Abstract
Coinfection of Mycoplasma gallisepticum (MG) and Escherichia coli (E. coli) is frequently reported in poultry farms. Baicalin possess various pharmacological properties such as anti-inflammatory, anticancer, and antioxidant, etc. However, the protective effects of baicalin against coinfection of MG and E. coli are still elusive. In this study, baicalin (450 mg/kg) treatment was started on day 13 after infection and continued for 5 d. Histopathological examination, qRT-PCR, ELISA, and molecular docking technique were used to evaluate the effects of baicalin on MG and E. coli coinfection in chicken lung and trachea. The results showed that coinfection caused severe lesions in the lung and tracheal tissues. However, baicalin treatment partially alleviated these lesions in coinfection group. Histopathological examination showed the alveolar spaces and mucosal layer thickening was restored and cilia gradually recovered with baicalin treatment compared in coinfection group and MG-infection group. Meanwhile, IL-17 singling pathway–related genes were significantly reduced (P < 0.05) in baicalin treatment group in lung, including IL-17C, TRAF6, NF-κB, CXCL1, CXCL2, MMP1, GM-CSF, and MUC5AC. The activities of cytokines and chemokines (CXCL1, CXCL2, MMP1, GMCSF, and MUC5AC) were decreased significantly (P < 0.05) in baicalin-treated group. The molecular docking of baicalin and NF-κB showed the highest fitness score and interaction. From these results, it has been suggested that baicalin proved effective against coinfection of MG and E. coli in chicken and provided scientific basis for further dose–response and drug–target interaction studies.
Key words: Mycoplasma gallisepticum, Escherichia coli, molecular docking, baicalin, IL-17
Introduction
Mycoplasmas are the smallest microorganism, belongs to class mollicutes, and lack of cell wall is the special distinguishing feature of these bacteria (Gharibi et al., 2018). Among mycoplasmas, Mycoplasma gallisepticum (MG) is well studied and devastating pathogen that caused chronic respiratory diseases in chickens (Beaudet et al., 2017; Chen et al., 2020) and results in significant economic losses to the poultry industry (Ishfaq et al., 2020a; Elliott et al., 2020). Previous studies reported coinfection of MG with other respiratory pathogenic microorganisms including Avian influenza virus, Infectious bronchitis virus, Escherichia coli (E. coli), and Mycoplasma synoviae (Dhillon and Kibenge, 1987; Stipkovits et al., 2012; Xiao et al., 2014; Sid et al., 2015; Bwala et al., 2018; Hutchinson, 2018; Canter et al., 2019). In the present study, we investigated the effect of MG and E. coli single or combined infection on chicken lung and trachea. E. coli comprises different pathogenic as well as harmless commensal variants (Leimbach et al., 2013; Mol et al., 2019). Recently, coinfection chicken model of E. coli and MG received much more attention for carrying research to mitigate the losses caused by coinfection. Some studies developed MG and E. coli chicken coinfection model for studying the pharmacokinetics of drugs (Xiao et al., 2015). Another study evaluated the effect of single or combined infection of MG and E. coli on chicken immune response induced by Newcastle disease virus vaccine (Awad et al., 2019). Our previous studies investigated coinfection of MG with E. coli that caused immunosuppression and inflammatory injury in chicken lung with the upregulation of interleukin-17 (IL-17) signaling pathway (Wu et al., 2019b), and elevated serum leukotriene C4 was considered as a biomarker for detecting respiratory infection in chickens (Wu et al., 2020). While, in the present study, we scrutinized the preventive effects of baicalin against coinfection of MG and E. coli in chickens.
With the increasing frequency of antibiotic-resistant infections, new antibiotics are critical for modern medicine (Rossiter et al., 2017). More and more natural products have been confirmed for their biological activity (Wang et al., 2018). Baicalin is a flavonoid compound extracted from the roots of Scutellaria baicalensis Georgi (Zhao et al., 2019), and possess potential pharmacological properties such as anticancer, anti-inflammatory, antioxidant, etc. (Dinda et al., 2017). Baicalin suppressed inflammation mainly through suppression of toll-like receptors and nuclear factor kappa-B (NF-κB) pathway (Ming et al., 2018). Earlier studies explained that baicalin interacted with various signaling pathways and ameliorated MG-induced inflammation in chicken thymus (Li et al., 2019), spleen (Ishfaq et al., 2019a), DF-1 cells (Wu et al., 2019a) and lungs (Ishfaq et al., 2019b). In addition, baicalin proved effective against E. coli or LPS-induced inflammation in various experimental animals (Nagaki et al., 2001; Cheng et al., 2017; Zhao et al., 2018). However, the effect of baicalin against coinfection of MG and E. coli in chicken is still not reported. Furthermore, there was no research on the molecular docking of baicalin and the corresponding target in chicken. Therefore, the objectives of the present study were to investigate the protective effects of baicalin against coinfection of MG and E. coli in chicken. The study could provide basis for further molecular studies to explore the mechanisms of baicalin for the prevention of coinfection in chickens.
Materials and methods
Bacterial Infection and Baicalin Administration
Mycoplasma gallisepticum strain Rlow was obtained from the Harbin Institute of Veterinary Medicine (Chinese Academy of Agricultural Sciences) and was grown in a Modified Hayflick medium, as described previously (Lu et al., 2017). The bacteria were used to challenge chickens at the density of 1 × 109 CCU/mL (color change unit per milliliter) in the culture medium. Escherichia coli O78 was isolated from chickens infected with colibacillosis in our laboratory and cultured in Nutrient Broth (Beijing Aoboxing Bio-tech Co., Ltd.). The concentration of E. coli was adjusted to 109 CFU/mL before infection. The detection of the density for MG and E. coli were consistent as explained in our previous study (Wu et al., 2020; Wu et al., 2019b). Baicalin (purity ≥ 98.0%) was bought from Huifeng Animal Health Co., Ltd. (Heilongjiang, China). Baicalin was orally administered at a dose of 450 mg/kg as mentioned earlier (Ishfaq et al., 2019b).
