Abstract
Antibiotics are one of the most important medical discoveries of the 20th century and will remain an essential tool for treating animal and human diseases in the 21st century. However, misuse of antibiotics imperils the development of animal husbandry and human health all over the world, and it is important to find reliable alternatives to antibiotics to reduce the use of antibiotics. In this study, 22 potential immunopotentiators were screened on the levels of apoptosis and inflammatory factor in duck embryo fibroblast cells (DEFs). The results indicated that interferon (IFN)-β and tumor necrosis factor-α gene transcriptions were significantly upregulated, while interleukin (IL)-2 and Bcl2 mRNA levels were significantly decreased during 22 immunopotentiators treatment. Besides, the expression level of IL-1β mRNA showed significant increase during dihydromyricetin, chlorogenic acid, naringin, imiquimod, thymopentin, β-D-Glucan, astragalus polysacharin, astragalus saponin I, astragalus flavone, curcumin, CpG-DNA-2, and LPS treatment. And the level of caspase 3 protein was significantly upregulated with treating chlorogenic acid, β-D-Glucan, astragalus polysacharin, astragalus flavone, curcumin, CpG-DNA-2, chicken IgG, LPS, and poly(I:C). These results indicated that chlorogenic acid, β-D-Glucan, astragalus flavone, CpG-DNA-2, and chicken IgG have the positive immune regulation effects on duck DEFs. Thus, the 5 immunopotentiators were chosen to further verify their immunomodulatory function in vivo. The results showed that the activity of serum AST was significantly downregulated during all immunopotentiators treatments excepting for β-D-Glucan, and the activities of serum IL12p40, IL-1β, IFN-α, and IFN-β were significantly increased compared with the control group. Five immunopotentiators also induced the duck's pattern recognition receptors and inflammatory factor gene expression. In addition, 5 immunopotentiators could facilitate the contents of serum caspase 3, iNOSm and COX2 and reduce the Bcl2. These results suggested that these 5 immunopotentiators could enhance duck innate immune responses. Taken together, our study not only screened out 5 kinds of duck innate immune immunopotentiators but also initially clarified their underlying mechanism of action, which provide a new insight for the development of efficient approaches to prevent the duck disease from pathogen infections.
Key words: duck, immunopotentiator, apoptosis, inflammatory cytokine, innate immunity
Introduction
Duck production is an important industry because of its high income and demand in the world. The data from the world Food and Agriculture Organization showed that the slaughtered meat ducks were valued at about 6.442 billion in 2019. However, with the rapid development of duck-raising technologies, especially the large-scale, intensive farming methods, the prevalence and harm of duck infectious diseases are becoming more and more serious, which seriously threatens animal production and human health (Casewell et al., 2003; Mathew et al., 2007; Millet and Maertens, 2011). At present, antibiotics are the main means to control the infectious diseases of ducks, but the overuse of antibiotics has not only become a breeding ground for drug-resistant bacteria but also caused pollution to the environment (Casewell et al., 2003). Therefore, reducing antibiotics in duck production has become an inevitable trend, which creates challenges for the animal feed and feed additive industries. Effective alternatives to antibiotics are urgently needed to help maintain current animal production levels without threatening public health, and this should stimulate new research (Dahiya et al., 2006; Millet and Maertens, 2011). Application of immunopotentiators to enhance duck's natural immune response is particularly concerned.
