Abstract
Low-egg-producing hens (LEPH) ovulate less frequently than high-egg-producing hens (HEPH) and exhibit differences in mRNA levels for components of the hypothalamo–pituitary–gonadal (HPG) axis, suggesting differential responsiveness to trophic stimulation. Ovulation frequency is governed by the production of the pituitary gonadotropins and feedback of the ovarian follicle steroid hormones, which are regulated by HPG axis stimulation and inhibition at the hypothalamic level. The pituitary and follicle cells from LEPH and HEPH were subjected to in vitro hormonal treatments to stimulate or inhibit the HPG axis, followed by expression analysis of mRNA levels for HPG axis genes and radioimmunoassays for steroid hormone production. Statistical analysis was performed using the mixed models procedure of SAS. The pituitary cells from HEPH showed upregulation of genes associated with ovulation stimulation, whereas cells from LEPH showed upregulation of genes associated with inhibition of ovulation. High-egg-producing hens’ follicle cells displayed a higher sensitivity and responsiveness to gonadotropin treatment. Level of egg production impacted ovulation-related gene expression in the pituitary cells as well as steroid hormone production in the follicle cells, with HEPH displaying a greater positive response to stimulation. These findings indicate that differences in egg production among turkey hens likely involve differential responsiveness of the cells within the HPG axis.
Key words: hypothalamo–pituitary–gonadal axis, gonadotropin production, steroid hormone production, avian, ovulation
Introduction
Differences in egg production rates among turkey hens in a flock result in low-egg-producing hens (LEPH) and high-egg-producing hens (HEPH). Low egg production in breeding hens costs the industry in lost poult production and is correlated with decreased ovulation frequency (Liu et al., 2001). Follicle ovulation in avian species is controlled by the hypothalamo–pituitary–gonadal (HPG) axis, which is composed of the hypothalamus, pituitary, and a single ovary. A preovulatory surge (PS) precedes each ovulation and consists of increased progesterone and luteinizing hormone (LH), produced by the ovary and pituitary, respectively (Paster, 1991). Steroid hormones, such as estradiol and progesterone, feedback on the HPG axis to regulate ovulation timing (reviewed in Ottinger and Bakst, 1995).
The HPG axis can be stimulated by gonadotropin-releasing hormone (GnRH) or inhibited by gonadotropin-inhibitory hormone (GnIH), both produced in the hypothalamus with the anterior pituitary as their target tissue. Neuron terminals containing GnRH extend into the external layer of the median eminence for neuropeptide release into the hypophysial portal vascular system (Bédécarrats, 2014). The neuron terminals containing GnIH also extend into the median eminence but also have direct contact with GnRH neurons, suggesting the capability of GnIH regulation of GnRH synthesis and release (Bédécarrats et al., 2016). Gonadotropin-releasing hormone or GnIH regulate pituitary gonadotropin production by binding to either gonadotropin-releasing hormone receptor (GnRHR) or gonadotropin-inhibitory hormone receptor (GnIHR), both located on pituitary gonadotrophs. Gonadotropin-releasing hormone receptor and GnIHR are G-protein-coupled receptors (GPCRs) present on pituitary gonadotroph cells, with GnRHR coupling to Gαs and Gαq and GnIHR coupling to Gαi (Tsutsui et al., 2006).
The ovary is composed of follicles in varying states of maturation, developing from quiescent primordial follicles to preovulatory follicles awaiting ovulation. Steroidogenesis occurs in ovarian follicles, with primary steroid production varying with follicle development and follicle cell type (Porter et al., 1989a). Most ovarian estradiol production occurs in the small white follicles (SWFs), which are slow-growing follicles that have yet to enter the preovulatory hierarchy (Johnson, 1992). Small white follicles comprise granulosa, theca interna, and theca externa cells. Ovarian progesterone production primarily occurs in the granulosa cells of the largest preovulatory follicle (F1G), which is the next follicle in line to ovulate (Bahr et al., 1983). The SWFs are mainly responsive to follicle-stimulating hormone (FSH), whereas F1G are responsive to LH. Follicle-stimulating hormone receptor (FSHR) and LH receptor (LHCGR) are also GPCRs that couple to Gαs to increase the transcription of genes involved in steroidogenesis through the cAMP-signaling pathway, such as steroidogenic acute regulatory protein (STAR) and aromatase (CYP19A1) (Li et al., 2014).
Previous studies comparing HPG axis gene expression of LEPH and HEPH found that HEPH displayed gene expression levels consistent with increased ovulation stimulation and decreased ovulation inhibition in the hypothalamus and pituitary. In addition, HEPH showed upregulation of genes related to progesterone production in the F1G and related to estradiol production in the SWFs (Brady et al., 2020). High-egg-producing hens showed decreased gene expression of GNIH in the hypothalamus, increased expression of both gonadotropin beta subunits in the pituitary, increased gene expression of STAR and cholesterol side chain cleavage enzyme (CYP11A1) in the F1G, and increased gene expression of 17β-hydroxysteroid dehydrogenase (HSD17B1) and CYP19A1 in the SWFs.
Based on previous gene expression differences between LEPH and HEPH, it was hypothesized that HEPH would show an increased sensitivity and responsiveness to GnRH treatment in the pituitary, to LH treatment in the F1G, and to FSH treatment in the SWFs, whereas LEPH would show an increased sensitivity and responsiveness to GnIH treatment in the pituitary. The present study sought to compare LEPH and HEPH in the responsiveness of isolated pituitary cells to GnRH and GnIH stimulation as well as in the responsiveness of the cells responsible for estradiol and progesterone production, SWF cells and F1G cells, to FSH and LH stimulation, respectively.
Materials and methods
Hen Selection
Females (200 hens) from a commercial line (Hybrid Turkey, Kitchener, Ontario) were housed at the Beltsville Agricultural Research Center in individual wire cages. Turkey hens were maintained under standard poultry management practices with artificial lighting (14L:10D) and were provided feed ad libitum to NRC standards. The hens were sampled at 35 wk of age. Daily egg records were used to calculate each hen's number of eggs per day (EPD) by dividing the total number of eggs produced by the number of days in production. Hens were classified as LEPH when EPD <0.6 and as HEPH when EPD >0.8. The egg production cutoffs were based on the distribution of flock egg production as previously described (Brady et al., 2020). Blood samples were taken from the wing vein immediately before sampling, collected in heparinized tubes, and fractionated by centrifugation (2,000 × g for 10 min at room temperature). Plasma samples were stored at −20°C before assessment through radioimmunoassays as described in the following. The pituitary, F1 follicle, and SWFs were isolated from 4 LEPH and 4 HEPH. All hens were sampled at the same time during the daily lighting schedule, sampled outside of the PS, and sampled on the second day of the hen's sequence, as previously described (Brady et al., 2019). The timing of the PS was estimated for each hen based on the oviposition–ovulation cycle as previously described (Brady et al., 2019). Plasma progesterone levels were examined to confirm correct sampling time during the ovulatory cycle. All animal procedures were approved by the Institutional Animal Care and Use Committees at the Beltsville Agricultural Research Center and at the University of Maryland.
Cell Isolation and Culture
All cell isolation procedures were performed using Minimum Essential Medium, Spinner modification (SMEM) or Dulbecco's Modified Eagle Medium (DMEM) as noted in the following. The medium was supplemented with 0.1% bovine serum albumen, 100-U/mL penicillin G (P), and 100-μg/mL streptomycin sulfate (S) (0.1% BSA and P/S).
