Abstract
Four experiments were conducted to estimate the optimal standardized ileal digestible (SID) level of branched-chain amino acids in low-protein diets during the starter, grower, and finisher periods, using the response surface methodology, and to study their effects on performance and mRNA expression of genes involved in the mechanistic target of rapamycin (mTOR) pathway of broiler chickens from 8 to 21 D of age. In experiments 1, 2, and 3, a total of 1,500 Cobb male broiler chickens were assigned to 15 diets of a central composite rotatable design (CCD) of response surface methodology containing 5 levels of SID Leu, Val, and Ile with 5 replicate pens of 20 birds each. A 3-factor, 5-level CCD platform was used to fit the second-order polynomial equation of broiler performance. In experiment 4, a total of 540 8-day-old Cobb male broiler chickens were distributed in a completely randomized 2 x 3 x 3 factorial arrangement with 2 SID Leu levels (1.28 or 1.83%), 3 SID Val levels (0.65, 0.90, or 1.20%), and 3 SID Ile levels (0.54, 0.79, or 1.09%) for a total of 18 treatments with 5 replicate cages of 6 birds each. High Leu levels impaired (P < 0.05) gain:feed when birds were fed marginal Val or Ile diets. However, gain:feed was restored when both Val and Ile were supplemented to reach adequate or high levels. High Leu levels increased (P < 0.05) mRNA expression of S6K1 and eEF2 genes only in birds fed high Ile levels. Dietary SID Leu, Val, and Ile levels required for gain:feed optimization in low-protein diets were estimated at 1.37, 0.94, and 0.87% during the starter period; 1.23, 0.82, and 0.75% during the grower period; and 1.15, 0.77, and 0.70% during the finisher phase, respectively. Higher Val and Ile levels are required to optimize the effect of Leu supplementation on mRNA expression of mTOR pathway genes in the pectoralis major muscle of broilers from day 1 to 21 after hatch.
Key words: branched-chain amino acid, broiler chicken, gene expression, performance, response surface methodology
Introduction
It has been well known that branched-chain amino acids (BCAA) (Val, Leu and Ile) are not only substrates for building block protein but they are also involved in intracellular signaling pathways on protein anabolism and stimulatory effects on protein synthesis. Among the 3 BCAA, Leu has been considered most effective in stimulating muscle protein synthesis by modulating the activation of mechanistic target of rapamycin (mTOR) and its downstream effectors, ribosomal protein S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E-binding protein-1 (4EBP1), which are components of the translation initiation machinery (Suryawan et al., 2008). In addition to its role in promoting the activation of S6K1 and 4EBP1, mTOR increases protein synthesis through eukaryotic translation elongation factor 2 (eEF2) activation (Proud, 2002), which is essential for translation elongation. High dietary Leu has been demonstrated to activate the mTOR signaling pathways in pigs and broilers (Suryawan et al., 2008; Torrazza et al., 2010; Deng et al., 2014; Chang et al., 2015). However, growth performance was not improved in a previous study with broilers fed high-Leu diets and graded Val levels, even though Leu supplementation increased the expression of genes involved in the mTOR signaling pathway of the pectoralis major muscle (Ospina-Rojas et al., 2019). The reduced blood availability of other amino acids than Val could have limited the protein synthesis. Positive Leu effects are limited by itself because in excess promotes its own oxidative degradation by stimulating the branched-chain α-keto acid dehydrogenase (BCKD) complex through its keto-acid, α-ketoisocaproate (Brosnan and Brosnan, 2006). The BCKD complex uses all 3 branched-chain α-keto acids as substrate and, once it is activated, promotes the irreversible catabolism of branched-chain α-keto acids to coenzyme A compounds. It was demonstrated that dietary Leu levels greater than recommended reduced the circulating concentrations of Val and Ile (D'Mello and Lewis, 1970; Wessels et al., 2016). Then, the assessment of Leu effects on mTOR pathway of protein synthesis should consider both dietary Val and Ile levels as BCAA could exhibit antagonistic interactions among each other (Harper et al., 1984).
Some feed ingredients have disproportionate high Leu levels compared with Val and Ile, which might create a BCAA imbalance in low-protein diets with a combination of some particular feed ingredients. Corn standardized ileal digestible (SID) Leu content is 11.6% relative to protein concentration, whereas it is 6.9% in soybean meal (Rostagno et al., 2011). Leucine concentration in sorghum is 3-fold higher than Val and Ile levels. In addition, corn gluten meal contains high SID Leu level in percentage of protein content (17%) but low values of Val and Ile (4%) (Rostagno et al., 2011). Protein-reduced diets decrease protein source inclusion and increase the proportion of dietary cereal ingredients, some of which have a high Leu level in relation to their protein contribution. Hence, Leu antagonism effect on other BCAA could be more significant for birds fed low-protein diets.
Dietary BCAA imbalance may affect broiler performance (D'Mello and Lewis, 1970; Allan and Baker, 1972; Smith and Austic, 1978; Ospina-Rojas et al., 2017, 2019), but other studies have indicated that excess Leu does not compromise broiler performance when protein levels are higher than broiler requirement (Burnham et al., 1992; Waldroup et al., 2002). Then, the ideal BCAA ratio in low-protein diets should be different from high-protein diets and should be studied. In previous studies, we estimated the ideal Val/Leu ratio in starter and grower diets (Ospina-Rojas et al., 2017, 2019), but we did not consider the effect of Ile levels to estimate the optimal BCAA ratio in low-protein diets. Several reports have estimated the Leu, Val, and Ile requirements separately without considering the interactions between them. The interaction effects of the BCAA, such as synergism or antagonism, could be evaluated using the response surface methodology which is an experimental procedure used to evaluate the nature of the response variable (performance) obtained from the simultaneous variation of explanatory variables (dietary SID levels of BCAA) and define the relationships between them (Baş and Boyacı, 2007). In addition to analyzing the effects of the explanatory variables, this experimental methodology generates a second-degree model that can be used to estimate the ideal dietary level to optimize performance. Based on these statements, the objective of this study was to estimate the optimal SID level of BCAA in low-protein diets during the starter (1 to 14 D), grower (14 to 28 D), and finisher (28 to 42 D) periods using the response surface methodology and to study their effects on performance and mRNA expression of genes involved in the mTOR pathway of broiler chickens from 8 to 21 D of age.
Materials and methods
Experimental Facility and Bird Husbandry
The broiler chickens were raised under standard husbandry conditions, and all experiments were performed in accordance with Brazil's National Council for the Control of Animal Experimentation guidelines and were authorized by the Ethics Committee for Animal Use of the Universidade Estadual de Maringá, PR, Brazil (Protocol No. 6075180815-CEUA/UEM).
In all experiments, chicks were obtained from a commercial hatchery after being vaccinated against Marek's disease and Newcastle disease and infectious bronchitis. Temperature was maintained at 32°C at placement and was gradually reduced to ensure comfort by using exhaust fans and evaporative cooling pads. Ventilation was accomplished by negative air pressure. The lighting program throughout the study consisted of 23 h of light (25 lux) and 1 h of dark. Feed and water were available to the birds ad libitum.
Amino acid analyses (method 994.12) and CP composition (method 968.06) were performed for corn, soybean meal, meat and bone meal, and wheat bran, before feed formulation, and also on representative samples of the experimental diets, as per AOAC (2006) procedures. After that, SID amino acid values of the used feedstuffs were estimated by applying the SID coefficients suggested by Rostagno et al. (2011). The basal diet with reduced protein content was formulated as per the nutritional recommendations for male broiler chickens (Rostagno et al., 2011), except for SID Leu, Val, and Ile levels (Table 1). The other experimental diets were obtained by supplementing the basal diet with L-Leu, L-Val, and L-Ile replacing the inert filler (kaolin).
Table 1.
Composition of the basal diets (as-fed basis) used in the experiments 1 (1–14 D), 2 (14–28 D), 3 (28–42 D), and 4 (8–21 D).
