Abstract
Since the coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a growing body of evidence indicates that besides common COVID-19 symptoms, patients may develop various neurological manifestations affecting both the central and peripheral nervous systems as well as skeletal muscles. These manifestations can occur prior, during and even after the onset of COVID-19 general symptoms. In this Review, we discuss the possible neuroimmunological mechanisms underlying the nervous system and skeletal muscle involvement, and viral triggered neuroimmunological conditions associated with SARS-CoV-2, as well as therapeutic approaches that have been considered for these specific complications worldwide.
Keywords: Coronavirus disease 2019 (COVID-19), Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Neuroimmunity, Guillain-Barré syndromes, Encephalitis, Myelitis
Abbreviations: angiotensin-converting enzyme 2, ACE-2; acute disseminated encephalomyelitis, ADEM; anti-epileptic drugs, AEDs; acute inflammatory demyelinating polyneuropathy;, AIDP; acute motor axonal neuropathy, AMAN; acute motor and sensory axonal neuropathy, AMSAN; acute necrotizing encephalopathy, ANE; activated partial thromboplastin time, aPTT; aquaporin-4, AQ4; acute respiratory distress syndrome, ARDS; blood-brain barrier;, BBB; creatinine kinase, CK; central nervous system, CNS; coronavirus disease 2019, COVID-19; C-reactive protein, CRP; cytokine release syndrome, CRS; cerebrospinal fluid, CSF; dipeptidyl peptidase 4, DPP4; diffusion-weighted imaging, DWI; electroencephalography, EEG; electromyography/nerve conduction study, EMG/NCS; erythrocyte sedimentation rate, ESR; fluid attenuated inversion recovery, FLAIR; Guillain-Barré syndrome, GBS; granulocyte-macrophage colony stimulating factor, GMCSF; hemagglutinating encephalomyelitis virus, HEV; intensive care unit, ICU; interleukin, IL; intravenous immunoglobulin, IVIG; olfactory receptor neurons, ORN; olfactory endothelia, OE; macrophage activation like syndrome, MAL; Middle East respiratory syndrome, MERS; Miller-Fisher syndrome, MFS; myelin oligodendrocyte glycoprotein, MOG; mouse hepatitis virus, MHV; nuclear factor κ-light-chain-enhancer of activated B cells, NF-κB; peripheral nervous system, PNS; personal protective equipment, PPE; prothrombin time, PT; real-time reverse transcription polymerase chain reaction, rRT-PCR; severe acute respiratory syndrome, SARS; severe acute respiratory syndrome coronavirus 2, SARS-CoV-2; secondary hemophagocytic lymphohistiocytosis, sHLH; single-stranded RNA, ss-RNA; toll-like receptors, TLRs; transmembrane protease, serine 2, TMPRSS2; tumor necrosis factor-α, TNF-α; TNF receptor-associated factor 6, TRAF6; TIR-domain-containing adapter-inducing interferon-β, TRIF; white blood cell, WBC; World Health Organization, WHO
Highlights
-
•
Patients with coronavirus disease 2019 (COVID-19) may variably develop neurological manifestations.
-
•
Neuroinflammation and autoimmunity may underlie COVID-19 neurological complications.
-
•
COVID-19 neuroimmunological complications include Guillain-Barré syndromes, myopathy, and encephalomyelitis.
-
•
Immunotherapy may variably improve outcome in COVID-19 related neuroimmunological complications.
1. Introduction
The first reports of an atypical pneumonia epidemic emerged out of Wuhan, China in December 2019, and by early January 2020 the World Health Organization (WHO) started reporting on the issue (World Health Organization (WHO), 2020). Cases were associated with a novel strain of coronavirus, retrieved from lower respiratory tract samples of 4 cases on 7 January 2020, which is from the same family of viruses that are associated with severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) (Zhu et al., 2020). Subsequently the virus was named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Gorbalenya et al., 2020), and the disease was classified as coronavirus disease 2019 (COVID-19), with clinical manifestations which ranged from asymptomatic to severe symptomatic disease, and a case fatality rate of 2.3% (Novel Coronavirus Pneumonia Emergency Response Epidemiology Team, 2020).
1.1. General virology
Coronaviridae family consists of corona viruses that can infect both animals and birds. α- and β-Coronaviruses subfamilies only infect mammals (Cui et al., 2019). Other sub-families are γ-coronaviruses and δ-coronaviruses that generally infect birds but some of the have the possibility to infect mammals too. Both α- and β-coronaviruses usually cause respiratory infections in humans and gastroenteritis in other animals. So far, all human coronaviruses have originated from animals. Among them SARS-CoV, SARS-CoV-2 and MERS-CoV most likely originated from bats. Generally, bats are the largest reservoir for α and β coronaviruses (Cui et al., 2019; Woo et al., 2012). The most common old known coronaviruses were causing the common cold. In 2002, SARS-CoV emerged for first time as a cause of a severe respiratory disease and it was followed by the appearance of MERS-CoV in 2013 and now SARS-COV-2 in 2019. They have gradually evolved to have a stronger affinity to their receptor and having higher pathogenicity. SARS-CoV-2 is a 29,903 bp positive-sense single-stranded RNA (ss-RNA) which is 79.5% identical to SARS-CoV and 96% identical to the bat coronavirus (Baig et al., 2020; Lu et al., 2020; Wu et al., 2020a; Wu et al., 2020b). It belongs to β-corona subgroup along with SARS-CoV and MERS-CoV, and they have the largest genome among RNA viruses with genome width of 26 to 32 kbp.
Common symptoms of COVID-19 have been reported to be dry cough, fever, fatigue, and shortness of breath (Centers for Disease Control and Prevention (CDC), 2020). Anosmia (Centers for Disease Control and Prevention (CDC), 2020, Moein et al., 2020) and ageusia (Centers for Disease Control and Prevention (CDC), 2020) were also later added as distinguishing symptoms. While the majority of cases of COVID-19 have mild symptoms, some cases lead to a cytokine storm followed by acute respiratory distress syndrome (ARDS) (Ye et al., 2020b), blood clots, septic shock, multiorgan failure, and ultimately the demise of the patient (Bikdeli et al., 2020; Cascella et al., 2020; Murthy et al., 2020). Viral exposure to the onset of symptoms is delayed by an average of five days, which may range from two to 14 days (Centers for Disease Control and Prevention (CDC), 2020).
1.2. Epidemiology and transmission mode
On 11 March 2020 the WHO declared COVID-19 as a pandemic. As of the date of finalizing this review, on 21 October 2020, 188 countries and territories have reported in excess of 41.07 million cases, which have resulted in more than 1,128,000 deaths, and about 28 million people who have recovered (Dong et al., 2020), as well as the effects on billions of livelihoods with widespread social and economic ramifications in every continent around the globe.
Early in the pandemic SARS-CoV-2 was thought to be spread through droplets and contaminated surfaces, while evidence aerosolized spread was limited (Pyankov et al., 2018). Later evidence emerged, suggesting that the pathogen may also be spread through aerosols (van Doremalen et al., 2020). The primary mode of transmission of SARS-CoV-2 is from person to person during close contact. Transmission is most often via micro-droplets, which are produced while coughing, sneezing, or talking (Centers for Disease Control and Prevention (CDC), 2020). Infection through contaminated surface contact followed by touching the face is now known to be less common (Centers for Disease Control and Prevention (CDC), 2020). Most micro-droplets fall to the ground or surfaces, and are not airborne over long distances (Centers for Disease Control and Prevention (CDC), 2020). The first three days after the onset of symptoms is the period in which SARS-CoV-2 is most contagious, however, transmission can occur prior to when symptoms appear, and people who do not show symptoms may be contagious (Centers for Disease Control and Prevention (CDC), 2020). The most common diagnosis of COVID-19 is via detecting the SARS-CoV-2 ribonucleic acid (RNA) through a real-time reverse transcription polymerase chain reaction (rRT-PCR), which is collected via a nasopharyngeal swab specimen (World Health Organization (WHO), 2020). Chest computerized tomography (CT) images have also been a reliable source for diagnosis, especially when infection is highly suspected based on symptoms and risk factors (Salehi et al., 2020).
While the primary mode of attack of the SARS-CoV-2 is reported to be through respiratory pathways, early in the pandemic, reports from Wuhan, China showed that some patients with COVID-19 also showed neurologic symptoms, such as headache, dizziness, and myalgia (Li et al., 2020). Ever since, a growing number of epidemiologic clinical studies as well as case series and reports worldwide have demonstrated that a considerable number of patients with COVID-19 may develop neurological symptoms and complications affecting both central and peripheral nervous system (CNS and PNS, respectively) as well as skeletal muscles. In this Review, we discuss possible mechanisms underlying the nervous system and skeletal muscle involvements in COVID-19 patients and neurological manifestations that can occur prior, during, and even after the onset of common COVID-19 symptoms. We also highlight therapeutic approaches that have been considered for management of these neurological complications, and the functional outcome in such patients.
2. Route to the nervous system
Previous studies on other coronaviruses suggest that neuronal retrograde, transcribrial, and hematogenous dissemination can be the possible pathways for SARS-CoV-2 to enter the central nervous system (CNS). Motor proteins (e.g. dynein and kinesins) moving along microtubules are shown to be involved in either retrograde or anterograde transportation of viruses (e.g. adenovirus and α-herpes viruses) through the sensory or motor nerve endings (Dodding and Way, 2011).
2.1. Olfactory pathway
The olfactory pathway is an example of such a neuronal pathway, which provides a unique and directly accessible gate of entry to the CNS from the periphery for a spectrum of viral families (e.g. Herpesviridae, Coronaviridae, Flaviviridae, Togaviridae, Bornaviridae, Bunyaviridae, Orthomyxoviridae, Paramyxoviridae, and Rhabdoviridae families) (Koyuncu et al., 2013). Olfactory nerves have bipolar neuronal structure that connects the nasal epithelium to CNS regions such as cortex, basal ganglia, and midbrain, which can be affected by the viruses (Netland et al., 2008). Notably, a recent multi-center study on 417 European patients with mild to moderate COVID-19 found olfactory dysfunction in 85.6% of the patients, of which 11.8% appeared before the other symptoms (Lechien et al., 2020). The onset of hyposmia and other neurological symptoms in patients with COVID-19 suggests the effect of SARS-CoV-2 on the olfactory system. A recent early postmortem study also revealed that SARS-CoV-2-related olfactory impairment seems to be restricted to olfactory bulbs (Coolen et al., 2020). Other viruses of the coronavirus family are also shown to involve the CNS through the olfactory tract in earlier stages of the infection (Desforges et al., 2019; Mori, 2015). SARS-CoV-2 attachment to olfactory receptor neurons may also trigger cytokine storm from accessory cells in olfactory system and exaggerated immunological response. Cytokine release might be contributing to the damage to olfactory sensory neurons (Jakhmola et al., 2020; Netland et al., 2008).
Contrasting evidence suggests that SARS-CoV-2 may not directly enter olfactory sensory neurons, but instead may target sustentacular, mucosal cells, Bowman's cells and olfactory stem cells in the human olfactory epithelium (Brann et al., 2020). Single cell transcriptional analysis demonstrated absence of angiotensin-converting enzyme 2 (ACE-2) receptors on multiple olfactory cells including glial cells and olfactory sensory neurons. Olfactory receptor neurons (ORN) and olfactory endothelia (OE) seem to be the point of viral entrance by anterograde vesicular axonal pathway (Jakhmola et al., 2020; Murthy et al., 2020). Infection of these non-neural cell types may be responsible for olfactory dysfunction in COVID-19 patients (Brann et al., 2020).
2.2. Pathways through other cranial nerves
Among other cranial nerves, trigeminal nerve and vagal nerve could be more plausible way of transmission. While SARS-CoV-2 involves lung and gastrointestinal tract very commonly, neuro-invasion through retrograde neuronal transport within vagal nerve afferents (Li et al., 2020; Toljan, 2020) has been postulated. Local peripheral nerves located in the enteric nervous system, may also get infected by other cells in the gastrointestinal tract (Bostancıklıoğlu, 2020; Lima et al., 2020; Wong et al., 2020b). Experimental studies have demonstrated this retrograde route for the influenza virus (Matsuda et al., 2004) and hemagglutinating encephalomyelitis virus (HEV) (Andries and Pensaert, 1980b). Moreover, trigeminal nerve, which usually supplies nociceptive cells in nasal cavity as well as sensory fibers in conjunctiva, might be a potential source of CNS involvement. Accordingly, SARS-CoV-2 RNA has been found in patients with conjunctivitis (Lima et al., 2020; Sun and Guan, 2020).
2.3. Hematogenous pathway
Hematogenous spread, through the destruction of the blood-brain barrier (BBB), has been proposed as yet another pathway of viral invasion to the brain, as found in influenza and other coronaviruses (Desforges et al., 2019; Koyuncu et al., 2013; Wang et al., 2010). This can be through the direct invasion of the CNS by SARS-CoV-2 or infected leukocytes entering the CNS (Bostancıklıoğlu, 2020). Additionally, SARS-CoV-2 can attack angiotensin-converting enzyme 2 (ACE-2) receptors that are present in the endothelial cells of blood vessels in the brain, disrupting the BBB and increasing the BBB permeability (Hamming et al., 2004), which ultimately encourages penetration into the CNS. The ability of the coronavirus to cross the BBB and infect mice and even primate CNS has been previously described in mouse hepatitis virus (MHV) models (Bleau et al., 2015; Cabirac et al., 1994; Cowley and Weiss, 2010). MHV studies demonstrated that coronaviruses might be capable of disrupting tight junctions of brain microvascular endothelial cells, leading to increase in permeability. MHV can cause myelitis, encephalitis and CNS demyelinating disease. Interestingly, MHV infected mice can be used as an experimental mouse model mimicking multiple sclerosis (Mecha et al., 2013) and causing demyelination in both brain and spinal cord. Viral-like particles of SARS-COV-2 were also found in post mortem brain endothelial capillary pericytes, supporting hematogenous CNS infection in COVID-19 patients. Moreover, the presence of SARS-CoV has been confirmed in the cerebrum of patients with SARS (Ding et al., 2004).
3. Mechanisms of CNS involvement
3.1. Direct infection
The first and foremost important indication of viral infectivity is receptor recognition and presenting a combination of amino acids for the strongest binding of virus-host receptor. If a new virus makes a stronger bond than a prior one, it would be chosen by natural selection. RNA viruses generally adapt to their hosts more rapidly, due to high mutation rates. Their high adaptation capacity favors them for transmitting between animals to humans (Wu et al., 2012). Coronavirus family are all spherical or oval and have spike (S) glycoproteins throughout their envelope, which gives them the shape of a crown under electron microscopy. Hence, they are named as Corona (crown) Viridae (Schoeman and Fielding, 2019; Wu et al., 2020b). The trimeric S proteins and their receptor biding domains have a similar 3D structure and homology in both SARS-CoV and SARS-CoV-2. They both have strong affinity toward the human ACE-2 receptor (Chen et al., 2020b; Li et al., 2005; Ziegler et al., 2020). A series of mutations led to a different affinity of SARS-CoV receptor domains toward human ACE-2 receptor. For example, there is a salt bridge between Lys31 and Glu35 under hydrophobic environment in human ACE-2 hot spot 31 (Yin et al., 2020). First in the civet SARS-CoV receptor binding domain, the 479 residues that counter reacts with hot spot 31 in ACE-2 was also a lysine (Yin et al., 2020). Lysine in both sides causes steric and electrostatic interference with civet-SARS-CoV and ACE-2 salt bridge counterpart. Later as result of a point mutation (K479N) at lysine residue was substituted by asparagine, which guaranteed a stronger interaction between SARS-CoV and ACE-2 and facilitated transmission of SARS-CoV to human. As such mutations happened through coronaviruses evolution, some of them were more advantageous for human-SARS-CoV-2 host interaction while still some are in favor of human-SARS-CoV. However, generally speaking, all the selected mutations in human-CoV enhance their interaction with human ACE-2 comparing to civet-CoV (Yin et al., 2020).
ACE-2 receptor is expressed in multiple tissues within human body including the CNS and skeletal muscle (Hamming et al., 2004). It is mainly detected over glial cells and neurons (Baig et al., 2020; Palasca et al., 2018). Thus, if the virus reaches out to CNS or PNS, neurons and glial cells would be potential targets. Within the brain, ACE-2 receptors are particularly present in the brainstem and medulla as part of reticular activating system function involved in regulation of cardiovascular system (Xia and Lazartigues, 2008). Interestingly though, expressing virus receptors, like ACE for SARS-CoV or dipeptidyl peptidase 4 (DPP4) for MERS-CoV, did not seem to be the only mechanism for the host cell infection with coronavirus family. It was first postulated that the level of receptor expression in the cell is the determinant factor for its infectability but marked infection of liver by coronavirus despite very low to undetectable level of ACE receptor suggested other mechanisms than ACE theory (Prabakaran et al., 2004; To and Lo, 2004). Another proposed pathway is through synaptic routes of nerve cells. HEV, which is also a member of β-Coronaviridae seems to infect CNS retrogradely via peripheral sensory nerves (Andries and Pensaert, 1980a; Hara et al., 2009). After infecting nerve cells HEV particles bud from endoplasmic reticulum-Golgi intermediate compartments. Afterward they form into virion vesicles through Golgi apparatus and finally are secreted into the surrounding matrix. Virions would then be up taken by adjacent nerve cells (Fig. 1 ) (Hara et al., 2009; Li et al., 2012; Steardo et al., 2020). Viral transport through olfactory nerve seems to be a feasible channel for introducing the virus from endothelium to olfactory nerves and bulb and finally passing to brain. Olfactory epithelial cells also express ACE-2. This pathway also explains the anosmia caused by SARS-CoV-2 infection. But whether interacting with ACE-2 receptor is the primary mechanism for anosmia commonly found in SARS-CoV infection or disruption of ciliary nasal epithelium similar to HCoV-229E is key, and is yet to be determined (Koyuncu et al., 2013; Lechien et al., 2020; Troyer et al., 2020; Wu et al., 2020b). Notably, when transgenic mice expressing human ACE-2 were infected intranasally by SARS-CoV, the viruses were found to enter the brain by day 4 post infection primarily via the olfactory bulb resulting in a rapid, transneuronal spread to the connected areas of the brain (Netland et al., 2008). Another coronavirus, HCoV-OC43, has demonstrated a similar behavior (Dube et al., 2018). In this case, the virus spreads to the piriformis cortex, brain stem, and spinal cord by day 4 post infection. Interestingly, administration of zinc sulfate, that causes degeneration of the olfactory sensory neurons, almost completely stopped the virus to gain entry to the CNS (Dube, Le Coupanec, 2018). Moreover, when transgenic mice expressing human DPP4 were infected intranasally by MERS-CoV, brain disease was observed, with the greatest involvement noted in the thalamus and brain stem (Li et al., 2016). The temporal course of brain tissue infection suggested retrograde virus spread from olfactory neurons. Altogether, these data support the critical role of the olfactory pathway and ACE-2 in the neuroinvasion process.
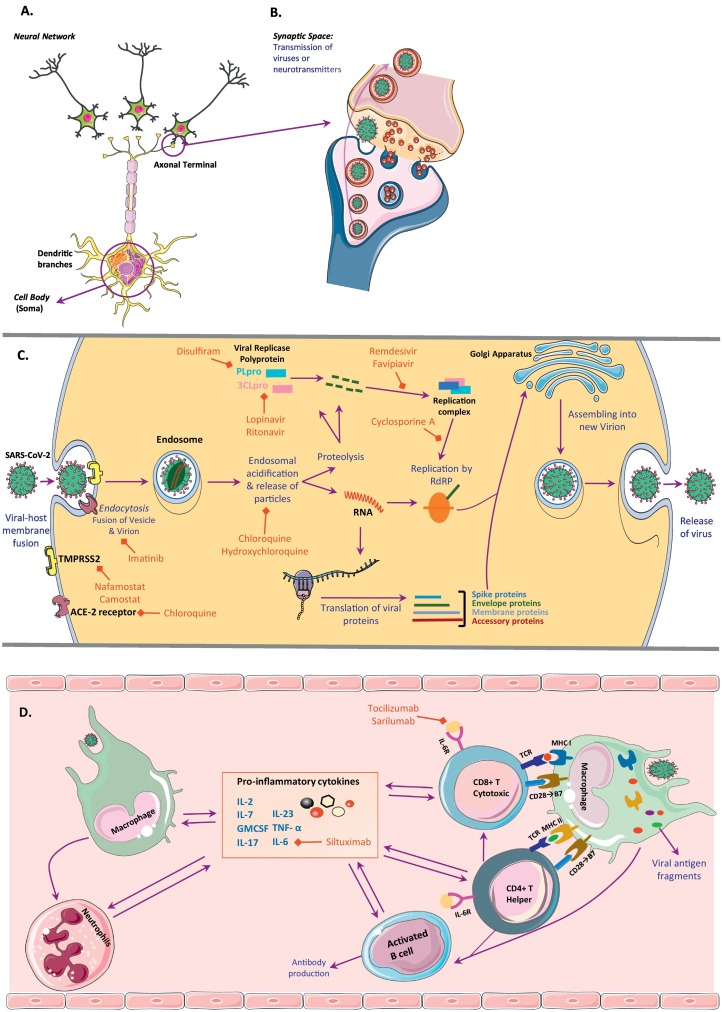
Fig. 1.