Isolated pituitaries were minced and dispersed in SMEM (0.1% BSA and P/S) using trypsin and collagenase (1 mg/mL of each) for 90 min at 37°C in a shaking water bath. After dispersion, the cells were filtered through 70 μm nylon mesh and washed twice with DMEM (0.1% BSA and P/S). The cells were diluted to a concentration of 200,000 cells/mL and plated in serum-free medium (DMEM/F12) supplemented with 0.1% bovine serum albumen, 5-μg/mL human insulin, 100-U/mL penicillin G, and 100-μg/mL streptomycin sulfate. The cells were plated in 24-well poly-L lysine–coated plates (Corning Life Sciences, Lowell, MA) at 100,000 cells/well and were allowed to attach for 2 h before treatment. Pituitary cells were treated with chicken GnRH-I or chicken GnIH (Phoenix Pharmaceuticals, Burlingame, CA) at 0, 10−9, 10−8, or 10−7 M for 6 or 24 h. Time points were selected to examine short- and long-term changes in mRNA levels due to treatment.
The F1 follicle was removed from the ovary and placed in ice-cold SMEM (0.1% BSA and P/S) until isolation of the granulosa cell layer. The follicle was drained of yolk, everted, and the granulosa cell layer was peeled off of the follicle wall. The granulosa cell layer was dispersed in SMEM (0.1% BSA and P/S) using trypsin (1 mg/mL) as previously described (Brady et al., 2019). After dispersion, the cells were filtered through a 70-μm nylon mesh and layered onto a 50% Percoll solution to remove the remaining yolk particles. The cells were washed twice with SMEM (0.1% BSA and P/S) and diluted to a density of 10,000 cells/mL for culture. The F1G cells were cultured in SMEM (0.1% BSA and P/S) in 12 × 75 mm polypropylene tubes (1 × 105 cells per tube). The cells were treated with ovine LH (National Hormone & Peptide Program, Torrance, CA) at 0, 1, 10, 100, or 1,000 ng/mL for 5 h as previously described (Porter et al., 1989a).
The SWFs were minced and dispersed in SMEM (0.1% BSA and P/S) using trypsin (1 mg/mL) for 60 min at 37°C in a shaking water bath. After dispersion, the cells were filtered through a 70-μm nylon mesh and layered onto a 50% Percoll solution to remove remaining red blood cells. The resulting SWF cells were washed twice with SMEM (0.1% BSA and P/S) and diluted to a density of 10,000 cells/mL for culture. The SWF cells were cultured in SMEM (0.1% BSA and P/S) in 12 × 75 mm polypropylene tubes (1 × 105 cells per tube). The cells were treated with porcine FSH (National Hormone & Peptide Program, Torrance, CA) at 0, 1, 10, 100, or 1,000 ng/mL for 5 h as previously described (Porter et al., 1989b).
The cells were maintained in a 37.5°C, 5% CO2 atmosphere during incubation. Pituitary cells were harvested at the completion of each incubation by retrypsinization, immediately frozen in liquid nitrogen, and stored at −80°C until RNA extraction. The media from the F1G and SWF cell cultures were recovered and stored at −20°C for progesterone and estradiol radioimmunoassays, respectively.
RT-qPCR
Total RNA was isolated from pituitary cell cultures with RNeasy Mini kits (Qiagen, Valencia, CA), including on-column deoxyribonuclease digestion. Quantification of RNA, RT, and RT-qPCR were performed as previously described (Brady et al., 2019) with the following exception. Reverse transcription reactions were performed on 50 ng total RNA with SuperScript III (Thermo Fisher Scientific, Waltham, MA) and an anchored oligo-dT primer (5′-CGGAATTCTTTTTTTTTTTTTTTTTTTTV-3′) (Integrated DNA Technologies, Skokie, IL). A pool of total RNA was made and the reaction conducted without reverse transcriptase as a control for genomic DNA contamination. Reactions were diluted to 40 μL before PCR analysis. PCR reactions (15 μL) were carried out as previously described using a CFX Connect Real-Time PCR System (Bio-Rad, Hercules, CA) (Brady et al., 2019). Data were normalized to phosphoglycerate kinase 1 (PGK1) and analyzed by the 2−ΔΔ Ct method. All PCR reactions for each gene in a given tissue were analyzed in a single run within a 96-well plate, allowing accurate performance of relative quantification without the need to include a reference control sample in each plate. Primers (Integrated DNA Technologies, Skokie, IL) for turkey PGK1, gonadotropin-releasing hormone receptor III (GNRHR), gonadotropin-inhibitory hormone receptor I (GNIHR), and luteinizing hormone beta-subunit (LHB) mRNA were designed and used with cycling parameters described previously (Brady et al., 2019). Data are presented as fold increase over levels in basal cells for each hormone treatment and time point. To calculate the ratio of GNIHR mRNA level to GNRHR mRNA level, average cycle threshold (Ct) values were normalized and log2-transformed before dividing GNIHR expression by GNRHR expression.
Radioimmunoassay
The RIAs used for progesterone and estradiol were coated tube kits (MP Biomedicals, Solon, OH). All protocols were performed as directed by the supplier. All samples were assayed in duplicate. All samples were measured in a single RIA for each hormone. Plasma samples were ether extracted and analyzed for progesterone to determine that hens were sampled outside of the PS. The culture media from the F1G and SWF cell cultures were assayed for progesterone and estradiol content, respectively. The standard curve was assessed for linearity as well as dilutional parallelism using serial plasma or culture media dilutions. The intra-assay coefficients of variation determined by pools run every 30 samples were 5.61% for progesterone and 6.63% for estradiol.
Statistics
All data were analyzed using SAS software (SAS Institute, Cary, NC). Normalized RT-qPCR data were log2-transformed before statistical analysis. A three-way ANOVA using the mixed models procedure (PROC MIXED) was conducted to compare the log2-transformed pituitary gene expression and the calculated GNIHR:GNRHR ratios between LEPH and HEPH. A two-way ANOVA using the mixed models procedure (PROC MIXED) was used to compare plasma hormone concentrations and culture media hormone concentrations between LEPH and HEPH. The least squares means for each group were compared using the test of least significant difference (PDIFF statement) when the ANOVA indicated an overall significance level of P < 0.05.
Results
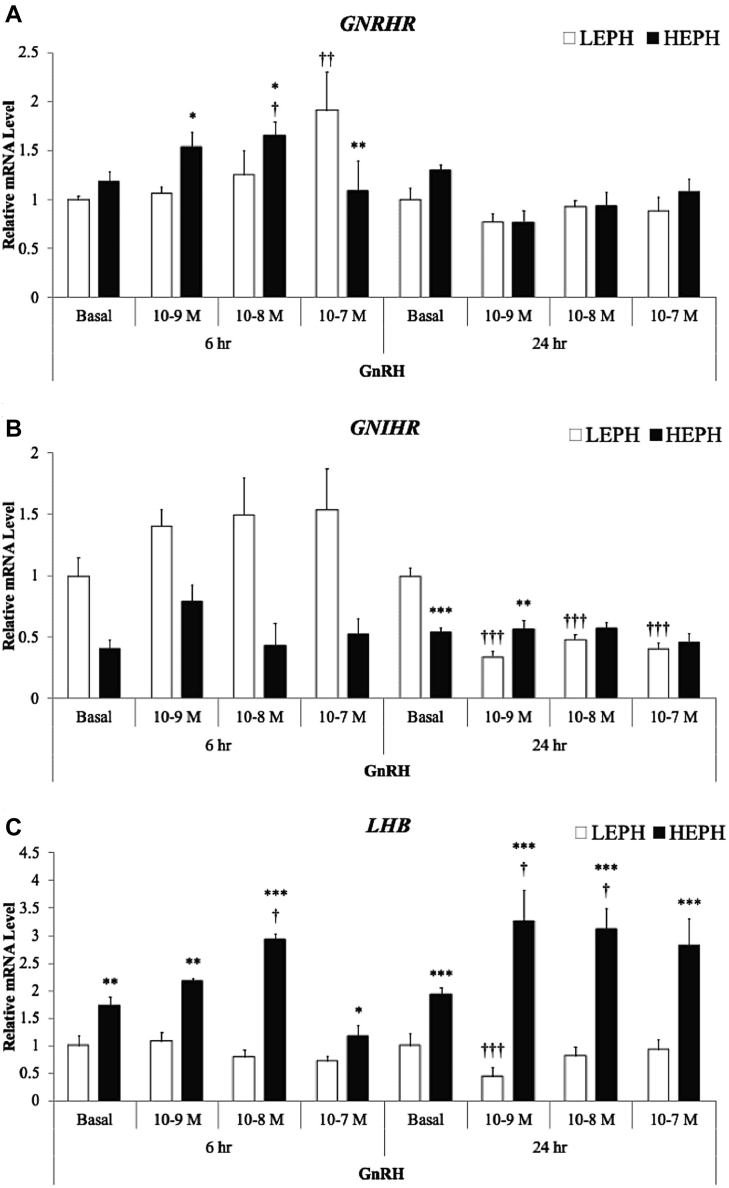
Expression of GNRHR in response to GnRH treatment is presented in Figure 1A. After GnRH treatment for 6 h, pituitary cells from HEPH showed higher GNRHR expression relative to cells from LEPH at 10−9 and 10−8 M GnRH, whereas cells from LEPH showed higher GNRHR expression relative to cells from HEPH at 10−7 M GnRH. Cells from LEPH showed increased GNRHR expression relative to the basal expression in response to GnRH treatment only at 10−7 M. Cells from HEPH showed increased GNRHR expression relative to the basal expression in response to GnRH treatment only at 10−8 M. Expression of GNRHR was not affected by GnRH treatment for 24 h.
