Abstract
Poultry colibacillosis has been one of the major causes behind economic losses in the poultry production; however, no effective method for its prevention has been developed so far. Vaccination against colibacillosis is capturing increasing interest. The aim of this study was to demonstrate benefits from using a live, aroA gene–deleted vaccine against colibacillosis in broiler chickens and its potential impact on reduced use of antibiotics, the efficacy of vaccination against infectious bronchitis (IB), and the structure and properties of Escherichia coli population in broilers under commercial farm conditions. In 2 experiments, carried out on 3 farms, broiler chickens of one chicken house from each farm were vaccinated against Escherichia coli (E. coli), whereas birds of other chicken houses of each farm were not vaccinated against E. coli. In experiment 1, which was carried out on 2 farms, for 3 consecutive production cycles, spray vaccination of day-old broilers against E. coli decreased the number of E. coli isolates from internal organs but not from the respiratory system in the sixth week of birds' life. In experiment 1, E. coli–vaccinated broilers did not receive the antimicrobials until 14 d after the vaccination. Escherichia coli isolates from the E. coli–vaccinated birds were more susceptible to the antimicrobials. Escherichia coli vaccination had no impact on the IB vaccination efficiency; it has reduced the mean number of days of the antimicrobial treatment and improved broiler production parameters. In experiment 2, chickens of both houses received the antimicrobials for the first 4 d of their life. Birds of chicken house 1 were vaccinated against E. coli on the ninth day of life, whereas birds of chicken house 2 were not vaccinated. In both houses, further antimicrobial usage was the same, and antimicrobials were not used until 14 d after E. coli vaccination. Similar to experiment 1, in experiment 2, vaccination decreased the number of E. coli isolates, and these isolates were more susceptible to the antimicrobials. Vaccination of broilers against E. coli should be considered in terms of routine immunoprophylaxis.
Key words: E. coli, vaccination, broiler chicken, field condition
Introduction
Poultry colibacillosis, induced by avian pathogenic Escherichia coli (APEC), usually appears as a secondary disease to the primary disorders of the homeostasis that can develop because of the dysfunction of the respiratory system, infectious or noninfectious immunosuppression, or other generalized infectious diseases (Dho-Mulin and Fairbrother, 1999). For years, the colibacillosis has been one of the major causes behind economic losses in the poultry production.
Up to date, no effective method for colibacillosis prevention has been developed. Its prevention and eradication is based on counteracting factors that increase the risk of its development in a flock (including, i.a., vaccinations against immunosuppressive diseases, improvement of environmental conditions, keeping hygienic standards at hatcheries, proper bird feeding, etc.) and while the disease has already appeared–on targeted antibiotic therapy established based on results of an antimicrobial susceptibility testing (Dho-Mulin and Fairbrother, 1999). Inactivated and subunit vaccines against colibacillosis are also available.
The control of APEC infections has to face also other challenges. The number of cases of infections with multidrug-resistant (MDR) and extended spectrum beta-lactamase (ESBL)-producing Escherichia coli (E. coli) has recently increased (Shrestha et al., 2017; Davis et al., 2018; Ibrahim et al., 2019). The MDR bacteria pose severe threat, especially in rapidly developing countries because of, i.a., the commonness of using antibiotics in the large-scale poultry production. This situation is also highly problematic, considering the restrictions imposed on the doses of antibiotics used per kilogram of live poultry and also considering the prospective withdrawal of certain active substances from the veterinary market in the upcoming years. The MDR bacteria pose also a direct risk to consumers as donors of antibiotic resistance genes to other bacteria (Nhung et al., 2017).
Escherichia coli infection usually proceeds through the respiratory system. It can develop into various forms, starting from local infections in individual systems and organs to a generalized form. Serotypes O1, O2, O78, O8, and O35 are considered to be particularly pathogenic to the poultry and are most often isolated from infected birds, although they are not the only serotypes isolated from colibacillosis cases in poultry (Dho-Mulin and Fairbrother, 1999).
Recently, the vaccination of poultry using genetically modified, live vaccines against colibacillosis is becoming more and more popular (Frommer et al., 1994; Kariyawasam et al., 2004). One of them is a commercially available vaccine based on O78:K80 E. coli strain with aroA gene deletion. La Ragione et al. (2013), as well as Rawiwet and Chansiripornchai (2009), have shown that a single vaccination with this vaccine on the first or the fifth day of birds' life ensured protection against mortality and significantly minimized the possibility of development of lesions, typical of colibacillosis, in chickens and turkeys after APEC experimental infection. In addition, they have demonstrated the cross-resistance to other E. coli serotypes, but achieved the greatest protection in the case of homologous infection. Galal et al. (2018) have additionally reported that chickens vaccinated against colibacillosis reached better BW gains after simultaneous experimental infection with infectious bronchitis virus (IBV) and E. coli, that their mortality rate was lower, and that the intensity of development of pathological lesions typical of colibacillosis decreased as well. In addition, they have shown no negative effect of the vaccination against colibacillosis on the effectiveness of vaccinations against infectious bronchitis (IB), Gumboro disease, or Newcastle disease.
Considering the above, a study was undertaken with the aim to demonstrate benefits from using a live vaccine against colibacillosis in broiler chicken flocks, in the context of its potential impact on the reduced use of antibiotics, production parameters, the efficacy of vaccination against IB, and on the structure and properties of E. coli population in broiler chickens reared under farm production conditions.
