Abstract
Heat stress as an environmental stressor causes abnormal bone remodeling and microarchitectural deterioration. The objective of this study was to investigate the effects of a Bacillus subtilis–based probiotic on bone mass of broilers subjected to cycling high ambient temperature. One hundred and twenty 1-day-old Ross 708 male broiler chicks were randomly assigned to 2 dietary treatments (12 pens per treatment): control diet and the control diet plus 250-ppm probiotic consisting of 3 strains of Bacillus subtilis. Room temperature was gradually decreased from 35°C on day 1 by 0.5°C/d until day 15, when ambient temperature was increased from 28°C to 32°C for 10 h (07:00 h–17:00 h) daily until day 44. Samples of blood, leg bones (tibia and femur), and brains (raphe nuclei and hypothalamus) were collected at day 43, while latency to lie test was conducted at day 44. Compared with controls, probiotic supplementation increased bone mineral content, weight, size, weight to length index, and reduced robusticity index in the tibia and femur (P < 0.05) of broilers subjected to heat stress. Serum concentrations of c-terminal telopeptide of type I collagen (CTX) were reduced (P = 0.02) by the probiotic supplementation, while ionized calcium, phosphate, and osteocalcin were not affected (P > 0.05). Moreover, tumor necrosis factor-α (TNF-α) in probiotic fed broilers was decreased (P = 0.003) without changes of plasma interleukin (IL)-6, IL-10, interferon-γ, and corticosterone concentrations. There were no treatment effects on the concentrations of peripheral serotonin and central serotonin and catecholamines (norepinephrine, epinephrine, and dopamine) as well as their metabolites. These results may indicate that dietary supplementation of Bacillus subtilis–based probiotic increases bone growth in broilers under a cyclic heating episode probably via inhibition of bone resorption, resulting from downregulation of the circulating TNF-α and CTX. Dietary probiotic supplementation may be a management strategy for increasing skeletal health of broilers under hot weather.
Key words: Bacillus subtilis, heat stress, bone, tumor necrosis factor-α, broiler
Introduction
High ambient temperatures, especially combined with high humidity, impose severe stress on broilers because of their limited ability to regulate heat loss by feathering and great metabolic rate resulting from selecting and breeding for fast growth (Geraert et al., 1996). Subsequently, heat stress (HS) causes detrimental effects in broilers ranging from reduced growth rate and carcass quality to eventually death, which leads to not only economic loss for poultry producers but also welfare concerns (Lara and Rostagno, 2013). Rapid growth–associated leg disorders commonly cause chronic pain and lameness in broilers (Caplen et al., 2013a). Reduced bone mass, such as ash content and bone volume, occurs in both broilers and turkeys exposed to high temperatures (Jankowski et al., 2015; Hosseini-Vashan et al., 2016). The underlying mechanisms for HS-induced bone developmental disorders in broilers are still being investigated, but the bone remodeling regulators including mineral metabolism, hormonal homeostasis, and immune factors are all directly impacted by HS.
HS-induced behavioral changes include decreased feeding and increased panting (Mack et al., 2013). In order to cope with hot rearing environments, depressed appetite in birds decreases metabolic heat production, which also reduces the availability of nutrients and minerals absorbed from the gastrointestinal tract, such as amino acids and calcium (Ca) required for bone health and body growth. Panting is the major cooling method used by birds to dissipate excess heat through evaporation of moisture in the upper respiratory tract. However, excessive rapid breathing may cause respiratory alkalosis, decreased ionized Ca (bioavailable Ca) in the blood. In addition, chronic HS in broilers can induce intestinal injury such as reduced height of villi, thinner gut mucosa, and decreased alkaline phosphatase activity (Hu et al., 2017), which further hampers intestinal Ca absorption. Therefore, a decrease in the bioavailability of circulating Ca may be a contributor toward reduced bone mineralization, strength, ash, and other indicators of bone traits in heat-stressed broilers (Jankowski et al., 2015; Hosseini-Vashan et al., 2016). HS also induces overactivation of the hypothalamic-pituitary-adrenal axis, leading to elevated blood corticosterone (CORT) (Quinteiro-Filho et al., 2012). Excess glucocorticoid negatively affects bone mass through inhibiting osteoblastogenesis, increasing osteoblast and osteocyte apoptosis (O'Brien et al., 2004), and promoting osteoclast survival (Jia et al., 2006). In addition, numerous studies have demonstrated that HS induces immunosuppression in broilers (Jahanian and Rasouli, 2015), accompanied by the changes in cytokines synthesis such as increased spleen concentrations of tumor necrosis factor-α (TNF-α) and interleukin (IL)-4 but decreased concentrations of interferon-γ (IFN-γ) and IL-2 (Xu et al., 2014). As a proinflammatory cytokine, TNF-α locally produced in bone is involved in bone resorption by directly enhancing osteoclastic activity (Schett, 2011) and indirectly acting through downregulation of osteoprotegerin-associated osteoclastogenesis (Boyce et al., 2005). In contrast to TNF-α, as a balance system, IFN-γ released by the mesenchymal stem cells and cells of immune origin within the bone microenvironment promotes bone formation in mice (Duque et al., 2011) as an exceptionally strong inhibitor of osteoclast differentiation (Schett, 2011). Considering the vital role of cytokines on bone cells and related metabolism (Inada and Miyaura, 2010; Schett, 2011), an alteration of bone homeostasis may be caused by the changes of the hypothalamic-pituitary-adrenal axis and cytokines of the immune system under HS.
Several probiotics have been reported to play a role in optimizing bone mass in various animals, including laying hens (Abdelqader et al., 2013; Yan et al., 2019b) and broilers (Houshmand et al., 2011; Sadeghi, 2014; Yan et al., 2019a). Although the mechanisms have not been not widely investigated in poultry, Bacillus subtilis–based probiotic has been reported to promote bone mass in broilers under a thermoneutral environment through increased circulating Ca levels and reduced bone resorption mediated by serotonin (5-HT)-suppressed sympathetic activity (Yan et al., 2018). In addition, the probiotic with Lactobacillus reuteri increases bone mass in rats, resulting from reduced intestinal TNF-α expression (McCabe et al., 2013). Therefore, probiotics could play a critical role in bone health and related mineralization in heat-stressed animals. The objective of this study was to investigate the effects of a Bacillus subtilis–based probiotic on bone mass of broilers subjected to cycling heat episodes. It was hypothesized that probiotic supplementation would improve bone mass in broilers under elevated temperatures.