| Item | Experiment 1 (1–14 D) | Experiment 2 (14–28 D) | Experiment 3 (28–42 D) | Experiment 4 (8–21 D) |
|---|---|---|---|---|
| Ingredient, % | ||||
| Corn | 66.08 | 66.62 | 72.26 | 68.33 |
| Soybean meal 45% | 10.47 | 9.20 | 7.04 | 16.53 |
| Wheat bran | 5.00 | 6.05 | 4.00 | 3.91 |
| Meat and bone meal | 6.83 | 5.73 | 5.02 | 4.99 |
| Soybean oil | 1.80 | 3.00 | 3.00 | 1.00 |
| Limestone | 0.12 | 0.26 | 0.29 | 0.23 |
| NaCl | 0.43 | 0.41 | 0.40 | 0.42 |
| Potassium chloride | 0.21 | 0.23 | 0.22 | 0.02 |
| Suppl, Min–Vit1 | 0.40 | 0.40 | 0.40 | 0.40 |
| Filler (kaolin)2 | 1.60 | 1.60 | 1.60 | 1.70 |
| L-Glu 99.4% | 3.50 | 3.50 | 3.50 | 0.51 |
| DL-Met 99% | 0.53 | 0.47 | 0.43 | 0.42 |
| L-Lys HCl 78.5% | 0.93 | 0.84 | 0.82 | 0.66 |
| L-Thr 98% | 0.39 | 0.34 | 0.32 | 0.26 |
| L-Val 98% | 0.28 | 0.17 | 0.06 | --- |
| L-Ile 98% | 0.25 | 0.19 | 0.05 | --- |
| L-Arg 99% | 0.55 | 0.49 | 0.49 | 0.32 |
| L-Trp 98% | 0.11 | 0.10 | 0.10 | 0.06 |
| Gly 97% | 0.52 | 0.40 | 0.00 | 0.24 |
| Calculated composition3 | ||||
| CP, % | 19.2 (19.0) | 17.8 (17.4) | 16.0 (16.2) | 18.0 (17.7) |
| ME, kcal/kg | 2,975 | 3,050 | 3,125 | 3,000 |
| Calcium, % | 0.87 | 0.78 | 0.69 | 0.82 |
| Chloride, % | 0.34 | 0.32 | 0.32 | 0.33 |
| Available phosphorus, % | 0.43 | 0.37 | 0.32 | 0.39 |
| Potassium, % | 0.59 | 0.58 | 0.58 | 0.59 |
| Sodium, % | 0.22 | 0.21 | 0.20 | 0.21 |
| SID Gly + Ser, % | 1.83 | 1.59 | 1.18 | 1.73 |
| SID Arg, % | 1.34 | 1.22 | 1.13 | 1.27 |
| SID Lys, % | 1.24 | 1.13 | 1.04 | 1.17 |
| SID Met + Cys, % | 0.90 | 0.82 | 0.76 | 0.85 |
| SID Thr, % | 0.81 | 0.73 | 0.68 | 0.76 |
| SID Trp, % | 0.21 | 0.20 | 0.19 | 0.20 |
| SID Leu, % | 1.10 | 1.00 | 0.98 | 1.28 |
| SID Val, % | 0.81 | 0.67 | 0.52 | 0.65 |
| SID Ile, % | 0.68 | 0.59 | 0.41 | 0.54 |
| Lys | 1.32 (1.27) | 1.21 (1.18) | 1.12 (1.09) | 1.27 (1.26) |
| Met + Cys | 0.96 (0.99) | 0.88 (0.90) | 0.82 (0.83) | 0.92 (0.92) |
| Leu | 1.18 (1.12) | 1.09 (1.09) | 1.06 (1.10) | 1.39 (1.43) |
| Val | 0.88 (0.87) | 0.74 (0.72) | 0.59 (0.52) | 0.73 (0.79) |
| Ile | 0.74 (0.69) | 0.65 (0.64) | 0.47 (0.51) | 0.61(0.58) |
| Arg | 1.41 (1.47) | 1.29 (1.36) | 1.19 (1.22) | 1.35 (1.40) |
Abbreviation: SID, standardized ileal digestible.
The vitamin and mineral premix contained per kilogram of diet: vitamin A (retinyl acetate), 2,654 μg; vitamin D3 (cholecalciferol), 125 μg; vitamin E (dl-α-tocopheryl acetate), 9.9 mg; vitamin K3 (menadione dimethyl pyrimidinol), 1.7 mg; vitamin B1 (thiamin mononitrate), 1.6 mg; vitamin B12 (cyanocobalamin), 16.7 μg; riboflavin, 5.3 mg; niacin (niacinamide), 36 mg; calcium pantothenate, 13 mg; folic acid, 0.8 mg; d-biotin, 0.1 mg; choline chloride, 270; BHT, 5.8; Fe (iron sulfate monohydrate), 50 mg; Cu (copper sulfate pentahydrate), 12 mg; I (calcium iodate), 0.9 mg; Zn (zinc oxide), 50 mg; Mn (manganous oxide), 60 mg; Se (sodium selenite), 0.2 mg; Co (cobalt sulfate), 0.2 mg.
Supplemental L-Leu, L-Val and L-Ile were added to the test diets at the expense of the inert filler (kaolin) to derive dietary treatments.
Analyzed values for total amino acids are in parentheses.
Experiments 1, 2, and 3
A total of 1,500 Cobb male broiler chickens were assigned to 15 diets of a central composite rotatable design of response surface methodology containing 5 levels of SID Leu, Val, and Ile with 5 replicate pens of 20 birds each. Three time periods were evaluated independently, 1 to 14 D (starter), 14 to 28 D (grower), and 28 to 42 D (finisher) of age. A common corn and soybean meal–based diet was formulated to satisfy all nutrient recommendations (Rostagno et al., 2011) and fed from placement to 13 D and to 27 D of age to evaluate the grower and finisher periods, respectively. Five dietary SID levels of Leu, Val, and Ile ranged as 1.10–1.70%, 0.81–1.21%, and 0.68–1.08% at the starter phase; 1.00–1.60%, 0.67–1.07%, and 0.59–0.99% at the grower period; and 0.98–1.58%, 0.52–1.12%, 0.41–1.01% at the finisher phase, respectively (Table 2). Broilers were raised on floor pens (2.0 × 1.0 m) with fresh wood shavings as litter (2.5 cm deep) and was equipped with 1 tube feeder of 48 cm and 1 nipple drinker (6 nipples/pen).
Table 2.
Dietary SID levels of Leu, Val, and Ile used in central composite design of response surface methodology and calculated and analyzed values of total branched-chain amino acids in the experiments 1 (1–14 D), 2 (14–28 D), and 3 (28–42 D).1
| Diet | Experiment 1 Starter (1 to 14 D) |
Experiment 2 Grower (14 to 28 D) |
Experiment 3 Finisher (28 to 42 D) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SID (%) |
Total levels (%) |
SID (%) |
Total levels (%) |
SID (%) |
Total levels (%) |
|||||||||||||
| Leu | Val | Ile | Leu | Val | Ile | Leu | Val | Ile | Leu | Val | Ile | Leu | Val | Ile | Leu | Val | Ile | |
| 1 | 1.55 | 1.11 | 0.98 | 1.63 (1.59) | 1.18 (1.24) | 1.04 (1.09) | 1.45 | 0.97 | 0.89 | 1.54 (1.59) | 1.05 (1.05) | 0.95 (1.01) | 1.43 | 0.97 | 0.86 | 1.51 (1.55) | 1.04 (1.01) | 0.92 (088) |
| 2 | 1.55 | 1.11 | 0.78 | 1.63 (1.60) | 1.18 (1.22) | 0.84 (0.81) | 1.45 | 0.97 | 0.69 | 1.54 (1.55) | 1.05 (1.11) | 0.75 (0.72) | 1.43 | 0.97 | 0.56 | 1.51 (1.52) | 1.04 (1.04) | 0.62 (0.65) |
| 3 | 1.55 | 0.91 | 0.98 | 1.63 (1.64) | 0.98 (1.01) | 1.04 (1,03) | 1.45 | 0.77 | 0.89 | 1.54 (1.52) | 0.85 (0.79) | 0.95 (0.93) | 1.43 | 0.67 | 0.86 | 1.51 (1.55) | 0.74 (0.77) | 0.92 (0.95) |
| 4 | 1.55 | 0.91 | 0.78 | 1.63 (1.60) | 0.98 (0.98) | 0.84 (0.79) | 1.45 | 0.77 | 0.69 | 1.54 (1.60) | 0.85 (0.88) | 0.75 (0.75) | 1.43 | 0.67 | 0.56 | 1.51 (1.56) | 0.74 (0.78) | 0.62 (0.65) |
| 5 | 1.25 | 1.11 | 0.98 | 1.33 (1.29) | 1.18 (1.15) | 1.04 (1.11) | 1.15 | 0.97 | 0.89 | 1.24 (1.19) | 1.05 (1.00) | 0.95 (0.97) | 1.13 | 0.97 | 0.86 | 1.21 (1.13) | 1.04 (1.01) | 0.92 (0.91) |
| 6 | 1.25 | 1.11 | 0.78 | 1.33 (1.33) | 1.18 (1.24) | 0.84 (0.85) | 1.15 | 0.97 | 0.69 | 1.24 (1.22) | 1.05 (1.04) | 0.75 (0.77) | 1.13 | 0.97 | 0.56 | 1.21 (1.20) | 1.04 (1.05) | 0.62 (0.65) |
| 7 | 1.25 | 0.91 | 0.78 | 1.33 (1.36) | 0.98 (1.02) | 0.84 (0.87) | 1.15 | 0.77 | 0.69 | 1.24 (1.24) | 0.85 (0.79) | 0.75 (0.76) | 1.13 | 0.67 | 0.56 | 1.21 (1.25) | 0.74 (0.72) | 0.62 (0.66) |
| 8 | 1.40 | 1.01 | 0.88 | 1.48 (1.51) | 1.08 (1.15) | 0.93 (0.91) | 1.30 | 0.87 | 0.79 | 1.39 (1.42) | 0.95 (0.94) | 0.85 (0.90) | 1.28 | 0.82 | 0.71 | 1.36 (1.36) | 0.89 (0.85) | 0.76 (0.79) |
| 9 | 1.40 | 1.01 | 0.68 | 1.48 (1.46) | 1.08 (1.10) | 0.74 (0.69) | 1.30 | 0.87 | 0.59 | 1.39 (1.35) | 0.95 (0.98) | 0.65 (0.64) | 1.28 | 0.82 | 0.41 | 1.36 (1.39) | 0.89 (0.90) | 0.47 (0.51) |
| 10 | 1.40 | 1.01 | 1.08 | 1.48 (1.43) | 1.08 (1.13) | 1.13 (1.12) | 1.30 | 0.87 | 0.99 | 1.39 (1.35) | 0.95 (0.97) | 1.06 (1.09) | 1.28 | 0.82 | 1.01 | 1.36 (1.39) | 0.89 (0.88) | 1.06 (1.08) |
| 11 | 1.40 | 1.21 | 0.88 | 1.48 (1.50) | 1.28 (1.25) | 0.94 (0.99) | 1.30 | 1.07 | 0.79 | 1.39 (1.37) | 1.15 (1.20) | 0.85 (0.87) | 1.28 | 1.12 | 0.71 | 1.36 (1.38) | 1.92 (1.89) | 0.77 (0.76) |
| 12 | 1.40 | 0.81 | 0.88 | 1.48 (1.48) | 0.88 (0.87) | 0.94 (0.96) | 1.30 | 0.67 | 0.79 | 1.39 (1.44) | 0.74 (0.72) | 0.85 (0.86) | 1.28 | 0.52 | 0.71 | 1.36 (1.35) | 0.59 (0.52) | 0.77 (0.79) |
| 13 | 1.10 | 1.01 | 0.88 | 1.18 (1.12) | 1.08 (1.14) | 0.94 (0.90) | 1.00 | 0.87 | 0.79 | 1.09 (1.09) | 0.95 (0.91) | 0.85 (0.88) | 0.98 | 0.82 | 0.71 | 1.06 (1.10) | 0.89 (0.92) | 0.77 (0.75) |
| 14 | 1.70 | 1.01 | 0.88 | 1.78 (1.77) | 1.08 (1.08) | 0.94 (0.94) | 1.60 | 0.87 | 0.79 | 1.70 (1.62) | 0.95 (0.92) | 0.85 (0.84) | 1.58 | 0.82 | 0.71 | 1.66 (1.59) | 0.89 (0.93) | 0.77 (0.75) |
| 15 | 1.25 | 0.91 | 0.98 | 1.33 (1.35) | 0.99 (0.95) | 1.04 (1.03) | 1.15 | 0.77 | 0.89 | 1.24 (1.26) | 0.85 (0.89) | 0.95 (0.96) | 1.13 | 0.67 | 0.86 | 1.21 (1.20) | 0.75 (0.77) | 0.92 (0.99) |
Abbreviation: SID, standardized ileal digestible.
