Abstract
Glycerol is the most widely used cryoprotectant for rooster sperm because it declines the mechanical damage to sperm during the freezing process. Despite its high molecular weight and viscosity, which may be cytotoxic, glycerol can cause damage to cells during the cryopreservation process, resulting in less fertility. Poloxamer 188 (P188) is an embryo cryopreservation supplement effective in many species and also for cell lines and plant cells. We tested the suitability of P188 in the cryopreservation of rooster sperm, considering post-thawing motility, abnormalities, membrane functionality (hypo-osmotic swelling test), mitochondrial activity, viability, apoptosis status, reactive oxygen species production, and ATP content after thawing and the fertility and hatchability after AI. We carried out a factorial experiment with glycerol concentrations of 2% glycerol (G2) and 8% glycerol (G8) and P188 concentrations of 0% (P0), 0.1% (P0.1), 0.5% (P0.5), and 1% (P1) as fixed effects, with replicate (seven) as a random effect. Interactions between glycerol and P188 were found, with G2P1 yielding higher quality and fertility. G8P0.5 yielded better in most parameters, however, not reaching G2P1. G2P1 showed significantly higher results for total and progressive motility, kinetic parameters (average path velocity, straight-line velocity, and linearity), membrane functionality, viability, mitochondrial activity, and ATP content and lower apoptosis, dead sperm, and reactive oxygen species production. G2P1 resulted in the highest percentages of fertilized and hatched eggs, with no effects in the hatched eggs ratio. Interestingly, G2 was less efficient in many parameters than G8 when combined with P0 and P0.1, being equivalent to G8 with P0.5 and superior to any G8 treatment as G2P1. In conclusion, P188 could improve rooster semen cryopreservation and allow reduction of glycerol in extenders, with a consequent impact in the poultry industry.
Key words: poloxamer, cryopreservation, rooster, sperm, glycerol
Introduction
Assisted reproductive technologies are crucial for intensively farmed breeds and endangered species, with small population sizes (Hezavehei et al., 2018). Cryopreservation allows spermatozoa to survive indefinitely, thereby enabling the extended use of the frozen samples and international transport. However, freezing and thawing can decrease sperm viability and motility, resulting in reduced fertility. This is due to a variety of factors such as ice crystal formation, high levels of dehydration, osmotic stress, changes in sperm structure, and morphological alterations (O'Neill et al., 2019; Pabon et al., 2019). Moreover, the generation of reactive oxygen species (ROS) and other oxidant species during the freeze-thawing process is a critical factor. Reactive oxygen species excess due to the imbalance between their production and the antioxidant defense systems results in oxidative stress, causing fertility loss (Shahin et al., 2020). In the case of poultry spermatozoa, sperm membranes are rich in unsaturated fatty acids, thus being prone to produce lipid peroxides in the presence of ROS (Thananurak et al., 2020; Najafi et al., 2020).
Dimethyl sulfoxide, glycerol, and other cryoprotectants play an essential role in preventing the harmful effects of a hypothermal situation. They prevent the formation of ice crystals, which can damage the inner and outer cell membranes. Glycerol is one of the most used cryoprotectants for sperm cryopreservation, including poultry sperm (Hezavehei et al., 2018). However, glycerol is toxic for sperm quality (Najafi et al., 2017), and it substantially decreases hen fertility if not removed before artificial insemination (AI). This process is both labor-intensive and increases osmotic stress on the rooster sperm during the dilution and reconcentration process. Moreover, glycerol has relatively large molecular weight and low membrane permeability compared with water, which causes its slower crossing through the plasma membrane than water. Therefore, when glycerol is added or removed, it causes cells to experience osmotic stress and, consequently, membrane damage by cell shrinkage and swelling (Gao and Zhou, 2012).
P188, also known as Pluronic F68, is a nonionic surfactant that protects cells against membrane rupture caused by various types of injuries (such as stroke, diffuse cortical depression, brain injury, intracranial hemorrhage, etc.) (Dong et al., 2019). Moreover, poloxamers have low toxicity. In experiments with animal models and humans, intravenously administered poloxamers resulted in a rapid clearance and few adverse effects (Singh-Joy and McLain, 2008; Hunter et al., 2010; Kerleta et al., 2010; Mansor et al., 2018). Indeed, P188 was approved by the Food and Drug Administration as a blood additive for transfusions 50 yr ago (Moloughney and Weisleder, 2012). The low toxicity and the surfactant properties of P188 make this polymer very useful in the cosmetic and pharmaceutical industry as a detergent; dispersing, emulsifying, and solubilizing agent; drug delivery compound; and for cell cryopreservation (Frim et al., 2004; Phillips and Haut, 2004; Natoli and Athanasiou, 2008; Yuhua et al., 2012; Wang et al., 2019).
A remarkable feature of P188 is its ability to repair damaged cell membranes (Hellung-Larsen et al., 2000; Riehm et al., 2018). These cytoprotective properties could explain not only its therapeutic effects but also its activity as a cryoprotectant (Zhao et al., 2005; Kerleta et al., 2010). Thus, it has found application in insect (Jordan et al., 1994) and human cell culture systems and cryopreservation (Hernández and Fischer, 2007; Kerleta et al., 2010) and plant tissue cryopreservation (Anthony et al., 1997; Lowe et al., 2001). Regarding assisted reproductive technologies, several studies have also shown that P188 has a protective effect on the cryopreservation of bovine and mouse embryos (Palasz et al., 2000).
To the best of our knowledge, there are no previous studies on the effect of P188 on sperm cryopreservation. Our objective was to test P188 as a supplement for rooster semen. We hypothesized that P188 at concentrations used for other cell types between 0.1% and 1.0% could also preserve sperm quality and fertility during cryopreservation in this important farm species. Considering the problems related to the use of glycerol in poultry semen cryopreservation and AI, there is a practical interest in reducing its content for preserving good sperm quality after freezing. Therefore, a second objective was to study its interactions with glycerol content in the freezing extender.
Materials and methods
Chemicals
All reagents listed in the following were obtained from Sigma Aldrich (St. Louis, MO) unless otherwise specified.