Materials and methods
Animals, Management, and Sample Collection
A total of one hundred and twenty 1-day-old Ross 708 male broiler chicks (Miller Poultry, Orland, IN) were used in this study. Chicks were randomly placed into 24 floor pens (152 cm × 81 cm) with 5 chicks each in an environmentally controlled room. The pens were randomly assigned to 2 dietary treatments (n = 12): control diet (Table 1) and the control diet plus 0.25 g/kg probiotic (Sporulin; Pacific Vet Group, Inc., Fayetteville, AR). Average BW were similar among all the pens (control: 34.78 ± 0.17 g, and probiotic: 34.92 ± 0.16 g, P = 0.55). The probiotic consists of 3 strains of Bacillus subtilis. The control diets were formulated according to Aviagen's formulations (2019) in mash. The probiotic mixed diets were prepared based on our previous studies (Yan et al., 2018), and the final dose was 1.0 × 106 spores/g of feed. All the diets were sampled for bacterial analysis before each feeding period.
Table 1.
The ration formulation.
| Item | Starter (Day 1–14) | Grower (Day 15–28) | Finisher (Day 29–44) |
|---|---|---|---|
| Ingredient, % | |||
| Corn | 52 | 52.3 | 62.8 |
| Soybean meal, 48% crude protein | 40 | 39.1 | 29.7 |
| Soybean oil | 3.59 | 4.97 | 4.11 |
| Sodium chloride | 0.51 | 0.46 | 0.43 |
| DL Methionine | 0.3 | 0.24 | 0.23 |
| L-Lysine HCL | 0.13 | — | 0.07 |
| Threonine | 0.06 | — | — |
| Limestone | 1.29 | 1.15 | 1.12 |
| Monocalcium phosphate | 1.75 | 1.48 | 1.17 |
| Vitamin/mineral premix1 | 0.35 | 0.35 | 0.35 |
| Calculated analyses | |||
| Crude protein % | 23.4 | 22.8 | 19.2 |
| ME kcal/kg | 3,050 | 3,151 | 3,200 |
| Ca % | 0.95 | 0.85 | 0.75 |
| Available P % | 0.5 | 0.44 | 0.36 |
| Methionine % | 0.66 | 0.59 | 0.53 |
| Methionine + cystine % | 1.04 | 0.97 | 0.86 |
| Lysine % | 1.42 | 1.29 | 1.09 |
| Threonine % | 0.97 | 0.89 | 0.74 |
| Na % | 0.22 | 0.20 | 0.19 |
Provided per kilogram of diet: vitamin A, 13,233 IU; vitamin D3, 6,636 IU; vitamin E, 44.1 IU; vitamin K, 4.5 mg; thiamine, 2.21 mg; riboflavin, 6.6 mg; pantothenic acid, 24.3 mg; niacin, 88.2 mg; pyridoxine, 3.31 mg; folic acid, 1.10 mg; biotin, 0.33 mg; vitamin B12, 24.8 μg; choline, 669.8 mg; iron from ferrous sulfate, 50.1 mg; copper from copper sulfate, 7.7 mg; manganese from manganese oxide, 125.1 mg; zinc from zinc oxide, 125.1 mg; iodine from ethylene diamine dihydroiodide, 2.10 mg; selenium from sodium selenite, 0.30 mg.
Each pen was equipped with 1 hanging feeder and drinker. Feed and water were provided ad libitum. Wood shavings were used as flooring material. Room temperature was gradually decreased from 35°C on day 1 by 0.5°C/d until 15 d of age at which time ambient temperature was increased from 28°C to 32°C for 10 h (07:00 h–17:00 h) daily until 44 d of age. The study was conducted in the summer months from June to July. Data loggers (HOBO; Onset Computer Corporation, Bourne, MA) were used for recording the room temperature and humidity, and the data were presented in Table 2. The lighting program was gradually decreased from 23 light:1 dark (01:00–02:00 h) at 30 lux up to day 7, then 20 light:4 dark (01:00–05:00 h) at 10 lux until the end of this study.
Table 2.
The temperature and humidity at different ages of birds.
| Age | Temperature (°C)1 |
Humidity (relative humidity %)1 |
||
|---|---|---|---|---|
| Day time (07:00–17:00 h) | Night time (17:00–07:00 h) | Day time (07:00–17:00 h) | Night time (17:00–07:00 h) | |
| Day 15–28 | 31.89 ± 0.61 | 26.43 ± 0.72 | 52.21 ± 1.72 | 55.02 ± 1.77 |
| Day 29–44 | 32.07 ± 0.45 | 26.74 ± 0.81 | 56.83 ± 1.39 | 59.16 ± 1.65 |
Mean ± SDs were presented.
The following samples were collected during the HS time period. At 44 d of age, 1 bird per pen was randomly picked, weighed, and then sedated using sodium pentobarbital (30 mg/kg of BW, i.v.). A total of 8 mL of blood was collected from each bird via cardiac puncture, with 5 mL placed into ice-cooled EDTA-coated plasma tube and 3 mL placed into a serum tube. For plasma, the blood samples were centrifuged at 1,500 × g at 4°C for 15 min. For serum, the blood samples were kept at room temperature for 30 min, and then centrifuged at 1,500 × g for 10 min. Samples of plasma and serum were then stored at −80°C until assayed. The birds were euthanized immediately after bleeding by cervical dislocation. The left tibia and femur were collected and placed in individual plastic bags, then kept at −20°C until assayed. The hypothalamus and raphe nuclei were dissected, immediately frozen on dry ice, and then stored at −80°C until assayed.
Bone Traits
The tibia and femur were measured for bone mineral density (BMD), bone mineral content (BMC), and bone area using a dual energy x-ray absorptiometry (Norland Medical Systems Inc., Fort Atkinson, WI) as previously described (Hester et al., 2013). The BMD was calculated as BMC divided by the area of the bone. After scanning, all the bones were boiled for 5 min, and then the soft tissues including meat, connective tissue, and the fibula bone were removed (Hall et al., 2003). Bone weight, length, width, and cortical bone thickness were determined using a digital micrometer (Coolant Proof Micrometer Series 293; Mitutoyo America Corp., Aurora, IL). Bone weight to length index and robusticity index were also calculated (Riesenfeld, 1972; Seedor et al., 1991). In addition, 2 broilers per pen were used to perform the latency to lie test at 44 d of age following the procedure descripted previously (Berg and Sanotra, 2003).
Blood Analyses
Serum concentrations of osteocalcin (OC), c-terminal telopeptide of type I collagen (CTX), ionized Ca, and phosphate (Pi) were determined using the commercial kits (MyBioSource, San Diego, CA; BioAssay Systems, Hayward, CA). Plasma samples were used for detecting concentrations of 5-HT, tryptophan, and cytokines of IL-6, IL-10, TNF-α, and IFN-γ using the commercial ELISA kits (MyBioSource, San Diego, CA). All the kits were used following the relative manufactural instructions. Plasma concentrations of CORT were measured using the commercial 125I CORT radioimmunoassay kit (MP Biomedicals, Orangeburg, NY) following the method described previously (Cheng et al., 2001).
