The coronavirus disease-2019, known as COVID-19, has affected >30 million people globally, leading to >900,000 deaths.1 Many health care systems have faced significant challenges in providing care for overwhelming numbers of patients due to resource constraints. The UK is one of the most affected countries, and, by 17 September 2020, 381,614 people were confirmed COVID-19 positive, 41,705 had died, and 13,710 had been admitted to critical care.1,2 Acute kidney injury was reported in 25% to 78% of critically ill patients and approximately 25% required renal replacement therapy (RRT).2,S1‒S3
London was the epicenter for infection in the UK and, as cases surged, there was an unprecedented increase in demand for RRT. This demand rapidly outstripped the commercial availability of RRT fluids, consequently leading to critical shortages in some parts of the world, including India, New York, and London.3,4,S4 In response, the UK National Health Service centralized the procurement process to oversee the supply chain and to allocate resources proportionately. However, ultimately, NHS procurement was only able to allocate fluids based on the available supply rather than on the overall patient need, which resulted in significant pressure on clinical services.
Existing renal and critical care services worked together closely to provide renal support, alternate modes of dialysis were explored, and, in some instances, patients were transferred to centers with greater RRT capacity. Indications for dialysis were also reviewed to allocate RRT in the most efficient manner. During the peak period, provision of renal support was adjusted daily, due to changes in patient numbers, dynamic changes in the supply chain, and availability of fluids and consumables.
Between March 3 and June 13 2020, 331 critically ill COVID patients were admitted to the expanded critical care units at Guy’s & St Thomas’ National Health Service Foundation Trust (GSTT). At the peak, there were 130 patients in the intensive care unit, of whom 44 required RRT. At this point, 34 continuous renal replacement therapy machines were available, but there was a major shortage of RRT fluids. Despite various actions, fluid shortages meant that continuous renal replacement therapy capacity was reached and a contingency working group was formed to develop a program for in-house production of dialysis solutions. The aim of this work was to report our experience of the manufacture and use of in-house dialysis solutions during the pandemic in critically ill patients with severe acute kidney injury. This will assist in preparations for future surges in both resource-rich and resource-poor countries.
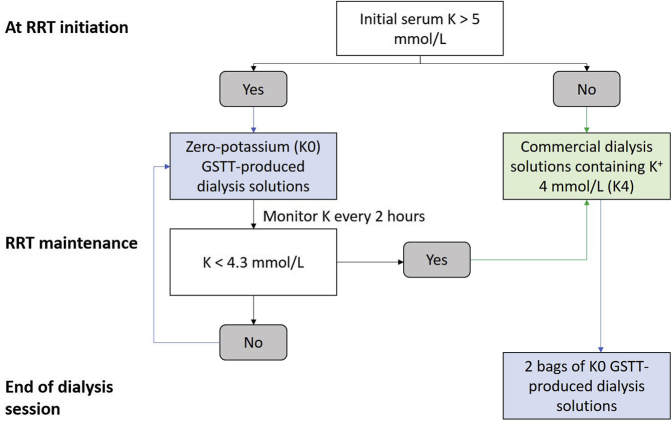
On April 16, 2020, an emergency multidisciplinary working group was formed with representation from pharmacy, renal critical care nursing, and medical staff with the goal of in-house production dialysis fluid for continuous venovenous hemodialysis. The formula, composition, and electrolyte concentration of the 2 selected dialysis solutions compared with commercial fluids are shown in Tables 1 and 2, formula 1 (low bicarbonate solution) and formula 2 (high bicarbonate solution). To minimize manipulations, the GSTT formulation did not contain any potassium (K), magnesium (Mg), or glucose. Calcium (Ca) was not added either, in order to prevent precipitation with bicarbonate and to use the fluid as calcium-free dialysate with regional citrate anticoagulation. The departmental protocol for administration of the solutions is shown in Figure 1. Nursing and medical staff protocols were adopted to ensure patient safety, including the need for at least 1 arterial blood gas, with pH, bicarbonate (HCO3), sodium (Na), K, ionized Ca (iCa) concentration, and glucose measured every 2 to 4 hours. Serum Mg and phosphate concentrations were measured routinely every day.
