Abstract
Wooden breast (WB) has arisen primarily in the breast muscle of commercial broilers. It is characterized by palpation of a rigid pectoralis major (p. major) muscle and is under severe oxidative stress and inflammation. Previous studies have shown that vitamin E (VE) has antioxidant properties and omega-3 (n-3) fatty acids have an anti-inflammatory effect. The objectives of this study were to identify the effects of VE and n-3 fatty acids on the severity of WB, morphological structure of the p. major muscle, expression of genes likely associated with WB and to determine the most beneficial supplementation period. A total of 210 Ross 708 broilers were randomly assigned into 7 treatments with 10 replicates of 3 birds each. The control group received a corn–soybean meal basal diet during the entire study (0–58 d). Supplementation of VE (200 IU/kg), n-3 fatty acids (n-6/n-3 ratio of 3.2:1), or combination of both were fed during the starter phase (0–10 d) or grower phase (11–24 d). All broilers were harvested at 58 d of age. Morphological assessment of the p. major muscle included myofiber width, perimysial and endomysial connective tissue space, overall morphological structure, and scoring of WB microscopically. Gene expression was measured using nanostring analysis. Genes associated with muscle development and growth factors, inflammation, extracellular matrix, and glucose metabolism were differentially expressed in the p. major muscle of the broilers supplemented with VE in the grower diet. Greater than 2 times more giant myofibers (≥70 μm) were found in the group supplemented with VE and n-3 fatty acids in the starter diet compared with the group fed VE in the grower diet (P = 0.02). Microscopic evaluation showed that VE supplementation in the grower diet had a 16.19% increase in muscle with no WB compared with the control group (P = 0.05). These data suggest that supplementation of VE during the grower phase may reduce the severity of WB in broilers.
Key words: broiler, muscle morphology, omega-3 fatty acid, vitamin E, wooden breast
Introduction
The demand for poultry meat among consumers has increased notably because of its lower cost, high protein, and convenience in cooking (Bordoni and Danesi, 2017; Mottet and Tempio, 2017). To meet consumer demand, broilers are continuously selected for rapid growth, improved feed conversion, and increased breast meat yield (Brewer et al., 2012; Chatterjee et al., 2019). Poultry meat production in 2019 is more than 5 times of that in 1970 (National Chicken Council, 2020). However, selection for rapid growth including the breast muscle has resulted in meat quality defects (Dransfield and Sosnicki, 1999; Petracci et al., 2013, 2015; Tijare et al., 2016). One of the primary myopathies, wooden breast (WB), has emerged within the broiler industry worldwide (Sihvo et al., 2014; Kuttappan et al., 2016; Tasoniero et al., 2016). Wooden breast is phenotypically characterized by a hard breast muscle on palpation (Sihvo et al., 2014). It is estimated that around 85% of the commercial broilers are at least mildly affected by the WB myopathy (Kuttappan et al., 2017). This myopathy has created considerable economic losses of over $200 million dollars per year because of the hardness of the breast muscle, lack of palatability, and product downgrades (Sihvo et al., 2014; Kuttappan et al., 2016).
Histologically, WB has moderate or severe myodegeneration along with different levels of myofiber necrosis (Papah et al., 2017), fibrosis (Sihvo et al., 2014; Velleman and Clark, 2015), and inflammatory cell accumulation (Sihvo et al., 2014, 2017). Gene expression analysis and metabolomics analysis have found that genes altered in WB muscle tissue are closely related with muscle development (Velleman and Clark, 2015; Abasht et al., 2016; Zambonelli et al., 2017), hypoxia and oxidative stress (Mutryn et al., 2015; Abasht et al., 2016; Brothers et al., 2019), response to inflammation (Mutryn et al., 2015; Zambonelli et al., 2017), calcium signaling (Mutryn et al., 2015; Zambonelli et al., 2017), and dysregulation of lipid and glucose metabolism (Abasht et al., 2016; Brothers et al., 2019). These findings are strongly suggestive that the WB myopathy is associated with oxidative stress and inflammation. Therefore, the severity of WB can be potentially decreased when oxidative stress and inflammation are reduced to levels that are not harmful to the breast muscle.
Oxidative stress is defined as an imbalance between oxidants and antioxidants in cells and tissues (Voljč et al., 2011). Reactive oxygen species are synthesized when oxygen is not reduced completely. Oxidative stress results when the antioxidant system is not able to remove reactive oxygen species properly (Panda and Cherian, 2014). Vitamin E (VE) is an antioxidant that removes free radicals and prevents oxidative stress (Kuttappan et al., 2012; Niki, 2016). DL-α-tocopherol acetate is the commonly used form of VE in the poultry industry and has high biological efficacy (Hosomi et al., 1997; Panda and Cherian, 2014). It can remove free radical intermediates by reacting with lipid radicals from the lipid peroxidation chain reaction (Niki et al., 1993). This thereby terminates the propagation reaction from continuing and prevents cell membrane oxidation (Niki et al., 1991). Wang et al. (2020) showed that supplementation of VE (200 IU/kg) in the starter diet (0–10 d) or grower diet (11–24 d) increased the percentage of birds without WB affected breast muscle compared with the control diet at 58 d of age. Additionally, omega-3 (n-3) polyunsaturated fatty acids can reduce inflammation and enhance muscle function (Calder, 2006; Ewaschuk et al., 2014; Yu et al., 2018). Synergistic effects of VE and n-3 fatty acids have been shown to reduce meat oxidation and inflammation (Taulescu et al., 2011; El-Samee et al., 2019). Thus, reducing oxidative stress and inflammation in broilers through VE and n-3 fatty acids administration will likely have beneficial implications on the structure of the pectoralis major (p. major) muscle, severity of the WB myopathy, and expression of genes related with muscle development, oxidative stress, and inflammation.
Although previous studies have identified that VE is an antioxidant (Kuttappan et al., 2012; Niki, 2016) and n-3 fatty acids have anti-inflammatory effects (Calder, 2006; Ewaschuk et al., 2014; Yu et al., 2018), there are no published studies showing the effects of VE and n-3 fatty acids on the morphological structure of the p. major muscle and expression of genes likely associated with WB. Therefore, the objectives of this study were to identify the effects of dietary VE and n-3 fatty acids independently or in combination when supplemented during the starter phase (0–10 d) or grower phase (11–24 d) on the severity of the WB myopathy. Assessment of the morphological structure of the p. major muscle, and expression of genes related with muscle formation and growth, growth factors, hypoxia, oxidative stress and inflammation, extracellular matrix, cell structure and migration, calcium regulation, and glucose metabolism in the p. major muscle were measured to determine the most beneficial dietary supplementation period to mitigate WB development in broilers.
Materials and methods
Birds and Experimental Diets
All bird activities were approved by the Institutional Animal Care and Use Committee of The Ohio State University. A total of 210 commercial Ross 708 broiler chicks were individually wing banded and placed into pens immediately after hatch. Broilers had ad libitum access to feed and water. Birds were assigned to 7 experimental groups in a completely randomized design (Figure 1). There were 10 pens per treatment, and each pen included 3 birds. The control group was fed a corn–soybean meal basal diet with VE (DL-α-tocopherol acetate, 10 IU/kg) and n-3 fatty acids (n-6/n-3 ratio of 30.2:1) at a standard level during the starter (0–10 d), grower (11–24 d), and finisher phases (25–58 d). Additional supplemental VE or n-3 fatty acids were fed during the starter or grower phases. For the starter dietary supplementation, starter VE, starter n-3, and starter VE and n-3 groups were fed the basal starter diet supplemented at concentrations of 200 IU/kg diet of VE, n-3 fatty acids with a n-6/n-3 ratio of 3.2:1, or a combination of both. The grower and finisher diets were the same as the control group. For the grower dietary supplementation, grower VE, grower n-3, and grower VE and n-3 groups were fed the basal grower diets supplemented at concentrations of 200 IU/kg diet of VE, n-3 fatty acids with a n-6/n-3 ratio of 3.2:1, or a combination of both. The starter and finisher diets were the same as the control group. Diets were formulated to meet or exceed all NRC (National Research Council, 1994) nutritional requirements and recommendations in Aviagen's Ross broiler production handbook (Aviagen, 2016). Feed ingredients and nutrient composition have been previously reported in Wang et al. (2020). At 58 d of age, all broilers were harvested in accordance with humane and commercial slaughter procedures. Samples of p. major muscle were obtained from each broiler for evaluation of p. major muscle morphology and gene expression.