Brain Monoamine Analyses
Monoamines and their metabolites of the left hypothalamus and raphe nuclei were analyzed using HPLC (UltiMate 3000 RSLCnano System; Thermo Fisher Scientific Inc., Waltham, MA). Each brain sample was weighed and homogenized with ice-cold 0.2-M perchloric acid at a 10:1 ratio (μL of perchloric acid:mg of sample). The homogenized mixture was centrifuged at 18,187 g for 15 min at 4°C. The resultant supernatant was drawn into a microcentrifuge tube and diluted 1:1 with mobile phase (MD-TM Mobile Phase, Thermo Fisher Scientific Inc., Waltham, MA). The mixture was then centrifuged at 18,187 g for 10 min at 4°C. The supernatant was filtered through a 0.2-μm polyvinylidene fluoride filter into a HPLC sample vial. The mobile phase flow rate was 0.8 mL/min. The concentrations of 5-HT, tryptophan, 5-hydroxyindoleacetic acid (5-HIAA), dopamine (DA), norepinephrine (NE), epinephrine (EP), 3,4-dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) were calculated from its reference curve made by using the relative standards. The 5-HIAA/5-HT, DOPAC/DA, and the HVA/DOPAC turnover ratios were additionally calculated as indexes of serotonergic and dopaminergic activities, respectively.
Statistical Analysis
A one-way ANOVA of the mixed model procedure of SAS 9.4 software (SAS Institute Inc., Cary, NC) was used for the data analysis with probiotic treatment as the fixed effect. The experiment unit was the pen (n = 12). Transformation of data was performed for normality when variances were not homogeneous (Steel et al., 1997). Statistical trends were similar for both transformed and untransformed data; therefore, the untransformed least square means and the SEM were presented. Statistical significance was set at P < 0.05, and tendency to a significant difference was set at 0.05 ≤ P ≤ 0.10.
Results
Bone Traits
Compared to controls, the inclusion of probiotic significantly increased the BMC and area of tibia in broilers subjected to elevated temperature (P = 0.001 and 0.003; Table 3), with a tendency of higher BMD (P = 0.07). A tendency of higher relative tibia weight was also noticed (P = 0.07). The width of tibia was also significantly increased by probiotic supplementation (P = 0.02), whereas the length and bone wall thickness (both medial and lateral) were not changed. The anatomical changes lead to greater weight/length but lower robusticity indexes in the tibia (P = 0.01 and 0.02) by the inclusion of dietary probiotic.
Table 3.
The effects of probiotic on bone traits of 43-day-old broilers subjected to daily cycling heating episodes.
| Item | Control | Probiotic | SEM | P value |
|---|---|---|---|---|
| Tibia | ||||
| BMD (g/cm2) | 0.167 | 0.176 | 0.001 | 0.07 |
| BMC (g) | 2.05 | 2.44 | 0.02 | 0.001 |
| Area (cm2) | 12.23 | 13.91 | 0.12 | 0.003 |
| Weight (g) | 6.50 | 9.04 | 0.22 | 0.01 |
| Relative weight1 (g/kg) | 3.37 | 4.09 | 0.09 | 0.07 |
| Length (mm) | 92.11 | 93.50 | 0.44 | 0.44 |
| Width (mm) | 8.17 | 9.31 | 0.11 | 0.02 |
| Medial thickness (mm) | 1.07 | 1.14 | 0.01 | 0.15 |
| Lateral thickness (mm) | 1.61 | 1.60 | 0.02 | 0.91 |
| Weight/length index (mg/mm) | 70.29 | 96.87 | 2.37 | 0.01 |
| Robusticity index (g, cm) | 5.00 | 4.52 | 0.04 | 0.02 |
| Femur | ||||
| BMD (g/cm2) | 0.142 | 0.145 | 0.001 | 0.56 |
| BMC (g) | 1.33 | 1.55 | 0.02 | 0.01 |
| Area (cm2) | 9.38 | 10.75 | 0.11 | 0.01 |
| Weight (g) | 5.42 | 7.45 | 0.22 | 0.03 |
| Relative weight1 (g/kg) | 2.80 | 3.38 | 0.09 | 0.13 |
| Length (mm) | 69.33 | 71.33 | 0.37 | 0.20 |
| Width (mm) | 8.51 | 9.19 | 0.09 | 0.07 |
| Medial thickness (mm) | 1.41 | 1.27 | 0.03 | 0.25 |
| Lateral thickness (mm) | 1.41 | 1.50 | 0.02 | 0.38 |
| Weight/length index (mg/mm) | 77.85 | 103.74 | 2.66 | 0.03 |
| Robusticity index (g, cm) | 3.99 | 3.68 | 0.03 | 0.03 |
Least square means were presented (n = 12).
Abbreviations: BMC, bone mineral content; BMD, bone mineral density.
Relative weight was calculated as bone weight in g divided by body weight in kg.
Similarly, the femoral BMC, area, and weight of probiotic-fed broilers were significantly increased compared with those of control diet-fed broilers (P < 0.05), with a tendency of wider width (P = 0.07). The weight to length index of femur was significantly higher, and the robusticity index was significantly lower (P = 0.03).
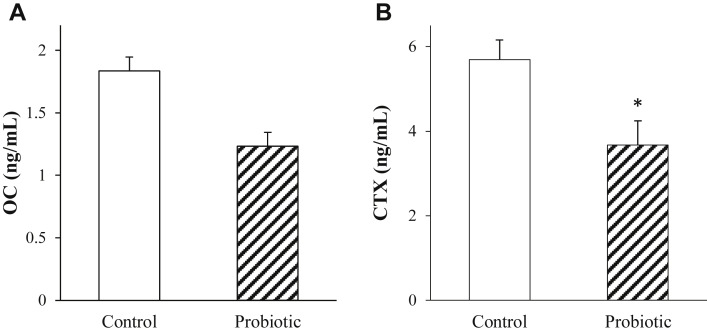
The results of latency to lie test at day 44 were similar between the probiotic-fed and the control broilers (probiotic: 122.42 ± 10.97 s; control:114.33 ± 10.97 s; P = 0.85). Compared with controls, serum concentrations of CTX (P = 0.02; Figure 1) but not OC were reduced in probiotic-fed broilers.
Figure 1.
The effects of probiotic on plasma concentrations of OC (A) and CTX (B) in 43-day-old broilers subjected to daily cycling heating episodes (n = 12). ∗Significant treatment differences P < 0.05. Abbreviations: CTX, c-terminal telopeptide of type I collagen; OC, osteocalcin.
Blood Parameters
Serum levels of Ca (P = 0.32) and phosphate (P = 0.14) were similar between probiotic-fed and control broilers (Table 4). There were also no treatment effects on the concentrations of blood 5-HT (P = 0.50), tryptophan (P = 0.93), and CORT (P = 0.42).
Table 4.