Table 1.
Fluid composition of each formula
| Composition | Formula 1 | Formula 2 |
|---|---|---|
| Sodium chloride 0.9% | 2 L | 2.5 L |
| Sodium bicarbonate 1.26% | 0.5 L | — |
| Sodium bicarbonate 8.4% | — | 0.1 L |
| Sterile water for injections | 0.5 L | 1 L |
| Total | 3 L | 3.6 L |
Table 2.
Comparison of electrolyte components, volume, and osmolarity between commercial fluids and GSTT formulas 1 and 2
| Electrolyte concentration (mmol/L) | Fluids compatible with citrate-based anticoagulation |
Fluids compatible with noncitrate anticoagulation |
Fluid based on GSTT formula 1 | Fluid based on GSTT formula 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CiCa K2 | CiCa K4 | Prism0Cal (Baxter) | Pureflow (NxStage) | MultiBic (Fresenius) | Prismasol (Baxter) | Accusol (Nikkiso) | Pureflow (NxStage) | |||
| Na+ | 133 | 133 | 140 | 140 | 140 | 140 | 140 | 140 | 128 | 135 |
| K+ | 2 | 4 | 2–4 | 0–4 | 0–4 | 0–4 | 0–4 | 0–4 | — | — |
| Mg2+ | 0.75 | 0.75 | 0.5–0.75 | 0.75 | 0.5 | 0.75 | 0.5 | 0.5–0.75 | — | — |
| Ca2+ | — | — | 0 | 0 | 1.5 | 1.25–1.75 | 1.75 | 0–1.5 | — | — |
| HCO3− | 20 | 20 | 22 | 25 | 35 | 32 | 35 | 35 | 25 | 28 |
| Cl− | 116.5 | 118.5 | 108–120.5 | 108.5–120.5 | 111–113 | 109–113 | 109.5–113.5 | 109–113 | 102.7 | 107 |
| PO42− | — | — | — | — | — | — | — | — | — | — |
| Glucose | 5.55 | 5.55 | 6.1 | 5.55 | 5.55 | 5.55 | 5.55 | 5.55 | — | — |
| Volume (L) | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3 | 3.6 |
| Theoretical osmolarity (mOsm/L) | 278 | 282 | 286–296 | 286–294 | 292–300 | 292–300 | 292–300 | 292–300 | 256 | 270 |
Ca2+, calcium; CiCa, citrate and calcium; Cl−, chloride; GSTT, Guy’s & St Thomas’ NHS Foundation Trust; HCO3−, bicarbonate; K+, potassium; Mg2+, magnesium; Na+, sodium; PO42−, phosphate.
Figure 1.
Departmental guideline for administration of GSTT dialysis solutions based on serum potassium. The dialysis bags were switched to 2 K0 bags toward the end of each RRT session to ensure the lowest acceptable potassium levels when RRT was discontinued and to prolong the time until RRT was necessary again. GSTT, Guy’s & St Thomas’ National Health Service Foundation Trust; RRT, renal replacement therapy.
GSTT has an approved aseptic preparation unit, used primarily for the preparation of bespoke adult parenteral nutrition solutions. This RRT fluid production process entailed the aseptic filling of parenteral nutrition bags from bulk sterile solutions in a European Union Good Manufacturing Practice Grade A environment.S5 (Supplementary Video) The time taken for the preparation of each bag was 4 minutes, and 80 minutes for each batch. In line with the department’s standard procedures, a stability test protocol was developed to confirm solution stability over a 7-day period (Supplementary Table S1). There was capacity to produce up to 60 bags of 3.6 L of GSTT formulation RRT fluid during working hours. The critical care pharmacy team worked closely with the critical care renal specialist nurses and the pharmacy manufacturing team to ensure judicious production and to minimize waste. Fluid manufacturing requirements were assessed every 2 or 3 days, depending on projection of ongoing needs.