Semen Collection, Processing, and Cryopreservation
Fifteen healthy Ross roosters (30 wk of age) of proven fertility were individually housed at the Education and Research Farm of Animal Science Department (University of Tabriz, Iran) in cages and used for semen collection. The birds were exposed to 15 h of light and 9 h of darkness and given commercial chicken breeder diet and ad libitum access to drinking water. The Tabriz University Animal Protection Committee approved all methods regarding rooster care and semen collection. Sperm samples were collected from the roosters twice per week using abdominal massage (Lotfi et al., 2017). The experiment was performed in 7 replicates on 7 collection days with a frequency of twice per week. Immediately after collection, the samples were sent to the laboratory within 5 min at 37°C. Samples not meeting the following conditions were discarded: ≥3 × 109 spermatozoa/mL, ≥90% normal morphology, and ≥80% motility. The remaining samples were then pooled to remove individual effects. The samples were divided into 8 aliquots and extended with Lake extender (composed of 8 g/L D-fructose, 3 g/L polyvinylpyrrolidone, 19.2 g/L sodium glutamate, 5 g/L potassium citrate, 0.7 g/L magnesium acetate, and 3.74 g/L glycine) supplemented either with 2 or 8% glycerol (G2 and G8, respectively) and either P188 concentrations of 0, 0.1, 0.5, or 1% (P0, P0.1, P0.5, and P1, respectively). Therefore, the sperm samples were frozen with the combinations G2P0, G2P0.1, G2P0.5, G2P1, G8P0, G8P0.1, G8P0.5, and G8P1, with G2P0 and G8P0 being considered as control. The extended semen was cooled gradually down to 4°C for 3 h. Then, 0.25 mL of semen straws was filled with the extended semen samples, placing them 4 cm above liquid nitrogen (LN2) for 7 min and then immersing them in LN2 for storage at −196°C until analysis. This experiment was repeated 7 times under the same condition. After 4 wk of storage, the samples were taken from LN2 and thawed in a water bath at 37°C for 30 s for analysis and AI.
Motility Analysis
Sperm motility was objectively assessed using a computer-assisted semen analysis system (12.3 CEROS, HamiltonThorne Biosciences, Beverly, MA) (Mehdipour et al., 2018). The evaluation of sperm (each sample was adjusted to 20 × 106 mL−1) motility and velocity parameters was performed using a Leja chamber (depth: 20 μm, 37°C) in a microscope with a warmed stage (37°C) × 10 magnification (negative phase contrast). For each semen sample, we recorded total and progressive motility and kinematic parameters: straight-line velocity (VSL, μm/s), curvilinear velocity (μm/s), average path velocity (VAP, μm/s), linearity (LIN, %; VSL/ curvilinear velocity), beat cross frequency (Hz), straightness (%; VSL/VAP), and amplitude of lateral head displacement (μm).
Abnormal Forms
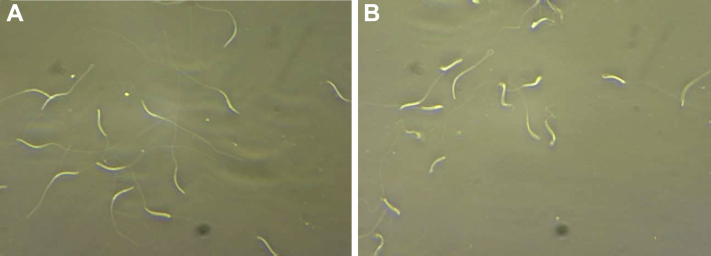
Abnormal forms were assessed using Hancock solution (Najafi et al., 2019b). Semen (10 μL) was placed on a slide and evaluated by phase-contrast microscopy at × 400 magnification. Spermatozoa (at least 200 per slide) were evaluated, and the percentage of total sperm abnormalities was determined (Figure 1A).
Figure 1.
Examples of spermatozoa as evaluated for (A) abnormal forms and (B) after submitting to the hypo-osmotic swelling (HOS) test for membrane functionality assessment.
Membrane Functionally (Hypo-osmotic Swelling Test)
The membrane functionally was determined with the hypo-osmotic swelling test (Mehdipour et al., 2017). Ten μL of thawed semen and 100 μL of hypo-osmotic swelling (HOS) solution (100 mOsm/kg; sodium citrate 1.9 mmol, fructose 5.0 mmol) were mixed and incubated for 30 min at 37°C. The samples were evaluated under a phase-contrast microscope at × 400 magnification, evaluating at least 200 spermatozoa (Figure 1B).
Determination of Mitochondrial Activity
Rhodamine 123 (R123) and propidium iodide (PI) were used to assess mitochondrial activity (Mehdipour et al., 2016). Five microliters of R123 (0.01 mg/mL stock) and PI solutions (1 mg/mL stock) were added to 250 μL of diluted semen and then incubated at 37°C in the dark for 15 min. We assessed the samples by flow cytometry, recording the percentage of viable spermatozoa with high mitochondrial potential (R123+/PI-) ratio.
Assessing Cell Viability and Apoptosis
Sperm cell viability and the apoptotic status were assessed using the Annexin V-FITC (AV) programmed cell death assay (Najafi et al., 2019a). Briefly, the sample was suspended in a calcium solution at a concentration of 10 × 106 mL−1, then followed by 10 μL of AV (stock solution; 0.01 mg/mL stock) was mixed with the semen sample, and incubated at room temperature (25°C) in the dark for 15 min. The viability staining was performed by adding 10 μL of PI (1 mg/mL stock) and then incubated for 10 min more. Then, the samples were analyzed by flow cytometry, recording the populations of live (AV-PI-), live-apoptotic (AV + PI-), and dead spermatozoa (PI+).
Flow Cytometry Analysis
Flow cytometry analysis was performed using a FACSCalibur (Becton Dickinson, San Jose, CA) with an argon-ion laser at 488 nm. Sperm cells were gated from debris by using a forward/side-scatter gate. Green fluorescence (Rhodamine 123 and Annexin V-FITC) was detected using the FL-1 detector using a band-pass filter (530/30 nm), and red fluorescence (PI) was detected using the FL-3 detector using a long-pass filter (610 nm). Acquisition and data analysis were carried out using the CellQuest 3.3 software (Becton Dickinson). At least 10,000 spermatozoa were assessed per sample.