Figure 1.
Relative pituitary expression of (A) gonadotropin-releasing hormone receptor (GNRHR), (B) gonadotropin-inhibitory hormone receptor (GNIHR), and (C) the beta subunit of luteinizing hormone (LHB) after gonadotropin-releasing hormone (GnRH) treatment in low-egg-producing hens (LEPH) and high-egg-producing hens (HEPH). Normalized data are presented relative to LEPH basal expression for each gene. Significant expression differences between LEPH and HEPH for a given condition are denoted with an asterisk (∗P ≤ 0.05, ∗∗P ≤ 0.01, and ∗∗∗P ≤ 0.001), whereas, significant differences between basal and a specific GnRH treatment for a given egg production group are denoted with a dagger (†P ≤ 0.05, ††P ≤ 0.01, and †††P ≤ 0.001).
Expression of GNIHR in response to GnRH treatment is presented in Figure 1B. After GnRH treatment for 6 h, the GNIHR expression was significantly affected by egg production level, but a response to GnRH treatment was not seen. After GnRH treatment for 24 h, pituitary cells from LEPH showed higher GNIHR expression relative to cells from HEPH under basal conditions, but HEPH demonstrated higher GNIHR expression at 10−9 M GnRH. Cells from LEPH showed decreased GNIHR expression relative to the basal expression in response to all GnRH treatments. Cells from HEPH did not show a response in GNIHR expression to GnRH treatment.
Expression of LHB in response to GnRH treatment is presented in Figure 1C. After GnRH treatment for 6 h, pituitary cells from HEPH showed higher LHB expression relative to cells from LEPH at all GnRH concentrations. Cells from LEPH did not show a response in LHB expression after GnRH treatment. Cells from HEPH showed increased LHB expression relative to the basal expression in response to GnRH treatment at 10−8 M and decreased expression relative to the basal expression at 10−7 M GnRH. After GnRH treatment for 24 h, pituitary cells from HEPH showed higher LHB expression relative to cells from LEPH at all GnRH concentrations. Cells from LEPH showed decreased LHB expression after GnRH treatment at 10−9 M. Cells from HEPH showed increased LHB expression relative to the basal expression in response to GnRH treatment at 10−9 and 10−8 M.
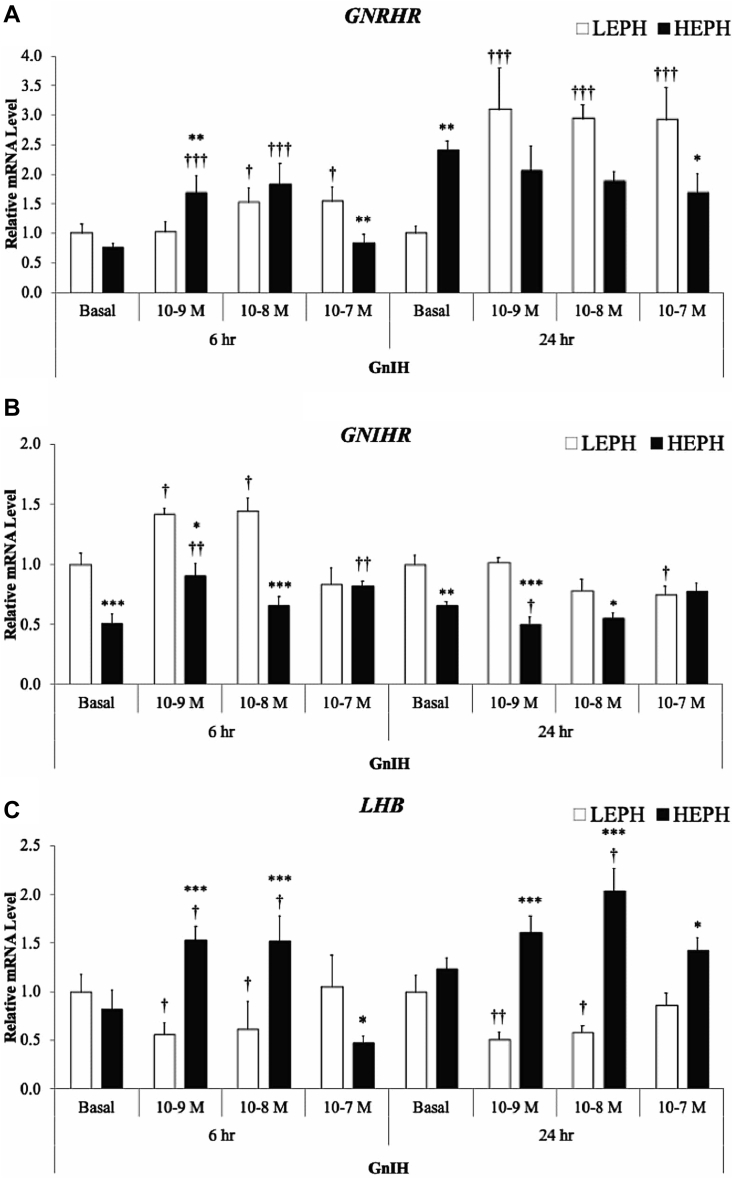
Expression of GNRHR in response to GnIH treatment is presented in Figure 2A. After GnIH treatment for 6 h, pituitary cells from HEPH showed higher GNRHR expression relative to cells from LEPH at 10−9 M GnIH, whereas cells from LEPH showed higher GNRHR expression relative to cells from HEPH at 10−7 M GnIH. Cells from LEPH showed increased GNRHR expression relative to the basal expression in response to GnIH treatment at 10−8 and 10−7 M. Cells from HEPH showed increased GNRHR expression relative to the basal expression in response to GnIH treatment at 10−9 and 10−8 M before returning to the basal expression. After GnIH treatment for 24 h, pituitary cells from HEPH showed higher GNRHR expression relative to cells from LEPH under basal conditions, whereas cells from LEPH showed higher GNRHR expression relative to cells from HEPH at 10−7 M GnIH. Cells from LEPH showed increased GNRHR expression relative to the basal expression in response to all the GnIH concentrations, whereas cells from HEPH did not show changes in GNRHR expression across GnIH treatments.
Figure 2.