A schematic neural network and synaptic space as well as pathways for intracellular SARS-CoV-2 cycle and its effect on both innate and advance immune response. (A) Neural network and main 3 parts of a neuron (cell body, dendritic branches and axon); (B) Synaptic space and transmission of neurotransmitters and SARS-CoV-2 viruses in similar manner; and (C) Pathways for viral fusion, viral RNA replication and assembly and finally excretion in any cell type including neurons. Virus enter the cell via endocytosis and then are lysed to their protein particles and nucleic acid through endosomal acidification. Viral genome is both replicated and translated to have all parts needed for reassembly of virions and excretion. RNA is replicated by help of RNA-dependent RNA polymerase (RdRP) and replication complex, which is made up from viral protein particles and viral replicase polyprotein. There are different medications blocking each step of the cascade which are outlined in red. (D) Depiction of the mechanisms of viral effects on both innate and advance immune response. There is an interplay between all immune cells types which leads to rapid increase in pro-inflammatory cytokine release and cytokine storm. As the initial immune response neutrophil number raise and macrophages as antigen presenting cells activate T helper and T cytotoxic cells via MHC class II and I respectively. T helper cells also help activating B cells and T cytotoxic cells. All these activated cells secrete pro-inflammatory cytokines including IL-6 and TNF-α. GMCSF, granulocyte-macrophage colony stimulating factor; TMPRSS2, transmembrane protease, serine 2. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To date, the full pathway for nerve and glial cells infections is not convincingly explained. However, in prior case reports and autopsies, SARS-CoV and MERS-CoV particles were found within neurons and glial cells as well as the cerebrospinal fluid (CSF) proving that cells in the nervous system can be infected (He et al., 2003; Lau et al., 2004; Li et al., 2016; Xu et al., 2005). After cell infection with coronavirus, the cell ultimate endpoint is death whether it would happen through autophagy, apoptosis, pyroptosis, elimination through innate immune cells, or other pathways (Varga et al., 2020; Yang and Shen, 2020). Viral antigens were detected in respiratory brain stem centers like nucleus of the solitary tract and nucleus ambiguous. Damage to these centers may be a contributor to cardiac or respiratory arrest (Li et al., 2020; Steardo et al., 2020; Xia and Lazartigues, 2010).
3.2. Neuroinflammation
Additional effect of SARS-CoV-2 on CNS is through systemic and local inflammatory response causing cytokines storming and immune cells reactivation (Shi et al., 2020; Steardo et al., 2020). Earlier epidemiologic studies showed that about 15% of cases might advance to severe disease and the rate is higher in people older than 65 years of age (Sun and Guan, 2020). Later some European countries like Italy showed higher case fatalities from what China and most of other countries witnessed (Onder et al., 2020). The determinant factors for progressing to severe stages are not completely understood; and is the most striking question.
There is always a tug-of-war between viruses and host immune response. Through years the host immune system either succeeds in clearing the pathogen or adapts in a way causing chronic viral infections. When a virus surpasses a species after years of co-evolution, the new host would respond to it with a more severe immune reaction that can even damage host tissues. Accordingly, possible severe immune response to SARS-CoV-2 is expected (Fung et al., 2020; Rahman et al., 2011; Wagstaff et al., 2013). There are several pathways proposed to be involved in human immune response toward SARS-CoV-2, and all include two general phases, innate and adaptive immune responses (Fig. 1). Innate immune response includes activation of neutrophils, macrophages and natural killer cells and adaptive response involves cytotoxic CD8+ cells, CD4+ T helper cells and B cells (Steardo et al., 2020). What has been observed so far in severe and fatal COVID-19 infection is a reduction in the absolute number of T cells as well as monocytes, eosinophils, and basophils. At the same time neutrophilic response is enhanced, leading to increased neutrophil-lymphocyte ratio. Despite absolute reduction in total number of T cells, including both CD4+ and CD8+ cells, the main reduction is among memory T helpers and regulatory cells, while naïve T cells and pro-inflammatory T helper 17 cells were even boosted in number (Karakike and Giamarellos-Bourboulis, 2019; Lagunas-Rangel, 2020; Qin et al., 2020; Xu et al., 2020e). Because of this pro-inflammatory cell shift, immune cells hyper-react by producing excess levels of inflammatory cytokines. Whether the cytokine storm is a part of the “cytokine release syndrome (CRS)”, or the “secondary haemophagocytic lymphohistiocytosis (sHLH)” also called “macrophage activation like syndrome (MAL)”, the outcome is a robust increase in the highly inflammatory cytokines such as interleukin (IL)-6, IL-2, IL-7, granulocyte-colony stimulating factor (GMCSF), and tumor necrosis factor-α (TNF-α) (Mehta et al., 2020; Zhang et al., 2020a).
CRS is commonly seen after car T cell therapy and sepsis following organ transplantation and is also reported after viral infections. Clinical features of CRS include headache, fever, encephalopathy, hypotension, coagulopathy, cytopenia and multiorgan failure (Le et al., 2018; Zhang et al., 2020a). Most of these features are shared with MAL, which also could occur secondary to infections and hematological malignancies (Karakike and Giamarellos-Bourboulis, 2019). One of the main outcomes commonly found in both CRS and MAL is an upsurge in IL-6, which is reported to be also augmented in moderate to severe cases of SARS-CoV-2 infection (Wan et al., 2020). Inflammatory conditions leading to increased IL-6 and TNF-α also might facilitate disruption of BBB (Ichiyama et al., 2002; Linker et al., 2008) which in turn might be responsible for encephalitis, acute necrotizing encephalopathy and demyelination in the CNS and even Guillain-Barré syndrome (GBS) in the PNS. There are several case reports of such complications due to SARS-CoV-2 infection as we discuss in the next sections (Alberti et al., 2020; McAbee et al., 2020; Moriguchi et al., 2020; Poyiadji et al., 2020; Zanin et al., 2020; Zhang et al., 2020c). Interestingly, tocilizumab (a recombinant, humanized monoclonal antibody against the IL-6 receptor), which is an FDA approved medication for treatment of T cell induced CRS, also showed some benefit over severe cases of COVID-19 infection (Le et al., 2018; Xu et al., 2020a). However, as of yet, there is not sufficient evidence to clarify the exact role of systemic inflammation versus local inflammation due to the direct viral infection or hypoxia, which is a common complication of SARS-CoV-2 infection.
3.3. Other mechanisms
The brain and the lungs have a close inter-relation. A disease process in one would potentially cause complications of the other (Abdennour et al., 2012). Brainstem centers for respiratory and cardiovascular systems are potential targets of SARS-CoV-2 and neural cell death in these centers might be responsible for a central cause of respiratory/cardiovascular arrest (Li et al., 2020; Steardo et al., 2020; Xia and Lazartigues, 2010). SARS-CoV-2 lung infection has been reported to cause an atypical form of ARDS, while patients usually show relatively well-preserved lung mechanics not matching the severity of hypoxemia. This may be due to the dysregulation of lung perfusion and hypoxic vasoconstriction, which may have a central cause as well (Gattinoni et al., 2020). On the other hand, through a process called “infectious toxic encephalopathy” usually seen in toxic metabolic disorders or acute infections, alveolar gas exchange problem might lead to anaerobic metabolism in brain cells, and cause CNS hypoxia. The hypoxemia and increased acidity within the brain causes cell swelling, interstitial edema, obstructive hydrocephalus, and increased intracranial hypertension leading to an altered mental status and even coma (Abdennour et al., 2012; Wu et al., 2020b). Hypoxic injury to the brain also may cause cerebrovascular accidents like stroke or seizures, and again would activate the loop of both local microglial activation and systemic inflammation (Liu and Mccullough, 2013; Wu et al., 2020b).
Another clinical and scientific significance of SARS-CoV-2 infection is widespread observation of hypercoagulable state indicated by elevated D-dimer level, prolongation of prothrombin time (PT), activated partial thromboplastin time (aPTT), and thrombocytopenia (Violi et al., 2020b). Coagulopathy was previously observed in infection with other Coronoviridae viruses including SARS and MERS (Giannis et al., 2020; Merad and Martin, 2020). Although there are some prospective studies currently looking at incidence of thrombotic events, early studies have already confirmed increased frequency of intravascular thrombosis leading to pulmonary embolism, myocardial infarction, ischemic strokes, and even cerebral venous sinus thrombosis. A thrombotic event was sometimes reported as the first presentation of COVID-19 infection (Hughes et al., 2020; Klok et al., 2020). In a retrospective study on 214 COVID-19 patients, about 6% presented with acute cerebrovascular events, mainly ischemic strokes (Cantador et al., 2020). A small number of stroke patients with COVID-19 infection presented with cerebral hemorrhage (Cantador et al., 2020). Recent investigation has also found that COVID-19 patients have increased serum Nox2 overactivation, which is an important trigger for vascular dysfunction through excess production of reactive oxygen species (Violi et al., 2020a). Interestingly, it was more up-regulated in more severe COVID-19 cases and also those with thrombotic complications (Violi et al., 2020a). One possible underlying mechanism is the reduced expression and function of ACE-2 in SARS-CoV-2 infected cells. ACE-2 regulates the cerebral blood flow and its altered signaling can lead to subsequent hypertension and predisposition to developing hemorrhagic stroke from arterial wall rupture (Sharifi-Razavi et al., 2020). Another possible mechanisms is the underlying coagulopathy induced by the infection with thrombocytopenia (Wang et al., 2020b).
4. Neurologic manifestations
Since the early phases of the global pandemic, numerous studies have demonstrated clinical symptoms and signs of COVID-19 which mainly include fever, cough, sore throat, dyspnea, diarrhea, nausea, vomiting, anorexia, and fatigue (Table 1 ). Accumulating evidence also indicates that patients with COVID-19 may variably develop neurological symptoms and complications (Table 1). An early study on 214 patients in Wuhan, China, found that about 36% of patients with COVID-19 had neurological manifestations including dizziness (16.8%), headache (13.1%), skeletal muscle injury (myalgia plus elevated creatinine kinase [CK], 10.7%), impaired consciousness (7.5%), ageusia (5.6%), anosmia (5.1%), stroke (2.8%), nerve pain (2.3%), visual impairment (1.4%), seizure (0.5%), and ataxia (0.5%) (Mao et al., 2020). An important finding was the significantly higher rate of neurological manifestations (in general) and impaired consciousness, stroke and skeletal muscle injury (in particular) in patients with severe COVID-19 infection than those with non-severe infection (Mao et al., 2020). Another study demonstrated that the major factor associated with neurologic complications was age over 60, which was also a strong risk factor for mortality (Xiong et al., 2020). When patients with COVID-19 infection were compared at the same level of severity, new-onset of neurologic critical events (e.g. impaired consciousness and stroke) was later found to increase the risk of death by six-fold (Xiong et al., 2020). Overall, the most common neurological symptoms described in COVID-19 patients are fatigue/malaise, myalgia, headache, impaired consciousness, dizziness, ageusia, and anosmia; and less common reported symptoms include visual impairment, nerve pain, occipital neuralgia, ataxia, tremor, and tic. Growing number of case reports and/or series indicate that a variety of neurological conditions and post-viral triggered autoimmune complications, as we discuss below, occur in association with SARS-CoV-2 infection which mainly include Guillain-Barré syndromes (GBSs) (Table 2 ), myopathy and rhabdomyolysis (Table 2), encephalopathy, meningoencephalitis, encephalomyelitis, and myelitis (Table 3 ).
Table 1.
Neurological symptoms and signs of COVID-19 in clinical studies.
| Region | Patients | Median (IQR) age, yr | Gender F (%) | Neurological Symptoms (%) | Other Symptoms (%) | Abnormal lab results (%) | CSF (% of cases) | Results of nervous system imaging or EEG (% of cases) | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Wuhan, China (total 799) | 113 death | 68 (62–77) | 30 (27) | Myalgia (19), headache (10), dizziness (9), impaired consciousness (22) | Fever (92), cough (70), fatigue (57), anorexia (27), dyspnea (62), chest tightness (49), pharyngalgia (4), hemoptysis (4), nausea (7), vomiting (5), abdominal pain (10) | Leukocytosis (50), lymphocytopenia (39); albumin (65) & ↓ K+ (12); ↑ AST (52), ALT (27), K+ (22), Na+ (18), D-dimer (35), LDH (82), CRP (60), IL-1β (9), IL-2R (81), IL-6 (100), IL-8 (28), IL-10 (70), TNF-α (77), pro-BNP (85) & cardiac troponin I (72). Higher ESR, ferritin & PT, and lower TSH in this group. |
NR | NR | (Chen et al., 2020b) |
| 161 recovery | 51 (37–66) | 73 (45) | Myalgia (24), headache (12), dizziness (7), impaired consciousness (1) | Fever (90), cough (66), fatigue (45), anorexia (22), dyspnea (31), chest tightness (30), pharyngalgia (5), hemoptysis (2), nausea (10), vomiting (6), abdominal pain (12) | Leukocytosis (4), lymphocytopenia (5), ↓ albumin (14) & K+ (11), ↑ AST (16), ALT (19), K+ (4), Na+ (2), D-dimer (2), LDH (14), CRP (14), IL-1β (12), IL-2R (37), IL-6 (60), IL-8 (8), IL-10 (19), TNF-α (47), pro-BNP (18) & cardiac troponin I (14). | NR | NR | ||
| Wuhan, China | 214 | Mean (SD) 52.7 (15.5) | 127 (59.3) | Dizziness (16.8), headache (13.1), skeletal muscle injury (10.7), impaired consciousness (7.5), ageusia (5.6), anosmia (5.1), stroke (2.8, ischemic [66.3], hemorrhagic [16.7]), nerve pain (2.3), visual change (1.4), ataxia (0.5), seizure (0.5) | Fever (61.7), cough (50), anorexia (31.8), diarrhea (19.2), throat pain (14.5), abdominal pain (4.7) | Lower lymphocyte & platelets, & higher BUN levels in cases with CNS symptoms; no differences in cases with PNS symptoms; higher CK, CRP, D-dimer & neutrophils with lower lymphocytes in cases with skeletal muscle injury. | NR | NR | (Mao, Jin, 2020) |
| Wuhan, China | 41 | 49 (41–58) | 11 (29) | Headache (8), myalgia or fatigue (44) | Fever (98), cough (76), hemoptysis (5), diarrhea (3), dyspnea (55) | Leukopenia (25), lymphocytopenia (63), thrombocytopenia (5), ↑ AST (37), creatinine (10), CK (33), LDH (73), troponin I (12) & procalcitonin (8); overall ↑ IL-1β, IL-1Rα, IL-7, IL-8, IL-9, IL-10, FGF, GCSF, GMCSF, IFNγ, IP10, MCP1, MIP1A, MIP1B, PDGF, TNFα, & VEGF. Higher PT & D-dimer in ICU cases than non-ICU. |
NR | NR | (Huang et al., 2020b) |
| Wuhan, China (total 710) | 52 critical ill | Mean (SD) 59.7 (13.3) | 17 (33) | Headache (6), myalgia (11.5), malaise (35) | Fever (98), cough (77), dyspnea (63.5), rhinorrhea (6), arthralgia (1), chest pain (2), vomiting (4), ARDS (67) | AKI (29), cardiac injury (23), liver dysfunction (29), hyperglycemia (35) | NR | NR | (Yang et al., 2020b) |
| Wuhan, China | 138 | 56 (42–68) | 63 (45.7) | Fatigue (69.6), myalgia (34.8), dizziness (9.4), headache (6.5) | Fever (98.6), cough (59.4), anorexia (39.9), dyspnea (31.2), pharyngalgia (17.4), diarrhea (10.1), nausea (10.1), vomiting (3.6), abdominal pain (2.2), ARDS (19.6) | Acute cardiac injury (7.2), AKI (3.6). Higher WBCs, neutrophils, D-dimer, CK, & creatine in ICU cases than non-ICU. |
NR | NR | Wan et al., 2020; Wang et al., 2020a) |
| Wuhan, China | 99 | Mean (SD) 55.5 (13.1) | 32 (32) | Myalgia (11), confusion (9), headache (8) | Fever (83), cough (82), dyspnea (31), sore throat (5), rhinorrhea (4), chest pain (2), diarrhea (2), nausea/vomiting (1), ARDS (17) | Leukocytosis (24), lymphocytopenia (35), thrombocytosis (4), thrombocytopenia (12), anemia (51), ↓ albumin (98), ↑ neutrophils (38), aPTT (6), PT (5), D-dimer (6), ALT (28), AST (35), BUN (6), CK (13), LDH (76), myoglobin (17), procalcitonin (6), IL-6 (52), ESR (23.4), ferritin (63) & CRP (86); hyperglycemia (51), AKI (3). | NR | NR | (Chen et al., 2020a) |
| Wuhan, China | 452 | 58 (47–67) | 217 (48) | Myalgia (21.4), confusion (0.7), headache (11.4), dizziness (8.1), fatigue (46.4) | Fever (92.6), cough (33.3), hemoptysis (2.6), dyspnea (50.8), rhinorrhea (1.8), pharyngalgia (4.8), anorexia (21), nausea/vomiting (9.2), diarrhea (26.7), abdominal pain (5) | Most cases: lymphocytopenia, ↑ procalcitonin, ESR, ferritin, CRP, TNF-α, IL-2R, & IL-6. ↓ helper T cells (CD3 + CD4+) and suppressor T cells (CD3 + CD8+). | NR | NR | (Qin, Zhou, 2020) |
| Wuhan, China | 140 | Median (range): 57 (25–87) | 69 (49.3) | Fatigue (75) | Fever (91.7), cough (75), chest tightness/dyspnea (36.7), nausea (17.3), diarrhea (12.9), anorexia (12.2), abdominal pain (5.8), belching (5), vomiting (5) | Leukocytosis (12.3), leukopenia (19.6), lymphocytopenia (75.4), ↑ D-dimer (43.2), CRP (91.9), amyloid A (90.2), procalcitonin (34.7) & CK (6.7). | NR | NR | (Zhang et al., 2020b) |
| Wuhan, China (fatal cases) | 85 | Mean (SD): 65.8 (14.2) | 23 (27.1) | Fatigue (58.8), myalgia (16.5), headache (4.7) | Fever (91.8), dyspnea (70.6), anorexia (56.5), cough (22.4), diarrhea (18.8), vomiting (4.7), abdominal pain (3.5), chest pain (2.4), pharyngalgia (2.4), ARDS (74.1) | Leukocytosis (44.7), leukopenia (11.8), ↑ neutrophils (60), neutropenia (12.9), lymphocytopenia (77.6), thrombocytosis (7.1), thrombocytopenia (41.2), ↓ albumin (78.8), ↑ D-dimer (65.9), aPTT (25.9), PT (25.9), fibrinogen (47.1), ALT (16.5), AST (32.9), BUN (49.4), creatinine (18.8), CK (36.5), LDH (82.4), procalcitonin (22.4) & CRP (91.8); hypoglycemia (3.5), hyperglycemia (78.8). | NR | NR | (Du et al., 2020) |
| Wuhan, China | 221 | Median (range): 55 (39–66.5) | 113 (51.1) | Fatigue (70.6), headache (7.7), | Fever (90.5), cough (61.5), anorexia (36.2), dyspnea (29), diarrhea (11.3), pharyngalgia (10), abdominal pain (2.3), ARDS (21.7) | Leukocytosis (10.4), leukopenia (33), lymphocytopenia (17.6), thrombocytosis (5.4), thrombocytopenia (13.4), ↑ procalcitonin (5.9). | NR | NR | (Zhang et al., 2020d) |
| Sichuan, Wuhan & Chongqing in China | 917 | Mean 48.7 (17.1) | 417 (44.8) | Impaired consciousness (2.7), stroke (1.1), muscle cramp (0.2), headache (0.2), occipital neuralgia (0.1), tremor/tic (0.2) | NR | NR | 3 cases: normal | Head CT scan (28 cases): new abnormal findings (32.1) including stroke (25%), brain tumor (3.6) & traumatic brain injury signs (3.5) | (Xiong, Mu, 2020) |
| Zhejiang, China | 645 | NR | 317 (49.1) | Myalgia (11), fatigue (18.3), headache (10.4) | Fever (83.7), cough (65.9), sputum (34.9), hemoptysis (1.7), sore throat (15.04), dyspnea (4). diarrhea (8.2), nausea or vomiting (3.4) | NR | NR | NR | (Zhang et al., 2020e) |
| Zhejiang, China | 91 | 50 (36.5–57) | 54 (59.34) | Fatigue (44), headache (7.7), myalgia (5.5), back discomfort (3.3) | Fever (71.4), cough (60.4), sputum (33), dyspnea (11), anorexia (25.3), diarrhea (23.1), nausea (12.1), vomiting (6.6) | Leukocytosis (4), leukopenia (15.4), lymphopenia (31.8), thrombocytopenia (11), ↑ CRP (53.8), procalcitonin (15.4), fibrinogen (24.2), D-dimer (24.2), ALT (7.7), AST (9.9) | NR | NR | (Qian et al., 2020) |
| Zhejiang, China | 62 | 41 (32–52) | 27 (43.5) | Headache (34), myalgia or fatigue (52) | Fever (77), cough (81), sputum (56), diarrhea (8), hemoptysis (3), dyspnea (3) | Leukopenia (31), lymphocytopenia (42), ↑ AST (16), procalcitonin (11) | NR | NR | (Xu et al., 2020c) |
| Guangdong, China | 90 | Mean (range): 50 (18–86) | 51 (56.7) |
Fatigue/weakness (21), myalgia (28), headache (4) | Fever (78), cough (63), sputum (12), sore throat (26), chills (7), diarrhea (6), nausea (6), vomiting (2) | Leukocytosis (3), leukopenia (21),↑ CRP (42) | NR | NR | (Xu et al., 2020b) |
| Wenzhou, China | 149 | Mean (SD): 45.11 (13.35) | 68 (45.6) | Headache (8.7), myalgia (3.4) | Fever (76.5), cough (58.4), sputum (32.2), dyspnea (1.3), sore throat (14.1), snotty (3.4), chest pain (3.4), chest tightness (10.8), chill (14.1), diarrhea (7.4), nausea or vomiting (1.3) | Leukocytosis (1.34), leukopenia (24.2), neutropenia (28.2), lymphocytopenia (35.6), thrombocytosis (5.4), thrombocytopenia (13.4), ↑ D-dimer (14.1), ALT (12.1), AST (18.1), LDH (30.2), CRP (23) & creatinine (28.9), & prolonged PT (11.4) or aPTT (26.9) | NR | NR | (Yang et al., 2020a) |
| Hubei, China | 137 | Median (range): 57 (20–83) | 76 (55.5) | Myalgia or fatigue (32.1%), headache (9.5) | Fever (81.8), cough (48.2), dyspnea (19), diarrhea (8), palpitation (7.3) | Leukocytosis (19), lymphocytopenia (72.3), ↑ CRP (83.9) | NR | NR | (Liu et al., 2020a) |
| Beijing, China | 13 | 34 (34–48) | 3 (23.1) | Myalgia 3 (23.1), headache (23.1) | Fever (92.3), cough (46.3), upper airway congestion (61.5), diarrhea (7.7), rhinorrhea (7.7) | Lymphocytosis & ↑ CRP (some cases) | NR | NR | (Chang et al., 2020) |
| Beijing, China | 262 | Median (range): 47.5 (1–94) | 216 (82.4) | Fatigue (26.3), headache (6.5), | Fever (82.1), cough (45.8), dyspnea (6.9) | NR | NR | NR | (Tian et al., 2020) |
| China | 1099 | 47 (35–58) | 459 (41.9) | Headache (13.6), fatigue (38.1), myalgia/arthralgia (14.9) | Admission fever (43.8), in-hospital fever (88.7), conjunctival congestion (0.8), nasal congestion (4.8), cough (67.8), sore throat (13.9), hemoptysis (0.9), dyspnea (18.7), nausea/vomiting (5), diarrhea (3.8), chills (11.5), tonsil swelling (2.1), ARDS (3.4) | Leukocytosis (5.9), leukopenia (33.7), lymphocytopenia (83.2), thrombocytopenia (36.2), AKI (0.5), DIC (0.1), rhabdomyolysis (0.2); ↑ CRP (most cases) & ↑ AST, ALT, CK & D-dimer (some cases). | NR | NR | (W-J and Z-Y, 2020) |
| China | 50 | Mean (range): 43.9 (3–85) | 21 (42) | Headache (10), myalgia (16), fatigue (16) | Fever (76), cough (40), sputum (14), sore throat (8), dyspnea (8), diarrhea (2) | Lymphocytopenia (28), ↑ CRP (52) | NR | NR | (Xu et al., 2020d) |
| South Korea | 28 | Mean (range): 42.6 (20–73 | 13 (46.1) | Myalgia (14.3), fatigue (10.7) | Fever (32.1), sore throat (32.1), cough (17.9), chills (17.9) | NR | NR | NR | (2020) |
| New Delhi, India | 21 | Mean (range): 40.3 (16–73) | 7 (33.3) | Headache (14.3) | Fever (42.9), cough (42.9), sore throat (23.8), dyspnea (4.8) | Leukopenia (4.8) | NR | NR | (Gupta et al., 2020) |
| South Iran | 113 | Mean (range): 53.75 (20–99) | 42 (37.2) | Fatigue (66.4), myalgia/arthralgia (61.1), headache (53.1), dizziness/vertigo (39.8), | Fever (59.3), cough (64.6), sputum (21.4), dyspnea (51.3), chest pain (38.1), sore throat (31.9), hemoptysis (6.2), chills (59.3), rhinorrhea (23), abdominal pain (21.2), diarrhea (22.1), nausea (42.5), vomiting (25.7), anorexia (66.4) | Leukocytosis (10.8), leukopenia (9), lymphocytopenia (12.6), thrombocytosis (11), thrombocytopenia (15.6), ↑ CRP (25), ESR (15.9), creatinine (35.5), & prolonged PT (77.9) or aPTT (45.8) | NR | NR | (Shahriarirad et al., 2020) |
| Istanbul, Turkey | 27 cases with brain MRI ⁎ | Median (range): 63 (34–87) | 6 (22) | Not specified | NR | NR | 5 cases: ↑ protein (80), normal WBC, glucose, IgG index & albumin (100), negative RT-PCR for SARS-CoV-2 (100) | Brain MRI: abnormal (44.4); cortical FLAIR signal abnormality (37), increased cortical DWI with corresponding low ADC values (26), subtle leptomeningeal enhancement (18), punctate cortical blooming artifact (11). | (Kandemirli, Dogan, 2020) |
| Strasbourg, France | 58 | 63 | NR | On ICU admission (14); After neuromuscular blockers/sedations hold (67): agitation (69), confusion (65), corticospinal tract signs (hyperreflexia, ankle clonus & Babinski signs) (67), dysexecutive syndrome (36) | Fever (16), ARDS (100) | NR | 7 cases: 0 WBC (100), + oligoclonal band with the same pattern in serum (29), ↑ IgG & protein (14), ↓ albumin (57), negative RT-PCR for SARS-CoV-2 (100) | Brain MRI (13 cases): leptomeningeal enhancement (62), bilateral frontotemporal hypoperfusion (11 cases, 100), ischemic stroke (23). EEG (8 cases): diffuse bifrontal slowing consistent with encephalopathy (12.5) |
(Helms, Kremer, 2020) |
ADC, Apparent diffusion coefficient; AKI, acute kidney injury, ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; CK, creatine kinase; CNS, central nervous system; CRP, C-reactive protein; DIC, disseminated intravascular coagulation; EEG, electroencephalography; ESR, erythrocyte sedimentation rate; F, female; FGF, fibroblast growth factors; GCSF, granulocyte colony-stimulating factor; GMCSF, granulocyte-macrophage colony-stimulating factor; ICU, intensive care unit; IFN-γ, interferon-γ; IL, interleukin; IP, induced protein; IQR, interquartile range; LDH, lactate dehydrogenase; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; PDGF, platelet derived growth factor; pro-BNP, pro-brain natriuretic peptide; PT, prothrombin time; RANTES, regulated on activation and normally T-cell expressed; SD, standard deviation; TNF-α, tumor necrosis factor-α; TSH, thyroid stimulating hormone; VEGF, vascular endothelial growth factor; WBC, white blood cell.
