Abstract
Context and background
Corona virus (COVID) has rapidly gained a foothold and caused a global pandemic. Particularists try their best to tackle this global crisis. New challenges outlined from various medical perspectives may require a novel design solution. Asymptomatic COVID-19 carriers show different health conditions and no symptoms; hence, a differentiation process is required to avert the risk of chronic virus carriers.
Objectives
Laboratory criteria and patient dataset are compulsory in constructing a new framework. Prioritisation is a popular topic and a complex issue for patients with COVID-19, especially for asymptomatic carriers due to multi-laboratory criteria, criterion importance and trade-off amongst these criteria. This study presents new integrated decision-making framework that handles the prioritisation of patients with COVID-19 and can detect the health conditions of asymptomatic carriers.
Methods
The methodology includes four phases. Firstly, eight important laboratory criteria are chosen using two feature selection approaches. Real and simulation datasets from various medical perspectives are integrated to produce a new dataset involving 56 patients with different health conditions and can be used to check asymptomatic cases that can be detected within the prioritisation configuration. The first phase aims to develop a new decision matrix depending on the intersection between ‘multi-laboratory criteria’ and ‘COVID-19 patient list’. In the second phase, entropy is utilised to set the objective weight, and TOPSIS is adapted to prioritise patients in the third phase. Finally, objective validation is performed.
Results
The patients are prioritised based on the selected criteria in descending order of health situation starting from the worst to the best. The proposed framework can discriminate among mild, serious and critical conditions and put patients in a queue while considering asymptomatic carriers. Validation findings revealed that the patients are classified into four equal groups and showed significant differences in their scores, indicating the validity of ranking.
Conclusions
This study implies and discusses the numerous benefits of the suggested framework in detecting/recognising the health condition of patients prior to discharge, supporting the hospitalisation characteristics, managing patient care and optimising clinical prediction rule.
Keywords: COVID-19, Laboratory characteristics, Respiratory, Prioritisation, Multi-criteria decision making, TOPSIS, Entropy
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic represents the biggest global shock in decades affecting all major life aspects [1,2]. Tentatively called as 2019 novel coronavirus (2019-nCoV), the virus has been officially named as Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) by the International Committee of Taxonomy of Viruses (ICTV) [86,87]. COVID-19 is caused by SARS-CoV-2 and has spread worldwide [3]. This illness is highly infectious and was announced by WHO as a global public health emergency [88]. By 24 September 2020, COVID-19 cases have reached over 32,310,000 worldwide with 797,914 deaths. With pursued global efforts, the number of cured patients has increased. However, new problems arise in the follow-up and re-examination of cured patients [4]. A recent retrospective review was conducted on medicine concerns regarding the readmission of patients with COVID-19. Many of them are readmitted because their real-time reverse transcription-polymerase chain reaction (RT-PCR) result of SARS-CoV-2 was positive again after their discharge [4,5]. The number of positive RT-PCR results of SARS-CoV-2 in recovered patients has recently increased [5,6], indicating that the actual virus is still not completely eliminated. With the reduction of drug and treatment course after discharge, the virus widely spreads in the body, resulting in the positive nucleic acid test. These patients are called asymptomatic carriers who could infect other people and become chronic virus carriers [4,7]. Prioritising infected patients and differentiating their critical health conditions while considering asymptomatic carriers are highly beneficial to laboratories and stakeholders and would support hospitalisation concerns in recognising health conditions [8], timely managing patients care [9], optimising clinical prediction rules [10,11], and enhancing decision-making for patients prior to discharge [4,12,13]. Additionally, early recognition and stratification according to priority levels upon admission to the emergency department (ED) are important for the quality and safety of emergency medicine [14,15]. Distinguishing mild from serious health situations for patients with COVID-19 can also provide recommended discharge guidelines for medical sectors [4,16]. During hospitalisation, the clinical data of patients include age, sex, symptoms, comorbidities, laboratory characteristics, treatments and outcomes [17]. However, multivariable adjustment of age and gender did not provide evidence for the association between prioritisation and detection of outcomes and health conditions of COVID-19 [17]. Symptoms and laboratory characteristics are the clinical respiratory signs in detecting COVID-19 infection. The main clinical manifestations (symptoms) collected are fever, cough, sputum, dyspnoea, fatigue, myalgia, anorexia and nausea [4,18,19]. Nevertheless, most chronic virus carriers have normal chest CT with no signs of viral infection and no symptoms; this occurrence produces a new challenge for health sectors [4]. Thus, symptoms are not the main basis for detecting health conditions, especially for asymptomatic carriers [4,19]. The challenges brought by this phenomenon are related to the differentiation among mild and critical patients with COVID-19 via multi-laboratory criteria [7,16,18,20]. Additionally, asymptomatic carriers show slight varying laboratory characteristics; hence, discriminating their health conditions from other infection patients increase the complexity of diagnosis [7]. In some cases, RT-PCR-positive chronic virus carriers tend to have normal health conditions during laboratory examination, and others exhibit abnormality [7].
When dealing with rapidly spreading diseases such as COVID-19, differentiation among chronic patients is a problematic but crucial task. The best prioritisation technique for asymptomatic carriers and the required criteria to achieve this goal must be established [21]. One possible way is to develop a new framework that can handle the progress above. However, the prioritisation of patients with COVID-19 including asymptomatic carriers to overcome these challenges remains a complex decision-making problem [22,23]. Particularly, the laboratory criteria collected from academic literature are numerous and widely vary. One study [19] used six criteria, and another [7] reported eight. One work [4] concluded 18 laboratory criteria that can be utilised to handle our aim. Among these 18 characteristics, the most important ones that can affect the priority process are not determined yet. Suitable approaches to evaluate and identify the importance laboratory criteria should be selected for prioritisation configuration. A clear demonstration of such a problem may cast doubt because the existing published works failed to produce a comprehensive patient dataset considering the laboratory criteria for this subject.
Detecting health conditions by using the most important criteria with respect to the appropriate weight allocated for each criteria is considered a multi-criteria decision matrix that can describe the specific problems of prioritisation [24,25]. Different weights are often given for various criteria which further increase the complexity of the task [26]. According to medical guidelines [4,7], each criterion has its reference range of examination results. The optimal range, in which some criteria are at low reference range and the others are at the high reference range, seriously affect the detection of health conditions [27,28]. This inverse relationship between criteria causes a trade-off. Thus, prioritisation is a complex multi-attribute decision-making problem, in which each patient is considered an alternative for the decision maker. In this circumstance, a new intelligent framework is essentially required to overcome these challenges. This work develops a method that can support precise differentiation and prioritisation methodology to solve these issues. The proposed technique is a result of literature review concentrated on works reporting using such method. Multi-criteria decision-making (MCDM) techniques are further discussed and analysed in the literature review section.
This study presents integration methods for prioritising and detecting the health conditions of patients with COVID-19 based on multi-laboratory criteria while considering asymptomatic carriers. The proposed technique evaluates discrimination procedures against asymptomatic carriers to give a possible recommendation prior to discharge from the hospitals to satisfy the requirements. The contributions of this research to the state-of-the-art are multi-faceted. In the first phase, identification using two feature selection approaches (i.e. data-driven and knowledge-driven) was implemented to select the eight most important laboratory criteria. All laboratory criteria were collected from literature for further analysis and discussion. Two datasets for patients with COVID-19 were then integrated based on the analysis of laboratory characteristics and clinical guiding principles [7,29] to produce a new dataset. Simulation data were generated based on reliable reference ranges and occurrence records validate by experts in the respiratory field with more than 10 experience years to include different health conditions. The outcome of the first phase is to develop a new decision matrix based on a crossover of (i) multi-laboratory characteristics criteria and (ii) lists of infected patients for patient prioritisation throughout the designed methodology. In the second phase, the objective weights of multi-criteria were extracted according to entropy. Third phase adapted TOPSIS for ranking. Finally, the outcome prioritisation results from an objective perspective were validated objectively. The proposed methodology could be set as a standard and guide when changing the used methods or including new criteria to develop another decision matrix. However, within the research context, the framework was presented as distinct in the respiratory field, particularly for COVID-19 to solve estimation issues from medical perspectives.
The rest of this paper is organised as follows. Section 2 describes the literature review with three clusters of research that address the gap analysis for the prioritisation COVID-19-infected patients. Section 3 presents the methodology phases. In Section 4, the results are presented and further discussed with sets of claim points to examine the importance of this study. Finally, Section 5 concludes the paper.
2. Literature review
An extensive literature search was conducted to identify articles that deal with prioritisation issues of infected patients with COVID-19. Clinical studies were reviewed comprehensively, and the need for a prioritisation method based on Artificial Intelligence (AI) was confirmed to provide a clear vision of prioritisation concepts contributing to this virus. Accordingly, MCDM techniques (i.e. TOPSIS and entropy) were reviewed as a recommended solution to solve this complex situation. This map-matching of review was developed to further explore the AI fields and create novel techniques for the prioritisation of coronavirus-infected patients. The analysis of directions is illustrated below.
-
•
Clinical studies of COVID-19 and patients’ prioritisation needs: this study examined issues of clinical studies that need the effects of the prioritisation process. In [30] highlighted the priorities need for the US Health Community to respond to COVID-19 to better understand the burden of COVID-19. To achieve this goal, medical experts need to expand testing to all health conditions of patients who have unexplained ARDS or severe pneumonia, and ultimately to patients who have mild symptoms consistent with COVID-19. The experts also need clear information regarding the operating characteristics of COVID-19 diagnostic tests to make the best clinical and public health decisions for the differentiation approach. In [7] reported “transmission of the COVID-19 from an asymptomatic carrier with normal chest computed tomography (CT) findings. In this study, six patients underwent chest CT imaging, and RT-PCR tests for COVID-19 were performed. The sequence of events suggests that the coronavirus may have been transmitted by the asymptomatic carrier”. The challenge highlighted in this study the patients have different levels of laboratory examination results and asymptomatic carrier conditions has the most optimal health situation among others. However, the priority concept to differentiate asymptomatic conditions among others can provide clear guidance to make decisions about the optimal health conditions for all patients. The research [21] highlighted an important question (from nine questions) about how transmission occurred for COVID-19 through asymptomatic. The study mentioned that the infected patients with mild symptoms are difficult to recognise. The possibility for transmission COVID-19 from asymptomatic to people is confirmed and the health conditions of such cases are varied. However, a method for differentiating such asymptomatic carriers among others can also solve the mentioned challenges. In [4], retrospectively analysed the clinical data and laboratory characteristics of eight readmission patients of positive RT-PCR test and provided reference for the management and follow-up of COVID-19. The phenomenon of positive RT-PCR results in recovered patients had arisen recently especially for asymptomatic carriers. However, the study presented the laboratory characteristics measurements of eight asymptomatic carriers and their health conditions were varied when they return to hospitals after discharge which increase the complexity. Yet, no method can detect their health conditions before and after discharge.