Herein we report the evaluation undertaken after the first 2 weeks of fluid production (April 17 to May 1, 2020). To assess clinical efficacy, we evaluated changes of serum electrolytes (Na, K, Ca, Mg, HCO3), acid-base status (pH, base excess) at baseline and 2, 4, and 6 hours after RRT initiation of all sessions. We further categorized patients according to whether they had received RRT with fluid based on formula 1 vs. formula 2, and citrate vs. noncitrate anticoagulation. For assessment of safety aspects, we evaluated the proportions of patients who developed arrhythmias, including atrial fibrillation, ventricular tachycardia, ventricular fibrillation, and significant metabolic disturbances, as specified by departmental protocols (serum K < 3.5 mmol/L, serum iCa < 1.0 mmol/L, serum Mg < 0.7 mmol/L, metabolic alkalosis defined as pH > 7.5, base excess > 5, or HCO3 concentration > 30 mmol/L, and blood glucose < 4 mmol/L) and requirement for additional electrolyte supplementations.
Results
Between April 17 and May 14, 2020, a total of 880 GSTT formulation dialysis bags were manufactured. We audited the use of 186 bags of fluid in 25 patients (total 42 sessions) between April 17 and May 1, 2020. Fluids based on formula 1 and formula 2 were used in 13 (31.0%) and 29 (69.0%) sessions, respectively. Thirty (71.4%) and 12 (28.6%) sessions were delivered using regional citrate anticoagulation and systemic heparin, respectively. The median duration for using K0 GSTT bags was 5 (interquartile range 2–8, range 1–23) hours and the median number of bags per session was 4 (interquartile range 2–5, range 1–15). The median blood flow rate was 100 (interquartile range 80–150, range 60–300) ml/min, and median prescribed dose was 26.67 (interquartile range 20.62–35.17, range 12.73–53.40) ml/kg per hour.
Clinical Data
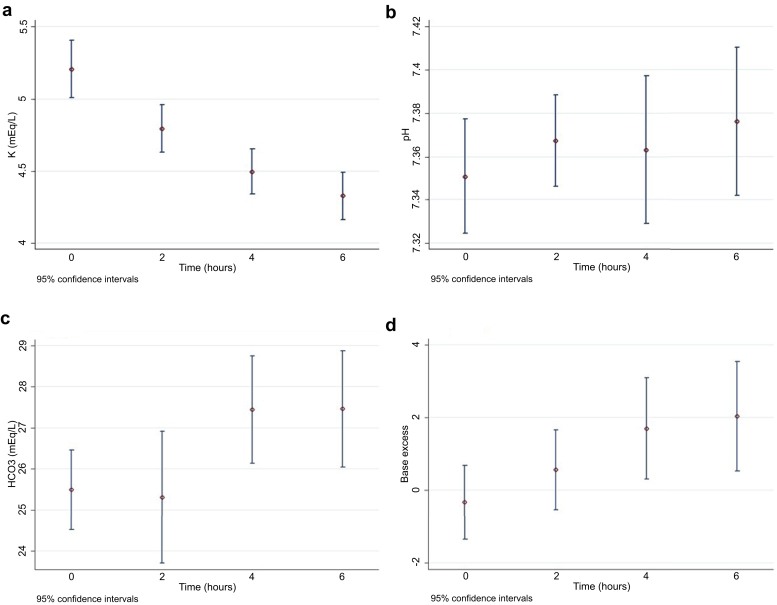
When using GSTT-produced fluid, serum potassium concentrations fell from 5.21 ± 0.63 to 4.33 ± 0.37 mmol/L over 6 hours (P < 0.001). There were also significant increases in pH, HCO3−, and base excess, and a decrease in chloride over 6 hours, but no significant changes in partial pressure of carbon dioxide, Na, lactate, iCa, glucose, or Mg (Table 3, Figure 2).
Table 3.