Relative pituitary expression of (A) gonadotropin-releasing hormone receptor (GNRHR), (B) gonadotropin-inhibitory hormone receptor (GNIHR), and (C) the beta subunit of luteinizing hormone (LHB) after gonadotropin-inhibitory hormone (GnIH) treatment in low-egg-producing hens (LEPH) and high-egg-producing hens (HEPH). Normalized data are presented relative to LEPH basal expression for each gene. Significant expression differences between LEPH and HEPH for a given condition are denoted with an asterisk (∗P ≤ 0.05, ∗∗P ≤ 0.01, and ∗∗∗P ≤ 0.001), whereas, significant differences between basal and a specific GnIH treatment for a given egg production group are denoted with a dagger (†P ≤ 0.05, ††P ≤ 0.01, and †††P ≤ 0.001).
Expression of GNIHR in response to GnIH treatment is presented in Figure 2B. After GnIH treatment for 6 h, pituitary cells from LEPH showed higher GNIHR expression relative to cells from HEPH at 0, 10−9, and 10−8 M GnIH. Cells from LEPH showed increased GNIHR expression relative to the basal expression in response to GnIH treatment at 10−9 and 10−8 M before returning to the basal expression. Cells from HEPH showed increased GNIHR expression relative to the basal expression in response to GnIH treatment at 10−9 and 10−7 M. After GnIH treatment for 24 h, pituitary cells from LEPH showed higher GNIHR expression relative to cells from HEPH at 0, 10−9, and 10−8 M GnIH. Cells from LEPH showed decreased GNIHR expression relative to the basal expression in response to GnIH treatment at 10−7 M. Cells from HEPH showed decreased GNIHR expression relative to the basal expression before returning to the basal expression in response to GnIH treatment at 10−9 M.
Expression of LHB in response to GnIH treatment is presented in Figure 2C. After GnIH treatment for 6 h, pituitary cells from HEPH showed higher LHB expression relative to cells from LEPH at 10−9 and 10−8 M GnIH, while LEPH showed higher LHB expression at 10−7 M GnIH. Cells from LEPH showed decreased LHB expression relative to the basal expression in response to GnIH treatment at 10−9 and 10−8 M before returning to the basal expression. Surprisingly, cells from HEPH showed increased LHB expression relative to the basal expression in response to GnIH treatment at 10−9 and 10−8 M before reducing expression at 10−7 M GnIH. Similarly, after GnIH treatment for 24 h, pituitary cells from HEPH showed increased LHB expression relative to cells from LEPH at 10−9, 10−8, and 10−7 M GnIH. Cells from LEPH showed decreased LHB expression relative to the basal expression in response to GnIH treatment at 10−9 and 10−8 M before returning to the basal expression. Cells from HEPH showed increased LHB expression relative to the basal expression in response to all GnIH concentrations.
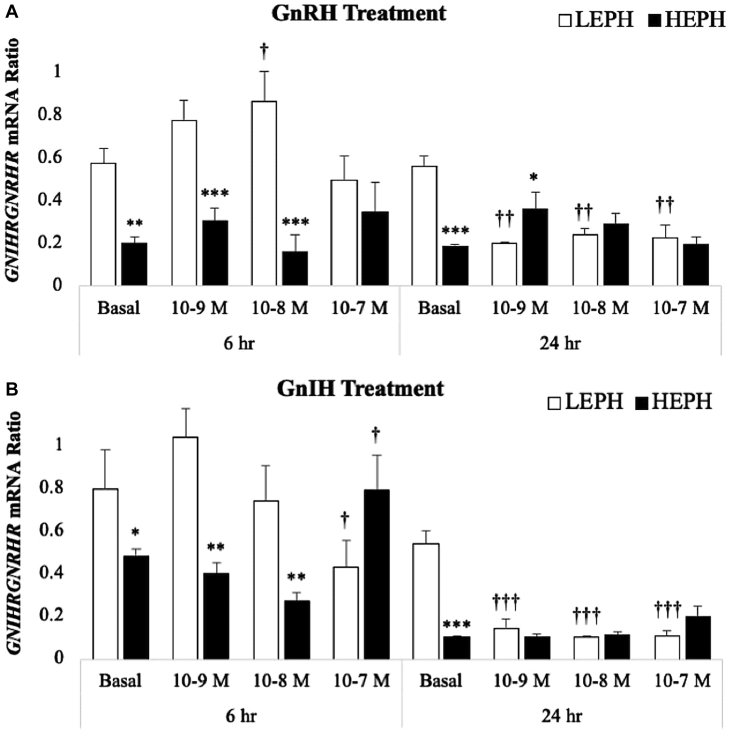
The calculated ratio of GNIHR expression to GNRHR expression after GnRH or GnIH treatment is presented in Figure 3A and Figure 3B, respectively. Under basal conditions, regardless of hormone treatment or time point, pituitary cells from LEPH exhibit an increased GNIHR:GNRHR compared with cells from HEPH. After both GnRH treatment and GnIH treatment for 6 h, the GNIHR:GNRHR ratio in the pituitary cells from HEPH was lower than that seen in the pituitary cells from LEPH at all treatment concentrations except at the highest treatment concentration of GnRH and GnIH. After GnRH treatment for 6 h, the GNIHR:GNRHR ratio in pituitary cells from LEPH further increased from the basal levels in response to GnRH treatment at 10−8 M, whereas the GNIHR:GNRHR ratio did not change significantly from the basal levels in pituitary cells from HEPH. Treatment with GnIH at the highest treatment concentration for 6 h decreased the GNIHR:GNRHR ratio in cells from LEPH from the basal levels but increased the GNIHR:GNRHR ratio from the basal levels in cells from HEPH. Interestingly, treatment with GnRH or GnIH at all treatment concentrations for 24 h reduced the GNIHR:GNRHR ratio in the pituitary cells from LEPH to a similar ratio seen in the pituitary cells from HEPH. On the other hand, treatment with GnRH or GnIH for 24 h did not significantly change the GNIHR:GNRHR ratio in pituitary cells from HEPH.
Figure 3.
Gonadotropin-inhibitory receptor (GNIHR) to gonadotropin-releasing hormone (GNRHR) mRNA ratio following (A) gonadotropin-releasing hormone (GnRH) treatment and (B) gonadotropin-inhibitory hormone (GnIH) treatment. Normalized mRNA levels were used to calculate the GNIHR:GNRHR ratio. Significant ratio differences between LEPH and HEPH for a given condition are denoted with an asterisk (∗P ≤ 0.05, ∗∗P ≤ 0.01, and ∗∗∗P ≤ 0.001), whereas, significant ratio differences between basal and a specific gonadotropin treatment for a given egg production group are denoted with a dagger (†P ≤ 0.05, ††P ≤ 0.01, and †††P ≤ 0.001).
F1G cell progesterone production in response to LH treatment is presented in Figure 4. Basal progesterone production from F1G cells from LEPH and HEPH did not differ significantly. F1G cells from HEPH responded to LH treatment with increased progesterone production compared with the basal levels at 10, 100, and 1,000 ng/mL, whereas F1G cells from LEPH did not respond to LH treatment with increased progesterone production compared to basal levels at the 4 experimental concentrations. Progesterone production differed significantly between F1G cells from LEPH and HEPH after treatment with 10, 100, and 1,000 ng/mL of LH.
Figure 4.
Progesterone production in F1 follicle granulosa cells (F1G) from low-egg-producing hens (LEPH) and high-egg-producing hens (HEPH) after luteinizing hormone (LH) treatment. Significant differences between LEPH and HEPH for a given condition are denoted with an asterisk (∗P ≤ 0.05, ∗∗P ≤ 0.01, and ∗∗∗P ≤ 0.001), whereas, significant differences between basal and a specific LH treatment for a given egg production group are denoted with a dagger (†P ≤ 0.05, ††P ≤ 0.01, and †††P ≤ 0.001).
