chickens from day 0 to 21 after hatching.
Materials and methods
The protocol of the animal experiment was reviewed and approved by the Purdue University Animal Care and Use Committee.
Experimental Birds, Housing, and Diets
A total of 240 male 0-day-old broiler chicks (Cobb 500, Siloam Springs, AR) were obtained from a commercial hatchery. The birds were individually tagged, weighed, and raised in heated battery brooders (model SB 4 T; Alternative Design Manufacturing, Siloam Springs, AR) with temperature and lighting maintained as previously described by Park et al. (2017). Birds were allotted to 3 dietary treatments (Table 1) in a randomized complete block design, consisting of 8 replicates and 10 birds per replicate. The diets contained 3 levels of α-amylase supplementation (0, 80, or 160 KNU/kg diet of Ronozyme HiStarch, DSM Nutritional Products, Switzerland). All diets were corn-soybean (SBM) based and formulated to meet breeder nutrient specifications. Mash diets and water were provided ad libitum throughout the experimental period. Titanium dioxide was used as an indigestible marker, and all diets contained phytase (Ronozyme HiPhos, DSM Nutritional Products, Switzerland) at 1,000 FYT/kg.
Table 1.
Ingredient and calculated nutrient composition of experimental diets, as-fed basis.
| Item | α-amylase, KNU/kg | ||
|---|---|---|---|
| Ingredients, g/kg | 0 | 80 | 160 |
| Corn | 555.6 | 535.6 | 515.6 |
| Soybean meal | 360.0 | 360.0 | 360.0 |
| Soybean oil | 5.5 | 5.5 | 5.5 |
| Monocalcium phosphate1 | 11.0 | 11.0 | 11.0 |
| Limestone2 | 13.0 | 13.0 | 13.0 |
| Salt | 3.0 | 3.0 | 3.0 |
| Vitamin-mineral premix3 | 3.0 | 3.0 | 3.0 |
| DL-Methionine | 2.0 | 2.0 | 2.0 |
| L-Lysine HCl | 1.9 | 1.9 | 1.9 |
| Amylase premix4 | 0.0 | 20.0 | 40.0 |
| Titanium dioxide premix5 | 25.0 | 25.0 | 25.0 |
| Phytase premix6 | 20.0 | 20.0 | 20.0 |
| Total | 1,000.0 | 1,000.0 | 1,000.0 |
| Calculated nutrients and energy | |||
| Crude protein, g/kg | 228.2 | 228.2 | 228.2 |
| ME, kcal/kg | 3,005.5 | 3,005.5 | 3,005.5 |
| Ca, g/kg | 7.8 | 7.8 | 7.8 |
| P, g/kg | 6.2 | 6.2 | 6.2 |
| Nonphytate P, g/kg | 3.6 | 3.6 | 3.6 |
| Ca:total P | 1.3 | 1.3 | 1.3 |
| Ca:nonphytate P | 2.2 | 2.2 | 2.2 |
| Starch, g/kg | 439.6 | 439.6 | 439.6 |
| Total amino acids, g/kg | |||
| Arg | 14.8 | 14.8 | 14.8 |
| His | 6.0 | 6.0 | 6.0 |
| Ile | 9.4 | 9.4 | 9.4 |
| Leu | 19.4 | 19.4 | 19.4 |
| Lys | 13.7 | 13.7 | 13.7 |
| Met | 5.5 | 5.5 | 5.5 |
| Cys | 3.7 | 3.7 | 3.7 |
| Phe | 10.7 | 10.7 | 10.7 |
| Tyr | 8.8 | 8.8 | 8.8 |
| Thr | 8.5 | 8.5 | 8.5 |
| Trp | 3.0 | 3.0 | 3.0 |
| Val | 10.4 | 10.4 | 10.4 |
| Met + Cys | 9.1 | 9.1 | 9.1 |
| Phe + Tyr | 19.5 | 19.5 | 19.5 |
| Analyzed composition | |||
| Amylase (KNU/kg)7 | LOQ | 61 | 134 |
16% Ca, 21% P.
38% Ca.
Supplied the following per kg diet: vitamin A, 5,484 IU; vitamin D3, 2,643 ICU; vitamin E, 11 IU; menadione sodium bisulfite, 4.38 mg; riboflavin, 5.49 mg; pantothenic acid, 11 mg; niacin, 44.1 mg; choline chloride, 771 mg; vitamin B12, 13.2 ug; biotin, 55.2 ug; thiamine mononitrate, 2.2 mg; folic acid, 990 ug; pyridoxine hydrochloride, 3.3 mg; I, 1.11 mg; Mn, 66.06 mg; Cu, 4.44 mg; Fe, 44.1 mg; Zn, 44.1 mg; Se, 300 ug.
Ronozyme HiStarch contained 600 KNU/g. 1 g of HiStarch added to 149 g of corn supplied 4 KNU/g of premix. 20 g premix delivered 80 KNU/kg of feed and 40 g premix delivered 160 KNU/kg of feed.
Prepared as 1 g titanium dioxide added to 4 g corn.
Ronozyme HiPhos contained 5,000 FYT/g. 1 g of HiPhos added to 99 g of ground corn, supplied 50 FYT/g of premix. 20 g delivered 1,000 FYT/kg of feed. 1,000 FYT/kg supplied 1.5 g P/kg and 1.7 g of Ca/kg.
LOQ = limit of quantification.
Sampling Procedures
On day 19 after hatching, trays under the cages were lined with waxed paper for a 3-d excreta collection. On day 21 after hatching, all birds per cage were individually weighed and euthanized by CO2 asphyxiation. Entire ileal and jejunal segments were excised from each bird. Specifically, each of the jejunum and ileum was divided into 2 sections of equal length, namely the anterior jejunum (AJ), posterior jejunum (PJ), anterior ileum (AI), and posterior ileum (PI). The digesta was collected from each section by flushing with distilled water into plastic containers and stored at −20°C before analysis. For viscosity measurement, the entire jejunal content from 1 bird per replicate with a median BW was gently squeezed into plastic tubes and stored at −20°C before analysis.