As can be seen from the above studies, the development of a methodology that allows for the accurate prioritise of the patients, considering all health conditions and the importance of laboratory characteristics of COVID-19 is quite and can be overcome by the MCDM framework. More about prioritisation approaches for different COVIVD-19 subjects using MCDM methods are discussed in below.
-
•
MCDM studies for the prioritisation of patients with COVID-19: a framework evaluating the performance of COVID-19 classifiers was proposed [31]. This framework can assist decision-makers in medical organisations to determine the optimal classifier for COVID-19 diagnosis. Another work [32] presented a real-time death assessment monitoring of COVID-19 using TOPSIS MCDM to select most important risk factors and applied GMDH to estimate death value within all confirmed cases. A study [33] investigated some elected activities and evaluated their importance and suggested that many activities should not be performed during the COVID 19 pandemic period. These activities were considered as criteria and then applied to an analytic hierarchy process (AHP) for calculating their weights and assigned ranks/priorities according to their effect. Our previous report [34] presented the prioritisation of patients with COVID-19 based on eight laboratory examinations tests; however, overcoming the mentioned challenges was hindered by the following limitations. (i) The evaluation and selection of important laboratory criteria were not discussed, thus raising questions for some experts. These criteria also have limitations in assessing accurate priority judgments, especially for patients with COVID-19 [4,19]. (ii) The study was unable to satisfy the acceptable sample size of patient dataset due to limited patient data (only six patients). (iii) The obtained results also lack a systematic validation guideline among the prioritisation scores. (iv) The study reported that a comprehensive analysis must be further conducted to overcome the above limitations. For this reason, the prioritisation results require a deepened intersection between importance laboratory criteria and a large patient dataset including additional asymptomatic carrier cases. The obtained results must be validated by defined methods. The description of relevant MCDM studies and the present work in term of aims, method and criteria used for COVID-19 case study are shown in Table 1 .
Table 1.
Description of MCDM studies for COVID-19 prioritisation.
| Ref. | Aim of the study | Method used | Criteria used | Case study |
|---|---|---|---|---|
| [31] | Evaluation and benchmarking methodology to select the best classifiers for COVID-19 diagnosis | Entropy–TOPSIS |
|
Public data sources from hospitals and physicians of chest X-ray and CT images for positive or suspected patients of COVID-19, MERS, SARS, and ARDS. |
| [32] |
|
TOPSIS GMDH (Group Method of Data Handling) | Important risk factors of COVID-19 | Confirmed and death cases collected from website of (WHO) and some Government report between 31-Dec-2019 to 05-Apr-2020. |
| [33] | Remedial activity prioritisation | AHP | Social distancing, Hygiene, Shared individual things, Needless of touch-things, items of daily fresh food, and Immunity/fitness | Guidelines and safety measures from WHO and governing organisations of diverse countries |
| [34] | COVID-19 patient prioritisation dependent on their health conditions | AHP and VIKOR | laboratory examinations (CRP, eosinophil ratios, eosinophils, white blood cell count, lymphocyte ratios, lymphocytes, neutrophil ratios, neutrophils) | Six patients with COVID-19 (included 1 Asymptomatic Carrier with COVID-19) |
| The presented Study | Prioritisation and detection of asymptomatic carriers with COVID-19 |
|
Multi-laboratory Characteristics (White blood cell count, count of Neutrophil, count of Lymphocyte, Haemoglobin, count of Blood platelet, Albumin, C-reactive protein, Interleukin 6) | 56 patients (included 8 asymptomatic carriers with COVID-19) |
Although the case study of [31] is COVID-19, the authors employed Entropy and TOPSIS methods to evaluate the diverse diagnostic models of machine learning where the SVM classifier was nominated as the most suitable diagnosis model for COVID19. Furthermore, the main objective of the study [32] is to identify the important risk aspect and continuous monitoring of death due to COVID-19. Result indicates that “contamination due to contact with the infected person” is the main accountable factor behind the pandemic COVID-19. However, as a conclusion from Table 1, there is no clear study presented a prioritisation method for patients with COVID-19 to overcome the mentioned challenges according to the topic of this study. Therefore, this study will focus on solving this challenge by using the appropriate methods based on decision making technique.
The recommendation solution for our study is to use MCDM that deals with decision problems with respect to decision criteria. MCDM has the potential to contribute to a fair, transparent and rational priority-setting process. More about MCDM studies and the review of needed methods (i.e. TOPSIS and Entropy) utilised with different fields are discussed in the below.
-
•
MCDM studies for prioritisation in other fields: MCDM is commonly adopted in numerous fields for diverse applications. It discoveries and ranks appropriate solutions to choice the appropriate alternative [35,36]. The research in [37] tried to highlight the difficulty of choosing the appropriate mHealth application and formed a model to compare and evaluate the performances of various mHealth applications depending on AHP and fuzzy TOPSIS. In [38], problem of health-care waste disposal were investigated and two models were applied and compared (VIKOR and TOPSIS) to prove their feasibility and effectiveness. Two distances were measured, geodesic distance and the probability distribution distance to determine the consensus degrees. The work in [39] assess the performance of Brazilian emergency clinics’ services TOPSIS and neural networks to inspect relevant factors in the socioeconomic, demographic and institutional areas as indicators of the performance levels accomplished. The outcome supply managerial insights concerning the performance of public hospitals and chances for superior resource apportionment in the healthcare field. The research in [40] assessed healthcare vulnerability utilising subjective and ambiguous environmental data for the health sector using the fuzzy TOPSIS for the impact of climate and air pollution in Korea. The work in [41] developed a classification arrangement that concentrated on practical concerns about TOPSIS that categorised into application areas, publication year, journal name, authors’ nationality, and integrating other MADM/MCDM methods. The authors found that TOPSIS methodology was applied successfully with many application domains. In [42], assessed the development of the best existing software solutions on the medical information systems' market by employing AHP-TOPSIS and reported that the best evaluated software isn't really the best for the usage and development. On the base of the eco‐environment, geographic and socio‐economic factors in Hunan province, the paper in [43] proposed a framework to select a site of inland NPPs of China by adopting many MCDM methods, however, the effectiveness of the TOPSIS was recommended in this study. The research in [44] described Hotel BEER project selection as an MCDM problem and Identified the optimal ranking to decrease energy consumption of building applying TOPSIS-based QUALIFLEX method to rank hotel projects. To prevent waste of research resources in the organisation, the work in [45] identified 191 topics and divided into many fields of healthcare along with planning and information technology using AHP- TOPSIS. The research in [46], proposed MCDM framework (i.e. entropy, AHP, and VIKOR) to aid the chemical manufacturing enterprise to select adequate sustainable supplier. The description of the other MCDM studies in term of aims, method and criteria used for various fields of study are shown in Table 2 .
Table 2.
Description of MCDM studies in prioritising other fields.
| Ref. | Aim of the study | Method used | Criteria used | Case study |
|---|---|---|---|---|
| [37] | Selection and evaluation of mHealth applications | AHP, fuzzy TOPSIS | User satisfaction, quality of information, compatibility, functionality, security, ease of access, responsiveness, and ease of learning and understanding | Numerical case example for different mHealth applications |
| [38] | settlement solution Drawing for an MAGDM problem through HFLTSs | VIKOR, TOPSIS | public approval, waste residuals, released-with-effects of health, reliability, treatment-usefulness, Net-cost-per-tonne | Example rely on the valuation of a health-care waste disposal management system defined in Shanghai, China case study [47] |
| [39] | Experimental guide on hospital services’ performance to estimate performance levels' prediction | TOPSIS, Neural Networks | Inputs – TOPSIS criteria Surgical-beds, beds for clinical, life-support equipment, image diagnosis equipment, No. of nurses, No. of physicians, Total No. of professionals | Public hospitals from 92 municipalities of Rio de Janeiro, for a period from 2008 to 2013 |
| [40] | -Vulnerability assessment of the health effects caused by climate variation -Validity of the vulnerability assessment results and the occurrence rate of cardiovascular patients |
Fuzzy TOPSIS | Meteorological, air pollution, vulnerable group, vulnerable environment, disease distribution, socioeconomic-capacity, health/medical-capacity, air-pollution control | Climate and air pollution data from South Korea in 2010 |
| [41] | Ranking variances between the TOPSIS and other MCDM approaches | TOPSIS | Combined-other-methods, year of publication, publication of journal, nationality of author | 266 papers about TOPSIS applications published since 2000 in 103 journals of scholarly |
| [45] | Research priorities ranking for organization of military health | AHP, TOPSIS | Time priority, accordance with organizational goals, applicability and usefulness | Cross-sectional study done in 2013 (clinics of a military health organization in Iran) |
| [42] | efficiency estimation of the health information systems in health care services and delivery | AHP-TOPSIS |
|
top 20 of the most used software solutions at the beginning of 2016 (from Electronic Health Record) |
| [43] | Selection of Power Plant Site of Inland Nuclear under Z‐numbers | Z -number ‐BWM, Z -number ‐DEMATEL, and Z -number ‐TOPSIS | Eco‐environment, physical geography, socio‐economics | Hunan Province of China |
| [44] | Rank the hotel BEER projects | Fuzzy TOPSIS-based QUALIFLEX method, (LD-PFOWA) | Project management, Project nuancing, EPC- innovation, supportable progress, measurement, verification, preparation of workable development strategy, contracting, external economics | Hotel BEER project selection problem for energy service company |
| [46] | Manage (Sustainable supplier selection) problems | Picture fuzzy exponential entropy, VIKOR | , environmental and social SSS evaluation criteria | Chemical manufacturing enterprise, China |
Nowadays the priority matter is considered very challenges for different kind of medical perspectives [[48], [49], [50]]. Recently, the newest trend regarding to MCDM methods use is to combine two or more methods to recoup the weaknesses in a single method [51]. Entropy and TOPSIS have become a commonly integrated MCDM method [52,51]. For these explanation the utilisation of weights and target information to acquire the relative distances, the ability to offer complete ranking results, trade-off smoothing by managing nonlinear relationships, the straightforwardness at which it tends to be easy programmable method, and the reasonableness to be joined with stochastic analysis. In addition, the measurement test of the COVID-19 dataset reflects a clear requirement for adding an entropy method to the objective function or finding the suitable weights [53]. One MCDM methodology to overcome all above issues to apply and require high-level of stages were patients’ data is also required as presented in next section.