Small white follicle cell estradiol production in response to FSH treatment is presented in Figure 5. Basal estradiol production from SWF cells from LEPH and HEPH did not differ significantly. Small white follicle cells from HEPH responded to FSH treatment with increased estradiol production compared with the basal levels at 10, 100, and 1,000 ng/mL, whereas SWF cells from LEPH only responded to FSH treatment with increased estradiol production compared with the basal levels at 100 and 1,000 ng/mL. Estradiol production differed significantly between SWF cells from LEPH and HEPH after treatment with 10, 100, and 1,000 ng/mL of FSH.
Figure 5.
Estradiol production in small white follicle (SWF) cells from low-egg-producing hens (LEPH) and high-egg-producing hens (HEPH) after follicle-stimulating hormone (FSH) treatment. Significant differences between LEPH and HEPH for a given condition are denoted with an asterisk (∗P ≤ 0.05, ∗∗P ≤ 0.01, and ∗∗∗P ≤ 0.001), whereas, significant differences between basal and a specific FSH treatment for a given egg production group are denoted with a dagger (†P ≤ 0.05, ††P ≤ 0.01, and †††P ≤ 0.001).
Discussion
The present study showed that pituitary, F1G, and SWF cells from LEPH and HEPH respond differently to HPG axis hormone stimulation and inhibition. Previous studies have focused on HPG axis hormone responses in the pituitary and ovarian cells during the initiation of egg production or during gonadal regression in both chicken and turkey hens (Porter et al., 1991b; Guémené and Williams, 1999). In addition, previous studies have compared ovarian responses with gonadotropin stimulation in broiler and layer line chicken hens (Hocking and McCormack, 1995). This is the first study to examine HPG axis hormone response differences in hens with differential egg production from the same flock. Differences in in vitro responses to stimulation and inhibition coupled with previously identified differences in HPG axis gene expression suggest core differences in the regulation and responsiveness of HPG axis function between LEPH and HEPH.
Under basal conditions, cells isolated from LEPH and HEPH exhibited several key differences that could ultimately impact the responsiveness of the cell to reproductive stimulation and inhibition. Across all time points, LEPH pituitary cells displayed increased basal mRNA levels for GNIHR. Increased GNIHR and GNIH mRNA levels in LEPH under basal conditions have been previously reported (Brady et al., 2020). The GNIHR mRNA levels have been inversely correlated with GNRHR mRNA levels in isolated chicken hen pituitaries across various time points of the reproductive cycle (Shimizu and Bedecarrats, 2006). Although not consistent at all basal time points, pituitary cells from HEPH did exhibit increased basal GNRHR mRNA levels compared to pituitary cells from LEPH. Although plasma estradiol was not measured in this study, our previous study found higher plasma estradiol levels in HEPH than in LEPH (Brady et al. 2020). Estradiol injection increased the equilibrium dissociation constant and maximum binding capacity of GnRHR in the pituitary of laying chicken hens (Kawashima et al. 1993). In addition, estradiol treatment of chicken pituitary cells in vitro decreased GNIHR mRNA levels (Maddineni et al., 2008). It is plausible that GNIHR regulation of GNRHR mRNA levels in LEPH and estradiol regulation of GnRHR and GNIHR levels/binding in HEPH could result in the significantly different ratios of the releasing factor receptors in the pituitary cells seen under basal conditions in the present study. The GnIHR:GnRHR ratio in pituitary cells has been shown to impact receptibility to both GnRH and GnIH during sexual maturation, initiation of egg lay, and cessation of egg lay (Shimizu and Bedecarrats, 2006). The fluctuation of this ratio during the ovulatory cycle and the possible role of this ratio in releasing factor sensitivity during the ovulatory cycle remains unclear and warrants further investigation. Previous studies in laying hens reported a GnIHR:GnRHR ratio of 1:2 in hens during peak egg production (Shimizu and Bedecarrats, 2006). In the present study, under basal conditions, pituitary cells from LEPH exhibited an average GNIHR:GNRHR ratio of 1:1.6, whereas pituitary cells from HEPH exhibited an average GNIHR:GNRHR ratio of 1:4. The differences in GNIHR:GNRHR ratios under basal conditions between hens with different egg production levels may lead to different levels of responsiveness to stimulatory and inhibitory inputs in these groups of hens.
High-egg-producing hens also tended to display increased basal LHB mRNA levels compared with LEPH, although this finding was not consistent across all basal wells and requires further experimental testing to draw conclusions. Inconsistencies in basal mRNA levels across similar time points could be due to variations in ovulation intervals between hens, whereas inconsistencies in basal mRNA levels across different time points may be due to time-related regulation of the genes examined. In the follicle cells examined, LEPH and HEPH did not differ in basal progesterone or estradiol production. However, in our previous study, we found that LEPH exhibited lower mRNA levels for several key genes involved in progesterone production in the F1G and in estradiol production in the SWFs when compared with HEPH (Brady et al. 2020). Higher expression of steroidogenic genes in HEPH under basal conditions may allow for a more rapid response in the follicle cells when stimulated by LH or FSH.
The GnRH stimulation of pituitary cells impacted mRNA levels for GNRHR, GNIHR, and LHB in both LEPH and HEPH, although cells from hens with different egg production levels showed dissimilar responses to GnRH treatment, which could ultimately result in differing ovulation frequencies. Short-term GnRH treatment increased GNRHR mRNA levels in pituitary cells from both groups of hens, but increases in cells from HEPH were seen at lower treatment concentrations compared with cells from LEPH. Although the effects of GnRH treatment on GNRHR expression have not been examined in avian species, a similar increase in GNRHR mRNA after a short-term GnRH treatment was seen in isolated rat pituitary cells (Janjic et al. 2019). The upregulation of GNRHR mRNA levels in HEPH pituitary cells at lower GnRH treatment concentrations could indicate that cells from HEPH are more responsive to GnRH treatment than cells from LEPH. In cells from HEPH, GNIHR mRNA levels were not impacted by GnRH treatment at either time point, but long-term GnRH treatment downregulated GNIHR in cells from LEPH, reducing GNIHR mRNA levels to those seen in cells from HEPH. The GnRH downregulation of GNIHR mRNA has also been reported in fish but the opposite trend is seen in rats (Choi et al. 2016; Sukhbaatar et al., 2014). A higher GnIHR:GnRHR ratio in pituitary cells from LEPH under basal conditions, as was seen at a transcriptional level in the present study, could lead to cells from LEPH being less responsive to GnRH treatment. In addition, in the present study, prolonged exposure to GnRH or GnIH drastically improved the GNIHR:GNRHR ratio in pituitary cells from LEPH to levels seen in pituitary cells from HEPH. The requirement of a long-term GnRH treatment to downregulate the high GNIHR mRNA levels seen in cells from LEPH may represent this ratio being adjusted to be more responsive to GnRH treatment. Pituitary cells from HEPH responded to short-term and long-term GnRH treatment with upregulation of LHB mRNA levels. This response has been previously shown in avian species, including desensitization at high GnRH concentrations (Proudman et al., 2006; Wilson et al., 1989; King et al., 1986). Pituitary cells from LEPH did not show upregulation of LHB mRNA after GnRH treatment. It is possible that increased basal expression of GNIHR in cells from LEPH prevented GnRH stimulation of LHB mRNA levels. In chickens, GnIHR signaling has been reported to block GnRHR signaling in pituitary gonadotrophs, as GnIHR has been shown to couple to Gai and block the activation of adenylyl cyclase normally seen during GnRHR coupling to Gas (Son et al., 2012; Shimizu and Bedecarrats, 2010). Increased responsiveness to GnRH in cells from HEPH, in terms of LHB and GNRHR expression, may result in increased ovulation rates in these hens, through increased gonadotropin production. Conversely, increased GNIHR expression in cells from LEPH may prevent GnRH stimulation, resulting in less frequent ovulation.