This is out of 50 of 235 (21%) ICU patients who developed neurological symptoms.
Table 2.
Case reports of Guillain-Barre syndrome (GBS) variants and skeletal muscle injury related to COVID-19.
| Region | Age, Gender | Neurological symptoms on admission (day from admission) | Other symptoms (onset day prior neurologic symptoms) | Admission serum labs (or day from admission) | CSF (day from admission) | Imaging or NCS/EMG (day from admission) | Treatments received | Outcome | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Miller-Fisher Syndrome | |||||||||
| Madrid, Spain | 50, M | Two-day anosmia, ageusia, right INO, right fascicular oculomotor palsy, perioral paresthesia, ataxia, & areflexia | Fever, cough, malaise, headache & lumbar pain (5 days prior) | Lymphocytopenia, ↑ CRP; positive anti-GD1b IgG antibody | ↑ Protein (80 mg/dL), 0 WBC, normal glucose, negative culture / COVID-19 rRT-PCR | Head CT scan: normal EMG/NCS: NR |
IVIG (0.4 g/kg/day for 5 days) | Favorable; recovery in 2 weeks with residual anosmia & ageusia | (Gutiérrez-Ortiz, Méndez, 2020) |
| Madrid, Spain | 39, M | Same-day ageusia, bilateral abducens palsy, & areflexia | Low-grade fever & diarrhea (3 days prior) | Leukopenia; normal LFT, RFT & cardiac enzymes | ↑ Protein (62 mg/dL), 2 WBC, normal glucose, negative culture / COVID-19 rRT-PCR | Head CT: normal. EMG/NCS: NR |
Supportive care | Favorable, complete recovery in 2 weeks | (Gutiérrez-Ortiz, Méndez, 2020) |
| Malaga, Spain | 51, F | Eleven-day radicular thoracic/lumbar back & all limbs pain; 7-day rapidly progressive lower limb weakness & binocular diplopia, left external rectus muscle & bifacial weakness, areflexia, & autonomic dysfunction (dry eyes/mouth, diarrhea, labile blood pressure) | Diarrhea, odynophagia & cough (15 days prior) | Positive SARS-CoV-2 IgG, negative COVID-19 rRT-PCR | ↑ Protein (70 mg/dL), 5 WBC, negative antiganglioside antibodies | EMG/NCS (day 4): asymmetric prolonged F wave latency for the lower limbs, low A-wave amplitude on the left leg, altered bilateral R1 responses in the Blink-Reflex, ↓ poor activity in right rectus-anterior femoral muscle & little spontaneous denervation activity in left rectus-anterior femoral (RAF) muscle on EMG, overall suggestive of demyelination in early stage. Repeat EMG/NCS (day 20): low F-wave amplitude & disintegrated morphology, similar alteration of Blink-Reflex & spontaneous denervation activity in bilateral RAF & left anterior tibialis. |
IVIG (0.4 g/kg/day for 5 days), gabapentin (total 900 mg/day) | Favorable | (Reyes-Bueno, García-Trujillo, 2020) |
| Acute Inflammatory Demyelinating Polyneuropathy (AIDP) | |||||||||
| Jingzhou, China | 61, F | One-day rapidly progressive ascending paraparesis & areflexia; evolving to tetraparesis & distal numbness (day 3) | No prior symptoms (had trip to Wuhan 7 days prior), later fever & cough (admission day 8) | Lymphocytopenia, thrombocytopenia, positive COVID-19 nasopharyngeal swab. | Day 4: ↑ protein level (124 mg/dL), 5 WBC, negative COVID-19 rRT-PCR. | EMG/NCS (day 5): prolonged left ulnar & bilateral tibial/peroneal distal motor latencies, absent ulnar/tibial/peroneal F waves, normal left median/ulnar & bilateral sural/superficial peroneal SNAPs, overall suggestive of demyelination. | IVIG (0.4 g/kg/day for 5 days); Day 8: arbidol, lopinavir & ritonavir | Favorable; complete recovery within 1 month | (Zhao, Shen, 2020a) |
| Northern Italy | 76, M | One-day lumbar pain followed & rapidly progressive paraparesis; evolving tetraplegia, areflexia, & ataxia (day 4) | Cough & anosmia (5 days prior), fever (prior IVIG) |
Lymphocytopenia, ↑ CRP, ketonuria; positive serum SARS-CoV-2 IgG (64.59 AU/mL), positive COVID-19 nasopharyngeal swab. | Day 5: normal with negative COVID-19 rRT-PCR. | Brain/Spine MRI: normal. EMG/NCS (day 2): prolonged tibial /ulnar distal motor latencies, ↓ tibial/ulnar CMAP amplitudes, slow tibial/ulnar motor conduction velocities, prolonged tibial F wave, normal sural/ulnar SNAPs, normal EMG, overall suggestive of demyelination. |
IVIG (0.4 g/kg/day for 5 days) | Incomplete recovery, upper limb improvement but unable to stand (one month later) | (Toscano et al., 2020) |
| Northern Italy | 61, M | One-day rapidly progressive paraplegia, lower limb paresthesia & areflexia; evolving to tetraplegia, bifacial weakness & dysphagia (day 3), & respiratory failure (day 4) | Cough, ageusia, & anosmia (7 days prior) | Lymphocytopenia, ↑ CRP, LDH & AST; ketonuria; negative anti-GM1, GQ1b & GD1b antibodies; positive serum SARS-CoV-2 IgG (50.92 AU/mL), negative COVID-19 nasopharyngeal swab. | Day 3: normal protein (40 mg/dL), 3 WBC, negative COVID-19 rRT-PCR and viral/bacterial panel. | EMG/NCS (day 3): prolonged tibial and normal ulnar distal motor latencies, ↓ tibial/ulnar CMAP amplitudes, slow tibial/ulnar motor conduction velocities, absent tibial F wave, ↓ ulnar SNAP amplitude with sural sparing, fibrillation potentials on EMG, overall suggestive of demyelination. | IVIG (0.4 g/kg/day for 5 days) & plasma exchange |
Poor, prolonged ICU stay (> 1 month), bacterial pneumonia during IVIG therapy delaying plasma exchange |
(Toscano, Palmerini, 2020) |
| Monza, Italy | 71, M | Three-day rapidly progressive distal paresthesia, lumbar pain, tetraparesis & areflexia | Low-grade fever (7 days prior), severe hypoxia on admission | Positive COVID-19 nasopharyngeal swab. | Admission: ↑ protein (54 mg/dL), 9 WBC, negative COVID-19 rRT-PCR. | Head CT scan: normal. EMG/NCS (on admission): absent bilateral sural SNAPs & tibial CMAP, prolonged peroneal motor distal latency, slow conduction velocity, & ↓ amplitude with temporal dispersion/conduction block, ulnar/radial CMAP temporal dispersion, slow radial CMAP conduction velocity, ↓ ulnar SNAP amplitude, normal radial SNAP, overall suggestive of demyelination. |
IVIG (0.4 g/kg/day; only received one dose), lopinavir, ritonavir, hydroxychloroquine | Death within 24 h due to progressive respiratory failure | (Alberti, Beretta, 2020) |
| Trento, Italy | 66, F | Three-day rapidly progressive paraplegia, upper limb distal & unilateral facial weakness, gait instability, & areflexia | Self-resolved mild fever & cough (10 days prior) | ↑ CRP (70.6 mg/L) & D-dimer (506 μg/L); normal CBCdiff, LFT, RFT, CK, PT, INR & LDH; positive COVID-19 nasopharyngeal swab | ↑ Protein (108 mg/dL), 0 WBC | EMG/NCS (day 7): diffuse prolonged left tibial/common peroneal & right median distal motor latencies, reduced distal CMAP amplitudes & slight slow conduction velocities, absent left tibial/common peroneal & right median F-waves, absent right median/ulnar/radial/sural SNAPs, overall suggestive of demyelination. | IVIG (0.4 g/kg/day for 5 days); lopinavir, ritonavir, hydroxychloroquine | Poor, progressive weakness, dysesthesia, intermittent confusion / psychomotor agitation, intubation due to respiratory failure, multiorgan failure, leg DVT & pneumonia | (Ottaviani et al., 2020) |
| Ravenna, Italy | 70, F | One-day progressive limbs weakness, distal limb paresthesia, gait instability, areflexia; evolving to respiratory failure & intubation (day 4) | Fever & cough (24 days prior) | Prior admission: positive COVID-19 nasopharyngeal swab. Admission: mild leukocytosis, normal D-dimer, CK, LFT, RFT, ESR, & CRP. |
Day 4: mild ↑ protein (48 mg/dL), 1 WBC; COVID-19 rRT-PCR not done. | EMG/NCS (day 4): prolonged left median/right ulnar/bilateral tibial distal latencies, absent right median CMAP, slow median/ulnar/tibial motor conduction velocities, absent median/ulnar/tibial F waves, absent left median/left ulnar/bilateral superficial peroneal SNAPs, ↓ right ulnar/sural SNAP amplitudes, neurogenic pattern on EMG, overall suggestive of demyelination | IVIG (0.4 g/kg/day for 5 days) | Poor, ICU stay & intubation due to respiratory failure | (Padroni et al., 2020) |
| Milan, Italy | 60s, M | Three-day progressive tetraparesis, distal paresthesia, areflexia; evolving to bifacial weakness, hypophonia and dysarthria (day 8) | Self-resolved fever, headache, myalgia followed by anosmia & ageusia (20 days prior) | ↑ IL-6, ferritin, LDH & fibrinogen; normal CBCdiff, CRP, CK, LFT & RFT; negative antiganglioside antibodies; negative COVID-19 nasopharyngeal swab, positive SARS-CoV-2 IgG. | Day 3: normal protein & WBC, negative COVID-19 rRT-PCR & other viral / bacterial panels. | Cervical spine MRI: normal EMG/NCS (day 5): prolonged right peroneal/median motor distal latencies, slow left tibial/bilateral peroneal/right ulnar motor conduction velocities, ↓ right median CMAP amplitude, abnormal temporal dispersion of peroneal CMAP, absent F waves, absent median/ulnar SNAPs with sural sparing, overall suggestive of demyelination. |
IVIG (0.4 g/kg/day for 5 days) | Incomplete with slow recovery | (Riva et al., 2020) |
| Zaragoza, Spain | 56, F | Acute hand paresthesia & gait instability; evolving to lumbar pain, progressive proximal paraparesis & areflexia (within 2 days of admission); following by tetraparesis, bifacial & bulbar weakness on IVIG. | Fever, cough & dyspnea (15 days prior) | Positive COVID-19 nasopharyngeal swab. | ↑ Protein (86 mg/dL), 3 WBC, negative COVID-19 rRT-PCR. | Spine MRI: brainstem and cervical meningeal enhancement. EMG/NCS (day 11): prolonged distal latencies and absent F waves, suggestive of demyelination. |
IVIG (0.4 g/kg/day for 5 days) | Initial worsening on IVIG but partial recovery in 7 days | (Sancho-Saldaña, Lambea-Gil, 2020) |
| Ciudad Real, Spain | 43, M | Acute rapidly progressive tetraparesis, distal paresthesia & areflexia; evolving to bifacial paresis & dysphagia (day 2) | Self-resolved diarrhea & cough (10 days prior) | Positive COVID-19 nasopharyngeal swab | Not done | EMG/NCS: prolonged distal motor latencies & slow sensory conduction velocities, prolonged F waves for right L5 and S1 roots, overall suggestive of demyelination | IVIG (0.4 g/kg/day for 5 days), hydroxychloroquine, lopinavir, ritonavir, amoxicillin, corticosteroids | Favorable | (Velayos Galán et al., 2020) |
| Paris, France | 64, M | Four-day rapidly progressive paraparesis, areflexia, distal hypoesthesia | Cough, dyspnea, diarrhea & fever (26 days prior) | Prior admission: positive COVID-19 nasopharyngeal swab. Admission: negative antiganglioside & anti-neuronal antibodies |
Day 6: ↑ protein (165 mg/dL), normal WBC, negative COVID-19 rRT-PCR | Head CT scan: normal. EMG/NCS (day 2): prolonged bilateral median & ulnar/left peroneal motor distal latencies, slow median/ulnar/peroneal/tibial motor conduction velocities and normal CMAP amplitudes, conduction blocks in bilateral peroneal/tibial CMAPs, absent SNAPS except for radial/ left median at palm, overall suggestive of demyelination. |
IVIG (0.4 g/kg/day for 5 days) | Favorable | (Arnaud et al., 2020) |
| La Tronche, France | 43, M | Four-day progressive ascending paraparesis, areflexia, limbs paresthesia & ataxia; evolving to right facial weakness (admission day) |
Self-resolved cough, asthenia, leg myalgia, acute anosmia, ageusia & diarrhea (21 days prior) | Normal CBCdiff & CRP; negative antiganglioside antibodies; positive COVID-19 nasopharyngeal swab | Admission: ↑ protein (94 mg/dL), 1 WBC, negative COVID-19 rRT-PCR. | Brain/spine MRI (day 3): multiple cranial neuritis (III, V, VI, VII, & VIII), radiculitis, & brachial/lumbar plexitis. EMG/NCS (day 5): bilateral peroneal conduction blocks, tibial/peroneal slow motor conduction velocities, sural sparing pattern, absent H-reflex, mildly prolonged F-waves, overall suggestive of demyelination |
IVIG (0.4 g/kg/day for 5 days) | Favorable, rapid improvement | (Bigaut, Mallaret, 2020) |
| La Tronche, France | 70, F | Three-day rapidly progressive tetraparesis, areflexia, forelimb / perioral paresthesia; evolving to respiratory failure (admission day) & left facial weakness (day 6) | Self-resolved diarrhea, mild asthenia & myalgia with continuous anosmia and ageusia (10 days prior) | Prior admission: positive COVID-19 nasopharyngeal swab. Admission: ↑ CRP (22 mg/L); negative antiganglioside antibodies |
Admission: ↑ protein (106 mg/dL), 6 WBC, negative COVID-19 rRT-PCR. | EMG/NCS (day 4): left median conduction block & temporal dispersion, prolonged median/ulnar motor distal latencies, diffuse slow, motor/sensory conduction velocities, neurogenic pattern on EMG, overall suggestive of demyelination. | IVIG (0.4 g/kg/day for 5 days) | Slow recovery | (Bigaut, Mallaret, 2020) |
| Lausanne / Geneva, Switzerland | 52, F | Acute lumbar pain, rapidly progressive proximal limb weakness, ataxia, distal paresthesia, dysgeusia & cacosmia; evolving to respiratory failure, dysautonomia & tetraplegia with areflexia (day 4) | Fever, cough, odynophagia, arthralgia & diarrhea (15 days prior) | Admission: normal CBCdiff, LFT & RFT, negative anti-GM1, GQ1b & GD1a antibodies. Day 14: positive serum SARS-CoV-2 IgM, positive COVID-19 nasopharyngeal swab (4th test) |
Day 2: ↑ protein (60 mg/dL), 3 WBC, negative COVID-19 rRT-PCR. |
Spine MRI: normal. EMG/NCS (day 4): prolonged tibial/peroneal/median/ulnar distal motor latencies & slow conduction velocities, absent F waves, no sural sparing, overall suggestive of demyelination Repeat EMG/NCS (days 7 & 14): slower conduction velocities & temporal dispersions. |
IVIG (0.4 g/kg/day for 5 days) | Favorable, initially worsening (day 4) while on IVIG, but recovery within 5 weeks | (Lascano et al., 2020) |
| Lausanne / Geneva, Switzerland | 63, F | Acute lower limb pain & weakness with normal reflexes; evolving to tetraparesis, distal paresthesia & areflexia (day 5) | Cough, shivering, odynophagia, dyspnea & chest pain (7 days prior) | Admission: negative COVID-19 nasopharyngeal swab; Day 7: positive COVID-19 nasopharyngeal swab. Mild lymphocytopenia, mild ↑ AST (65 U/L), normal RFT. |
Day 6: normal protein (40 mg/dL), 2 WBC; COVID-19 rRT-PCR not done. |
EMG/NCS (day 9): conduction block in tibial/peroneal/ulnar CMAPs, absent F waves, normal insertional/spontaneous activity on EMG, overall suggestive of demyelination. | IVIG (0.4 g/kg/day for 5 days), 5-day amoxicillin & clarithromycin (pneumonia) | Favorable, complete motor recovery residual distal paresthesia & areflexia (5 weeks) | (Lascano, Epiney, 2020) |