Viscosity Measurements
The jejunal digesta was thawed on ice, and approximately 10 g of sample per replicate was placed in a 50-mL plastic centrifuge tube, vortexed for 10 s, and centrifuged at 10,000 × g for 10 min at 4°C. The supernatant was transferred into a 2-mL sample cup and placed in a water bath (Precision, GCA Corp., College Park, MD) that had been preheated to 40°C until the temperature of the sample equilibrated with that of the water in the water bath. The viscosity, in centipoise (cP), of these samples was determined using a viscometer (Vibro viscometer, model SV-1A, A&D Instruments Ltd., Oxfordshire, United Kingdom).
Intestinal Morphological Analysis
Mid-jejunal segments were collected from 1 bird per replicate with a median BW, flushed with ice-cold 10% phosphate-buffered saline (VWR International, Radnor, PA) and fixed in 10% neutral buffered formalin (VWR International, Radnor, PA) for approximately 30 d. Subsequently, the samples were dehydrated with ethanol (VWR International, Radnor, PA), cleared with Sub-X (Polysciences, Inc., Warrington, PA) and placed in paraffin (Polyfin paraffin, Sigma Polysciences, St. Louis, MO). The segments (5 μm) were stained with hematoxylin and eosin at the Purdue Histology and Phenotyping Laboratory (Purdue University, West Lafayette, IN). The villus height and crypt depth were measured from 5 complete, vertically oriented villi per slide, and subsequently, the villus height-to-crypt depth ratio was calculated. All measurements were performed under a binocular light microscope (National Optical and Scientific Instruments, Inc., Schertz, TX).
Chemical Analyses
The intestinal digesta and excreta samples were freeze-dried for 96 h and subsequently ground to pass through a 0.5-mm screen (Retsch ZM 100, GmbH, Haan, Germany). Diets, intestinal digesta, and excreta samples were analyzed for DM analysis by drying overnight at 105°C (Precision Scientific Co., Chicago, IL; method 934.01; AOAC, 2006). The nitrogen content of the samples was subsequently determined by combustion (TruMac N; LECO Corp., St. Joseph, MI; method 990.03; AOAC, 2000) with EDTA as a calibration standard. The gross energy (GE) concentration in diets, ileal digesta, and excreta samples was determined by using a isoperibol bomb calorimeter (Parr 1261; Parr 105 Instrument Co., Moline, IL). The Megazyme total starch determination kit (method 996.11; AOAC, 2000) was used to analyze samples for starch. Titanium concentration was measured on a UV spectrophotometer following the method of Short et al. (1996).
The apparent digestibility of nutrients in the intestinal digesta and excreta was calculated with the index method, according to the following equation:
where AD is the apparent digestibility of nutrients, TiI is the titanium concentration in diets; TiO is titanium concentration in the output (intestinal digesta or excreta); NO is the concentration of nutrients in the intestinal digesta or excreta; and NI is the concentration of a nutrient in the diet.
All digestibility values are expressed as grams per kilogram of DM.
The digestible energy and apparent metabolizable energy (AME) (kcal/kg DM) of the diet was calculated as the product of the coefficient and GE concentrations (kcal/kg) in the diet. The AMEn was calculated by correcting for 0 N retention using a factor of 8.22 kcal/g (Hill and Anderson, 1958), as described by Zhang and Adeola (2017).