Determination of ATP in Sperm
ATP was measured by the method followed by Kamali Sangani et al. (2016). Five microliters of each sample was first diluted in 750 μL of Lake buffer. Then 5 μL of the sample was pipetted into 190 μL of perchloric acid. The tubes were centrifuged at 12,600 × g for 2 min. The upper phase (180 μL) was transferred to another tube and neutralized by adding 10.7 μL of 2 mol KCl, 58.7 μL of 1 mol KOH, 10.7 μL of saturated Tris, and 1 μg/mL of red phenol. Just before measuring the tubes, we added 100 μL of luciferin–luciferase reconstituted reagent (in 100 mmol glycine, 20 mmol MgSO4, pH 7.4). Standards were prepared from the ATP standard using serial dilutions to obtain concentrations of 10−7 to 10−12 mol. The ATP content was expressed as pmol ATP per 106 sperm.
Determination of ROS
Reactive oxygen species were measured by the method followed by Kamali Sangani et al. (2016). In brief, the semen samples were incubated for 20 min in 250 μL of the phosphate buffered saline (PBS) at 37°C. The samples were centrifuged at 300 × g for 7 min, and the supernatant was removed. Three milliliters of PBS was added to the pellet, centrifuging at 300 × g for 7 min. The sperm concentration was adjusted to 20 × 106 mL−1 by diluting with PBS. Then, 10 μL of luminol (5 mmol, 5-amino-2,3-dihydro-1,4-phthalazinedione; Sigma Chemical, St. Louis, MO) was added to 400 μL of sample, and then the tubes were placed in an Orion II Microplate Luminometer (Berthold Detection Systems Gmbh, Germany). The results were expressed as 103 counted photons per minute (cpm) per 106 spermatozoa.
Artificial Insemination
Artificial insemination was performed using the method proposed by Najafi et al. (2018) with minor alterations. To evaluate fertility, 240 Ross 308 breeder hens (28 wk of age) were divided into 8 groups of 30 hens. Each group of hens was kept in separate cages (70 × 70 × 85 cm) and inseminated with thawed semen from the 8 experimental treatments. Glycerol was removed using a discontinuous Accudenz gradient, which contained a 30% (0.5 mL) layer under a 12% (5.0 mL) layer. After centrifugation (1,200 × g, 20 min), the extender remained above the 12% layer, whereas the spermatozoa formed a band between the 12 and 30% layers. The semen band was recovered and resuspended with 500 μL of glycerol-free Lake extender. The AI was carried out using 100 × 106 sperm from each treatment. Eggs were collected for up to 5 days after the last insemination. After that, the eggs were set in an incubator for 18 D at 37.7°C and then in a hatcher for 3 D. The fertility was evaluated on the 7th D of incubation by candling. After 21 D of incubation, the hatched eggs were counted. We obtained the fertility ratio as fertilized eggs to total eggs, the hatching ratio as hatched eggs to total eggs, and the hatched eggs ratio as hatched eggs to fertilized eggs.
Statistical Analysis
The experiment was performed in 7 replicates. Data were analyzed in the R statistical environment (Team, 2019) by linear mixed-effects models, with the glycerol and P188 treatments as fixed effects and the replicate as the grouping factor of the random part of the model. Results were expressed as mean ± 95% CI, except as otherwise indicated. Fertility data were analyzed as binomial (fertilized, hatched, not fertilized, or not hatched, vs. total number of eggs) by general linear models. Signification threshold was established at P < 0.05.
Results
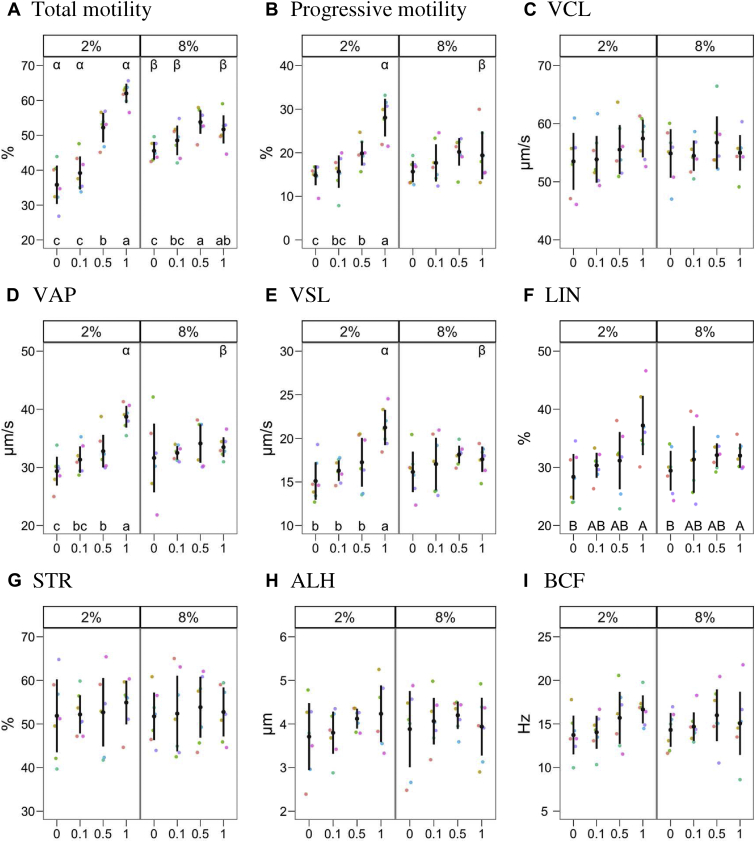
Figure 2 shows the effects of the treatments on computer-assisted semen analysis parameters. The analysis showed a significant interaction of glycerol × P188 for total and progressive motility, VAP, and VSL. In general, G2 with the lower P188 concentrations 0 and 0.1% presented the most depressed total motility (P < 0.05). P188 had a significant effect in both glycerol treatments, with maximum motility for P1 in G2 (P < 0.05) and for P0.5 in G8 (P < 0.05). For progressivity, VAP, and VSL, the P188 effect was significant only at G2, with P1 yielding higher values than the other concentrations (P < 0.05). Linearity did not show a glycerol × P188 interaction, with P188 being significant as a main effect and P1 showing the highest overall value (P < 0.05). We did not detect any significant effect for the other kinematic parameters.
Figure 2.