| Lausanne / Geneva, Switzerland | 61, F | Four-day rapidly progressive lower limb weakness, distal paresthesia, dizziness, dysphagia, bifacial weakness & areflexia; evolving to dysautonomia (one day prior admission) | Fever, cough, myalgia, headache, vasovagal syncope, diarrhea, nausea & vomiting (22 days prior) | Prior to admission: positive COVID-19 nasopharyngeal swab. Admission: lymphocytopenia, hyponatremia, normal LFT & RFT. |
Day 1: ↑ protein (140 mg/dL), 4 WBC, negative COVID-19 rRT-PCR. | Brain MRI: normal. Spine MRI: lumbosacral nerve root enhancement. EMG/NCS (day 4): prolonged peroneal/median motor distal latencies, slow tibial/peroneal/median/ulnar conduction velocities, ↓ tibial/peroneal/median CMAP amplitudes, absent F waves, overall suggestive of demyelination. |
IVIG (0.4 g/kg/day for 5 days), duloxetine | Favorable, residual allodynia & mild lower limb weakness after 5 weeks | (Lascano, Epiney, 2020) |
| Geneva, Switzerland | 70s, M | Four-day rapidly progressive paraparesis, distal allodynia & areflexia; evolving to voiding problem & constipation | myalgia, fatigue & cough (6 days prior) | Prior admission: positive COVID-19 nasopharyngeal swab. | Day 1: ↑ protein, normal WBC, negative COVID-19 rRT-PCR, negative antiganglioside antibodies. | Spine MRI: normal. EMG/NCS (day 1): sensorimotor demyelinating polyneuropathy with sural sparing pattern, absent or prolonged F waves in tested nerves. |
IVIG (0.4 g/kg/day for 5 days) | Favorable within 11 days | (Coen et al., 2020) |
| Selters, Germany | 54, F | Ten-day progressive proximal>distal paraparesis, four limbs numbness & paresthesia, gait instability, & areflexia; evolving to paraplegia & dysphagia (day 2). | No symptoms; transient anosmia/ageusia (14 days prior); exposed to a case with PCR-positive COVID-19 | Prior admission: positive COVID-19 nasopharyngeal swab (3 weeks prior), Admission: normal CRP, CBCdiff, TSH, electrolytes & vitamin B12 level; negative repeat COVID-19 nasopharyngeal swab. | ↑ Protein (140 mg/dL), normal WBC; negative serology, Lyme antibody & COVID-19 rRT-PCR | Cervical spine MRI: normal. EMG/NCS (admission day): prolonged distal motor latencies and temporal dispersion of bilateral common peroneal nerve CMAPs, normal bilateral tibial nerve F wave latencies with pathological intermediate latency responses (complex A waves), overall suggestive of demyelination. |
IVIG (0.4 g/kg/day for 5 days) | Favorable, complete recovery, unchanged repeat EMG/NCS (14 days later) | (Scheidl et al., 2020) |
| Pittsburgh, USA | 72, M | One-day rapidly progressive ascending weakness, areflexia, distal paresthesia; evolving to respiratory failure and intubation (day 3), dysautonomia with labile blood pressure & tachycardia (day 4) with tetraplegia (day 6) | Self-resolved diarrhea, anorexia & chills (7 days prior) | Admission: leukocytosis, normal LFT, RFT, CK, & Lyme antibody; negative anti-GM1, GD1b, GQ1b and acetylcholine receptor binding, voltage-gated Ca2+ channel, ANA, & ANCA antibodies; positive COVID-19 nasopharyngeal swab. Day 8: SIADH with hyponatremia Day 28: negative COVID-19 nasopharyngeal swab. |
Day 8: ↑ protein (313 mg/dL), 1 WBC, negative COVID-19 rRT-PCR & other viral / bacterial panels. | Head CT scan: normal. EMG/NCS (day 13): prolonged right ulnar & bilateral tibial/peroneal motor distal latencies with slow conduction velocities, absent F waves, ↓ right ulnar/peroneal CMAP amplitudes, absent right ulnar/bilateral sural SNAPs, normal EMG with poor effort, overall suggestive of demyelination. |
IVIG (0.4 g/kg/day for 4 days) | Poor, prolonged ICU (> 1 month) stay | (Su et al., 2020) |
| Bursa, Turkey | 53, F | 3-day history of dysarthria associated with progressive weakness and numbness of the lower extremities 3-day history of dysarthria associated with progressive weakness and numbness of the lower extremities Three-day dysarthria & progressive lower limbs weakness & numbness, & areflexia |
No symptoms prior, mild fever (day 5 after neurological symptoms) | Admission: mild neutropenia, normal electrolytes, LFT, RFT & CRP. Day 5: mild lymphocytopenia, ↑ CRP, positive COVID-19 nasopharyngeal swab. |
Day 7: normal protein (32.6 mg/dL), 0 WBC, negative COVID-19 rRT-PCR. | Thoracic/lumbar spine MRI: asymmetrical thickening and hyperintensity of post-ganglionic roots supplying the brachial and lumbar plexuses in STIR sequences. EMG/NCS: conduction blocks and temporal dispersion in right median/ulnar/peroneal CMAPs, normal right median/ulnar/peroneal F waves with decreased persistence, normal right sural/median/ulnar SNAPs, overall suggestive of demyelination. |
Plasma exchange (5 sessions every other day), hydroxychloroquine | Favorable, recovery within 2 weeks | (Oguz-Akarsu et al., 2020) |
| Acute Motor and Sensory Axonal Neuropathy (AMSAN) | |||||||||
| Northern Italy | 77, F | Same-day rapidly progressive tetraplegia, facial weakness, areflexia, upper limb paresthesia (36 h later), & respiratory failure (day 6) | Fever, cough & ageusia (7 days prior) | Lymphocytopenia, ↑ CRP & LDH, ketonuria; negative anti-GM1, GQ1b & GD1b antibodies; positive COVID-19 nasopharyngeal swab. | Day 2: normal. Day 10: ↑ protein (101 mg/dL), 4 WBC, negative COVID-19 rRT-PCR in both days. |
Brain MRI: normal. Spine MRI: caudal nerve roots enhancement. EMG/NCS (day 3): ↓ tibial/ulnar CMAP amplitudes, absent tibial/ulnar F waves, ↓ ulnar SNAP amplitude with sural sparing & fibrillation potentials on EMG, overall suggestive of AMSAN. |
2 cycles of IVIG (0.4 g/kg/day for 5 days) | Poor; persistent tetraplegia & dysphagia | (Toscano, Palmerini, 2020) |
| Northern Italy | 23, M | Two-day progressive bifacial weakness & areflexia, evolving to lower limb paresthesia, ageusia & sensory ataxia | Fever & sore throat (10 days prior) | Lymphocytopenia, ↑ CRP, ferritin, LDH & AST; positive COVID-19 nasopharyngeal swab | Day 3: ↑ protein (123 mg/dL), 0 WBC, negative COVID-19 rRT-PCR. | Brain MRI: focal contrast enhancement at the internal acoustic meatus (bilateral facial nerve). Spinal MRI: normal. EMG/NCS (day 12): ↓ tibial/facial but normal ulnar CMAP amplitudes, prolonged ulnar distal latency, absent tibial F waves, ↓ ulnar SNAP amplitude with sural sparing, & fibrillation potentials on EMG, overall suggestive of AMSAN. |
IVIG (0.4 g/kg/day for 5 days) | Favorable; residual ataxia & facial weakness | (Toscano, Palmerini, 2020) |
| Casablanca, Morocco | 70, F | Ten-day rapidly progressive tetraplegia, distal limbs paresthesia, areflexia | Self-resolved cough (3 days prior) | Lymphocytopenia, positive COVID-19 nasopharyngeal swab. | ↑ protein (100 mg/dL), normal WBC, COVID-19 rRT-PCR not done. | EMG/NCS (day 10): markedly ↓ or absent motor and sensory nerve amplitudes in all four limbs, relatively normal conduction velocities and latencies, fibrillation potentials on EMG, overall suggestive of AMSAN. | IVIG (0.4 g/kg/day for 5 days), hydroxychloroquine, azithromycin | No improvement after one week | (El Otmani et al., 2020) |
| Sari, Iran | 65, M | Five-day rapidly progressive ascending tetraparesis, bifacial weakness, areflexia, & distal limbs numbness | Fever, cough, intermittent dyspnea (14 days prior) | ↑ ESR & CRP, normal LFT, RFT & electrolytes, positive COVID-19 nasopharyngeal swab | Not done | Cervical spine MRI: only mild herniation of 2 intervertebral discs. EMG/NCS (day 9): ↓ bilateral median/ulnar/tibial CMAP amplitudes, absent bilateral peroneal CMAP, absent tibial F waves, absent bilateral median/ulnar/right sural SNAPs, ↓ recruitment on EMG, overall suggestive of AMSAN. |
IVIG (0.4 g/kg/day for 5 days) | NR | (Sedaghat and Karimi, 2020) |
| Acute Motor Axonal Neuropathy (AMAN) | |||||||||
| Northern Italy | 55, M | Two-day rapidly progressive tetraparesis, limb paresthesia, neck pain, & areflexia; evolving to bifacial weakness & respiratory failure (day 5) | Fever & cough (12 days prior) | Lymphocytopenia, ↑ CRP, LDH, AST & GGT, ketonuria; negative anti-GM1, GQ1b & GD1b antibodies; positive serum SARS-CoV-2 IgG (32.5 U/mL), positive COVID-19 nasopharyngeal swab. | Day 3: ↑ protein (193 mg/dL), 0 WBC, negative COVID-19 rRT-PCR. | Brain MRI: normal. Spinal MRI: contrast enhancement of caudal nerve roots. EMG/NCS (day 11): ↓ tibial/ulnar CMAP amplitudes, absent tibial/ulnar F waves, normal ulnar/sural SNAPs, & fibrillation potentials on EMG, overall suggestive of AMAN. |
2 cycles of IVIG (0.4 g/kg/day for 5 days) | Poor; prolonged (> 1 month) ICU stay due to neuromuscular respiratory failure & tetraplegia |
(Toscano, Palmerini, 2020) |
| Unspecified GBS variant (No EMG/NCS available) | |||||||||
| Madrid, Spain | 61, M | Same-dame right facial weakness; evolving to bifacial weakness next day | Fever & cough (10 days prior) | Prior admission: positive COVID-19 nasopharyngeal swab. | Mild ↑ protein (44 mg/dL), 0 WBC, negative COVID-19 rRT-PCR. | Head CT scan & Brain MRI: normal. | Low dose oral prednisone, hydroxychloroquine, lopinavir, ritonavir | Favorable, recovery after 2 weeks | (Juliao Caamaño and Alonso, 2020) |
| Pamplona, Spain | 76, F | Ten-day radicular lumbar pain, progressive tetraparesis, distal hypoesthesia, areflexia; evolving to bulbar weakness & respiratory failure within 4–12 h of admission | Fever & cough (8 days prior) | Prior admission: positive COVID-19 nasopharyngeal swab. Admission: mild thrombocytopenia, ↑ fibrinogen & D-dimer |
Not done | Head/cervical spine CT scan: only vertebral bodies degenerative signs. | Supportive | Death within 12 h of admission due to respiratory failure | (Marta-Enguita, Rubio-Baines, 2020) |
| Pittsburgh, USA | 54, M | Two-day rapidly progressive ascending paraparesis & numbness, lower limbs areflexia, upper limbs hyporeflexia, later urinary retention | Fever & cough (7 days prior), Clostridium difficile colitis diarrhea (2 days prior), dyspnea & intubation | Normal CBCdiff & electrolytes, positive COVID-19 nasopharyngeal swab | Not done | Thoracic/lumbar MRI: normal. | IVIG (0.4 g/kg/day for 5 days), hydroxychloroquine | Favorable, residual lower limb weakness | (Virani et al., 2020) |
| Skeletal Muscle Injury | |||||||||
| Wuhan, China | 60, M | Admission day 9: proximal lower limb weakness, myalgia & tenderness | Fever & cough (6 days prior), continued fever till admission day 6 | Admission: leukopenia, ↑ CRP (111 mg/L), ↑ LDH (280 U/L), normal CK, LFT, RFT, positive COVID-19 nasopharyngeal swab. Day 7: ↑ CRP (206 mg/L). Day 9: ↑↑ myoglobin (>12,000 μg/L), CK (11,842 U/L), LDH (2347 U/L), ALT (111 U/L) & AST (213 U/L), normal RFT & electrolytes. Day 12: negative COVID-19 nasopharyngeal swab. |
NR | NR | Admission: opinavir, moxifloxacin & interferon nebulization; Day 6: meropenem & methylprednisolone Day 9: IV fluid, plasma transfusion, IVIG & supportive care |
Favorable; recovery within few days | (Jin and Tong, 2020) |
| New York, USA | 88, M | Acute progressive proximal lower limb weakness & myalgia | Low-grade fever & tachypnea (on admission) | ↑↑ CK (13,581 U/L), ↑ LDH (364 U/L), positive COVID-19 nasopharyngeal swab; AKI (day 7) | NR | NR | IV fluid, hydroxychloroquine | Favorable; ↓ CK within 6 days (368 U/L) | (Suwanwongse and Shabarek, 2020) |
| New York, USA | 75, F | Four-day generalized weakness; evolving to lethargy, acute encephalopathy, & respiratory distress requiring ICU admission (day 3) | Concurrent ↓ appetite | Admission: ↑ CK (2767 U/L), ↑ troponin (0.663 ng/mL), normal EKG, hypernatremia (152 mM/L), AST (198 U/L), ALT (63 U/L), BUN (31 mg/dL) & creatinine (1.2 mg/dL); normal CBCdiff. Day 2: positive COVID-19 nasopharyngeal swab, ↑ LDH (497 U/L), CRP (37 mg/L), D-dimer (573 μg/L) & ferritin (2134 μg/L) |
NR | NR | Day 2: azithromycin, hydroxychloroquine, vancomycin & cefepime. Day 3: IV fluid, supportive care in ICU |
Favorable | (Chan et al., 2020) |
| New York, USA | 71, M | On admission: intermittent leg twitching with tingling/numbness at the lateral upper thigh radiating down to the posterior mid-calf. | No prior symptoms, fever on admission; Day 2: spike fever and AKI Day 3: tachypnea, tachycardia, AF with RVR, AKI & ARDS requiring intubation |
Admission: ↑ CK (1859 U/L), BUN (78 mg/dL), creatinine (3.6 mg/dL), troponin (0.249 ng/mL), LDH (538 U/L), CRP (18.8 mg/L), D-dimer (989 μg/L) & ferritin (1003 μg/L); normal CBCdiff; EKG: new AF, positive COVID-19 nasopharyngeal swab. Day 2: ↑↑ creatinine (5.6 mg/dL) |
NR | Head CT scan: old right lacunar infarct. | Admission: doxycycline, ceftriaxone, hydroxychloroquine, IV fluid; heparin and metoprolol for new AF. Day 3: hemodialysis for AKI |
Poor, prolonged ICU stay & intubated. | (Chan, Farouji, 2020) |
| New York, USA | 16, M | Four-day generalized myalgias, fatigue, & 2-day dark-colored urine; evolving to continued myalgia (day 4) | Fever, concurrent dyspnea on exertion (4 days), pharyngeal erythema & abdominal pain (on exam) | Mild leukocytosis & thrombocytopenia; ↑ AST (839 U/L) & ALT (157 U/L); normal creatinine, GGT, & electrolytes; positive COVID-19 nasopharyngeal swab, ↑↑↑ CK (427,656 U/L). Repeat CK (296,396 U/L), hyponatremia (130 mM/L), ↓ albumin (3.2 g/dL), normal creatinine & ferritin, ↑ procalcitonin (0.22 μg/L), LDH (2184 U/L), CRP (24.9 mg/L), troponin (0.58 ng/mL) & HgA1C (8.2%). |
NR | NR | IV fluid with sodium bicarbonate & KCl |
Favorable; recovery with ↓ CK (6526 U/L) and no myalgia at discharge (day 12) | (Gefen, Palumbo, 2020) |
AIDP, acute inflammatory demyelinating polyneuropathy; AKI, acute kidney injury, ALT, alanine aminotransferase; AMAN, acute motor axonal neuropathy; AMSAN, acute motor and sensory axonal neuropathy; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; CBCdif, complete blood counts with differential; CK, creatine kinase; CMAP, compound motor action potential; CRP, C-reactive protein; COVID-19, coronavirus disease 2019; CSF, cerebrospinal fluid; EMG/NCS, electromyography/nerve conduction study; ESR, erythrocyte sedimentation rate; F, female; IL, interleukin; INO, internuclear ophthalmoparesis; INR, international normalized ratio; IVIG, intravenous immunoglobulin; LDH, lactate dehydrogenase; LFT, liver function test; M, male; NR, not reported; PT, prothrombin time; RFT, renal function test; rRT-PCR, real-time reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SIADH, syndrome of inappropriate antidiuretic hormone secretion; SNAP, sensory nerve action potential; WBC, white blood cell.
Table 3.
Case reports of seizure, encephalitis, meningoencephalitis, and CNS demyelination related to COVID-19.