Statistical Analyses
Data were analyzed as a randomized complete block design using the GLM procedures of SAS (SAS Inst. Inc., Cary, NC). The initial body weight was used as the blocking criterion. An α level of 0.05 was considered significant.
Results
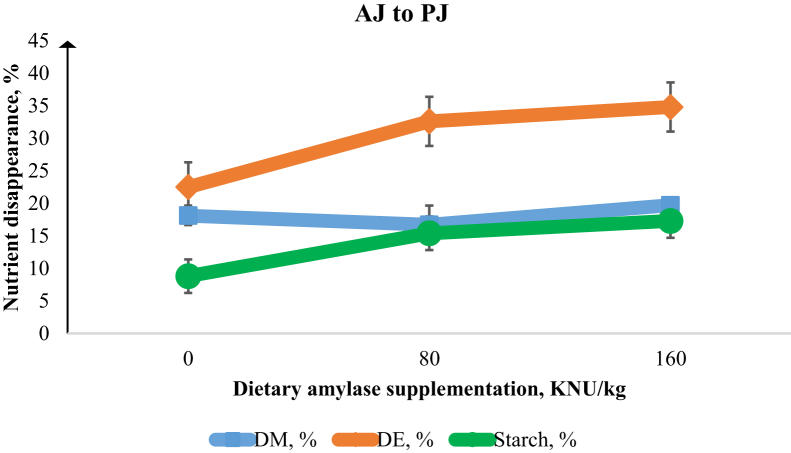
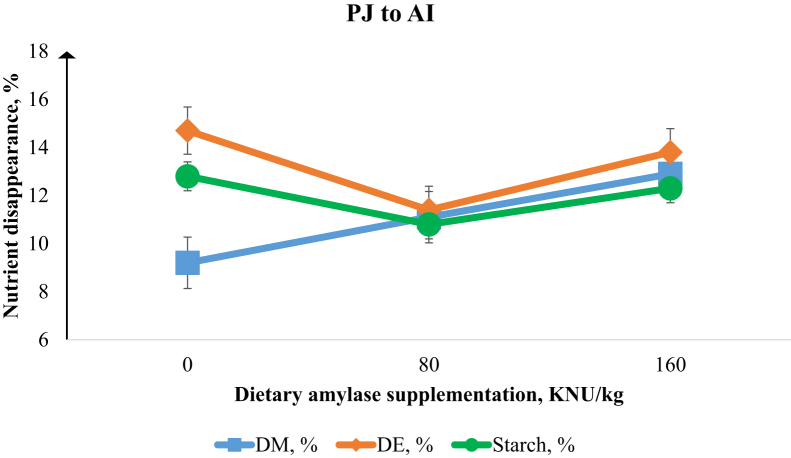
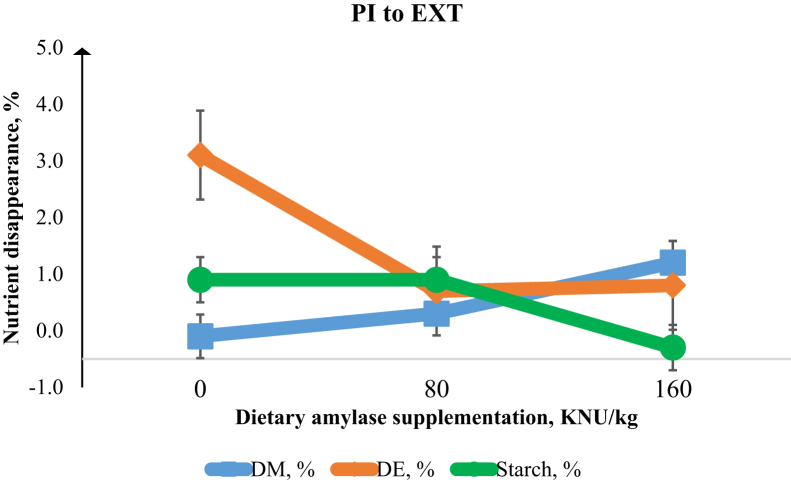
The effect of α-amylase supplementation on growth performance of broiler chickens is presented in Table 2. Increasing dietary α-amylase supplementation led to linear and quadratic increases (P < 0.01) in the BW at 21 d and linear increases (P < 0.01) in BW gain from 0 to 21 d. Feed efficiency in the overall period of the study (day 0–21) was linearly improved (P < 0.01) with increasing levels of α-amylase. Table 3 shows that increasing dietary α-amylase supplementation resulted in linear increases in the digestibility of starch at the PJ (P < 0.05), AI (P < 0.01), PI (P < 0.01), and total tract (P < 0.05). There was a quadratic response (P < 0.01) of increasing α-amylase supplementation on starch digestibility in the PJ and AJ. There were linear and quadratic increases (P < 0.01) in the digestibility of energy (DE; %) at the PJ, AI, and PI sites associated with increasing α-amylase concentration. There was no effect of α-amylase supplementation on AME (kcal/kg). However, there was a tendency (P = 0.06) for improvement in AMEn (kcal/kg) as a result of α-amylase supplementation. Increasing α-amylase supplementation resulted in linear (P < 0.01) and quadratic (P < 0.05) responses in the villus height and linearly reduced (P < 0.05) the viscosity in the jejunum (Table 4). Figure 1, Figure 2, Figure 3, Figure 4 show the nutrient disappearance in the gastrointestinal tract of birds fed diets supplemented with α-amylase. As digesta flows from the AJ to PJ site, an increasing α-amylase supplementation resulted in linear improvements (P < 0.01) in the disappearance of starch and DE. In contrast, increasing α-amylase supplementation resulted in a linear decrease (P < 0.01) in starch disappearance with digesta flow from the PI to the total tract site.
Table 2.
Effect of graded amylase supplementation on growth performance of broiler chickens.1
| Item | α-amylase, KNU/kg |
SEM | Linear | Quadratic | ||
|---|---|---|---|---|---|---|
| 0 | 80 | 160 | ||||
| BW, g | ||||||
| Day 0 | 36.3 | 36.3 | 36.3 | 0.02 | 0.416 | 0.636 |
| Day 7 | 124.0 | 128.4 | 126.2 | 2.27 | 0.497 | 0.258 |
| Day 14 | 381.8 | 395.7 | 392.0 | 6.78 | 0.304 | 0.306 |
| Day 21 | 806.0 | 884.0 | 870.4 | 11.96 | 0.002 | 0.007 |
| Day 0 to 7 | ||||||
| BW gain, g/bird | 87.7 | 92.1 | 89.9 | 2.27 | 0.501 | 0.256 |
| Feed intake, g/bird | 128.1 | 129.4 | 116.8 | 11.86 | 0.508 | 0.638 |
| Gain:feed, g/kg | 694.5 | 755.9 | 826.1 | 60.73 | 0.149 | 0.958 |
| Day 0 to 14 | ||||||
| BW gain, g/bird | 345.5 | 359.4 | 355.7 | 6.78 | 0.305 | 0.316 |
| Feed intake, g/bird | 435.8 | 452.8 | 426.5 | 18.56 | 0.727 | 0.356 |
| Gain:feed, g/kg | 798.5 | 808.4 | 839.2 | 33.64 | 0.407 | 0.803 |
| Day 0 to 21 | ||||||
| BW gain, g/bird | 769.6 | 837.1 | 834.1 | 14.69 | 0.007 | 0.070 |
| Feed intake, g/bird | 1,073.8 | 1,094.6 | 1,049.6 | 28.20 | 0.554 | 0.357 |
| Gain:feed, g/kg | 718.3 | 766.7 | 796.1 | 13.36 | 0.001 | 0.571 |
Data are least square means of 8 replicate cages per diet.
Table 3.