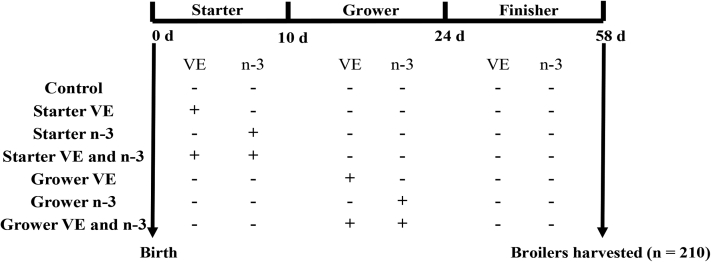
Figure 1.
Timeline of the experimental design. Broilers in the control group were fed diets with standard level (−) of Vitamin E (VE: 10 IU/kg) and n-3 fatty acids (n-6/n-3 ratio of 30.2:1) during the entire study (0–58 d). Broilers in starter VE, starter n-3, and starter VE and n-3 groups were fed diets with increased level (+) of VE (200 IU/kg), n-3 fatty acids (n-6/n-3 ratio of 3.2:1), or combination of both during the starter phase (0–10 d). Broilers in grower VE, grower n-3, and grower VE and n-3 groups were fed diets with increased level of VE, n-3 fatty acids, or combination of both during the grower phase (11–24 d). All broilers were harvested at 58 d of age (n = 210).
Pectoralis Major Muscle Morphology
Samples for muscle histology were collected according to Velleman et al. (2003b). In brief, after the skin was removed, muscle fibers in the anterior portion of the muscle were dissected following the fiber orientation and tied to wooden applicator sticks to prevent contraction. Tissue samples were fixed in 10% (vol/vol) buffered formalin (pH 7.0) and stored at 4°C. Histological samples were processed with dehydration in a graded series of alcohol and cleared using Pro-Par Clearant (Anatech, Battle Creek, MI), and paraffin embedded based on the method of Jarrold et al. (1999). Samples were then cross sectioned at a 5 μm thickness and mounted on Starfrost Adhesive slides (Mercedes Medical, Sarasota, FL). Each slide contained 4 sections. The sections were stained with hematoxylin and eosin and imaged with a QImaging digital camera (QImaging, Burnaby, BC, Canada) attached to an Olympus IX 70 microscope (Olympus America, Mellville, NY).
Four photomicrographs from each sample were taken for p. major muscle morphology score and WB myopathy score evaluation. The p. major muscle morphology score was evaluated as described by Velleman et al. (2003b). Samples were scored using a 1 to 5 scale by 4 trained panelists. A score of 1 was given to samples with limited or no perimysial or endomysial connective tissue spacing and excessive myofiber degradation and necrosis. A score of 5 was given to samples with well-structured muscle fiber bundles and myofibers with ample perimysial and endomysial connective tissue spacing. Scores of 2 to 4 were intermediate. Wooden breast myopathy scores were recorded based on the degree of fibrosis, necrosis, and immune cell infiltration, with a score of zero representing no necrosis, fibrosis, or immune cell infiltration, a score of 1 representing minimal necrosis, fibrosis, and immune cell infiltration, a score of 2 being intermediate, and a score of 3 representing severe necrosis, fibrosis, and immune cell infiltration.
Myofiber diameter and perimysial and endomysial width were measured from 4 photomicrographs. At least 20 measurements were taken in each photomicrograph using Image Pro software (Media Cybernectics, Bethesda, MD). Myofiber widths were grouped into the following categories based on Clark et al. (2017): small (fiber width < 20 μm), intermediate (20 μm ≤ fiber width < 40 μm), large (40 μm ≤ fiber width < 70 μm), and giant fibers (fiber width ≥ 70 μm).
Nanostring nCounter Gene Expression Analysis
About 0.50 g of p. major muscle samples were collected and maintained in RNAlater (Ambion, Grand Island, NY). After 24 h, RNAlater was removed and the samples were stored at −20°C until RNA extraction. Total RNA was extracted from p. major muscle samples using RNAzol RT (Molecular Research Center, Cincinnati, OH) according to manufacturer's protocol. Gene expression analysis was completed by Nanostring nCounter Analysis (Nanostring Technologies, Seattle, WA) following the procedure described in Geiss et al. (2008). Genes whose expression is associated with muscle formation and growth, growth factors, hypoxia, oxidative stress and inflammation, extracellular matrix, cell structure and migration, calcium regulation, and glucose metabolism were selected as target sequences to be measured (Table 1). Codesets containing reporter and capture probes for target sequences were designed by Nanostring (Nanostring Technologies, Seattle, WA). The RNA samples were hybridized to the codsets, incubated for 16 h, and digitally analyzed for quantification.
Table 1.
List of genes analyzed by Nanostring nCounter gene expression analysis.
| Accession number | Symbol | Gene name |
|---|---|---|
| Muscle formation and growth | ||
| NM_204214.2 | MYOD1 | Myogenic differentiation 1 |
| NM_204184.1 | MYOG | Myogenin |
| NM_205065.1 | PAX7 | Paired box 7 |
| Growth factors | ||
| NM_001001461.1 | MSTN | Myostatin |
| NM_001318456.1 | TGFB1 | Transforming growth factor beta 1 |
| NM_205433.1 | FGF2 | Fibroblast growth factor 2 |
| Hypoxia, oxidative stress, and inflammation | ||
| NM_204297.1 | HIF1A | Hypoxia inducible factor 1 subunit alpha |
| NM_001318460.1 | TRPA1 | Transient receptor potential cation channel subfamily A member 1 |
| NM_001163245.1 | GPX7 | Glutathione peroxidase 7 |
| NM_204524.1 | IL1B | Interleukin 1, beta |
| XM_025153162 | SELE | Selectin E |
| Extracellular matrix | ||
| XM_025144131.1 | COL1A1 | Collagen type 1 alpha 1 chain |
| NM_205380.2 | COL3A1 | Collagen type 3 alpha 1 chain |
| NM_001162399.3 | COL4A1 | Collagen type 4 alpha 1 chain |
| NM_001030747.2 | DCN | Decorin |
| NM_001007869.1 | SDC4 | Syndecan-4 |
| NM_001305060.2 | GPC1 | Glypican-1 |
| Cell structure and migration | ||
| NM_001039254.2 | ITGB1 | Integrin subunit beta 1 |
| NM_204127.1 | ACTN1 | Actin, alpha 1 |
| Calcium regulation | ||
| XM_015272496.1 | RYR1 | Ryanodine receptor 1 |
| Glucose metabolism | ||
| NM_205284.1 | LDHA | Lactate dehydrogenase A |
| Housekeeping genes | ||
| NM_204902.2 | HMGB1 | High mobility group box 1 |
| NM_204861.1 | ANPEP | Alanyl aminopeptidase, membrane |
| NM_001007479.1 | RPL4 | Ribosomal protein L4 |
| XM_424881.6 | FNTA | Farnesyltransferase, CAAX box, alpha |
Statistical Analysis
Data of fiber width, perimysial and endomysial width, and morphology score were analyzed as a completely randomized design using PROC MIXED procedure of SAS version 9.4 software (SAS Institute INC., Cary, NC). Wooden breast score was analyzed with PROC GENMOD procedure of SAS. Individual pen was identified as the experimental unit. Dietary treatments were used as a fixed effect. Least square means were estimated with the LSMEANS procedure and separated with the PDIFF option. Significance was accepted at P ≤ 0.05. Gene expression is presented as fold changes and was analyzed with the Nanostring nSolver version 4.0 software (Nanostring Technologies, Seattle, WA). Fold change for each gene was calculated as the ratio between each dietary treatment and the control group. If ratio was higher than 1, fold change was equal to the ratio. If ratio was lower than 1, fold change was the negative inverse of the ratio. Fold differences of gene expression among the dietary treatments were calculated as fold changes. If one of the fold changes were positive and another was negative, the fold difference of gene expression between the 2 treatments was calculated as the percentage of the multiplication of their fold changes subtracted from 100%. If the fold changes were both positive or negative, the fold difference of gene expression between the 2 treatments was calculated as the percentage of the division of their fold changes subtracted from 100%. Heatmap was generated by RStudio version 3.5.2 with the R pheatmap package (RStudio INC., Boston, MA).