| Region | Age, Gender | Neurological symptoms on admission (day from admission) | Other symptoms (onset day prior neurologic symptoms) | Admission serum labs (or day from admission) | CSF (day from admission) | Imaging or EEG (day from admission) | Treatments received | Outcome | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Seizure | |||||||||
| Madrid, Spain | 26 days, M | Two paroxysmal episodes: 1st, several-minute upward eyes rolling & generalized hypertonia associated with a feeding; 2nd, several-minute generalized hypertonia & facial cyanosis during sleep. No abnormal movements. | 12-h fever, rhinorrhea, & vomiting | Mild ↑ CK (380 U/L), LDH (390 U/L) & fibrinogen (4.18 mg/dL); normal CBCdiff, BMP, LFTs, CRP, & BCx/UCx; positive COVID-19 nasopharyngeal swab | Normal | Cranial ultrasound: normal. cEEG: continuous background patters with sleep-wake cycles without electrical and clinical seizures. |
Six-day hospitalization with supportive care | Favorable | (Chacón-Aguilar et al., 2020) |
| New York, USA | 6 weeks, M | Two brief 10–15 s episodes of upward gaze & bilateral leg stiffening. No abnormal movements. | Fever & cough | Leukopenia (5.07 K/μL), ↑ procalcitonin (0.21 ng/mL), normal BMP; positive for rhinovirus / enterovirus PCR; positive COVID-19 nasopharyngeal swab | Normal | Brain MRI: normal. cEEG: Excess of temporal sharp transients for age & intermittent vertex delta slowing with normal sleep-wake cycling. |
One-day hospitalization | Favorable | (Dugue et al., 2020) |
| Brooklyn, USA | 72, M | Altered mental status requiring intubation (admission); followed by multiple episodes of generalized tonic colonic movements (day 3) | Dyspnea | Lymphocytopenia (0.5 K), leukopenia (4 K), ↑ CRP (61 mg/L) & LDH (230 U/L), negative nasopharyngeal swab for influenza A and B, positive COVID-19 nasopharyngeal swab | NR | Head CT scan: chronic microvascular ischemic changes with no acute changes. Brain MRI: not done due to patient unstable condition. cEEG: Six left temporal seizures & left temporal sharp waves which were epileptogenic. |
Transient oseltamivir; hydroxychloroquine, azithromycin, vancomycin, piperacillin tazobactam, versed & levetiracetam with additional valproate | Death due to cardiac arrest (day 5) | (Sohal and Mossammat, 2020) |
| Encephalitis / Meningoencephalitis | |||||||||
| Yamanashi, Japan | 24, M | Multiple generalized seizures, unconsciousness & neck stiffness | Fever & generalized fatigue (9 days prior); headache & sore throat (5 days prior) | ↑ WBC (mainly neutrophils) & CRP; negative HSV1/VZV IgM; negative COVID-19 nasopharyngeal swab | Day 3: ↑ pressure (320 mmH2O), 12 WBC (10 mononuclear), positive COVID-19 rRT-PCR | Head CT scan: normal. Brain DWI MRI: hyperintensity along the wall of inferior horn of right lateral ventricle; FLAIR MRI: hyperintense signal changes in the right mesial temporal lobe & hippocampus with slight hippocampal atrophy with no contrast enhancement, suggestive of right lateral ventriculitis & encephalitis mainly on right mesial lobe & hippocampus; T2-weighted image: pan-paranasal sinusitis. |
Transient ceftriaxone, vancomycin, acyclovir & steroids; levetiracetam; favipiravir | Poor; ongoing (>15 days) ICU stay due to encephalitis & bacterial pneumonia | (Moriguchi, Harii, 2020) |
| Wuhan, China | NR, M | Acute confusion, nuchal rigidity, Kernig, Brudzinski & Babinski signs. | Fever, dyspnea & myalgia (12 days prior) | ↓ WBC (lymphocytopenia), positive COVID-19 nasopharyngeal swab. | Mild ↑ pressure (220 mmHg), normal protein (80 mg/dL), 1 WBC, negative COVID-19 rRT-PCR, IgM & IgG | Head CT scan: normal. | Supportive care, mannitol infusion | Favorable; complete recovery within 14 days | (Ye, Ren, 2020a) |
| Nanjing, China | 64, M | Acute lethargy & unresponsiveness, positive ankle clonus (left>right), left Babinski & Chaddock signs, & mild neck stiffness | Fever, cough (13 days prior), insomnia & myalgia (11 days prior) | ↑ CRP (10.74 mg/L), normal WBC, positive COVID-19 nasopharyngeal swab. | Day 6: pressure (200 mmH2O), normal protein (27.5 mg/dL), 1 WBC, negative COVID-19 rRT-PCR | Head CT scan: normal. | NR | Favorable; recovery within 14 days | (Yin, Feng, 2020) |
| Brescia, Italy | 60, M | Five-day progressive irritability, confusion, asthenia, cognitive fluctuations; evolving to severe akinetic mutism, positive palmomental & glabella reflexes, & nuchal rigidity | Fever & cough (3 days after onset of neurologic symptoms) | ↑ D-dimer (968 μg/L); normal CBCdiff, CRP, fibrinogen & ferritin; negative autoimmune encephalitis antibody panel; positive COVID-19 nasopharyngeal swab | Admission: ↑ protein (69.6 mg/dL), 18 WBC (100% lymphocytes), negative culture/viral/COVID-19 rRT-PCR. Repeat one day after steroid: ↑protein (127.2 mg/dL), 18 WBC (100% lymphocytes); ↑ IL-6, IL-8, TNF-α & β2-microglobulin; normal tau & neurofilament light; negative culture / viral / COVID-19 rRT-PCR |
Head CT scan: normal. Brain MRI: normal. EEG: generalized slowing with decreased reactivity to acoustic stimuli. |
Lopinavir, ritonavir, hydroxychloroquine, ampicillin & acyclovir, 5-day methylprednisolone (1 g/day), post-discharge oral prednisone with rapid taper | Favorable; residual mild disinhibition & fluctuating alertness; normal exam on day 11 | (Pilotto, Odolini, 2020) |
| Varese, Italy | 22, F | Admission: acute loss of consciousness, hypocapnia & hypoxia, requiring intubation. Day 15: acute flaccid paraparesis, lower hyperreflexia, urine/bowel incontinence, & lower limbs hypoesthesia |
Dyspnea & fever (concurrent) | Leukocytosis (28.7 K), ↑ CRP (136.1 mg/L), D-dimer (>9000 μg/L), glucose (744 mg/dL), LDH (729 U/L) & AST (144 U/L); normal RFT; positive COVID-19 nasopharyngeal swab | Day 18: mild ↑ protein (53 mg/dL), normal WBC, negative COVID-19 rRT-PCR. | Head CT / CT angiogram (admission): a tiny right frontal parenchymal hemorrhage; no vascular malformations. Brain & Spine MRI: Only a late subacute phase tiny frontal hemorrhage (8 mm of maximum diameter) |
Antiviral & immunomodulatory therapies | Favorable; recovery after 15 days | (Giorgianni et al., 2020) |
| Barcelona, Spain | 25, M | One-day headache, left-sided paresthesia, & ipsilateral paresis, evolving to confusion & agitation (12h) | Concurrent fever | Mild ↑ D-dimer (600 μg/L), normal CBCdiff | ↑ protein (105.5 mg/dL), IL-1β (14.8 pg/mL), IL-6 (190 pg/mL) & ACE (15.5 U/L); normal WBC, negative COVID-19 rRT-PCR. | Head CT scan / brain MRI: normal. | Acyclovir, ampicillin, & ceftriaxone | Favorable, recovery within 1 day | (Bodro et al., 2020) |
| Barcelona, Spain | 49, M | 7-day myalgia with acute difficulty naming objects, temporospatial disorientation, confusion, & agitation | Persistent fever, myalgias & cough (7 days prios) | Mild lymphocytopenia, mild ↑ D-dimer (600 μg/L), LDH (254 U/L) & ferritin (428 μg/L) | ↑ protein (115.5 mg/dL), IL-6 (25 pg/mL) & ACE (10.9 U/L); normal WBC, negative COVID-19 rRT-PCR. | Head CT scan / brain MRI (day 2): normal. | Acyclovir, ampicillin, & ceftriaxone | Favorable, recovering within 3 days | (Bodro, Compta, 2020) |
| Lausanne, Switzerland | 64, F | Acute psychosis, tonic-clonic seizure followed by disorientation, attention deficit, verbal and motor perseverations, bilateral grasping, alternating with psychotic symptoms (hyper-religiosity with mystic delusions, visual hallucinations). | Mild fatigue, myalgia, cough (5 days prior) | Positive COVID-19 nasopharyngeal swab | ↑ Protein (466 mg/dL), 17 WBC, negative culture, viral PCR & COVID-19 rRT-PCR & anti-NMDA antibody |
Brain MRI: normal. Admission EEG: nonconvulsive, focal status epilepticus (abundant bursts of anterior low-medium voltage irregular spike-and waves superimposed on an irregularly slowed theta background) Repeat EEG at 56 h: normal. |
IV clonazepam & valproate | Favorable; recovery within 96 h | (Bernard-Valnet, Pizzarotti, 2020) |
| Lausanne, Switzerland | 67, F | Intense wake-up headache followed by drowsiness and confusion, motor perseverations, bilateral grasping, aggressiveness and left hemianopia and sensory hemineglect | Mild respiratory symptoms with confirmed COVID-19 (17 days prior) | Positive COVID-19 nasopharyngeal swab | ↑ Protein (461 mg/dL), 21 WBC, negative culture, viral PCR & COVID-19 rRT-PCR | Brain MRI: normal. | Transient ceftriaxone, amoxicillin & acyclovir |
Favorable; recovery within 24 h | (Bernard-Valnet, Pizzarotti, 2020) |
| Istanbul, Turkey | 6 cases (22–59 age range), one F | Encephalopathy, failure in gaining consciousness, developing agitation & delirium upon extubation | ARDS requiring intubation | Leukocytosis (11–42 K); ↑ CRP (32–732 mg/L), D-dimer (730–7930 μg/L), LDH (271–1110 U/L), ferritin (555–5235 μg/L), ↑ IL-6 (3 patients checked, 481–9192 pg/mL) | ↑ Protein (5 cases, 57–131 mg/dL), 0 WBC, normal glucose, ↑ IgG (5 cases checked), negative oligoclonal bands & culture / viral PCR / COVID-19 rRT-PCR. | Brain MRI (3 cases): cortical/white matter hyperintensities, contrast enhancement, and sulcal hemorrhagic features, compatible with meningoencephalitis. Brain MRI (3 cases): normal |
Plasmapheresis | Early gain of consciousness in 4 patients; one death due to COVID-19 worsening & cardiac arrest; prolonged ICU stay in one case due to CMV infection | (Dogan, Kaya, 2020) |
| Boca Raton, USA | 74, M (originally from Netherland) | Headache & progressive altered mental status | Fever & cough (one day prior) | Negative blood culture, urinalysis & Influenza A and B tests; positive COVID-19 nasopharyngeal swab | Mild ↑ protein (68 mg/dL), 4 WBC; negative culture, viral panel & COVID-19 rRT-PCR negative | Head CT scan: a left temporal region encephalomalacia, related to prior history of embolic stroke. EEG: bilateral slowing & focal slowing in the left temporal region with sharply countered waves. |
Hydroxychloroquine, lopinavir, ritonavir, vancomycin, meropenem & acyclovir. | Poor; critically ill | (Filatov, Sharma, 2020) |
| Telford, UK | 40, M (originally from Nigeria) | On admission day 3: gait instability; evolving to diplopia, oscillopsia, limb ataxia, right arm numbness, hiccups, bifacial/tongue weakness, upbeat nystagmus & normal reflexes (day 4) | Fever, dyspnea & malaise (10 days prior) | Admission: marginally ↑ CRP (50 mg/L), ALT (88 U/L) & GGT (107 U/L); normal CBCdiff; positive COVID-19 nasopharyngeal swab. Later: ↑ LFTs; Liver ultrasound: an inflammatory diffusely hypoechoic liver with a raised periportal & pericholecystic echogenicity. |
Normal protein (42.3 mg/dL), 0 WBC; COVID-19 rRT-PCR not done (low volume tap) | Brain/cervical spine MRI: hyperintensity in the right inferior cerebellar peduncle, extending to involve a small portion of the upper cord, measuring 13 mm in maximum cross-sectional area and 28 mm in longitudinal extent, swelling at the affected tissue and associated microhemorrhage; suggestive of inflammatory rhombencephalitis & myelitis. |
Supportive care, gabapentin (300 mg twice daily) | Favorable; recovery with residual oscillopsia & ataxia | (Wong, Craik, 2020a) |
| New York, USA | 74, M | Acute confusion following falls; evolving to combative behavior in hospital. | Admission: fever Day 5: new atrial fibrillation |
Admission: thrombocytopenia (122K), positive COVID-19 nasopharyngeal swab. Day 3: ↑ D-dimer (740 μg/L) Day 7: ↑ CRP (183.5 mg/L), ferritin (2837 μg/L) Day 10: ↑ D-dimer (5850 μg/L) |
NR | Head CT scan: unchanged nonspecific patchy subcortical & periventricular hypodensities. Brain autopsy: 80 to 110 nm viral particles in frontal lobe brain sections with Pleomorphic spherical viral-like particles observed in small vesicles of endothelial cell, distended cytoplasmic vacuoles containing enveloped viral particle in neural cell bodies, positive COVID-19 rRT-PCR. |
Admission: Hydroxychloroquine (4 days), enoxaparin S.C. Day 5: tocilizumab & metoprolol |
Death at day 1 due to pneumonia and respiratory failure related to COVID-19 | (Paniz-Mondolfi et al., 2020) |
| Los Angeles, USA | 41, F | Same-day headache, new onset seizure, lethargy, neck stiffness & photophobia | Admission: fever | ↑ lactic acid (4.8 mM/L); normal WBC, CK, LFT & RFT; negative nasopharyngeal swab for influenza A and B viruses; positive COVID-19 nasopharyngeal swab. | ↑ Protein (100 mg/dL), 70 WBC (100% lymphocytes), negative culture & viral PCR, positive COVID-19 rRT-PCR, | Head CT scan: normal. EEG: generalized slowing with no epileptic discharges. |
Transient ceftriaxone, vancomycin & acyclovir; levetiracetam; hydroxychloroquine | Favorable; recovery within 12 days | (Duong et al., 2020, Huang, Jiang, 2020b) |
| Acute necrotizing hemorrhagic encephalopathy | |||||||||
| Detroit, USA | Late 50, F | Three-day altered mental status | Fever & cough (concurrent) | Positive COVID-19 nasopharyngeal swab | Limited data due to traumatic LP, negative culture & viral PCR; unable to test COVID-19 rRT-PCR. | Head CT scan: symmetric hypoattenuation within the bilateral medial thalami. Head CT angiogram / venogram: normal. Brain MRI: hemorrhagic rim enhancing lesions within the bilateral thalami, medial temporal lobes, and subinsular regions. |
IVIG | NR | (Poyiadji, Shahin, 2020) |
| Acute CNS demyelination: Brain and/or Spinal Cord | |||||||||
| Wuhan, China | 66, M | Same-day rapidly progressive paraparesis, hyporeflexia, bladder & bowel incontinence, sensory loss below T10 level | Fever & fatigue (6 days prior) | Prior admission: positive COVID-19 nasopharyngeal swab. Admission: Leukocytosis (11.8 K), lymphocytopenia; ↑ IL-6 (56.7 pg/mL), CRP (278 mg/L), amyloid (1844 mg/L), procalcitonin (4.33 ng/mL), AST (50 U/L) & ALT (56.4 U/L); normal CK, LDH, troponin & RFT |
Not done | Head CT scan: bilateral basal ganglia and paraventricular lacunar infarction & brain atrophy. Brain MRI: not done. |
Ganciclovir, lopinavir, ritonavir, moxifloxacin, meropenem, glutathione, dexamethasone, IVIG (15 g/day for 7 days), mecobalamin & pantoprazole | Favorable; partial recovery after 7 days | (Zhao et al., 2020b) |
| Denmark | 28, F | Severn-day persistent lumbosacral pain, progressive ascending paresthesia/sensory loss mid-chest below the nipple line, bilateral upper extremities, and tip of tongue; evolving to urinary retention, nausea & vomiting in 48 h; Lhermitte's sign & wide-based gait | Cough, fever, lumbar pain, myalgias & rhinorrhea (7 days prior) | Prior admission: positive COVID-19 nasopharyngeal swab | ↑ Protein (60 mg/dL) & 125 WBC; | Spine MRI: Widespread elongated signal changes throughout the spinal cord to the conus medullaris and involving the medulla, overall suggestive of longitudinally extensive acute transverse myelitis | Prednisone & 2 plasmapheresis sessions | Favorable; improvement within 8 days | (Sarma and Bilello, 2020) |
| Marseille, France | 54, F | Admission: altered mental status Day 10: slight improvement in mental status, but right hemiplegia |
Fever, asthenia, dyspnea (8 days prior) | Admission: ↑ CRP (346 mg/L), LFTs & ferritin, positive COVID-19 nasopharyngeal swab | Admission: traumatic LP but normal protein and WBCs, negative culture/viral/ COVID-19 rRT-PCR. Repeat (day 9): Unchanged. |
Head CT scan (day 2): hypodense lesions involving supratentorial white matter & pallidum bilaterally. EEG: slowed background activity. Brain MRI (day 7): Multiple supratentorial punctiform and tumefactive lesions involving the white matter bilaterally and showing hypersignal on coronal FLAIR, axial T2-weighted, & DWI with low ADC. Some periventricular lesions or involving the corpus callosum with a mass effect on the left lateral ventricle. No intracranial vessels abnormalities. Repeat MRI (day 10): unchanged, but all homogenous contrast enhancement without any sign of hemorrhage in all lesions. Spine MRI: normal. |
Hydroxychloroquine, azithromycin, amoxicillin/clavulanic acid; Day 12: steroid therapy after negative COVID-19 nasopharyngeal swab | NR | (Brun, Hak, 2020) |
| Brescia, Italy | 54, F | Acute unconsciousness at home; evolving to hypoxia & intubation | No symptoms; anosmia & ageusia (several days prior) | Lymphocytopenia, ↑ CRP (41.3 mg/L) & fibrinogen (520 mg/dL); positive COVID-19 nasopharyngeal swab | Normal, negative COVID-19 rRT-PCR | Head CT scan: normal. EEG: two seizures starting from right frontotemporal region & diffusing in homologous contralateral hemisphere. Brain MRI: numerous periventricular white matter alterations, confluent with each other & compatible with demyelinating lesions, adjacent to the temporal, frontal & occipital horns & to the trigones, hyperintense in T2, without restriction of diffusion & without contrast enhancement. Cervical/thoracic spine MRI: numerous focal hyperintense intramedullary signal alterations in T2 & without contrast enhancement, located at the bulb-medullary junction, at C2 and from C3 to T6 levels. |
Hydroxychloroquine, lacosamide, levetiracetam, phenytoin, dexamethasone (10-day 20 mg/day & 10-day 10 mg/day) | Favorable, recovery after 12 days | (Zanin, Saraceno, 2020) |
| Genova, Italy | 64, F | Acute bilateral vision impairment, right leg numbness, headache, mild irritability, bilateral RAPD, ageusia & anosmia, right abdominal sensory level, left lower limb hyperreflexia & Babinski sign. | Flu-like symptoms, & persistent ageusia & anosmia (25 days prior) | Negative COVID-19 nasopharyngeal swab, positive anti-SARS-CoV-2 IgG, negative AQ4 & anti-MOG antibody | Mild ↑ protein (45.2 mg/dL), 22 WBC (T-lymphocytic), negative COVID-19 rRT-PCR | Brain/spine MRI: multiple T1 post-contrast enhancing lesions of the brain, associated with a single spinal cord lesion at T8 level & with bilateral optic nerve enhancement, suspicion about ADEM. Follow-up MRI: a partial improvement with a reduction in the number of contrast-enhancing lesions. |
Methylprednisolone (1 g/day for 5 days) oral prednisone (75 mg/day), IVIG (2 g/kg for 5 days) | Favorable; improvement after 14 days of treatment | (Novi, Rossi, 2020) |
| Esslingen, Germany | 60, M | Two-day urinating problem, progressive spastic paraparesis, hypesthesia below T9 level & Babinski's sign | Respiratory symptoms (8 days prior) | Prior admission: positive COVID-19 nasopharyngeal swab Admission: normal CRP, negative COVID-19 nasopharyngeal swab |
Admission: ↑ protein (79.3 mg/dL), 16 WBC (lymphocytic), no oligoclonal band. Repeat: ↑ protein (117.7 mg/dL), 27 WBC (lymphocytic); negative COVID-19 rRT-PCR both times, negative SARS-CoV-2 IgG. Day 12: ↑ protein (73.4 mg/dL), 3 WBC |
Brain MRI: normal. Spine MRI: T2 signal hyperintensity of the thoracic spinal cord at T9 level, suggestive of acute transverse myelitis. Spine MRI (day 6): patchy hyperintensity of the thoracic myelin at T9-T10 & T3-T5 level, suggestive of transverse myelitis. |
Transient acyclovir & ceftriaxone; Day 7: methylprednisolone (100 mg/day) with taper after discharge | Favorable; recovery within 13 days with slight spastic paraparesis & hypesthesia below T9 level | (Munz, Wessendorf, 2020) |
| Ann Arbor, USA | 61, F | Two-day progressive distal limbs paresthesia & paraparesis; evolving to sensory loss till abdomen level, constipation & lower hyporeflexia on admission | Rhinorrhea & chills (5 days prior) | Lymphocytopenia, positive COVID-19 nasopharyngeal swab | Day 11: ↑ Protein (87 mg/dL), 3 WBC, no oligoclonal band, negative encephalitis antibody panel, negative COVID-19 rRT-PCR. Day 20 (repeat): ↑ Protein (153 mg/dL), 1 WBC, negative COVID-19 rRT-PCR. |
Spine MRI: Extensive ill-defined patchy hyperintense signal throughout the central aspect of the spinal cord on STIR, T2-weighted axial indicating mild enlargement of the spinal cord caliber & hyperintense signal without contrast enhancement. | Methylprednisolone (1 g/day for 5 days) & plasmapheresis (5 sessions) | Poor, incomplete recovery | (Valiuddin, Skwirsk, 2020) |
| Dubai, UAE | 32, M | One-day progressive paraparesis, lower hyporeflexia & urinating problem | Fever & flu-like symptoms (2 days prior); PE (one day after admission) | Normal ESR, WBC & ferritin; ↓ Hgb (10.7 g/dL); ↑ CRP (42 mg/L), D-dimer (2000 μg/L) & procalcitonin (0.13 μg/L); mild ↑ CK (252 U/L), prolonged PT (16.8 s), aPTT (51.3 s) & INR (1.33), positive Lupus anticoagulant antibody; positive COVID-19 nasopharyngeal swab | NR | Spine MRI: Diffuse hyperintensity predominantly in cervical, dorsal, & lumbar gray matter, mild enlargement & swelling of cervical cord, no cord or nerve root enhancement; DWI & ADC showing restricted diffusion, overall suggestive of acute myelitis. | Methylprednisolone (1 g/day for 5 days), acyclovir, & enoxaparin | Favorable; improvement after steroid therapy | (AlKetbi, AlNuaimi, 2020) |
| Acute Necrotizing Myelitis | |||||||||
| Terrassa, Spain | 68, F | Seven-day radicular neck pain, left hand numbness/weakness, & imbalance; evolving to both hand weakness / numbness & paraparesis with sphincter incontinence (few days after steroid therapy) | Fever & cough (8 days prior) | Negative AQ4 & anti-MOG antibody; positive COVID-19 nasopharyngeal swab | ↑ Protein (283 mg/dL), 75 WBC (98% lymphocytes), no oligoclonal bands, negative neuronal surface antibodies, | Brain MRI: normal. Spine MRI: T2-hyperintensity extending from the medulla oblongata to C7, involving most of the cord with diffuse patchy enhancing lesions, suggesting acute transverse myelitis. Repeat MRI (7 days): transversally & caudally progression until T6 level with similar enhancement & a new area of central necrosis at the T1 level with peripheral enhancement. Repeat MRI (after plasmapheresis): significant decreases of both myelitis extension & enhancement, with necrosis area in evolution. |
2 cycles of methylprednisolone (1 g/day for 5 days) with oral prednisone taper, plasma exchange | Favorable; slow recovery | (Sotoca and Rodríguez-Álvarez, 2020) |
ACE, angiotensin-converting enzyme; ADC, apparent diffusion coefficient; ADEM, acute disseminated encephalomyelitis; ALT, alanine aminotransferase; aPTT, activated partial-thromboplastin time; AQ4, antiaquaporin-4; BCx, blood culture; ARDS, acute respiratory distress syndrome; AST, Aspartate transaminase; CBCdiff, complete blood count with differential; CK, creatine kinase; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; DWI, diffusion-weighted imaging; EEG, electroencephalography; ESR, erythrocyte sedimentation rate; F, female; FLAIR, fluid attenuated inversion recovery; HSV, herpes simplex virus; ICU, intensive care unit; IL, interleukin; INR, international normalized ratio; IVIG, intravenous immunoglobulin; LDH, lactate dehydrogenase; LFT, liver function test; LP, lumbar puncture; M, male; RAPD, relative afferent pupillary defect; MOG, myelin oligodendrocyte glycoprotein; NR, not reported; RFT, renal function test; rRT-PCR, real-time reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF-α, tumor necrosis factor-α; UCx, urine culture; VZV, varicella zoster virus.
4.1. Guillain-Barré syndromes
GBSs characteristically manifest with acute (< 4 weeks) ascending muscle weakness accompanied by decreased/absent deep tendon reflexes, mild-moderate sensory loss, occasionally cranial nerve involvement, and radicular or muscle pain. Although GBSs are commonly demyelinating (i.e. acute inflammatory demyelinating polyneuropathy [AIDP]), primary axonal injury may occur, known as acute motor and sensory axonal neuropathy (AMSAN) or acute motor axonal neuropathy (AMAN). Miller-Fisher syndrome (MFS) is another GBS variant which is characterized by the triad of ophthalmoplegia, gait ataxia, and areflexia (Rocha Cabrero and Morrison, 2020). GBS is considered as an autoimmune neurologic disease that can be triggered by a variety of viruses. About 70% of cases may have a viral illness 1–3 weeks prior to neurologic symptoms (Wakerley and Yuki, 2013). GBS outbreak has been observed with viral epidemics including those with coronaviruses (i.e. MERS-CoV and SARS-CoV) (Kim et al., 2017; Wakerley and Yuki, 2013). The first case of SARS-CoV-2 related GBS was reported from the major COVID-19 hotspot, Wuhan, China (Zhao et al., 2020a). The patient was a 61 years old woman who, one week after her trip from Wuhan, developed rapidly progressive ascending limb weakness over 3 days accompanied by areflexia and later distal sensory changes. The CSF study (day 4) revealed albuminocytologic dissociation and electromyography/nerve conduction study (EMG/NCS) on day 5 showed a demyelinating neuropathy at early stage. Laboratory results on admission were notable for lymphocytopenia and thrombocytopenia. She was treated with intravenous immunoglobulin (IVIG). Notably, she developed fever, cough, and pneumonia eight days after the onset of neurological symptoms. The SARS-CoV-2 rRT-PCR was positive in oropharyngeal swabs at that time. The patient had a full recovery after 3 weeks and the repeat test for COVID-19 was negative (Zhao, Shen, 2020a).