Efficacy of dietary α-amylase supplementation on nutrient digestibility in different intestinal sites of the broiler chicken.1
| Intestinal site | α-amylase, KNU/kg |
SEM | Linear | Quadratic | ||
|---|---|---|---|---|---|---|
| 0 | 80 | 160 | ||||
| Anterior jejunum | ||||||
| DMD, % | 42.6 | 43.3 | 36.8 | 2.12 | 0.077 | 0.188 |
| DE, % | 32.3 | 30.1 | 26.2 | 2.14 | 0.063 | 0.769 |
| DE, kcal/g | 1.417 | 1.307 | 1.175 | 0.095 | 0.093 | 0.923 |
| N, % | 49.8 | 47.4 | 49.0 | 1.92 | 0.781 | 0.413 |
| Starch, % | 71.1 | 69.6 | 66.0 | 1.71 | 0.053 | 0.629 |
| Posterior jejunum | ||||||
| DMD, % | 60.7 | 60.0 | 56.6 | 0.87 | 0.005 | 0.227 |
| DE, % | 54.8 | 62.6 | 61.1 | 1.06 | 0.001 | 0.003 |
| DE, kcal/g | 2.403 | 2.724 | 2.736 | 0.047 | <0.001 | 0.017 |
| N, % | 68.7 | 65.3 | 66.3 | 1.24 | 0.189 | 0.177 |
| Starch, % | 79.9 | 85.0 | 83.3 | 0.90 | 0.018 | 0.008 |
| Anterior ileum | ||||||
| DMD, % | 69.8 | 74.1 | 74.9 | 0.49 | 0.639 | 0.035 |
| DE, % | 69.6 | 74.1 | 74.9 | 0.50 | <0.001 | 0.009 |
| DE, kcal/g | 3.048 | 3.221 | 3.354 | 0.022 | <0.001 | 0.477 |
| N, % | 76.8 | 76.9 | 76.5 | 0.74 | 0.757 | 0.816 |
| Starch, % | 92.8 | 95.8 | 95.7 | 0.25 | <0.001 | <0.001 |
| Posterior ileum | ||||||
| DMD, % | 74.5 | 73.5 | 72.3 | 0.39 | 0.001 | 0.861 |
| DE, % | 72.9 | 76.0 | 76.3 | 0.38 | <0.001 | 0.008 |
| DE, kcal/g | 3.194 | 3.306 | 3.417 | 0.017 | <0.001 | 0.977 |
| N, % | 81.7 | 79.4 | 80.3 | 0.67 | 0.189 | 0.077 |
| Starch, % | 97.1 | 97.8 | 98.9 | 0.24 | <0.001 | 0.420 |
| Total tract | ||||||
| DMD, % | 74.4 | 73.8 | 73.5 | 1.17 | 0.586 | 0.910 |
| AME, % | 75.9 | 76.7 | 77.0 | 1.02 | 0.460 | 0.877 |
| AME, kcal/g | 3.328 | 3.335 | 3.452 | 0.045 | 0.072 | 0.335 |
| N, % | 72.9 | 73.1 | 73.9 | 0.17 | 0.568 | 0.829 |
| AMEn, % | 70.6 | 71.5 | 71.7 | 0.94 | 0.428 | 0.781 |
| AMEn, kcal/g | 3.095 | 3.109 | 3.213 | 0.041 | 0.063 | 0.388 |
| Starch, % | 98.1 | 98.7 | 98.7 | 0.17 | 0.038 | 0.147 |
Abbreviations: AME, apparent metabolizable energy; DE, digestibility energy; DMD, DM digestibility.
Data are least square means of 8 replicate cages per diet.
Table 4.
Villus height, crypt depth, villus height-to-crypt depth ratio and viscosity of the jejunal digesta of broiler chickens fed diets supplemented with graded levels of α-amylase.1
| Item | α-amylase, KNU/kg |
SEM | Linear | Quadratic | ||
|---|---|---|---|---|---|---|
| 0 | 80 | 160 | ||||
| Villus height, μm | 709.6 | 914.3 | 937.8 | 30.82 | <0.001 | 0.031 |
| Crypt depth, μm | 99.8 | 115.9 | 107.6 | 7.87 | 0.495 | 0.225 |
| VH:CD | 7.5 | 8.1 | 9.0 | 0.73 | 0.179 | 0.872 |
| Viscosity | 3.0 | 2.8 | 2.8 | 0.06 | 0.023 | 0.388 |
Abbreviations: CD, crypt depth; VH, villus height.
Data are least square means of 8 replicate cages per diet.
Figure 1.
Disappearance of DM, digestible energy (DE), and digesta starch from the anterior jejunum (AJ) to the posterior jejunum (PJ) in the broiler chicken intestine. There were linear increases (P < 0.01) in starch (%) and DE (%) disappearance with increasing α-amylase supplementation. Error bars are the SEM of 8 observations.
Figure 2.
Disappearance of DM, digestible energy (DE), and digesta starch from the posterior jejunum (PJ) to the anterior ileum (AI) in the broiler chicken intestine. There was a linear increase (P < 0.05) in DM (%) and quadratic response (P < 0.05) in DE (%) disappearance with increasing α-amylase supplementation. Error bars are the SEM of 8 observations.
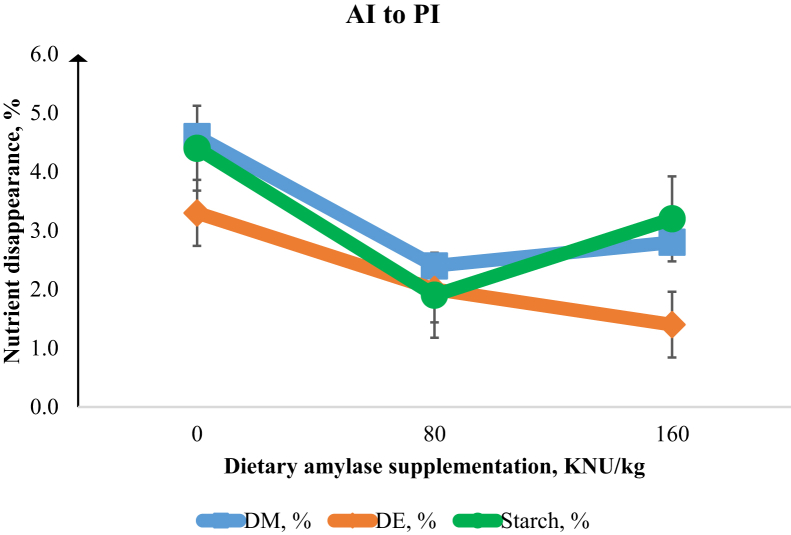
Figure 3.