3. Methodology
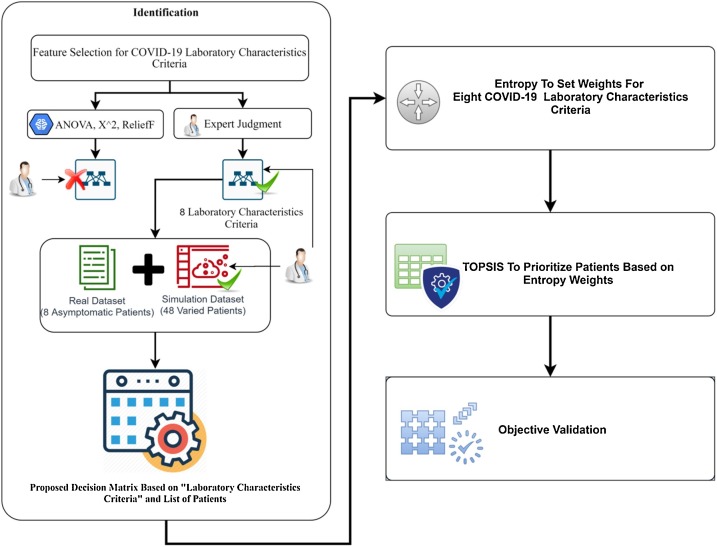
The development methodology for decision-making solution in this study can be divided into three phases. The first phase is identification for the laboratory criteria, the new dataset with diversity emergency levels, and the propose decision matrix for prioritisation process. First phase engagement and outcome are to be utilised for the other phases. The second phase proposes a particular methodology to set objective weights to the multi-laboratory criteria for COVID-19 based on Entropy method. The aim of this phase is to investigate the most effected criteria for this virus. For the third phase, prioritise the all emergency situations of the infected patients with COVID-19 based on integrated Entropy-TOPSIS methods. The aim is to investigate ranking results of patients with COVID-19 and to compare the obtained results with the results presented in previous studies. Finally, the validation of the results objectively is presented in phase four. In this way, transferring the physicians' preferences and experiences to an expert system can be proven to solve the mentioned challenges. The structure of the research methodology phases is shown in Fig. 1 .
Fig. 1.
Methodology phases.
3.1. Phase 1: identification
Three essential stages have been presented in this phase. First, feature selection approaches for COVID-19 laboratory criteria is evaluated and discussed based on real dataset to present the eight important criteria that affect the prioritisation process for patients with COVID-19. Second, the real and simulated dataset for patients with COVID-19 depend on the selected criteria is presented. Third, the examined selected criteria, and patients of COVID-19 from the new dataset (real and simulated) are utilised to propose patients’ prioritisation decision matrix (DM).
3.1.1. Feature selection for COVID-19 laboratory characteristics criteria
In this paper, feature selection is carried out by selecting the critical laboratory criteria that form the root cause for the problem under consideration, which is the prioritisation of patients with COVID-19. The real COVID-19 dataset in the study [4] is used for experimentation. This criterion set includes 18 laboratory characteristics for eight patients. As mentioned previously, the study of [4] was significantly showed the extent of comprehensive coverage in the largest number of laboratory criteria. Two commonly approaches were employed for feature selection that comprise use of automatic feature selection mechanisms (i.e. data-driven) or expert judgment (i.e. knowledge-driven) [54,55]. Mean imputation also was used to replace missing values within the data with the mean value of that feature/variable. Because of contrasts in their implicit processes, the two common feature selection approaches may have their novel predispositions that conceivably lead to dissimilar order viability. The ANOVA, , and ReliefF algorithms [[56], [57], [58], [59], [60]], are used as a base to reduce the number of features (criteria) that are most valuable to a model. The results of the three algorithms are presented in Fig. 2 . Besides, to achieve knowledge-driven approach, specialists in the respiratory field with more than 10 years of experience give a subjective judgment for the importance of the important laboratory criteria. Table 3 concludes the most important eight criteria results for both approaches.
Fig. 2.
The obtained results from three automatic feature selection algorithms.
Table 3.
Criteria selected by three feature selection algorithms and expert judgment.
| Data-Driven Approaches |
Knowledge-Driven Approach |
||||||
|---|---|---|---|---|---|---|---|
| Criteria Selected | Criteria Selected | Criteria Selected | Criteria Selected | ||||
| ANOVA | Albumin | Albumin | Relief | Alanine aminotransferase | Expert Judgment | White blood cell count | |
| Alanine aminotransferase | Aspartate aminotransferase | Total bilirubin | Neutrophil count | ||||
| Aspartate aminotransferase | White blood cell count | Albumin | Lymphocyte count | ||||
| White blood cell count | Creatinine | Aspartate aminotransferase | Haemoglobin | ||||
| Total bilirubin | Interleukin 6 | White blood cell count | Blood platelet count | ||||
| Creatinine | Neutrophil count | Creatinine | Albumin | ||||
| Interleukin 6 | C-reactive protein | Interleukin 6 | C-reactive protein | ||||
| Lactic dehydrogenase | D dimer | Lactic dehydrogenase | Interleukin 6 | ||||
In this study, the effectiveness criteria resulted from the two feature selection approaches were empirically evaluate in Fig. 2 and Table 3. The results of our evaluation suggest that the criteria elected by experts can improve the prioritisation process of the patients, while the subsets of features that selected by an automatic feature selection mechanism cannot predict the majority of the important criteria. This study critically examines the assumptions behind and the main justifications for this result. Firstly, the automatic feature selection mechanism can be defined as the procedure of selecting a certain feature from a massive collection of features residing within the data [61]. However, the experiments for the feature selection algorithms was utilised on a limited number of eight patients with COVID-19′ data related to the study of [4]. Secondly, Fig. 2 shows variances among the features obtained from the three algorithms. Thirdly, the use of traditional algorithms for selecting features is dependent fundamentally on the correlation between features or their bond to the target feature. In our case, all features in principles that have the same importance and do not depend on the correlation of one feature to another or all the features with a specific target. Nevertheless, the medical expert didn’t satisfy the results obtained from feature selection mechanisms and no clear evidence that these criteria would actually affect the emergency health situation of patients with COVID-19. Therefore, adopting the expert opinion in feature selection is considered an optimal choice than using the traditional algorithms. The 8 selected criteria from the expert judgment that presented in Table 3 will be utilised for the next section.
3.1.2. Real and simulation dataset for COVID-19 laboratory characteristics
In this research, we base our study on real and simulated dataset of patients with COVID-19. As mentioned previously, the real data for 8 infected patients are derived from the study of [4]. The rang of age for these eight patients was 26–72 years. There were five females and three males. One patient had hypothyroidism and anther had obsolete pulmonary tuberculosis. The eight patients in this dataset were asymptomatic infection and his RT-PCR of SARS-CoV-2 tested positive. The patients had no symptoms and their chest CT was almost normal with no viral infection signs. The dataset for the COVID-19 in the literature either presented with limited number of patients or lack to use sufficient laboratory criteria that affect the prioritisation process [62,63]. Most of the studies did not include enough laboratory patients’ data. To reduce this gap, we propose simulated dataset to increase the number of patients to 56 based on medical perspective and dependent on reliable laboratory reference rages. Therefore, a specialised expert in the respiratory field with more than ten experience years also gives a subjective judgment and validated the simulated laboratory characteristics measures. The development of consensus among experts to review the new dataset at the same time is challenging, and the process between data generation and data validation can take a long time. Therefore, this study is content with only 48 of patients' data generation. In these contexts, the real and simulated laboratory characteristics dataset is presented Table 4 . The first eight patients are considering the real dataset, while the other measures are the simulated dataset. The reference range for each criterion is considered a guidance medical indicator for the patient’s emergency level and also shown in the same table. Therefore, the simulation dataset adopted based on the mentioned reference ranges to include different emergency levels such as mild, sever, and critical. Asymptomatic carriers infection marked with (*) sign based on derived dataset as mentioned in study of [4]. For more description about the laboratory criteria the reader referred to references [18,[64], [65], [66], [67], [68], [69]].
Table 4.