Materials and methods
Ethical Statement
According to the information obtained from the Local Ethics Committee in Olsztyn, no special approval was necessary for the experiments performed under the field conditions. All animal procedures, vaccination, and sample collection were performed during the standard veterinary inspection and observations.
Experiment 1
Experiment 1 was performed in parallel at 2 broiler chicken farms. Farm 1 (F1) had 2 chicken houses: F1-K1 and F1-K2, with stock density of 40,000 and 60,000 broiler chickens, respectively, whereas farm 2 (F2) comprised 3 chicken houses: F2-K1, F2-K2, and F2-K3, with stock density of 15,000 broiler chickens each. At F2, the experiment was conducted in F2-K1 and F2-K2. The experiment began in January 2019, initially from F1 settling with chicks in the mid of January 2019, and afterward, its scope was extended onto F2 that was settled with chicks in the mid of February 2019. The experiment was continued throughout 3 consecutive production cycles at both farms. Broiler chickens were vaccinated against colibacillosis using a live vaccine on the first day of life, with a coarse spray in a dose recommended by the producer. In practice, the broiler chickens were vaccinated immediately after they had been transported onto the farm, in transport baskets. Ten to fifteen minutes after E. coli vaccination, they were spilled out from the baskets onto the litter. Throughout the experiment 1, only birds from K1 from both farms were vaccinated (F1-K1 and F2-K1), whereas chicken houses F1-K2 and F2-K2 served as control objects—birds nonvaccinated against colibacillosis. Throughout the experiment, the birds from chicken houses F1-K1 and F2-K1 did not receive any antibiotics at least till the 14th day of rearing. In turn, birds from chicken houses F1-K2 and F2-K2 could receive antibiotics starting from the first day of life, accordingly to the indications of their clinical condition. During the experiment, all birds from both farms were vaccinated against IB on the first day of life in hatcheries, using vaccines based on H-120 and 1/96 strains, with coarse spray in doses recommended by the producer and against Gumboro disease (infectious bursal disease) around the 16th day of life (established based on the Deventer formula), using a vaccine based on the Winterfield strain, administered with drinking water. During the experiment, production results were registered at F1 throughout the 3 production cycles (FCR, BW, mortality, culled birds, condemnation, veterinary expenses—including the cost of the E. coli vaccine in F1-K1). At F1 and F2, swabs from internal organs, such as the peritoneum, liver, heart, lungs, jejunum, hip joint, hock, trachea, and suborbital sinus, were collected for microbiological analyses from 3 birds from both K1 and K2 houses, immediately after birds were euthanized for diagnostic purposes, in the third and sixth week of rearing. Individual swabs were transferred for microbiological analyses, with semiquantitative determination of the E. coli colony count. Each isolated E. coli strain was cultured individually to determine its susceptibility to a panel of 20 antimicrobials. Antimicrobial usage was recorded through the experiment at both farms and in each chicken house individually. In addition, in the third production cycle, 23 blood samples were collected from birds from F1-K1 and F1-K2 in the sixth week of life and from 23 birds from F2-K1 and F2-K2 in the third and sixth weeks of life, for IBV serological analyses. The scheme of experiment 1 is summarized in Table 1.
Table 1.
Summary of the layout of experiments 1 and 2.
| Analysis type3 |
Experiment 11 |
Experiment 22 |
|||||
|---|---|---|---|---|---|---|---|
| Production cycle 1 |
Production cycle 2 |
Production cycle 3 |
Cycle 1 |
||||
| Farm 1 | Farm 2 | Farm 1 | Farm 2 | Farm 1 | Farm 2 | Farm 3 | |
| E. coli isolation and enumeration | At 3 and 6 wk4 | At 3 and 6 wk | At 3 and 6 wk | At 3 and 6 wk | At 3 and 6 wk | At 3 and 6 wk | At 6 wk |
| E. coli susceptibility evaluation | At 3 and 6 wk | At 3 and 6 wk | At 3 and 6 wk | At 3 and 6 wk | At 3 and 6 wk | At 3 and 6 wk | At 6 wk |
| Production parameters | Average5 | ND6 | Average | ND | Average | ND | ND |
| Days of antimicrobial usage | Number of days | Number of days | Number of days | Number of days | Number of days | Number of days | ND |
| Serology | ND | ND | ND | ND | At 6 wk | At 3 and 6 wk | ND |
In each experiment, the same samples were collected from both chicken houses (K1—E. coli vaccinated and K2—control, not vaccinated) at farms F1, F2, and F3.
Abbreviation: E. coli, Escherichia coli.
In experiment 1 birds were vaccinated against E. coli (K1) on the first day of life. In vaccinated birds, antimicrobial usage was prohibited for 14 d after E. coli vaccination. Control birds (K2) received antibiotics from day 1 of their life. In other stages of the production cycles, the antibiotic usage was the same in both groups.
In experiment 2 both the control and E. coli–vaccinated birds receive the same antimicrobials during the entire production cycle. Both groups received Linco-Spectin for the first 4 d of life. Birds in K1 were vaccinated against E. coli on the ninth day of life. Further antimicrobial usage was prohibited in both groups until the 23rd day of life (14 d after E. coli vaccination in K1).