Overall 30 cases of GBS variants have been reported worldwide in patients with confirmed COVID-19 since the pandemic (Table 2), with mostly typical manifestation of rapidly progressive flaccid limbs weakness and areflexia, with and without facial muscle weakness, and distal paresthesia or numbness. Cranial nerve involvements have been observed in 5 (17%) cases (Bigaut et al., 2020; Gutiérrez-Ortiz et al., 2020; Reyes-Bueno et al., 2020; Sancho-Saldaña et al., 2020). Either unilateral or bifacial weakness was present in 12 (40%) cases. In 28 patients, COVID-19 symptoms were variably present between 26 and 2 days prior to the onset of GBS symptoms, including cough (70%), fever (57%), gastrointestinal symptoms (e.g. diarrhea, nausea and vomiting, 33%), dyspnea (17%), myalgia (17%), ageusia (20%), anosmia (20%), fatigue/malaise (13%). Ageusia and anosmia were also present on admission in 17% and 10% of these cases, respectively. Three cases (all from Spain) had MFS (Gutiérrez-Ortiz, Méndez, 2020, Reyes-Bueno, García-Trujillo, 2020). Among 26 patients that underwent CSF study, elevated protein levels (44 to 313 mg/dL) with normal leukocyte counts (i.e. albuminocytologic dissociation) were found in 22 (85%) patients. Positive serum anti-GD1b IgG antibody was reported in one patient with MFS (Gutiérrez-Ortiz, Méndez, 2020). However, serum and CSF anti-ganglioside antibodies (including anti-GM1, GQ1b, and GD1b antibodies) checked in 10 and 2 cases, respectively, were negative (Table 2). Variable degrees of leukocytopenia (mainly lymphocytopenia; 40%) and elevated acute phase reactants (e.g. erythrocyte sedimentation rate [ESR], C-reactive protein [CRP], ferritin, or fibrinogen; 40%) were also reported among these 30 cases. Except for five patients (two with MFS) that did not undergo EMG/NCS, 20 cases (including one with MFS) (80%) had demyelinating features (i.e. AIDP), 4 (16%) had AMSAN, and one (4%) had AMAN in EMG/NCS. This implies that different GBS variants could occur in association with SARS-CoV-2. Except for 4 patients (including one MFS and one AIDP case who received plasma exchange therapy), all other patients received a 5-day course of IVIG (0.4 mg/kg/day, one patient received only one dose) with an additional cycle for 2 patients. Overall, the outcome was favorable with partial to complete recovery in 18 (60%) cases. Two patients passed away due to severe, progressive respiratory failure within 24 h after initiation of IVIG (Alberti, Beretta, 2020, Marta-Enguita et al., 2020).
An important finding in these reported cases is that COVID-19 rRT-PCR was negative in all those 23 checked CSF samples, indicating no active intrathecal SARS-CoV-2 replication or root infection. This finding combined with relatively favorable outcome post-IVIG therapy and positive anti-GD1b antibody in one case may suggest an underlying autoimmune process triggered by post-SARS-CoV-2 viral infection in these cases. There is evidence that the SARS-CoV-2 S protein can bind to sialic acid-containing glycoprotein and gangliosides on cell surfaces (Fantini et al., 2020), increasing its viral transmissibility. Therefore, it is possible that cross-reactivity between epitopes within the SARS-CoV-2 S protein-bearing gangliosides and surface peripheral nerve glycolipids may occur, serving as an underlying mechanism in SARS-CoV-2 triggered autoimmune GBS. Accordingly, most GBS variants (AIDP, AMAN, AMSAN, and MFS) have been reported in SARS-Cov-2 patients (Table 2). Checking anti-ganglioside antibodies in future cases may provide more detailed information about this hypothesis. It is also noteworthy that some of the reported GBS cases received hydroxychloroquine in addition to IVIG or plasma exchange therapy. Chloroquine is shown to bind to sialic acids and GM1 gangliosides preventing binding to SARS-CoV-2 S protein, thereby inhibiting virus entry to the cells. Therefore, adjunctive therapy with chloroquine in SARS-CoV-2-associated GBS could be an interesting consideration in future studies.
4.2. Skeletal muscle injury
An early clinical study from Wuhan (Mao, Jin, 2020) reported that skeletal muscle injury, defined as myalgia plus hyperCKemia, were present in about 11% of patients with COVID-19. Although case reports of rhabdomyolysis with CK levels as high as >11,000 U/L have been reported in COVID-19 patients (Table 2) (Gefen et al., 2020; Jin and Tong, 2020; Suwanwongse and Shabarek, 2020), association of SARS-CoV-2 with either viral or necrotizing autoimmune myositis is still elusive. Two reported cases may indirectly suggest a SARS-CoV-2 triggered necrotizing autoimmune myositis (Jin and Tong, 2020, Suwanwongse and Shabarek, 2020). The first case is a man, aged 88 years, from New York, presenting with acute, painful bilateral proximal lower limb weakness and hyperCKemia (13,581 U/L) (Suwanwongse and Shabarek, 2020) who was found COVID positive and started on hydroxychloroquine, and his weakness and CK levels improved one week later. The second case is a man, aged 60 years, from Wuhan, with 6-day COVID-positive pneumonia and fever who 7 days later, despite improvement in his clinical condition, developed painful proximal muscle weakness with hyperCKemia (11,842 U/L) and elevated CRP, and benefited from IVIG therapy (Jin and Tong, 2020). A more recent study also reported six intensive care unit (ICU)-admitted cases (age between 51 and 72 years old) with COVID-19 who had acute flaccid quadriplegia (Madia et al., 2020). EMG/NCS showed myopathic features in all of these patients and CK levels were normal to mildly elevated (highest level of 1274 U/L), suggesting the presence of critically illness myopathy (Madia, Merico, 2020). Overall, these observations may necessitate pursuing more investigations such as muscle biopsy and antibody screening in some COVID-19 patients with signs of skeletal muscle injury, as treatment with IVIG may potentially improve functional outcomes in these patients. Notably, ACE-2 is shown to be expressed in skeletal muscles (Cabello-Verrugio et al., 2015); thus, evaluation of direct SARS-CoV-2 infection of skeletal muscle fibers would be a highly interesting topic for future studies.
4.3. Encephalopathy
Human coronavirus infections have been associated with encephalopathies in the past and are known to have human neurotropic and neuroinvasive potentials, mainly through the olfactory bulb or hematogenous route, causing inflammation, and demyelination (Desforges, Le Coupanec, 2019). The underlying pathophysiology is still not well understood, and includes abnormal host immune responses with autoimmunity and/or direct CNS damage due to viral replication and infiltration (Desforges, Le Coupanec, 2019). More in details, ACE-2 and transmembrane protease, serine 2 (TMPRSS2) are documented co-receptors for SARS-CoV-2 entry and they are expressed in the oligodendrocytes, suggesting a direct involvement of white matter in case of encephalitis related to COVID-19 infection (Needham et al., 2020; Sellner et al., 2020). A retrospective study from Turkey (Kandemirli et al., 2020) included 235 patients in ICU, 50 of which (21%) developed neurological symptoms. The authors collected MRI data from 27/50 patients to show neurotropism related to COVID-19. The findings included cortical FLAIR (fluid attenuated inversion recovery) abnormalities (37%) with cortical diffusion restriction, leptomeningeal enhancement, or cortical blooming artifact in a non-specific pattern. Less frequently subcortical and deep white matter FLAIR lesions were reported. Unfortunately, the findings were not correlated with the patients' symptomatology. CSF data were also obtained in half of the patients with cortical FLAIR abnormalities, showing elevated proteins, normal cell count, glucose level, IgG index, oligoclonal bands and albumin as well as negative rRT-PCR for SARS-CoV-2 (Kandemirli et al., 2020). Another report from UK described a patient with fever and respiratory symptoms who developed progressive unsteady gait, diplopia, limb ataxia, altered sensation in the right arm, hiccups, and dribbling when eating, found to have a rhomboencephalitis in the MRI with involvement of the right inferior cerebellar peduncle (Table 3). CSF showed normal protein, normal white blood cell (WBC) counts and negative bacterial culture (Wong et al., 2020a). Unfortunately, the SARS-CoV-2 PCR test was not administered, and there were no results for the myelin oligodendrocyte glycoprotein and aquaporin 4 antibodies sent as part of the workup.
The presence of brain inflammatory changes related to COVID-19 was also confirmed by neuropathology findings of foci of perivascular lymphocytes, focal leptomeningeal inflammation in brain specimens of 18 encephalopathic patients, although these findings were reported as rare and did not support an underlying diagnosis of encephalitis. Immunohistochemical analyses to detect SARS-CoV-2 by rRT-PCR performed in the tissues were negative, and the virus was detected at low levels in only 5 patients, possibly as a result of viral direct infiltration in the brain or viral RNA coming from blood (Solomon et al., 2020).
Increasing evidence indicates that encephalopathy is one of the several presenting symptoms or complications of COVID-19. Encephalitis represents an inflammatory process of the brain and surrounding tissues and its symptomatology can include altered mental status, headache, behavioral changes, psychiatric disturbances in association with focal neurological signs (e.g. paresthesia, weakness, etc.). On the other end, meningitis is an inflammatory process of the meninges and spinal cord and gives typical symptoms such as fever, headache, photophobia, phonophobia and neck stiffness. Seizures could also be part of the encephalitis and meningitis presentation (Asadi-Pooya, 2020; Sohal and Mossammat, 2020). The severity of the above symptoms can vary and sometimes it is difficult to make a proper diagnosis particularly in patients with mild symptoms. To add complexity in the diagnosis and management of encephalopathies, there is the inability to distinguish the underlying process (infectious or toxic-metabolic) only based on the symptoms. Indeed, many patients with severe COVID-19 infection may present with altered mental status from the toxic metabolic processes due to hypoxia, electrolyte derangements, metabolic disturbances, and multiorgan failure, without necessarily presenting involvement of the CNS. Moreover, two cases of acute necrotizing encephalopathy (ANE) in patients with COVID-19 positivity from nasopharyngeal and oropharyngeal swab, but without CSF PCR for SARS-CoV-2 data, were reported in the literature (Poyiadji, Shahin, 2020, Radmanesh et al., 2020). ANE is characterized by neuroinflammation secondary to cytokine storm with multifocal symmetric lesions in the gray and white matter without direct viral damage. In addition, there is a report of possible demyelinating lesion in the white matter and globus pallidus in a 54-year-old woman admitted initially for respiratory distress due to COVID-19 infection with only history of mild elevated blood pressure under treatment. Her Glasgow Coma Scale score was 14 with altered sensorium but her neurological exam was non-focal on admission, then rapidly deteriorated and required endotracheal intubation and received hydroxychloroquine in addition to azithromycin and amoxicillin/clavulanic acid. Sedation was discontinued two days later but the patient remained obtunded for a long period afterwards, and this prompt neuroimaging and further investigations. Brain MRI eventually revealed bilateral asymmetric restricted diffusion lesions without hemorrhage or enhancement in the supratentorial periventricular white matter and globus pallidus, without involvement of the thalamus, striatum and posterior fossa. Subsequent MRI obtained 2 days later showed homogeneous contrast enhancement of the lesions, brain vascular images were negative. CSF studies, performed twice (on admission and 9 days after), were reportedly unremarkable, including rRT-PCR for SARS-CoV-2. The patient was treated with steroid for suspected demyelination twelve days later after her hospitalization upon negative nasopharyngeal PCR. The patient reported residual right-side hemiplegia and there are no data about her response to steroids, which could be supportive of the diagnosis of demyelination (Brun et al., 2020). Despite that, the images were suspicious of demyelination, which has been described associated with coronavirus, both in murine animal models (Wu et al., 2000), and in a pediatric patient with acute disseminated encephalomyelitis (ADEM) (Yeh et al., 2004). However, other diagnoses could not be completely excluded in the case described above.
In a retrospective observational case series from Wuhan (Mao, Jin, 2020) that collected data from 214 patients with laboratory-confirmed diagnosis, 36.4% had neurological manifestations. More in detail, CNS manifestation was present in 24.8% of the patients, and in particular 7.5% had encephalopathy. The authors noticed that CNS manifestation were significantly more common in patients with severe infection compared to non-severe infection, with encephalopathy present in 14.8% of cases versus 2.4% (P < 0.001), respectively. The patients with severe infection were older, with higher blood pressure and with less typical symptoms such as fever or cough on admission. Furthermore, those patients were more prone to develop neurological symptoms few days after the admission, with associated higher mortality rate. They also had a marked inflammatory response with higher levels of WBC counts, neutrophil counts, blood urea nitrogen, D-dimer and CRP, and reduced lymphocyte and platelet counts than in those with less severe infection, pointing to a multiorgan involvement and immunosuppression as underlying pathogenic mechanism for the neurological manifestations. The study did not further investigate the etiology of the encephalopathy, either toxic-metabolic or infective. Other case reports described encephalopathic patients who first presented to the hospital with new onset of seizure as manifestation of underlying meningoencephalitis in the setting of COVID-19 infection (Asadi-Pooya, 2020, Moriguchi, Harii, 2020, Sohal and Mossammat, 2020).
As of now, there are no CSF or serologic biomarkers available worldwide to help diagnosing cases of COVID-19 with CNS involvement (Baig, 2020, Kandemirli, Dogan, 2020); therefore, proper neurological evaluation of encephalopathic COVID-19 patients can help early diagnosis, better tailor the treatment, and possibly improve the outcome. The workup for encephalopathic patients should not only include detailed documentation of the neurological symptoms but also electrophysiological studies (i.e. electroencephalography or EEG), CSF analysis, and perhaps brain imaging (Asadi-Pooya and Simani, 2020; Liu et al., 2020b; Oxley et al., 2020). Moreover, seizures should be suspected in case of altered mental status in patients with COVID-19 since cases with clinical or subclinical seizures or status epilepticus have been reported, either as a direct consequence of the brain damage from the virus or secondary toxic-metabolic derangements (Asadi-Pooya, 2020). Anti-epileptic drugs (AEDs) should be administered in patients with seizure as initial presentation, to prevent further episodes, for a period of 6 weeks and then taper and discontinue the AED in 1–2 weeks (Asadi-Pooya, 2020). Since these COVID-19 patients are usually critically ill, intravenous formulations and AEDs with less side effect on respiratory and cardiac status are recommended, such as levetiracetam and brivaracetam. Moreover, since some patients may require extracorporeal membrane oxygenation which affects the pharmacokinetic of highly protein-bound AEDs, phenytoin and valproic acid should be avoided (Asadi-Pooya, 2020).
4.3.1. Viral vs autoimmune meningoencephalitis
The diagnosis of COVID-19 meningoencephalitis is based on clinical and laboratory studies such as CSF characteristics and possibly detection of the virus in the CSF. The first case of COVID-19 with associated laboratory-confirmed viral encephalitis was reported in Beijing in a patient with altered mental status, seizures, persistent hiccups, hyperreflexia, meningeal irritation and slow pupillary response (Table 3). Notably, CSF studies showed normal range WBC, glucose and protein, but an increased opening pressure and positive PCR for SARS-CoV-2 (Oxley, Mocco, 2020, Sun and Guan, 2020). This case report was published in Chinese and it seems to lack further clinical and laboratory data to corroborate the diagnosis. A recent paper discussed about a woman with encephalitis and no respiratory symptoms with SARS-CoV-2 positivity in both nasopharyngeal swab and CSF (Huang et al., 2020a). Another case of meningoencephalitis from COVID-19 in Japan (Moriguchi, Harii, 2020) described a young patient who presented with headache, fatigue, fever and few day later was found unconscious with an episode of generalized tonic seizure while transported to the hospital. He had clear meningeal signs, pleocytosis in the blood, negative CT scan of the head, with SARS-CoV-2 detected only in the CSF but not in the nasopharyngeal swab. Interestingly CSF showed elevated opening pressure and 12 WBC mainly mononuclear; MRI showed DWI (diffusion-weighted imaging) positivity along the wall of the inferior horn of the right ventricle and FLAIR abnormalities in the right mesial temporal lobe and hippocampus. These are the only three encephalitis cases based on our knowledge that were associated with CSF viral detection. It is noteworthy that false positivity of the PCR has been reported given the risk of sample contamination from shed airborne virus with this diagnostic technique (Needham, Chou, 2020).
On the contrary, there is also a case report of a patient who presented with fever, cough and typical multiple ground-glass opacities on CT of the lungs, who later developed focal neurological symptoms and altered mental status suggestive of meningoencephalitis (Yin et al., 2020). Notably, the throat swab was positive for COVID-19 but the CSF PCR was negative (Yin et al., 2020). Other CSF results suggestive of viral infection were the elevated opening pressure and proteins(Yin et al., 2020). The patient was monitored, treated with antivirals and supportive care. Over time the lung infections improved, he started requiring less oxygen supplementation and concomitantly his neurological exam improved consistently. At that time two further throat swab were done and resulted negative. Other case reports of patients with suspected viral meningoencephalitis with positive nasopharyngeal swab but negative CSF PCR for SARS-CoV-2 have been reported in the literature. In the majority of the reports, CSF showed increased cells, mainly lymphocytes, and elevated protein levels (Bernard-Valnet et al., 2020; Dogan et al., 2020). More in details, a case series of 53 ICU patients reported 29 subjects intubated for severe ARDS and no improvement of their mental status or agitated delirium after extubation with subsequent neurological workup (Dogan et al., 2020). Neurological involvement was diagnosed in 6 of the 29 intubated patients (20.6%). These patients had increased levels of acute-phase reactants such as ferritin, CRP, IL-6, fibrinogen. MRI findings showed white matter and cortical abnormalities and contrast enhancement compatible with meningoencephalitis in 3/6 patients. CSF data revealed elevated proteins without pleocytosis in all cases with negative PCR for viruses including SARS-CoV-2. An underlying autoimmune etiology was suspected for both MRI positive and negative patients, and they underwent treatment with plasmapheresis. Improvements of the clinical status were observed in 5/6 patients, and MRI findings were reversible in all 3 patients with positive MRI. Another case from Italy (Pilotto et al., 2020) described a 60-year old man with mild respiratory symptoms that developed akinetic mutism and nuchal rigidity. MRI and EEG were negative for any abnormalities. CSF showed elevated protein level and lymphocytic pleocytosis, as well as increased IL-6, IL-8 and TNF-α but negative for SARS-CoV-2 and for other neurotropic viruses. COVID-19 infection was established by nasopharyngeal swab. The patient was started on antibiotic and antiviral coverage initially, as well as hydroxychloroquine. An improvement was seen after high dose of steroids were initiated. Patient was discharged after 5 days of IV steroids with oral prednisone taper with normal neurological examination.
Although the exact pathogenetic mechanism of autoimmune encephalitis in the setting of COVID-19 is unclear, it may be related to cytokine storm with direct damage to the BBB and increased leukocyte migration to the brain (Sohal and Mossammat, 2020), as well as dysregulation of viral immunity mediated by molecular mimicry (Pilotto et al., 2020). A trial with immunomodulatory therapies can be crucial to diagnose autoimmune encephalitis. Further case reports of patients with suspected viral or autoimmune meningoencephalitis and detailed description of their presentation and workup are also needed. The cases reported above showed that CSF PCR may be not reliable for the diagnosis since SARS-CoV-2 dissemination in the brain can be transient and its CSF titer may be extremely low (Ye et al., 2020a). Furthermore, the test is not widely available. A proper neurological examination, EEG, CSF studies, and brain imaging are for now the only tools that can guide to the diagnosis of COVID-19-associated meningoencephalitis, and appropriate treatment should be initiated promptly.
4.3.2. Toxic metabolic encephalopathy
A retrospective study of 799 patients with COVID-19 (Chen, Wu, 2020b) reported altered mental status on hospital admission in 22% of patients who expired and 1% among those who recovered from the infection. This hints toward a possible negative prognostic factor related to encephalopathy as initial presentation. Headache without associated neurological symptoms or signs was reported in 10% of the deceased patients, versus 12% of the recovered patients. Metabolic derangements were more common in deceased patients than in recovered patients, and 20% of the deceased patients suffered from what was classified as hypoxic encephalopathy related to pulmonary inflammation. In this study neurological symptoms other than headache, neurological signs, and seizures were not reported or considered as possible manifestation of the disease. Furthermore, no laboratory studies were carried to rule out a viral encephalitis/meningitis, underlying seizures, and non-convulsive status epilepticus. It is possible that some of the cases described to have toxic encephalopathy were indeed suffering from a viral encephalitis.
One of the first case reports published at the beginning of the pandemic (Filatov et al., 2020) reported the case of a 74-year old man with chronic obstructive disease (COPD), atrial fibrillation and prior cardioembolic stroke in the left posterior cerebral artery. He presented to the emergency department with fever and cough, initial negative workup for pneumonia, and initially discharged with possible COPD exacerbation. He was readmitted to the emergency department after 24 h with severe altered mental status and headache. Neurology was consulted at that time and a full work up included a CT scan of the head, EEG and CSF studies were conducted. The head CT scan showed the old stroke, related encephalomalacia; and CSF was not suggestive for infection, although SARS-CoV-2 PCR was not performed. EEG showed diffuse slowing and focal sharply contoured waves in the left temporal region which raised the suspicion for subclinical seizures. He eventually tested positive for COVID-19. He was treated with AEDs as well as hydroxychloroquine, lopinavir, and ritonavir. Unfortunately, there is no follow-up report about his response to the treatments. This case report is an example of COVID-19 related toxic metabolic encephalopathy and epileptic activity from lowered seizure threshold secondary to severe underlying metabolic process (Delanty et al., 1998).
Another study from France (Helms et al., 2020) showed that 84% of patients with ARDS and COVID-19 presented with neurological signs such as agitation (69%), corticospinal tract signs (67%) and dysexecutive syndrome with inattention, disorientation, or poorly organized movements in response to command (36%). MRI of the brain was performed in 13 of the 54 patients reported in the study. Notably, these patients did not have any focal signs suggestive for stroke, although 23% had an underlying ischemic stroke, 62% had leptomeningeal enhancement, and all the patients who underwent perfusion imaging (11; 100%) had bilateral frontotemporal hypoperfusion. A small proportion of the patients (8 out of 54) had an EEG that showed non-specific changes or bilateral frontal slowing; and a smaller proportion (7) had CSF studies showing no cells or other signs of infection as well as negative RT-PCR assays for SARS-CoV-2 in all the samples. It is unclear which patients underwent CSF analysis. Unfortunately, this report lacks a detailed description of the patients and their workup, and it is possible that some of them, particularly those with focal neurological symptoms and leptomeningeal enhancement on MRI, were experiencing a viral involvement of the CNS.