Real and simulated dataset of multi-laboratory characteristics measurement for patients with COVID-19.
| Multi-Laboratory Characteristics Measurement |
White blood cell count | Neutrophil count | Lymphocyte count | Haemoglobin | Blood platelet count | Albumin | C-reactive protein | Interleukin 6 |
|---|---|---|---|---|---|---|---|---|
| Reference ranges | (3.5–10.5 × 109/L) | (2.0–7.5 × 109 /L) | (1.5–4.5 × 109 /L) | (138–172 g/L) | (150–400 × 109 /L) | (40–55 g/L) | mg/L (<8) | (0–7 pg/mL) |
| Patient-1 * | 5.57 | 2.94 | 2.37 | 134 | 268 | 43.3 | 5 | 1.78 |
| Patient-2 * | 5.69 | 3.16 | 1.61 | 120 | 225 | 46.9 | 2.7 | 2.03 |
| Patient-3 * | 5.83 | 4.18 | 1.19 | 135 | 345 | 46.5 | 0.4 | 1.55 |
| Patient-4 * | 6.5 | 3.82 | 1.99 | 128 | 159 | 38.4 | 3.9 | 3.27 |
| Patient-5 * | 5.53 | 1.94 | 2.82 | 140 | 189 | 41 | 2 | 6.84 |
| Patient-6 * | 5.71 | 59.2 | 1.59 | 152 | 166 | 42.6 | 1.6 | 3.83 |
| Patient-7 * | 5.97 | 4.24 | 1.35 | 107 | 216 | 40.5 | 2.6 | 2.97 |
| Patient-8 * | 4.97 | 2.89 | 1.44 | 149 | 174 | 45.3 | 2.6 | 1.5 |
| Patient-9 | 9.441 | 2.9706 | 2.098 | 137.06 | 175.44 | 54.672 | 5.245 | 3.096 |
| Patient-10 | 4.196 | 6.9314 | 3.147 | 139.73 | 344.688 | 51.456 | 3.147 | 1.032 |
| Patient-11 | 9.441 | 5.9412 | 4.196 | 152.19 | 331.272 | 43.952 | 3.147 | 1.032 |
| Patient-12 | 6.294 | 5.9412 | 4.196 | 127.27 | 293.088 | 51.456 | 6.294 | 5.16 |
| Patient-13 | 10.49 | 6.9314 | 3.147 | 142.4 | 307.536 | 52.528 | 4.196 | 1.032 |
| Patient-14 | 8.392 | 3.9608 | 3.147 | 132.61 | 202.272 | 47.168 | 7.343 | 1.032 |
| Patient-15 | 7.343 | 4.951 | 3.147 | 127.27 | 340.56 | 55.744 | 3.147 | 1.032 |
| Patient-16 | 6.294 | 4.951 | 2.098 | 140.62 | 223.944 | 42.88 | 3.147 | 2.064 |
| Patient-17 | 10.49 | 5.9412 | 2.098 | 134.39 | 211.56 | 58.96 | 6.294 | 2.064 |
| Patient-18 | 9.441 | 6.9314 | 3.147 | 141.51 | 315.792 | 42.88 | 3.147 | 7.224 |
| Patient-19 | 4.196 | 4.951 | 3.147 | 124.6 | 309.6 | 47.168 | 4.196 | 6.192 |
| Patient-20 | 6.294 | 1.9804 | 3.147 | 133.5 | 195.048 | 51.456 | 1.049 | 5.16 |
| Patient-21 | 5.245 | 5.9412 | 3.147 | 141.51 | 214.656 | 53.6 | 2.098 | 4.128 |
| Patient-22 | 6.294 | 4.951 | 4.196 | 127.27 | 177.504 | 55.744 | 2.098 | 2.064 |
| Patient-23 | 4.196 | 6.9314 | 4.196 | 131.72 | 272.448 | 55.744 | 3.147 | 3.096 |
| Patient-24 | 9.441 | 2.9706 | 2.098 | 122.82 | 196.08 | 53.6 | 7.343 | 2.064 |
| Patient-25 | 8.392 | 5.9412 | 2.098 | 146.85 | 373.584 | 55.744 | 6.294 | 5.16 |
| Patient-26 | 4.196 | 1.9804 | 4.196 | 137.95 | 173.376 | 55.744 | 6.294 | 2.064 |
| Patient-27 | 4.196 | 5.9412 | 2.098 | 141.51 | 328.176 | 47.168 | 5.245 | 4.128 |
| Patient-28 | 7.343 | 3.9608 | 3.147 | 124.6 | 392.16 | 52.528 | 1.049 | 3.096 |
| Patient-29 | 9.441 | 6.9314 | 3.147 | 137.95 | 365.328 | 58.96 | 6.294 | 1.032 |
| Patient-30 | 4.196 | 6.9314 | 3.147 | 146.85 | 357.072 | 48.24 | 5.245 | 4.128 |
| Patient-31 | 7.343 | 2.9706 | 3.147 | 131.72 | 184.728 | 47.168 | 6.294 | 1.032 |
| Patient-32 | 8.392 | 5.9412 | 4.196 | 141.51 | 362.232 | 49.312 | 4.196 | 6.192 |
| Patient-33 | 5.245 | 1.9804 | 3.147 | 122.82 | 195.048 | 42.88 | 5.245 | 1.032 |
| Patient-34 | 9.441 | 1.9804 | 2.098 | 123.71 | 293.088 | 48.24 | 4.196 | 1.032 |
| Patient-35 | 6.294 | 3.9608 | 4.196 | 151.3 | 306.504 | 42.88 | 5.245 | 5.16 |
| Patient-36 | 10.49 | 1.9804 | 3.147 | 152.19 | 398.352 | 43.952 | 6.294 | 2.064 |
| Patient-37 | 7.343 | 5.9412 | 4.196 | 144.18 | 376.68 | 42.88 | 3.147 | 2.064 |
| Patient-38 | 7.343 | 6.9314 | 2.098 | 145.07 | 334.368 | 58.96 | 7.343 | 7.224 |
| Patient-39 | 6.294 | 3.9608 | 2.098 | 146.85 | 404.544 | 54.672 | 4.196 | 1.032 |
| Patient-40 | 6.294 | 4.951 | 2.098 | 129.05 | 288.96 | 52.528 | 2.098 | 2.064 |
| Patient-41 | 10.49 | 6.9314 | 3.147 | 126.38 | 367.392 | 49.312 | 5.245 | 7.224 |
| Patient-42 | 4.196 | 5.9412 | 3.147 | 143.29 | 180.6 | 54.672 | 2.098 | 6.192 |
| Patient-43 | 4.196 | 3.9608 | 2.098 | 145.96 | 203.304 | 58.96 | 3.147 | 2.064 |
| Patient-44 | 9.441 | 4.951 | 2.098 | 141.51 | 278.64 | 54.672 | 2.098 | 6.192 |
| Patient-45 | 7.343 | 2.9706 | 2.098 | 123.71 | 299.28 | 48.24 | 7.343 | 3.096 |
| Patient-46 | 10.49 | 5.9412 | 4.196 | 145.07 | 198.144 | 55.744 | 1.049 | 5.16 |
| Patient-47 | 9.441 | 6.9314 | 2.098 | 143.29 | 286.896 | 54.672 | 5.245 | 6.192 |
| Patient-48 | 7.343 | 2.9706 | 4.196 | 150.41 | 312.696 | 58.96 | 3.147 | 1.032 |
| Patient-49 | 8.392 | 6.9314 | 2.098 | 126.38 | 266.256 | 56.816 | 1.049 | 5.16 |
| Patient-50 | 5.245 | 4.951 | 2.098 | 143.29 | 177.504 | 52.528 | 6.294 | 1.032 |
| Patient-51 | 6.294 | 3.9608 | 4.196 | 123.71 | 359.136 | 45.024 | 6.294 | 3.096 |
| Patient-52 | 6.294 | 1.9804 | 2.098 | 149.52 | 261.096 | 51.456 | 6.294 | 7.224 |
| Patient-53 | 5.245 | 5.9412 | 4.196 | 149.52 | 362.232 | 46.096 | 7.343 | 1.032 |
| Patient-54 | 8.392 | 6.9314 | 3.147 | 153.08 | 238.392 | 48.24 | 4.196 | 4.128 |
| Patient-55 | 4.196 | 6.9314 | 2.098 | 136.17 | 327.144 | 47.168 | 5.245 | 4.128 |
| Patient-56 | 8.392 | 3.9608 | 3.147 | 125.49 | 402.48 | 55.744 | 6.294 | 6.192 |
3.1.3. Propose DM
This section presents the proposal of patients’ prioritisation DM. New DM based approach can give guidance on prioritisation for all emergency health conditions. The asymptomatic carriers and other emergency levels such mild, sever, and critical for the infected patients must be evaluated and distinguished based on the selected criteria. In this regard, the DM is revealed in Table 5 .
Table 5.
Prioritisation DM.
| Multi-Laboratory Characteristics Criteria | C1 | C 2 | C 3 | C 4 | C 5 | C 6 | C 7 | C 8 |
|---|---|---|---|---|---|---|---|---|
| Patients | ||||||||
| Patient-1 | C1 / P1 | C2 / P1 | C3/P1 | C4 / P1 | C5 / P1 | C6 / P1 | C7/ P1 | C8 / P1 |
| Patient-2 | C1 / P2 | C2 / P2 | C3/P2 | C4 / P2 | C5 / P2 | C6 / P2 | C7 / P2 | C8 / P2 |
| Patient-3 | C1 / P3 | C2 / P3 | C3/P3 | C4 / P3 | C5 / P3 | C6 / P3 | C7 / P3 | C8 / P3 |
| . | . | . | . | . | . | . | . | . |
| . | . | . | . | . | . | . | . | . |
| Patient-56 | C1 / P56 | C2 / P56 | C3 / P56 | C4 / P56 | C5 / P56 | C6 / P56 | C7 / P56 | C8 / P56 |
“C1= White blood cell count, C2= Neutrophil count, C3= Lymphocyte count, C4= Haemoglobin, C5= Blood platelet count, C6= Albumin, C7= C-reactive protein, C8= Interleukin 6, P = Patient”.
The proposed DM constructed based on intersection between “Multi-Laboratory Characteristics criteria” and “COVID-19 infected patients list”. However, according to the problems of patients with COVID-19′ prioritisation that mentioned in the introduction part, the integration between the two decision-making methods (i.e. Entropy and TOPSIS) considered a substantial approach to decrease the problem complexity.