Although GnIH treatment resulted in similar expression patterns for GNRHR and GNIHR in pituitary cells from both groups of hens, LHB showed opposite responses in cells from LEPH and HEPH. Upregulation of GNRHR mRNA levels required higher treatment concentration of GnIH and longer exposure to GnIH in LEPH. While GNIHR and GNRHR mRNA levels were inversely correlated in isolated pituitaries, the impact of in vitro GnIH treatment on GNRHR mRNA levels has not been determined, although in vitro activation of GnIHR through GnIH binding has been shown to reduce GnRHR function in chicken pituitary cells (Bédécarrats et al., 2009). It is plausible that GnIH treatment causes a decrease in GnRHR function through increased GnIHR signaling and that pituitary cells compensate for the reduced function by upregulating GNRHR mRNA levels. If this hypothesis is correct, the requirement of higher GnIH treatment concentrations and longer-term GnIH exposure to upregulate GNRHR mRNA levels seen in pituitary cells from LEPH would suggest that LEPH are not as capable of compensating for reduced GnRHR function as HEPH. Short-term GnIH treatment has been shown to increase GNIHR expression in the chicken (Maddineni et al., 2008). Cells from LEPH and HEPH both showed short-term upregulation of GNIHR after GnIH treatment, although cells from LEPH displayed higher overall GNIHR mRNA levels than cells from HEPH. Expression of GNIHR after long-term exposure to GnIH has not been assessed in avian species but in the present study resulted in depressed GNIHR mRNA levels in cells from both groups of hens. However, higher GnIH treatment concentrations were required to depress GNIHR mRNA levels in cells from LEPH. Downregulation of several G-protein-coupled receptors after prolonged ligand exposure has been previously reported and could account for the downregulation of GNIHR mRNA levels seen in pituitary cells from both groups of hens after long-term GnIH treatment (reviewed in Gainetdinov et al., 2004). If this hypothesis is correct, it would suggest that the GnIHR in pituitary cells from HEPH is desensitized at a lower concentration of GnIH than is seen in cells from LEPH, possibly leading to the downregulation of HPG axis inhibitory pathways at lower circulating concentrations of GnIH in HEPH. Conflicting reports within and between species exist on the effect of GnIH on gonadotropin production and release. In the quail, GnIH has been shown to depress LHB mRNA levels and LH release (Tsutsui et al., 2000; Ubuka et al., 2006). In studies examining sexually mature chickens, one study found that GnIH did not decrease LH release, whereas another study found that GnIH decreased LH release but not LHB mRNA levels (Ciccone et al., 2004; Maddineni et al., 2008). A reduction in LHB mRNA levels was seen in cells from LEPH after GnIH treatment but, surprisingly, upregulation of LHB mRNA levels in response to GnIH treatment was seen in cells from HEPH. This is the first report of GnIH increasing LHB mRNA levels and is also the first study to examine the effects of GnIH in a turkey hen. Chicken GnIH was utilized in this study and shares 90% similarity at the peptide level to the proposed turkey GnIH sequence, whereas the chicken and putative turkey GnIHR protein sequences shares 94.5% similarity (Ubuka and Tsutsui, 2014). Although issues with GnIH bioactivity are not likely, due to the high conservation of the ligand and receptor sequences, the species differences should be noted. Another possible explanation for the opposite responses of LHB to GnIH in hens with differing egg production levels are structural or functional differences in the GNIHR receptor resulting in alternative coupling or additional factors which prevent the repression of LHB transcription in HEPH downstream of coupling to Gai. It should also be noted that GnIH treatment was only evaluated at the mRNA level in this study and that the impact of GnIH treatment on LH release may be different from the observed effects on LHB mRNA levels. If GnIH upregulates LHB mRNA levels and LH release in HEPH, this could be a possible mechanism to decrease ovulation intervals and increase egg production rates.
A dose-dependent progesterone production response to LH treatment was seen in F1G cells from HEPH, whereas cells from LEPH did not respond to LH treatment in terms of progesterone production. Results seen in cells from HEPH are consistent with previously published studies in chicken and turkey hens (Bakst et al., 1983; Porter et al., 1991a). Progesterone production from the F1G cells is imperative for the PS to occur and induce ovulation. Lack of response to LH stimulation in F1G cells from LEPH may contribute to decreased ovulation rates seen in this group of hens. The lack of response seen in F1G cells from LEPH could be due to the decreased basal mRNA levels of STAR and cholesterol side-chain cleavage enzyme (CYP11A1) reported in LEPH (Brady et al. 2020). STAR and CYP11A1 are required for progesterone production, with transport of cholesterol into the inner mitochondrial membrane by STAR as the rate-limiting step of progesterone production (Johnson et al., 2002). The lack of response seen in F1G cells from LEPH could also be due to decreased LHCGR expression. Previous studies did not observe differences in LHCGR mRNA levels in F1G cells isolated from LEPH and HEPH, although mRNA levels and protein levels are often not analogous (Brady et al. 2020). An estradiol production response to FSH treatment was seen in cells isolated from the SWFs of LEPH and HEPH. However, SWF cells from HEPH responded at a lower dose of FSH treatment and responded with significantly higher estradiol production when compared with cells from LEPH. Results seen in HEPH were also consistent with previous studies in chicken and turkey SWFs (Etches and Cheng, 1981; Porter et al., 1989b). Similar to F1G cells from LEPH, SWF cells from LEPH were also found to exhibit decreased mRNA levels of all 3 key genes involved in estradiol production, which could slow the response of SWF cells from LEPH to FSH stimulation (Brady et al., 2020). Differences in FSHR mRNA levels in SWF cells from LEPH and HEPH were not found in previous studies (Brady et al., 2020); however, differences in FSHR protein levels cannot be ruled out and could contribute to the response difference to FSH treatment that were seen in hens with different egg production levels. Follicle cells from LEPH and HEPH appear to respond differently to gonadotropin stimulation, impacting the steroid hormone production capabilities in these groups of hens. Increased progesterone and estradiol production in ovarian cells from HEPH may lead to increased egg production levels and decreased ovulation intervals through local ovarian effects or through HPG axis feedback at the hypothalamic and pituitary levels through regulation of releasing factors, releasing factor receptors, and gonadotropins.
The HPG axis response differences between LEPH and HEPH seen in the present study may also be explained on a more global level by genetic variance in regions impacting the regulation of key reproductive genes, by regulation of the HPG axis through hormones or transcription factors not examined in this study, or by differences in ovulation intervals leading to stage differences in the ovulatory cycle at sampling. Single-nucleotide polymorphisms (SNPs) are the most common type of genetic variation and can impact transcription, translation, and post-translational function of a gene product. Recent studies in chickens have found SNPs associated with increased egg production localized to key genes of the HPG axis, such as GNIH and GNRHR (Fatemi et al., 2012; Hu et al., 2015). Investigation into the localization and functional effects of significant SNPs between hens with differing egg production levels may help to understand the noted differences of hormone responsiveness between LEPH and HEPH. Several additional factors that were not examined in this study have been shown previously to impact HPG axis function at the neuroendocrine and ovarian levels in avian species, such as activins/inhibins, thyroid hormones, androgens, and prolactin. Activins/inhibins, thyroid hormones, and androgens act locally at the ovarian level to regulate steroidogenesis and feedback at the neuroendocrine level to regulate releasing factors and gonadotropin production (Lovell et al., 2001; Rangel et al., 2009; Sechman et al., 2009). In addition, prolactin is of particular interest in turkey hens because of the role it plays in broodiness and ovarian regression (Chaiseha and El Halawani, 2015). Finally, possible differences in the amount of time between ovulations have not been established between LEPH and HEPH. Previously, HEPH were found to have longer durations of egg lay and shorter durations without egg lay, but differences in ovulation intervals could also contribute to the egg production levels seen (Brady et al. 2020). Changes in hypothalamic releasing factor activity and receptibility have been established during the ovulatory cycle in avian and mammalian species (Gibson et al., 2008; Bédécarrats et al., 2016). If ovulation intervals are different between LEPH and HEPH, HEPH would have been further along in the ovulatory cycle than LEPH, which could allow HEPH to be more receptive to the stimulatory effects of the HPG axis while leaving LEPH more susceptible to the inhibitory effects of the HPG axis. This also could help explain inconsistencies among basal LHB mRNA levels in HEPH. If the ovulation interval is shorter in HEPH, sampling of hens closer to the PS, with elevated LHB mRNA levels yet basal plasma progesterone levels, would be more likely in HEPH than in LEPH.