Results
Pectoralis Major Muscle Myofiber Width and Perimysial and Endomysial Space
Pectoralis major muscle myofiber width from all treatments is shown in Table 2. The average myofiber width and percentage of intermediate myofiber width (20 μm ≤ fiber width < 40 μm) were not significantly different among the dietary treatments (P > 0.05). However, there was a trend that broilers supplemented with n-3 fatty acids during the grower phase (11–24 d) had a 42% increase of small myofibers (fiber width < 20 μm) compared with VE supplemented broilers in the starter phase (0–10 d; P = 0.09). Broilers fed dietary VE (200 IU/kg) in the starter phase had 1.14 times of large myofibers (40 μm ≤ fiber width < 70 μm) than broilers fed dietary n-3 fatty acids (n-6/n-3 ratio of 3.2:1) in the grower phase (P = 0.03). Meanwhile, 2.10 times of giant myofibers with widths greater than 70 μm were identified in the group supplemented with VE and n-3 fatty acids in the starter phase compared with the group supplemented with VE in the grower phase (P = 0.02). There was no significant difference of the dietary treatments in perimysial or endomysial connective tissue space in any of the treatment groups as shown in Table 3 (P > 0.05).
Table 2.
Effect of vitamin E and omega-3 fatty acids on fiber width of pectoralis major muscle of broilers.
| Item | Treatments1 |
SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Starter VE | Starter n-3 | Starter VE and n-3 | Grower VE | Grower n-3 | Grower VE and n-3 | |||
| Average fiber width, μm | 45.87 | 47.54 | 46.55 | 47.90 | 45.36 | 46.75 | 46.75 | 0.42 | 0.71 |
| Fiber width <20 μm, % | 6.25 | 4.47 | 6.17 | 5.64 | 5.97 | 6.33 | 6.75 | 0.33 | 0.68 |
| 20 μm ≤ Fiber width <40 μm, % | 30.41 | 28.41 | 29.62 | 29.34 | 32.04 | 31.74 | 29.43 | 0.70 | 0.83 |
| 40 μm ≤ Fiber width <70 μm, % | 55.38a,b | 58.85a | 55.10a,b | 52.46a,b | 56.02a,b | 51.74 b | 54.70a,b | 0.79 | 0.38 |
| Fiber width ≥70 μm, % | 7.96a,b | 8.27a,b | 9.11a,b | 12.56a | 5.97 b | 10.19a,b | 9.12a,b | 0.66 | 0.34 |
a-bValues within a row without a common letter are significantly different (P ≤ 0.05).
Broilers in the control group were fed diets with standard level of vitamin E (VE; 10 IU/kg) and omega-3 (n-3) fatty acids (n-6/n-3 ratio of 30.2:1) during the entire study (0–58 d). Supplementation of additional VE (200 IU/kg), n-3 fatty acids (n-6/n-3 ratio of 3.2:1), or combination of both was performed during the starter phase (0–10 d) or grower phase (11–24 d).
Table 3.
Effect of vitamin E and omega-3 fatty acids on perimysial and endomysial connective tissue space and morphology score of pectoralis major muscle of broilers.
| Item | Treatments1 |
SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Starter VE | Starter n-3 | Starter VE and n-3 | Grower VE | Grower n-3 | Grower VE and n-3 | |||
| Perimysium, μm | 21.97 | 23.37 | 21.46 | 21.09 | 22.34 | 20.33 | 19.9 | 0.77 | 0.93 |
| Endomysium, μm | 7.16 | 7.08 | 7.05 | 7.35 | 7.05 | 6.83 | 7.26 | 0.11 | 0.97 |
| Morphology score2 | 2.70a,b | 2.57a,b | 2.68a,b | 2.65a,b | 2.85a | 2.46b | 2.57a,b | 0.05 | 0.48 |
a-bValues within a row without a common letter are significantly different (P ≤ 0.05).
Broilers in the control group were fed diets with standard level of vitamin E (VE; 10 IU/kg) and omega-3 (n-3) fatty acids (n-6/n-3 ratio of 30.2:1) during the entire study (0–58 d). Supplementation of additional VE (200 IU/kg), n-3 fatty acids (n-6/n-3 ratio of 3.2:1), or combination of both was performed during the starter phase (0–10 d) or grower phase (11–24 d).
Scoring scale of 1 to 5 was used for pectoralis major muscle morphology evaluation. Samples with limited or no perimysial or endomysial connective tissue space, and excessive myofiber degradation were given a score of 1. Samples with morphology score of 5 have ample perimysial and endomysial connective tissue spacing and well-structured muscle fibers. Score of 2 to 4 are intermediate.
Morphology Score and Wooden Breast Score
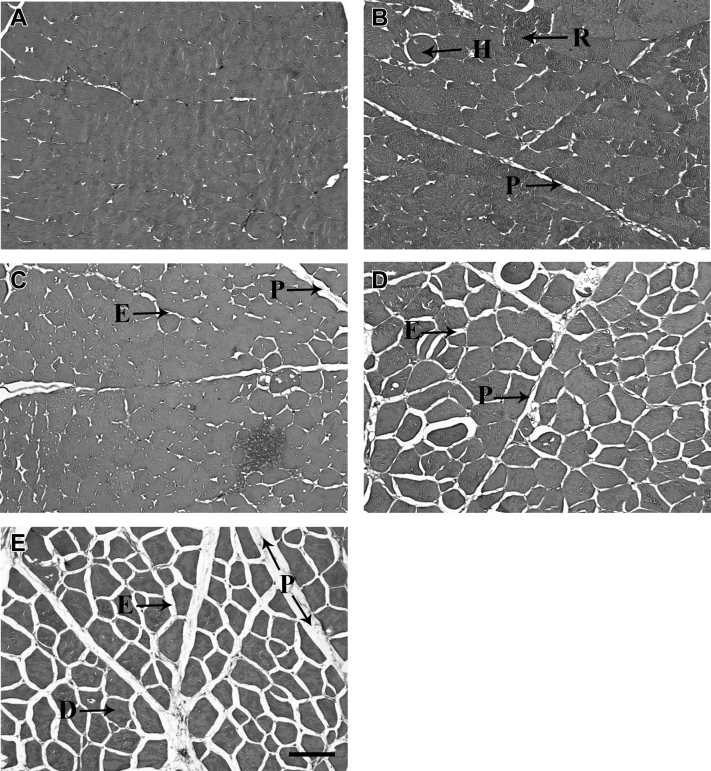
Figure 2 shows representative photomicrographs of the p. major muscle histology with morphology scores of 1 to 5. Figure 2A shows samples with a score of 1 with indistinct myofibers and limited or no perimysial or endomysial connective tissue space. Figure 2B shows samples with a score of 2 with excessive regenerating and hypertrohic fibers and limited perimysial or endomysial connective tissue space. Figure 2C shows samples with a score of 3 with intermediate regenerating fibers and perimysial or endomysial connective tissue space. Figure 2D shows samples with a score of 4 with minimal regenerating fibers and ample perimysial and endomysial connective tissue space. Figure 2E shows samples with a score of 5 with distinct myofibers and ample perimysial and endomysial connective tissue space. Vitamin E supplementation during the grower phase significantly increased morphology scores by 16% compared with the group supplemented with n-3 fatty acids in the grower phase (P = 0.04; Table 3).
Figure 2.
Representative photomicrographs of pectoralis major muscle with morphology score of 1 (A), 2 (B), 3 (C), 4 (D), and 5 (E). Samples with limited or no perimysial or endomysial connective tissue space, and excessive myofiber degradation were given a score of 1. Samples with morphology score of 5 have ample perimysial and endomysial connective tissue spacing and well-structured muscle fibers. Score of 2 to 3 are intermediate. Scale bar = 100 μm. Abbreviations: D, distinct myofiber; E, endomysial connective tissue; H, hypertrohic myofiber; P, perimysial connective tissue; R, regenerating myofiber.