The effects of probiotic on serum parameters of 43-day-old broilers subjected to daily cycling heating episodes.
| Item | Control | Probiotic | SEM | P value |
|---|---|---|---|---|
| Calcium (mg/dL) | 11.86 | 11.34 | 0.13 | 0.32 |
| Phosphate (mg/dL) | 2.06 | 2.19 | 0.02 | 0.14 |
| 5-Hydroxytryptamine (ng/mL) | 35.81 | 31.33 | 11.44 | 0.50 |
| Tryptophan (umol/L) | 68.77 | 68.37 | 24.97 | 0.93 |
| Corticosterone (ng/mL) | 2.99 | 2.19 | 0.80 | 0.42 |
Least square means were presented (n = 12).
Brain Monoamines and Metabolites
In the raphe nuclei, the concentrations of 5-HT (P = 0.60), its precursor tryptophan (P = 0.81), and metabolite 5-HIAA (P = 0.46) were not affected by probiotic supplementation (Table 5). In addition, probiotic supplementation had no effects on the concentrations of catecholamines (NE [P = 0.83], EP [P = 0.66], and DA [P = 0.79]) and DA metabolites (DOPAC [P = 0.74] and HVA [P = 0.95]). The metabolic ratios of 5-HT, 5HIAA/5-HT (P = 0.51), and DA, DOPAC/DA (P = 0.3), were not different between treatments (P = 0.51).
Table 5.
The effects of probiotic on catecholamines, 5-HT, and respective metabolites in the raphe nuclei of 43-day-old broilers subjected to daily cycling heating episodes (n = 12).
| Item | Control | Probiotic | SEM | P value |
|---|---|---|---|---|
| Catecholamine system | ||||
| DA (ng/g) | 106.69 | 103.08 | 2.00 | 0.79 |
| NE (ng/g) | 921.93 | 946.96 | 23.34 | 0.83 |
| EP (ng/g) | 156.46 | 161.41 | 5.71 | 0.66 |
| DOPAC (ng/g) | 52.32 | 53.29 | 0.71 | 0.74 |
| HVA (ng/g) | 156.39 | 155.82 | 2.40 | 0.95 |
| DOPAC/DA | 0.49 | 0.53 | 0.01 | 0.30 |
| HVA/DOPAC | 1.48 | 1.56 | 0.04 | 0.64 |
| 5-HT system | ||||
| TRP (ng/g) | 5753.77 | 5887.75 | 141.30 | 0.81 |
| 5-HT (ng/g) | 472.39 | 448.69 | 10.97 | 0.60 |
| 5-HIAA (ng/g) | 376.39 | 411.81 | 11.69 | 0.46 |
| 5-HIAA/5-HT | 0.84 | 0.95 | 0.04 | 0.51 |
Least square means were presented (n = 12).
Abbreviations: 5-HIAA, 5-hydroxyindoleacetic acid; 5-HT, 5-hydroxytryptamine; DA, dopamine; DOPAC, 3,4-Dihydroxyphenylacetic acid; EP, epinephrine; HVA, homovanillic acid; NE, norepinephrine; TRP, tryptophan.
In the hypothalamus, there was no treatment effects on the concentrations of 5-HT and catecholamines, except for 5-HT and DA metabolites (Table 6). A tendency for higher concentrations of DOPAC (P = 0.10) and HVA (P = 0.07) was found in probiotic-fed broilers compared with controls. Probiotic-fed broilers also had a tendency for higher concentrations of 5-HIAA (P = 0.08), resulting in a higher 5-HIAA to 5-HT ratio (P = 0.07).
Table 6.
The effects of probiotic on catecholamines, 5-HT, and respective metabolites in the hypothalamus of 43-day-old broilers subjected to daily cycling heating episodes.
| Item | Control | Probiotic | SEM | P value |
|---|---|---|---|---|
| Catecholamine system | ||||
| DA (ng/g) | 301.77 | 295.84 | 6.76 | 0.37 |
| NE (ng/g) | 1673.75 | 1780.36 | 28.71 | 0.11 |
| EP (ng/g) | 273.22 | 322.92 | 7.39 | 0.83 |
| DOPAC (ng/g) | 88.25 | 97.36 | 1.31 | 0.10 |
| HVA (ng/g) | 235.71 | 268.94 | 4.27 | 0.07 |
| DOPAC/DA | 0.30 | 0.33 | 0.01 | 0.23 |
| HVA/DOPAC | 0.80 | 0.92 | 0.02 | 0.19 |
| 5-HT system | ||||
| TRP (ng/g) | 5041.90 | 5609.00 | 107.84 | 0.21 |
| 5-HT (ng/g) | 966.84 | 954.74 | 13.43 | 0.82 |
| 5-HIAA (ng/g) | 275.33 | 328.64 | 7.21 | 0.08 |
| 5-HIAA/5-HT | 0.28 | 0.35 | 0.01 | 0.07 |
Least square means were presented (n = 12).
Abbreviations: 5-HIAA, 5-hydroxyindoleacetic acid; 5-HT, 5-hydroxytryptamine; DA, dopamine; DOPAC, 3,4-dihydroxyphenylacetic acid; EP, epinephrine; HVA, homovanillic acid; NE, norepinephrine; TRP, tryptophan.
Cytokines
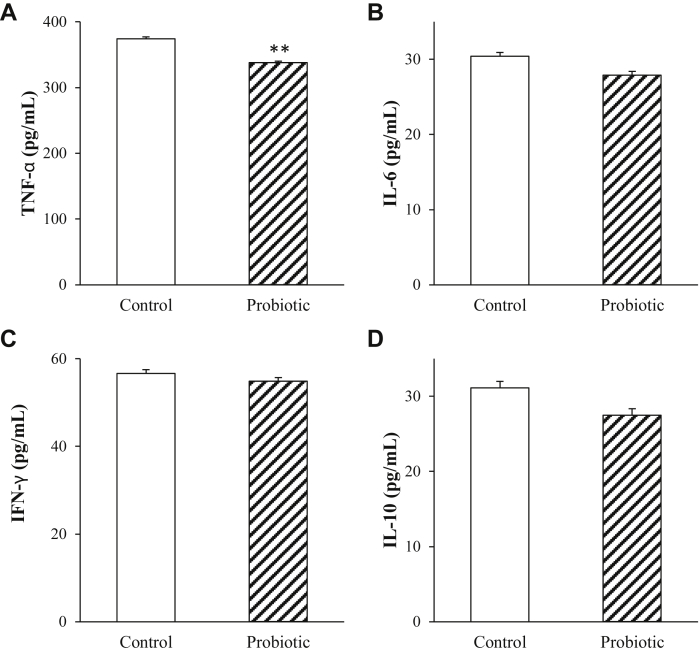
The concentrations of TNF-α were significantly decreased in broilers fed diet supplemented with the probiotic (P = 0.003; Figure 2) without effects on the concentrations of IL-6 (P = 0.22), IL-10 (P = 0.31), and IFN-γ (P = 0.62) compared to control broilers.
Figure 2.