Immunopotentiators, also known as immunostimulant, can effectively increase animal resistance to infectious disease, not by enhancing specific immune responses but by enhancing nonspecific defense mechanisms. Therefore, application of these immunopotentiators to increase the immunocompetency and disease resistance of animals has aroused great attention and extensive research (Sakai, 1999). When the activity of the immune system is enhanced, the immunopotentiators' effect can be observed by promotion in the activity of macrophages, granulocytes lymphocytes, and so on (Jucker, 2001; Ueno et al., 2007). Recent studies have been reported in mouse (Saito et al., 1983), fish (Chen and Ainsworth, 2006; Jang et al., 2006), and pig (Hiss and Sauerwein, 2003). Hiss and Sauerwein (2003) have showed that treatment with β-glucan could enhance the pig's pattern recognition receptors (PRRs) titres to enhance the immunity of weaned piglets. Jang et al. (2006) reported that in vitro treatment with glycyrrhizin enhanced the respiratory burst activity of macrophages and the proliferative responses of lymphocytes from rainbow trout. Besides, phytoremedies seem to be a significant source as immunomodulatory agents. Ayurveda documents the role of “Rasayana” as a “system of rejuvenation” (Ali, 1998). Therefore, it seems to be fruitful to explore immunopotentiators for potential immunomodulatory activity in ducks.
The immunomodulatory mechanisms of immunopotentiators are complex. When treated with immunopotentiators, the body usually shows enhanced phagocytic cell activities. The activities of phagocytic cells can be detected by phagocytosis, killing, and chemotaxis. Enhancement of pathogen killing is most important in the macrophages treated with immunopotentiators (Sakai et al., 1993; Chen and Ainsworth, 2006). And the process of immunostimulation is desired to police up normal immunological functions (Ganeshpurkar and Saluja, 2017). This response is triggered by a proinflammatory signal from various components, of which macrophages are an essential part (Zhong et al., 2016). However, the exact mechanisms by which immunopotentiators trigger innate immune responses, leading to beneficial adaptive immune responses, are unknown.
The innate immune system plays an essential role in the early defense against pathogen infection. To screen the effect immunopotentiators have in enhancing the duck innate immune responses, first, we investigated the effects of 22 immunopotentiators in vitro cultures of duck embryo fibroblast cells (DEFs), in which the mRNA expression and protein levels of a panel of cytokines and apoptosis-inducing factors were studied by using quantitative real-time PCR (qRT-PCR) and Western blot. Then, the duckling application was tested in vivo, in which the selected immunopotentiators were injected in ducklings to investigate their effects on biochemical parameters, cytokines, and apoptosis-inducing factor gene expression and protein secretion in ducklings. The present study could provide a novel strategy for reliable alternatives to antibiotics in duck as well as in other animals.
Materials and methods
Ethics Approval and Consent to Participate
The animal experiment was reviewed and approved by the Institutional Animal Care and Use Committee of Yangzhou University (approval number: 151-2014). Procedures were performed in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals (Yangzhou University, China, 2012) and the Standards for the Administration of Experimental Practices (Jiangsu, China, 2008). We also confirm that all efforts were made to minimize suffering.
Cells and Reagents
DEFs were obtained from the American Type Culture Collection (VA). Cell cultures were grown in eagle's minimum essential medium (American Type Culture Collection) containing 10% fetal bovine serum (Gibco, NY). Cell cultures were maintained in an incubator at 37°C with 95% humidity and 5% of CO2. Saline was purchased from Kelun Pharmaceutical Co., Ltd. (Henan, China), dihydromyricetin, ribavirin, chlorogenic acid, naringen, imiquimod, thymopentin, thymosin α1, propolis, β-D-Glucan, astragalus polysacharin, astragalus saponin I, astragalus flavone, lycium barbarum polysaccharide, and levamisole were purchased from Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Mannan peptide was purchased from National Institutes for Food and Drug Control (Beijing, China); curcumin and LPS were purchased from Sigma-Aldrich (St. Louis, MO); CpG-DNA-1 (5′-ATGGCGTTGACGTTT-3′) and CpG-DNA-2 (5′-GATATGCGACCGATT-3′) were obtained from TSINGKE Biological Technology (Beijing, China); human IgG and chicken IgG were purchased from Solarbio (Beijing, China); and poly(I:C) was purchased from InvivoGen (San Diego, CA). All reagents were dissolved in sterile water, and the stock solution was kept at −20°C and protected from light until use.