What types of laboratory analyses were performed at different stages of experiments 1 and 2.
Samples were collected at the third and/or sixth week of birds' life.
Data were analyzed as an average value of each production parameter for each chicken house.
Not done.
Experiment 2
Experiment 2 was performed at farm F3 comprising 2 chicken houses: F3-K1 and F3-K2, with a stock density of 40,000 and 60,000 broiler chickens, respectively. As in experiment 1, the vaccination against colibacillosis was performed in the birds from F3-H1, but on the ninth day of birds' life. Since the first day of life, the birds from both chicken houses were administered Linco-Spectin (Zoetis, Parsippany, NJ) with drinking water for 4 consecutive days. The vaccination against E. coli was performed after the Linco-Spectin withdrawal period (5 d in the case of chicken meat). After the vaccination, the birds from both chicken houses were not administered any antibiotics for the subsequent 14 d. In the remaining production period, the scheme of antibiotics administration was the same for birds from F3-K1 and F3-K2. The remaining vaccination program was the same as in experiment 1. In the scope of experiment 2, analogously to experiment 1, in the sixth week of birds' life, swabs were collected from internal organs from 3 birds from chicken houses F3-K1 and F3-K2 for microbiological analyses. The scheme of experiment 2 is summarized in Table 1.
Birds and E. coli vaccine
In each experiment and production cycle, all chicken houses at each farm were settled with Ross 308 broiler chicks of both sexes, purchased from one hatchery and from one hatch. In addition, in experiment 1, the eggs and eventually chicks that were used to settle the F2 were provided for the hatchery from one reproduction flock. A live attenuated vaccine against E. coli (Zoetis) was used in both experiments. Its active substance (accordingly to the characteristics of the medical product) consists of viable E. coli bacteria with aroA gene deletion, type O78, strain EC34195. The vaccine strain, from the same batch as the vaccines used in the experiment, was analyzed according to the analogous antimicrobial susceptibility testing procedure as the one used for the E. coli isolated in the course of both experiments. Feed and water were given to the birds ad libitum.
Microbiological Analyses
The swabs were preincubated in a Tryptic Soy Broth (Argenta, UK) at 40.5°C. After 24 h, further incubation was proceeded on Columbia agar with 5% addition of defibrinated sheep blood, and MacConkey Agar media (Argenta, UK). Plates were incubated at 40.5°C for 24 h. After morphological and biochemical evaluation, E. coli isolates were evaluated for their susceptibility to a panel of 20 antimicrobials, according to the Clinical and Laboratory Standards Institute guidelines (Sweeney et al., 2018). The identification of E. coli was performed based on the following biochemical criteria: catalase (positive), oxidase (negative), glucose and lactose fermentation (positive), indole (positive), urease (negative), hydrogen sulfide (negative), citrate (negative), pyrrolidonyl arylamidase (negative), and beta galactosidase (positive). The following antimicrobials were used to determine the susceptibility of E. coli strains: amoxycillin (amx), amoxycillin + clavulanic (amc), ceftiofur (cef), clindamycin (cli), colistin sulfate (cst), doxycycline (doxy), enrofloxacin (enr), erythromycin (ery), gentamycin (gen), florfenicol (ff), flumequine (fluq), lincomycin/spectinomycin (ls), marbofloxacin (mbf), neomycin (nm), norfloxacin (nor), oxytetracycline (otc), penicillin G (pen), sulfamethoxazole/trimethoprim (sxt), tetracycline (tet), and tylosin (tyl) (Argenta, UK). The susceptibility of E. coli isolates was evaluated in a 4-level scale (0-3, where 0 denotes no susceptibility to a given antimicrobial). The strains found sensitive to 40% (8 or more) of the antimicrobials tested, with a susceptibility level of at least 1 or higher, were referred to as multisensitive strains. In contrast, strains that were sensitive to less than 40% of the antimicrobials were referred to as multiresistant.
Results of microbiological analyses from experiments 1 and 2 were presented as the mean number of E. coli isolates from the internal organs of birds from experimental or control groups, in various periods of the experiment (the third or sixth week of birds' life). In addition, for experiment 1, the results were summarized as the mean number of E. coli isolates from each internal organ, in total for birds from the vaccinated or control groups from both farms. Results of antimicrobial susceptibility testing were presented as the mean percentage of multisensitive strains among all E. coli isolates from internal organs. These results were summarized separately in particular production cycles for the vaccinated and for the control groups from farms F1, F2 (in the third and sixth weeks of birds' life), and F3 (in the sixth week).
Serological Analyses
A commercial kit of ELISA IBV Ab Tests (IDEXX Laboratories) was used to determine the titer of anti-IBV–specific IgY in broiler serum. Particular stages of the tests were performed with an Eppendorf epMotion 5075 LH automatic pipetting station (Eppendorf, Germany), a BioTek ELx405 automatic multi-well plate washer (BioTek), and a BioTek EL×800 plate reader. The mean geometric titer of antibodies and CV% were computed for each group in each sampling period.
Production Results
Production results recorded at F1 in the 3 consecutive productive cycles were used to calculate earnings per broiler chicken. The calculation was made using the following formula: [(livestock price ∗ final BW) - %mortality and condemnation] – [(veterinary expenses/number of birds) + (final BW ∗ FCR ∗ price of a kilogram of feed)]. Results were presented as a difference in earning/broiler chicken between F1-K1 and F1-K2 separately for each of the 3 production cycles.