The effects of probiotic on systemic immune cytokines in 43-day-old broilers subjected to daily cycling heating episodes (n = 12). A, tumor necrosis factor-α (TNF-α); B, interleukin-6 (IL-6); C, interferon-γ (IFN-γ); D, interleukin-10 (IL-10). ∗∗Significant treatment differences P < 0.01.
Discussion
The current heat episode 32°C/10 h/d (Lu et al., 2017; Mohammed et al., 2018) or similar ambient conditions 32°C to 33°C for 8 h/d (Cheng et al., 2019) have been reported to induce HS in broilers. In one of our parallel studies, heat-stressed broilers showed the signs of distress including panting, wing spreading, and squatting close to the ground; and these HS-associated behaviors were reduced by the dietary inclusion of the probiotic (Wang et al., 2018).
Numerous studies have demonstrated the function of probiotics on bone health in humans and various animals (Quach and Britton, 2017; McCabe and Parameswaran, 2018) including poultry (Houshmand et al., 2011; Ziaie et al., 2011; Abdelqader et al., 2013; Fuentes et al., 2013; Sadeghi, 2014; Yan et al., 2019b). However, the bone-promoting effects of probiotics in poultry under HS have not been widely evaluated, with one reported increased bone mass of the tibia, femur, and humerus in broilers after consuming a synbiotic mixed diet for 6 wk (Yan et al., 2019a). In the present study, our results showed that the dietary inclusion of the probiotic, Bacillus subtilis, increased the size, weight, and BMC of tibia and femur in 43-day-old broilers under HS, but the changes in BMD and bone wall thickness were not significant. The increase in BMC in broilers fed the probiotic diet is most likely due to increased body weight as a result of nutrient absorption and improved intestinal integrity. As reported by one of our parallel studies, probiotic supplementation had no effect on feed consumption of broilers but improved group averaged body weight (Wang et al., 2018). It has been reported elsewhere that the inclusion of probiotics ameliorates the negative effect of heat on gut health in broilers (Song et al., 2014) and laying hens (Deng et al., 2012). In the present study, the concentrations of Ca and phosphate in broilers may be increased as a result of feeding the probiotic, but it could occur in bones without reflection in the blood. Bone Ca was not measured in the present study; however, highly positive correlation between BMC and bone Ca has been reported for tibia and humerus in laying hens (Robison and Karcher, 2019). The effect of probiotic on serum Ca levels under thermoneutral condition has also been reported previously (Yan et al., 2018), and Ca levels were increased in probiotic-fed broilers at day 14 but not at day 43. On the other hand, these results are not in line with our previous findings that the Bacillus subtilis–based probiotic supplementation significantly promoted BMD of the femur and tibia under a thermoneutral condition (Yan et al., 2018). Possibly, the elevated cycling temperature investigated in the present study impaired the effectiveness of the probiotic to promote intestinal Ca absorption, affecting the deposition of Ca along with phosphate into bone tissues. HS-reduced Ca resorption in the intestine has been found in laying hens (Hansen et al., 2004).
The results collected from the latency to lie test suggest that probiotic supplementation does not improve lameness of broilers reared under elevated temperatures. The test has routinely been used as an indirect measure of the leg strength, especially for assessing the severity of lameness in broilers (Aydin et al., 2015). Under thermoneutral conditions, probiotics prophylactically can reduce incidences of lameness in wire-reared broilers (Wideman et al., 2012). The reasons without the treatment effect found in this study are unclear but could be related to water cooling effects. Heat-stressed broilers may lie down in water quickly for cooling regardless of water-associated stimulations, making it more difficult to show diet treatment effects. The hypothesis may further support that animals' adaption to stressors is based on their biological needs, such as HS vs. water stimulation, which is based on if the stressor affects animal survival and reproduction (Chrousos and Gold, 1992). This hypothesis will be tested in future studies.
Osteoblasts synthesize and release OC to facilitate bone mineralization and growth. Another role of OC is to assist with maintaining Ca balance between the tissue organs and blood (Zoch et al., 2016) under the influences of the Ca-regulating hormones. In the present study, there was no treatment effect on serum OC, which may contribute to the findings of constant levels of circulating Ca between probiotic-fed broilers and controls. Besides increased availability of minerals and nutrients needed for bone formation, other pathways, such as reduced bone resorption, may also be involved in stimulating bone growth and bone size traits in probiotic-fed broilers under the HS condition. Serum CTX is often used as a biomarker of bone resorption for evaluating bone turnover in humans because circulating levels of CTX are positively correlated with osteoclastic activity (Song, 2017). In the present study, the lower concentration of serum CTX as compared to controls may indicate that bone resorption is reduced in HS broilers fed probiotic diet, by which it may facilitate bone growth.
The effect of 5-HT on bone remodeling is dependent on its source, central or peripheral synthesis. In the brain, 5-HT is synthesized by the raphe serotonergic neurons, while in the peripheral, 5-HT is synthesized by the intestinal enterochromaffin cells (Fouquet et al., 2019). Brain 5-HT, acting as a neurotransmitter, stimulates bone formation and inhibits bone resorption, promoting bone development and increasing bone mass, whereas peripheral 5-HT, acting as a hormone, has an opposite effect on bone remodeling, resulting in inhibition of bone formation (Yadav et al., 2009). In our previous study, we revealed that the reduced bone resorption in probiotic-fed broilers reared under thermoneutral conditions is mediated by 5-HT–induced reduction of sympathetic activity (Yan et al., 2018). According to the proposed underlying regulation, similar to mammals (Koed and Linnet, 2000), probiotics upregulate 5-HT synthesis in the raphe nuclei which is then transported and released in the terminal areas within the hypothalamus, inhibiting NE synthesis. The reduced sympathetic outflow in turn contributes to reduced bone resorption. The endogenous sympathetic outflow such as releasing NE regulates bone remodeling, leading to reduced bone formation and increased bone resorption (Ma et al., 2013). A greater sympathetic activity has been used as a risk factor for stress-induced bone deterioration in humans (Kim et al., 2018) and mice (Baldock et al., 2014). However, this proposed mechanism is not upheld under the condition of cycling heating episodes as there are no significant effects of probiotic on both the peripheral and central 5-HT concentrations as well as the central catecholamines (NE, EP, and DA) and related metabolites. Other biological systems rather than the 5-HT and catecholamine systems may be involved in the regulation of bone remodeling in broilers under the current rearing conditions.