Changes of electrolytes, acid-base status, and glucose from baseline until 6 hours (n = 42)
| Hour | 0 (n = 42) | 2 (n = 40)a | 4 (n = 24)a | 6 (n = 21)a | P value |
|---|---|---|---|---|---|
| pH | 7.35 ± 0.08 | 7.37 ± 0.07b | 7.36 ± 0.08c | 7.38 ± 0.08d | 0.002b |
| PCO2 (kPa) | 6.37 ± 1.51 | 6.18 ± 1.10 | 6.66 ± 1.70 | 6.46 ± 1.28 | 0.75 |
| Na (mmol/L) | 141.31 ± 5.17 | 141.59 ± 4.07 | 140.84 ± 3.65 | 141.13 ± 2.92 | 0.65 |
| K (mmol/L) | 5.21 ± 0.63 | 4.80 ± 0.51b | 4.50 ± 0.38c | 4.33 ± 0.37d | <0.001 |
| Cloride (mmol/L) | 101.38 ± 3.00 | 101.22 ± 2.84 | 100.69 ± 2.76 | 99.66 ± 2.40d | <0.001 |
| HCO3 (mmol/L) | 25.49 ± 3.10 | 25.31 ± 5.01 | 27.44 ± 3.02c | 27.46 ± 3.10d | 0.02 |
| BE | −0.33 ± 3.26 | 0.56 ± 3.42b | 1.70 ± 3.21c | 2.03 ± 3.31d | <0.001 |
| Lactate (mmol/L) | 1.32 ± 0.50 | 1.38 ± 0.38 | 1.38 ± 0.46 | 1.34 ± 0.42 | 0.84 |
| iCa (mmol/L) | 1.13 ± 0.09 | 1.12 ± 0.06 | 1.11 ± 0.07 | 1.12 ± 0.04 | 0.48 |
| Glucose (mmol/L) | 9.84 ± 2.90 | 9.25 ± 3.11 | 8.60 ± 2.44 | 8.50 ± 2.14 | 0.17 |
| Mg (mmol/L) | 1.22 ± 0.22 | — | — | 1.13 ± 0.28e | 0.15 |
BE, base excess; iCa, ionized calcium; PCO2, partial pressure of carbon dioxide.
Bold P values are statistically significant.
Laboratory data were not obtained after switching to commercial fluids.
P < 0.05 for time 0 vs. 2.
P < 0.05 for time 0 vs. 4.
P < 0.05 for time 0 vs. 6.
Magnesium was measured once daily. Therefore, the values represent the levels at 24 hours. There were no missing data for magnesium levels.
Figure 2.
Changes of potassium (a), pH (b), bicarbonate (c), and base excess (d) at baseline (n = 42), and at 2 (n = 40), 4 (n = 24), and 6 (n = 21) hours.
Hypomagnesemia developed in 1 session and there were 3 episodes of hypocalcemia. Mg and Ca were administered as an “as-required” prescription in 16 and 4 from 42 sessions, respectively (Table 4).
Table 4.
Complications and electrolyte supplementation (n = 42)
| Number of sessions with ≥1 adverse event (total n = 42) | |
|---|---|
| Complications | |
| Hypokalemia (K <3.5 mmol/L) | 0 |
| Hypomagnesemia (Mg <0.7 mmol/L) | 1 |
| Hypocalcemia (iCa <1.0 mmol/L) | 3 |
| Hypoglycemia (BG <4 mmol/L) | 0 |
| Metabolic alkalosis (BE >5, pH >7.5, HCO3 >30 mmol/L) | 8 |
| Supplementation | Session |
| K | 0 |
| Mg | 16 |
| Ca | 4 |
BE, base excess; BG, blood glucose; iCa, ionized calcium.