Statistics
Statistical analysis was performed with GraphPad Prism 6.05 with the use of Mann–Whitney U test. Differences were considered statistically significant at P < 0.05.
Results
Number of E. coli Isolates
The mean number of E. coli isolates from the internal organs of E. coli–vaccinated and control birds from F1 and F2 (experiment 1), and F3 (experiment 2) is summarized in Table 2. At each stage of both experiments, the number of E. coli isolates was lower in the vaccinated than in the nonvaccinated birds. A significant decrease in the number of E. coli isolates was recorded in E. coli–vaccinated chickens at both farms of experiment 1 in the sixth week of birds' life.
Table 2.
Average number of E. coli isolates from E. coli–vaccinated (vacc.) and control (not vacc.) chickens at F1 and F2 in third and sixth weeks of birds' life.
| Week | Farm and chicken house (E. coli vaccinated or not vaccinated) |
|||
|---|---|---|---|---|
| F1 vacc. | F1 not vacc. | F2 vacc. | F2 not vacc. | |
| 3 | 5.33 | 8 | 8.67 | 10.33 |
| 6 | 5.331 | 7.67 | 7.331 | 10 |
Abbreviation: E. coli, Escherichia coli.
Significant difference in the number of E. coli isolates in vaccinated birds, at different sampling points, in comparison to the control chickens on a farm.
In experiment 1, a decrease was also noted in the mean number of E. coli isolates from particular internal organs of the E. coli–vaccinated chickens compared with the nonvaccinated birds (Table 3). This decrease was observed practically for all organs, except for the suborbital sinus and lungs.
Table 3.
Average number of E. coli isolates from different internal organs of E. coli–vaccinated and control (not vaccinated) chickens.
| Sample | Average number of E. coli isolates |
|
|---|---|---|
| Not vaccinated | Vaccinated | |
| Peritoneum | 0.5 | 0.25 |
| Liver | 0.5 | 0 |
| Heart | 0.25 | 0.08 |
| Lungs | 1 | 1 |
| Hip joint | 0.42 | 0 |
| Hock | 0.25 | 0 |
| Intestinum | 3 | 2.92 |
| Suborbital sinus | 0.67 | 0.83 |
| Trachea | 2.33 | 1.58 |
The average value was calculated based on the results from all 3 production cycles and 2 samplings (in third and sixth weeks of birds' life) at both F1 and F2 during experiment 1.
Abbreviation: E. coli, Escherichia coli.
E. coli Antimicrobial Susceptibility Profile
The percentage of multisensitive E. coli strains among all isolates from internal organs, in experiments 1 and 2, is summarized in Table 4. In the third and sixth weeks of birds' life, the mean percentage of multisensitive strains reached 62.7 and 66.67% in the vaccinated birds as well as 12.5 and 13.33% in the nonvaccinated birds from F1, respectively. In F2, the mean percentage of these strains reached 64.33 and 74% in the vaccinated birds as well as 3.67 and 0% in the nonvaccinated birds. The mean percentage of the multisensitive strains was statistically significantly higher in both the third and the sixth weeks of birds' life in the E. coli–vaccinated chickens than the nonvaccinated ones from both farms in experiment 1. In turn, in experiment 2, 75% of E. coli strains isolated from the vaccinated birds were multisensitive, whereas in the nonvaccinated birds, their percentage accounted for 0%.
Table 4.
Average percentage of multisusceptible E. coli stains within all isolated E. coli strains in vaccinated (vacc.) and control, not vaccinated (not vacc.), birds in experiments 1 (F1 and F2) and 2 (F3).
| Farm | Week | Chicken house | Percent of susceptible isolates |
Average | ||
|---|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 3 | ||||
| F1 | 3 | Vacc. | 0 | 88 | 100 | 62.671 |
| Not vacc. | 37.5 | 0 | 0 | 12.5 | ||
| 6 | Vacc. | 25 | 100 | 75 | 66.671 | |
| Not vacc. | 40 | 0 | 0 | 13.33 | ||
| F2 | 3 | Vacc. | 33 | 60 | 100 | 64.331 |
| Not vacc. | 0 | 0 | 11 | 3.67 | ||
| 6 | Vacc. | 60 | 62 | 100 | 741 | |
| Not vacc. | 0 | 0 | 0 | 0 | ||
| F3 | 6 | Vacc. | 75 | - | - | - |
| Not vacc. | 0 | - | - | - | ||
Abbreviation: E. coli, Escherichia coli.
Significant difference in percent of multisusceptible E. coli isolates from vaccinated birds, at different sampling points, in comparison to the control chickens on a farm.