Disappearance of DM, digestible energy (DE), and digesta starch from the anterior ileum (AI) to the posterior ileum (PI) in the broiler chicken intestine. Increasing α-amylase supplementation resulted in linear and quadratic responses (P < 0.05) in DM (%) disappearance. There was a linear decrease (P < 0.05) in DE (%) and a quadratic response (P < 0.01) in starch (%) disappearance with increasing α-amylase supplementation. Error bars are the SEM of 8 observations.
Figure 4.
Disappearance of DM, digestible energy (DE), and digesta starch from the posterior jejunum (PI) to excreta (EXT) in the broiler chicken intestine. There was linear decrease (P < 0.01) in starch (%) disappearance with increasing α-amylase supplementation. Error bars are the SEM of 8 observations.
Discussion
The need to improve and optimize the efficiency of starch digestion is an integral part of animal nutrition. Starch digestion in the digestive tract of livestock is affected by both intrinsic and external factors (Silveira et al., 2007; Witt et al., 2010), and although starch is mainly digested in the small intestine (Wiseman, 2006), variations along discrete intestinal regions may impact the overall starch utilization and animal performance, and also the intestinal microbiota activities (Bolhuis et al., 2008; Zijlstra et al., 2012). In the present study, we examined differences in starch and energy digestibility at different intestinal sites and the extent to which exogenous amylase supplementation may influence these digestibility responses.
The results from the present study showed that exogenous α-amylase supplementation improved the overall BW gain and feed efficiency of the birds at 21 d after hatching. This observation is similar to previous reports in which α-amylase was either supplemented separately (Jiang et al., 2008) or included in a cocktail (Olukosi et al., 2007), which improved the weight gain and feed efficiency of the 21-day-old birds. Similarly, Stefanello et al. (2019) reported improvements in growth performance when broiler chickens were fed corn-SBM–based diets supplemented with graded concentrations of α-amylase. The improvement in growth performance may be associated with the observed increases in starch and energy digestibility in the gastrointestinal tract as a result of α-amylase supplementation and corroborates several previous reports for corn-SBM–based diets (Gracia et al., 2003; Cowieson et al., 2019; Stefanello et al., 2019; Woyengo et al., 2019). Although broiler chickens have high innate capacity to digest dietary starch, as observed in the present study, it could be limited by several factors such as inadequacies in endogenous amylases, the nature of the starch crystals and issues around extraction of glucose from the lumen via Na-dependent transport systems. Krogdahl and Sell (1989) suggested that poultry develop an increased capacity to digest starch as the intestinal tract matures, by increasing pancreatic amylase production in response to elevated starch intake. However, Noy and Sklan (1995) found that production of amylase in the pancreas is not clearly correlated with the levels of starch digestion. Comparing birds at 14 and 42 d of age, they found that although starch intake increased by over 200%, pancreatic amylase output increased by only 95%. Croom et al. (1999) previously noted that intestinal mass and pancreatic tissue become increasingly smaller proportion of the metabolic weight of birds as they grow older. This has led to the assumption that birds may be responsive to augmentation of endogenous amylase systems with exogenous microbial amylase supplementation. While the cooperativity of exogenous and endogenous amylase is not entirely clear, previous work by Pedersen et al. (2015) showed extensive pore formation and collapse of starch granule structure when only pancreatin was used concurrently with exogenous bacterial amylase and vice versa. This could partly explain the improvements in intestinal starch digestibility in the birds as a result of exogenous amylase supplementation in the present study. Given that the jejunum is the site with the largest capacity for nutrient absorption in birds, it is possible that the exogenous amylase action on starch degradation upregulated the extraction of glucose monomers from the lumen via Na-dependent transport systems, which resulted in a linear increase in starch disappearance from the AJ to PJ sites.
Interestingly, there was no effect of exogenous amylase on starch and energy digestibility in the AJ compared with other intestinal sites. There was a noteworthy tendency for a decrease in starch and energy digestibility with increasing α-amylase supplementation in the AJ. This suggests a delayed effect of the exogenous amylase on nutrient digestibility or an ineffective mixing of substrates and digestive enzymes. This asynchrony in response could also be due to variations in the digesta transit and retention times within the intestinal segments. Alternatively, it is possible that the relatively higher concentration of pancreatic amylase, which is secreted into the duodenum, carried over to the AJ, essentially masking any additional effect of the exogenous amylase. Another possibility is that the exogenous amylase works best in the posterior sections of the gastrointestinal tract but downregulates the pancreatic amylase output, which thus resulted in lower starch digestibility in the AJ.
Furthermore, the present study showed a shift in the site of starch digestibility from the distal to the proximal intestinal segments, and this corroborates a previous report by Svihus (2014). At the end of the AJ, approximately 70% of dietary starch have been digested, which further confirms the high innate ability of the chicken to digest starch, as previously described (Moran, 1982). The differences in the digestibility indices, which diminished with digesta flow toward the distal parts, suggest high variation in digestion rates within the intestine. Weurding et al. (2001) posited that although the amount of starch digested at different intestinal sites differs, variations in the rates of digestion may have metabolic consequences that influence feed efficiency. For example, Weurding (2002) observed that slowly digestible starch in broiler diets benefits feed efficiency, and Liu et al. (2014) noted the benefit of starch–protein digestibility dynamics on improvements in feed efficiency. This mechanism is not entirely clear but may be associated with energy metabolism in intestinal epithelial cells. Although glucose is a more effective energy source for the enterocytes in the intestinal tract, amino acids (notably glutamine) are readily catabolized as an alternative energy source, especially in the absence of glucose. Therefore, higher rates of starch digestion in proximal intestinal regions may be deleterious to amino acid digestion and overall feed efficiency of the bird. However, contrary to this, birds fed the control diet had higher starch disappearance toward the more distal intestinal regions but had lower feed efficiency than the enzyme-supplemented groups. Although this observation remains unclear, it could be attributed to the relatively lower starch and energy digestibility of the control birds, further limited by a decrease in the absorptive capacity in the jejunum, compared with the enzyme-supplemented groups. Given the role of the jejunum as the site of maximal intestinal absorption, improvements in nutrient digestion and absorptive capacity by supplemental enzymes could favor growth performance of the birds.