Results (mean ± 95% CI) for CASA parameters. The figures show glycerol concentrations (2 and 8%) and poloxamer 188 (P188) concentrations (0, 0.1, 0.5, 1%). When an interaction was found between both factors (total and progressive motility, VAP, and VSL), the levels were compared within each level of the other. Latin lowercase letters indicate differences among P188 concentrations within each glycerol concentration, whereas Greek letters show differences between glycerol concentrations within each treatment (P < 0.05). Capital Latin letters indicate no interaction and that P188 concentrations differ over glycerol concentrations. Plots with no letters indicate neither a significant effect of the factors nor interaction. Abbreviations: VSL, straight-line velocity (μm/s); VAP, average path velocity (μm/s); VCL, curvilinear velocity (μm/s); LIN, linearity (%); STR, straightness (%); ALH, mean amplitude of the lateral head displacement (μm); BCF, mean of the beat cross frequency (Hz); CASA, computer-assisted semen analysis.
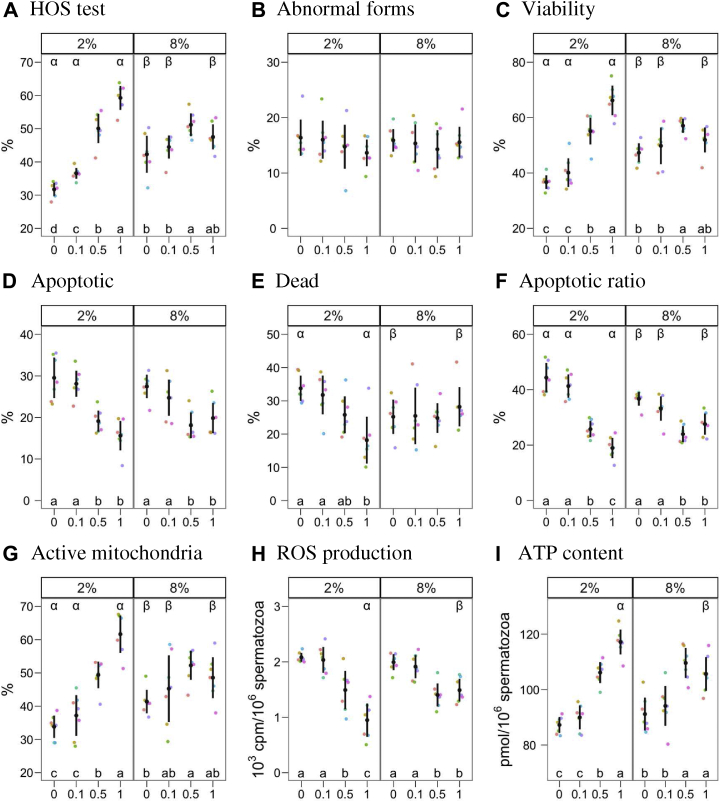
The result of abnormal forms, HOS, cell viability, apoptosis, mitochondrial activity, ROS, and ATP content is presented in Figure 3. We found a significant glycerol × P188 interaction for the results of all these variables, except for the proportion of the abnormal forms, which was not affected by either factor or their interaction (P < 0.05). For the HOS, viability, mitochondrial activity, and ATP content tests, the G2P1 combination yielded the highest values (P < 0.05). For these variables, G2P1 was significantly higher than G8P1, and for HOS test, viability and mitochondrial viability P0 and P0.1 at G2 displayed significantly lower average values than at G8. Similarly to motility, P0.5 showed higher values at G8 for these variables, being significantly higher than G8P0 in all cases, and G8P0.1 for HOS test, viability, and ATP content (P < 0.05).
Figure 3.
Results (mean ± 95% CI) for physiological parameters. The figures show glycerol concentrations (2 and 8%) and poloxamer 188 (P188) concentrations (0, 0.1, 0.5, 1%). When an interaction was found between both factors (HOS test, viability, apoptosis, dead sperm, apoptotic ratio, active mitochondria, ROS production, and ATP content), the levels were compared within each level of the other. Latin lowercase letters indicate differences among P188 concentrations within each glycerol concentration, whereas Greek letters show differences between glycerol concentrations within each treatment (P < 0.05). Plots with no letters indicate neither a significant effect of the factors nor interaction. Abbreviations: HOS, hypo-osmotic swelling; ROS, reactive oxygen species.
The proportion of apoptotic and dead spermatozoa, the apoptotic ratio, and ROS production followed the opposite trend. P1 was significantly lower than P0 and P0.1 at G2 (P < 0.05). In parallel, G2P1 was significantly lower than G8P1 for these variables, with G2P0 being significantly higher than G8P0 for dead spermatozoa (P < 0.05).
Table 1 summarizes the fertility results. The interaction of glycerol × P188 was significant both for the fertility and hatchability ratios (P < 0.05). The highest values were achieved with the G2P1 combination (P < 0.05). P1 was also significantly higher at G2 than at G8. The hatched eggs ratio (hatched/fertilized) was not affected by either any of the main factors or their interaction.
Table 1.
Effect of poloxamer 188 on fertility and hatchability rates of rooster semen after freeze-thawing. Each experimental group contained 195 eggs initially.
| Glycerol (%) |
2 |
8 |
||||||
|---|---|---|---|---|---|---|---|---|
| Poloxamer 188 (%) | 0 | 0.1 | 0.5 | 1 | 0 | 0.1 | 0.5 | 1 |
| Fertilized eggs (N) | 68 | 79 | 104 | 130 | 88 | 92 | 115 | 97 |
| Fertilized eggs (%) | (34.8)a,α | (40.5)a,b | (53.3)b | (66.7)c,α | (45.1)a,β | (47.2)a,b | (59.0)b | (49.7)a,b,β |
| Hatched eggs (N) | 43 | 51 | 69 | 94 | 56 | 60 | 77 | 64 |
| Hatched eggs (%) | (22.1)a | (26.2)a,b | (35.4)b,c | (48.2)c,α | (28.7) | (30.8) | (39.5) | (32.8)β |
| Hatched eggs ratio (%) | 63.2 | 64.6 | 66.4 | 72.3 | 63.6 | 65.2 | 67.0 | 66.0 |
Numbers are absolute counts of eggs, with percentages (ratio respect to the initial egg count) between parentheses, except for the hatched eggs ratio (hatched/fertilized as percentage). Different Latin superscript letters indicate significant differences between poloxamer 188 concentrations within each glycerol concentration (P < 0.05). Different Greek superscript letters within each row indicate significant differences between glycerol treatments within each poloxamer 188 concentration (P < 0.05).