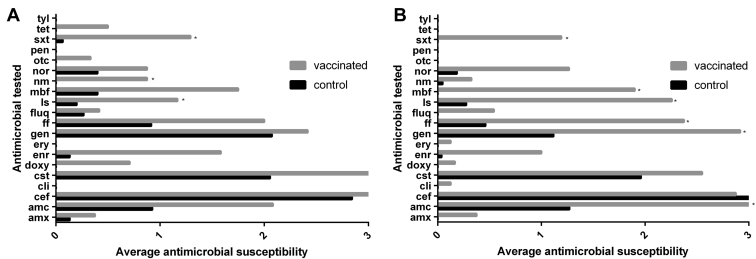
Figure 1 presents the mean susceptibility to particular antimicrobials exhibited by E. coli isolates from E. coli–vaccinated and nonvaccinated birds from farm 1 (Figure 1A) and farm 2 (Figure 1B). At F1, the susceptibility of E. coli isolates from the vaccinated birds was significantly higher to neomycin, sulfamethoxazole/trimethoprim and lincomycin/spectinomycin, whereas at F2, the susceptibility of these isolates was significantly higher to amoxycillin + clavulanic acid, gentamycin, lincomycin/spectinomycin, florfenicol, marbofloxacin, sulfamethoxazole/trimethoprim.
Figure 1.
Average susceptibility of E. coli isolates from vaccinated and control birds, at F1 (A) and F2 (B) in the sixth week of birds' life, to 20 tested antimicrobials. Antimicrobials used in our study are as follows: amoxycillin (amx), amoxycillin + clavulanic (amc), ceftiofur (cef), clindamycin (cli), colistin sulfate (cst), doxycycline (doxy), enrofloxacin (enr), erythromycin (ery), gentamycin (gen), florfenicol (ff), flumequine (fluq), lincomycin/spectinomycin (ls), marbofloxacin (mbf), neomycin (nm), norfloxacin (nor), oxytetracycline (otc), penicillin G (pen), sulfamethoxazole/trimethoprim (sxt), tetracycline (tet), tylosin (tyl). ∗ Significant difference in susceptibility of E. coli isolates from vaccinated birds in comparison to the control chickens on a farm. E. coli, Escherichia coli.
Table 5 presents selected elements of the spectrum of vaccine strain susceptibility compared with the field isolates of E. coli obtained from the vaccinated birds in experiments 1 and 2. None of the E. coli isolates from the vaccinated broiler chickens revealed the same spectrum of susceptibility as the vaccine strain did, at any of the stages of both experiments.
Table 5.
Average susceptibility of isolated E. coli stains to clindamycin (Cli) and oxytetracycline (Oct) in each production cycle in E. coli–vaccinated birds.
| Farm | Week | Antimicrobial | Average susceptibility of E. coli isolates in vaccinated chicken houses in each cycle |
||
|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 3 | |||
| F1 | 3 | Cli | 0 | 0 | 0 |
| Otc | 0 | 0 | 1.33 | ||
| 6 | Cli | 0 | 0 | 0 | |
| Otc | 0 | 1 | 0 | ||
| F2 | 3 | Cli | 0 | 0 | 0 |
| Otc | 0 | 0 | 0 | ||
| 6 | Cli | 0 | 0.38 | 0 | |
| Otc | 0 | 0 | 0 | ||
| F3 | 6 | Cli | 0 | ND1 | ND |
| Otc | 0 | ND | ND | ||
The results of E. coli vaccine strains revealed that average susceptibility of this strain to clindamycin (Cli) and oxytetracycline (Oct) was 3. Field E. coli isolates were not susceptible to both antimicrobials at any point of the experiment.
Abbreviation: E. coli, Escherichia coli.
Not done.
Average Number of Days of Antimicrobial Treatment
The data concerning the number of days of antibiotic treatment of broilers are presented in Table 6 as a total number of days of treatment and the percentage reduction in the number of days the antimicrobials were used during each production cycle at F1-K1 and F2-K1 in comparison to F1-K2 and F2-K2, respectively. During 3 production cycles, an average reduction in the number of days of antimicrobial treatment reached 10.31 and 40.57% in the E. coli–vaccinated birds at F1 and F2, respectively, in comparison to the control birds.
Table 6.
Percentage reduction in the number of days of antimicrobial usage in E. coli–vaccinated birds in comparison to the control, not vaccinated, chickens at F1 and F2 recorded during experiment 1.
| Farm | Cycle | Number of days of antimicrobial usage in not-vaccinated birds | Number of days of antimicrobial usage in E. coli–vaccinated birds | Percent reduction in the number of days of antimicrobial usage in K1 |
|---|---|---|---|---|
| F1 | 1 | 19 | 14 | −26.32 |
| 2 | 20 | 16 | −20% | |
| 3 | 13 | 15 | +15.38%1 | |
| Average | 17.33 | 15 | −10.31 | |
| F2 | 1 | 11 | 8 | −27.27% |
| 2 | 14 | 7 | −50% | |
| 3 | 9 | 5 | −44.44% | |
| Average | 11.33 | 6.67 | −40.57 |
Abbreviation: E. coli, Escherichia coli.
In production cycle number 3 at F1 an incident of hip joints inflammation associated with Enterococcus spp. infection occurred in E. coli–vaccinated birds, which resulted in higher antimicrobial usage.
Serological Evaluation
Results of serological examination are summarized in Table 7. In experiment 1, no statistically significant differences were demonstrated during the third production cycle in the mean titer of antibodies against IBV between the E. coli–vaccinated and nonvaccinated birds, at any of the experimental farms.
Table 7.