Experimental Groupings
Eighty (1-day-old) White Leghorn chickens were purchased from Chia Chau Chicken Farm (Harbin, China) and were assigned randomly to 4 groups namely A (control group), B (baicalin-alone group), C (coinfection group), and D (coinfection + baicalin group). Each group was randomly assigned 20 chickens (6 chickens were divided into experimental groups in 3 replicates), housed in a positive-pressure fiberglass isolator, and provided with antibacterial-free balanced feed and fresh drinking water ad libitum. The antibacterial-free chicken feed was purchased from Lenong Feed Co., Ltd. (Harbin, Heilongjiang). The treatments are as follows: (A) control group; (B) baicalin-alone treated group (the baicalin treatment started on day 13 and continued for 5 d, once in a day at a dose of 450 mg/kg); (C) coinfection group (0.2 mL of MG medium (1 × 109 CCU/mL) was injected into the left caudal thoracic air sac on the seventh day, and 0.1 mL of E. coli bacteria (109 CFU/mL) was injected intraperitoneally at day 10); (D) coinfection + baicalin-treated group (after the aforementioned coinfection route, the baicalin treatment started on day 13 and continued for 5 d, once in a day at a dose of 450 mg/kg). At the 18th d, 20 chickens from each group were humanely sacrificed to avoid pain and suffering of chickens. Lung and tracheal samples in each group were collected for further analysis. The protocol for this experiment was approved by Institutional Animal Care and Use Committee of Northeast Agricultural University (Heilongjiang province, China) in accordance with Laboratory animal-Guideline for ethical review of animal welfare (GB/T 35892–2018, National Standards of the People's Republic of China).
Microscopic Lung and Tracheal Examination
Microscopic examination of lung and tracheal tissues were carried out as previously described (Wu et al., 2019a). The lung and tracheal tissues were fixed in 10% formalin, dehydrated, and immersed in transparent samples of wax, then cut into slices (4 mm) and stained with hematoxylin and eosin. The sections were observed under light microscope (Nikon E100, 40X magnification, Tokyo, Japan).
Extraction of Total RNA and Quantitative Real-Time PCR Analysis
The lung tissue samples were homogenized for 2 min at a low frequency of 65 Hz using an automatic tissue homogenizer machine (Shanghai Jingxin Industrial Development Co., Ltd.). Total RNA was extracted using TRIzol reagent (Invitrogen Inc., Carlsbad, CA) as previously described (Ishfaq et al., 2020b), and the reverse transcription of cDNA was performed in accordance with the manufacturer's instructions (Takara Biomedical Technology (Beijing) Co., Ltd.). In accordance with our previous study (Wu et al., 2019b), 13 genes in IL-17 signaling pathway were selected for further experimental analyses, which had showed significant changes in coinfection of MG and E. coli. The primer sequences are shown in Table 1. Quantitative real-time PCR (qRT-PCR) was performed to analyze gene expression using a LightCycler96 (Roche, Basel, Switzerland). Each sample was analyzed in triplicate. The fold change in gene expression was calculated using the △△cycle time (Ct) method (Schmittgen and Livak, 2008) after the expression level was normalized with GAPDH gene taken as internal standard.
Table 1.
Primers used in qRT-PCR analysis.
| Name | Sense strand/sense primer (5′–3′) | Antisense strand/antisense primer (5′–3′) |
|---|---|---|
| IL-17C | CGAGGACGAGGACCGCTACC | CACGGATGTAATCCACGTCGAAGG |
| CIKS | GCCGTGGTCAGAATATACCGATCC | GTCCTCAGGAGCATCATCCAAGC |
| TRAF6 | CACAGAGGAGACGCAGGGATA | AACAGATCGGGCACTCGTATTT |
| AP-1 | AAGCAGAGATGATGCACTGGAAGC | TGGATGTGATGCTGGTGTTGGATG |
| CXCL1 | TGGCTCTTCTCCTGATCTCAATG | GCACTGGCATCGGAGTTCA |
| CXCL2 | GCCCTCCTCCTGGTTTCAG | TGGCACCGCAGCTCATT |
| CXCL8 | CCAAGCACACCTCTCTTCCA | GCAAGGTAGGACGCTGGTAA |
| GMCSF | CCGTTTCAGGAACCAGAGAG | GTCTGGCTGCTGGACATTTT |
| MUC5AC | AAGACGGCATTTATTTCTCCAC | TCATTACCAACAAGCCAGTGA |
| MMP1 | ATTTGATGCCATTACCACTT | ACTTCATCCCTTTCAATGTTCT |
| MMP3 | ATCAGGCTCTACAGTGGTG | ATGGGATACATCAAGGCAC |
| C/EBPβ | ATTACGAGGCGGACTGTTTGG | CGGGTGAGGCTGATGTAGGTG |
| NF-κB | AGAAAAGCTGGGTCTTGGCA | CCATCTGTGTCAAAGCAGCG |
Determination of Cytokines and Chemokines by ELISA
Six lung samples from each group were cut and weighed (100 mg). About 500 mL of cold PBS (PH 7.4) was added, and the mixture was homogenized for 2 min at a low frequency of 65 Hz using the automatic tissue homogenizer machine followed by centrifugation (3,000 × g for 20 min) at 4°C. The supernatant was collected, and the samples were loaded on a 96-well microtiter plate in duplicate along with a blank control sample. Enzyme-linked immunosorbent assay (ELISA) for CXCL1, CXCL2, MMP1, GM-CSF, and MUC5AC were carried out in accordance with the manufacturer's instructions (Beijing Cheng Lin Biological Technology Co., Ltd.). The readings were taken at a wavelength of 450 nm on an iMARKTM microplate reader (Bio-Rad Co., Ltd. Shanghai, China).