Discussion
For the first time, we have reported the successful use of poloxamer 188 (P188) for freezing of spermatozoa. Poloxamer 188 has shown beneficial proven roles in freezing of stem cells, embryo, and plant cells. In our particular experiment on rooster semen, we not only achieved an improvement in sperm quality and fertility by adding P188 but also found interactions with the glycerol content in the extender. This opens new avenues of research on this particular supplement and the formulation of new extenders for poultry semen.
Poloxamer 188 is an effective cell protector, possibly due to its stabilizing and repairing effects on biological membranes (Curry et al., 2004; Moloughney and Weisleder, 2012). This effect has been observed in many cell lines and could explain the protective effects observed in the viability/apoptosis assay. Thus, P188 protects cells from chemical and physical stress that causes cell death (Hellung-Larsen et al., 2000). In our study, we have reported an improvement of sperm membrane functionality and integrity and a decrease of apoptotic markers when supplementing 0.5 and 1% P188, most notably with the glycerol 2% combination. These events were associated with the improvement of several motility parameters, possibly resulting from enhanced viability, mitochondrial activity, and ATP content. Our findings follow previous reports on other mammal cell types, in which P188 contributed to the preservation of sperm viability after cryopreservation (Hernández and Fischer, 2007). In another study, P188 could block the entire range of inflammation, coagulation, and apoptotic responses after injury (Hunter et al., 2010). In some studies, the effects of P188 have been attributed to modulating gene expression, favoring prosurvival vs. proapoptotic genes (Doğan et al., 2013). This would not be the case in spermatozoa. The red blood model, a cell type with no gene expression too, would be more comparable. Indeed, poloxamers (especially P188) prevented red blood cell hemolysis during freeze-thawing in several studies (Armstrong et al., 1995; Toth et al., 2000; Cancelas et al., 2017).
Different steps of cryopreservation (extension, cooling, freezing, and thawing) can cause changes in the sperm plasma membrane structure, leading to severe disruptions in the cell. In a mouse model for amyotrophic lateral sclerosis, P188 restored membrane integrity after damage induced by oxidative stress, by interacting with the cell membranes (Riehm et al., 2018). However, not only is P188 adsorbed to the cell membrane (Al-Rubeai et al., 1993), it also incorporates into the phospholipid bilayer and reduces plasma membrane fluidity by directly interacting with the plasma membrane (Ramírez and Mutharasan, 1990; Hellung-Larsen et al., 2000). P188 stimulates transient “pores” of the membrane by affecting lipid–lipid and lipid–protein interactions and thereby increases membrane resistance to physical force (Palasz et al., 2000; Riehm et al., 2018).
Furthermore, the insertion of P188 increases the resistance of the bilayer to mechanical rupture (Houang et al., 2017). Guzniczak et al. (2018) studied the effect of P188 on the mechanical properties of cells. They observed that cells responded to P188 within the first 3 h of incubation and became stiffer without affecting cell size. Pettitt and Buhr (1998) speculated that the hydrophobic portion of poloxamer could serve as an “anchor,” allowing it to protect cells from the effects of a harsh hydrodynamic environment. The results in other cell types suggest that the composition of the plasma membrane could modulate the interactions of P188. This composition is quite particular in spermatozoa (Thananurak et al., 2020), and it could explain why 0.1% P188 did not show significant improvements.
In contrast, we obtained the best outcomes with 0.5% P188 with 8% glycerol and 1% P188 with 2% glycerol. Here, we have to highlight the interaction of the P188 concentration with the glycerol concentration. The 8% glycerol showed higher post-thawing quality and fertility in control (0% poloxamer). The 2% glycerol results improved when adding P188, up to the point that G2P1 yielded the best results. Glycerol not only acts as a cryoprotectant but also diffuses across the membranes and interacts with other molecules, helping to stabilize them during the cryopreservation process (Sieme et al., 2016). There is a balance between the protective and toxic effects of glycerol (Hezavehei et al., 2018). It was evident that in the absence of P188, glycerol at 2% might not offer enough protection during the freezing process. Many studies have suggested that glycerol at low concentrations is not sufficient to adequately protect rooster spermatozoa during cryopreservation (Phillips et al., 1996; Blanch et al., 2014). For instance, rooster semen frozen using 4% glycerol exhibited the lowest quality, which increased with its concentration up to 8% (Blanch et al., 2014). Thus, P188 seems to have a synergic effect with glycerol, possibly due to its membrane-stabilizing properties, explaining the increase in sperm quality with the rising poloxamer concentration up to the maximum 1% tested here. This situation seems to be similar for 8% glycerol, but after a maximum positive effect of P188 around 0.5%, the toxic effects of glycerol might prevail, abolishing this synergy at 1%.
The effects of P188 go beyond stabilizing the plasma membrane. Poloxamer 188 inhibits cell aggregation upon interacting with the membranes (Hellung-Larsen et al., 2000). Sperm aggregation can often be a problem, especially in thawed samples. Indeed, 0.5 and 1% P188 treatments could exert a part of the positive effect on motility by preventing this issue.
We have also observed better preservation of mitochondrial activity with the higher proportions of P188 (G2P1 and G8P0.5) and a concomitant higher ATP content possibly associated. The mitochondrion has a crucial function in the spermatozoon because the oxidative phosphorylation and ATP production occur in this organelle. However, this process also produces ROS, which reduce the NADH pool, resulting in the loss of efficiency of the electron transfer chain, leading to ATP depletion (Martin Munoz et al., 2018; Nesci et al., 2020). Thus, the increase of ATP content and reduction in ROS production could be a consequence of mitochondrial protection by P188, resulting in better motility, increased sperm survival, and lower presence of apoptotic markers. Bird spermatozoa are especially sensitive to oxidative stress because of the composition of their plasma membrane (Thananurak et al., 2020). Therefore, they could benefit from this kind of protection resulting from P188.
These events might also be associated with the higher sperm viability and lower proportion of spermatozoa with apoptotic features. Poloxamer 188 could also protect mitochondria by inhibiting the outer membrane permeabilization. In other cell models (Wang et al., 2017), the treatment with P188 inhibited Bcl-2–associated X protein translocation from the cytosol to mitochondria, the release of cytochrome c from this organelle, and the activation of caspases, mediators of cell death also in spermatozoa (Aitken et al., 2016; Del Olmo et al., 2016). Therefore, the reduced incidence of apoptosis and the increase in sperm survival and functionality could also be associated with the improvement of mitochondrial performance. This better mitochondrial functionality could also explain the rise of ATP content and the lower levels of ROS. The increase in ATP content might also contribute to improved motility.