Analyzed values for total amino acids are in parentheses.
Broiler Performance
Growth performance was evaluated from day 1 to 14, 14 to 28, and 28 to 42 after hatch in the experiments 1, 2, and 3, respectively. Broilers and experimental diets were weighed weekly for performance evaluation (feed intake, BW gain, and gain:feed). Broiler mortality was recorded twice daily.
Experiment 4
At the placement, chicks were distributed across 25 floor pens (30 birds/pen), each floor pen (0.13 m2/bird) had fresh wood shavings and was equipped with 1 tube feeder and 3 nipple drinkers. On day 8, 540 birds were randomly placed into 90 grower battery cages (6 birds/cage) with raised wire floors (0.08 m2/bird), and each battery cage was fitted with an adjustable trough feeder and drinker. Birds were assigned 1 of 18 dietary treatments (5 replications/treatment) from 8 to 21 D of age with varying concentrations of SID Leu, Val, and Ile. A factorial arrangement of treatments consisted of 2 SID levels of Leu (1.28 or 1.83%), 3 SID levels of Val (0.65, 0.90 or 1.20%), and 3 SID levels of Ile (0.54, 0.79 or 1.09%). Growth was measured from day 8 to 21 after hatch. Chickens and experimental diets were weighed at 8, 15, and 21 D of age to evaluate the feed intake, BW gain, and gain:feed.
Gene Expression Analysis
A factorial arrangement of 2 SID Leu levels (1.28 or 1.83%), 2 SID Val levels (0.65 or 1.20%), and 2 SID Ile levels (0.54 or 1.09%) was used for gene expression analysis. At 21 D of age, 5 birds per treatment (8 treatments) were slaughtered in the morning, and samples of muscle (pectoralis major) were collected immediately to determine the mRNA expression of mTOR, S6K1, and eEF2 genes. Total RNA was extracted with the use of TRIzol reagent (Invitrogen, Carlsbad CA) by following the manufacturer's directions in the proportion of 1 mL per 100 mg of tissue. The RNA concentration was measured using a spectrophotometer at a wavelength of 260 nm (NanoDrop 2000, Thermo Fisher Scientific, MA). RNA integrity was evaluated in a 1% agarose gel stained with SYBR safe (Life Technologies, CA) and visualized with UV light. From the concentration measured, 1 μg of total RNA was incubated with DNAse I (Invitrogen Corporation, Brazil), and subsequently, DNAse I was inactivated by adding EDTA and heating at 65°C for 5 min.
The preparation of cDNA was performed with the SuperScript III First-Strand Synthesis Super Mix kit and Oligo-d(T) (Invitrogen Corporation, Brazil) and RNAse-out (Invitrogen Corporation, Brazil) for mRNA degradation as per the guidelines of manufacturer. The reaction was performed at 42°C for 15 min, 50°C for 50 min, and 70°C for 15 min. The cDNA quantification was estimated using the Nanodrop spectrophotometer. The samples were stored at −80 until further analysis.
For the real-time PCR reactions, the cDNA was diluted to obtain a solution of 200 ng/μl. The fluorescent dye SYBR Green (SYBR GREEN PCR Master Mix, Applied Biosystems) was used. The real-time PCR analysis was performed by the StepOne Real-Time PCR system (Applied Biosystems, Carlsbad, CA). Thermal cycling parameters for all genes were the following: hot-start, 95°C for 10 min, followed by 40 cycles of denaturation and annealing/extension, 95°C for 15 s, 60°C for 1 min, and then, melting curve, 65–95°C. All analyses were performed with 1 μL of cDNA (200 ng) and both sense and antisense oligonucleotides (1 μL of 5 μmol primer stock solution each) in a final volume of 25 uL. A duplicated with negative control was added to all the plates to control possible contamination in the reactions.
The oligonucleotide primers used for amplification of target genes (mTOR, S6K1, and eEF2; Table 3) were based on the nucleotide sequences used by Lee and Aggrey (2016) as per chicken sequences (GenBank access number: XM_417614; NM_001030721; and NM_205368, respectively). The glyceraldehyde-3-phosphate dehydrogenase gene was used as a reference gene as described by Gilbert et al. (2007). The expression of target and reference genes of each sample was run simultaneously in duplicate on the same PCR plate, and the average of cycle threshold (CT) value was used to calculate 2−ΔCT for analysis of relative quantification (Livak and Schmittgen, 2001) and data were expressed by arbitrary units (AU). The efficiency of target and reference genes was calculated from a standard curve plate of serial dilutions (from 25 to 400 ng) of cDNA pools.
Table 3.
Primer sequences of genes in chickens for real-time PCR analyses.
| Gene | Amplicon (bp) | Annealing temperature (°C) | Primer sequence (5′- 3′) |
|---|---|---|---|
| mTOR1 | 119 | 59 | S: TTGGGTTTGCTTTCTGTGGCTGTC A: ACAGACTTCTGCCTCTTGTGAGCA |
| S6K11 | 123 | 63 | S: TTTGCCTCCCTACCTCACACAAGA A: AAGAACGGGTGAGCCTGAACTTCT |
| eEF21 | 99 | 59 | S: AGCCAATCCAAAGGACCATCCTCA A: ACTGATCAACACCAACCAGACCGA |
| GAPDH2 | 73 | 53 | S: GCCGTCCTCTCTGGCAAAG A: TGTAAACCATGTAGTTCA |
Abbreviations: A, antisense; eEF2, eukaryotic translation elongation factor 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; mTOR, mechanistic target of rapamycin; S, sense.
Nucleotide sequences used by Lee and Aggrey (2016).
Nucleotide sequences used by Gilbert et al. (2007).
Statistical Analysis
A 3-factor 5-level central composite rotatable design was used in experiments 1, 2, and 3. Data were analyzed using the RSREG procedure of SAS software (SAS Inst. Inc., Cary, NC) to fit a quadratic response surface model. Linear and quadratic terms for the SID Leu, Val, and Ile levels and the interaction among these variables were evaluated. A second-order polynomial equation was generated as described by Box et al. (1987):
where Y is the response variable, xi represents the input variables (SID Leu, Val, and Ile levels), β0 is the constant term, βi is the coefficients of the linear parameters, βii is the coefficients of the quadratic parameter, βij is the coefficients of the interaction parameters, and ε is the residual error. The RSREG procedure displays the canonical coefficients, indicating the effect of each input variable on the curvature direction at the optimal response (stationary point) in the predicted model. The input variable with the largest influence on the direction of the stationary point was considered the more important factor during the modeling process. A ridge analysis was applied to compute the optimal response for performance using RIDGE MAX when the total model was significant (P < 0.05). A reduced model with significant terms was fitted when the total model and cross product (interactions) were not detected (P > 0.05). Recommended Leu, Val, and Ile needs were established by estimating 95% of the minimum or maximum responses. In experiment 4, data were analyzed using the GLM procedure of SAS Institute (2009). Data were analyzed by 3-way ANOVA to determine the main effects (SID Leu, Val, and Ile levels) and their interaction. Differences among means were separated using a Tukey's multiple range tests by the LSMEANS procedure of SAS.
Results
Experiments 1, 2 and 3
Linear, quadratic, cross product, and total model terms were not significant (P > 0.05) for feed intake of broilers fed SID Leu, Val, and Ile during the starter (1 to 14 D), grower (14 to 28 D), and finisher (28 to 42 D) periods (Table 4, Table 5, Table 6). Dietary SID Leu, Val, and Ile levels resulted in quadratic effects (P < 0.05) on BW gain during the starter and grower phases, but the cross product and total model were not significant (P > 0.05). Therefore, a reduced model was fitted without the interaction terms as follows:
Table 4.