Predictive Models and Molecular Docking
Total 5 target proteins were selected considering their key roles in IL-17 signaling pathway: TNF receptor–associated factor (TRAF6), SEFIR domain–containing protein, and positive regulation of I-kappaB kinase/NF-κB signaling (CIKS). Nuclear factor kappa-B p105 subunit: P105 is the precursor of the p50 subunit of NF-κB, which binds to the kappa-B consensus sequence 5′-GGRNNYYCC-3′, located in the enhancer region of genes involved in immune response and acute phase reactions. Transcription factor AP-1: transcription factor that recognizes and binds to the enhancer heptamer motif 5′-TGA[CG]TCA-3′, may bind to the USP28 promoter (AP-1) and CCAAT/enhancer-binding protein beta (C/EBPβ), and it is an important transcriptional activator regulating the expression of genes involved in immune and inflammatory responses. The sequence of these 5 proteins was obtained from the UniProt databases (Universal Protein Resource) (UniProt Consortium, 2018). The UniProtKB IDs were as follows: TRAF6, E1C626; CIKS, F1NQU5; NF-κB, Q04861; AP-1, P18870; and C/EBPβ, Q05826.
As the 3D structure of these 5 proteins (Gallus gallus) has not been elucidated yet, method of comparative modeling was used for their 3D structure. This alignment was submitted to the SWISS-MODEL server (Arnold et al., 2006; Guex et al., 2009) to construct the model using the alignment mode. Furthermore, PyMol (Delano, 2002) (version 2.3) software package was used to erase the heteroatoms, water molecules, and inhibitor present in the structure and saved as a PDB file. The 3D structures of the ligand molecule (baicalin) was built, optimized, and stored as a Mol2 file with the help of the TCMSP databases (Ru et al., 2014). The format of proteins and ligand was converted to the PDBQT files by the Open Babel (The Open Babel Package, version 2.3.1) (O'Boyle et al., 2011) before docking. The nonbonding interaction of ligand–protease was calculated using Autodock Vina software package (Trott and Olson, 2010) for docking analysis. After docking, the interaction of the 5 proteins and baicalin with the lowest affinity score for the receptors were selected for further analysis.
Statistical Analysis
Data are presented as mean results ± SD. All the experiments were performed in triplicates (n = 3) unless otherwise mentioned. The significance was determined using one-way ANOVA followed by Dunnett T3 test. The data were analyzed by using the GraphPad Prism (version 5.01). Values with P < 0.05 were considered statistically significant. Heatmaps were made by Heatmap Illustrator software (1.03.7).
Results
Histological Examination of Chicken Lung and Trachea
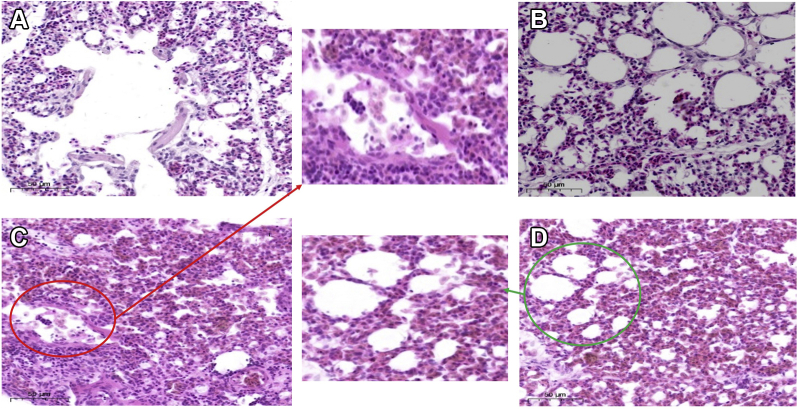
The photomicrographs of histopathological examination are shown in Figure 1. It has been examined that the alveolar structure was complete and the single-layer ciliated columnar epithelium structure was clearly visible in the control group (Figure 1A). The alveolar structure of group B was intact, with no obvious lesions (Figure 1B). It was clearly observed that the alveolar cavity was reduced and substantial lesions occurred, accompanied by a large amount of inflammatory cell infiltration (Figure 1C) in the coinfection group. In the baicalin treatment group (group D), the alveolar interval was widened and the capillaries were dilated and congested (Figure 1D).
Figure 1.
Histopathological examination of chicken lung (100×). (A) Control group. (B) Baicalin group. (C) Coinfection group, the red circle shows the alveolar cavity shrinking, deformed and filled with a large number of inflammatory cells. (D) Baicalin treatment group, the green circle shows the alveolar cavity structure gradually recovered.
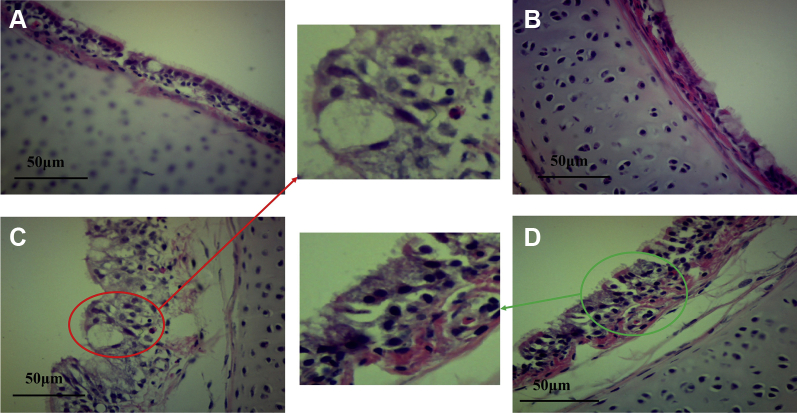
Histological assessment of tracheal tissues (Figure 2) showed that the structure of the mucosal epithelium and lamina propria was tight, and the cilia are evenly arranged in the control group (Figure 2A) and the baicalin-alone group (Figure 2B). Although in the coinfection group (Figure 2C), hyperplasia occurred in mucosal layer, the number of goblet cells increased and cilia were almost completely missing. The tracheal pathological characteristics after baicalin treatment improved significantly, the mucosal layer thickening was reduced and the cilia gradually recovered (Figure 2D).
Figure 2.
Histological assessment of tracheal tissues (100×). (A) Control group. (B) Baicalin group. (C) Coinfection group, the red circle shows the mucosal layer hyperplasia, goblet cells increased. (D) Baicalin treatment group, the green circle shows the mucosal layer thickening was reduced and the cilia gradually recovered.