The immune system and bone health are tightly linked (Criscitiello et al., 2015), especially via the innate immune-bone axis (Charles and Nakamura, 2014). The activity of the RANKL/RANK axis is regulated by a variety of cytokines. For instance, IFN-γ, the main Th1 cytokine, functions to inhibit osteoclastogenesis (Pappalardo and Thompson, 2013). IL-10 causes immune response to inhibit osteoclastic bone resorption and regulate osteoblastic bone formation (Fujioka et al., 2015). In addition, some proinflammatory cytokines, such as IL-6 (Yokota et al., 2014) and TNF-α (de Vries et al., 2016), also involve in bone remodeling through regulation of osteoclast formation. HS has been shown to suppress immunity (Jahanian and Rasouli, 2015), including a rapid change of circulating cytokines. For instance, a study reported increased TNF-α and IL-4 levels but decreased IFN-γ and IL-2 levels in the spleen of chickens exposed to cycling heat for 16 h daily (4 h of 23.9°C–37°C, 8 h of 37°C, and 4 h of 37°C–23.9°C) for 4 wk (Xu et al., 2014). Our results showed that compared with controls, probiotic supplementation reduced the plasma concentrations of TNF-α in HS broilers. Consequently, the lowered levels of TNF-α may decrease osteoclast formation, which was paralleled with the reduced serum CTX concentrations, a bone resorption indicator, in the present study. The reduced TNF-α expression was correlated to facilitated bone development and growth in probiotic-fed broilers. In line with our findings, reduced TNF-α concentration or gene expression has been considered as the major reason of improved bone mass in germ-free mice or mice fed probiotic supplementations (Sjogren et al., 2012; McCabe et al., 2013). In addition, probiotics, such as Bacillus licheniformis, reduce HS-induced elevation of both serum TNF-α and CORT concentrations (Deng et al., 2012). In the present study, however, there was no probiotic effects on serum CORT concentrations, which may suggest that the probiotic regulates bone traits in broilers reared under HS mainly through the downregulation of the circulating proinflammatory TNF-α cytokine via the gut-microbiota-immune-bone axis.
Dietary supplementation of Bacillus subtilis–based probiotic promoted bone development, resulting in larger and heavier leg bones in broilers under cycling heat episodes. Mechanistically, the probiotic may inhibit HS-induced osteoclastogenesis by suppressing TNF-α expression to reduce bone resorption. The current findings suggest that dietary probiotic supplementation has a potential value as a management strategy for increasing skeletal health in broilers, especially for those reared at the tropical and subtropical locations.
Acknowledgments
This study was funded by the National Institute of Food and Agriculture - Agriculture and Food Research Initiative (NIFA-AFRI), United States Department of Agriculture (USDA) (grant number 2017-67015-26567); National Natural Science Foundation of China (grant number 31702153), and Scientific Research Foundation from Zhejiang A&F University (grant number 2017FR020). The probiotic was donated by the Novus International, Inc. (Saint Charles, MO). The authors thank J. Y. Hu, U. T. Mahmoud, G. T. Cao, X. H. Huang, Y. N. Wu, and Z. Y. Xu of the USDA-ARS Livestock Behavior Research (West Lafayette, IN) for technical assistance.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement of the USDA. The USDA is an equal opportunity provider and employer.
Conflicts of Interest Statement: The authors did not provide a conflict of interest statement.
Contributor Information
Fei-fei Yan, Email: yanff@zafu.edu.cn.
Heng-wei Cheng, Email: Heng-Wei.Cheng@ars.usda.gov.
References
- Abdelqader A., Irshaid R., Al-Fataftah A.R. Effects of dietary probiotic inclusion on performance, eggshell quality, cecal microflora composition, and tibia traits of laying hens in the late phase of production. Trop. Anim. Health Prod. 2013;45:1017–1024. doi: 10.1007/s11250-012-0326-7. [DOI] [PubMed] [Google Scholar]
- Aviagen. 2019. Ross 708 broiler nutrition specifications. Accessed Sep. 2020. https://en.aviagen.com/assets/Tech_Center/Ross_Broiler/RossBroilerNutritionSpecs2019-EN.pdf.
- Aydin A., Bahr C., Berckmans D. Automatic classification of measures of lying to assess the lameness of broilers. Anim. Welf. 2015;24:335–343. [Google Scholar]
- Baldock P.A., Lin S., Zhang L., Karl T., Shi Y., Driessler F., Zengin A., Hormer B., Lee N.J., Wong I.P., Lin E.J., Enriquez R.F., Stehrer B., During M.J., Yulyaningsih E., Zolotukhin S., Ruohonen S.T., Savontaus E., Sainsbury A., Herzog H. Neuropeptide y attenuates stress-induced bone loss through suppression of noradrenaline circuits. J. Bone Miner. Res. 2014;29:2238–2249. doi: 10.1002/jbmr.2205. [DOI] [PubMed] [Google Scholar]
- Berg C., Sanotra G. Can a modified latency-to-lie test be used to validate gait-scoring results in commercial broiler flocks? Anim. Welf. 2003;12:655–659. [Google Scholar]
- Boyce B.F., Li P., Yao Z., Zhang Q., Badell I.R., Schwarz E.M., O'Keefe R.J., Xing L. TNF-alpha and pathologic bone resorption. Keio J. Med. 2005;54:127–131. doi: 10.2302/kjm.54.127. [DOI] [PubMed] [Google Scholar]
- Caplen G., Baker L., Hothersall B., McKeegan D.E., Sandilands V., Sparks N.H., Waterman-Pearson A.E., Murrell J.C. Thermal nociception as a measure of non-steroidal anti-inflammatory drug effectiveness in broiler chickens with articular pain. Vet. J. 2013;198:616–619. doi: 10.1016/j.tvjl.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles J.F., Nakamura M.C. Bone and the innate immune system. Curr. Osteoporos. Rep. 2014;12:1–8. doi: 10.1007/s11914-014-0195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.F., Chen Y.P., Chen R., Su Y., Zhang R.Q., He Q.F., Wang K., Wen C., Zhou Y.M. Dietary mannan oligosaccharide ameliorates cyclic heat stress-induced damages on intestinal oxidative status and barrier integrity of broilers. Poult. Sci. 2019;98:4767–4776. doi: 10.3382/ps/pez192. [DOI] [PubMed] [Google Scholar]
- Cheng H.W., Dillworth G., Singleton P., Chen Y., Muir W.M. Effects of group selection for productivity and longevity on blood concentrations of serotonin, catecholamines, and corticosterone of laying hens. Poult. Sci. 2001;80:1278–1285. doi: 10.1093/ps/80.9.1278. [DOI] [PubMed] [Google Scholar]