Effect of SID Leu, Val, and Ile content in low-protein diets on growth performance of male broiler from 1 to 14 D of age (experiment 1).1
| Treatment | SID amino acids (%) |
Feed intake (g) | BW gain (g) | Gain:feed (g/g)2 | ||
|---|---|---|---|---|---|---|
| Leu | Val | Ile | ||||
| 1 | 1.55 | 1.11 | 0.98 | 477 | 371 | 779 |
| 2 | 1.55 | 1.11 | 0.78 | 472 | 371 | 787 |
| 3 | 1.55 | 0.91 | 0.98 | 447 | 380 | 851 |
| 4 | 1.55 | 0.91 | 0.78 | 483 | 383 | 795 |
| 5 | 1.25 | 1.11 | 0.98 | 472 | 380 | 805 |
| 6 | 1.25 | 1.11 | 0.78 | 471 | 377 | 801 |
| 7 | 1.25 | 0.91 | 0.78 | 477 | 377 | 792 |
| 8 | 1.40 | 1.01 | 0.88 | 471 | 397 | 842 |
| 9 | 1.40 | 1.01 | 0.68 | 471 | 373 | 792 |
| 10 | 1.40 | 1.01 | 1.08 | 476 | 377 | 793 |
| 11 | 1.40 | 1.21 | 0.88 | 474 | 377 | 796 |
| 12 | 1.40 | 0.81 | 0.88 | 479 | 373 | 780 |
| 13 | 1.10 | 1.01 | 0.88 | 482 | 374 | 777 |
| 14 | 1.70 | 1.01 | 0.88 | 470 | 373 | 794 |
| 15 | 1.25 | 0.91 | 0.98 | 468 | 372 | 794 |
| SEM |
9.36 |
6.29 |
14.92 |
|||
|
Response Surface |
P-Value |
|||||
| Linear | 0.45 | 0.99 | 0.29 | |||
| Quadratic | 0.91 | 0.02 | 0.01 | |||
| Cross product (Leu x Val x Ile) | 0.23 | 0.40 | 0.06 | |||
| Total model | 0.57 | 0.15 | 0.01 | |||
Abbreviation: SID, standardized ileal digestible.
Values are means of 5 replicate pens per treatment (treatments = 15; n = 75).
Values corrected for mortality.
Table 5.
Effect of SID Leu, Val, and Ile content in low-protein diets on growth performance of male broiler from 14 to 28 D of age (experiment 2).1
| Treatment | SID amino acids (%) |
Feed intake (g) | BW gain (g) | Gain:feed (g/g)2 | ||
|---|---|---|---|---|---|---|
| Leu | Val | Ile | ||||
| 1 | 1.45 | 0.97 | 0.89 | 1,587 | 1,022 | 644 |
| 2 | 1.45 | 0.97 | 0.69 | 1,594 | 1,068 | 670 |
| 3 | 1.45 | 0.77 | 0.89 | 1,623 | 1,039 | 640 |
| 4 | 1.45 | 0.77 | 0.69 | 1,579 | 1,021 | 647 |
| 5 | 1.15 | 0.97 | 0.89 | 1,545 | 1,045 | 676 |
| 6 | 1.15 | 0.97 | 0.69 | 1,588 | 1,009 | 635 |
| 7 | 1.15 | 0.77 | 0.69 | 1,542 | 1,000 | 648 |
| 8 | 1.30 | 0.87 | 0.79 | 1,590 | 1,097 | 690 |
| 9 | 1.30 | 0.87 | 0.59 | 1,599 | 1,043 | 652 |
| 10 | 1.30 | 0.87 | 0.99 | 1,568 | 1,020 | 650 |
| 11 | 1.30 | 1.07 | 0.79 | 1,583 | 1,014 | 641 |
| 12 | 1.30 | 0.67 | 0.79 | 1,593 | 1,040 | 653 |
| 13 | 1.00 | 0.87 | 0.79 | 1,603 | 1,040 | 648 |
| 14 | 1.60 | 0.87 | 0.79 | 1,550 | 1,005 | 649 |
| 15 | 1.15 | 0.77 | 0.89 | 1,591 | 1,029 | 647 |
| SEM |
22.34 |
18.55 |
8.51 |
|||
|
Response Surface |
P-Value |
|||||
| Linear | 0.97 | 0.99 | 0.95 | |||
| Quadratic | 0.96 | 0.03 | 0.01 | |||
| Cross product (Leu x Val x Ile) | 0.17 | 0.27 | 0.04 | |||
| Total model | 0.76 | 0.15 | 0.01 | |||
Abbreviation: SID, standardized ileal digestible.
Values are means of 5 replicate pens per treatment (treatments = 15; n = 75).
Values corrected for mortality.
Table 6.
Effect of SID Leu, Val, and Ile content in low-protein diets on growth performance of male broiler from 28 to 42 D of age (experiment 3).1
| Treatment | SID amino acids (%) |
Feed intake (g) | BW gain (g) | Gain:feed (g/g)2 | ||
|---|---|---|---|---|---|---|
| Leu | Val | Ile | ||||
| 1 | 1.43 | 0.97 | 0.86 | 2,500 | 1,447 | 580 |
| 2 | 1.43 | 0.97 | 0.56 | 2,477 | 1,385 | 560 |
| 3 | 1.43 | 0.67 | 0.86 | 2,482 | 1,345 | 541 |
| 4 | 1.43 | 0.67 | 0.56 | 2,387 | 1,157 | 485 |
| 5 | 1.13 | 0.97 | 0.86 | 2412 | 1,399 | 580 |
| 6 | 1.13 | 0.97 | 0.56 | 2,501 | 1,421 | 569 |
| 7 | 1.13 | 0.67 | 0.56 | 2,499 | 1,444 | 578 |
| 8 | 1.28 | 0.82 | 0.71 | 2,470 | 1,472 | 596 |
| 9 | 1.28 | 0.82 | 0.41 | 2,450 | 1,289 | 527 |
| 10 | 1.28 | 0.82 | 1.01 | 2,414 | 1,330 | 550 |
| 11 | 1.28 | 1.12 | 0.71 | 2,438 | 1,276 | 525 |
| 12 | 1.28 | 0.52 | 0.71 | 2,436 | 1,332 | 545 |
| 13 | 0.98 | 0.82 | 0.71 | 2,441 | 1,320 | 542 |
| 14 | 1.58 | 0.82 | 0.71 | 2,465 | 1,199 | 488 |
| 15 | 1.13 | 0.67 | 0.86 | 2,469 | 1,425 | 578 |
| SEM |
47.76 |
38.95 |
14.89 |
|||
|
Response Surface |
P-Value |
|||||
| Linear | 0.97 | 0.01 | 0.001 | |||
| Quadratic | 0.85 | 0.001 | 0.001 | |||
| Cross product (Leu x Val x Ile) | 0.13 | 0.01 | 0.02 | |||
| Total model | 0.65 | 0.001 | 0.001 | |||
Abbreviation: SID, standardized ileal digestible.
Values are means of 5 replicate pens per treatment (treatments = 15; n = 75).
Values corrected for mortality.
BW gain (1 to 14 D) = −1,030.94 + 694.08∗Leu − 247.79∗Leu2 + 1,032.60∗Val – 512.03∗Val2 + 946.56∗Ile − 535.28∗Ile2, R2 = 0.71.
BW gain (14 to 28 D) = −2,314.75 + 1,966.92∗Leu – 756.85∗Leu2 + 2,766.99∗Val – 1,588.92∗Val2 + 2,324.22∗Ile – 1,474.67∗Ile2; R2 = 0.70.
Significant quadratic regressions, cross product, and total model were found (P < 0.05) for BW gain during the finisher phase and a polynomial equation was fitted to the data:
BW gain (28 to 42 D) = −1,621.31 + 3,392.27∗Leu + 1,294.30∗Val + 1,497.46∗Ile − 2,570.22∗Leu2 − 2,071.78∗Val2 − 2,008.67∗Ile2 + 2,113.33∗LeuVal + 1,625.11∗LeuIle −717.11∗ValIle; R2 = 0.83.
In the canonical analysis (Table 7), all canonical coefficients (eigenvalues) were negative for BW gain in all growth periods; thus, the stationary points were a maximum response. Among the eigenvalues of greater magnitude for BW gain during the starter (−29.085437), grower (−90.142648), and finisher (−343.729259) phases, Leu was the amino acid with the largest eigenvector. Thus, Leu is the most important variable in the fitted models for BW gain; therefore, the direction of stationary point curvature was determined by Leu. Based on the fitted models, BW gain can be maximized with dietary digestible Leu, Val, and Ile levels of 1.33, 0.96, and 0.84% during the starter phase; 1.23, 0.83, and 0.75% during the grower period; and 1.16; 0.77, and 0.68% during the finisher phase, respectively.
Table 7.
Eigenvectors of Leu, Val, and Ile levels for BW gain and gain:feed of broiler from 1 to 14 D, 24 to 28 D, and 28 to 42 D of age.
| Eigenvalues | Eigenvectors |
||
|---|---|---|---|
| Leu | Val | Ile | |
| 1 to 14 D (experiment 1) | |||
| BW gain | |||
| −13.927675 | −0.589620 | 0.753419 | 0.291048 |
| −21.180221 | 0.437732 | −0.004757 | 0.899093 |
| −29.085437 | 0.678778 | 0.657524 | −0.326990 |
| Gain:feed | |||
| −0.024179 | 0.610054 | −0.621257 | 0.491807 |
| −0.067139 | −0.229480 | 0.455557 | 0.860120 |
| −0.078448 | 0.758402 | 0.637580 | −0.135348 |
| 14 to 28 D (experiment 2) | |||
| BW gain | |||
| −34.642313 | 0.539044 | 0.389961 | −0.746567 |
| −65.875039 | −0.493047 | 0.864725 | 0.095684 |
| −90.142648 | 0.682888 | 0.316515 | 0.658394 |
| Gain:feed | |||
| −0.018204 | −0.655612 | 0.122933 | 0.745024 |
| −0.037750 | 0.331890 | 0.933157 | 0.138083 |
| −0.056847 | 0.678249 | −0.337795 | 0.652589 |
| 28 to 42 D (experiment 3) | |||
| BW gain | |||
| −102.866999 | 0.680217 | 0.627754 | 0.378456 |
| −151.963741 | 0.070879 | −0.570214 | 0.818433 |
| −343.729259 | −0.729576 | 0.529888 | 0.432364 |
| Gain:feed | |||
| −0.043902 | 0.618231 | 0.732308 | 0.285509 |
| −0.059180 | 0.079991 | −0.419979 | 0.904002 |
| −0.114252 | −0.781916 | 0.318222 | 0.536044 |
Abbreviation: SID, standardized ileal digestible.