Quantitative RT-PCR Verification on IL-17 Signaling in the Lung
Based on our previous study on IL-17 signaling pathway activation caused by coinfection, we screened 13 genes to explore the therapeutic effect of baicalin, including upstream (IL-17C, TRAF6, CIKS, NF-κB, AP-1, and C/EBPβ) and downstream genes (CXCL1, CXCL2, CXCL8, MMP1, MMP3, GM-CSF, and MUC5AC). The mRNA expression of genes was used to produce a heatmap for the relationships between the 3 groups, as shown in Figure 3. The heatmap shows that coinfection significantly activated the IL-17 signaling compared with the control group, and the downstream products were significantly reduced in baicalin-treated group. Among these upstream genes, IL-17C, TRAF6, and NF-κB show significant differences. Meanwhile, the downstream genes (CXCL1, CXCL2, MMP1, GM-CSF, and MUC5AC) showed significant differences and were selected for further investigation.
Figure 3.
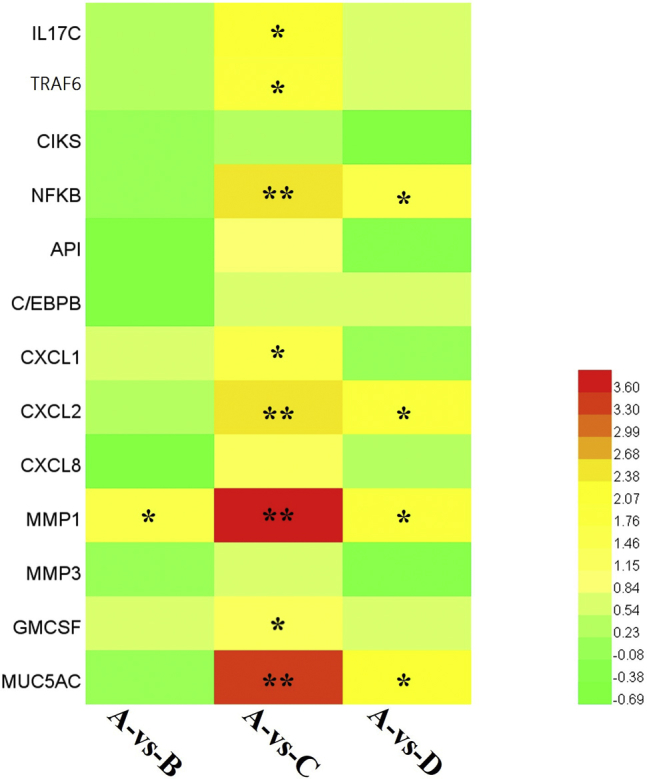
The heatmap of 13 IL-17 signaling-related genes from qPCR. A bright red indicates stronger upregulation and a green color indicates no significant change in expression. The values with star differ significantly (with ∗ 0.01 < P < 0.05) or very significantly (with ∗∗P < 0.01) compared with the control group.
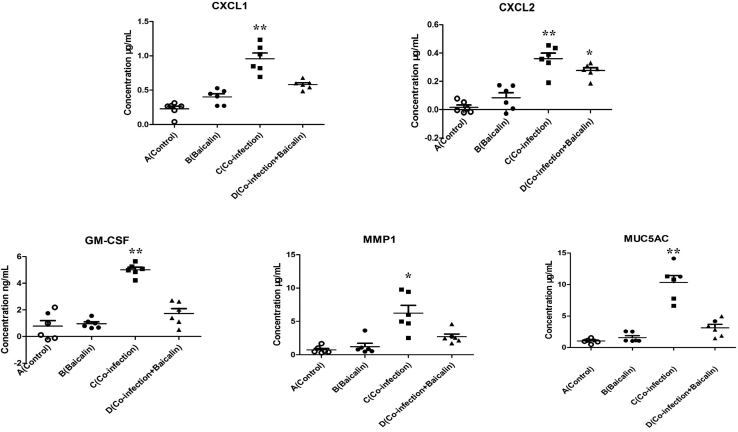
Influence of Baicalin Treatment on Cytokines and Chemokines in the Lung
To determine the effects of baicalin treatment, the influence of the 4 groups was determined by measuring the concentrations of CXCL1, CXCL2, MMP1, GMCSF, and MUC5AC in the lungs by using a sandwich ELISA. The results showed that compared with the control group, an increase in the expression of explosive IL-17 related cytokines and chemokines (P < 0.01) has been noted in the coinfection group, and the expression of cytokines and chemokines decreased significantly in baicalin-treated group (Figure 4). However, there was no significant increase (P > 0.05) in the baicalin control group compared with the control group.
Figure 4.
Scatter plot of IL-17 downstream cytokines and chemokines (CXCL1, CXCL2, MMP1, GM-CSF, and MUC5AC) detected by ELISA in the lung tissues. The values with star differ significantly (with ∗ 0.01 < P < 0.05) or very significantly (with ∗∗P < 0.01) compared with the control group.
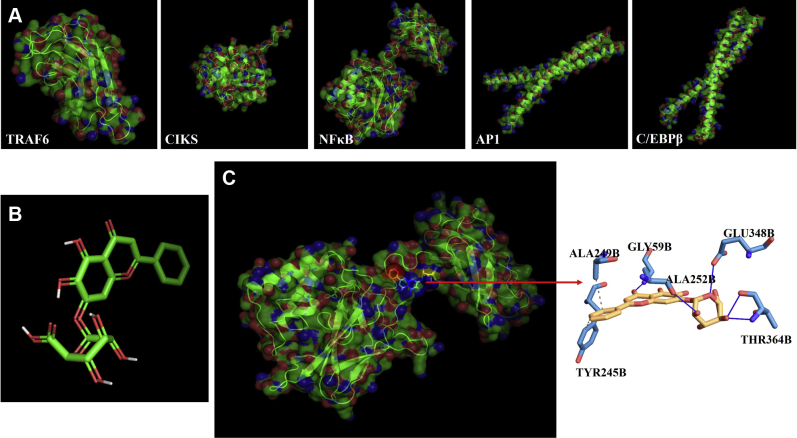
The Three-Dimensional Models and Molecular Docking
The three-dimensional models of the 5 proteins were shown in Figure 5A. The percentages of the best sequence identity were as follows: TRAF6, 84.81%; CIKS, 20.28%; NF-κB, 92.92%; AP-1, 98.39%; and C/EBPβ, 94.81%. The previous study found that the protein comprising the same family and containing at least 30% identity in sequence alignment can be a suitable template (Khan et al., 2016). The structure of baicalin is shown in Figure 5B. The molecular docking was performed to find out the best candidates based on their binding scores by using AutoDock Vina. The predicted binding affinity is in kcal/mol (energy). The docking scores were as follows: TRAF6, −6.1 kcal/mol; CIKS, −6.1 kcal/mol; NF-κB, −7.3 kcal/mol; AP-1, −5.7 kcal/mol; and C/EBPβ, −6.2 kcal/mol. As the higher fitness score indicates the better docking interaction between ligand and protein, therefore, we chose the docking interaction of baicalin and NF-κB for further study because of its higher score and interaction.