Results of IBV serological evaluation in E. coli–vaccinated and control, not vaccinated, birds at F1 and F2 during experiment 1.
| Farm | Week | Parameter | Chicken house |
|
|---|---|---|---|---|
| Vaccinated | not vaccinated | |||
| F1 | 3 | Gmean | ND1 | ND1 |
| CV% | ND1 | ND1 | ||
| 6 | Gmean | 1.392 | 1.201 | |
| CV% | 77.8 | 68.7 | ||
| F2 | 3 | Gmean | 140 | 143 |
| CV% | 169.9 | 113.4 | ||
| 6 | Gmean | 1.373 | 1.585 | |
| CV% | 61.5 | 74.5 | ||
All the birds (both E. coli–vaccinated and not vaccinated) were vaccinated on day 1 with live, attenuated H-120 and 1/96 IBV vaccines via coarse spray in the hatchery. Escherichia coli–vaccinated birds were vaccinated against E. coli on a farm right after their placement in the appropriate chicken house. 15 minutes after E. coli vaccination birds were released from transport boxes.
Abbreviation: E. coli, Escherichia coli.
Not done.
Average Earnings
The difference in earnings per bird obtained between the E. coli–vaccinated and the nonvaccinated birds at F1 in experiment 1 is shown in Table 8. In the 3 subsequent production cycles, the average earnings per bird in the E. coli–vaccinated chicken house was 0.14 PLN (approximately 0.031 EUR, if 1 EUR = 4.52 PLN and 0.037 USD, if 1 USD = 3,76 PLN) higher than the nonvaccinated chicken house (the calculation takes account of the costs of vaccine and vaccination against E. coli).
Table 8.
Average difference in earnings per bird in F1-K1 (E. coli vaccinated) in comparison to F1-K2 (not vaccinated) chicken house.
| Farm | Production cycle | Earning per bird in K1 vs K2 (PLN) |
|---|---|---|
| F 1 | 1 | −0.081 |
| 2 | 0.459 | |
| 3 | 0.048 | |
| Average | 0.141 |
Earnings were calculated with the use on following formula: [(sale price1 average final BW) - %mortality and condemnation] – [(veterinary expenses/number of birds) + (average final BW1 FCR1 cost of 1 kg of feed)].
Earnings per bird in the E. coli–vaccinated chicken house was 0.14 PLN (approximately 0.031 EUR, if 1 EUR = 4.52 PLN and 0.037 USD, if 1 USD = 3.76 PLN) higher.
Discussion
Avian pathogenic Escherichia coli is considered a primary or secondary pathogen of poultry. The key points in controlling avian colibacillosis are management interventions, infection controls, and vaccination strategies (Dho-Mulin and Fairbrother, 1999; Kabir, 2010).
The development of resistance is a complex process associated with the presence of resistance-encoding genes that are found inside plasmids or chromosomal genetic material. Bacterial antimicrobial resistance develops naturally over time. The unprecedented increase of antimicrobial-resistant organisms is linked to the massive use of antimicrobial agents for disease control and prevention (Shrestha et al., 2017; Davis et al., 2018; Ibrahim et al., 2019). This type of practice allows antimicrobial drugs to eliminate sensitive bacterial strains and select strains with genetic traits that can resist antimicrobials, which provides favorable conditions for selected strain persistence and spread at the farm level (Castanon, 2007). However, this phenomenon is determined not only by these differences. As it turns out, a high correlation has been demonstrated between the increased incidence of MDR E. coli in food products from poultry and the practices and procedures applied in a processing plant manufacturing these products (Davis et al., 2018). This highlights that proper on and outside farm management could be effective in controlling the spread of antimicrobial resistance pathogens in poultry production (McEwen and Fedorka-Cray, 2002; Davis et al., 2018).
Antimicrobial-resistant Escherichia coli strains pose a serious problem to the public health because these strains could be passed to humans via the food chain or by direct contact with infected birds. In addition, resistant E. coli may act as transporters for antimicrobial-resistant genes to other pathogens (Dho-Mulin and Fairbrother, 1999; Akond, 2009).
One of the key elements discussed for a long time in the context of minimizing further multiresistance spread among pathogens include fast diagnostics, targeted therapy, quarantine, and—last but not least—the necessity of restricting the use of antimicrobials, particularly in the sector of industrial animal production (Dho-Mulin and Fairbrother, 1999). Considering the restoration of the susceptibility to antimicrobials, interesting results have also been provided from experiments into poultry coccidiosis. As it turned out, the introduction of a population of pathogen with a wide spectrum of susceptibility to antimicrobials into the resistant population can, with time, contribute to the increased susceptibility of the latter through the transmission of susceptibility genes (Chapman and Jeffers, 2014). In the context of coccidiosis, this can be achieved on a wide scale in the poultry industry by vaccinating birds with live vaccines containing Eimeria species being highly susceptible to coccidiostats (Chapman, 1994; Peek and Landman, 2003; Mathis and Broussard, 2006; Chapman and Jeffers, 2015).