In the present study, 1% P188, together with a reduced glycerol concentration of 2%, improved sperm quality and fertility after thawing rooster semen. While this study raises many intriguing questions regarding the effects of poloxamers at the cellular and molecular levels on poultry spermatozoa, the practical outcomes are very interesting for the industry. Thus, P188 is a supplement with excellent prospects for the poultry sector, having low toxicity, having low cost, and being easy to add to existing extenders. The results are enticing for future work because higher P188 concentrations could yield further benefits at low glycerol levels, helping reduce the problems associated with the use of high levels of this efficient cryoprotectant in bird semen.
Conclusion
We have demonstrated that P188 is an effective supplement for the freezing extender in rooster semen cryopreservation. However, its effects depend on the concentration of glycerol in the sample. Eight percent glycerol yielded higher sperm quality and fertility than 2%, whereas a combination of 2% glycerol and 1% P188 achieved the highest values. The importance of these results is twofold. First, as a supplement for cryofreezing extenders, P188 could be safely used for improving fertility owing to the application of semen by AI in the poultry industry. Second, P188 allows reducion of glycerol concentrations in semen extenders, a well-known toxic cryoprotectant for AI.
Acknowledgments
This research is supported by a research grant of the University of Tabriz (number 5782).
Conflict of Interest Statement: None of the authors have any conflict of interest to declare.
References
- Aitken R.J., Gibb Z., Baker M.A., Drevet J., Gharagozloo P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2016;28:1–10. doi: 10.1071/RD15325. [DOI] [PubMed] [Google Scholar]
- Al-Rubeai M., Emery A.N., Chalder S., Goldman M.H. A flow cytometric study of hydrodynamic damage to mammalian cells. J. Biotechnol. 1993;31:161–177. doi: 10.1016/0168-1656(93)90158-j. [DOI] [PubMed] [Google Scholar]
- Anthony P., Lowe K.C., Azhakanandam K., Davey M.R., Power J.B. Qualitative assessment of rice : novel strategies for improving post-thaw. Proc. Int. Symp. Held Nottingham. 1997;24 [Google Scholar]
- Armstrong J.K., Meiselman H.J., Fisher T.C. Inhibition of red blood cell-induced platelet aggregation in whole blood by a nonionic surfactant, poloxamer 188 (Rheothrx® Injection) Thromb. Res. 1995;79:437–450. doi: 10.1016/0049-3848(95)00134-d. [DOI] [PubMed] [Google Scholar]
- Blanch E., Tomas C., Casares L., Gomez E.A., Sansano S., Gimenez I., Moce E. Development of methods for cryopreservation of rooster sperm from the endangered breed “Gallina Valenciana de Chulilla” using low glycerol concentrations. Theriogenology. 2014;81:1174–1180. doi: 10.1016/j.theriogenology.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Cancelas J.A., Rugg N., Nestheide S., Hill S.E., Emanuele R.M., McKenzie D.S. The purified vepoloxamer prevents haemolysis in 42-day stored, DEHP/PVC-free red blood cell units. Blood Transfus. 2017;15:165. doi: 10.2450/2017.0351-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry D.J., Wright D.A., Lee R.C., Kang U.J., Frim D.M. Surfactant poloxamer 188—related decreases in inflammation and tissue damage after experimental brain injury in rats. J. Neurosurg. Pediatr. 2004;101:91–96. doi: 10.3171/ped.2004.101.2.0091. [DOI] [PubMed] [Google Scholar]
- Del Olmo E., García-Álvarez O., Maroto-Morales A., Ramón M., Jiménez-Rabadán P., Iniesta-Cuerda M., Anel-Lopez L., Martinez-Pastor F., Soler A.J., Garde J.J. Estrous sheep serum enables in vitro capacitation of ram spermatozoa while preventing caspase activation. Theriogenology. 2016;85:351–360. doi: 10.1016/j.theriogenology.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Doğan A., Yalvaç M.E., Yılmaz A., Rizvanov A., Şahin F. Effect of F68 on cryopreservation of mesenchymal stem cells derived from human tooth germ. Appl. Biochem. Biotechnol. 2013;171:1819–1831. doi: 10.1007/s12010-013-0472-z. [DOI] [PubMed] [Google Scholar]
- Dong H., Qin Y., Huang Y., Ji D., Wu F. Poloxamer 188 rescues MPTP-induced lysosomal membrane integrity impairment in cellular and mouse models of Parkinson’s disease. Neurochem. Int. 2019;126:178–186. doi: 10.1016/j.neuint.2019.03.013. [DOI] [PubMed] [Google Scholar]
- Frim D.M., Wright D.A., Curry D.J., Cromie W., Lee R., Kang U.J. The surfactant poloxamer-188 protects against glutamate toxicity in the rat brain. Neuroreport. 2004;15:171–174. doi: 10.1097/00001756-200401190-00033. [DOI] [PubMed] [Google Scholar]
- Gao D., Zhou X. Current Frontiers in Cryobiology. Tech Rijeka; Croatia: 2012. Prevention of lethal osmotic injury to cells during addition and removal of cryoptotective agents: theory and technology; pp. 101–136. [Google Scholar]
- Guzniczak E., Jimenez M., Irwin M., Otto O., Willoughby N., Bridle H. Impact of poloxamer 188 (Pluronic F-68) additive on cell mechanical properties, quantification by real-time deformability cytometry. Biomicrofluidics. 2018;12:44118. doi: 10.1063/1.5040316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellung-Larsen P., Assaad F., Pankratova S., Saietz B.L., Skovgaard L.T. Effects of Pluronic F-68 on Tetrahymena cells: protection against chemical and physical stress and prolongation of survival under toxic conditions. J. Biotechnol. 2000;76:185–195. doi: 10.1016/s0168-1656(99)00188-1. [DOI] [PubMed] [Google Scholar]