Duckings and Sample Collection
One hundred and fifty one-day-old healthy Shaoxing ducklings were obtained from Guiliu Breeding Company (Zhoukou, Henan, China). The ducklings were randomly divided into 6 groups, each containing 25 ducklings. Then the 6 groups of ducklings were daily given subcutaneous injection in the neck with 0.5 mL of saline, 0.4 mL chlorogenic acid (1 mg/mL), 0.7 mL β-D-Glucan (1 mg/mL), 0.4 mL astragalus flavone (5 mg/mL), 0.5 mL CpG-DNA-2 (10 μg/mL), and 0.5 mL chicken IgG (1 mg/mL) for 3 d continuously. In the 18th d, after injection, 5 ducks were selected from each group and put to death by injection with pentobarbital sodium (100 mg/kg) and cervical dislocation for serum and bursa tissues collection. Then the samples were immediately snap-frozen in liquid nitrogen and stored at −80°C until needed, and the bursa tissues were placed also in a 4% formaldehyde solution for situ hybridization analysis.
Cellular Treatment
To compare the immunomodulatory activity of 22 immunopotentiators, DEFs were seeded in 6-well plates and grown for 24 h, then they were respectively incubated with 10 mg/mL of dihydromyricetin, 1 mg/mL of ribavirin, 10 mg/mL of chlorogenic acid, 1 mg/mL of naringen, 1 mg/mL of imiquimod, 1 mg/mL of mannan peptide, 5 mg/mL of thymopentin, 1 mg/mL of thymosin α1, 1 mg/mL of propolis, 1 mg/mL of β-D-Glucan, 1 mg/mL of astragalus polysacharin, 1 mg/mL of astragalus saponin I, 40 mg/mL of astragalus flavone, 1 mg/mL of lycium barbarum polysaccharide, 20 μmol/L of curcumin, 10 mg/mL of levamisole, 10 μmol/L of CpG-DNA-1, 10 μmol/L of CpG-DNA-2, 1 mg/mL of human IgG, 1 mg/mL of chicken IgG, 1 mg/mL of LPS, and 1 mg/mL of poly(I:C) for 24 h. Then DEFs from each group were collected for Western blot and qRT-PCR analysis. Saline-treated DEFs were used as control.
Enzyme-Linked Immunoassay
Enzyme-linked immunoassay (ELISA) analysis (double antibody sandwich method) was used to determine the levels of duck serum IL-12p40, IL-1β, IFN-α, and IFN-β. Duck blood was gathered, and supernatants were collected after centrifugation at 3,000 rpm for 20 min at 4°C and subjected to ELISA for detection of duck serum IL-12p40, IL-1β, IFN-α, and IFN-β. The concentration of IL-12p40, IL-1β, IFN-α, and IFN-β in the samples were measured using a multifunctional microplate reader (Tecan Infinite M200 PRO; Switzerland) and determined by comparing the optical density of the samples to the standard curve.
Situ Hybridization
IL-1β, IFN-α, and IFN-β expression in bursa were determined using digoxigenin-labeled locked nucleic acid probes (Exiqon, Woburn, MA). In in situ hybridization, the slides were incubated at 37°C for 2 min and fixed in 4% paraformaldehyde on ice for 15 min and then washed twice for 5 min in 1× phosphate-buffered saline (PBS). The fixed sections were subjected to acetylation for 10 min, followed by twice washing with PBS. The slides were then prehybridized for 2 h at room temperature, and IL-1β, IFN-α, and IFN-β hybridization was then carried out at 48°C overnight. After stringent washing using 5× saline sodium citrate for 10 min and 0.2× saline sodium citrate for 1 h, the slides were incubated in a blocking solution for 1 h at room temperature and then incubated with alkaline phosphatase–conjugated antibody to digoxigenin (Roche Diagnostics, Indianapolis, IN) at 4°C overnight. The next day, the slides were brought to room temperature and washed twice in PBS, followed by twice washing with alkaline phosphatase buffer, and then incubated in nitro tetrazolium blue chloride and 5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt in the dark until the prospective intensity of staining was reached.