3.2. Phase 2: setting weights for laboratory characteristics COVID-19 criteria
A detailed description of weighting attribute based on Entropy is presented in the steps below. In general, evaluation criteria can be categorised into two types: benefit criteria and cost criteria. Benefit criterion means that a bigger value is more valuable whereas cost criteria are just the opposite. From a medical point of view consideration, all criteria of laboratory characteristics are considered benefit criteria except (C7=C-reactive protein) which considered a cost criterion. “The data in have different dimensions, thus it needs to be normalised when needed within Entropy method in order to transform various criterion dimensions into the non-dimensional criterion, which allows comparison across the criteria” [70]. The problem of determination of evaluation criteria weights objectively can be solved by Entropy method. In MCDM problems “one of the toughest jobs is to assign weights accurately to the different criteria with respect to which the alternatives are to be ranked” [71,72]. According to [73]“The Entropy method is a generic form of Monte Carlo simulation which is applied in complicated estimation and optimisation problems for minimising the error”. Besides, TOPSIS is used in the next phase for complete the prioritisation process based on the constructed weight from Entropy method. For this, the flowchart of integrated Entropy-TOPIP methods is illustrated in Fig. 3 . Furthermore, Entropy method steps' details are explained in below:
Fig. 3.
Flowchart of integrated Entropy-TOPSIS methods.
STEP 1: Establish a Matrix between Criteria and Alternatives
The system can be viewed as MCDM with (m) and (n) that represent alternatives and criteria respectively. This can be represents in matrix using Eq. (1) [74]:
The system can be viewed as MCDM with (m) and (n) that represent alternatives and criteria respectively. This can be represents in matrix using Eq. (1) [74]:
| (1) |
Where “A1, A2, Am” represent alternatives “C1, C2, ...,Cn” are the evaluation criteria and the performance evaluation of alternatives Ai under Cn criterion is represented by xij, and wn represents the weight of the criterion Cn that fulfilling weights' summation equal one.
STEP 2: Normalised DM for each Criterion
There is a need to normalise the data in DM to transform different criterion dimensions into the non-dimensional criterion; this allows comparison across the criteria. Normalised DM is computed by Eq. (2) [75]:
| (2) |
STEP 3: Determinations of Evaluation Criteria Weights
The entropy value ej can be measured for each criterion using Eq. (3) [76]:
| (3) |
STEP 4: Calculating the Diversity Degree
The degree of information diversity implicated by every criterion should be computed in this step using Eq. (4) [76]:
| (4) |
STEP 5: Calculate Weights
For each criterion weights is given by Eq. (5) [76]:
| (5) |
3.3. Phase 3: prioritisation COVID-19 patients
TOPSIS is used in the prioritisation process due to it is suitability for dealing with several attributes and to identify the most suitable alternatives (Asymptomatic carriers). The available alternatives (infected patients) are scored in descending order, and the most urgent patients are ranked depend on TOPSIS. The aggregate score provides an idea on which patients should be given more urgent attention than others. As with other ranking options, relying on people to rank the most urgent case is always possible. TOPSIS assigns the rank to each patient depend on geometric distance from negative and positive ideal solutions. High-emergency patients would have “the shortest geometric distance to the ideal positive solution and the longest geometric distance to the ideal negative solution according to this technique (i.e. highest value amongst others)” [77]. The steps of TOPSIS [77] are illustrated as follows:
Step 1: Construct the normalised decision matrix
This process attempts to transform the dimensions of various attributes (vital features) into non-dimensional attributes and perform a comparison. The matrix()_(m*n) is normalised from()_(m*n) to the matrix R=()_(m*n)via the normalisation method as shown in Eq. (6)”:
| (6) |
This step will produce a new matrix R, where R is as follows:
| (7) |
Step 2: Construct the weighted (scoring points) and normalised decision matrix
In this process, “the weights for each attribute are calculated according to the Entropy method, a set of weights from the decision-maker is accommodated to the normalised decision matrix; the resulting matrix can be calculated by multiplying each column of the normalised decision matrix R with its associated weight ” . The set of the weights is equal to1, as illustrated in Eq. (8).
| (8) |
This step will produce a new matrix V, where V is as follows:
| (9) |
Step 3: Locate the ideal and negative ideal solutions
In this process, the two alternatives, namely, “A* (ideal alternative) and A- (negative ideal alternative)”, are determined by the Eq.s(10) and(11), respectively:
| (10) |
| (11) |
where" is “a subset of , which presents the benefit attribute (i.e. offering an increasing utility with its higher values), and is the complement set of , the opposite could also be added for the cost attribute as denoted by ” .
Step 4: Compute the separation measurement depend on the Euclidean distance.
A separation measurement is performed by calculating the distance between each alternative in V and the ideal vector A* with the Euclidean distance which is assumed by Eq.(12) as follows:
| (12) |
For each alternative in ‘V from the negative ideal A-’, the separation measurement is given by Eq. (13) as follows:
| (13) |
At the end of step 4, the values S_(i*) and S_(i-) for each alternative were calculated. The two values characterise the distance between each alternative and both the ideal and negative ideal.
Step 5: Compute the closeness to the ideal solution.
The closeness of A_i to the ideal solution A* is defined as described in Eq. (14):
| (14) |
“C_ (i*) = 1, if and only if (A_i = A*). Similarly, C(i*) = 0, if and only if (A_i = A-).”.
Step 6: Optimise patients according to their closeness to the ideal solution.
“The set of patients〖 A〗_i can now be optimised in the descending order of 〖C〗_ (i*); the highest value indicates the optimal performance”.
3.4. Phase 4: objective validation
The results are validated by employing the objective validation in accordance with previously described methods presented in [78,79]. The following steps were conducted to ensure that the results were statistically ranked:
-
1
Final prioritisation results are categorised into four equal groups. Each group contains on fourteen patients. However, the number of the alternative number within each group does not affect the validation result [80,81].
-
2
The mean ± standard deviation (M ± STD) of each group is obtained on the basis of the normalisation of the patient datasets. The 1st group is statistically proven to be the highest amongst all other groups. The 2nd group should prove to be lower than or equal to those of the 1st group. The 3rd group must be lower than those of the 1st and 2nd groups or equal to those of the 2nd group. The 4th group should be lower than those of the 1st, 2nd and 3rd groups or equal to those of the 3rd group [82].
Eq. (15) indicates the mean () that represents the average as the sum of all the observed results from the sample divided by the total number (n):
| (15) |
Eq. (16) presents the measurement of the standard deviation to quantify the variation amount or dispersion of a set of data values.
| (16) |
4. Results and discussion
This section outlines the results of the prioritisation framework. First of all, the results of objective criteria weights of the eight multi-laboratory characteristics based on the Entropy method is presented. Then after, TOPSIS are adopted to provide the final rank for patients considering the weights of multi-criteria for laboratory characteristics. Furthermore, the results of the objective validation are presented. Finally, the discussion of the results based on the integrated AHP-TOPSIS methods is presented. The sequences of results described as follows.
4.1. Weighting result of laboratory characteristics based on Entropy
After applied all steps illustrated in Section “Entropy for Setting Objective Weights”, the sequence results and the final laboratory characteristics weights are listed in Table 6 . The weight chart is presented in Fig. 4 .
Table 6.
Multi-laboratory criteria and weights according to entropy.
| Multi-laboratory Criteria | C1 | C 2 | C 3 | C 4 | C 5 | C 6 | C 7 | C 8 |
|---|---|---|---|---|---|---|---|---|
| Entropy | 0.00117 | 0.00086 | 0.00099 | 0.00131 | 0.00110 | 0.00129 | 0.00143 | 0.00043 |
| Degree of Diversity | 0.99883 | 0.99914 | 0.99901 | 0.99869 | 0.99890 | 0.99871 | 0.99857 | 0.99957 |
| Final Weights | 0.12499 | 0.12503 | 0.12501 | 0.12497 | 0.12500 | 0.12497 | 0.12496 | 0.12508 |
Fig. 4.
Multi-laboratory characteristics weights chart based on Entropy.
4.2. TOPSIS prioritisation results
In this stage, TOPSIS is used to prioritisation patients with COVID-19 and can characterise the most appropriate option. In addition, the Entropy method will derive the overall weights. The set of patients are ranked in descending order starting from the critical health condition ending with the mild condition. The prioritisation process for the 56 patients reported in this section. The final results of integrated Entropy-TOPSIS are illustrated in Table 7 based on objective weight by Entropy method. The first eight patients are the asymptomatic carriers and marked with (*) to be further discussed.
Table 7.
Patients Prioritisation results based on integrated Entropy-TOPSIS.
| Patients | C_(i*) Final Score |
Prioritisation order | Patients | C_(i*) Final Score |
Prioritisation orders |
|---|---|---|---|---|---|
| Patient.1* | 0.11576353 | 55 | Patient.29 | 0.18974366 | 35 |
| Patient.2* | 0.1566184 | 47 | Patient.30 | 0.18813267 | 37 |
| Patient.3* | 0.22474058 | 19 | Patient.31 | 0.21778208 | 22 |
| Patient.4* | 0.14284989 | 52 | Patient.32 | 0.11366058 | 56 |
| Patient.5* | 0.22652304 | 17 | Patient.33 | 0.19366277 | 33 |
| Patient.6* | 0.80139593 | 1 | Patient.34 | 0.24772896 | 10 |
| Patient.7* | 0.15106112 | 48 | Patient.35 | 0.20135777 | 31 |
| Patient-8* | 0.1316362 | 53 | Patient.36 | 0.18079573 | 42 |
| Patient.9 | 0.17348503 | 44 | Patient.37 | 0.18082765 | 40 |
| Patient.10 | 0.25244966 | 9 | Patient.38 | 0.17047042 | 46 |
| Patient.11 | 0.18080876 | 41 | Patient.39 | 0.22404457 | 20 |
| Patient.12 | 0.25842548 | 5 | Patient.40 | 0.21198729 | 24 |
| Patient.13 | 0.24421914 | 12 | Patient.41 | 0.24169691 | 13 |
| Patient.14 | 0.23267098 | 16 | Patient.42 | 0.14509157 | 50 |
| Patient.15 | 0.25791067 | 6 | Patient.43 | 0.20241879 | 30 |
| Patient.16 | 0.18318402 | 38 | Patient.44 | 0.25718751 | 7 |
| Patient.17 | 0.18164462 | 39 | Patient.45 | 0.20116892 | 32 |
| Patient.18 | 0.14366012 | 51 | Patient.46 | 0.22327042 | 21 |
| Patient.19 | 0.19245807 | 34 | Patient.47 | 0.27143174 | 2 |
| Patient.20 | 0.20735241 | 28 | Patient.48 | 0.21150921 | 25 |
| Patient.21 | 0.27041505 | 3 | Patient.49 | 0.18849281 | 36 |
| Patient.22 | 0.20981639 | 26 | Patient.50 | 0.21729405 | 23 |
| Patient.23 | 0.17246897 | 45 | Patient.51 | 0.20559879 | 29 |
| Patient.24 | 0.23783985 | 15 | Patient.52 | 0.20858765 | 27 |
| Patient.25 | 0.25383109 | 8 | Patient.53 | 0.22601953 | 18 |
| Patient.26 | 0.14894843 | 49 | Patient.54 | 0.25965293 | 4 |
| Patient.27 | 0.17511999 | 43 | Patient.55 | 0.24675968 | 11 |
| Patient.28 | 0.23956294 | 14 | Patient.56 | 0.12628451 | 54 |
As shown in the Table 7, the patients’ final score are assigned for each according to TOPSIS method. The highest score indicates that the patient in worst health condition. On the contrary, the lowest score indicated that this patient is the best health condition among others. The patients gain their orders based on descending phenomena [77]. Further, the results will be discussed in terms of claim points in section (4.5).