Metabolic alkalosis developed in 8 of 42 sessions and was more common in patients receiving citrate (regional citrate anticoagulation) and fluid based on formula 2 (Na 135/HCO3 28) (Supplementary Tables S2 and S3, Supplementary Figures S1 and S2). When alkalosis occurred, there were 3 implemented troubleshooting strategies. First, blood flow rate was decreased to reduce citrate load. Second, we switched the fluid bags to K4 commercial solutions. Third, we used a K0 GSTT bag and a K4 commercial bag in combination to reduce the total HCO3 concentration. All methods corrected metabolic alkalosis successfully unless it was suspected to be secondary to significant clogging in the circuit, in which case the treatment was stopped. There was mild and transient metabolic acidosis in 1 patient receiving noncitrate anticoagulation, which was rapidly corrected after switching to K4 solutions. Hypoglycemia and arrhythmias related to the RRT solution were not observed.
Stability and Sterility
All results passed the standard assays for stability and bacteriostatic sterility at 4 °C and 25 °C for 7 days (Supplementary Appendix S1).
Discussion
This evaluation has clearly confirmed the safety, feasibility, and efficacy of in-house dialysis fluid production for the management of critically ill patients requiring RRT. The fluids had satisfactory electrolyte concentrations, sterility, and stability over 7 days at room temperature. In particular, with regard to efficacy, hyperkalemia, which was a major clinical problem in the reported cohort of COVID-19 critical care patients, was corrected. The most common side effect was metabolic alkalosis, especially with regional citrate anticoagulation and fluids based on formula 2 (high HCO3 solution).
During the COVID-19 pandemic, several options were utilized to compensate for shortages of RRT fluid and consumables.5,S6,S7 Utilization of RRT was based on patients’ needs, local expertise, and availability of staff and equipment. Prolonged intermittent RRT for a duration of 8 to 12 hours permitted a single machine to be used for 2 or 3 patients per day. In 2 of our intensive care units with reverse osmosis systems, intermittent hemodialysis was provided for patients who were hemodynamically stable. Although acute peritoneal dialysis is another option, as less infrastructure and equipment are required and anticoagulant is not needed,6,7 Peritoneal dialysis was not an option due to lack of experience in our center and a high proportion of critically ill patients who required ventilation in the prone position. Other strategies included optimization of vascular access and blood flow rate, intensified anticoagulation to prolong filter life, and adjustment of RRT dose once metabolic control was achieved to conserve RRT fluids.
Production of in-house fluids is common in settings where continuous renal replacement therapy consumables and fluids are not always available and in health care systems in which resources are limited and expensive commercial RRT fluids are not an option. We decided to pursue this option as a rescue strategy to maintain RRT capacity during the COVID-19 supersurge. Although we were able to manufacture fluids in bulk quantities, our limitation was the number of bags that could be produced daily balanced by the large number of patients requiring RRT. As a result, we remained partially dependent on the supply of commercial fluids. In addition, our use of relatively basic RRT fluids that did not contain any Mg or Ca meant that more frequent monitoring and supplementation was required. As expected, this increased the bedside workload and associated clinical concern, particularly for bedside nurses with varying RRT experience who were already working in a stressful environment in pandemic conditions. To offset this, renal critical care nurses and the renal critical care physicians provided enhanced support to the clinical teams. This facilitated physicians’ and nurses’ knowledge and understanding of the effects of the dialysis solutions on serum electrolytes, acid-base balance, glucose, and their interactions with citrate with protocols to make adjustments to accommodate new bags. Overall, the introduction of an in-house dialysis solution required significant training and constant feedback from the clinical team under close supervision within a strong clinical governance process.
Our assessments confirmed that the GSTT RRT fluid formulations achieved significant reductions in serum K concentrations. Although this was a desired effect, we acknowledge that acute fluctuations in serum potassium can cause deviations in transmembrane potential of cardiac and skeletal muscle and may lead to arrhythmia and paralysis.S8 An increase in serum HCO3 concentration can also stimulate a shift of K into cells and further lower the serum K level.S9 Previous observational studies showed an increased risk of arrhythmia or death in chronic hemodialysis patients when using lower dialysate K.S10–S14 In contrast, some studies demonstrated a decreased risk of mortality in patients with a serum K of >5 mmol/L who used dialysate containing <2 mmol/L of K concentration.S15,S16 We selected only hyperkalemic patients with an average baseline serum K of 5.2 mmol/L, and instructed the clinical staff to monitor electrolytes as frequently as every 2 hours so that the K0 bags could be changed promptly to K4 bags once serum K levels fell. In addition, the dialysate flow rate is only ∼50 ml/min during prolonged intermittent RRT and ∼30 ml/min during continuous venovenous hemodialysis in a 60-kg patient, as opposed to 500 to 800 ml/min in intermittent hemodialysis, which may cause less aggressive potassium removal. Later, we adjusted the prescription by hanging 1 bag of K0 and 1 bag of K4 together on the balancing scale. We did not observe any serious episodes of hypokalemia or cardiac arrhythmias.