Figure 5.
Three-dimensional models of the 5 proteins (A) and baicalin template (B). (C) Nonbonding interactions of baicalin with the main protease of NF-κB (pose predicted by AutoDock Vina; the interaction analysis was analyzed by Protein–Ligand Interaction Profiler). The blue solid line represents hydrogen bond, and the gray dotted line represents hydrophobic interactions.
Molecular Interaction of Baicalin With NF-κB
The ligand (baicalin)-receptor (NF-κB) complex was generated by PyMol and the interaction analysis was analyzed by Protein–Ligand Interaction Profiler (Salentin et al., 2015) as shown in (Figure 5C). Baicalin was surrounded by 5 hydrogen bonds (GLY59B, ALA252B, THR346B, and GLU348B) and 2 hydrophobic bonds (TYR245B and ALA249B) interacting with the receptor protein.
Discussion
Previous studies demonstrated that MG-induced histopathological changes accompanied with severe inflammatory response in chicken lung and trachea (Bao et al., 2020; Ishfaq et al., 2020a). Similarly, avian pathogenic E. coli also induced pathological changes and caused severe lung damage (Peng et al., 2019). Several studies examined histopathological changes in lungs and mucosal respiratory epithelium with increased inflammation in coinfection of MG with other respiratory pathogens including E. coli (Stipkovits et al., 2012; Xiao et al., 2014; Sid et al., 2015; Wu et al., 2020). In the present study, increased expressions of inflammatory cytokines were observed in chicken lung in MG or E. coli single or mixed infection group. However, baicalin partially ameliorated histopathological changes in MG or E. coli single or combined infection group. These findings are in line with previous studies that baicalin alleviated histopathological and suppressed the increased expression of inflammatory markers in chicken lung during E. coli or MG infection (Ding et al., 2016; Ishfaq et al., 2019b; Wu et al., 2019a). These findings aid in understanding the therapeutic role of baicalin in coinfection. However, further studies are needed to investigate the dose–response, bioavailability, administration route and dose optimization studies of baicalin in chicken coinfection models.
Researchers reported that respiratory pathogen–induced lung damage was associated with changes in inflammatory cytokines and/or chemokines and signaling pathways (Melvin and Bomberger, 2016), including IRF1, STAT1, and NOD-like receptor ligands (Feng et al., 2016), CXCL8 and C/EBPβ (Huang et al., 2017), and IL-17 (Kurai et al., 2013; Jungnickel et al., 2017), were commonly reported. In the present study, key inflammatory mediators including upstream and downstream mediators were selected based on our previous findings (Wu et al., 2019b), and their mRNA and protein expression were examined by qRT-PCR and ELISA assays, respectively. Our results indicated that IL-17C, TRAF6, NF-κB, CXCL1, CXCL2, MMP1, GM-CSF, and MUC5AC gene mRNA expression were significantly enhanced in coinfection group. The proteins expression results were in consistence and CXCL1, CXCL2, MMP1, GMCSF, and MUC5AC gene protein expression were increased in coinfection group. Meanwhile, baicalin significantly reduced the expression of these inflammatory markers both at mRNA and protein level. The previous study showed that baicalin effectively protects against allergic asthma in mice by regulating the immunological imbalance of Th17 responses (Xu et al., 2017), and baicalin significantly reduced related gene expressions, including IL-6, TNF, CXCL1, CXCL10, MMP3, MMP13, and Nos2 in a knee osteoarthritis mice model (Xing et al., 2017). Our findings showed that baicalin has a therapeutic effect on coinfection through the IL-17 signaling pathway. Hence, we conducted in vitro studies on the molecular docking of baicalin with IL-17-related target proteins for further research.
In the present study, docking results were as follows: TRAF6, −6.1 kcal/mol; CIKS, −6.1 kcal/mol; NF-κB, −7.3 kcal/mol; AP-1, −5.7 kcal/mol; and C/EBPβ, −6.2 kcal/mol. The higher fitness score indicates the better docking interaction between ligand and protein, so the interaction of NF-κB with baicalin has the strongest binding ability among IL-17 upstream-related proteins. Previous studies showed that baicalin protects against chronic gastritis in rat by inhibiting Akt/NF-κB pathway, and the molecular docking analysis showed baicalin had affinity with NF-κB (p65, PDB ID: 3QXY) (Ji et al., 2019). The interaction between baicalin and P2Y12 receptor (PDB ID: 4PY0) were determined by molecular docking (Sheng et al., 2018), which demonstrated fit interaction (−8.4 kcal/mol). In silico analysis using molecular docking demonstrated close interactions between baicalin and chikungunya virus envelope protein with considerably strong binding affinity of −9.7 kcal/mol (Oo et al., 2018). Baicalin was found to have a wide range of antiviral, antibacterial, and anti-inflammatory effects through the method of molecular docking. Our study provides a new insight through molecular docking that how baicalin inhibits IL-17 inflammatory storm caused by coinfection in chicken. Nevertheless, future interaction studies are required to scrutinize the complex relationship between baicalin and its molecular targets that could provide better understanding of ligand-binding interaction and signaling pathways, and these finding could facilitate the treatment of various inflammatory diseases.