In summary, HEPH displayed increased responsiveness to GnRH in pituitary cells, to LH in F1G cells, and to FSH in SWF cells. On the other hand, pituitary cells from LEPH displayed increased responsiveness to the inhibitory properties of GnIH, whereas pituitary cells from HEPH showed paradoxical positive responses to GnIH treatment. These findings demonstrate that HPG axis responsiveness differs between LEPH and HEPH, with LEPH favoring the inhibitory pathways of the axis and HEPH favoring the stimulatory pathways of the axis. Understanding how HPG axis hormone responsiveness ultimately impacts egg production on a molecular level would be imperative to improve the reproductive efficiency of LEPH in the turkey industry. Dissecting the egg-production–associated differences in responsiveness of HPG axis to hormonal stimulation and inhibition, as well as the crosstalk between these 2 pathways, requires a further understanding of the role of GnRH/GnIH and their associated receptors in the regulation of the ovulatory cycle, the influences of pathways outside of the HPG axis on this regulation, and species-specific impacts on the function and feedback mechanisms of GnRH and GnIH.
Acknowledgments
Funding was provided by Agriculture and Food Research Initiative Competitive Grant no. 2019-67015-29472 from the USDA National Institute of Food and Agriculture to T.E. Porter.
Conflict of Interest Statement: The authors declare that they have no conflicts of interest.
References
- Bahr J.M., Wang S.C., Huang M.Y., Calvo F.O. Steroid concentrations in isolated theca and granulosa layers of preovulatory follicles during the ovulatory cycle of the domestic hen. Biol. Reprod. 1983;29:326–334. doi: 10.1095/biolreprod29.2.326. [DOI] [PubMed] [Google Scholar]
- Bakst M., Scanes C., Phillips A. Steroidogenic and morphological responses of hen granulosa cells to luteinizing hormone in vitro. Scan. Electron Microsc. Pt. 1983;4:1931–1938. [PubMed] [Google Scholar]
- Brady K., Porter T.E., Liu H.C., Long J.A. Characterization of gene expression in the hypothalamo-pituitary-gonadal axis during the preovulatory surge in the Turkey hen. Poult. Sci. 2019;98:7041–7049. doi: 10.3382/ps/pez437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady K., Porter T.E., Liu H.C., Long J.A. Characterization of the hypothalamo-pituitary-gonadal axis in low and high egg producing Turkey hens. Poult. Sci. 2020;99:1163–1173. doi: 10.1016/j.psj.2019.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédécarrats G.Y. Control of the reproductive axis: Balancing act between stimulatory and inhibitory inputs. Poult. Sci. 2014;94:810–815. doi: 10.3382/ps/peu042. [DOI] [PubMed] [Google Scholar]
- Bédécarrats G.Y., McFarlane H., Maddineni S.R., Ramachandran R. Gonadotropin-inhibitory hormone receptor signaling and its impact on reproduction in chickens. Gen. Comp. Endocrinol. 2009;163:7–11. doi: 10.1016/j.ygcen.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Bédécarrats G.Y., Baxter M., Sparling B. An updated model to describe the neuroendocrine control of reproduction in chickens. Gen. Comp. Endocrinol. 2016;227:58–63. doi: 10.1016/j.ygcen.2015.09.023. [DOI] [PubMed] [Google Scholar]
- Chaiseha Y., El Halawani M.E. Brooding. In: Scanes C.G., editor. Sturkie's Avian Physiology. 6th rev. Academic Press; Cambridge, MA: 2015. pp. 717–738. [Google Scholar]
- Choi Y.J., Kim N.N., Habibi H.R., Choi C.Y. Effects of gonadotropin inhibitory hormone or gonadotropin-releasing hormone on reproduction-related genes in the protandrous cinnamon clownfish, Amphiprion melanopus. Gen. Comp. Endocrinol. 2016;235:89–99. doi: 10.1016/j.ygcen.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Ciccone N.A., Dunn I.C., Boswell T., Tsutsui K., Ubuka T., Ukena K., Sharp P.J. Gonadotrophin inhibitory hormone depresses gonadotrophin alpha and follicle-stimulating hormone beta subunit expression in the pituitary of the domestic chicken. J. Neuroendocrinol. 2004;16:999–1006. doi: 10.1111/j.1365-2826.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- Etches R.J., Cheng K.W. Changes in the plasma concentrations of luteinizing hormone, progesterone, oestradiol and testosterone and in the binding of follicle-stimulating hormone to the theca of follicles during the ovulation cycle of the hen (Gallus domesticus) J. Endocrinol. 1981;91:11–22. doi: 10.1677/joe.0.0910011. [DOI] [PubMed] [Google Scholar]
- Fatemi S.A., Mehrabani-Yeganeh H., Nejati-Javaremi A., Niknafs S.H. Association of neuropeptide Y and gonadotrophin-releasing hormone receptor gene SNPs with breeding value for growth and egg production traits in Mazandaran native chickens. Genet. Mol. Res. 2012;11:2539–2547. doi: 10.4238/2012.July.10.9. [DOI] [PubMed] [Google Scholar]
- Gainetdinov R.R., Premont R.T., Bohn L.M., Lefkowitz R.J., Caron M.G. Desensitization of G protein-coupled receptors and neuronal functions. Annu. Rev. Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Gibson E.M., Humber S.A., Jain S., Williams W.P., Zhao S., Bentley G.E., Tsutsui K., Kriegsfeld L.J. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinol. 2008;149:4958–4969. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guémené D., Williams J.B. LH responses to chicken luteinizing hormone-releasing hormone I and II in laying, incubating, and out of lay Turkey hens. Domest. Anim. Endocrinol. 1999;17:1–15. doi: 10.1016/s0739-7240(99)00020-x. [DOI] [PubMed] [Google Scholar]
- Hocking P.M., McCormack H.A. Differential sensitivity of ovarian follicles to gonadotrophin stimulation in broiler and layer lines of domestic fowl. J. Reprod. Fertil. 1995;105:49–55. doi: 10.1530/jrf.0.1050049. [DOI] [PubMed] [Google Scholar]
- Hu Y.D., Huang Q.K., Zhu Q., Lan D., Feng Z.Q., Zhang L., Lan X., Ye L., Liu Y.P., He M., Pu H.B. Identification and association of single-nucleotide polymorphisms in gonadotropin-inhibitory hormone (GnIH) gene with egg production traits in Erlang mountainous chickens. Genet. Mol. Res. 2015;14:294–303. doi: 10.4238/2015.January.23.3. [DOI] [PubMed] [Google Scholar]
- Janjic M.M., Prévide R.M., Fletcher P.A., Sherman A., Smiljanic K., Abebe D., Bjelobaba I., Stojilkovic S.S. Divergent expression patterns of pituitary gonadotropin subunit and GnRH receptor genes to continuous GnRH in vitro and in vivo. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-56480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.L. Some Characteristics of small white ovarian follicles: Implications for Recruitment and Atresia in the domestic hen. Ornis. Scand. 1992;23:233–237. [Google Scholar]