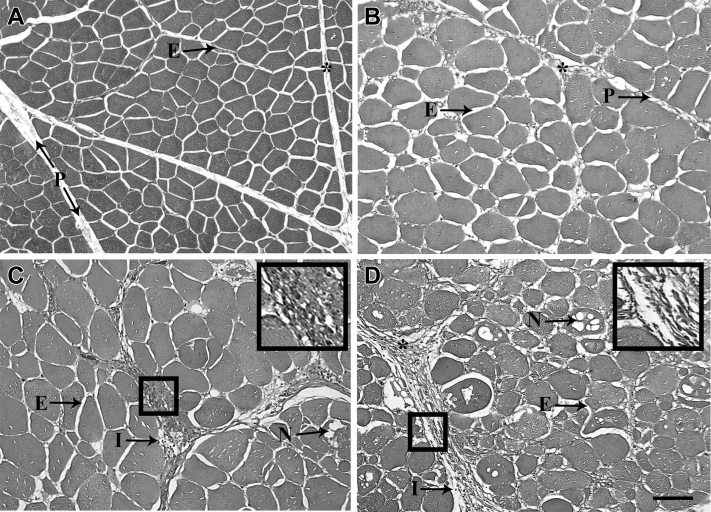
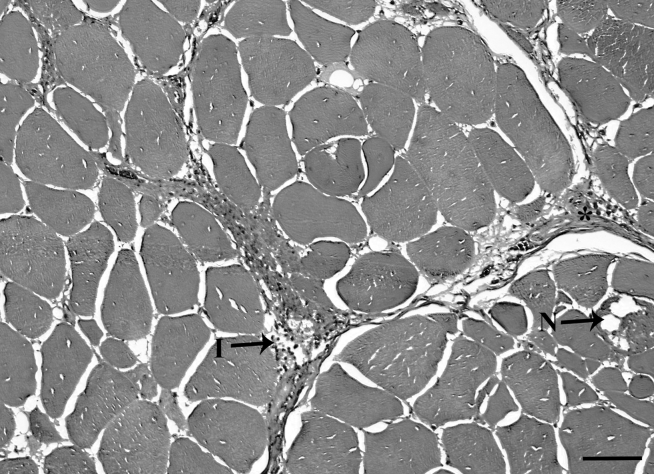
Representative images of WB scores of 0 to 3 are shown in Figure 3. Figure 3A shows samples with a score of 0 with no necrosis, fibrosis, or immune cell infiltration, indicating no WB myopathy in the p. major muscle. Figure 3B shows samples with a score of 1 with minimal necrosis, fibrosis, and immune cell infiltration, indicating mild WB myopathy. Figure 3C shows samples with a score of 2 shows samples with intermediate necrosis, fibrosis, and immune cell infiltration, indicating moderate WB myopathy. Figure 3D shows samples with a score of 3 with extensive fibrosis, necrosis, and immune cell infiltration, indicating severe WB myopathy. Figure 4 shows extensive mononucleated inflammatory cell infiltration associated with WB myopathy.
Figure 3.
Representative photomicrographs of pectoralis major muscle samples with wooden breast (WB) score of 0 (A), 1 (B), 2 (C), and 3 (D). The WB myopathy score were evaluated based on the degree of fibrosis, necrosis, and immune cell infiltration, with a score of zero representing no necrosis, fibrosis, or immune cell infiltration, a score of 1 representing minimal, a score of 2 being intermediate, and a score of 3 representing severe necrosis, fibrosis, and immune cell infiltration. Scale bar = 100 μm. The boxes contain enlargements of the collagen. Abbreviations: ∗, Collagen; E, endomysial connective tissue; I, immune cell infiltration; N, necrotic myofibers; P, perimysial connective tissue.
Figure 4.
Immune cell infiltration associated with wooden breast myopathy. Scale bar = 100 μm. Abbreviations: ∗, collagen; I, immune cell infiltration; N, necrotic myofibers.
Table 4 shows the WB score of broiler p. major muscle in the dietary treatments. The percentage of WB score zero was increased 16.19% in the broilers supplemented with VE during the grower phase compared with the control group (P = 0.05). Supplementation of n-3 fatty acids in the grower diet increased the percentage of WB score 1 compared with the group supplemented with n-3 fatty acids in the starter diet (P = 0.03) and VE in the grower diet (P = 0.01). When the broilers were fed dietary n-3 fatty acids during the starter phase, the percentage of WB score 2 was increased compared with the control group (P = 0.04). Supplementation of n-3 fatty acids in the starter diet decreased the percentage of WB score 3 compared with supplementation of VE in the grower diet (P = 0.02).
Table 4.
Effect of vitamin E and omega-3 fatty acids on wooden breast score of pectoralis major muscle of broilers.
| Item | Treatments1 |
SEM | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | Starter VE | Starter n-3 | Starter VE and n-3 | Grower VE | Grower n-3 | Grower VE and n-3 | ||
| Wooden breast score2 | ||||||||
| Score 0, % | 70.85b | 65.82b | 70.16b | 70.37b | 82.32a | 58.88b | 68.71b | 0.05 |
| Score 1, % | 16.75a,b,c,d,e | 26.67a,b | 14.68c,d,e | 20.38a,b,c,d | 7.00e | 27.78a | 21.42a,b,c | 0.04 |
| Score 2, % | 8.26b | 4.01b | 14.16a | 4.81b | 3.67b | 8.52a,b | 7.60b | 0.02 |
| Score 3, % | 4.14a,b | 3.50a,b | 1.00b | 4.44a,b | 7.01a | 4.82a,b | 2.27a,b | 0.02 |
a-eValues within a row without a common letter are significantly different (P ≤ 0.05).
Broilers in the control group were fed diets with standard level of vitamin E (VE; 10 IU/kg) and omega-3 (n-3) fatty acids (n-6/n-3 ratio of 30.2:1) during the entire study (0–58 d). Supplementation of additional VE (200 IU/kg), n-3 fatty acids (n-6/n-3 ratio of 3.2:1), or combination of both was performed during the starter phase (0–10 d) or grower phase (11–24 d).
Wooden breast (WB) scores were based on the degree of fibrosis, necrosis, and immune cell infiltration. Score 0 = none WB, score 1 = mild WB, score 2 = moderate WB, score 3 = severe WB.
Nanostring nCounter Gene Expression
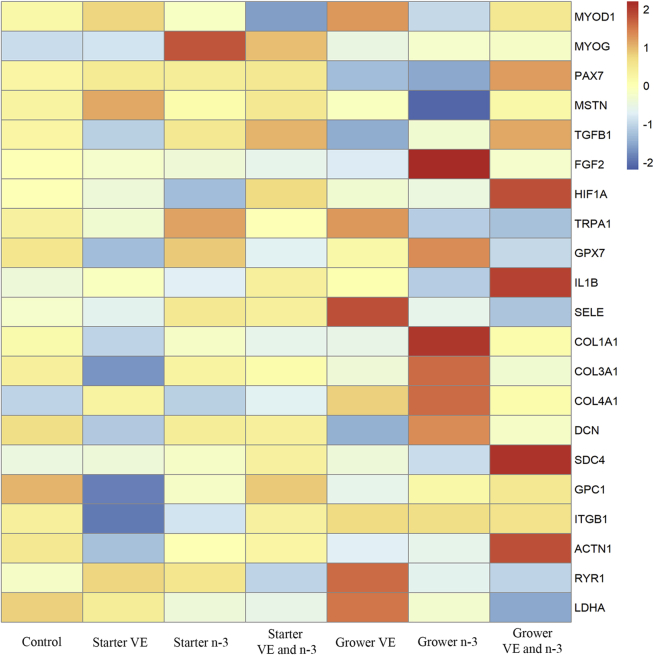
Figure 5 depicts the gene expression heatmap of p. major muscle of the broilers supplemented with different dietary treatments. The data reveal differential gene expression among treatments. Normalized gene expression abundance is color-coded according to the legend. Redness represents upregulation of genes, whereas blueness represents downregulation of genes compared with the control group. Gene fold changes are in Table 5. In terms of muscle formation and growth, supplemental VE in the grower diet had a 14% increase of expression of myogenic differentiation factor 1 (MYOD1; P = 0.04) compared with VE and n-3 fatty acids supplementation in the starter diet. Expression of transforming growth factor beta 1 (TGFB1) in the broilers supplemented with VE and n-3 fatty acids during the starter phase was 1.40 times of expression in VE supplemented broilers during the grower phase (P = 0.02). With regard to genes associated with hypoxia, oxidative stress, and inflammation, broilers fed VE supplementation in the grower diet had an 1.41 times of expression of transient receptor potential cation channel subfamily A member 1 (TRPA1) compared with the broilers fed supplemental n-3 fatty acids during the grower phase (P = 0.05). Expression of selectin E (SELE) was increased when broilers were fed dietary VE in the grower diet compared with the control diet (P = 0.02), dietary VE in the starter diet, or dietary n-3 fatty acids in the grower diet (P = 0.01). Extracellular matrix membrane associated gene, syndecan-4 (SDC4), had a 1.34 times increase in expression in p. major muscle of broilers supplemented with VE and n-3 fatty acids in the grower diet compared with the control group (P = 0.03). Expression of lactate dehydrogenase A (LDHA), an enzyme involved in glucose metabolism, expression was increased 1.17 times in the VE group supplemented during the grower phase compared with the n-3 fatty acids group supplemented during the starter phase (P = 0.05).