Although the present study showed that exogenous amylase substantially shifts the site of starch digestion to the PJ (about 15% improvement from the AJ to PJ), there were also localized improvements in the more distal regions. This suggests a protein-sparing effect of the exogenous amylase by generating more sustained circulating levels of glucose to the lower small intestine, which would spare amino acids from catabolism and therefore increase feed efficiency and energy utilization. About 98% of the dietary starch was digested at the end of the ileum, which is consistent with previous reports (Svihus, 2001; Hetland et al., 2003; Svihus et al., 2004; Zelenka and Ceresnakova 2005). Although relatively high, exogenous amylase supplementation led to a linear increase in starch digestibility at the PI. This high capacity of broiler chickens for starch digestion suggests a balance of the gut absorptive capacity with postabsorptive tissue metabolism. Croom et al. (1999) previously suggested that intensive genetic selection for growth in broiler chickens may have uncoupled the intestinal nutrient delivery from increased postabsorption nutrient demand, and therefore, absorption of nutrients could be a potential rate-limiting factor in survival, growth, and feed conversion in birds. However, the improvement in starch digestion by the exogenous amylase, at the end of the ileum, is marginal (about 1.8%) and may not fully explain the increased feed efficiency and body weight responses of the birds. Although this remains unclear, it is possible that variations in starch digestion rates along specific intestinal sections (Weurding, 2002), and improvement in the absorptive capacity of the jejunum by the exogenous amylase via an increased villi length, could enhance the utilization of other nutrients in the diet (e.g. dietary fat and protein). For instance, Jiang et al. (2008) noted that supplemental amylase increased the amylase, protease, and trypsin activity in the duodenum and jejunum, which marked implications on growth performance. Furthermore, it has been reported that supplemental amylases could improve fat digestibility (Yuan et al., 2017). This suggests that growth performance responses by exogenous enzymes may not always be solely related to greater degradation of the target substrates (Vieira et al., 2015) and could partly explain our observation.
The undigested starch fractions, which contains predominantly resistant starch, may also serve as substrate for the exogenous amylase. Schramm et al. (2016) noted a significant increase (75 vs. 81%) in the digestibility of the resistant starch fraction in a corn-SBM–based diets not supplemented vs. supplemented with an exogenous amylase. This could possibly explain the improvements observed in the total tract starch digestibility with exogenous amylase administration, which corresponds to slight increases in AME (kcal/g) and AMEn (kcal/g). This observation is consistent with previous reports by Svihus (2011) and Stefanello et al. (2019) of a strong correlation between AME and total tract starch digestibility. Although undigested starch may also serve as substrate for bacteria present in the hind gut, starch fermentation is energetically less efficient than enzymatic starch digestion in the small intestine (Dierick et al., 1989). In addition, Kussaibati et al. (1982) reported a similarity in the undigested starch fraction between conventional and germ-free chicks. Therefore, this undigested portion could provide more substrate for the exogenous enzyme. However, it is possible that even when glucose is successfully produced from starch in the hind gut, it may exceed the absorptive capacity of the bird, consequently resulting in no changes in energy utilization.
The present study showed a reduction in the viscosity of the jejunal digesta in response to an increasing α-amylase supplementation. This is contrary to previous reports that show a lack of effect of α-amylase supplementation on intestinal digesta viscosity (Gracia et al., 2003). The reason for this observation is not clear as corn-SBM–based diets are low in nonstarch polysaccharides and should not present viscosity issues when compared with barley or wheat. However, starch is an extremely heterogeneous structure (Tester et al., 2004), and the ratio between amylose and amylopectin in starch determines whether starch may be categorized as high amylose or waxy. Waxy starch, which has a high proportion of amylopectin relative to amylose, tend to be more amorphous and soluble. However, the chain length and organization of internal unit chains of amylopectin influence the gelatinization and pasting properties of starch (Vamadevan and Bertoft, 2020), which could contribute to viscosity (Klaochanpong et al., 2015). Pirgozliev et al. (2010) previously reported a reduced growth performance and higher viscosity of the jejunal digesta when birds were fed a maize–starch mixture with a lower amylose content. Although modest, the reduction in viscosity in the present study could be as a result of the disruption in the structure and composition of the native starch granule.
It is well established that viscosity of intestinal content interferes with digestion and absorption (Cowieson, 2010). Therefore, it is not far-fetched to assume that the improvements in starch and energy digestibility by exogenous amylase may have been partially mediated by reducing the digesta viscosity and a greater access to digestive enzymes. Furthermore, exogenous amylase increased the villus height in the jejunal tissue by about 30%. It is safe to assume that this increase in absorptive capacity would have marked implications on nutrient utilization in the chicken intestine, as was observed in the study.
In conclusion, the present study showed significant improvements in the growth performance and nutrient utilization of broiler chickens fed diets supplemented with α-amylase. Although the digestibility of starch and energy varied with the intestinal site, the efficacy of the α-amylase supplementation was greater within the jejunum compared with other intestinal regions. Given the potential impact of feed form on bird's responses, the results from this study would require careful interpretation. Although pelleted feed, as opposed to the mash, increases feed consumption and efficiency in birds, factors such as variations in pellet quality could affect the digestibility of starch, and other nutrients. Moreover, the pelleting process remains a potentially aggressive process on the stability of exogenous feed enzymes. Therefore, further studies are suggested to evaluate and compare the influence of exogenous amylase on the dynamics of intestinal starch digestion using pelleted feed.