In conclusion, baicalin partially ameliorated abnormal morphological changes in single or combined coinfection group in chicken lung and trachea. In addition, the increased level of NF-κB and its downstream genes were significantly reduced in chicken lung. Moreover, docking revealed higher interaction between NF-κB and baicalin. These results provide basis for further molecular studies to investigate the interaction between baicalin and targets.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31973005 and 31772801).
Conflict of Interest Statement: The authors declare no conflicts of interest and no competing financial interests.
Contributor Information
Muhammad Ishfaq, Email: ishfaqmuhammad@neau.edu.cn.
Jichang Li, Email: lijichang@neau.edu.cn.
References
- Arnold K., Bordoli L., Kopp J., Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- Awad N.F.S., Abd El-Hamid M.I., Hashem Y.M., Erfan A.M., Abdelrahman B.A., Mahmoud H.I. Impact of single and mixed infections with Escherichia coli and Mycoplasma gallisepticum on Newcastle disease virus vaccine performance in broiler chickens: an in vivo perspective. J. Appl. Microbiol. 2019;127:396–405. doi: 10.1111/jam.14303. [DOI] [PubMed] [Google Scholar]
- Bao J., Wu Z., Ishfaq M., Miao Y., Li R., Clifton A.C., Ding L., Li J. Comparison of experimental infection of normal and immunosuppressed chickens with mycoplasma gallisepticum. J. Comp. Pathol. 2020;175:5–12. doi: 10.1016/j.jcpa.2019.12.001. [DOI] [PubMed] [Google Scholar]
- Beaudet J., Tulman E.R., Pflaum K., Liao X., Kutish G.F., Szczepanek S.M., Silbart L.K., Geary S.J. Transcriptional profiling of the chicken tracheal response to virulent Mycoplasma gallisepticum strain R low. Infect. Immun. 2017;85 doi: 10.1128/IAI.00343-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bwala D.G., Solomon P., Duncan N., Wandrag D.B.R., Abolnik C. Assessment of Mycoplasma gallisepticum vaccine efficacy in a co-infection challenge model with QX-like infectious bronchitis virus. Avian Pathol. 2018;47:261–270. doi: 10.1080/03079457.2018.1440064. [DOI] [PubMed] [Google Scholar]
- Canter J.A., Tulman E.R., Beaudet J., Lee D.H., May M., Szczepanek S.M., Geary S.J. Transcriptional and pathological host responses to coinfection with virulent or attenuated mycoplasma gallisepticum and low-pathogenic avian influenza A virus in chickens. Infect. Immun. 2019;88 doi: 10.1128/IAI.00607-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Li J., Zhang W., Shah S.W.A., Ishfaq M. Mycoplasma gallisepticum triggers immune damage in the chicken thymus by activating the TLR-2/MyD88/NF-kappaB signaling pathway and NLRP3 inflammasome. Vet. Res. 2020;51:52. doi: 10.1186/s13567-020-00777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P., Wang T., Li W., Muhammad I., Wang H., Sun X., Yang Y., Li J., Xiao T., Zhang X. Baicalin alleviates lipopolysaccharide-induced liver inflammation in chicken by suppressing TLR4-mediated NF-kappaB pathway. Front. Pharmacol. 2017;8:547. doi: 10.3389/fphar.2017.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano W.L. Vol 30. DeLano Scientific; San Carlos, CA: 2002. (The PyMol Molecular Graphics System. Pages 442–454 in). [Google Scholar]
- Dhillon A.S., Kibenge F.S. Adenovirus infection associated with respiratory disease in commercial chickens. Avian Dis. 1987;31:654–657. [PubMed] [Google Scholar]
- Dinda B., Dinda S., DasSharma S., Banik R., Chakraborty A., Dinda M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017;131:68–80. doi: 10.1016/j.ejmech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Ding X.M., Pan L., Wang Y., Xu Q.Z. Baicalin exerts protective effects against lipopolysaccharide-induced acute lung injury by regulating the crosstalk between the CX3CL1-CX3CR1 axis and NF-kappaB pathway in CX3CL1-knockout mice. Int. J. Mol. Med. 2016;37:703–715. doi: 10.3892/ijmm.2016.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K.E.C., Branton S.L., Evans J.D., Leigh S.A., Kim E.J., Olanrewaju H.A., Pharr G.T., Pavlidis H.O., Gerard P.D., Peebles E.D. Growth and humoral immune effects of dietary original XPC in layer pullets challenged with Mycoplasma gallisepticum. Poult. Sci. 2020;99:3030–3037. doi: 10.1016/j.psj.2020.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M., Dai M., Xie T., Li Z., Shi M., Zhang X. Innate immune responses in ALV-J infected chicks and chickens with hemangioma in vivo. Front. Microbiol. 2016;7:786. doi: 10.3389/fmicb.2016.00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharibi D., Ghadimipour R., Mayahi M. Detection of Mycoplasma gallisepticum and Mycoplasma synoviae among commercial poultry in Khouzestan province, Iran. Arch. Razi. Inst. 2018;73:139–146. doi: 10.22092/ari.2018.116164. [DOI] [PubMed] [Google Scholar]
- Guex N., Peitsch M.C., Schwede T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis. 2009;30 Suppl 1:S162–S173. doi: 10.1002/elps.200900140. [DOI] [PubMed] [Google Scholar]
- Huang Z.W., Lien G.S., Lin C.H., Jiang C.P., Chen B.C. p300 and C/EBPbeta-regulated IKKbeta expression are involved in thrombin-induced IL-8/CXCL8 expression in human lung epithelial cells. Pharmacol. Res. 2017;121:33–41. doi: 10.1016/j.phrs.2017.04.020. [DOI] [PubMed] [Google Scholar]