Figure 5.
Heatmap for pectoralis major muscle of broilers with different early posthatch dietary treatments. The heatmap was generated by RStudio with the R pheatmap package (RStudio INC., Boston, MA) using the expression of each gene (in rows) and treatments (in columns). The normalized expression values are color-coded according to the legend. Broilers in the control group were fed diets with standard level of Vitamin E (VE) (10 IU/kg) and n-3 fatty acids (n-6/n-3 ratio of 30.2:1) during the entire study (0–58 d). Broilers in starter VE, starter n-3, and starter VE and n-3 groups were fed diets with increased level of VE (200 IU/kg), n-3 fatty acids (n-6/n-3 ratio of 3.2:1), or combination of both during the starter phase (0–10 d). Broilers in grower VE, grower n-3, and grower VE and n-3 groups were fed diets with increased level of VE, n-3 fatty acids, or combination of both during the grower phase (11–24 d). All broilers were harvested at 58 d of age (n = 210).
Table 5.
Effect of vitamin E and omega-3 fatty acids on relative expression (fold change1) of genes in pectoralis major muscle of broilers.
| Item | Treatments2 |
||||||
|---|---|---|---|---|---|---|---|
| Starter VE | Starter n-3 | Starter VE and n-3 | Grower VE | Grower n-3 | Grower VE and n-3 | ||
| Muscle formation and growth | |||||||
| MYOD1 | Myogenic differentiation 1 | 1.03 | −1.01 | −1.09 | 1.05 | −1.06 | 1.01 |
| MYOG | Myogenin | −1.00 | 1.13 | 1.20 | 1.05 | 1.08 | 1.09 |
| PAX7 | Paired box 7 | 1.02 | 1.01 | 1.01 | −1.06 | −1.07 | 1.05 |
| Growth factors | |||||||
| MSTN | Myostatin | 1.02 | −1.01 | −1.02 | −1.04 | −1.16 | −1.06 |
| TGFB1 | Transforming growth factor beta 1 | −1.21 | 1.03 | 1.10 | −1.27 | −1.13 | 1.13 |
| FGF2 | Fibroblast growth factor 2 | −1.02 | −1.06 | −1.09 | −1.07 | 1.11 | −1.05 |
| Hypoxia, oxidative stress and inflammation | |||||||
| HIF1A | Hypoxia inducible factor 1 subunit alpha | −1.01 | −1.07 | 1.05 | −1.01 | −1.02 | 1.11 |
| TRPA1 | Transient receptor potential cation channel subfamily A member 1 | −1.08 | 1.10 | −1.12 | 1.13 | −1.25 | −1.22 |
| GPX7 | Glutathione peroxidase 7 | −1.08 | 1.02 | −1.05 | −1.01 | 1.03 | −1.07 |
| IL1B | Interleukin 1, beta | 1.05 | 1.01 | 1.17 | 1.09 | −1.14 | 1.31 |
| SELE | Selectin E | −1.07 | 1.06 | 1.03 | 1.29 | −1.09 | −1.19 |
| Extracellular matrix | |||||||
| COL1A1 | Collagen type 1 alpha 1 chain | −1.11 | −1.02 | −1.11 | −1.08 | 1.13 | 1.02 |
| COL3A1 | Collagen type 3 alpha 1 chain | −1.13 | 1.00 | −1.03 | −1.05 | 1.05 | −1.02 |
| COL4A1 | Collagen type 4 alpha 1 chain | 1.05 | 1.00 | 1.02 | 1.07 | 1.09 | 1.04 |
| DCN | Decorin | −1.1 | −1.01 | −1.03 | −1.12 | 1.02 | −1.03 |
| SDC4 | Syndecan-4 | −1.00 | 1.02 | 1.11 | 1.03 | −1.09 | 1.34 |
| GPC1 | Glypican-1 | −1.09 | −1.04 | 1.00 | −1.04 | −1.03 | 1.01 |
| Cell structure and migration | |||||||
| ITGB1 | Integrin subunit beta 1 | −1.06 | −1.04 | −1.00 | 1.01 | 1.01 | 1.01 |
| ACTN1 | Actin, alpha 1 | −1.21 | −1.08 | −1.05 | −1.14 | −1.14 | 1.13 |
| Calcium regulation | |||||||
| RYR1 | Ryanodine receptor 1 | 1.05 | 1.03 | −1.05 | 1.09 | −1.03 | −1.04 |
| Glucose metabolism | |||||||
| LDHA | Lactate dehydrogenase A | −1.02 | −1.08 | −1.12 | 1.08 | −1.07 | −1.18 |
The fold change for each gene was calculated as the ratio between treatments and control group. If ratio is higher than 1, fold change is equal to the ratio. If ratio is lower than 1, fold change is the negative inverse of the ratio.
Broilers in the control group were fed diets with standard level of vitamin E (VE; 10 IU/kg) and omega-3 (n-3) fatty acids (n-6/n-3 ratio of 30.2:1) during the entire study (0–58 d). Supplementation of additional VE (200 IU/kg), n-3 fatty acids (n-6/n-3 ratio of 3.2:1), or combination of both was performed during the starter phase (0–10 d) or grower phase (11–24 d).
Discussion
A previous study identified the effect of VE and n-3 fatty acids on phenotypic WB severity (Wang et al., 2020) and found that supplementation of dietary VE during the starter phase or grower phase reduced the incidence of WB myopathy. However, some birds that were phenotypically identified as normal by palpation on microscopic evaluation of the morphological structure of the p. major muscle had damage consistent with WB (Velleman and Clark, 2015). Therefore, the present study examined the effects of dietary supplemented VE and n-3 fatty acids independently or in combination during the starter phase (0–10 d) or the grower phase (11–24 d) on p. major muscle structure and expression of genes likely associated with WB in a meat-type commercial broiler line. Specifically, the p. major muscle morphological structure and expression of genes related to muscle formation and growth, growth factors, hypoxia, oxidative stress and inflammation, extracellular matrix, cell structure and migration, calcium regulation, and glucose metabolism were examined.
More than 2 times more giant myofibers were identified in the broilers supplemented with VE and n-3 fatty acids during the starter phase compared with VE supplementation during the grower phase. The giant myofibers are closely related with the process of muscle growth and development. By the time of hatch, postnatal myofiber growth is dependent on a myogenic stem cell population called satellite cells as myoblasts have withdrawn from the cell cycle, and muscle fiber formation is complete at hatch (Smith, 1963). Satellite cells fuse with multinucleated myofibers, donate their nuclei, and increase protein synthesis capabilities (Moss and Leblond, 1971). This results in muscle growth through hypertrophy or the enlargement of existing muscle fibers. Selection for increased breast muscle mass frequently results in large and giant myofibers, which restrict available space for the circulatory system and are thus more prone to oxidative stress associated with the WB myopathy (Dransfield and Sosnicki, 1999; Velleman et al., 2003a). This is consistent with the WB score showing that broilers supplemented with VE and n-3 fatty acids in the starter diet had a higher severity of WB than the broilers fed VE in the grower diet.
Broilers supplemented with VE in the grower diet had the most improved muscle morphological structure. The same results were found in histological WB severity showing that VE supplementation during the grower phase had increased number of p. major muscles with no WB. This is consistent with previous study suggesting that VE supplementation reduced the incidence and severity of phenotypic WB myopathy (Wang et al., 2020). The improved morphology indicates that the samples had more distinct myofibers and lower level of myofiber degeneration. The degeneration process starts with disruption of sarcolemma (Straub et al., 1998). Necrosis is thereby initiated as calcium influx from the sarcoplasmic reticulum which results in activation of a calcium-dependent protease called calpain (Dargelos et al., 2008). Immune cells such as heterophils and macrophages are recruited to help clear the damaged myofibers (Brigitte et al., 2010; Chazaud, 2016). This reactivates satellite cell–mediated regeneration process by reentering the cell cycle, undergoing proliferation, differentiation, and fusion with existing muscle fibers (Snow, 1977, 1978; Straub et al., 1998). When the satellite cells are not able to repair the damaged myofibers to its original structure, fibrosis with connective tissue replacing the myofibers is amplified and the severity of WB myopathy can be enhanced (Sihvo et al., 2014). Velleman et al. (2018) have found smaller fibers present in WB affected muscle. This is also consistent with the current study that more small myofibers were found in the n-3 fatty acid supplementation group during the grower phase along with a higher severity of WB compared with the VE supplementation group during the starter phase.