Acknowledgments
This research was supported by funding from DSM Nutritional Products, Kaiseraugst, Switzerland. The authors thank Cobb-Vantress, Monticello, KY, for donating the chicks and Pat Jaynes for her technical assistance.
Conflicts of Interest Statement: The authors did not provide a conflict of interest statement.
References
- AOAC. 17th ed. Assoc. Off. Anal. Chem.; Arlington, VA: 2000. Official Methods of Analysis. [Google Scholar]
- AOAC. 18th ed. Assoc. Off. Anal. Chem.; Gaithersburg, MD: 2006. Official Methods of Analysis. [Google Scholar]
- Bolhuis J.E., Vanden Brand H., Staals S., Zandstra T.M., Alferink S.J.J., Heetkamp M.J.W., Gerrits W.J.J. Effects of fermentable starch and straw-enriched housing on energy partitioning of growing pigs. Animal. 2008;2:1028–1036. doi: 10.1017/S175173110800222X. [DOI] [PubMed] [Google Scholar]
- Cowieson A.J. Strategic selection of exogenous enzymes for corn/soy-based poultry diets. J. Poult. Sci. 2010;47:1–7. [Google Scholar]
- Cowieson A.J., Vieira S.L., Stefanello C. Exogenous microbial amylase in the diets of poultry: what do we know? J. Appl. Poult. Res. 2019;28:556–565. [Google Scholar]
- Croom W.J., Brake J., Coles B.A., Havenstein G.B., Christensen V.L., McBride B.W., Peebles E.D., Taylor I.R. Is intestinal absorption capacity rate-limiting for performance in poultry? J. Appl. Poult. Res. 1999;8:242–252. [Google Scholar]
- Dierick N.A., Vervaeke I.J., Demeyer D.I., Decuypere J.A. Approach to the energetic importance of fibre digestion in pigs. I. Importance of fermentation in the overall energy supply. Anim. Feed. Sci. Tech. 1989;23:141–167. [Google Scholar]
- Gracia M.I., Aran’ıbar M.J., L’azaro R., Medel P., Mateos G.G. Alpha-amylase supplementation of broiler diets based on corn. Poult. Sci. 2003;82:436–442. doi: 10.1093/ps/82.3.436. [DOI] [PubMed] [Google Scholar]
- Hetland H., Svihus B., Krogdahl A. Effects of oat hulls and wood shavings on digestion in broilers and layers fed diets based on whole or ground wheat. Br. Poult. Sci. 2003;44:275–282. doi: 10.1080/0007166031000124595. [DOI] [PubMed] [Google Scholar]
- Hill F.W., Anderson D.L. Comparison of metabolizable energy and productive energy determinations with growing chicks. J. Nutr. 1958;64:587–603. doi: 10.1093/jn/64.4.587. [DOI] [PubMed] [Google Scholar]
- Hong D., Burrows H., Adeola O. Addition of enzyme to starter and grower diets for ducks. Poult. Sci. 2002;81:1842–1849. doi: 10.1093/ps/81.12.1842. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Zhou Y., Lu F., Han Z., Wang T. Effects of different levels of supplementary alpha-amylase on digestive enzyme activities and pancreatic amylase mRNA expression of young broilers. Asian-Australas. J. Anim. Sci. 2008;21:97–102. [Google Scholar]
- Klaochanpong N., Puttanlek C., Rungsardthong V., Puncha-arnon S., Uttapap D. Physicochemical and structural properties of debranched waxy rice, waxy corn and waxy potato starches. Food Hydrocolloid. 2015;45:218–226. [Google Scholar]
- Kocher A., Choct M., Ross G., Broz J., Chung T.K. Effects of enzyme combinations on apparent metabolizable energy of corn-soybean meal-based diets in broilers. J. Appl. Poult. Res. 2003;12:275–283. [Google Scholar]
- Krogdahl A., Sell J.L. Influence of age on lipase, amylase, and protease activities in pancreatic tissue and intestinal contents of young turkeys. Poult. Sci. 1989;68:1561–1568. doi: 10.3382/ps.0681561. [DOI] [PubMed] [Google Scholar]
- Kussaibati R., Guillaume J., Leclercq B. The effects of the gut microflora on the digestibility of starch and proteins in young chicks. Ann. de zootechnie, INRA/EDP Sci. 1982;31:483–488. [Google Scholar]
- Liu S.Y., Selle P.H., Cowieson A.J. Influence of steam-pelleting temperatures and grain variety of finely-ground, sorghum-based broiler diets on small intestinal starch and nitrogen digestion dynamics in broiler chickens. Int. J. Poult. Sci. 2014;13:308–315. [Google Scholar]
- Moran E.T. Starch digestion in fowl. Poult. Sci. 1982;61:1257–1267. doi: 10.3382/ps.0611257. [DOI] [PubMed] [Google Scholar]
- Noy Y., Sklan D. Digestion and absorption in the young chick. Poult. Sci. 1995;74:366–373. doi: 10.3382/ps.0740366. [DOI] [PubMed] [Google Scholar]
- Olukosi O.A., Cowieson A.J., Adeola O. Age-related influence of a cocktail of xylanase, amylase, and protease or phytase individually or in combination in broilers. Poult. Sci. 2007;86:77–86. doi: 10.1093/ps/86.1.77. [DOI] [PubMed] [Google Scholar]
- Olukosi O.A., Adeola O. Whole body nutrient accretion, growth performance and total tract nutrient retention responses of broilers to supplementation of xylanase and phytase individually or in combination in wheat-soybean meal-based diets. J. Poult. Sci. 2008;45:192–198. [Google Scholar]
- Park C.S., Helmbrecht A., Htoo J.K., Adeola O. Comparison of amino acid digestibility in full-fat soybean, two soybean meals, and peanut flour between broiler chickens and growing pigs. J. Anim. Sci. 2017;95:3110–3119. doi: 10.2527/jas.2017.1404. [DOI] [PubMed] [Google Scholar]