4.4. Acute myelitis
Acute myelitis is a known complication of viral infections, mainly attributed as an autoimmune response, although it could also be an early manifestation of other neuro-immunological disorders such as multiple sclerosis and neuromyelitis optica spectrum disorders. Little is known about association between SARS-CoV-2 and acute myelitis. Up to date, 7 cases of acute myelitis, alone (4 cases) or combined with brain involvement (3 cases), have been reported in relation to COVID-19 (AlKetbi et al., 2020, Munz et al., 2020, Novi et al., 2020, Sarma and Bilello, 2020, Valiuddin et al., 2020, Wong, Craik, 2020a, Zanin, Saraceno, 2020). Six patients (28–64 years age range, 57.1% female) variably had symptoms of COVID-19 (fever, dyspnea, malaise, chills, and rhinorrhea) 25 to 2 days prior the onset of neurologic symptoms of myelitis (Table 3). SARS-CoV-2 rRT-PCR were checked in the CSF of 5 patients and all were negative. Overall, the functional outcome was favorable in 6 (85.7%) patients after treatment with either steroids or plasmapheresis. Additionally, a recent case report from Spain (Sotoca and Rodríguez-Álvarez, 2020) has presented a 68 years old woman who developed a 7-day radicular neck pain, right facial numbness, left hand numbness and weakness, gait instability, and general hyperreflexia. She had fever and cough 8 days prior these symptoms. The cervical spine demonstrated a T-2 hyperintensity extending from the medulla oblongata to C7, suggestive of acute transverse myelitis. She had negative blood testing for anti-antiaquaporin-4 (AQ4), −myelin oligodendrocyte glycoprotein (MOG), and antiphospholipid antibodies, but had elevated protein level and pleocytosis in the CSF study with no oligoclonal band, normal IgG index, no anti-neuronal surface antibody, and negative SARS-CoV-2 PCR. However, nasopharyngeal swab for SARS-CoV-2 rRT-PCR was positive. She was treated with 5-day methylprednisolone (1 g/day). Few days later her symptoms worsened, and she developed bladder/bowel incontinence, bilateral hands weakness and paresthesia, and paraparesis. The repeated spinal MRI showed a new area of central necrosis at the T1 level with peripheral enhancement. This case was indeed the first case of acute necrotizing myelitis in association with COVID-19. She was additionally treated with plasmapheresis and 5-day methylprednisolone (1 g/day) followed by slow tapering oral prednisone with favorable outcome after 4 weeks. The exact underlying mechanism in acute necrotizing myelitis is still elusive; however, a post-viral triggered autoimmune cytokine storm has been suggested (Kansagra and Gallentine, 2011). Thus, this may imply in the case of SARS-CoV-2, especially with observed clinical improvement after steroid and plasmapheresis therapy.
5. Psychiatric manifestations
There is growing evidence about psychiatric manifestations as potential complication of SARS-CoV-2 infection. Accordingly, a worldwide exacerbation of mental health disorders during the pandemic has been reported, which includes but not limited to delirium, cognitive impairment, mood alterations, psychosis and suicide (Orsini et al., 2020). More in details, delirium has been noticed more in 90% of COVID-19 patients whose conditions require ICU level of care versus 70–75% rates documented in the past (Kotfis et al., 2020). Cognitive dysfunctions have been reported to be possibly a direct consequence of COVID-19 infection to the CNS, and in particular to the hippocampus, which appears to be vulnerable to coronaviruses infections, with possible acceleration of hippocampal degeneration as occurs in Alzheimer's disease (Ritchie et al., 2020). Cognitive impairment can be also a consequence of acute respiratory syndrome and relative hypoxia which have been associated to cerebral atrophy and ventricular enlargement (Hopkins et al., 2006) and worsening attention, executive functions and verbal memory (Hopkins et al., 2005). Anxiety, depression, post-traumatic stress disorder, insomnia and obsessive-compulsive symptomatology appear to be very common in COVID-19 survivors, particularly in females, and with worsened scores on psychopathological measures in those with previous psychiatric comorbidities (Mazza et al., 2020). A recent study (Brown et al., 2020) reports an incidence of psychosis in infected patients between 0.9% and 4% versus a median value of 15.2 (7.7–43.0) per 100,000 previously described (McGrath et al., 2004). Increased rates of suicide have been also reported, with possible contributing factors found in the social isolation/distancing, economic recession and social discrimination (Thakur and Jain, 2020).
It is unclear whether the above psychiatric symptoms are a direct consequence of the CNS viral infection (i.e. viral meningoencephalitis), cerebrovascular accidents, hypoxia, and the immunological and inflammatory responses, which may play important roles in major depressive disorder (Ghasemi et al., 2019; Wohleb et al., 2016) and psychosis (Ferrando et al., 2020), or whether they are related to increased psychosocial stress of this severe and potentially fatal disease and difficulties accessing to health care related to the pandemic infection (Zhou and Yao, 2020). This has been posing increased challenges in the treatment of infected patients, especially those in the ICU, requiring accurate multidisciplinary approaches and early interventions to decrease overall morbidity and mortality (Ojeahere et al., 2020).
6. Therapeutic strategies
As described in above sections, several treatment approaches have been used to treat manifestations or consequences of the SARS-CoV-2 − related nervous system injury, such as IVIG for GBS and skeletal muscle injury, IV/oral steroids and plasmapheresis for autoimmune encephalitis and acute myelitis, and AEDs for seizures. With regards to medications aimed to modulate the immune response to viral infection and to induce viral clearance, antimalarial drugs (e.g. hydroxychloroquine), dexamethasone, RNA-dependent RNA polymerase inhibitors (e.g. remdesivir), HIV-1 protease inhibitors (lopinavir/ritonavir), and biological agents like tocilizumab, interferons and convalescent plasma have shown some beneficial effects (Chibber et al., 2020). Among these, the FDA granted Emergency Use Authorization for remdesivir as an emergency medication for severely ill hospitalized adult and pediatric patients with proved or suspected SARS-CoV-2 infection (Lamb, 2020). This drug, which has a broad-spectrum antiviral activity against several RNA viruses, can inhibit SARS-CoV-2 replication, alleviate symptoms, fasten the recovery rate, and reduce mortality rate (Frediansyah et al., 2020). More in details, the final report of the Adaptive Covid-19 Treatment Trial (ACTT-1), a double-blind randomized placebo-controlled trial of intravenous remdesivir in affected adults with evidence of lower respiratory tract infection, showed a median recovery time of 10 days (95% confidence interval [CI], 9 to 11) versus 15 days (95% CI, 13 to 18) in those in the placebo group. Mortality estimates by day 15 were 6.7% with remdesivir versus 11.9% with placebo and those by day 29 were 11.4% with remdesivir and 15.2% with placebo (Paladugu and Donato, 2020). Given the lack of head to head comparison, it is unclear if remdesivir offers a superior benefit over dexamethasone, which is widely available and less expensive (McCreary and Angus, 2020). The Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial has shown that dexamethasone resulted in lower 28-day mortality in COVID-19 patients receiving either invasive mechanical ventilation or oxygen alone at randomization but not in those receiving no respiratory support (Horby et al., 2020). The authors of the ACTT-1 trial also adjusted the data for glucocorticoid use suggesting that the benefit of dexamethasone may be additive to that of remdesivir (Beigel et al., 2020). However, it is still unclear whether remdesivir or dexamethasone have beneficial effects on the neurological manifestation of COVID-19.
With regards to possible therapeutic strategies aimed to ameliorate the neuronal damage mediated by COVID-19, high doses of melatonin seem promising in immunomodulation and reducing neuroinflammation, with no direct effect on viral replication or transcription. Melatonin seems to act via an anti-inflammatory, anti-oxidative and immune-enhancing mechanism with ability to restore the BBB hemostasis (Romero et al., 2020).
There are ongoing worldwide clinical trials for the development of a vaccine to prevent COVID-19, which is not currently available and that poses some challenges due to safety, efficacy, and long-lasting effects without further risks of re-infection, particularly in the elderly population (Jamwal et al., 2020). The COVID-19 pandemic with its variety of manifestations, not only pulmonary or neurological, is an international public health emergency that requires efforts from all countries to develop effective drugs and vaccines as early as possible.
7. Conclusion
Evolving data indicates that patients with COVID-19 may variably develop neurologic manifestations prior, during and even after the onset of common COVID-19 symptoms. The commonly reported neurological symptoms and signs include dizziness, headache, myalgia, fatigue, impaired consciousness and confusion, ageusia, anosmia, neuropathic or radicular pain, occipital neuralgia, visual impairment, seizure, and ataxia. Based on a growing number of case reports and series, both the CNS, PNS and skeletal muscles can be involved in COVID-19 presenting with a variety of neuroimmunological conditions including GBSs, myopathy and rhabdomyolysis, encephalopathy, meningoencephalitis, encephalomyelitis, and acute myelitis. The exact etiology of these complications remains to be fully elucidated. However, suggested mechanisms are direct SARS-CoV-2 infection to the nervous system, neuroinflammation, post-viral triggered autoimmune response, hypercoagulability, and metabolic or hypoxic injury. In general, therapeutic strategies for COVID-19 are based on three main directions: (i) targeting SARS-CoV-2 with antivirals, neutralizing antibodies or convalescent plasma therapy, (ii) targeting inflammatory storm using immunomodulatory medications and cytokine inhibitors, and (iii) developing vaccines to prevent the disease manifestation (for comprehensive review see (Vabret et al., 2020)). However, it is still too early to find out whether even with successful treatment of the active infection, post-viral triggered autoimmune neurological complications of COVID-19 (e.g. GBS, myositis, CNS demyelination, and myelitis) will be also lowered in frequency and/or severity. Additional studies are clearly needed to address this issue.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
No conflict of interest exists in relation to the submitted manuscript.
References
- Coen M., Jeanson G., Culebras Almeida L.A., Hübers A., Stierlin F., Najjar I. Guillain-Barré syndrome as a complication of SARS-CoV-2 infection. Brain Behav. Immun. 2020;87:111–112. doi: 10.1016/j.bbi.2020.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong L., Xu P., Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in Downtown Los Angeles, early April 2020. Brain Behav. Immun. 2020;S0889-1591(20) doi: 10.1016/j.bbi.2020.04.024. (30509–2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. [Google Scholar]
- Ritchie K., Chan D., Watermeyer T. The cognitive consequences of the COVID-19 epidemic: collateral damage? Brain Commun. 2020;2:fcaa069. doi: 10.1093/braincomms/fcaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdennour L., Zeghal C., Deme M., Puybasset L. Interaction brain-lungs. Ann Fr Anesth Reanim. 2012;31(6):e101–e107. doi: 10.1016/j.annfar.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Alberti P., Beretta S., Piatti M., Karantzoulis A., Piatti M.L., Santoro P. Guillain-Barré syndrome related to COVID-19 infection. Neurol. Neuroimmunol. Neuroinflam. 2020;7 doi: 10.1212/NXI.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlKetbi R., AlNuaimi D., AlMulla M., AlTalai N., Samir M., Kumar N. Acute myelitis as a neurological complication of Covid-19: A case report and MRI findings. Radiol Case Rep. 2020;6(15):1591–1595. doi: 10.1016/j.radcr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andries K., Pensaert M. Immunofluorescence studies on the pathogenesis of hemagglutinating encephalomyelitis virus infection in pigs after oronasal inoculation. Am. J. Vet. Res. 1980;41:1372–1378. [PubMed] [Google Scholar]
- Andries K., Pensaert M.B. Immunofluorescence studies on the pathogenesis of hemagglutinating encephalomyelitis virus infection in pigs after oronasal inoculation. Am. J. Vet. Res. 1980;41:1372–1378. [PubMed] [Google Scholar]
- Arnaud S., Budowski C., Ng Wing Tin S., Degos B. Post SARS-CoV-2 Guillain-Barré syndrome. Clin. Neurophysiol. 2020;131:1652–1654. doi: 10.1016/j.clinph.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi-Pooya A.A. Seizures associated with coronavirus infections. Seizure. 2020;79:49–52. doi: 10.1016/j.seizure.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi-Pooya A.A., Simani L. Central nervous system manifestations of COVID-19: A systematic review. J. Neurol. Sci. 2020;413:116832. doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A.M. Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci. Ther. 2020;26:499–501. doi: 10.1111/cns.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the Treatment of Covid-19 - Final report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007764. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard-Valnet R., Pizzarotti B., Anichini A., Demars Y., Russo E., Schmidhauser M. Two patients with acute meningoencephalitis concomitant with SARS‐CoV‐2 infection. Eur. J. Neurol. 2020 doi: 10.1111/ene.14298. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigaut K., Mallaret M., Baloglu S., Nemoz B., Morand P., Baicry F. Guillain-Barré syndrome related to SARS-CoV-2 infection. Neurol. Neuroimmunol. Neuroinflamm. 2020;7 doi: 10.1212/NXI.0000000000000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleau C., Filliol A., Samson M., Lamontagne L. Brain invasion by mouse hepatitis virus depends on impairment of tight junctions and beta interferon production in brain microvascular endothelial cells. J. Virol. 2015;89:9896–9908. doi: 10.1128/JVI.01501-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodro M., Compta Y., Llansó L., Esteller D., Doncel-Moriano A., Mesa A. Increased CSF levels of IL-1β, IL-6, and ACE in SARS-CoV-2–associated encephalitis. Neurol. Neuroimmun. Neuroinflam. 2020;7 doi: 10.1212/NXI.0000000000000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostancıklıoğlu M. Temporal correlation between neurological and gastrointestinal symptoms of SARS-CoV-2. Inflamm. Bowel Dis. 2020;26(8):e89–e91. doi: 10.1093/ibd/izaa131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann D., Tsukahara T., Weinreb C., Logan D.W., Datta S.R. Non-neural expression of SARS-CoV-2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID-19 patients. Sci. Adv. 2020;6(31):eabc5801. doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E., Gray R., Lo Monaco S., O’Donoghue B., Nelson B., Thompson A. The potential impact of COVID-19 on psychosis: a rapid review of contemporary epidemic and pandemic research. Schizophr. Res. 2020;222:79–87. doi: 10.1016/j.schres.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun G., Hak J.F., Coze S., Kaphan E., Carvelli J., Girard N. COVID-19-white matter and globus pallidum lesions: demyelination or small-vessel vasculitis? Neurol. Neuroimmunol. Neuroinflamm. 2020;7 doi: 10.1212/NXI.0000000000000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello-Verrugio C., Morales M.G., Rivera J.C., Cabrera D., Simon F. Renin-angiotensin system: an old player with novel functions in skeletal muscle. Med. Res. Rev. 2015;35:437–463. doi: 10.1002/med.21343. [DOI] [PubMed] [Google Scholar]
- Cabirac G.F., Soike K.F., Butunoi C., Hoel K., Johnson S., Cai G.-Y. Springer; 1994. Coronavirus JHM OMP1 Pathogenesis in Owl Monkey CNS and Coronavirus Infection of Owl Monkey CNS Via Peripheral Routes. Coronaviruses; pp. 347–352. [DOI] [PubMed] [Google Scholar]
- Cantador E., Nunez A., Sobrino P., Espejo V., Fabia L., Vela L. Incidence and consequences of systemic arterial thrombotic events in COVID-19 patients. J. Thromb. Thrombolysis. 2020;50(3):543–547. doi: 10.1007/s11239-020-02176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. StatPearls Publishing; Treasure Island, FL: 2020. Features, Evaluation and Treatment Coronavirus (COVID-19). StatPearls. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) 2020. Coronavirus Disease 2019 (COVID-19) [Google Scholar]
- Chacón-Aguilar R., Osorio-Cámara J.M., Sanjurjo-Jimenez I., González-González C., López-Carnero J., Pérez-Moneo-Agapito B. COVID-19: Fever syndrome and neurological symptoms in a neonate. An. Pediatr. (Engl Ed) 2020;92(6):373–374. doi: 10.1016/j.anpede.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.H., Farouji I., Abu Hanoud A., Slim J. Weakness and elevated creatinine kinase as the initial presentation of coronavirus disease 2019 (COVID-19) Am. J. Emerg. Med. 2020;38(7):1548.e1–1548.e3. doi: 10.1016/j.ajem.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D., Lin M., Wei L., Xie L., Zhu G., Dela Cruz C.S. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan. Jama. 2020;323:1092–1093. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibber P., Haq S.A., Ahmed I., Andrabi N.I., Singh G. Advances in the possible treatment of COVID-19: a review. Eur. J. Pharmacol. 2020;883:173372. doi: 10.1016/j.ejphar.2020.173372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen T., Lolli V., Sadeghi N., Rovaï A., Trotta N., Taccone F.S. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology. 2020 doi: 10.1212/WNL.0000000000010116. [DOI] [PubMed] [Google Scholar]
- Cowley T.J., Weiss S.R. Murine coronavirus neuropathogenesis: determinants of virulence. J. Neuro-Oncol. 2010;16:427–434. doi: 10.1007/BF03210848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delanty N., Vaughan C.J., French J.A. Medical causes of seizures. Lancet. 1998;352:383–390. doi: 10.1016/S0140-6736(98)02158-8. [DOI] [PubMed] [Google Scholar]
- Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dubé M. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12 doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodding M.P., Way M. Coupling viruses to dynein and kinesin-1. EMBO J. 2011;30:3527–3539. doi: 10.1038/emboj.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan L., Kaya D., Sarikaya T., Zengin R., Dincer A., Akinci I.O. Plasmapheresis treatment in COVID-19-related autoimmune meningoencephalitis: Case series. Brain Behav. Immun. 2020;87:155–158. doi: 10.1016/j.bbi.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am. J. Respir. Crit. Care Med. 2020;201(11):1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube M., Le Coupanec A., Wong A.H.M., Rini J.M., Desforges M., Talbot P.J. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J. Virol. 2018;92 doi: 10.1128/JVI.00404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugue R., Cay-Martínez K.C., Thakur K.T., Garcia J.A., Chauhan L.V., Williams S.H. Neurologic manifestations in an infant with COVID-19. Neurology. 2020;94(24):1100–1102. doi: 10.1212/WNL.0000000000009653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Otmani H., El Moutawakil B., Rafai M.A., El Benna N., El Kettani C., Soussi M. Covid-19 and Guillain-Barré syndrome: more than a coincidence! Rev. Neurol. (Paris) 2020;176:518–519. doi: 10.1016/j.neurol.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini J., Di Scala C., Chahinian H., Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob. Agents. 2020;55(5):105960. doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando S.J., Klepacz L., Lynch S., Tavakkoli M., Dornbush R., Baharani R. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? Psychosomatics. 2020;61:551–555. doi: 10.1016/j.psym.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov A., Sharma P., Hindi F., Espinosa P.S. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12 doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frediansyah A., Nainu F., Dhama K., Mudatsir M., Harapan H. Remdesivir and its antiviral activity against COVID-19: A systematic review. Clinical epidemiology and global health. 2020 doi: 10.1016/j.cegh.2020.07.011. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung S.-Y., Yuen K.-S., Ye Z.-W., Chan C.-P., Jin D.-Y. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg. Microbes Infect. 2020;9:558–570. doi: 10.1080/22221751.2020.1736644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020;201(10):1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefen A.M., Palumbo N., Nathan S.K., Singer P.S., Castellanos-Reyes L.J., Sethna C.B. Pediatric COVID-19-associated rhabdomyolysis: a case report. Pediatr. Nephrol. 2020:1–4. doi: 10.1007/s00467-020-04617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi M., Claunch J., Niu K. Pathologic role of nitrergic neurotransmission in mood disorders. Prog. Neurobiol. 2019;173:54–87. doi: 10.1016/j.pneurobio.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. 2020;104362 doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgianni A., Vinacci G., Agosti E., Cariddi L.P., Mauri M., Baruzzi F. Transient acute-onset tetraparesis in a COVID-19 patient. Spinal Cord. 2020:1–3. doi: 10.1038/s41393-020-0493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N., Agrawal S., Ish P., Mishra S., Gaind R., Usha G. Clinical and epidemiologic profile of the initial COVID-19 patients at a tertiary care Centre in India. Monaldi Arch. Chest Dis. 2020;90 doi: 10.4081/monaldi.2020.1294. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Ortiz C., Méndez A., Rodrigo-Rey S., San Pedro-Murillo E., Bermejo-Guerrero L., Gordo-Mañas R. Miller fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95(5):e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y., Hasebe R., Sunden Y., OCHIAI K., Honda E., Sakoda Y. Propagation of swine hemagglutinating encephalomyelitis virus and pseudorabies virus in dorsal root ganglia cells. J. Vet. Med. Sci. 2009;71:595–601. doi: 10.1292/jvms.71.595. [DOI] [PubMed] [Google Scholar]
- He L., Ding Y., Che X., Zhang Q., Huang Z., Wang H. Expression of the monoclonal antibody against nucleocapsid antigen of SARS-associated coronavirus in autopsy tissues from SARS patients. Di 1 jun yi da xue xue bao. 2003;23:1128–1130. [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins R.O., Weaver L.K., Collingridge D., Parkinson R.B., Chan K.J., Orme J.F., Jr. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2005;171:340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- Hopkins R.O., Gale S.D., Weaver L.K. Brain atrophy and cognitive impairment in survivors of acute respiratory distress syndrome. Brain Inj. 2006;20:263–271. doi: 10.1080/02699050500488199. [DOI] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.H., Jiang D., Huang J.T. SARS-CoV-2 Detected in Cerebrospinal Fluid by PCR in a Case of COVID-19 Encephalitis. Brain Behav. Immun. 2020;87:149. doi: 10.1016/j.bbi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C., Nichols T., Pike M., Subbe C., Elghenzai S. Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur. J. Case Rep. Int. Med. 2020;7 doi: 10.12890/2020_001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama T., Shoji H., Kato M., Sawaishi Y., Ozawa H., Matsubara T. Cerebrospinal fluid levels of cytokines and soluble tumour necrosis factor receptor in acute disseminated encephalomyelitis. Eur. J. Pediatr. 2002;161:133–137. doi: 10.1007/s00431-001-0888-2. [DOI] [PubMed] [Google Scholar]