qRT-PCR Analysis
Total RNA was isolated using TRIzol reagent (TAKARA, Dalian, China). qRT-PCR assays were performed using the ABI 7500 System (Applied Biosystems, Foster City, CA). Briefly, 1 μg of total RNA was used with gDNase (TIANGEN, Beijing, China) for reverse transcription according to the manufacturer's protocol. The amplification mixture (20 μL) contained 2 μL of cDNA, 0.4 μL of 10-μmol/L each primer, 10 μL of SYBR qPCR Master Mix, and 7.2 μL of RNA-free H2O. The primers are listed in Table 1. The reaction conditions included 1 cycle at 95°C for 5 min, followed by 35 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s and a final incubation at 72°C for 10 min. β-Actin was used as internal control to normalize gene expression studies. Each qRT-PCR reaction was carried out for 3 biological replicates. Relative changes in the levels of genes of interest were determined by the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Table 1.
Primers used in the study.
| Primer name | Primer sequence (5’→3′) | Annealing temperature (°C) | Application |
|---|---|---|---|
| qCOX-2-F | CACGCTCTGATTGTTGCC | 60 | qRT-PCR |
| qCOX-2-R | AGGATTTGTAGGGATGGG | ||
| qiNOS-F | CCACCAGGAGATGTTGAATATGTC | 60 | qRT-PCR |
| qiNOS-R | AGGATTTGTAGGGATGGG | ||
| qCaspase3-F | CTACTGCTCCAGGCTATTACT | 60 | qRT-PCR |
| qCaspase3-R | ACACTCTGCGATTTACACG | ||
| qBcl2-F | TCTCGCAGAGGGGATAC | 60 | qRT-PCR |
| qBcl2-R | ACATCTGGGCGAAGTCC | ||
| qIFN-β-F | CACCTCCTCCAACACCTCTT | 60 | qRT-PCR |
| qIFN-β-F | TGGAGGAAGTGTTGGATGCT | ||
| qIL-1β-F | TGGGCATCAAGGGCTACAAG | 60 | qRT-PCR |
| qIL-1β-R | GCTGTCGATGTCCCTCATGAC | ||
| qIL-2-F | GCCAAGAGCTGACCAACTTC | 60 | qRT-PCR |
| qIL-2-R | ATCGCCCACACTAAGAGCAT | ||
| qTNF-α-F | CACAGGACAGCCTATGCCAACAA | 60 | qRT-PCR |
| qTNF-α-R | CTGAACTGGGCGGTCATAAAATAC | ||
| qRIG-I-F | AGCAGCAGGCATAACTAAACTCA | 60 | qRT-PCR |
| qRIG-I-R | TCACTGTCAACATCTTTGGCATTA | ||
| qTLR3-F | GCAACACTCCGCCTAAGTATCA | 60 | qRT-PCR |
| qTLR3-R | CAGTAGAAAGCTATCCTCCACCCT | ||
| qTLR7-F | GACAACCTTTCCCAGAGCATTC | 60 | qRT-PCR |
| qTLR7-R | ACAGCCTTTTCCTCAGCCTAAC | ||
| qIFN-α-F | TCCTCCAACACCTCTTCGAC | 60 | qRT-PCR |
| qIFN-α-R | GGGCTGTAGGTGTGGTTCTG | ||
| β-actin-F | ATGTCGCCCTGGATTTCG | 60 | qRT-PCR |
| β-actin-R | CACAGGACTCCATACCCAAGAA |
Western Blot
For Western blot, total protein was obtained from tissues and DEFs using lysis buffer (Beyotime, Shanghai, China) following the manufacturer's instructions. Whole-cell extracts (30 μg) were separated by SDS-PAGE, transferred onto a polyvinylidene difluoride membrane (Millipore, Danvers, MA), blocked with 5% nonfat milk in TBS-Tween-20 (10-mmol/L Tris-HCl, pH 7.5, 150-mmol/L NaCl, 0.1% Tween-20), and incubated overnight at 4°C with specific primary antibodies against Bcl-2 (1:500; Abcam, Cambridge, UK), caspase 3 (1:1000; Abcam), TNF-α (1:500; Abcam), iNOS (1:1000; Abcam), COX2 (1:500; Bioybyt, Shanghai, China), and GAPDH (1:10000; Abcam). Then membranes were incubated for 1 h with appropriate horseradish peroxidase-conjugated secondary antimouse IgG, antirabbit IgG, and antigoat IgG (1:5000; Thermo Fisher Scientific, Bohemia, NY). Protein levels were quantified using the Image J software.