Hypomagnesemia is also a potential side effect of using Mg-free dialysis fluid. It is a well-known risk factor for arrhythmia and has potentiating effects on hypokalemia, as it promotes intracellular shifts in K.S17 Mg removal during hemodialysis increases with lower Mg in dialysate,S18 but in our study hypomagnesemia occurred in only 1 session.
Hypoglycemia did not occur in any of our patients. In contrast, hyperglycemia was common, as previously reported in the literature.S19 Fluids based on formula 2 caused more alkalemia. This may have been due to the HCO3 concentration being 28 mmol/L in our fluids combined with citrate administration in patients whose acid-base was normal. Decreasing the blood flow rate can also reduce the citrate load and prevent alkalemia. The possibility of low osmolality and the risk of hypotension was considered with fluids based on formula 1, but we did not observe any hyponatremia or hemodynamic instability. It is important to acknowledge that other formulas for in-house production of dialysis have been described in previous studies, some of which had higher sodium and bicarbonate concentrations (Supplementary Table S4).8,S21–S27
The electrolyte concentrations in our fluids remained stable over 7 days at 4° C and 25° C. Fortunately, our hospital is equipped with an aseptic unit, which allowed us to produce the fluids in a sterile environment. In settings where an aseptic technique cannot be ensured, the RRT fluid bags should be safe to stay at room temperature for 24 hours with a monitoring protocol similar to ours.
We successfully used the in-house‒produced fluids for 4 weeks until the number of COVID-19 patients declined and the supply of commercial fluids was sufficient. When reflecting back on our experience and planning for a possible second wave, we are confident that our process was safe and efficient, and the protocol was feasible and effective at both the patient and organizational levels. Specialist clinical oversight and frequent monitoring were essential in avoiding complications. In preparation for a future crisis, we are now developing training modules on different RRT modalities, RRT prescriptions, monitoring, and complication management for nursing and medical staff, including junior doctors. Other strategies to prepare for a future RRT surge include installation of additional reverse osmosis points to increase intermittent hemodialysis capacity in critical care. Another technique is to produce RRT fluids with intermittent hemodialysis machines.9,S28 This process may allow greater volumes of fluid to be produced and at a lower cost. However, the environment in which the fluid is produced may not be conducive to asepsis. Moreover, the intermittent hemodialysis cartridges containing the electrolyte mix are not designed for this purpose and regulatory approval would be needed.
In conclusion, we have described our experience with manufacturing in-house aseptic RRT fluid in the COVID-19 pandemic setting as a rescue strategy when faced with a shortage of commercial RRT fluids. We confirmed the safety, feasibility, and efficacy of in-house dialysis fluid production for the management of critically ill patients requiring RRT. Other health care systems and critical care centers may need to consider this option in times of crisis and the data we have presented will hopefully be useful to clinical teams.
Disclosure
All authors declared no conflicts of interest.
Acknowledgments
NL was trained in Thailand and expresses her gratitude to the staff at the Division of Nephrology and Excellence Center for Critical Care Nephrology, King Chulalongkorn Memorial Hospital, Bangkok, where custom-mix dialysis fluids are commonly prescribed and produced daily by bedside nurses. All authors thank all the health care professionals involved, especially those who provided RRT during the pandemic.
Footnotes
Supplementary Methods.
Supplementary References.