Genes associated with muscle formation and growth factors were differentially expressed in the dietary treatments. When broilers were fed dietary VE in the grower diet, expression of MYOD1 was increased compared with the group supplemented with VE and n-3 fatty acids in the starter diet. This indicates increased proliferation and regeneration levels in the broilers, as regeneration has been shown to result in increased expression of MYOD1 (Füchtbauer and Westphal, 1992). Higher regeneration levels are also indicated by decreased expression of TGFB1, which is a strong inhibitor of myogenic proliferation and differentiation (Massague et al., 1986; Rizzino, 1988). With higher proliferation potential, satellite cell-mediated repair is likely increased leading to a reduced severity of WB. This is consistent with Velleman and Clark (2015) who reported that expression of TGFB1 was decreased when broilers were not affected with WB myopathy compared with affected birds.
With regard to hypoxia and oxidative stress and inflammation, expression of TRPA1 and SELE were increased in the VE supplementation group during the grower phase. The TRPA1 is a subfamily of transient receptor potential cation channel regulators of calcium level (Zurborg et al., 2007) and mediate inflammation (Bautista et al., 2006; Moilanen et al., 2012). It is activated by G protein-coupled receptors leading to increased intracellular calcium (Hardie et al., 2001; Bandell et al., 2004). Dysregulated calcium homeostasis results in degeneration (Petracci et al., 2015). The SELE is an endothelial leukocyte adhesion molecule (Fries et al., 1993; De Luca et al., 1994) and is involved in chronic and acute inflammation processes in muscle (Lundberg, 2000; Ley, 2003). It is stimulated by cytokines and then recruits leukocytes to inflammatory sites (Ley et al., 1998; Patel et al., 2002). Adhesions between endothelial cells and extracellular matrix help them interact with each other and exchange information thereby improving migration and proliferation (Ruoslahti and Pierschbacher, 1987). Increased migration and proliferation are suggestive of a greater degree of myofiber regeneration. This may also explain why broilers fed additional VE in the grower diet had a reduced severity of WB as SELE was increased compared with the control group suggesting the presence of myofiber regeneration. Changes in expression level of genes related with myofiber regeneration and muscle ion homeostasis have been identified in Zambonelli et al. (2017). They found that the differentially expressed genes in WB affected breasts were involved in muscle development and calcium signaling pathway. Altered calcium levels have also been found in p. major muscle of 2-wk-old broilers before WB phenotype has been detected at market age (Lake et al., 2019).
Expression of SDC4 was increased in the broilers fed VE and n-3 fatty acids in the grower diet compared with the control group. Syndecan 4 is a transmembrane heparan sulfate proteoglycan and is involved in focal adhesion formation and cell migration (Longley et al., 1999; Couchman, 2003). It regulates focal adhesion by activating protein kinase C alpha through the SDC4 cytoplasmic domain (Woods and Couchman, 1992; Lee et al., 1998; Lim et al., 2003; Shin et al., 2013). Downstream signaling is then initiated and activates Ras homolog family member A to modulate focal adhesion, cell migration, and proliferation (Woods et al., 2000; Dovas et al., 2006). Increased expression of SDC4 in the group supplemented with VE and n-3 fatty acids in the grower diet is suggestive of cell migration which is necessary for the repair and regeneration process.
Another differential expressed gene is LDHA which was increased in the broilers supplemented with VE in the grower diet compared with n-3 fatty acids supplementation in the starter diet. The LDHA is an enzyme regulating conversion between lactate and pyruvate (Cahn et al., 1962). Lactic acid is produced by glycolytic metabolism as p. major muscle is composed of type 2B fibers, which is an anaerobic muscle fiber. Higher muscle mass as a result of selection restricts available space for circulatory system (Dransfield and Sosnicki, 1999). With insufficient circulatory system, lactic acid cannot be removed sufficiently and will be retained in the muscle decreasing pH and result in muscle damage (Velleman et al., 2003a). Abasht et al. (2019) has identified dysregulation of lipid and glycolytic metabolism in the breast muscle of the broilers with high feed efficiency, which are more prone to have WB. Higher levels of LDHA has a greater potential of lactate and pyruvate conversion and thus reducing the severity of WB. This has also been identified in Zhao et al. (2020) that LDHA expression level was decreased in WB affected tissues.
In conclusion, VE supplementation during the grower phase (11–24 d) showed a beneficial effect on improving muscle morphology and reducing the severity of WB myopathy. Genes related with muscle development and growth factors, response to inflammation, extracellular matrix, and glucose metabolism were differentially expressed because of early posthatch dietary treatments. Overall, muscle morphological results are consistent with changes in gene expression indicating a positive effect of VE supplementation during the grower phase on reducing the incidence and severity of WB myopathy. The current research represents an initial study evaluating the effect of VE and n-3 fatty acids on morphological structure of the p. major muscle and expression of genes likely associated with WB. Future research needs to be focused on determining the most beneficial supplementation concentration and administration period of VE on reducing the severity of the WB myopathy.
Acknowledgments
This study was supported by US Poultry and Egg grant (No. 710) to S.G.V. and S.K.J. and the China Scholarship Council (No. 201706350026) to J.W. The authors would like to thank Janet McCormick for technical assistance.
Conflict of Interest Statement: The authors did not provide any conflict of interest statement.
References
- Abasht B., Mutryn M.F., Michalek R.D., Lee W.R. Oxidative stress and metabolic perturbations in Wooden Breast disorder in chickens. PLoS One. 2016;11:1–16. doi: 10.1371/journal.pone.0153750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abasht B., Zhou N., Lee W.R., Zhuo Z., Peripolli E. The metabolic characteristics of susceptibility to wooden breast disease in chickens with high feed efficiency. Poult. Sci. 2019;98:3246–3256. doi: 10.3382/ps/pez183. [DOI] [PubMed] [Google Scholar]
- Aviagen . Aviagen; Huntsville, AL: 2016. Ross Broiler Management Manual. [Google Scholar]
- Bandell M., Story G.M., Hwang S.W., Viswanath V., Eid S.R., Petrus M.J., Earley T.J., Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bautista D.M., Jordt S.E., Nikai T., Tsuruda P.R., Read A.J., Poblete J., Yamoah E.N., Basbaum A.I., Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bordoni A., Danesi F. Poultry Quality Evaluation. Woodhead Publishing; Cambridge, United Kingdom: 2017. Poultry meat nutritive value and human health; pp. 279–290. [Google Scholar]
- Brewer V.B., Kuttappan V.A., Emmert J.L., Meullenet J.F.C., Owens C.M. Big-bird programs: effect of phase-feeding, strain, sex, and debone time on meat quality of broilers. Poult. Sci. 2012;91:248–254. doi: 10.3382/ps.2011-01705. [DOI] [PubMed] [Google Scholar]
- Brigitte M., Schilte C., Plonquet A., Baba-Amer Y., Henri A., Charlier C., Tajbakhsh S., Albert M., Gherardi R.K., Chrétien F. Muscle resident macrophages control the immune cell reaction in a mouse model of notexin-induced myoinjury. Arthritis Rheum. 2010;62:268–279. doi: 10.1002/art.27183. [DOI] [PubMed] [Google Scholar]