- Hutchinson E.C. Influenza virus. Trends. Microbiol. 2018;26:809–810. doi: 10.1016/j.tim.2018.05.013. [DOI] [PubMed] [Google Scholar]
- Ishfaq M., Chen C., Bao J., Zhang W., Wu Z., Wang J., Liu Y., Tian E., Hamid S., Li R., Ding L., Li J. Baicalin ameliorates oxidative stress and apoptosis by restoring mitochondrial dynamics in the spleen of chickens via the opposite modulation of NF-kappaB and Nrf2/HO-1 signaling pathway during Mycoplasma gallisepticum infection. Poult. Sci. 2019;98:6296–6310. doi: 10.3382/ps/pez406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishfaq M., Zhang W., Ali Shah S.W., Wu Z., Wang J., Ding L., Li J. The effect of Mycoplasma gallisepticum infection on energy metabolism in chicken lungs: through oxidative stress and inflammation. Microb. Pathog. 2020;138:103848. doi: 10.1016/j.micpath.2019.103848. [DOI] [PubMed] [Google Scholar]
- Ishfaq M., Zhang W., Liu Y., Wang J., Wu Z., Waqas Ali Shah S., Li R., Miao Y., Chen C., Li J. Baicalin attenuated Mycoplasma gallisepticum-induced immune impairment in the chicken Bursa of fabricius through modulation of autophagy and inhibited inflammation and apoptosis [e-pub ahead of print] J. Sci. Food Agric. 2020 doi: 10.1002/jsfa.10695. accessed Sep. 30, 2020. [DOI] [PubMed] [Google Scholar]
- Ishfaq M., Zhang W., Hu W., Waqas Ali Shah S., Liu Y., Wang J., Wu Z., Ahmad I., Li J. Antagonistic effects of baicalin on Mycoplasma gallisepticum-induced inflammation and apoptosis by restoring energy metabolism in the chicken lungs. Infect. Drug Resist. 2019;12:3075–3089. doi: 10.2147/IDR.S223085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W., Liang K., An R., Wang X. Baicalin protects against ethanol-induced chronic gastritis in rats by inhibiting Akt/NF-kappaB pathway. Life Sci. 2019;239:117064. doi: 10.1016/j.lfs.2019.117064. [DOI] [PubMed] [Google Scholar]
- Jungnickel C., Schmidt L.H., Bittigkoffer L., Wolf L., Wolf A., Ritzmann F., Kamyschnikow A., Herr C., Menger M.D., Spieker T., Wiewrodt R., Bals R., Beisswenger C. IL-17C mediates the recruitment of tumor-associated neutrophils and lung tumor growth. Oncogene. 2017;36:4182–4190. doi: 10.1038/onc.2017.28. [DOI] [PubMed] [Google Scholar]
- Khan F.I., Wei D.Q., Gu K.R., Hassan M.I., Tabrez S. Current updates on computer aided protein modeling and designing. Int. J. Biol. Macromol. 2016;85:48–62. doi: 10.1016/j.ijbiomac.2015.12.072. [DOI] [PubMed] [Google Scholar]
- Kurai D., Nakagaki K., Wada H., Saraya T., Kamiya S., Fujioka Y., Nakata K., Takizawa H., Goto H. Mycoplasma pneumoniae extract induces an IL-17-associated inflammatory reaction in murine lung: implication for mycoplasmal pneumonia. Inflammation. 2013;36:285–293. doi: 10.1007/s10753-012-9545-3. [DOI] [PubMed] [Google Scholar]
- Leimbach A., Hacker J., Dobrindt U. E. coli as an all-rounder: the thin line between commensalism and pathogenicity. Curr. Top. Microbiol. Immunol. 2013;358:3–32. doi: 10.1007/82_2012_303. [DOI] [PubMed] [Google Scholar]
- Li J., Qiao Z., Hu W., Zhang W., Shah S.W.A., Ishfaq M. Baicalin mitigated Mycoplasma gallisepticum-induced structural damage and attenuated oxidative stress and apoptosis in chicken thymus through the Nrf2/HO-1 defence pathway. Vet. Res. 2019;50:83. doi: 10.1186/s13567-019-0703-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Xie D., Chen Y., Tian E., Muhammad I., Chen X., Miao Y., Hu W., Wu Z., Ni H., Xin J., Li Y., Li J. TLR2 mediates autophagy through ERK signaling pathway in Mycoplasma gallisepticum-infected RAW264.7 cells. Mol. Immunol. 2017;87:161–170. doi: 10.1016/j.molimm.2017.04.013. [DOI] [PubMed] [Google Scholar]
- Melvin J.A., Bomberger J.M. Compromised defenses: exploitation of epithelial responses during viral-bacterial co-infection of the respiratory tract. PLoS Pathog. 2016;12:e1005797. doi: 10.1371/journal.ppat.1005797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming J., Zhuoneng L., Guangxun Z. Protective role of flavonoid baicalin from Scutellaria baicalensis in periodontal disease pathogenesis: a literature review. Complement. Ther. Med. 2018;38:11–18. doi: 10.1016/j.ctim.2018.03.010. [DOI] [PubMed] [Google Scholar]
- Mol N., Peng L., Esnault E., Quere P., Haagsman H.P., Veldhuizen E.J.A. Avian pathogenic Escherichia coli infection of a chicken lung epithelial cell line. Vet. Immunol. Immunopathol. 2019;210:55–59. doi: 10.1016/j.vetimm.2019.03.007. [DOI] [PubMed] [Google Scholar]
- Nagaki Y., Hayasaka S., Kadoi C., Nakamura N., Hayasaka Y. Effects of scutellariae radix extract and its components (baicalein, baicalin, and wogonin) on the experimental elevation of aqueous flare in pigmented rabbits. Jpn. J. Ophthalmol. 2001;45:216–220. doi: 10.1016/s0021-5155(01)00330-6. [DOI] [PubMed] [Google Scholar]
- O'Boyle N.M., Banck M., James C.A., Morley C., Vandermeersch T., Hutchison G.R. Open babel: an open chemical toolbox. J. Cheminform. 2011;3:33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oo A., Rausalu K., Merits A., Higgs S., Vanlandingham D., Bakar S.A., Zandi K. Deciphering the potential of baicalin as an antiviral agent for Chikungunya virus infection. Antiviral. Res. 2018;150:101–111. doi: 10.1016/j.antiviral.2017.12.012. [DOI] [PubMed] [Google Scholar]