- Hernández Y.G., Fischer R.W. Serum-free culturing of mammalian cells-adaptation to and cryopreservation in fully defined media. Altex-alternatives Anim. Exp. 2007;24:110–116. doi: 10.14573/altex.2007.2.110. [DOI] [PubMed] [Google Scholar]
- Hezavehei M., Sharafi M., Kouchesfahani H.M., Henkel R., Agarwal A., Esmaeili V., Shahverdi A. Sperm cryopreservation: a review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online. 2018;37:327–339. doi: 10.1016/j.rbmo.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Houang E.M., Bates F.S., Sham Y.Y., Metzger J.M. All-atom molecular dynamics-based analysis of membrane-stabilizing copolymer interactions with lipid bilayers probed under constant surface tensions. J. Phys. Chem. B. 2017;121:10657–10664. doi: 10.1021/acs.jpcb.7b08938. [DOI] [PubMed] [Google Scholar]
- Hunter R.L., Luo A.Z., Zhang R., Kozar R.A., Moore F.A. Poloxamer 188 inhibition of ischemia/reperfusion injury: evidence for a novel anti-adhesive mechanism. Ann. Clin. Lab. Sci. 2010;40:115–125. [PubMed] [Google Scholar]
- Jordan M., Eppenberger H.M., Sucker H., Widmer F., Einsele A. Interactions between animal cells and gas bubbles: the influence of serum and Pluronic F68 on the physical properties of the bubble surface. Biotechnol. Bioeng. 1994;43:446–454. doi: 10.1002/bit.260430603. [DOI] [PubMed] [Google Scholar]
- Kamali Sangani A., Masoudi A.A., Vaez Torshizi R. Association of mitochondrial function and sperm progressivity in slow-and fast-growing roosters. Poult. Sci. 2016;96:211–219. doi: 10.3382/ps/pew273. [DOI] [PubMed] [Google Scholar]
- Kerleta V., Andrlik I., Braunmüller S., Franke T., Wirth M., Gabor F. Poloxamer 188 supplemented culture medium increases the vitality of Caco-2 cells after subcultivation and freeze/thaw cycles. Altex-alternatives Anim. Exp. 2010;27:191–197. [PubMed] [Google Scholar]
- Lotfi S., Mehri M., Sharafi M., Masoudi R. Hyaluronic acid improves frozen-thawed sperm quality and fertility potential in rooster. Anim. Reprod. Sci. 2017;184:204–210. doi: 10.1016/j.anireprosci.2017.07.018. [DOI] [PubMed] [Google Scholar]
- Lowe K.C., Anthony P., Davey M.R., Power J.B. Beneficial effects of Pluronic F-68 and artificial oxygen carriers on the post-thaw recovery of cryopreserved plant cells. Artif. Cells, Blood Substitutes. Biotechnol. 2001;29:297–316. doi: 10.1081/bio-100104232. [DOI] [PubMed] [Google Scholar]
- Mansor M.H., Najberg M., Contini A., Alvarez-Lorenzo C., Garcion E., Jérôme C., Boury F. Development of a non-toxic and non-denaturing formulation process for encapsulation of SDF-1α into PLGA/PEG-PLGA nanoparticles to achieve sustained release. Eur. J. Pharm. Biopharm. 2018;125:38–50. doi: 10.1016/j.ejpb.2017.12.020. [DOI] [PubMed] [Google Scholar]
- Martin Munoz P., Anel-López L., Ortiz-Rodríguez J.M., Álvarez M., de Paz P., Balao da Silva C., Rodríguez Martinez H., Gil M.C., Anel L., Peña F.J. Redox cycling induces spermptosis and necrosis in stallion spermatozoa while the hydroxyl radical (OH•) only induces spermptosis. Reprod. Domest. Anim. 2018;53:54–67. doi: 10.1111/rda.13052. [DOI] [PubMed] [Google Scholar]
- Mehdipour M., Daghigh Kia H., Moghaddam G., Hamishehkar H. Effect of egg yolk plasma and soybean lecithin on rooster frozen-thawed sperm quality and fertility. Theriogenology. 2018;116:89–94. doi: 10.1016/j.theriogenology.2018.05.013. [DOI] [PubMed] [Google Scholar]
- Mehdipour M., Daghigh-Kia H., Najafi A., Dodaran H.V., Garcia-Alvarez O. Effect of green tea (Camellia sinensis) extract and pre-freezing equilibration time on the post-thawing quality of ram semen cryopreserved in a soybean lecithin-based extender. Cryobiology. 2016;73:297–303. doi: 10.1016/j.cryobiol.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Mehdipour M., Daghigh-Kia H., Nazari M., Najafi A. Effect of lecithin nanoliposome or soybean lecithin supplemented by pomegranate extract on post-thaw flow cytometric, microscopic and oxidative parameters in ram semen. Cryobiology. 2017;78:34–40. doi: 10.1016/j.cryobiol.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Moloughney J.G., Weisleder N. Poloxamer 188 (p188) as a membrane resealing reagent in biomedical applications. Recent Pat. Biotechnol. 2012;6:200–211. doi: 10.2174/1872208311206030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi A., Daghigh-Kia H., Dodaran H.V., Mehdipour M., Alvarez-Rodriguez M. Ethylene glycol, but not DMSO, could replace glycerol inclusion in soybean lecithin-based extenders in ram sperm cryopreservation. Anim. Reprod. Sci. 2017;177:35–41. doi: 10.1016/j.anireprosci.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Najafi A., Daghigh-Kia H., Hamishehkar H., Moghaddam G., Alijani S. Effect of resveratrol-loaded nanostructured lipid carriers supplementation in cryopreservation medium on post-thawed sperm quality and fertility of roosters. Anim. Reprod. Sci. 2019;201:32–40. doi: 10.1016/j.anireprosci.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Najafi A., Taheri R.A., Mehdipour M., Martínez-Pastor F., Rouhollahi A.A., Nourani M.R. Improvement of post-thawed sperm quality in broiler breeder roosters by ellagic acid-loaded liposomes. Poult. Sci. 2019;98:440–446. doi: 10.3382/ps/pey353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi A., Daghigh-Kia H., Mehdipour M., Hamishehkar H., Alvarez-Rodriguez M. Effect of quercetin loaded liposomes or nanostructured lipid carrier (NLC) on post-thawed sperm quality and fertility of rooster sperm. Theriogenology. 2020;152:122–128. doi: 10.1016/j.theriogenology.2020.04.033. [DOI] [PubMed] [Google Scholar]