Dietary Leu, Val, and Ile levels resulted in significant (P < 0.05) quadratic, cross product, and total model terms for gain:feed during all growth periods. Therefore, polynomial equations were fitted as follows:
G:F (1 to 14 D) = −3.99 + 2.14∗Leu + 4.33∗Val + 2.52∗Ile − 0.64∗Leu2 − 1.38∗Val2 − 1.42∗Ile2 + 0.73∗LeuVal + 0.47∗LeuIle − 0.61∗ValIle; R2 = 0.73;
G:F (14 to 28 D) = −1.64 + 1.49∗Leu + 1.38∗Val + 1.91∗Ile − 0.42∗Leu2 − 0.99∗Val2 − 0.88∗Ile2 − 0.60∗LeuIle + 0.09∗LeuVal + 0.30∗ValIle; R2 = 0.67.
G:F (28 to 42 D) = −0.80 + 1.55∗Leu + 0.50∗Val + 0.71∗Ile − 0.97∗Leu2 − 0.74∗Val2 − 0.71∗Ile2 + 0.67∗LeuVal + 0.36∗LeuIle − 0.14∗ValIle; R2 = 0.82.
As canonical coefficients were positive for gain:feed, the stationary point was represented by a maximum response. The last eigenvalues presented the highest value for gain:feed during the starter (−0.078448), grower (−0.056847), and finisher (−0.114252) periods. Among the eigenvalues mentioned, the largest eigenvector corresponded to the Leu as observed in BW gain models, followed by Ile in grower and finisher phases and by Val during the starter period. As per the fitted models, the optimization of gain:feed can be achieved using dietary SID Leu, Val, and Ile levels of 1.37, 0.94, and 0.87% during the starter period; 1.23, 0.82 and 0.75% during the grower period; and 1.15, 0.77 and 0.70% during the finisher phase, respectively.
Experiment 4
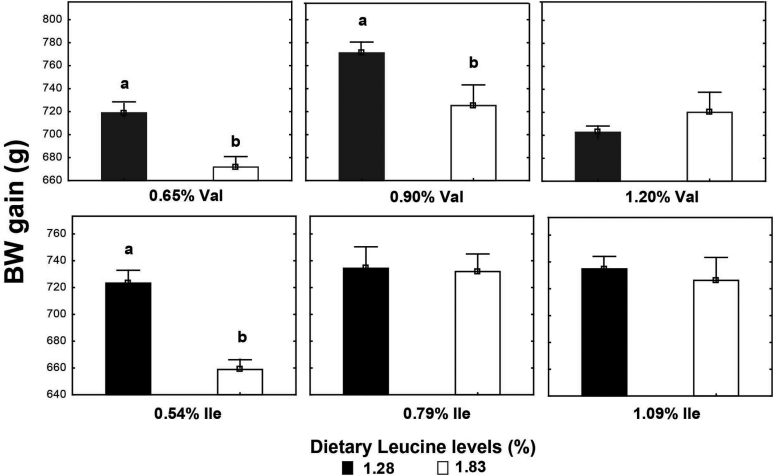
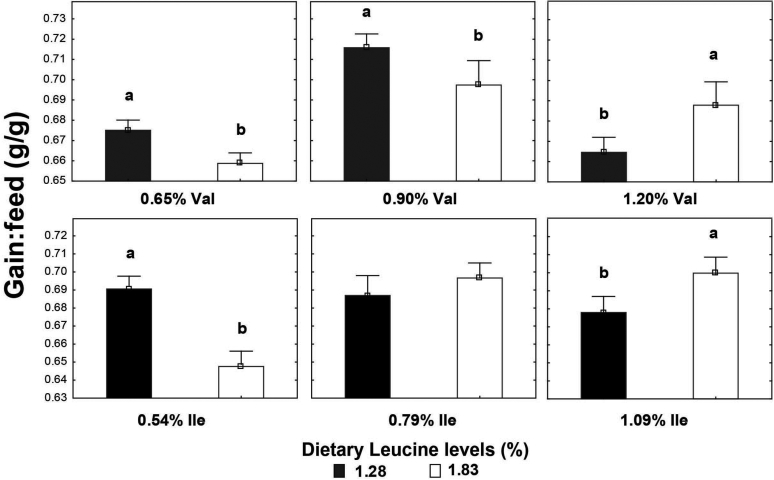
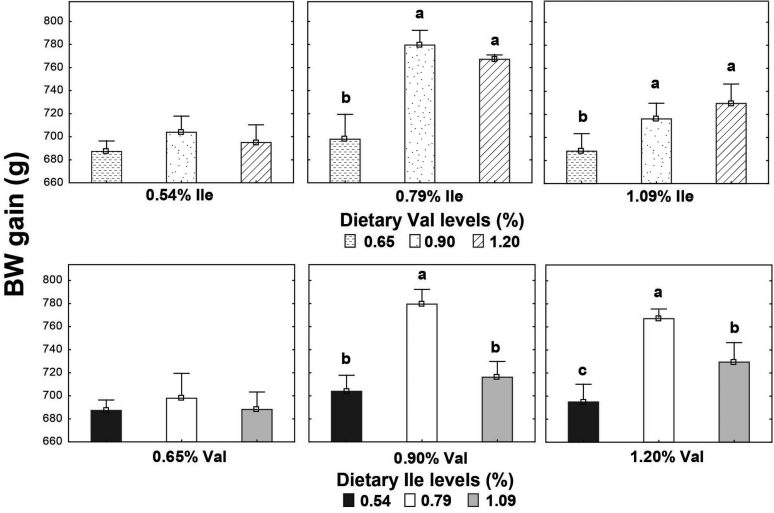
High Leu levels reduced (P < 0.05) feed intake and BW gain, but gain:feed was not affected (P > 0.05) (Table 8). Adequate Val levels resulted in better (P < 0.05) BW gain and gain:feed compared to high and low Val levels. BW gain and gain:feed were higher (P < 0.05) in birds fed high and adequate Ile levels than broilers receiving low Ile levels. There was an interaction (P < 0.05) between SID Leu, Val, and Ile levels on gain:feed. High Leu levels impaired (P < 0.05) gain:feed when birds were fed marginal Val or Ile diets. However, gain:feed was restored when both Val and Ile were supplemented to reach adequate or high levels. Interactions between SID Leu and Val levels and between SID Leu and Ile concentrations were found (P < 0.05) on BW gain and gain:feed. Levels of 1.83% Leu impaired (P < 0.05) BW gain and gain:feed in low and adequate Val levels but not in high Val concentrations (Figures 1 and 2). Likewise, high Leu levels did not impair BW gain and gain:feed in adequate and high Ile levels. There was an interaction (P < 0.05) between SID Val and Ile levels on BW gain. BW gain did not respond to Val or Ile supplementation when 1 of these amino acids remain in deficient levels (Figure 3).
Table 8.
Effect of SID Leu, Val, and Ile content in low-protein diets on growth performance of male broiler from 8 to 21 D of age (experiment 4).1
| SID amino acids (%) |
Feed intake (g) | BW gain (g) | Gain:feed (g/g)2 | ||
|---|---|---|---|---|---|
| Leu | Val | Ile | |||
| 1.28 | 0.65 | 0.54 | 1,036 | 705 | 681b,c,d,e,f |
| 1.28 | 0.65 | 0.79 | 1,070 | 719 | 671c,d,e,f |
| 1.28 | 0.65 | 1.09 | 1,090 | 734 | 673b,c,d,e,f |
| 1.28 | 0.90 | 0.54 | 1,070 | 750 | 702a,b,c,d |
| 1.28 | 0.90 | 0.79 | 1,078 | 791 | 734a |
| 1.28 | 0.90 | 1.09 | 1,085 | 772 | 712a,b,c |
| 1.28 | 1.20 | 0.54 | 1,038 | 715 | 689a,b,c,d,e |
| 1.28 | 1.20 | 0.79 | 1,058 | 693 | 656d,e,f |
| 1.28 | 1.20 | 1.09 | 1,078 | 699 | 649e,f |
| 1.83 | 0.65 | 0.54 | 1,037 | 661 | 647e,f |
| 1.83 | 0.65 | 0.79 | 1,035 | 689 | 666c,d,e,f |
| 1.83 | 0.65 | 1.09 | 991 | 656 | 663c,d,e,f |
| 1.83 | 0.90 | 0.54 | 984 | 670 | 657d,e,f |
| 1.83 | 0.90 | 0.79 | 1,079 | 768 | 713a,b,c |
| 1.83 | 0.90 | 1.09 | 1,057 | 763 | 722a,b |
| 1.83 | 1.20 | 0.54 | 1,038 | 661 | 638e,f |
| 1.83 | 1.20 | 0.79 | 1,040 | 739 | 711a,b,c |
| 1.83 | 1.20 | 1.09 | 1,074 | 760 | 708a,b,c |
| SEM | 26.61 | 14.83 | 10.78 | ||
| SID Leu levels (%) | |||||
| 1.28 | 1,067a | 731a | 685 | ||
| 1.83 | 1,037b | 706b | 681 | ||
| SEM | 8.87 | 4.94 | 3.59 | ||
| SID Val levels (%) | |||||
| 0.65 | 1,043 | 695b | 667b | ||
| 0.90 | 1,059 | 748a | 707a | ||
| 1.20 | 1,054 | 711b | 675b | ||
| SEM | 10.86 | 6.05 | 4.40 | ||
| SID Ile levels (%) | |||||
| 0.54 | 1,034 | 691b | 669b | ||
| 0.79 | 1,060 | 733a | 692a | ||
| 1.09 | 1,062 | 731a | 688a | ||
| SEM |
10.86 |
6.05 |
4.40 |
||
| Effects |
P-value |
||||
| Leu | 0.02 | 0.03 | 0.37 | ||
| Val | 0.57 | 0.01 | 0.001 | ||
| Ile | 0.12 | 0.001 | 0.02 | ||
| Leu x Val | 0.44 | 0.02 | 0.04 | ||
| Leu x Ile | 0.69 | 0.001 | 0.001 | ||
| Val x Ile | 0.63 | 0.04 | 0.12 | ||
| Leu x Val x Ile | 0.23 | 0.10 | 0.02 | ||
a–fMeans in columns followed by different superscript letters are statistically different (P < 0.05).