4.3. Validation results
In this section, and as explained in phase 4, the objective validation can be constructed by dividing the prioritisation results into four equal groups. Each group comprised 14 patients. Mean ± STD is calculated for each group based on the normalisation scores generated by the TOPSIS process to ensure that the prioritised patients undergo systematic ranking. The prioritisation results presented in Table 7 above are visualised in graphical formats (Fig. 5 ) after categorised into four groups based on descending patients' scores to further discuss their comparisons.
Fig. 5.
Results of four groups of patients. (A)1stgroup.(B) 2ndgroup.(C) 3rdgroup.(D)4thgroup.
Initial observation of the ranking results of the four patient groups show that the patient groups are systematically distributed as the ranking results of the 2nd group start from the end of the ranking results of the 1st group and so on for other groups. As shown in Fig. 6 , the statistical analysis was performed among the groups, and Eq.s (15) and (16) are applied to obtain the M ± STD. In the 1st group, the value is M = 0.20 ± 0.017. The 1st group was the highest-scoring among the four groups. The 2nd and 3rd groups had the same values of M = 0.018 ± 0.003 and had lower scores than the 1st group but higher scores than the 4th group. The 4th group had a value of M = 0.016 ± 0.002 and considered the lowest scores among the four groups. Thus, the statistical results indicate that the ranking results underwent systematic ranking.
Fig. 6.
Bar Charts for Mean Results for Four Groups of Patients.
4.4. Discussion and claim points
From the medical perspective, the selected laboratory criteria have been commonly circulated in diagnostic virology and have generated few false-positive results [83]. The chance to recognise the health situation for infected patients with COVID-19 dependent on included eight criteria are considered highly significant [83,7]. The criteria weights are constructed by utilising real patients’ data by Entropy for the first time in this study as shown in Table 6. To acquire using the aim higher process to implement a prioritisation of patients in COVID-19, we present a new simulation dataset by an expert based on a standard medical reference ranges as shown in Table 4. The generation process of such patients’ laboratory data included several runs intended to surpass limitations founded in the literature about the limited data samples of patients with COVID-19. At this point, the reporting results of the patients’ prioritisation can meet our research question by the TOPSIS method especially for the cases of patients (1) to (8) (Asymptomatic Carriers) which gain different orders as shown in Table 7. However, the short claim points below may verify the significance and advantages of the prioritisation technique and possibly affect the decision satisfaction of clinical staff about asymptomatic carriers in real life.
-
•
Detection based prioritisation for asymptomatic carriers: according to the medical analysis reported in [4], the eight patients had no symptoms and most of their laboratory measures had normal and others were slightly high therefore they represent a new challenge to hospitals and medical staff as mentioned [4]. Besides, similar problems also reported in numerous studies as in [19,7]. The presented methodology carried out the prioritisation of patients with different health conditions and can identify a score for each case. In these contexts, the detection and differentiation process for such asymptomatic carriers among other infected patients is accomplished.
-
•
Estimation of health conditions management using MCDM: on one hand, TOPSIS is prioritising patients in descending order, the maximum C_(i*) value will be the most critical health condition among others and vice versa. On the other hand, Patient 6 is one of the asymptomatic carriers of COVID-19 and gains order 1 with a highest score value (0.80139593) and this gives conclusive evidence that he has the worst healthy situation. After analysing his laboratory characteristics data by medical expert, we noticed that his measure in C2 is (59.2), while the reference rage of such criteria is (2.0–7.5 × 109 /L) which considered a very critical situation that put him in the first prioritisation order. For this reason, the reported mild classification for asymptomatic carriers has been refuted by our results. After reviewed the historical medication of several asymptomatic carriers in published articles, they were returned to the hospital after they discharge because of their deteriorating health. On the contrary, Patients 2, 7, 4, and 8 gain the minimum C_ (i*) value and come within last orders because of their laboratory characteristics data were normal. Furthermore, the experts give their subjective judgment for the overall ranking results especially for the first ten and last ten patients’ orders and satisfy the health conditions based on ranks. For this, a patient management path must incorporate the presented framework to consider the above significant implications of our prioritisation results.
-
•
Effects of the selected Laboratory characteristics for hospitalisation: the recovery of COVID-19 might take a long period of time; the course of some patients had persisted more than 90 days from the onset of the first symptom. According to our results, the detection of the health condition justifies the importance of handling laboratory characteristics measurements more than symptoms for hospitalisation characteristics. The presented framework can support the specialists in this filed after create a repository to include the historical patients’ data of the 8 laboratory criteria, then, the emergency level for each patient condition can be detected and recognised.
-
•
Support Clinical rule towards patients’ discharge: Although the RT-PCR test is highly recommended to detect the infected patients towards discharge decision from the hospital, a high proportion of false-negative of RT-PCR test results might be occurred due to several reasons such as the source/method of samples collected, sample transportation, test operation…etc. [84,85]. Thus, the discharge of patients cannot be considered only by RT-PCR positive tests. In this context, the functionality of the presented DM is integrated the laboratory measurements for reporting the patient health condition. So, the discharge decision must incorporate the prioritisation orders where the health condition could be recognised by our new technique.
Our proposed methodology can differentiate mild, serious or critical condition of infected patients and place them in a queue based on integrated decision-making. The increasing number of infected patients with this virus confuses the global medical policy on how to detect and prioritise all health conditions. The integrated entropy–TOPSIS can be effectively utilised to overcome these challenges. Specialists and interested parties in this field can benefit from the proposed method. Including additional criteria or changing the weights by using another MCDM method can be accomplished by following the conceptual framework.
5. Conclusion
This paper presents a new framework that addresses the challenges of prioritisation for COVID-19-infected patients based on multi-laboratory criteria based on integrated MCDM. The most effective criteria for such domain are investigated for the first time by using two approaches, and the weight for each criterion is assigned by objective MCDM (i.e. entropy). Patient prioritisation is achieved using a new dataset (real and simulation) established with TOPSIS, and the obtained results undergo systematic ranking through defined objective validation. The proposed methodology has the resilience to adaptive or extended/additional criteria and even changes the subjective judgment from the medical experts by following the same phases described in this study. The claim points of the results are discussed to transfer knowledge between multi-dimension domains (i.e. artificial intelligence and medical). This study has the following limitations. The data of asymptomatic carriers are limited to eight patients, and the simulation patient’s data are only from 48 patients due to consensus challenges among experts. In future works, the dataset sample could be increased to show the effectiveness of the priority process in deep analysis. Other MCDM methods for setting the weights and ranking patients can also be employed to establish a conclusion and construct a COVID-19 triage method. In these contexts, the machine learning algorithm can be utilised as a supervised learning method to predict the severity of COVID-19 infections depending on laboratory data and rapid diagnosis for the selection of appropriate treatment protocols. The most preferable algorithm with high accuracy should be selected.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants of the study.