- Chrousos G.P., Gold P.W. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Criscitiello C., Viale G., Gelao L., Esposito A., De Laurentiis M., De Placido S., Santangelo M., Goldhirsch A., Curigliano G. Crosstalk between bone niche and immune system: osteoimmunology signaling as a potential target for cancer treatment. Cancer Treat. Rev. 2015;41:61–68. doi: 10.1016/j.ctrv.2014.12.001. [DOI] [PubMed] [Google Scholar]
- de Vries T.J., Yousovich J., Schoenmaker T., Scheres N., Everts V. Tumor necrosis factor-alpha antagonist infliximab inhibits osteoclast formation of peripheral blood mononuclear cells but does not affect periodontal ligament fibroblast-mediated osteoclast formation. J. Periodontal Res. 2016;51:186–195. doi: 10.1111/jre.12297. [DOI] [PubMed] [Google Scholar]
- Deng W., Dong X.F., Tong J.M., Zhang Q. The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult. Sci. 2012;91:575–582. doi: 10.3382/ps.2010-01293. [DOI] [PubMed] [Google Scholar]
- Duque G., Huang D.C., Dion N., Macoritto M., Rivas D., Li W., Yang X.F., Li J., Lian J., Marino F.T., Barralet J., Lascau V., Deschenes C., Ste-Marie L.G., Kremer R. Interferon-gamma plays a role in bone formation in vivo and rescues osteoporosis in ovariectomized mice. J. Bone Miner. Res. 2011;26:1472–1483. doi: 10.1002/jbmr.350. [DOI] [PubMed] [Google Scholar]
- Fouquet G., Coman T., Hermine O., Cote F. Serotonin, hematopoiesis and stem cells. Pharmacol. Res. 2019;140:67–74. doi: 10.1016/j.phrs.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Fuentes C., Orozco L., Vicente J., Velasco X., Menconi A., Kuttappan V., Kallapura G., Latorre J., Layton S., Hargis B., Téllez G. Effect of a lactic acid bacterium based probiotic, Floramax-B11®, on performance, bone qualities, and morphometric analysis of broiler chickens: an economic analysis. Biol. Syst. Open Access. 2013;2:113. [Google Scholar]
- Fujioka K., Kishida T., Ejima A., Yamamoto K., Fujii W., Murakami K., Seno T., Yamamoto A., Kohno M., Oda R., Yamamoto T., Fujiwara H., Kawahito Y., Mazda O. Inhibition of osteoclastogenesis by osteoblast-like cells genetically engineered to produce interleukin-10. Biochem. Biophys. Res. Commun. 2015;456:785–791. doi: 10.1016/j.bbrc.2014.12.040. [DOI] [PubMed] [Google Scholar]
- Geraert P.A., Padilha J.C., Guillaumin S. Metabolic and endocrine changes induced by chronic heat exposure in broiler chickens: growth performance, body composition and energy retention. Br. J. Nutr. 1996;75:195–204. doi: 10.1079/bjn19960124. [DOI] [PubMed] [Google Scholar]
- Hall L.E., Shirley R.B., Bakalli R.I., Aggrey S.E., Pesti G.M., Edwards H.M., Jr. Power of two methods for the estimation of bone ash of broilers. Poult. Sci. 2003;82:414–418. doi: 10.1093/ps/82.3.414. [DOI] [PubMed] [Google Scholar]
- Hansen K.K., Beck M.M., Scheideler S.E., Blankenship E.E. Exogenous Estrogen Boosts circulating Estradiol concentrations and calcium Uptake by Duodenal tissue in heat-stressed hens. Poult. Sci. 2004;83:895–900. doi: 10.1093/ps/83.6.895. [DOI] [PubMed] [Google Scholar]
- Hester P.Y., Enneking S.A., Haley B.K., Cheng H.W., Einstein M.E., Rubin D.A. The effect of perch availability during pullet rearing and egg laying on musculoskeletal health of caged White Leghorn hens. Poult. Sci. 2013;92:1972–1980. doi: 10.3382/ps.2013-03008. [DOI] [PubMed] [Google Scholar]
- Hosseini-Vashan S.J., Golian A., Yaghobfar A. Growth, immune, antioxidant, and bone responses of heat stress-exposed broilers fed diets supplemented with tomato pomace. Int. J. Biometeorol. 2016;60:1183–1192. doi: 10.1007/s00484-015-1112-9. [DOI] [PubMed] [Google Scholar]
- Houshmand M., Azhar K., Zulkifli I., Bejo M.H., Meimandipour A., Kamyab A. Effects of non-antibiotic feed additives on performance, tibial dyschondroplasia incidence and tibia characteristics of broilers fed low-calcium diets. J. Anim. Physiol. Anim. Nutr. (Berl) 2011;95:351–358. doi: 10.1111/j.1439-0396.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- Hu F., Gao X., She R., Chen J., Mao J., Xiao P., Shi R. Effects of antimicrobial peptides on growth performance and small intestinal function in broilers under chronic heat stress. Poult. Sci. 2017;96:798–806. doi: 10.3382/ps/pew379. [DOI] [PubMed] [Google Scholar]
- Inada M., Miyaura C. Cytokines in bone diseases. Cytokine and postmenopausal osteoporosis. Clin. Calcium. 2010;20:1467–1472. [PubMed] [Google Scholar]
- Jahanian R., Rasouli E. Dietary chromium methionine supplementation could alleviate immunosuppressive effects of heat stress in broiler chicks. J. Anim. Sci. 2015;93:3355–3363. doi: 10.2527/jas.2014-8807. [DOI] [PubMed] [Google Scholar]
- Jankowski J., Mikulski D., Tatara M.R., Krupski W. Effects of increased stocking density and heat stress on growth, performance, carcase characteristics and skeletal properties in turkeys. Vet. Rec. 2015;176:21. doi: 10.1136/vr.102216. [DOI] [PubMed] [Google Scholar]
- Jia D., O'Brien C.A., Stewart S.A., Manolagas S.C., Weinstein R.S. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592–5599. doi: 10.1210/en.2006-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.J., Kwak M.K., Kim J.S., Lee S.H., Koh J.M. Higher sympathetic activity as a risk factor for skeletal deterioration in pheochromocytoma. Bone. 2018;116:1–7. doi: 10.1016/j.bone.2018.06.023. [DOI] [PubMed] [Google Scholar]
- Koed K., Linnet K. Opposing changes in serotonin and norepinephrine transporter mRNA levels after serotonin depletion. Eur. Neuropsychopharmacol. 2000;10:501–509. doi: 10.1016/s0924-977x(00)00121-8. [DOI] [PubMed] [Google Scholar]
- Lara L.J., Rostagno M.H. Impact of heat stress on poultry production. Animals (Basel) 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., He X., Ma B., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Chronic heat stress impairs the quality of Breast-Muscle meat in broilers by affecting redox status and energy-Substance metabolism. J. Agric. Food Chem. 2017;65:11251–11258. doi: 10.1021/acs.jafc.7b04428. [DOI] [PubMed] [Google Scholar]
- Ma Y., Krueger J.J., Redmon S.N., Uppuganti S., Nyman J.S., Hahn M.K., Elefteriou F. Extracellular norepinephrine clearance by the norepinephrine transporter is required for skeletal homeostasis. J. Biol. Chem. 2013;288:30105–30113. doi: 10.1074/jbc.M113.481309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack L.A., Felver-Gant J.N., Dennis R.L., Cheng H.W. Genetic variations alter production and behavioral responses following heat stress in 2 strains of laying hens. Poult. Sci. 2013;92:285–294. doi: 10.3382/ps.2012-02589. [DOI] [PubMed] [Google Scholar]