Abbreviation: SID, standardized ileal digestible.
Values are means of 5 replicate cages per treatment (treatments = 18; n = 90).
Values corrected for mortality.
Figure 1.
Interaction effect of SID Leu and Val levels and Leu and Ile concentrations on BW gain. Results are presented as means ± SEM. a,bMeans not sharing a lowercased letter differ significantly by Tukey's test at P < 0.05 level.
Figure 2.
Interaction effect of SID Leu and Val levels and Leu and Ile concentrations on gain:feed. Results are presented as means ± SEM. a,bMeans not sharing a lowercased letter differ significantly by Tukey's test at P < 0.05 level.
Figure 3.
Interaction effect of SID Val and Ile levels on BW gain. Results are presented as means ± SEM. a,bMeans not sharing a lowercased letter differ significantly by Tukey's test at P < 0.05 level.
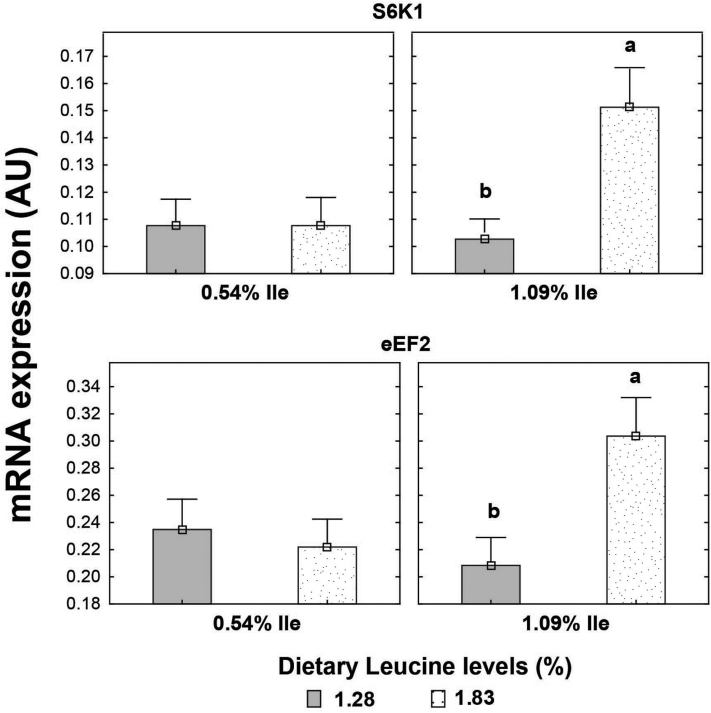
Higher Leu levels increased (P < 0.05) mRNA expression of mTOR and S6K1 genes in muscle tissue but not that of the eEF2 gene (P > 0.05) (Table 9). Main effects of dietary Val and Ile levels did not affect (P > 0.05) mRNA expression of the genes investigated. There was an interaction (P < 0.05) between SID Leu, Val, and Ile levels on mRNA expression of eEF2 gene. Higher mRNA expression of eEF2 gene was found (P < 0.05) in birds fed high dietary Leu, Val, and Ile levels than in those receiving low dietary levels of these amino acids. Likewise, there was an interaction (P < 0.05) between SID Leu and Ile levels on mRNA expression of S6K1 and eEF2 genes. High Leu levels increased (P < 0.05) on mRNA expression of S6K1 and eEF2 genes in birds fed high Ile levels but not in those fed low Ile levels (Figure 4).
Table 9.
Effect of SID Leu, Val, and Ile content in low-protein diets on mRNA expression of mTOR, S6K1, and eEF2 genes of the pectoralis major muscle of broiler chickens at 21 D of age (Experiment 4)1.
| SID amino acids (%) |
mTOR | S6K1 | eEF2 | ||
|---|---|---|---|---|---|
| Leu | Val | Ile | |||
| 1.28 | 0.65 | 0.54 | 0.0032 | 0.099 | 0.175c |
| 1.28 | 0.65 | 1.09 | 0.0035 | 0.110 | 0.230a,b,c |
| 1.28 | 1.2 | 0.54 | 0.0045 | 0.114 | 0.280a,b |
| 1.28 | 1.2 | 1.09 | 0.0029 | 0.095 | 0.187b,c |
| 1.83 | 0.65 | 0.54 | 0.0044 | 0.110 | 0.238a,b,c |
| 1.83 | 0.65 | 1.09 | 0.0044 | 0.130 | 0.285a,b |
| 1.83 | 1.2 | 0.54 | 0.0047 | 0.105 | 0.206b,c |
| 1.83 | 1.2 | 1.09 | 0.0050 | 0.172 | 0.322a |
| SEM | 0.0007 | 0.016 | 0.032 | ||
| SID Leu levels (%) | |||||
| 1.28 | 0.0035b | 0.105b | 0.218 | ||
| 1.83 | 0.0046a | 0.129a | 0.263 | ||
| SEM | 0.0003 | 0.008 | 0.022 | ||
| SID Val levels (%) | |||||
| 0.65 | 0.0039 | 0.113 | 0.232 | ||
| 1.2 | 0.0043 | 0.122 | 0.249 | ||
| SEM | 0.0003 | 0.008 | 0.022 | ||
| SID Ile levels (%) | |||||
| 0.54 | 0.0039 | 0.108 | 0.225 | ||
| 1.09 | 0.0043 | 0.127 | 0.256 | ||
| SEM |
0.0003 |
0.008 |
0.022 |
||
| Effects |
P-value |
||||
| Leu | 0.01 | 0.03 | 0.10 | ||
| Val | 0.37 | 0.42 | 0.46 | ||
| Ile | 0.52 | 0.09 | 0.18 | ||
| Leu x Val | 0.86 | 0.39 | 0.53 | ||
| Leu x Ile | 0.38 | 0.04 | 0.03 | ||
| Val x Ile | 0.39 | 0.70 | 0.38 | ||
| Leu x Val x Ile | 0.21 | 0.10 | 0.02 | ||
a,b,cMeans in columns followed by different superscript letters are statistically different (P < 0.05).
Abbreviations: eEF2, eukaryotic translation elongation factor 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; mTOR, mechanistic target of rapamycin.
Data are means of 5 replicate cages per treatment (treatments = 8; n = 40). Samples were analyzed in duplicate.
Figure 4.
Interaction effect of SID Leu and Ile levels on mRNA expression of S6K1 and eEF2 genes of the pectoralis major muscle of broiler chickens at 21 D of age. Results are presented as means ± SEM. AU = arbitrary units. a,bMeans not sharing a lowercased letter differ significantly by Tukey's test at P < 0.05 level.
Discussion
Leucine was the most influential BCAA in the fitted models for BW gain and gain:feed as per canonical analysis. Thus, predicted optimal levels of BCAA were mainly affected by Leu levels in low-protein diets. However, several studies estimated Val and Ile requirements without considering dietary Leu levels, which may be the main factor of variation in Val and Ile requirement estimations. High Leu plasma concentration activates the metabolic pathway that oxidizes all of the BCAA (Brosnan and Brosnan, 2006). Then, Leu decreases plasma concentrations of both Val and Ile (D'Mello and Lewis, 1970; Wessels et al., 2016). Previous researches showed a strong interrelationship between Leu and other BCAA in low-protein diets (D'Mello and Lewis, 1970; Allan and Baker, 1972; Smith and Austic, 1978; Gloaguen et al., 2011; Ospina-Rojas et al., 2017) where Leu levels greater than recommended values increased the optimal dietary levels of Val or Ile. Therefore, the interactions between BCAA are important to be considered in low-protein diets. As per the fitted models for BW gain and gain:feed, the optimization response can be achieved by using dietary SID Leu, Val, and Ile levels of 1.33–1.37%, 0.94–0.96%, and 0.84–0.87% during the starter period; 1.23%, 0.82–0.83%, and 0.75% during the grower period; and 1.15–1.16%, 0.77%, and 0.68–0.70% during the finisher phase, respectively. Using the predicted levels, the optimal average ratios for Leu:Val:Ile in low-protein diets were estimated at 100:69:62 from 1 to 14 D, 100:67:61 from 14 to 28, and 100:67:60 from 28 to 42 D. In the literature, the estimated Val and Ile to Leu ratios ranged from 67 to 78 and 58 to 69, respectively, as per the nutritional recommendations proposed by researchers who studied the combined BCAA requirements (Farran and Thomas, 1990; Faridi et al., 2014; Sedghi et al., 2015).
Branched-chain amino acid concentrations show a fast increase in the bloodstream after protein intake and are promptly available for protein synthesis in skeletal muscle as BCAA do not have hepatic first-pass metabolism as other amino acids (Brosnan and Brosnan, 2006). The initial step of BCAA catabolism takes place in the muscle owing to the high activity of BCAA aminotransferase, the first enzyme in the BCAA catabolism pathway, whereas BCAA aminotransferase hepatic activity is low (Harper et al., 1984). The BCAA aminotransferase reaction involves the reversible transfer of Leu, Val, and Ile to form the corresponding branched-chain α-keto acids, α-ketoisocaproate (KIC), α-ketoisovalerate, and α-keto-β-methylvalerate, respectively.