- Jakhmola S., Indari O., Chatterjee S., Jha H.C. SARS-CoV-2, an underestimated pathogen of the nervous system. SN Comprehen. Clin. Med. 2020:1–10. doi: 10.1007/s42399-020-00522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamwal S., Gautam A., Elsworth J., Kumar M., Chawla R., Kumar P. An updated insight into the molecular pathogenesis, secondary complications and potential therapeutics of COVID-19 pandemic. Life Sci. 2020;257:118105. doi: 10.1016/j.lfs.2020.118105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M., Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg. Infect. Dis. 2020;26 doi: 10.3201/eid2607.200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliao Caamaño D.S., Alonso Beato R. Facial diplegia, a possible atypical variant of Guillain-Barré syndrome as a rare neurological complication of SARS-CoV-2. J. Clin. Neurosci. 2020;77:230–232. doi: 10.1016/j.jocn.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandemirli S.G., Dogan L., Sarikaya Z.T., Kara S., Akinci C., Kaya D. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology. 2020;201697 doi: 10.1148/radiol.2020201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansagra S.M., Gallentine W.B. Cytokine storm of acute necrotizing encephalopathy. Pediatr. Neurol. 2011;45:400–402. doi: 10.1016/j.pediatrneurol.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Karakike E., Giamarellos-Bourboulis E.J. Macrophage activation-like syndrome: a distinct entity leading to early death in sepsis. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.E., Heo J.H., Kim H.O., Song S.H., Park S.S., Park T.H. Neurological complications during treatment of Middle East respiratory syndrome. J. Clin. Neurol. 2017;13:227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotfis K., Williams Roberson S., Wilson J., Pun B., Ely E.W., Jeżowska I. COVID-19: what do we need to know about ICU delirium during the SARS-CoV-2 pandemic? Anaesthesiol. Intensive Ther. 2020;52:132–138. doi: 10.5114/ait.2020.95164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyuncu O.O., Hogue I.B., Enquist L.W. Virus infections in the nervous system. Cell Host Microbe. 2013;13:379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunas-Rangel F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.25819. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb Y.N. Remdesivir: first approval. Drugs. 2020;80:1355–1363. doi: 10.1007/s40265-020-01378-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascano A.M., Epiney J.B., Coen M., Serratrice J., Bernard-Valnet R., Lalive P.H. SARS-CoV-2 and Guillain-Barré syndrome: AIDP variant with favorable outcome. Eur. J. Neurol. 2020 doi: 10.1111/ene.14368. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K.-K., Yu W.-C., Chu C.-M., Lau S.-T., Sheng B., Yuen K.-Y. Possible central nervous system infection by SARS coronavirus. Emerg. Infect. Dis. 2004;10:342. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le R.Q., Li L., Yuan W., Shord S.S., Nie L., Habtemariam B.A. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23:943. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Otorhinolaryngol. 2020:1–11. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- Li Y.-C., Bai W.-Z., Hirano N., Hayashida T., Hashikawa T. Coronavirus infection of rat dorsal root ganglia: ultrastructural characterization of viral replication, transfer, and the early response of satellite cells. Virus Res. 2012;163:628–635. doi: 10.1016/j.virusres.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Wohlford-Lenane C., Perlman S., Zhao J., Jewell A.K., Reznikov L.R. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J. Infect. Dis. 2016;213:712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima M., Siokas V., Aloizou A.-M., Liampas I., Mentis A.-F.A., Tsouris Z. Unraveling the possible routes of SARS-COV-2 invasion into the central nervous system. Curr. Treat. Options Neurol. 2020;22:1–15. doi: 10.1007/s11940-020-00647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker R.A., Lühder F., Kallen K.-J., Lee D.-H., Engelhardt B., Rose-John S. IL-6 transsignalling modulates the early effector phase of EAE and targets the blood-brain barrier. J. Neuroimmunol. 2008;205:64–72. doi: 10.1016/j.jneuroim.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Liu F., Mccullough L.D. Inflammatory responses in hypoxic ischemic encephalopathy. Acta Pharmacol. Sin. 2013;34:1121–1130. doi: 10.1038/aps.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Pan M., Xiao Z., Xu X. Neurological manifestations of the coronavirus (SARS-CoV-2) pandemic 2019-2020. J. Neurol. Neurosurg. Psychiatry. 2020;91(6):669–670. doi: 10.1136/jnnp-2020-323177. [DOI] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madia F., Merico B., Primiano G., Cutuli S.L., De Pascale G., Servidei S. Acute myopathic quadriplegia in COVID-19 patients in the intensive care unit. Neurology. 2020 doi: 10.1212/WNL.0000000000010280. [DOI] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marta-Enguita J., Rubio-Baines I., Gastón-Zubimendi I. Fatal Guillain-Barre syndrome after infection with SARS-CoV-2. Neurologia (Barcelona, Spain) 2020;35:265–267. doi: 10.1016/j.nrl.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K., Park C., Sunden Y., Kimura T., Ochiai K., Kida H. The vagus nerve is one route of transneural invasion for intranasally inoculated influenza a virus in mice. Vet. Pathol. 2004;41:101–107. doi: 10.1354/vp.41-2-101. [DOI] [PubMed] [Google Scholar]
- Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAbee G.N., Brosgol Y., Pavlakis S., Agha R., Gaffoor M. Encephalitis associated with COVID-19 infection in an 11 year-old child. Pediatr. Neurol. 2020;109:94. doi: 10.1016/j.pediatrneurol.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreary E.K., Angus D.C. Efficacy of Remdesivir in COVID-19. Jama. 2020;324:1041–1042. doi: 10.1001/jama.2020.16337. [DOI] [PubMed] [Google Scholar]
- McGrath J., Saha S., Welham J., El Saadi O., MacCauley C., Chant D. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med. 2004;2:13. doi: 10.1186/1741-7015-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecha M., Carrillo-Salinas F.J., Mestre L., Feliú A., Guaza C. Viral models of multiple sclerosis: neurodegeneration and demyelination in mice infected with Theiler’s virus. Prog. Neurobiol. 2013;101–102:46–64. doi: 10.1016/j.pneurobio.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020:1–8. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moein S.T., Hashemian S.M.R., Mansourafshar B., Khorram-Tousi A., Tabarsi P., Doty R.L. Smell dysfunction: a biomarker for COVID-19. Int. Forum Allergy Rhinol. 2020;10(8):944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I. Transolfactory neuroinvasion by viruses threatens the human brain. Acta Virol. 2015;59:338–349. doi: 10.4149/av_2015_04_338. [DOI] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz M., Wessendorf S., Koretsis G., Tewald F., Baegi R., Krämer S. Acute transverse myelitis after COVID-19 pneumonia. J. Neurol. 2020:1–2. doi: 10.1007/s00415-020-09934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy S., Gomersall C.D., Fowler R.A. Care for Critically ill Patients with COVID-19. JAMA. 2020;323(15):1499–1500. doi: 10.1001/jama.2020.3633. [DOI] [PubMed] [Google Scholar]
- Needham E.J., Chou S.H., Coles A.J., Menon D.K. Neurological implications of COVID-19 infections. Neurocrit. Care. 2020;32(3):667–671. doi: 10.1007/s12028-020-00978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82:7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novi G., Rossi T., Pedemonte E., Saitta L., Rolla C., Roccatagliata L. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol. Neuroimmun. Neuroinflam. 2020;7 doi: 10.1212/NXI.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguz-Akarsu E., Ozpar R., Mirzayev H., Acet-Ozturk N.A., Hakyemez B., Ediger D. Guillain-Barré syndrome in a patient with minimal symptoms of COVID-19 infection. Muscle Nerve. 2020;62(3):E54–E57. doi: 10.1002/mus.26992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeahere M.I., de Filippis R., Ransing R., Karaliuniene R., Ullah I., Bytyçi D.G. Management of psychiatric conditions and delirium during the COVID-19 pandemic across continents: lessons learned and recommendations. Brain Behav. Immun. Health. 2020;9:100147. doi: 10.1016/j.bbih.2020.100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- Orsini A., Corsi M., Santangelo A., Riva A., Peroni D., Foiadelli T. Challenges and management of neurological and psychiatric manifestations in SARS-CoV-2 (COVID-19) patients. Neurol. Sci. 2020;41:2353–2366. doi: 10.1007/s10072-020-04544-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani D., Boso F., Tranquillini E., Gapeni I., Pedrotti G., Cozzio S. Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): a case report from an Italian COVID-hospital. Neurol. Sci. 2020;41(6):1351–1354. doi: 10.1007/s10072-020-04449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P. Large-vessel stroke as a presenting feature of Covid-19 in the young. NEJM. 2020;382 doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padroni M., Mastrangelo V., Asioli G.M., Pavolucci L., Abu-Rumeileh S., Piscaglia M.G. Guillain-Barré syndrome following COVID-19: new infection, old complication? J. Neurol. 2020:1–3. doi: 10.1007/s00415-020-09849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladugu S., Donato A.A. Remdesivir improved time to recovery in adults hospitalized with COVID-19 and lower respiratory tract involvement. Ann. Intern. Med. 2020;173:Jc4. doi: 10.7326/ACPJ202007210-005. [DOI] [PubMed] [Google Scholar]
- Palasca O., Santos A., Stolte C., Gorodkin J., Jensen L.J. TISSUES 2.0: an integrative web resource on mammalian tissue expression. Database. 2018;2018 doi: 10.1093/database/bay003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniz-Mondolfi A., Bryce C., Grimes Z., Gordon R.E., Reidy J., Lednicky J. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J. Med. Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A., Odolini S., Stefano Masciocchi S., Comelli A., Volonghi I., Gazzina S. Steroid-responsive encephalitis in Covid-19 disease. Ann. Neurol. 2020 doi: 10.1002/ana.25783. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020;201187 doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabakaran P., Xiao X., Dimitrov D.S. A model of the ACE2 structure and function as a SARS-CoV receptor. Biochem. Biophys. Res. Commun. 2004;314:235–241. doi: 10.1016/j.bbrc.2003.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyankov O.V., Bodnev S.A., Pyankova O.G., Agranovski I.E. Survival of aerosolized coronavirus in the ambient air. J. Aerosol Sci. 2018;115:158–163. doi: 10.1016/j.jaerosci.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian G.-Q., Yang N.-B., Ding F., Ma A.H.Y., Wang Z.-Y., Shen Y.-F. Epidemiologic and Clinical Characteristics of 91 Hospitalized Patients with COVID-19 in Zhejiang, China: A retrospective, multi-centre case series. QJM. 2020:hcaa089. doi: 10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Clinical Infectious Diseases; China: 2020. Dysregulation of Immune Response in Patients with COVID-19 in Wuhan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmanesh F., Rodriguez-Pla A., Pincus M.D., Burns J.D. Severe cerebral involvement in adult-onset hemophagocytic lymphohistiocytosis. J. Clin. Neurosci. 2020;76:236–237. doi: 10.1016/j.jocn.2020.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S., Khan Z.K., Jain P. The tug-of-war between dendritic cells and human chronic viruses. Int. Rev. Immunol. 2011;30:341–365. doi: 10.3109/08830185.2011.561506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Bueno J.A., García-Trujillo L., Urbaneja P., Ciano-Petersen N.L., Postigo-Pozo M.J., Martínez-Tomás C. Miller-fisher syndrome after SARS-CoV-2 infection. Eur. J. Neurol. 2020 doi: 10.1111/ene.14383. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva N., Russo T., Falzone Y.M., Strollo M., Amadio S., Del Carro U. Post-infectious Guillain-Barré syndrome related to SARS-CoV-2 infection: a case report. J. Neurol. 2020:1–3. doi: 10.1007/s00415-020-09907-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- W-J Guan, Z-Y Ni. Hu Y, Liang W-h, Ou C-q, He J-x, et al. clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha Cabrero F., Morrison E.H. StatPearls Publishing; Treasure Island (FL): 2020. Miller Fisher Syndrome. StatPearls. [Google Scholar]
- Romero A., Ramos E., López-Muñoz F., Gil-Martín E., Escames G., Reiter R.J. Coronavirus Disease 2019 (COVID-19) and Its Neuroinvasive Capacity: Is It Time for Melatonin? Cell. Mol. Neurobiol. 2020:1–12. doi: 10.1007/s10571-020-00938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients. AJR Am. J. Roentgenol. 2020:1–7. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- Sancho-Saldaña A., Lambea-Gil Á., Liesa J.L.C., Caballo M.R.B., Garay M.H., Celada D.R. Guillain-Barré syndrome associated with leptomeningeal enhancement following SARS-CoV-2 infection. Clin. Med. (Lond). 2020;20(4):e93–e94. doi: 10.7861/clinmed.2020-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma D, Bilello LA. A Case Report of Acute Transverse Myelitis Following Novel Coronavirus Infection. Clin. Pract. Cases Emerg. Med. 2020;4:In Press. [DOI] [PMC free article] [PubMed]
- Scheidl E., Canseco D.D., Hadji-Naumov A., Bereznai B. Guillain-Barre syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J. Peripheral Nervous System. 2020;25(2):204–207. doi: 10.1111/jns.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaghat Z., Karimi N. Guillain Barre syndrome associated with COVID-19 infection: A case report. J. Clin. Neurosci. 2020;76:233–235. doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellner J., Taba P., Öztürk S., Helbok R. The need for neurologists in the care of COVID-19 patients. Eur. J. Neurol. 2020 doi: 10.1111/ene.14257. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahriarirad R., Khodamoradi Z., Erfani A., Hosseinpour H., Ranjbar K., Emami Y. Epidemiological and clinical features of 2019 novel coronavirus diseases (COVID-19) in the South of Iran. BMC Infect. Dis. 2020;20:427. doi: 10.1186/s12879-020-05128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Razavi A., Karimi N., Rouhani N. COVID-19 and intracerebral hemorrhage: causative or coincidental? New Microbes New Infect. 2020;35:100669. doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal S., Mossammat M. COVID-19 Presenting with Seizures. IDCases. 2020;20:e00782. doi: 10.1016/j.idcr.2020.e00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon I.H., Normandin E., Bhattacharyya S., Mukerji S.S., Keller K., Ali A.S. Neuropathological Features of Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotoca J., Rodríguez-Álvarez Y. COVID-19-associated acute necrotizing myelitis. Neurol. Neuroimmun. Neuroinflam. 2020;7 doi: 10.1212/NXI.0000000000000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steardo L., Steardo L., Jr., Zorec R., Verkhratsky A. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. 2020:e13473. doi: 10.1111/apha.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X.W., Palka S.V., Rao R.R., Chen F.S., Brackney C.R., Cambi F. SARS-CoV-2-associated Guillain-Barré syndrome with dysautonomia. Muscle Nerve. 2020 doi: 10.1002/mus.26988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T., Guan J. Novel coronavirus and the central nervous system. Eur. J. Neurol. 2020 doi: 10.1111/ene.14227. (In press) [DOI] [PubMed] [Google Scholar]
- Suwanwongse K., Shabarek N. Rhabdomyolysis as a Presentation of 2019 Novel Coronavirus Disease. Cureus. 2020;12 doi: 10.7759/cureus.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur V., Jain A. COVID 2019-suicides: A global psychological pandemic. Brain Behav. Immun. 2020;88:952–953. doi: 10.1016/j.bbi.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tian S., Hu N., Lou J., Chen K., Kang X., Xiang Z. Characteristics of COVID-19 infection in Beijing. J. infect. 2020;80:401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K., Lo A.W. Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS-CoV) and its putative receptor, angiotensin-converting enzyme 2 (ACE2) J. Pathol. 2004;203:740–743. doi: 10.1002/path.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toljan K. Letter to the Editor Regarding the Viewpoint “Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanism”. ACS Chem. Neurosci. 2020;11:1192–1194. doi: 10.1021/acschemneuro.0c00174. [DOI] [PubMed] [Google Scholar]
- Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G. Guillain-Barré Syndrome Associated with SARS-CoV-2. N. Engl. J. Med. 2020;382(26):2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020;87:34–39. doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M. Immunology of COVID-19: Current State of the Science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiuddin H., Skwirsk B., Paz-Arabo P. Acute Transverse Myelitis Associated with SARS-CoV-2: A Case-Report. Brain Behav. Immun. Health. 2020;100091 doi: 10.1016/j.bbih.2020.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayos Galán A., Del Saz Saucedo P., Peinado Postigo F., Botia Paniagua E. Guillain-Barré syndrome associated with SARS-CoV-2 infection. Neurologia (Barcelona, Spain) 2020;35:268–269. doi: 10.1016/j.nrl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violi F., Oliva A., Cangemi R., Ceccarelli G., Pignatelli P., Carnevale R. Nox2 activation in Covid-19. Redox Biol. 2020;101655:36. doi: 10.1016/j.redox.2020.101655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violi F., Pastori D., Cangemi R., Pignatelli P., Loffredo L. Hypercoagulation and Antithrombotic Treatment in Coronavirus 2019: A New Challenge. Thrombosis Hemostasis. 2020;120:949–956. doi: 10.1055/s-0040-1710317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virani A., Rabold E., Hanson T., Haag A., Elrufay R., Cheema T. Guillain-Barré Syndrome associated with SARS-CoV-2 infection. IDCases. 2020:e00771. doi: 10.1016/j.idcr.2020.e00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff L., Kolahgar G., Piddini E. Competitive cell interactions in cancer: a cellular tug of war. Trends Cell Biol. 2013;23:160–167. doi: 10.1016/j.tcb.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Wakerley B.R., Yuki N. Infectious and noninfectious triggers in Guillain-Barré syndrome. Expert. Rev. Clin. Immunol. 2013;9:627–639. doi: 10.1586/1744666X.2013.811119. [DOI] [PubMed] [Google Scholar]
- Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) MedRxiv. 2020 (In press) [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.Y., Li X.L., Yan Z.R., Sun X.P., Han J., Zhang B.W. Potential neurological symptoms of COVID-19. Ther. Adv. Neurol. Disord. 2020;13 doi: 10.1177/1756286420917830. (1756286420917830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Le T.Q., Kurihara N., Chida J., Cisse Y., Yano M. Influenza virus-cytokine-protease cycle in the pathogenesis of vascular hyperpermeability in severe influenza. J. Infect. Dis. 2010;202:991–1001. doi: 10.1086/656044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb E.S., Franklin T., Iwata M., Duman R.S. Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci. 2016;17:497–511. doi: 10.1038/nrn.2016.69. [DOI] [PubMed] [Google Scholar]
- Wong P.F., Craik S., Newman P., Makan A., Srinivasan K., Crawford E. Lessons of the month 1: A case of rhombencephalitis as a rare complication of acute COVID-19 infection. Clin. Med. (Lond) 2020;20(3):293–294. doi: 10.7861/clinmed.2020-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.H., Lui R.N., Sung J.J. Covid-19 and the digestive system. J. Gastroenterol. Hepatol. 2020;35:744–748. doi: 10.1111/jgh.15047. [DOI] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Lam C.S., Lau C.C., Tsang A.K., Lau J.H. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012;86:3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) Novel Coronavirus – China. 2020. https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/;
- Wu G.F., Dandekar A.A., Pewe L., Perlman S. CD4 and CD8 T cells have redundant but not identical roles in virus-induced demyelination. J. Immunol. 2000;165:2278–2286. doi: 10.4049/jimmunol.165.4.2278. [DOI] [PubMed] [Google Scholar]
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K., Peng G., Wilken M., Geraghty R.J., Li F. Mechanisms of host receptor adaptation by severe acute respiratory syndrome coronavirus. J. Biol. Chem. 2012;287:8904–8911. doi: 10.1074/jbc.M111.325803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H., Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J. Neurochem. 2008;107:1482–1494. doi: 10.1111/j.1471-4159.2008.05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H., Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr. Hypertens. Rep. 2010;12:170–175. doi: 10.1007/s11906-010-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W., Mu J., Guo J., Lu L., Liu D., Luo J. New onset neurologic events in people with COVID-19 infection in three regions in China. Neurology. 2020 doi: 10.1212/WNL.0000000000010034. [DOI] [PubMed] [Google Scholar]
- Xu J., Zhong S., Liu J., Li L., Li Y., Wu X. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin. Infect. Dis. 2005;41:1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Han M., Li T., Sun W., Wang D., Fu B. Proceedings of the National Academy of Sciences. 2020. Effective treatment of severe COVID-19 patients with tocilizumab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Yu C., Qu J., Zhang L., Jiang S., Huang D. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur. J. Nucl. Med. Mol. Imaging. 2020;47:1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;m606:368. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y–H—, Dong J‐H, An W‐M, Lv X‐Y, Yin X‐P, Zhang J‐Z. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J. infect. 2020;80:394–400. doi: 10.1016/j.jinf.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N., Shen H.-M. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in covid-19. Int. J. Biol. Sci. 2020;16:1724. doi: 10.7150/ijbs.45498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Cao Q., Qin L., Wang X., Cheng Z., Pan A. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):A multi-center study in Wenzhou city, Zhejiang, China. J. infect. 2020;80:388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Ren Y., Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav. Immun. 2020;88:945–946. doi: 10.1016/j.bbi.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J. infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E.A., Collins A., Cohen M.E., Duffner P.K., Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113:e73–e76. doi: 10.1542/peds.113.1.e73. [DOI] [PubMed] [Google Scholar]
- Yin R., Feng W., Wang T., Chen G., Wu T., Chen D. Concomitant neurological symptoms observed in a patient diagnosed with coronavirus disease 2019. J. Med. Virol. 2020 doi: 10.1002/jmv.25888. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanin L., Saraceno G., Panciani P.P., Renisi G., Signorini L., Migliorati K. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020:1–4. doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020;105954 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- Zhang T., Hirsh E., Zandieh S., Rodricks M.B. COVID-19-Associated Acute Multi-infarct Encephalopathy in an Asymptomatic CADASIL Patient. Neurocrit. Care. 2020 doi: 10.1007/s12028-020-01119-7. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Hu C., Luo L., Fang F., Chen Y., Li J. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J. Clin. Virol. 2020;104364:127. doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Cai H., Hu J., Lian J., Gu J., Zhang S. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int. J. Infect. Dis. 2020;94:81–87. doi: 10.1016/j.ijid.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K., Huang J., Dai D., Feng Y., Liu L., Nie S. Acute myelitis after SARS-CoV-2 infection: a case report. medRxiv. 2020 (In press) [Google Scholar]
- Zhou X., Yao B. Social support and acute stress symptoms (ASSs) during the COVID-19 outbreak: deciphering the roles of psychological needs and sense of control. Eur. J. Psychotraumatol. 2020;11:1779494. doi: 10.1080/20008198.2020.1779494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 2020;181:1016-35.e19. [DOI] [PMC free article] [PubMed]