- Pedersen N.R., Schimler P., Fangel V.T. 2015. Ronozyme HiStarch and pancreatin: visualization of the synergistic effect of Ronozyme HiStarch and pancreatin on pelleted and milled corn and sorghum. Novozymes, Internal report. [Google Scholar]
- Pirgozliev V.R., Rose S.P., Bedford M.R. The effect of amylose: amylopectin ratio in dietary starch on growth performance and gut morphology in broiler chickens. Archiv für Geflügelkunde. 2010;74:21–29. [Google Scholar]
- Ravindran V. Feed enzymes: the science, practice, and metabolic realities. J. Appl. Poult. Res. 2013;22:628–636. [Google Scholar]
- Schramm V.G., Durau J.F., Massuquetto A., Zavelinski V.A.B., Fascina V.B., Maiorka A. Amylase improves digestibility of pelleted diets. Poult. Sci. 2016;95:11. doi: 10.1016/j.psj.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short F.J., Gorton P., Wiseman J., Boorman K.N. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996;59:215–221. [Google Scholar]
- Silveira C., Oba M., Yang W.Z., Beauchemin K.A. Selection of barley grain affects ruminal fermentation, starch digestibility, and productivity of lactating dairy cows. J. Dairy Sci. 2007;90:2860–2869. doi: 10.3168/jds.2006-771. [DOI] [PubMed] [Google Scholar]
- Stefanello C., Vieira S.L., Soster P., Santos B.D., K Dalmoro Y., Favero A., Cowieson A.J. Utilization of corn-based diets supplemented with an exogenous α-amylase for broilers. Poult. Sci. 2019;98:5862–5869. doi: 10.3382/ps/pez290. [DOI] [PubMed] [Google Scholar]
- Svihus B. Research note: a consistent low starch digestibility observed in pelleted broiler chicken diets containing high levels of different wheat varieties. Anim. Feed Sci. Technol. 2001;92:45–49. [Google Scholar]
- Svihus B. Limitations to wheat starch digestion in growing broiler chickens: a brief review. Anim. Prod. Sci. 2011;51:583–589. [Google Scholar]
- Svihus B. Starch digestion capacity of poultry. Poult. Science. 2014;93:2394–2399. doi: 10.3382/ps.2014-03905. [DOI] [PubMed] [Google Scholar]
- Svihus B., Kløvstad K.H., Perez V., Zimonja O., Sahlström S., Schüller R.B., Jeksrud W.K., Prestløkken P. Nutritional effects of pelleting of broiler chicken diets made from wheat ground to different coarsenesses by the use of roller mill and hammer mill. Anim. Feed Sci. Technol. 2004;117:281–293. [Google Scholar]
- Tester R.F., Karkalas J., Qi X. Starch–Composition, fine structure and architecture. J. Cereal Sci. 2004;39:151–165. [Google Scholar]
- Vamadevan V., Bertoft E. Observations on the impact of amylopectin and amylose structure on the swelling of starch granules. Food Hydrocolloid. 2020;103:105663. [Google Scholar]
- Vieira S.L., Stefanello C., Rios H.V., Serafini N., Hermes R.G., Sorbara J.O.B. Efficacy and metabolizable energy equivalence of an α-amylase-β-glucanase complex for broilers. Braz. J. Poult. Sci. 2015;17:227–235. [Google Scholar]
- Weurding R.E., Veldman A., Veen W.A.G., Van der Aar P.J., Verstegen M.W.A. Starch digestion rate in the small intestine of broiler chickens differs among feedstuffs. J. Nutr. 2001;131:2329–2335. doi: 10.1093/jn/131.9.2329. [DOI] [PubMed] [Google Scholar]
- Weurding R.E. Wageningen Universiteit (Wageningen University); Wageningen, Netherlands: 2002. Kinetics of Starch Digestion and Performance of Broiler Chickens. [Google Scholar]
- Wiseman J. Variations in starch digestibility in nonruminants. Anim. Feed Sci. Technol. 2006;130:66–77. [Google Scholar]
- Wiseman J., Nicol N.T., Norton G. Relationship between apparent metabolisable (AME) values and in vivo/in vitro starch digestibility of wheat for broilers. W. Poult. Sci. J. 2000;54:305–318. [Google Scholar]
- Witt T., Gidley M.J., Gilbert R.G. Starch digestion mechanistic information from the time evolution of molecular size distributions. J. Agric. Food Chem. 2010;58:8444–8452. doi: 10.1021/jf101063m. [DOI] [PubMed] [Google Scholar]
- Woyengo T.A., Bogota K.J., Noll S.L., Wilson J. Enhancing nutrient utilization of broiler chickens through supplemental enzymes. Poult. Sci. 2019;98:1302–1309. doi: 10.3382/ps/pey452. [DOI] [PubMed] [Google Scholar]
- Yuan J., Wang X., Yin D., Wang M., Yin X., Lei Z., Guo Y. Effect of different amylases on the utilization of cornstarch in broiler chickens. Poult. Sci. 2017;96:1139–1148. doi: 10.3382/ps/pew323. [DOI] [PubMed] [Google Scholar]
- Zelenka J., Ceresnakova Z. Effect of age on digestibility of starch in chickens with different growth rate. Czech J. Anim. Sci. 2005;50:411–415. [Google Scholar]
- Zhang F., Adeola O. Energy values of canola meal, cottonseed meal, bakery meal, and peanut flour meal for broiler chickens determined using the regression method. Poult. Sci. 2017;96:397–404. doi: 10.3382/ps/pew239. [DOI] [PubMed] [Google Scholar]
- Zijlstra R.T., Jha R., Woodward A.D., Fouhse J., van Kempen T.A. Starch and fiber properties affect their kinetics of digestion and thereby digestive physiology in pigs. J. Anim. Sci. 2012;90:49–58. doi: 10.2527/jas.53718. [DOI] [PubMed] [Google Scholar]