The second enzyme of BCAA catabolism, BCKD is a key multienzyme complex involved in the irreversible degradation of the 3 branched-chain α-keto acids and is regulated by the phosphorylation–dephosphorylation mechanism. Phosphorylation mediated by BCKD kinase results in inactivation, whereas dephosphorylation by BCKD phosphatase activates the enzyme (Holeček, 2018). Dietary Leu increases the KIC formation, α-keto acid of Leu, which inhibits BCKD kinase. As a result of BCKD kinase inhibition, the BCKD phosphatase activates the BCKD complex activity by dephosphorylation of E1α subunit (Harris et al., 2005). Therefore, a high Leu level can stimulate the catabolism of Val and Ile by increasing the intracellular concentration of KIC and promoting the activation of BCKD complex. The BCKD complex activation by Leu could explain the fact that Leu was found to be the most influential BCAA in the fitted models and that optimal dietary Val and Ile levels rely on Leu levels in low-protein diets. The α-keto acids of Val and Ile, α-ketoisovalerate and α-keto-β-methylvalerate, also inhibit BCKD kinase but with lower efficacy than that of KIC (Brosnan and Brosnan, 2006). For this reason, excess Val or Ile leads to small reductions in plasma concentrations of the other 2 BCAA (D'Mello and Lewis, 1970; Smith and Austic, 1978), indicating that Val, Ile, or their α-keto acids do not increase significantly the BCAA catabolism.
Levels of 1.83% Leu impaired BW gain and gain:feed in low and adequate Val levels, whereas growth performance of birds fed adequate Ile levels was not reduced by high Leu levels (1.83%). This result supports the affirmation that the antagonistic effect of Leu is more potent on Val than on Ile in birds. Dietary Leu levels of 1.83% reduced BW gain compared with birds fed diets with 1.28% Leu, probably because of reduced feed intake. Some studies pointed out that feed intake reduction is the main cause of lower growth performance of broilers and pigs fed high Leu levels or BCAA imbalance diets (Ueda et al., 1981; Farran et al., 2002; Wiltafsky et al., 2010; Wessels et al., 2016). High Leu levels mostly affected the feed intake in previous studies performed in our laboratory where SID Leu levels ranged from 1.0 to 1.96% from 1 to 21 D (Ospina-Rojas et al., 2019), and 1.0 to 1.80% from 22 to 42 D (Ospina-Rojas et al., 2017). In experiments 1, 2, and 3, feed intake was not affected by dietary Leu levels that ranged from 1.10 to 1.70%; 1.0 to 1.60%; and 0.98 to 1.58% during the starter, grower, and finisher periods, respectively. However, the increased SID Leu levels from 1.28 to 1.83% decreased the feed intake of broilers from 8 to 21 D (experiment 4). These results suggest that there is a breakpoint level where the SID Leu concentration may impair the feed intake in low-protein diets.
Levels of 1.83% Leu impaired gain:feed when birds were fed marginal Val or Ile diets. However, gain:feed was restored when both Val and Ile were supplemented to reach adequate or high levels. This finding supports the statement that the antagonistic effects of high Leu are more evident when the other BCAA are lower than the broiler requirement. Likewise, higher dietary levels of Val and Ile are required to optimize broiler performance in high Leu levels. Branched-chain amino acids share a structurally similar side chain and are actively transported into cells by the same carrier system, the system-L transporter, which it is primarily expressed as 2 different isoforms: LAT1 and LAT2 (L-type amino acid transporters 1 and 2) in organs that play an important role in BCAA homeostasis and utilization, such as the intestine, kidney, liver, and muscle (Zhang et al., 2018). Therefore, higher levels of Val and Ile could compete with Leu for transport into cells and reducing the negative effects of high Leu levels on broiler performance. In addition, Val and Ile supplementation could restore plasma concentration of these amino acids already oxidized by BCKD complex activation as a result of high Leu and KIC concentrations. Broilers fed high dietary protein content with amino acid levels greater than their minimum requirement did not exhibit reduced performance by high Leu levels (Burnham et al., 1992; Waldroup et al., 2002), whereas the Leu antagonism has been more evident in low-protein diets where some amino acids could be at minimal dietary levels (D'Mello and Lewis, 1970; Allan and Baker, 1972; Smith and Austic, 1978; Ospina-Rojas et al., 2017, 2019). Then, the reduction magnitude of dietary protein may influence the bird response to SID Leu levels. Level inclusion of corn increases as soybean meal decreases in protein-reduced diets. Valine and Ile supplementation allows reduction of dietary protein and may decrease Leu levels, but special attention should be paid to the BCAA ratio in low-protein diets formulated with particular combinations of feed ingredients and marginally adequate levels of Ile and Val owing to disproportionally high Leu levels in some ingredients relative to other BCAA. In some cases, relative Leu content could be high (low Val- and Ile-to-Leu ratios) even in low-protein diets that might increase optimal dietary Val and Ile levels but broiler recommendations of Val and Ile are kept at the same levels in a least-cost feed formulation. Low-protein diets without Leu supplementation resulted in good broiler performance when Val and Ile requirements were fully met. This result indicates that Val and Ile supplementation with adequate Leu:Val:Ile ratios could be a strategy for supporting broiler growth by reducing dietary Leu content and improving BCAA balance in low-protein diets.
It has been known that Leu can enhance muscle protein synthesis by activating the mTOR and its downstream effectors, S6K1 and 4EBP1, which are 2 proteins involved in mRNA translation initiation into protein (Lynch et al., 2002). Leu-supplemented diets increased mRNA expression of mTOR and S6K1 genes in muscle tissue, which is in accordance with data reported by other researchers in broilers and pigs (Suryawan et al., 2008; Torrazza et al., 2010; Deng et al., 2014; Chang et al., 2015; Ospina-Rojas et al., 2019). In addition to muscle tissue, Leu has shown to increase mRNA expression of genes related to the mTOR pathway in the small intestine (Chang et al., 2015). These findings are in contrast with the observations of Zeitz et al. (2019a,b) where the mTOR pathway was not affected in broilers fed high-Leu diets. This might be due to their basal diets that had no marginal Leu levels, and some of them that had Leu content higher than the broiler recommendation, which could limit the impact of dietary graded Leu levels on broiler response.
In addition to the mTOR's role in stimulating the mRNA translation initiation through S6K1 and 4EBP1, mTOR promotes the elongation machinery of mRNA translation through eEF2 activation (Proud, 2009). Although an increased activity of mTOR with Leu supplementation has been reported, there is no evidence of Leu direct effect on eEF2 activity by phosphorylation at the post-translational (Deldicque et al., 2008; Wilson et al., 2010) or transcriptional level because we found no effect of Leu levels on mRNA expression of eEF2. Similarly, Val and Ile levels did not affect the mRNA expression of eEF2. Previously, we also found that neither Leu nor Val changed mRNA expression of eEF2 (Ospina-Rojas et al., 2019).
The compiled results indicated that negative effects of higher Leu levels than recommended values are more pronounced in low-protein diets with marginal levels of Val and Ile. Higher dietary Val and Ile levels are required to minimize the antagonistic effect of excess Leu on broiler performance with an optimal ratio for Leu:Val:Ile in low-protein diets of 100:69:62 from 1 to 14 D, 100:67:61 from 14 to 28, and 100:67:60 from 28 to 42 D. Dietary amino acids at levels greater than broiler requirement in high-protein diets reduce the negative effect of high Leu levels, and hence, the ideal BCAA ratios in diets with excess protein levels are different from those in low-protein diets. Further studies are required to understand and estimate the BCAA relationships at different dietary protein levels. The concentration of SID Leu relative to protein content is almost 2-fold higher in corn than in soybean meal. As corn inclusion increases in low-protein diets, Val and Ile supplementation in these diets is important for supporting broiler growth by improving BCAA balance and counteracting the metabolic pathway activation that oxidizes all of the BCAA.
In conclusion, SID Leu, Val, and Ile levels required for gain:feed optimization were estimated at 1.37, 0.94, and 0.87% during the starter period; 1.23, 0.82, and 0.75% during the grower period; and 1.15, 0.77, and 0.70% during the finisher phase, respectively. For optimal BW gain, recommended SID Leu, Val, and Ile levels were estimated at 1.33, 0.96, and 0.84% for the starter phase; 1.23, 0.83, and 0.75% for the grower period; and 1.16; 0.77, and 0.68% for the finisher phase, respectively. Leucine was the most important BCAA in the fitted models for broiler performance, and it affects the dietary levels of Val and Ile. Consequently, dietary Leu content should be considered in estimation of the ideal level of Val and Ile in low-protein diets. Higher Val and Ile levels optimize the effect of Leu supplementation on mRNA expression of mTOR pathway genes in the pectoralis major muscle of broilers from day 1 to 21 after hatch.