- McCabe L.R., Irwin R., Schaefer L., Britton R.A. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J. Cell. Physiol. 2013;228:1793–1798. doi: 10.1002/jcp.24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe L.R., Parameswaran N. Advances in probiotic regulation of bone and mineral metabolism. Calcif. Tissue Int. 2018;102:480–488. doi: 10.1007/s00223-018-0403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A.A., Jacobs J.A., Murugesan G.R., Cheng H.W. Effect of dietary synbiotic supplement on behavioral patterns and growth performance of broiler chickens reared under heat stress. Poult. Sci. 2018;97:1101–1108. doi: 10.3382/ps/pex421. [DOI] [PubMed] [Google Scholar]
- O'Brien C.A., Jia D., Plotkin L.I., Bellido T., Powers C.C., Stewart S.A., Manolagas S.C., Weinstein R.S. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145:1835–1841. doi: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- Pappalardo A., Thompson K. Activated gammadelta T cells inhibit osteoclast differentiation and resorptive activity in vitro. Clin. Exp. Immunol. 2013;174:281–291. doi: 10.1111/cei.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach D., Britton R.A. Gut microbiota and bone health. Adv. Exp. Med. Biol. 2017;1033:47–58. doi: 10.1007/978-3-319-66653-2_4. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Gomes A.V., Pinheiro M.L., Ribeiro A., Ferraz-de-Paula V., Astolfi-Ferreira C.S., Ferreira A.J., Palermo-Neto J. Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella Enteritidis. Avian Pathol. 2012;41:421–427. doi: 10.1080/03079457.2012.709315. [DOI] [PubMed] [Google Scholar]
- Riesenfeld A. Metatarsal robusticity in bipedal rats. Am. J. Phys. Anthropol. 1972;36:229–233. doi: 10.1002/ajpa.1330360211. [DOI] [PubMed] [Google Scholar]
- Robison C.I., Karcher D.M. Analytical bone calcium and bone ash from mature laying hens correlates to bone mineral content calculated from quantitative computed tomography scans. Poult. Sci. 2019;98:3611–3616. doi: 10.3382/ps/pez165. [DOI] [PubMed] [Google Scholar]
- Sadeghi A.A. Bone mineralization of broiler chicks Challenged with Salmonella enteritidis fed diet Containing probiotic (Bacillus subtilis) Probiotics Antimicrob. Proteins. 2014;6:136–140. doi: 10.1007/s12602-014-9170-6. [DOI] [PubMed] [Google Scholar]
- Schett G. Effects of inflammatory and anti-inflammatory cytokines on the bone. Eur. J. Clin. Invest. 2011;41:1361–1366. doi: 10.1111/j.1365-2362.2011.02545.x. [DOI] [PubMed] [Google Scholar]
- Seedor J.G., Quartuccio H.A., Thompson D.D. The bisphosphonate alendronate (MK-217) inhibits bone loss due to ovariectomy in rats. J. Bone Miner. Res. 1991;6:339–346. doi: 10.1002/jbmr.5650060405. [DOI] [PubMed] [Google Scholar]
- Sjogren K., Engdahl C., Henning P., Lerner U.H., Tremaroli V., Lagerquist M.K., Backhed F., Ohlsson C. The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. 2012;27:1357–1367. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Xiao K., Ke Y.L., Jiao L.F., Hu C.H., Diao Q.Y., Shi B., Zou X.T. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 2014;93:581–588. doi: 10.3382/ps.2013-03455. [DOI] [PubMed] [Google Scholar]
- Song L. Calcium and bone metabolism Indices. Adv. Clin. Chem. 2017;82:1–46. doi: 10.1016/bs.acc.2017.06.005. [DOI] [PubMed] [Google Scholar]
- Steel R.G., Torrie J.H., Dickey D.A. McGraw Hill Book Co.; New York, NY: 1997. Principles and Procedures of Statistics: A Biometrical Approach. [Google Scholar]
- Wang W.C., Yan F.F., Hu J.Y., Amen O.A., Cheng H.W. Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. J. Anim. Sci. 2018;96:1654–1666. doi: 10.1093/jas/sky092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wideman R.F., Jr., Hamal K.R., Stark J.M., Blankenship J., Lester H., Mitchell K.N., Lorenzoni G., Pevzner I. A wire-flooring model for inducing lameness in broilers: evaluation of probiotics as a prophylactic treatment. Poult. Sci. 2012;91:870–883. doi: 10.3382/ps.2011-01907. [DOI] [PubMed] [Google Scholar]
- Xu D., Li W., Huang Y., He J., Tian Y. The effect of selenium and polysaccharide of Atractylodes macrocephala Koidz. (PAMK) on immune response in chicken spleen under heat stress. Biol. Trace Elem. Res. 2014;160:232–237. doi: 10.1007/s12011-014-0056-y. [DOI] [PubMed] [Google Scholar]
- Yadav V.K., Oury F., Suda N., Liu Z.W., Gao X.B., Confavreux C., Klemenhagen K.C., Tanaka K.F., Gingrich J.A., Guo X.E., Tecott L.H., Mann J.J., Hen R., Horvath T.L., Karsenty G. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F.F., Mohammed A.A., Murugesan G.R., Cheng H.W. Effects of a dietary synbiotic inclusion on bone health in broilers subjected to cyclic heat stress episodes. Poult. Sci. 2019;98:1083–1089. doi: 10.3382/ps/pey508. [DOI] [PubMed] [Google Scholar]
- Yan F.F., Murugesan G.R., Cheng H.W. Effects of probiotic supplementation on performance traits, bone mineralization, cecal microbial composition, cytokines and corticosterone in laying hens. Animal. 2019;13:33–41. doi: 10.1017/S175173111800109X. [DOI] [PubMed] [Google Scholar]
- Yan F.F., Wang W.C., Cheng H.W. Bacillus subtilis based probiotic improved bone mass and altered brain serotoninergic and dopaminergic systems in broiler chickens. J. Funct. Foods. 2018;49:501–509. [Google Scholar]
- Yokota K., Sato K., Miyazaki T., Kitaura H., Kayama H., Miyoshi F., Araki Y., Akiyama Y., Takeda K., Mimura T. Combination of tumor necrosis factor alpha and interleukin-6 induces mouse osteoclast-like cells with bone resorption activity both in vitro and in vivo. Arthritis Rheumatol. 2014;66:121–129. doi: 10.1002/art.38218. [DOI] [PubMed] [Google Scholar]
- Ziaie H., Bashtani M., Torshizi M.A.K., Naeeimipour H., Farhangfar H., Zeinali A. Effect of antibiotic and its alternatives on morphometric characteristics, mineral content and bone strength of tibia in Ross broiler chickens. Glob. Vet. 2011;7:315–322. [Google Scholar]
- Zoch M.L., Clemens T.L., Riddle R.C. New insights into the biology of osteocalcin. Bone. 2016;82:42–49. doi: 10.1016/j.bone.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]