Figure S1. Changes of pH and bicarbonate between baseline and 6 hours in patients on citrate (n = 16) and heparin anticoagulation (n = 5).
Figure S2. Changes of pH and bicarbonate between baseline and 6 hours in patients using fluids based on GSTT formulas 1 (n = 6) and 2 (n = 15).
Figure S3. GSTT dialysis solutions.
Table S1. Dialysis solution testing methods.
Table S2. Changes of electrolytes and acid-base status between baseline and 6 hours in patients on citrate (n = 16) and heparin anticoagulation (n = 5).
Table S3. Changes of electrolytes and acid-base status between baseline and 6 hours in patients using fluids based on GSTT formula 1 (n = 6) and formula 2 (n = 15).
Table S4. Available custom-mix dialysis solutions.
Supplementary Appendix S1. Stability assay of the dialysis solution at 4 °C and 25 °C to determine the shelf-life.
Supplementary Video. Production of in-house dialysis solutions using aseptic technique.
Supplementary Material
Supplementary Methods.
Supplementary References.
Figure S1. Changes of pH and bicarbonate between baseline and 6 hours in patients on citrate (n = 16) and heparin anticoagulation (n = 5).
Figure S2. Changes of pH and bicarbonate between baseline and 6 hours in patients using fluids based on GSTT formulas 1 (n = 6) and 2 (n = 15).
Figure S3. GSTT dialysis solutions.
Table S1. Dialysis solution testing methods.
Table S2. Changes of electrolytes and acid-base status between baseline and 6 hours in patients on citrate (n = 16) and heparin anticoagulation (n = 5).
Table S3. Changes of electrolytes and acid-base status between baseline and 6 hours in patients using fluids based on GSTT formula 1 (n = 6) and formula 2 (n = 15).
Table S4. Available custom-mix dialysis solutions.
Supplementary Appendix S1. Stability assay of the dialysis solution at 4 °C and 25 °C to determine the shelf-life.
Supplementary Video. Production of in-house dialysis solutions using aseptic technique.
References
- 1.John Hopkins University & Medicine Coronavirus Resource Center COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University (JHU) 2020. https://coronavirus.jhu.edu/map.html Available at:
- 2.Intensive Care National Audit & Research Centre ICNARC report on COVID-19 in critical care. September 7, 2020. https://www.icnarc.org/our-audit/audits/cmp/reports Available at:
- 3.Goldfarb D.S., Benstein J.A., Zhdanova O. Impending shortages of kidney replacement therapy for COVID-19 patients. Clin J Am Soc Nephrol. 2020;15:880–882. doi: 10.2215/CJN.05180420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramachandran R., Jha V. Adding insult to injury: kidney replacement therapy during COVID-19 in India. Kidney Int. 2020;98:238–239. doi: 10.1016/j.kint.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostermann M., Lumlertgul N., Forni L.G., Hoste E. What every intensivist should know about COVID-19 associated acute kidney injury. J Crit Care. 2020;60:91–95. doi: 10.1016/j.jcrc.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sourial M.Y., Sourial M.H., Dalsan R. Urgent peritoneal dialysis in patients with COVID-19 and acute kidney injury: a single-center experience in a time of crisis in the United States. Am J Kidney Dis. 2020;76:401–406. doi: 10.1053/j.ajkd.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Shamy O., Patel N., Abdelbaset M.H. Acute start peritoneal dialysis during the COVID-19 pandemic: outcomes and experiences. J Am Soc Nephrol. 2020;2:377–380. doi: 10.1681/ASN.2020050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgner A., Ikizler T.A., Dwyer J.P. COVID-19 and the inpatient dialysis unit: managing resources during contingency planning pre-crisis. Clin J Am Soc Nephrol. 2020;15:720–722. doi: 10.2215/CJN.03750320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleveland Clinic An in-house solution to address a national shortage of dialysate [video] https://consultqd.clevelandclinic.org/an-in-house-solution-to-address-a-national-shortage-of-dialysate-video Available at:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.