Declaration of Competing Interest
The authors report no declarations of interest.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.artmed.2020.101983.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Richardson S., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA - J Am Med Assoc. 2020:E1–E8. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monshi M.M.A., Poon J., Chung V. Deep learning in generating radiology reports: a survey. Artif. Intell. Med. 2020;106 doi: 10.1016/j.artmed.2020.101878. p. 101878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao H., Ruan L., Liu J., Liao W. The clinical characteristic of eight patients of COVID-19 with positive RT-PCR test after discharge. J. Med. Virol. 2020 doi: 10.1002/jmv.26017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.feng Zhang J., Yan K., hua Ye H., Lin J., jun Zheng J., Cai T. SARS-CoV-2 turned positive in a discharged patient with COVID-19 arouses concern regarding the present standards for discharge. Int. J. Infect. Dis. 2020;97:212–214. doi: 10.1016/j.ijid.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lan L., et al. Positive RT-PCR test results in patients recovered from COVID-19. JAMA - J Am Med Assoc. 2020;323(15):1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai Y., et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA - J Am Med Assoc. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Killerby M.E., et al. Characteristics associated with hospitalisation among patients with COVID-19 — metropolitan Atlanta, Georgia, March–april 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):790–794. doi: 10.15585/mmwr.mm6925e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook T.M., El-Boghdadly K., McGuire B., McNarry A.F., Patel A., Higgs A. Consensus guidelines for managing the airway in patients with COVID-19: guidelines from the difficult airway society, the association of anaesthetists the intensive care society, the faculty of intensive care medicine and the royal college of anaesthetist. Anaesthesia. 2020;75(6):785–799. doi: 10.1111/anae.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Qaness M.A.A., Ewees A.A., Fan H., El Aziz M.A. Optimization method for forecasting confirmed cases of COVID-19 in China. Appl. Sci. (Basel) 2020;9(3):674. doi: 10.3390/JCM9030674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christenson J., et al. A clinical prediction rule for early discharge of patients with chest pain. Ann Emerg Med. 2006;47(1):1–10. doi: 10.1016/j.annemergmed.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Petrilli C.M., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christenson J., et al. Early discharge of patients with presumed opioid overdose: development of a clinical prediction rule. Acad. Emerg. Med. 2000;7(10):1110–1118. doi: 10.1111/j.1553-2712.2000.tb01260.x. [DOI] [PubMed] [Google Scholar]
- 14.Acampora G., Cook D.J., Rashidi P., Vasilakos A.V. A survey on ambient intelligence in healthcare. Proc. IEEE. 2013;101(12):2470–2494. doi: 10.1109/JPROC.2013.2262913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocha A., et al. Innovations in health care services: the CAALYX system. Int. J. Media Inf. Lit. 2013;82(11):e307–20. doi: 10.1016/j.ijmedinf.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Ai T., et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. p. 200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng Y., et al. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog. 2020;16(4) doi: 10.1371/journal.ppat.1008520. p. e1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen C., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA - J Am Med Assoc. 2020 doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parsons L.M., et al. Laboratory diagnosis of tuberculosis in resource-poor Countries: challenges and opportunities. Clin Microbiol. Rev. 2011;24(2):314–350. doi: 10.1128/CMR.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuen K.S., Ye Z.W., Fung S.Y., Chan C.P., Jin D.Y. SARS-CoV-2 and COVID-19: the most important research questions. Cell. Biosci. 2020;10(1) doi: 10.1186/s13578-020-00404-4. p. 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akdag H., Kalaycı T., Karagöz S., Zülfikar H., Giz D., Akdag D., et al. The evaluation of hospital service quality by fuzzy MCDM, Applied Soft computing. Appl. Soft .Comput. 2014;23:239–248. [Google Scholar]
- 23.Khan A.M.R., Prasad P.N., Rajamanoharane S. A decision-making framework for service quality measurements in hospitals. Int. J. Enterp. Netw. Manag. 2010;4(1):80. doi: 10.1504/IJENM.2010.034478. [DOI] [Google Scholar]
- 24.Kovalchuk S.V., Krotov E., Smirnov P.A., Nasonov D.A., Yakovlev A.N. Distributed data-driven platform for urgent decision making in cardiological ambulance control. Future Gener. Comput. Syst. 2018;79:144–154. doi: 10.1016/j.future.2016.09.017. [DOI] [Google Scholar]
- 25.Chen H., Qin R. Decision making in service industries: a practical approach. CRC Press; 2012. Revenue management of transportation infrastructure during the service life using real options; pp. 257–278. [Google Scholar]
- 26.Yas Q.M., Zaidan A.A., Zaidan B.B., Rahmatullah B., Abdul Karim H. Comprehensive insights into evaluation and benchmarking of real-time skin detectors: review, open issues & challenges, and recommended solutions. Meas. J. Int. Meas. Confed. 2018;114:243–260. doi: 10.1016/j.measurement.2017.09.027. [DOI] [Google Scholar]
- 27.Wei J., et al. 2019 novel coronavirus (Covid-19) pneumonia: serial computed tomography findings. Korean J. Radiol. 2020;21(4):494–497. doi: 10.3348/kjr.2020.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu J., et al. Clinical characteristics of imported cases of COVID-19 in Jiangsu Province: a multicenter descriptive study. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization . 2020. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. [Google Scholar]
- 30.Adalja A.A., Toner E., Inglesby T.V. Priorities for the US health community responding to COVID-19. JAMA - J Am Med. Assoc. 2020;323(14):1343–1344. doi: 10.1001/jama.2020.3413. [DOI] [PubMed] [Google Scholar]
- 31.Mohammed M.A., et al. Benchmarking methodology for selection of optimal COVID-19 diagnostic model based on entropy and TOPSIS methods. IEEE Access. 2020;8:99115–99131. doi: 10.1109/ACCESS.2020.2995597. [DOI] [Google Scholar]
- 32.Majumder P., Biswas P., Majumder S. Application of new TOPSIS approach to identify the most significant risk factor and continuous monitoring of death of COVID-19. Electron J. Gen. Med. 2020;17(6) em234.” 2020. [Google Scholar]
- 33.Singh R., Avikal S. COVID-19: a decision-making approach for prioritisation of preventive activities. Int. J. Healthc. Manag. 2020;13(3):257–262. doi: 10.1080/20479700.2020.1782661. [DOI] [Google Scholar]
- 34.Albahri A.S., et al. Multi-biological laboratory examination framework for the prioritisation of patients with COVID-19 based on integrated AHP and group VIKOR methods. Int. J. Inf. Technol .Decis. Mak. 2020 [Google Scholar]
- 35.Campos M., et al. A methodology based on multiple criteria decision analysis for combining antibiotics in empirical therapy. Artif. Intell. Med. 2020;102 doi: 10.1016/j.artmed.2019.101751. p. 101751. [DOI] [PubMed] [Google Scholar]
- 36.Qu Y., Yue G., Shang C., Yang L., Zwiggelaar R., Shen Q. Multi-criterion mammographic risk analysis supported with multi-label fuzzy-rough feature selection. Artif. Intell. Med. 2019;100 doi: 10.1016/j.artmed.2019.101722. p. 101722. [DOI] [PubMed] [Google Scholar]
- 37.Rajak M., Shaw K. Evaluation and selection of mobile health (mHealth) applications using AHP and fuzzy TOPSIS. Technol Soc. 2019;59 doi: 10.1016/j.techsoc.2019.101186. p. 101186. [DOI] [Google Scholar]
- 38.Wu Z., Xu J., Jiang X., Zhong L. Two MAGDM models based on hesitant fuzzy linguistic term sets with possibility distributions: VIKOR and TOPSIS. Inf. Sci. (Ny) 2019;473:101–120. doi: 10.1016/j.ins.2018.09.038. [DOI] [Google Scholar]
- 39.Araujo C.A.S., Wanke P., Siqueira M.M. A performance analysis of Brazilian public health: TOPSIS and neural networks application. Int .J. Product Perform Manag. 2018;67(9):1526–1549. doi: 10.1108/IJPPM-11-2017-0319. [DOI] [Google Scholar]
- 40.Bae H.J., Kang J.E., Lim Y.R. Assessing the health vulnerability caused by climate and air pollution in Korea using the fuzzy TOPSIS. Sustain. 2019;11(10) doi: 10.3390/su11102894. p. 2894. [DOI] [Google Scholar]
- 41.Behzadian M., Khanmohammadi Otaghsara S., Yazdani M., Ignatius J. A state-of the-art survey of TOPSIS applications. Expert Syst. Appl. 2012;39(17):13051–13069. doi: 10.1016/j.eswa.2012.05.056. [DOI] [Google Scholar]
- 42.Rađenović Ž., Veselinović I. Integrated AHP-TOPSIS method for the assessment of health management information systems efficiency. Econ. Themes. 2017;55(1):121–142. doi: 10.1515/ethemes-2017-0008. [DOI] [Google Scholar]
- 43.Heng-ming P., Xiao-kang W., Tie-li W., Ya-hua L., Jian-qiang W. A multi-criteria decision support framework for inland nuclear power plant site selection under Z-Information: A case study in hunan province of China. Mathematics. 2020;8(2) doi: 10.3390/math8020252. p. 252. [DOI] [Google Scholar]
- 44.Wang L., Zhang H.-Y., Wang J.-Q., Wu G.-F. Picture fuzzy multi-criteria group decision-making method to hotel building energy efficiency retrofit project selection. RAIRO - Oper Res. 2020;54(1):211–229. doi: 10.1051/ro/2019004. [DOI] [Google Scholar]
- 45.Bahadori M., Izadi M., Karamali M., Teymourzadeh E., Yaghoubi M. Research priorities in a military health organization using multi criteria decision making techniques. J. Mil. Med. 2014;16(1):37–44. [Google Scholar]
- 46.Peng J., Tian C., Zhang W., Zhang S., Wang J. An integrated multi-criteria decision-making framework for sustainable supplier selection under picture fuzzy environment. Technol. Econ. Dev. Econ. 2020;26(3):573–598. doi: 10.3846/tede.2020.12110. [DOI] [Google Scholar]