Statistical Analysis
Data were represented as mean ± SD. Statistical differences were assessed by t test analysis using SPSS 13.0 and GraphPad Prism 8 software. P < 0.05 was considered statistically significant.
Results
Screening of Immunopotentiators Based on Apoptosis-Inducing Factors and Cytokines of DEFs in mRNA Levels
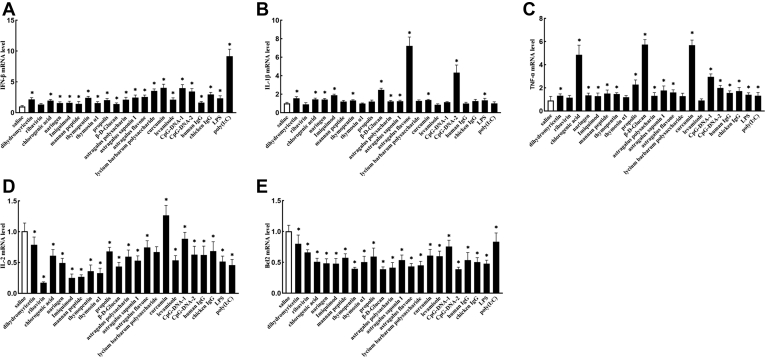
To determine the effects of 22 immunopotentiators on DEFs, we coincubated cell cultures with 22 immunopotentiators, and the mRNA levels of apoptosis and inflammatory factors were analyzed by qRT-PCR. In the case of DEFs, treatments with 22 immunopotentiators caused a significant high accumulation of IFN-β levels (Figure 1A). As for the mRNA levels of IL-1β, differences were significant in dihydromyricetin, chlorogenic acid, naringin, imiquimod, thymopentin, β-D-Glucan, astragalus polysacharin, astragalus saponin I, astragalus flavone, curcumin, CpG-DNA-2, and LPS treatments when compared with those in the control group (Figure 1B). Besides, the TNF-α mRNA level was significantly upregulated in all treated groups, except for groups of ribavirin, thymosin αl, lycium barbarum polysaccharide, and levamisole (Figure 1C). Interestingly, IL-2, an anti-inflammatory cytokine, was significantly increased in curcumin group, while other treated groups showed opposite results that the IL-2 mRNA level was significantly downregulated, when compared with those in the control group (Figure 1D). In addition, the expression level of Bcl2 mRNA was significantly decreased during treating with 22 immunopotentiators (Figure 1E).
Figure 1.
Effects of 22 kinds of immunopotentiators on the mRNA expression of apoptosis and cytokine gene in DEFs. mRNA Expressions of IFN-β (A), IL-1β (B), TNF-α (C), IL-2 (D), and Bcl2 (E) after treatment of DEFs with immunopotentiators for 24 h. Results are presented as the mean ± SD of 3 independent experiments. Asterisks (∗) represent significant differences.