- Brothers B.K., Zhuo Z., Papah M., Abasht B. RNA-seq analysis reveals spatial and sex differences in pectoralis major muscle of broiler chickens contributing to difference in susceptibility to wooden breast disease. Front. Physiol. 2019;10:764. doi: 10.3389/fphys.2019.00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn R.D., Kaplan N.O., Levine L., Zwilling E. Nature and development of lactic dehydrogenases. Science. 1962;136:962–969. doi: 10.1126/science.136.3520.962. [DOI] [PubMed] [Google Scholar]
- Calder P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- Chatterjee R.N., Bhattacharya T.K., Paul S.S. Breeding poultry for improved input use efficiency and nutrient quality of products. Indian J. Genet. 2019;79:204–207. [Google Scholar]
- Chazaud B. Inflammation during skeletal muscle regeneration and tissue remodeling: application to exercise-induced muscle damage management. Immunol. Cell Biol. 2016;94:140–145. doi: 10.1038/icb.2015.97. [DOI] [PubMed] [Google Scholar]
- Clark D.L., Walter K.G., Velleman S.G. Incubation temperature and time of hatch impact broiler muscle growth and morphology. Poult. Sci. 2017;96:4085–4095. doi: 10.3382/ps/pex202. [DOI] [PubMed] [Google Scholar]
- Couchman J.R. Syndecans: proteoglycan regulators of cell-surface microdomains? Nat. Rev. Mol. Cell Biol. 2003;4:926–937. doi: 10.1038/nrm1257. [DOI] [PubMed] [Google Scholar]
- Dargelos E., Poussard S., Brulé C., Daury L., Cottin P. Calcium-dependent proteolytic system and muscle dysfunctions: a possible role of calpains in sarcopenia. Biochimie. 2008;90:359–368. doi: 10.1016/j.biochi.2007.07.018. [DOI] [PubMed] [Google Scholar]
- De Luca L.G., Johnson D.R., Whitley M.Z., Collins T., Pober J.S. cAMP and tumor necrosis factor competitively regulate transcriptional activation through and nuclear factor binding to the cAMP-responsive element/activating transcription factor element of the endothelial leukocyte adhesion molecule-1 (E-selectin) promoter. J. Biol. Chem. 1994;269:19193–19196. [PubMed] [Google Scholar]
- Dovas A., Yoneda A., Couchman J.R. PKC-α-dependent activation of RhoA by syndecan-4 during focal adhension formation. J. Cell Sci. 2006;119:2837–2846. doi: 10.1242/jcs.03020. [DOI] [PubMed] [Google Scholar]
- Dransfield E., Sosnicki A.A. Relationship between muscle growth and poultry meat quality. Poult. Sci. 1999;78:743–746. doi: 10.1093/ps/78.5.743. [DOI] [PubMed] [Google Scholar]
- El-Samee L.D.A., El-Wardany I., Abdel-Fattah S.A., El-Azeem N.A.A., Elsharkawy M.S. Dietary omega-3 and antioxidants improve long-chain omega-3 and lipid oxidation of broiler meat. Bull. Natl. Res. Cent. 2019;43:45. [Google Scholar]
- Ewaschuk J.B., Almasud A., Mazurak V.C. Role of n-3 fatty acids in muscle loss and myosteatosis. Appl. Physiol. Nutr. Metab. 2014;39:654–662. doi: 10.1139/apnm-2013-0423. [DOI] [PubMed] [Google Scholar]
- Fries J.W., Williams A.J., Atkins R.C., Newman W., Lipscomb M.F., Collins T. Expression of VCAM-1 and E-selectin in an in vivo model of endothelial activation. Am. J. Clin. Pathol. 1993;143:725. [PMC free article] [PubMed] [Google Scholar]
- Füchtbauer E.M., Westphal H. MyoD and myogenin are coexpressed in regenerating skeletal muscle of the mouse. Dev. Dyn. 1992;193:34–39. doi: 10.1002/aja.1001930106. [DOI] [PubMed] [Google Scholar]
- Geiss G.K., Bumgarner R.E., Birditt B., Dahl T., Dowidar N., Dunaway D.L., Fell H.P., Ferree S., George R.D., Grogan T., James J.J., Maysuria M., Mitton J.D., Oliveri P., Osborn J.L., Peng T., Ratcliffe A.L., Webster P.J., Davidson E.H., Hood L., Dimitrov K. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat. Biotechnol. 2008;26:317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- Hardie R.C., Raghu P., Moore S., Juusola M., Baines R.A., Sweeney S.T. Calcium influx via TRP channels is required to maintain PIP2 levels in Drosophila photoreceptors. Neuron. 2001;30:149–159. doi: 10.1016/s0896-6273(01)00269-0. [DOI] [PubMed] [Google Scholar]
- Hosomi A., Arita M., Sato Y., Kiyose C., Ueda T., Igarashi O., Arai H., Inoue K. Affinity for α-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Letters. 1997;409:105–108. doi: 10.1016/s0014-5793(97)00499-7. [DOI] [PubMed] [Google Scholar]
- Jarrold B.B., Bacon W.L., Velleman S.G. Expression and localization of the proteoglycan decorin during the progression of cholesterol induced atherosclerosis in Japanese quail: implications for interaction with collagen type I and lipoproteins. Atherosclerosis. 1999;146:299–308. doi: 10.1016/s0021-9150(99)00154-9. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Goodgame S.D., Bradley C.D., Mauromoustakos A., Hargis B.M., Waldroup P.W., Owens C.M. Effect of different levels of vitamin E (DL-α-tocopherol acetate) on the occurence of various degrees of white striping on broiler breast fillets. Poult. Sci. 2012;91:3230–3235. doi: 10.3382/ps.2012-02397. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Hargis B.M., Owens C.M. White striping and woody breast myopathies in the modern poultry industry: a review. Poult. Sci. 2016;95:2724–2733. doi: 10.3382/ps/pew216. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Owens C.M., Coon C., Hargis B.M., Vazquez-Anon M. Incidence of broiler breast myopathies at 2 different ages and its impact on selected raw meat quality parameters. Poult. Sci. 2017;96:3005–3009. doi: 10.3382/ps/pex072. [DOI] [PubMed] [Google Scholar]
- Lake J.A., Papah M.B., Abasht B. Increased expression of lipid metabolism genes in early stages of Wooden Breast links myopathy of broilers to metabolic syndrome in humans. Genes. 2019;10:746. doi: 10.3390/genes10100746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D., Oh E.S., Woods A., Couchman J.R., Lee W. Solution structure of a syndecan-4 cytoplasmic domain and its interaction with phosphatidylinositol 4, 5-bisphosphate. J. Biol. Chem. 1998;273:13022–13029. doi: 10.1074/jbc.273.21.13022. [DOI] [PubMed] [Google Scholar]
- Ley K. The role of selectins in inflammation and disease. Trends. Mol. Med. 2003;9:263–268. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Ley K., Allietta M., Bullard D.C., Morgan S. Importance of E-selectin for firm leukocyte adhesion in vivo. Circ. Res. 1998;83:287–294. doi: 10.1161/01.res.83.3.287. [DOI] [PubMed] [Google Scholar]
- Lim S.T., Longley R.L., Couchman J.R., Woods A. Direct binding of syndecan-4 cytoplasmic domain to the catalytic domain of protein kinase Cα (PKCα) increases focal adhesion localization of PKCα. J. Biol. Chem. 2003;278:13795–13802. doi: 10.1074/jbc.M208300200. [DOI] [PubMed] [Google Scholar]
- Longley R.L., Woods A., Fleetwood A., Cowling G.J., Gallagher J.T., Couchman J.R. Control of morphology, cytoskeleton and migration by syndecan-4. J. Cell Sci. 1999;112:3421–3431. doi: 10.1242/jcs.112.20.3421. [DOI] [PubMed] [Google Scholar]
- Lundberg I.E. The role of cytokines, chemokines, and adhesion molecules in the pathogenesis of idiopathic inflammatory myopathies. Curr. Rheumatol. Rep. 2000;2:216–224. doi: 10.1007/s11926-000-0082-y. [DOI] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Endo T., Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc. Natl. Acad. Sci. 1986;83:8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moilanen L.J., Laavola M., Kukkonen M., Korhonen R., Leppänen T., Högestätt E.D., Zygmunt P.M., Nieminen R.M., Moilanen E. TRPA1 contributes to the acute inflammatory response and mediates carrageenan-induced paw edema in the mouse. Sci. Rep. 2012;2:1–6. doi: 10.1038/srep00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss F.P., Leblond C.P. Satellite cells as the source of nuclei in muscles of growing rats. Anat. Rec. 1971;170:421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Mottet A., Tempio G. Global poultry production: current state and future outlook and challenges. Worlds Poult. Sci. J. 2017;73:245–256. [Google Scholar]