- 47.Liu H.-C., Wu J., Li P. Assessment of health-care waste disposal methods using a VIKOR-based fuzzy multi-criteria decision making method. Waste Manag. 2013;33(12):2744–2751. doi: 10.1016/j.wasman.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Tinetti M., et al. Challenges and strategies in patients’ health priorities-aligned decision-making for older adults with multiple chronic conditions. PLoS One. 2019;14(6) doi: 10.1371/journal.pone.0218249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martínez V., Navarro C., Cano C., Fajardo W., Blanco A. DrugNet: network-based drug-disease prioritisation by integrating heterogeneous data. Artif. Intell. Med. 2015;63(1):41–49. doi: 10.1016/j.artmed.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Miao F., et al. Continuous blood pressure measurement from one-channel electrocardiogram signal using deep-learning techniques. Artif. Intell. Med. 2020;108 doi: 10.1016/j.artmed.2020.101919. p. 101919. [DOI] [PubMed] [Google Scholar]
- 51.Alao M.A., Ayodele T.R., Ogunjuyigbe A.S.O., Popoola O.M. Multi-criteria decision based waste to energy technology selection using entropy-weighted TOPSIS technique: the case study of Lagos, Nigeria. Energy. 2020;201 doi: 10.1016/j.energy.2020.117675. p. 117675. [DOI] [Google Scholar]
- 52.Gao X., Yan X., Gao P., Gao X., Zhang S. Automatic detection of epileptic seizure based on approximate entropy, recurrence quantification analysis and convolutional neural networks. Artif Intell Med. 2020;102 doi: 10.1016/j.artmed.2019.101711. p. 101711. [DOI] [PubMed] [Google Scholar]
- 53.Jalal A., Khalid N., Kim K. Automatic recognition of human interaction via hybrid descriptors and maximum entropy markov model using depth sensors. Entropy. 2020;22(8) doi: 10.3390/E22080817. p. 817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng T.H., Wei C.P., Tseng V.S. Vol. 2006. 2006. Feature selection for medical data mining: comparisons of expert judgment and automatic approaches; pp. 165–170. (Proceedings - IEEE Symposium on Computer-Based Medical Systems). [DOI] [Google Scholar]
- 55.Iglesias N., Juarez J.M., Campos M. Comprehensive analysis of rule formalisms to represent clinical guidelines: selection criteria and case study on antibiotic clinical guidelines. Artif. Intell. Med. 2020;103 doi: 10.1016/j.artmed.2019.101741. p. 101741. [DOI] [PubMed] [Google Scholar]
- 56.Feng W., Dauphin G., Huang W., Quan Y., Liao W. New margin-based subsampling iterative technique in modified random forests for classification. Knowledge-Based Syst. 2019;182 doi: 10.1016/j.knosys.2019.07.016. p. 104845. [DOI] [Google Scholar]
- 57.Zamani Esfahlani F., Visser K., Strauss G.P., Sayama H. A network-based classification framework for predicting treatment response of schizophrenia patients. Expert. Syst. Appl. 2018;109:152–161. doi: 10.1016/j.eswa.2018.05.005. [DOI] [Google Scholar]
- 58.Zhang J., Chen M., Zhao S., Hu S., Shi Z., Cao Y. ReliefF-based EEG sensor selection methods for emotion recognition. Sensors. 2016;16(10) doi: 10.3390/s16101558. p. 1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reyes O., Pérez E., Luque R.M., Castaño J., Ventura S. A supervised machine learning-based methodology for analyzing dysregulation in splicing machinery: an application in cancer diagnosis. Artif Intell Med. 2020;108 doi: 10.1016/j.artmed.2020.101950. p. 101950. [DOI] [PubMed] [Google Scholar]
- 60.Alberdi A., Aztiria A., Basarab A. On the early diagnosis of Alzheimer’s Disease from multimodal signals: a survey. Artif. Intell. Med. 2016;71:1–29. doi: 10.1016/j.artmed.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 61.Li Y., Li T., Liu H. Recent advances in feature selection and its applications. Knowl Inf Syst. 2017;53(3):551–577. [Google Scholar]
- 62.Hanratty B., Burton J.K., Goodman C., Gordon A.L., Spilsbury K. Covid-19 and lack of linked datasets for care homes: The pandemic has shed harsh light on the need for a live minimum dataset. BMJ. 2020;369 doi: 10.1136/bmj.m2463. British Medical Journal Publishing Group. [DOI] [PubMed] [Google Scholar]
- 63.Hall L.O., Paul R., Goldgof D.B., Goldgof G.M. Finding Covid-19 from chest X-rays using deep learning on a small dataset. arXiv Prepr. arXiv2004.02060. 2020 http://arxiv.org/abs/2004.02060 [Online]. Available: [Google Scholar]
- 64.Larsson S., Thelander U., Friberg S. C-reactive protein (CRP) levels after elective orthopedic surgery. Clin. Orthop. Relat. Res. 1992;(275):237–242. doi: 10.1097/00003086-199202000-00035. [DOI] [PubMed] [Google Scholar]
- 65.Shine B., de Beer F.C., Pepys M.B. Solid phase radioimmunoassays for human C-reactive protein. Clin.Chim. Acta. 1981;117(1):13–23. doi: 10.1016/0009-8981(81)90005-X. [DOI] [PubMed] [Google Scholar]
- 66.Tai P.C., Spry C.J.F. Studies on blood eosinophils. I. Patients with a transient eosinophilia. Clin. Exp. Immunol. 1976;24(3):415–422. [PMC free article] [PubMed] [Google Scholar]
- 67.Shanafelt T.D., et al. MBL or CLL: which classification best categorizes the clinical course of patients with an absolute lymphocyte count ≥ 5 × 109 L-1 but a B-cell lymphocyte count < 5 × 109 L-1? Leuk. Res. 2008;32(9):1458–1461. doi: 10.1016/j.leukres.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Al-Gwaiz L.A., Babay H.H. The diagnostic value of absolute neutrophil count, band count and morphologic changes of neutrophils in predicting bacterial infections. Med. Princ. Pract. 2007;16(5):344–347. doi: 10.1159/000104806. [DOI] [PubMed] [Google Scholar]
- 69.Tajiri J., Noguchi S. Antithyroid drug-induced agranulocytosis: Special reference to normal white blood cell count agranulocytosis. Thyroid. 2004;14(6):459–462. doi: 10.1089/105072504323150787. [DOI] [PubMed] [Google Scholar]
- 70.Vitoriano R.P., Botti L.C.L. Using cross entropy optimization to model active galactic nuclei light curves from VLBA MOJAVE images. Astrophys. J. 2018;854(1) doi: 10.3847/1538-4357/aaa4f8. p. 59. [DOI] [Google Scholar]
- 71.Eghbali-Zarch M., Tavakkoli-Moghaddam R., Esfahanian F., Sepehri M.M., Azaron A. Pharmacological therapy selection of type 2 diabetes based on the SWARA and modified MULTIMOORA methods under a fuzzy environment. Artif Intell. Med. 2018;87:20–33. doi: 10.1016/j.artmed.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 72.Abdel-Basset M., El-hoseny M., Gamal A., Smarandache F. A novel model for evaluation Hospital medical care systems based on plithogenic sets. Artif. Intell. Med. 2019;100 doi: 10.1016/j.artmed.2019.101710. p. 101710. [DOI] [PubMed] [Google Scholar]
- 73.Singh P. A neutrosophic-entropy based adaptive thresholding segmentation algorithm: a special application in MR images of Parkinson’s disease. Artif. Intell. Med. 2020;104 doi: 10.1016/j.artmed.2020.101838. p. 101838. [DOI] [PubMed] [Google Scholar]
- 74.Ozernoy V.M. Proceedings of the XIth International Conference on MCDM, 1–6 August 1994; Coimbra, Portugal; 1997. no. 103–112. Springer Science & Business Media. [Google Scholar]
- 75.Munda G. Vol. 78. Springer Science & Business Media; 2005. p. 23. (Multiple criteria decision analysis: State of the art surveys). [Google Scholar]
- 76.Nikunj Agarwal H.G. Entropy based multi-criteria decision making method under fuzzy environment and unknown attribute weights. Glob. J. Technol. Optim. 2015;06(03):13–20. doi: 10.4172/2229-8711.1000182. [DOI] [Google Scholar]
- 77.Georgopoulos V.C., Stylios C.D. Vol. 302. Springer; Berlin, Heidelberg: 2013. (Fuzziness and medicine: philosophical reflections and application systems in health care). [Google Scholar]
- 78.Kalid N., et al. Based on real time remote health monitoring systems: a new approach for prioritisation ‘Large scales data’ patients with chronic heart diseases using body sensors and communication technology. J. Med. Syst. 2018;42(4) doi: 10.1007/s10916-018-0916-7. p. 69. [DOI] [PubMed] [Google Scholar]
- 79.Almahdi E.M., Zaidan A.A., Zaidan B.B., Alsalem M.A., Albahri O.S., Albahri A.S. Mobile-based patient monitoring systems: a prioritisation framework using multi-criteria decision-making techniques. J. Med. Syst. 2019;43(7) doi: 10.1007/s10916-019-1339-9. p. 219. [DOI] [PubMed] [Google Scholar]
- 80.Abdulkareem K.H., et al. A new standardisation and selection framework for real-time image dehazing algorithms from multi-foggy scenes based on fuzzy Delphi and hybrid multi-criteria decision analysis methods. Neural. Comput. Appl. 2020 doi: 10.1007/s00521-020-05020-4. [DOI] [Google Scholar]
- 81.Abdulkareem K.H., et al. A novel multi-perspective benchmarking framework for selecting image dehazing intelligent algorithms based on BWM and group VIKOR techniques. Int. J. Inf. Technol. Decis. Mak. 2020;19(03):909–957. doi: 10.1142/s0219622020500169. [DOI] [Google Scholar]
- 82.Qader M.A., Zaidan B.B., Zaidan A.A., Ali S.K., Kamaluddin M.A., Radzi W.B. A methodology for football players selection problem based on multi-measurements criteria analysis. Meas. J. Int. Meas. Confed. 2017;111:38–50. doi: 10.1016/j.measurement.2017.07.024. [DOI] [Google Scholar]
- 83.Corman V.M., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu K., et al. Management of COVID-19: the Zhejiang experience. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(2):147–157. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Y., et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J. Med. Virol. 2020;92(7):903–908. doi: 10.1002/jmv.25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Albahri O.S., Al-Obaidi J.R., Zaidan A.A., Albahri A.S., Zaidan B.B., Salih M.M., et al. Helping doctors hasten COVID-19 treatment: Towards a rescue framework for the transfusion of best convalescent plasma to the most critical patients based on biological requirements via ml and novel MCDM methods. Comput. methods and programs in biomed. 2020;196:105617. doi: 10.1016/j.cmpb.2020.105617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Albahri O.S., Zaidan A.A., Albahri A.S., Zaidan B.B., Abdulkareem K.H., Al-Qaysi Z.T., et al. Systematic review of artificial intelligence techniques in the detection and classification of COVID-19 medical images in terms of evaluation and benchmarking: Taxonomy analysis, challenges, future solutions and methodological aspects. J. Infect. and Public Health. 2020 doi: 10.1016/j.jiph.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Albahri A.S., Hamid R.A. Role of biological Data Mining and Machine Learning Techniques in Detecting and Diagnosing the Novel Coronavirus (COVID-19): A Systematic Review. J. Med. Syst. 2020;44(7) doi: 10.1007/s10916-020-01582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.