Screening of Immunopotentiators Based on the Protein Level of Apoptosis Inducing Factors and Cytokines in DEFs
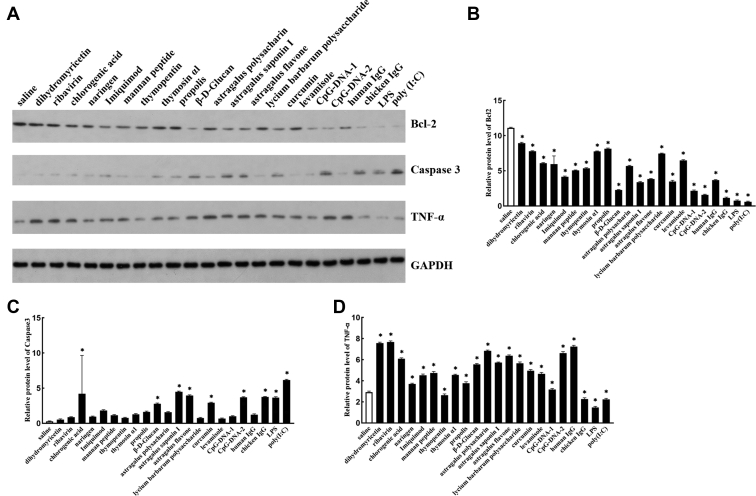
To further determine the effect of 22 immunopotentiators in DEFs, we investigated the protein level of apoptosis and inflammatory factor with Western blot. The results indicated that compared with the control group, the Bcl2 levels were significantly decreased during treating with 22 immunopotentiators (Figures 2A, 2B). A significant increase in the index of caspase 3 protein was observed in the groups of chlorogenic acid, β-D-Glucan, astragalus saponin I, astragalus flavone, curcumin, CpG-DNA-2, chicken IgG, LPS, and poly(I:C) (Figures 2A, 2C). Besides, all immunopotentiators could significantly promote the production of TNF-α (Figures 2A, 2D). These observations indicated that in some extent, the apoptosis and inflammatory progressions in DEFs were affected by these immunopotentiators, especially treating with chlorogenic acid, β-D-Glucan, astragalus flavone, CpG-DNA-2, and chicken IgG.
Figure 2.
Effects of 22 kinds of immunopotentiators on the protein expression of apoptosis and cytokines in DEFs (A). Protein expressions of Bcl2 (B), caspase 3 (C), and TNF-α (D) after treatment of DEFs with immunopotentiators for 24 h. Results are presented as the mean ± SD. Asterisks (∗) represent significant differences.
Biochemical Markers in Serum Samples of Ducklings
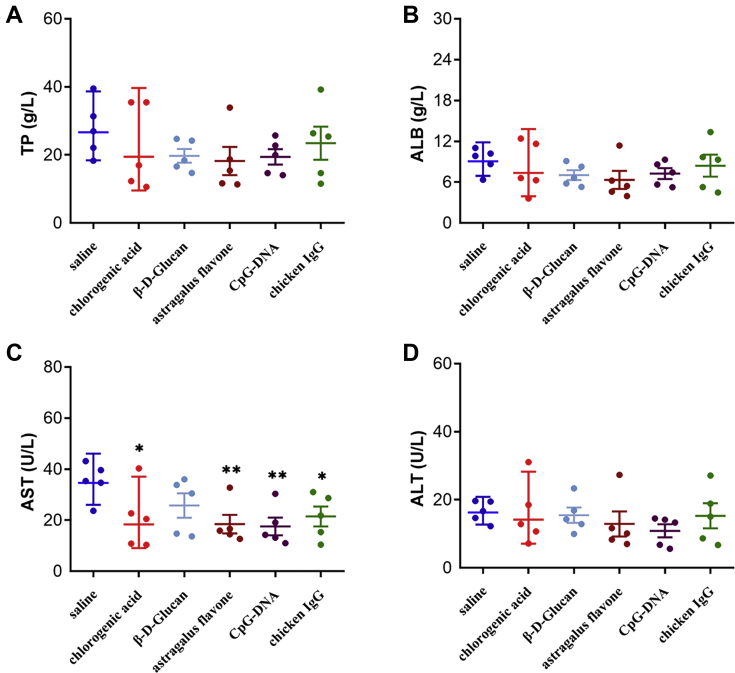
To further verify whether the screened 5 immunopotentiators could enhance the immunity of ducklings, serum samples were collected to detect the levels of TP, ALB, AST, and ALT. As shown in Figure 3, the activities of serum TP, ALB, and ALT in 5 treated groups showed no significant difference compared with the control group (Figures 3A, 3B, 3D). However, the activities of AST in groups of chlorogenic acid, astragalus flavone, CpG-DNA, and chicken IgG were significantly higher than those in the control group (Figure 3C).