- Peng L.Y., Yuan M., Song K., Yu J.L., Li J.H., Huang J.N., Yi P.F., Fu B.D., Shen H.Q. Baicalin alleviated APEC-induced acute lung injury in chicken by inhibiting NF-kappaB pathway activation. Int. Immunopharmacol. 2019;72:467–472. doi: 10.1016/j.intimp.2019.04.046. [DOI] [PubMed] [Google Scholar]
- Rossiter S.E., Fletcher M.H., Wuest W.M. Natural products as platforms to overcome antibiotic resistance. Chem. Rev. 2017;117:12415–12474. doi: 10.1021/acs.chemrev.7b00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru J., Li P., Wang J., Zhou W., Li B., Huang C., Li P., Guo Z., Tao W., Yang Y., Xu X., Li Y., Wang Y., Yang L. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salentin S., Schreiber S., Haupt V.J., Adasme M.F., Schroeder M. PLIP: fully automated protein-ligand interaction profiler. Nucleic Acids Res. 2015;43:W443–W447. doi: 10.1093/nar/gkv315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sheng X., Wang J., Guo J., Xu Y., Jiang H., Zheng C., Xu Z., Zhang Y., Che H., Liang S., Zhu G., Li G. Effects of baicalin on diabetic cardiac autonomic neuropathy mediated by the P2Y12 receptor in rat stellate ganglia. Cell. Physiol. Biochem. 2018;46:986–998. doi: 10.1159/000488828. [DOI] [PubMed] [Google Scholar]
- Sid H., Benachour K., Rautenschlein S. Co-infection with multiple respiratory pathogens contributes to increased mortality rates in Algerian poultry flocks. Avian Dis. 2015;59:440–446. doi: 10.1637/11063-031615-Case.1. [DOI] [PubMed] [Google Scholar]
- Stipkovits L., Egyed L., Palfi V., Beres A., Pitlik E., Somogyi M., Szathmary S., Denes B. Effect of low-pathogenicity influenza virus H3N8 infection on Mycoplasma gallisepticum infection of chickens. Avian Pathol. 2012;41:51–57. doi: 10.1080/03079457.2011.635635. [DOI] [PubMed] [Google Scholar]
- Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UniProt Consortium T. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2018;46:2699. doi: 10.1093/nar/gky092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Jin X., Li Q., Sawaya A., Le Leu R.K., Conlon M.A., Wu L., Hu F. Propolis from different geographic origins decreases intestinal inflammation and Bacteroides spp. populations in a model of DSS-induced colitis. Mol. Nutr. Food Res. 2018;62:e1800080. doi: 10.1002/mnfr.201800080. [DOI] [PubMed] [Google Scholar]
- Wu Z., Chen C., Miao Y., Liu Y., Zhang Q., Li R., Ding L., Ishfaq M., Li J. Baicalin attenuates Mycoplasma gallisepticum-induced inflammation via inhibition of the TLR2-NF-kappaB pathway in chicken and DF-1 cells. Infect. Drug Resist. 2019;12:3911–3923. doi: 10.2147/IDR.S231908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Chen C., Zhang Q., Bao J., Fan Q., Li R., Ishfaq M., Li J. Arachidonic acid metabolism is elevated in Mycoplasma gallisepticum and Escherichia coli co-infection and induces LTC4 in serum as the biomarker for detecting poultry respiratory disease. Virulence. 2020;11:730–738. doi: 10.1080/21505594.2020.1772653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Ding L., Bao J., Liu Y., Zhang Q., Wang J., Li R., Ishfaq M., Li J. Co-infection of Mycoplasma gallisepticum and Escherichia coli triggers inflammatory injury involving the IL-17 signaling pathway. Front. Microbiol. 2019;10:2615. doi: 10.3389/fmicb.2019.02615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Sun J., Chen Y., Zou M., Zhao D.H., Liu Y.H. Ex vivo pharmacokinetic and pharmacodynamic analysis of valnemulin against Mycoplasma gallisepticum S6 in Mycoplasma gallisepticum and Escherichia coli co-infected chickens. Vet. J. 2015;204:54–59. doi: 10.1016/j.tvjl.2015.01.020. [DOI] [PubMed] [Google Scholar]
- Xiao X., Zhao D.H., Yang X., Shi W., Deng H., Ma J., Zhang S., Liu Y.H. Mycoplasma gallisepticum and Escherichia coli mixed infection model in broiler chickens for studying valnemulin pharmacokinetics. J. Vet. Pharmacol. Ther. 2014;37:99–102. doi: 10.1111/jvp.12065. [DOI] [PubMed] [Google Scholar]
- Xing D., Gao H., Liu Z., Zhao Y., Gong M. Baicalin inhibits inflammatory responses to interleukin-1beta stimulation in human chondrocytes. J. Interferon Cytokine Res. 2017;37:398–405. doi: 10.1089/jir.2017.0030. [DOI] [PubMed] [Google Scholar]
- Xu L., Li J., Zhang Y., Zhao P., Zhang X. Regulatory effect of baicalin on the imbalance of Th17/Treg responses in mice with allergic asthma. J. Ethnopharmacol. 2017;208:199–206. doi: 10.1016/j.jep.2017.07.013. [DOI] [PubMed] [Google Scholar]
- Zhao T., Tang H., Xie L., Zheng Y., Ma Z., Sun Q., Li X. Scutellaria baicalensis Georgi. (Lamiaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019;71:1353–1369. doi: 10.1111/jphp.13129. [DOI] [PubMed] [Google Scholar]
- Zhao Q.Y., Yuan F.W., Liang T., Liang X.C., Luo Y.R., Jiang M., Qing S.Z., Zhang W.M. Baicalin inhibits Escherichia coli isolates in bovine mastitic milk and reduces antimicrobial resistance. J. Dairy Sci. 2018;101:2415–2422. doi: 10.3168/jds.2017-13349. [DOI] [PubMed] [Google Scholar]