- Najafi A., Taheri R.A., Mehdipour M., Farnoosh G., Martinez-Pastor F. Lycopene-loaded nanoliposomes improve the performance of a modified Beltsville extender broiler breeder roosters. Anim. Reprod. Sci. 2018;195:168–175. doi: 10.1016/j.anireprosci.2018.05.021. [DOI] [PubMed] [Google Scholar]
- Natoli R.M., Athanasiou K.A. P188 reduces cell death and IGF-I reduces GAG release following single-impact loading of articular cartilage. J. Biomech. Eng. 2008;130 doi: 10.1115/1.2939368. [DOI] [PubMed] [Google Scholar]
- Nesci S., Spinaci M., Galeati G., Nerozzi C., Pagliarani A., Algieri C., Tamanini C., Bucci D. Sperm function and mitochondrial activity: an insight on boar sperm metabolism. Theriogenology. 2020 doi: 10.1016/j.theriogenology.2020.01.004. [DOI] [PubMed] [Google Scholar]
- O’Neill H.C., Nikoloska M., Ho H., Doshi A., Maalouf W. Improved cryopreservation of spermatozoa using vitrification: comparison of cryoprotectants and a novel device for long-term storage. J. Assist. Reprod. Genet. 2019;36:1713–1720. doi: 10.1007/s10815-019-01505-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabon D., Meseguer M., Sevillano G., Cobo A., Romero J.L., Remohi J., de Los Santos M.J. A new system of sperm cryopreservation: evaluation of survival, motility, DNA oxidation, and mitochondrial activity. Andrology. 2019;7:293–301. doi: 10.1111/andr.12607. [DOI] [PubMed] [Google Scholar]
- Palasz A.T., Thundathil J., De La Fuente J., Mapletoft R.J. Effect of reduced concentrations of glycerol and various macromolecules on the cryopreservation of mouse and cattle embryos. Cryobiology. 2000;41:35–42. doi: 10.1006/cryo.2000.2262. [DOI] [PubMed] [Google Scholar]
- Pettitt M.J., Buhr M.M. Extender components and surfactants affect boar sperm function and membrane behavior during cryopreservation. J. Androl. 1998;19:736–746. [PubMed] [Google Scholar]
- Phillips D.M., Haut R.C. The use of a non-ionic surfactant (P188) to save chondrocytes from necrosis following impact loading of chondral explants. J. Orthop. Res. 2004;22:1135–1142. doi: 10.1016/j.orthres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Phillips J.J., Bramwell R.K., Graham J.K. Cryopreservation of rooster sperm using methyl cellulose. Poult. Sci. 1996;75:915–923. doi: 10.3382/ps.0750915. [DOI] [PubMed] [Google Scholar]
- Ramírez O.T., Mutharasan R. The role of the plasma membrane fluidity on the shear sensitivity of hybridomas grown under hydrodynamic stress. Biotechnol. Bioeng. 1990;36:911–920. doi: 10.1002/bit.260360906. [DOI] [PubMed] [Google Scholar]
- Riehm J.J., Wang L., Ghadge G., Teng M., Correa A.M., Marks J.D., Roos R.P., Allen M.J. Poloxamer 188 decreases membrane toxicity of mutant SOD1 and ameliorates pathology observed in SOD1 mouse model for ALS. Neurobiol. Dis. 2018;115:115–126. doi: 10.1016/j.nbd.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahin M.A., Khalil W.A., Saadeldin I.M., Swelum A.A., El-Harairy M.A. Comparison between the effects of adding Vitamins, Trace Elements, and nanoparticles to SHOTOR extender on the cryopreservation of Dromedary Camel Epididymal spermatozoa. Anim. 2020;10 doi: 10.3390/ani10010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieme H., Oldenhof H., Wolkers W.F. Mode of action of cryoprotectants for sperm preservation. Anim. Reprod. Sci. 2016;169:2–5. doi: 10.1016/j.anireprosci.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Singh-Joy S.D., McLain V.C. Safety assessment of poloxamers 101, 105, 108, 122, 123, 124, 181, 182, 183, 184, 185, 188, 212, 215, 217, 231, 234, 235, 237, 238, 282, 284, 288, 331, 333, 334, 335, 338, 401, 402, 403, and 407, poloxamer 105 benzoate, and poloxamer 182 dibenzoate as used in cosmetics. Int. J. Toxicol. 2008;27:93–128. doi: 10.1080/10915810802244595. [DOI] [PubMed] [Google Scholar]
- Team, R. C. R Foundation for Statistical Computing; Vienna, Austria: 2019. R: A Language and Environment for Statistical Computing, version 3.0. 2. [Google Scholar]
- Thananurak P., Chuaychu-noo N., Thélie A., Phasuk Y., Vongpralub T., Blesbois E. Different concentrations of cysteamine, ergothioneine, and serine modulate quality and fertilizing ability of cryopreserved chicken sperm. Poult. Sci. 2020;99:1185–1198. doi: 10.1016/j.psj.2019.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K., Wenby R.B., Meiselman H.J. Inhibition of polymer-induced red blood cell aggregation by poloxamer 188. Biorheology. 2000;37:301–312. [PubMed] [Google Scholar]
- Wang J.C., Bindokas V.P., Skinner M., Emrick T., Marks J.D. Mitochondrial mechanisms of neuronal rescue by F-68, a hydrophilic Pluronic block co-polymer, following acute substrate deprivation. Neurochem. Int. 2017;109:126–140. doi: 10.1016/j.neuint.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Markham A., Thomas S.J., Wang N., Huang L., Clemens M., Rajagopalan N. Solution stability of poloxamer 188 under stress conditions. J. Pharm. Sci. 2019;108:1264–1271. doi: 10.1016/j.xphs.2018.10.057. [DOI] [PubMed] [Google Scholar]
- Yuhua S., Ligen L., Jiake C., Tongzhu S. Effect of Poloxamer 188 on deepening of deep second-degree burn wounds in the early stage. Burns. 2012;38:95–101. doi: 10.1016/j.burns.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Zhao M.A., Xhu Y.Z., Dhital S.P., Khu D.M., Song Y.S., Wang M.Y., Lim H.T. An efficient cryopreservation procedure for potato (Solanum tuberosum L.) utilizing the new ice blocking agent, Supercool X1000. Plant Cell Rep. 2005;24:477–481. doi: 10.1007/s00299-005-0970-8. [DOI] [PubMed] [Google Scholar]