- Johnson A.L., Solovieva E.V., Bridgham J.T. Relationship between steroidogenic acute regulatory protein expression and progesterone production in hen granulosa cells during follicle development. Biol. Reprod. 2002;67:1313–1320. doi: 10.1095/biolreprod67.4.1313. [DOI] [PubMed] [Google Scholar]
- Kawashima M., Kamiyoshi M., Tanaka K. Estrogen receptor binding in the hen hypothalamus and pituitary during the ovulatory cycle. Poult. Sci. 1993;72:839–847. doi: 10.3382/ps.0720839. [DOI] [PubMed] [Google Scholar]
- King J.A., Davidson J.S., Millar R.P. Desensitization to gonadotropin-releasing hormone in perifused chicken anterior pituitary cells. Endocrinol. 1986;119:1510–1518. doi: 10.1210/endo-119-4-1510. [DOI] [PubMed] [Google Scholar]
- Li L., Zhang Z., Peng J., Wang Y., Zhu Q. Cooperation of luteinizing hormone signaling pathways in preovulatory avian follicles regulates circadian clock expression in granulosa cell. Mol. Cell. Biochem. 2014;394:31–41. doi: 10.1007/s11010-014-2078-3. [DOI] [PubMed] [Google Scholar]
- Liu H.K., Nestor K.E., Long D.W., Bacon W.L. Frequency of luteinizing hormone surges and egg production rate in Turkey hens. Biol. Reprod. 2001;64:1769–1775. doi: 10.1095/biolreprod64.6.1769. [DOI] [PubMed] [Google Scholar]
- Lovell T.M., Knight P.G., Groome N.P., Gladwell R.T. Changes in plasma inhibin A levels during sexual maturation in the female chicken and the effects of active immunization against inhibin alpha-subunit on reproductive hormone profiles and ovarian function. Biol. Reprod. 2001;64:188–196. doi: 10.1095/biolreprod64.1.188. [DOI] [PubMed] [Google Scholar]
- Maddineni S., Ocón-grove O.M., Krzysik-Walker S.M., Hendricks G.L., Proudman J.A., Ramachandran R. Gonadotrophin-Inhibitory hormone receptor expression in the chicken pituitary gland: Potential influence of sexual maturation and ovarian steroids. J. Neuroendocrinol. 2008;20:1078–1088. doi: 10.1111/j.1365-2826.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- Ottinger M., Bakst M. Endocrinology of the avian reproductive system. J. Avian Med. Surg. 1995;9:242–250. [Google Scholar]
- Paster M.B. Avian reproductive endocrinology. Vet. Clin. North Am. Small Anim. Pract. 1991;21:1343–1359. doi: 10.1016/s0195-5616(91)50143-1. [DOI] [PubMed] [Google Scholar]
- Porter T.E., Hargis B.M., Silsby J.L., El Halawani M.E. Differential steroid production between theca interna and theca externa cells: a three-cell model for follicular steroidogenesis in avian species. Endocrinol. 1989;125:109–116. doi: 10.1210/endo-125-1-109. [DOI] [PubMed] [Google Scholar]
- Porter T.E., Hargis B.M., Silsby J.L., El Halawani M.E. Enhanced progesterone and testosterone secretion and depressed estradiol secretion in vitro from small white follicle cells of incubating Turkey hens. Gen. Comp. Endocrinol. 1989;74:400–405. doi: 10.1016/s0016-6480(89)80037-1. [DOI] [PubMed] [Google Scholar]
- Porter T.E., Hargis B.M., Silsby J.L., El Halawani M.E. Characterization of dissimilar steroid productions by granulosa, theca interna and theca externa cells during follicular maturation in the Turkey (Meleagris gallopavo) Gen. Comp. Endocrinol. 1991;84:1–8. doi: 10.1016/0016-6480(91)90058-e. [DOI] [PubMed] [Google Scholar]
- Porter T.E., Silsby J.L., Behnke E.J., Knapp T.R., El Halawani M.E. Ovarian steroid production in vitro during gonadal regression in the Turkey. I. Changes associated with incubation behavior. Biol. Reprod. 1991;45:581–586. doi: 10.1095/biolreprod45.4.581. [DOI] [PubMed] [Google Scholar]
- Proudman J.A., Scanes C.G., Johannsen S.A., Berghman L.R., Camp M.J. Comparison of the ability of the three endogenous GnRHs to stimulate release of follicle-stimulating hormone and luteinizing hormone in chickens. Dom. Anim. Endocrinol. 2006;31:141–153. doi: 10.1016/j.domaniend.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Rangel P.L., Rodríguez A., Rojas S., Sharp P.J., Gutierrez C.G. Testosterone stimulates progesterone production and STAR, P450 cholesterol side-chain cleavage and LH receptor mRNAs expression in hen (Gallus domesticus) granulosa cells. Reprod. 2009;138:961–969. doi: 10.1530/REP-09-0071. [DOI] [PubMed] [Google Scholar]
- Sechman A., Pawlowska K., Rzasa J. Influence of triiodothyronine (T(3)) on secretion of steroids and thyroid hormone receptor expression in chicken ovarian follicles. Dom. Anim. Endocrinol. 2009;37:61–73. doi: 10.1016/j.domaniend.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Bédécarrats G.Y. Identification of a novel pituitary-specific chicken gonadotropin-releasing hormone receptor and its splice variants. Biol. Reprod. 2006;75:800–808. doi: 10.1095/biolreprod.105.050252. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Bédécarrats G.Y. Activation of the chicken gonadotropin-inhibitory hormone receptor reduces gonadotropin releasing hormone receptor signaling. Gen. Comp. Endocrinol. 2010;167:331–337. doi: 10.1016/j.ygcen.2010.03.029. [DOI] [PubMed] [Google Scholar]
- Son Y.L., Ubuka T., Millar R.P., Kanasaki H., Tsutsui K. Gonadotropin-inhibitory hormone inhibits gnrh-induced gonadotropin subunit gene transcriptions by inhibiting AC/cAMP/PKA-dependent ERK pathway in LβT2 Cells. Endocrinol. 2012;153:2332–2343. doi: 10.1210/en.2011-1904. [DOI] [PubMed] [Google Scholar]
- Sukhbaatar U., Kanasaki H., Mijiddorj T., Oride A., Miyazaki K. Expression of gonadotropin-inhibitory hormone receptors in mouse pituitary gonadotroph LβT2 cells and hypothalamic. Endocr. J. 2014;61:25–34. doi: 10.1507/endocrj.ej13-0238. [DOI] [PubMed] [Google Scholar]
- Tsutsui K., Saigoh E., Ukena K., Teranishi H., Fujisawa Y., Kikuchi M., Ishii S., Sharp P.J. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem. Biophys. Res. Commun. 2000;275:661–667. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- Tsutsui K., Ubuka T., Yin H., Osugi T., Ukena K., Bentley G.E., Wingfield J.C. Mode of action and functional significance of avian gonadotropin-inhibitory hormone (GnIH): a review. J. Exp. Zool. A. Comp. Exp. Biol. 2006;305:801–806. doi: 10.1002/jez.a.305. [DOI] [PubMed] [Google Scholar]
- Ubuka T., Ukena K., Sharp P.J., Bentley G.E., Tsutsui K. Gonadotropin-inhibitory hormone inhibits gonadal development and maintenance by decreasing gonadotropin synthesis and release in male quail. Endocrinol. 2006;147:1187–1194. doi: 10.1210/en.2005-1178. [DOI] [PubMed] [Google Scholar]
- Ubuka T., Tsutsui K. Evolution of gonadotropin-inhibitory hormone receptor and its ligand. Gen. Comp. Endocrinol. 2014;209:148–161. doi: 10.1016/j.ygcen.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Wilson S.C., Cunningham F.J., Chairil R.A., Gladwell R.T. Maturational changes in the LH response of domestic fowl to synthetic chicken LHRH-I and -II. J. Endocrinol. 1989;123:311–318. doi: 10.1677/joe.0.1230311. [DOI] [PubMed] [Google Scholar]