Acknowledgments
We would like to thank National Counsel of Technological and Scientific Development (CNPq) - Brazil for their support for this research.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Allan N.K., Baker D.H. Quantitative efficacy of dietary isoleucine and valine for chick growth as influenced by variable quantities of excess dietary leucine. Poult. Sci. 1972;51:1292–1298. doi: 10.3382/ps.0511292. [DOI] [PubMed] [Google Scholar]
- AOAC . 18th ed. Association of Official Analytical Chemists; Arlington, VA: 2006. Official Methods of Analysis. [Google Scholar]
- Baş D., Boyacı İ.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007;78:836–845. [Google Scholar]
- Box G.E.P., Hunter W.G., Hunter J.S. Wiley; New York, NY: 1987. Statistics for Experimenters: An Introduction to Design, Data Analysis and Model Building. [Google Scholar]
- Brosnan J.T., Brosnan M.E. Branched-chain amino acids: enzyme and substrate regulation. J. Nutr. 2006;136:207S–211S. doi: 10.1093/jn/136.1.207S. [DOI] [PubMed] [Google Scholar]
- Burnham D., Emmans G.C., Gous R.M. Isoleucine requirements of the chicken: the effect of excess leucine and valine on the response to isoleucine. Br. Poult. Sci. 1992;33:71–87. doi: 10.1080/00071669208417445. [DOI] [PubMed] [Google Scholar]
- Chang Y., Cai H., Liu G., Chang W., Zheng A., Zhang S., Liao R., Liu W., Li Y., Tian J. Effects of dietary leucine supplementation on the gene expression of mammalian target of rapamycin signaling pathway and intestinal development of broilers. Anim. Nutr. 2015;1:313–319. doi: 10.1016/j.aninu.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deldicque L., Sanchez Canedo C., Horman S., De Potter I., Bertrand L., Hue L., Francaux M. Antagonistic effects of leucine and glutamine on the mTOR pathway in myogenic C2C12 cells. Amino Acids. 2008;35:147–155. doi: 10.1007/s00726-007-0607-z. [DOI] [PubMed] [Google Scholar]
- Deng H., Zheng A., Liu G., Chang W., Zhang S., Cai H. Activation of mammalian target of rapamycin signaling in skeletal muscle of neonatal chicks: effects of dietary leucine and age. Poult. Sci. 2014;93:114–121. doi: 10.3382/ps.2013-03287. [DOI] [PubMed] [Google Scholar]
- D’Mello J.P.F., Lewis D. Amino acid interactions in chick nutrition. 2. Interrelationships between leucine, isoleucine, and valine. Br. Poult. Sci. 1970;11:313–323. doi: 10.1080/00071667008415821. [DOI] [PubMed] [Google Scholar]
- Faridi A., Golian A., Heravi Mousavi A., France J. Bootstrapped neural network models for analyzing the responses of broiler chicks to dietary protein and branched chain amino acids. Can. J. Anim. Sci. 2014;94:79–85. [Google Scholar]
- Farran M.T., Thomas O.P. Dietary requirements of leucine, isoleucine, and valine in male broilers during the starter period. Poult. Sci. 1990;69:757–762. doi: 10.3382/ps.0690757. [DOI] [PubMed] [Google Scholar]
- Farran M.T., Barbour E.K., Ashkarian V.M. Effect of excess leucine in low protein diet on ketosis in 3-week-old male broiler chicks fed different levels of isoleucine and valine. Anim. Feed Sci. Technol. 2002;103:171–176. [Google Scholar]
- Gilbert E.R., Li H., Emmerson D.A., Jr Webb K.E., Wong E.A. Developmental regulation of nutrient transporter and enzyme mRNA in the small intestine of broiler chicks. Poult. Sci. 2007;86:1739–1753. doi: 10.1093/ps/86.8.1739. [DOI] [PubMed] [Google Scholar]
- Gloaguen M., Le Floc’h N., Brossard L., Barea R., Primot Y., Corrent E., Van Milgen J. Response of piglets to the valine content in diet in combination with the supply of other branched chain amino acids. Animal. 2011;5:1734–1742. doi: 10.1017/S1751731111000760. [DOI] [PubMed] [Google Scholar]
- Harper A.E., Miller R.H., Block K.P. Branched-chain amino acid metabolism. Ann. Rev. Nutr. 1984;4:409–454. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- Harris R.A., Joshi M., Jeoung N.H., Obayashi M. Overview of the molecular and biochemical basis of branched-chain amino acid catabolism. J. Nutr. 2005;135:1527S–1530S. doi: 10.1093/jn/135.6.1527S. [DOI] [PubMed] [Google Scholar]
- Holeček M. Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018;15:33. doi: 10.1186/s12986-018-0271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Aggrey S.E. Transcriptomic differences of genes in the avian target of rapamycin (avTOR) pathway in a divergent line of meat-type chickens selected for feed efficiency. Genet. Mol. Res. 2016;30:15. doi: 10.4238/gmr.15027120. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔC(T) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lynch C.J., Patson B.J., Anthony J., Vaval A., Jefferson L.S., Vary T.C. Leucine is a direct-acting nutrient signal that regulates protein synthesis in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2002;283:E503–E513. doi: 10.1152/ajpendo.00084.2002. [DOI] [PubMed] [Google Scholar]
- Ospina-Rojas I.C., Murakami A.E., Duarte C.R.A., Nascimento G.R., Garcia E.R.M., Sakamoto M.I., Nunes R.V. Leucine and valine supplementation of low-protein diets for broiler chickens from 21 to 42 days of age. Poult. Sci. 2017;96:914–922. doi: 10.3382/ps/pew319. [DOI] [PubMed] [Google Scholar]
- Ospina-Rojas I.C., Murakami A.E., Duarte C.R.A., Pozza P.C., Rossi R.M., Gasparino A. Performance, diameter of muscle fibers, and gene expression of mechanistic target of rapamycin in pectoralis major muscle of broilers supplemented with leucine and valine. Can. J. Anim. Sci. 2019;99:168–178. [Google Scholar]
- Proud mTORC1 signalling and mRNA translation. Biochem. Soc. Trans. 2009;37:227–231. doi: 10.1042/BST0370227. [DOI] [PubMed] [Google Scholar]
- Proud C.G. Regulation of mammalian translation factors by nutrients. Eur. J. Biochem. 2002;269:5338–5349. doi: 10.1046/j.1432-1033.2002.03292.x. [DOI] [PubMed] [Google Scholar]
- Rostagno H.S., Albino L.F.T., Donzele J.L., Gomes P.C., de Oliveira R.F., Lopes D.C., Ferreira A.S., Barreto S.L.T., Euclides R.F. 3rd ed. UFV; Viçosa, Minas Gerais, Brazil: 2011. Brazilian Tables for Poultry and Swine: Feed Composition and Nutritional Requirements. [Google Scholar]
- SAS Institute . SAS Inst. Inc.; Cary, NC: 2009. SAS Proprietary Software, Release 9.2. [Google Scholar]
- Sedghi M., Golian A., Kolahan F., Afsar A. Optimisation of broiler chicken responses from 0 to 7 d of age to dietary leucine, isoleucine and valine using Taguchi and mathematical methods. Br. Poult. Sci. 2015;56:696–707. doi: 10.1080/00071668.2015.1096323. [DOI] [PubMed] [Google Scholar]
- Smith T.K., Austic R.E. The branched-chain amino acid antagonism in chicks. J. Nutr. 1978;108:1180–1191. doi: 10.1093/jn/108.7.1180. [DOI] [PubMed] [Google Scholar]
- Suryawan A., Jeyapalan A.S., Orellana R.A., Wilson F.A., Nguyen H.V., Davis T.A. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am. J. Physiol. Endocrinol. Metab. 2008;295:E868–E875. doi: 10.1152/ajpendo.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrazza R.M., Suryawan A., Gazzaneo M.C., Orellana R.A., Frank J.W., Nguyen H.V., Fiorotto M.L., El-Kadi S., Davis T.A. Leucine supplementation of a low-protein meal increases skeletal muscle and visceral tissue protein synthesis in neonatal pigs by stimulating mTOR-dependent translation initiation. J. Nutr. 2010;140:2145–2152. doi: 10.3945/jn.110.128421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H., Yabuta S., Yokota H., Tasaki I. Involvement of feed intake and feed utilization in the growth retardation of chicks given the excessive amounts of leucine, lysine, phenylalanine or methionine. Nutr. Rep. Int. 1981;24:135–144. [Google Scholar]
- Waldroup P.W., Kersey J.H., Fritts C.A. Influence of branched chain amino acid balance in broiler diets. Int. J. Poult. Sci. 2002;1:136–144. [Google Scholar]
- Wessels A.G., Kluge H., Hirche F., Kiowski A., Schutkowski A., Corrent E., Bartelt J., König B., Stangl G.I. High leucine diets stimulate cerebral branched-chain amino acid degradation and modify serotonin and ketone body concentrations in a pig model. PLoS One. 2016;11:e0150376. doi: 10.1371/journal.pone.0150376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson F.A., Suryawan A., Gazzaneo M.C., Orellana R.A., Nguyen H.V., Davis T.A. Stimulation of muscle protein synthesis by prolonged parenteral infusion of leucine is dependent on amino acid availability in neonatal pigs. J. Nutr. 2010;140:264–270. doi: 10.3945/jn.109.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltafsky M.K., Pfaffl M.W., Roth F.X. The effects of branched-chain amino acid interactions on growth performance, blood metabolites, enzyme kinetics and transcriptomics in weaned pigs. Br. J. Nutr. 2010;103:964–976. doi: 10.1017/S0007114509992212. [DOI] [PubMed] [Google Scholar]
- Zeitz J.O., Käding S.C., Niewalda I.R., Most E., Dorigam J.C.P., Eder K. The influence of dietary leucine above recommendations and fixed ratios to isoleucine and valine on muscle protein synthesis and degradation pathways in broilers. Poult. Sci. 2019;98:6772–6786. doi: 10.3382/ps/pez396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz J.O., K¨ading S.C., Niewalda I.R., Machander V., de Paula Dorigam J.C., Eder K. Effects of leucine supplementation on muscle protein synthesis and degradation pathways in broilers at constant dietary concentrations of isoleucine and valine. Arch. Anim. Nutr. 2019;73:75–87. doi: 10.1080/1745039X.2019.1583519. [DOI] [PubMed] [Google Scholar]
- Zhang Z.Y., Monleon D., Verhamme P., Staessen J.A. Branched-chain amino acids as critical switches in health and disease. Hypertension. 2018;72:1012–1022. doi: 10.1161/HYPERTENSIONAHA.118.10919. [DOI] [PubMed] [Google Scholar]