Results obtained in experiment 1 indicate that the use of live, attenuated, deletion vaccine against colibacillosis contributed to a reduction in the amount of E. coli in the population of birds. These results are in agreement with findings of El-Mawgoud et al. (2020), who demonstrated that live E. coli spray vaccination of broiler chickens reduced the APEC colonization in the heart and liver of the birds after experimental infection. Therefore, it cannot be excluded that spray vaccination, via protection in the gate of infection, contributes to a reduced number of infections among birds. This was confirmed by the fact that the number of E. coli isolates from all internal organs, except for the lungs and suborbital sinuses, was lower in the E. coli–vaccinated than in the nonvaccinated birds. In addition, in our study, the vaccination contributed to a successive increase of E. coli strain susceptibility to the antimicrobials tested. With few exceptions, 100% of E. coli strains isolated from the nonvaccinated birds exhibited multiresistance. The mean susceptibility of E. coli strains isolated from the vaccinated birds, determined for the entire experimental period, reached 62.67% and 66.67 at F1 as well as 66.33% and 74% at F2 in the third and sixth weeks of birds' life, respectively. In addition, it is worth emphasizing that in experiment 1, the susceptibility of E. coli strains isolated from the vaccinated birds generally increased with each production cycle. Interestingly, none of the E. coli isolates from the vaccinated birds had the same spectrum of susceptibility as the vaccine strain used in the study. Explicit explanation of these dependencies is difficult; however, it seems that the results obtained should not be attributed to the selection of resistant strains through antibiotic treatment in the first days of birds life. This hypothesis was supported by results obtained in experiment 2 according to which, despite antimicrobial treatment from the first till the fourth day of birds' life, in the sixth week, 75% of multisensitive strains were identified in the vaccinated birds, while no such strains (0%) were found in the nonvaccinated chickens among E. coli isolates. In addition, in experiment 2, 25% (1/4) of the E. coli strains isolated from the vaccinated birds were still sensitive to Linco-Spectin, and this susceptibility was the same as that of the vaccine strain (level 1). At the same time, none of the E. coli isolates from the nonvaccinated birds was susceptible to this antibiotic. Likewise, in experiment 1, none of the E. coli isolates obtained in experiment 2 had the same spectrum of susceptibility as the vaccine strain had. The phenomena associated with the possibility of transmission of antimicrobial susceptibility and resistance genes between the vaccine strain and field E. coli strains require further investigations.
Results from experiment 1 indicate that the vaccination against E. coli in the first day of chickens' life had no negative effect on the effectiveness of vaccination against IB, as no differences were demonstrated in the mean titer of antibodies against IBV in the third and/or sixth week of birds' life, at any of the farms, between the vaccinated and nonvaccinated birds in the third production cycle. These results correspond with findings reported by Galal et al. (2018), who concluded that live E. coli vaccination was not negatively affecting the immune response against different concurrent viral vaccines such as infectious bursal disease, and moreover, improved the immune response against some others such as Newcastle disease virus, H5 avian influenza, and IBV.
After in-depth analysis of expenditures and incomes, including the mean final BW of birds, averaged FCR, cost of feed, livestock price, percentage of dead and culled birds, number of condemnations, veterinary expenditures including costs of E. coli vaccine in the case of vaccinated birds, and average balance of profits gained in the 3 subsequent production cycles at F1 was, on average, by 0.14 PLN (approx. 0.031 EUR or 0.037 USD) higher per broiler chicken than the nonvaccinated birds. The above, coupled with the fact that clinical observations made in the experiment, which indicated a higher effectiveness of justified and undertaken antimicrobial treatment in the E. coli–vaccinated birds, support our opinion that vaccination of broiler chickens against E. coli using a live deletion vaccine should be considered in terms of routine immunoprophylaxis.
Conclusion
Although no clinical cases of colibacillosis were diagnosed at any of the farms throughout the experiments 1 and 2, still the vaccination performed seems to minimize also the incidence of the subclinical forms of infection with E. coli, which by reducing the metabolic effort of the body contributes to the improvement of overall production results. It is also worth emphasizing that, in experiment 1, the number of days of antibiotic treatment in the chicken houses vaccinated against colibacillosis decreased by 10.31 and 40.57% at F1 and F2, respectively. The above explains the effectiveness of vaccination in 2 ways. Apart from the observed effect on the increased susceptibility of E. coli isolates to the antimicrobials tested, in the E. coli–vaccinated birds, the reduced use of antibiotics may in the long term contribute to the permanent improvement of the situation with MDR E. coli. On the other hand, the restricted use of antimicrobials perfectly inscribes into the currently prevailing trends aimed at minimizing their use or their complete withdrawal from poultry production. As it turns out, these restrictions should not be identified with achieving worse economical results in broiler chickens' production.
Acknowledgements
The project is financially supported by the Ministry of Science and Higher Education in the range of the program entitled "Regional Initiative of Excellence" for the years 2019-2022, Project No. 010/RID/2018/19, amount of funding 12.000.000 PLN. The authors would like to thank Ewa Szczucińska from the Department of Microbiology and Clinical Immunology, University of Warmia and Mazury, Olsztyn, Poland, for the help with microbiological study.
Conflict of Interest Statement: The authors declare that there is no conflict of interest.