- Mutryn M.F., Brannick E.M., Fu W., Lee W.R., Abasht B. Characterization of a novel chicken muscle disorder through differential gene expression and pathway analysis using RNA-sequencing. BMC Genomics. 2015;16:399. doi: 10.1186/s12864-015-1623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirement of Poultry: Ninth Revised Edition. [Google Scholar]
- National Chicken Council . National Chicken Council; Washington, DC: 2020. Statistics: U.S. Broiler Production. [Google Scholar]
- Niki E. Oxidative stress and antioxidants: distress or eustress? Arch. Biochem. Biophys. 2016;595:19–24. doi: 10.1016/j.abb.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Niki E., Noguchi N., Gotoh N. Dynamics of lipid peroxidation and its inhibition by antioxidants. Biochem. Soc. Trans. 1993;21:313–317. doi: 10.1042/bst0210313. [DOI] [PubMed] [Google Scholar]
- Niki E., Yamamoto Y., Komuro E., Sato K. Membrane damage due to lipid oxidation. Am. J. Clin. Nutr. 1991;53:201S–205S. doi: 10.1093/ajcn/53.1.201S. [DOI] [PubMed] [Google Scholar]
- Panda A.K., Cherian G. Role of vitamin E in counteracting oxidative stress in poultry. J. Poult. Sci. 2014;51:109–117. [Google Scholar]
- Papah M.B., Brannick E.M., Schmidt C.J., Abasht B. Evidence and role of phlebitis and lipid infiltration in the onset and pathogenesis of Wooden Breast Disease in modern broiler chickens. Avian Pathol. 2017;46:623–643. doi: 10.1080/03079457.2017.1339346. [DOI] [PubMed] [Google Scholar]
- Patel K.D., Cuvelier S.L., Wiehler S. Selectins: critical mediators of leukocyte recruitment. Semin. Immunol. 2002;14:73–81. doi: 10.1006/smim.2001.0344. [DOI] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Bonfiglio A., Cavani C. Occurrence of white striping under commercial conditions and its impact on breast meat quality in broiler chickens. Poult. Sci. 2013;92:1670–1675. doi: 10.3382/ps.2012-03001. [DOI] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Soglia F., Cavani C. Meat quality in fast-growing broiler chickens. Worlds Poult. Sci. J. 2015;71:363–374. [Google Scholar]
- Rizzino A. Transforming growth factor-β: Multiple effects on cell differentiation and extracellular matrices. Dev. Biol. 1988;130:411–422. doi: 10.1016/0012-1606(88)90337-5. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M.D. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Shin J., McFarland D.C., Velleman S.G. Migration of Turkey muscle satellite cells is enhanced by the syndecan-4 cytoplasmic domain through the activation of RhoA. Mol. Cell Biochem. 2013;375:115–130. doi: 10.1007/s11010-012-1534-1. [DOI] [PubMed] [Google Scholar]
- Sihvo H.K., Lindén J., Airas N., Immonen K., Valaja J., Puolanne E. Wooden Breast myodegeneration of pectoralis major muscle over the growth period in broilers. Vet. Pathol. 2017;54:119–128. doi: 10.1177/0300985816658099. [DOI] [PubMed] [Google Scholar]
- Sihvo H.K., Immonen K., Puolanne E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2014;51:619–623. doi: 10.1177/0300985813497488. [DOI] [PubMed] [Google Scholar]
- Smith J.H. Relation of body size to muscle cell size and number in the chicken. Poult. Sci. 1963;42:283–290. [Google Scholar]
- Snow M.H. Myogenic cell formation in regenerating rat skeletal muscle injured by mincing. II. An autoradiographic study. Anat. Rec. 1977;188:201–217. doi: 10.1002/ar.1091880206. [DOI] [PubMed] [Google Scholar]
- Snow M.H. An autoradiographic study of satellite cell differentiation into regenerating myotubes following transplantation of muscles in young rats. Cell Tissue Res. 1978;186:535–540. doi: 10.1007/BF00224941. [DOI] [PubMed] [Google Scholar]
- Straub V., Duclos F., Venzke D.P., Lee J.C., Cutshall S., Leveille C.J., Campbell K.P. Molecular pathogenesis of muscle degeneration in the δ-sarcoglycan- deficient hamster. Am. J. Pathol. 1998;153:1623–1630. doi: 10.1016/s0002-9440(10)65751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijare V.V., Yang F.L., Kuttappan V.A., Alvarado C.Z., Coon C.N., Owens C.M. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poult. Sci. 2016;95:2167–2173. doi: 10.3382/ps/pew129. [DOI] [PubMed] [Google Scholar]
- Tasoniero G., Cullere M., Cecchinato M., Puolanne E., Dalle Zotte A. Technological quality, mineral profile, and sensory attributes of broiler chicken breasts affected by white striping and wooden breast myopathies. Poult. Sci. 2016;95:2707–2714. doi: 10.3382/ps/pew215. [DOI] [PubMed] [Google Scholar]
- Taulescu C., Mihaiu M., Bele C., Matea C., Dan S.D., Mihaiu R., Lapusan A. Antioxidant effect of vitamin E and selenium on omega-3 enriched oultry meat. Bul. Univ. Agric. Sci. Vet. Med. 2011;68:293–300. [Google Scholar]
- Velleman S.G., Clark D.L. Histopathologic and myogenic gene expression changes associated with Wooden Breast in broiler breast muscles. Avian Dis. 2015;59:410–418. doi: 10.1637/11097-042015-Reg.1. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., Clark D.L., Tonniges J. The effect of the Wooden Breast myopathy on sarcomere structure and organization. Avian Dis. 2018;62:28–35. doi: 10.1637/11766-110217-Reg.1. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., Anderson J.W., Coy C.S., Nestor K.E. Effect of selection for growth rate on muscle damage during Turkey breast muscle development. Poult. Sci. 2003;82:1069–1074. doi: 10.1093/ps/82.7.1069. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., Anderson J.W., Nestor K.E. Possible maternal inheritance of breast muscle morphology in turkeys at sixteen weeks of age. Poult. Sci. 2003;82:1479–1484. doi: 10.1093/ps/82.10.1479. [DOI] [PubMed] [Google Scholar]
- Voljč M., Frankič T., Levart A., Nemec M., Salobir J. Evaluation of different vitamin E recommendations and bioactivity of α-tocopherol isomers in broiler nutrition by measuring oxidative stress in vivo and the oxidative stability of meat. Poult. Sci. 2011;90:1478–1488. doi: 10.3382/ps.2010-01223. [DOI] [PubMed] [Google Scholar]
- Wang J., Clark D.L., Jacobi S.K., Velleman S.G. Effect of vitamin E and omega-3 fatty acids early post-hatch supplementation on reducing the severity of wooden breast myopathy in broilers. Poult. Sci. 2020;99:2108–2119. doi: 10.1016/j.psj.2019.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A., Couchman J.R. Protein kinase C involvement in focal adhesion formation. J. Cell Sci. 1992;101:277–290. doi: 10.1242/jcs.101.2.277. [DOI] [PubMed] [Google Scholar]
- Woods A., Longley R.L., Tumova S., Couchman J.R. Syndecan-4 binding to the high affinity heparin-binding domain of fibronectin drives focal adhesion formation in fibroblasts. Arch. Biochem. Biophys. 2000;374:66–72. doi: 10.1006/abbi.1999.1607. [DOI] [PubMed] [Google Scholar]
- Yu C., Tan S., Wang Z., Yu Z., Zhuang S. Omega-3 polyunsaturated fatty acids reduce intestinal inflammation and enhance intestinal motility associated with reduced nitric oxide production in chronic kidney disease. Clin. Nutr. 2018;37:S92–S93. [Google Scholar]
- Zambonelli P., Zappaterra M., Soglia F., Petracci M., Sirri F., Cavani C., Davoli R. Detection of differentially expressed genes in broiler pectoralis major muscle affected by White Striping - wooden Breast myopathies. Poult. Sci. 2017;95:2771–2785. doi: 10.3382/ps/pew268. [DOI] [PubMed] [Google Scholar]
- Zhao D., Kogut M.H., Genovese K.J., Hsu C.Y., Lee J.T., Farnell Y.Z. Altered expression of lactate dehydrogenase and monocarboxylate transporter involved in lactate metabolism in broiler wooden breast. Poult. Sci. 2020;99:11–20. doi: 10.3382/ps/pez572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurborg S., Yurgionas B., Jira J.A., Caspani O., Heppenstall P.A. Direct activation of the ion channel TRPA1 by Ca 2+ Nat. Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]