References
- Akond M. Antibiotic Resistance of Escherichia Coli isolated from poultry and poultry environment of Bangladesh. Am. J. Environ. Sci. 2009;5:47–52. [Google Scholar]
- Castanon J.I.R. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007;86:2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Sensitivity of field isolates of Eimeria to monensin following the use of a coccidiosis vaccine in broiler chickens. Poult. Sci. 1994;73:476–478. doi: 10.3382/ps.0730476. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Jeffers T.K. Vaccination of chickens against coccidiosis ameliorates drug resistance in commercial poultry production. Int. J. Parasitol. Drugs Drug Resist. 2014;4:214–217. doi: 10.1016/j.ijpddr.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H.D., Jeffers T.K. Restoration of sensitivity to salinomycin in Eimeria following 5 flocks of broiler chickens reared in floor-pens using drug programs. Poult. Sci. 2015;94:943–946. doi: 10.3382/ps/pev077. [DOI] [PubMed] [Google Scholar]
- Davis G.S., Waits K., Nordstrom L., Grande H., Weaver B., Papp K., Horwinski J., Koch B., Hungate B.A., Liu C.M., Price L.B. Antibiotic-resistant Escherichia coli from retail poultry meat with different antibiotic use claims. BMC Microbiol. 2018;18:174. doi: 10.1186/s12866-018-1322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dho-Moulin M., Fairbrother J.M. Avian pathogenic Escherichia coli (APEC) Vet. Res. 1999;30:299–316. [PubMed] [Google Scholar]
- El-Mawgoud A.I.A., El-Nahass E.S., Shany S.A.S., EL-Sawah A.A., Dahshan A.H.M., Nasef S.A., Ali A. Efficacy of live attenuated vaccine and commercially available Lectin against avian pathogenic E. coli infection in broiler chickens. Vet. Sci. 2020;7:65. doi: 10.3390/vetsci7020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommer A., Freidlin P.J., Bock R.R., Leitner G., Chaffer M., Heller E.D. Experimental vaccination of young chickens with a live, nonpathogenic strain of Escherichia coli. Avian Pathol. 1994;23:425–433. doi: 10.1080/03079459408419013. [DOI] [PubMed] [Google Scholar]
- Galal H.M., Tawfek A.M., Abdrabou M.I., Hessain A.M., Alhaaji A.H., Kabli S.A., Elbehiry A., Alwarhi W.A., Moussa I.M. Recent Approaches for control of E. coli and respiratory complex in Middle East. Saudi. J. Biol. Sci. 2018;25:1302–1307. doi: 10.1016/j.sjbs.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim R.A., Cryer T.L., Lafi S.Q., Basha E., Good L., Tarazi Y.H. Identification of Escherichia coli from broiler chickens in Jordan, their antimicrobial resistance, gene characterization and the associated risk factors. BMC Vet. Res. 2019;15:159. doi: 10.1186/s12917-019-1901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir S.M.L. Avian colibacillosis and salmonellosis: a closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int. J. Environ. Res. Public Health. 2010;7:89–114. doi: 10.3390/ijerph7010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariyawasam S., Wilkie B.N., Gyles C.L. Construction,characterization, and evaluation of the vaccine potential of three genetically defined mutants of avian pathogenic Escherichia coli. Avian Dis. 2004;48:287–299. doi: 10.1637/7093. [DOI] [PubMed] [Google Scholar]
- La Ragione R.M., Woodward M.J., Kumar M., Rodenberg J., Fan H., Wales A.D., Karaca K. Efficacy of a live attenuated Escherichia coli O78∶K80 vaccine in chickens and turkeys. Avian Dis. 2013;57:273–279. doi: 10.1637/10326-081512-Reg.1. [DOI] [PubMed] [Google Scholar]
- Mathis G.F., Broussard C. Increased level of Eimeria sensitivity to diclazuril after using a live coccidial vaccine. Avian Dis. 2006;50:321–324. doi: 10.1637/7455-101305R1.1. [DOI] [PubMed] [Google Scholar]
- McEwen S.A., Fedorka-Cray P.J. Antimicrobial use and resistance in animals. Clin. Infect. Dis. 2002;34:93–106. doi: 10.1086/340246. [DOI] [PubMed] [Google Scholar]
- Nhung N.T., Chansiripornchai N., Carrique-Mas J.J. Antimicrobial resistance in bacterial poultry pathogens: a Review. Front. Vet. Sci. 2017;4:126. doi: 10.3389/fvets.2017.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek H.W., Landman W.J.M. Resistance to anticoccidial drugs of Dutch avian Eimeria spp. field isolates originating from 1996, 1999 and 2001. Avian Pathol. 2003;32:391–401. doi: 10.1080/0307945031000121149. [DOI] [PubMed] [Google Scholar]
- Rawiwet V., Chansiripornchai N. The efficacy of Escherichia coli aroA-live vaccine in broilers against avian E. coli serotype O78 infection. Thai J. Vet. Med. 2009;39:337–342. [Google Scholar]
- Shrestha A., Bajracharya A.M., Subedi H., Turha R.S., Kafle S., Sharma S., Neupane S., Chaudhary D.K. Multi-drug resistance and extended spectrum beta lactamase producing Gram negative bacteria from chicken meat in Bharatpur Metropolitan, Nepal. BMC Res. Notes. 2017;10:574. doi: 10.1186/s13104-017-2917-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M.T., Diaz-Campos D.V., Bowden R., Fritsche T.R., Hayes J., Langston C., Lubbers B., Martin-Jimenez T., Miller C., Pallotta C., Papich M.G., Parkinson A., Schwarz S., Traczewski M.M. Clinical and Laboratory Standards Institute, VET01. Wayne, PA; 2018. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; pp. 1–156. [Google Scholar]