Figure 3.
Serum biochemical markers TP (A), ALP (B), AST (C), and ALT (D) in 6 groups. Results are presented as the mean ± SD (n = 5). Asterisks (∗) represent significant differences.
Cytokine Activity in Serum Samples of Ducklings
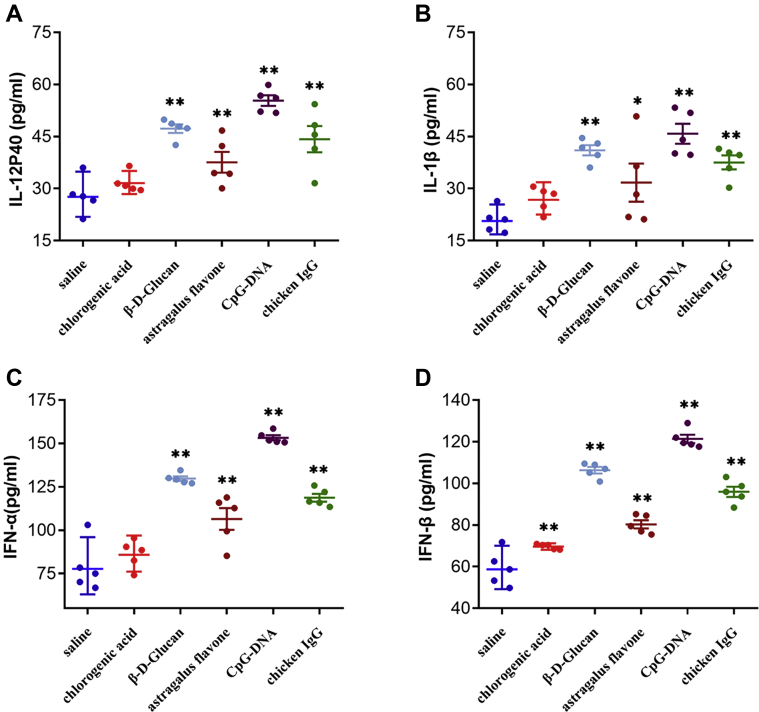
In order to investigate the changes of cellular immunity in ducklings during immunopotentiators treatments, the ELISA analysis was used to detect the changes of serum IL-12p40, IL-1β, and other cytokine levels. In groups of β-D-Glucan, astragalus flavone, CpG-DNA, and chicken IgG, the expression levels of IL-12p40, IL-1β, IFN-α, and IFN-β displayed a similar upregulation pattern, with significant increase (Figures 4A–D). While in ducklings treated with chlorogenic acid, only the level of IFN-β was significantly increased (Figure 4D).
Figure 4.
Effect of different immunopotentiators treatment on the cytokine in the ducklings. All assays were repeated at least 5 times, and data are shown as mean ± SD (n = 5) from one representative experiment. The expression is shown for (A) IL-12p40, (B) IL-1β, (C) IFN-α, and (D) IFN-β.
Expression of PRRs and Inflammatory Factor Genes
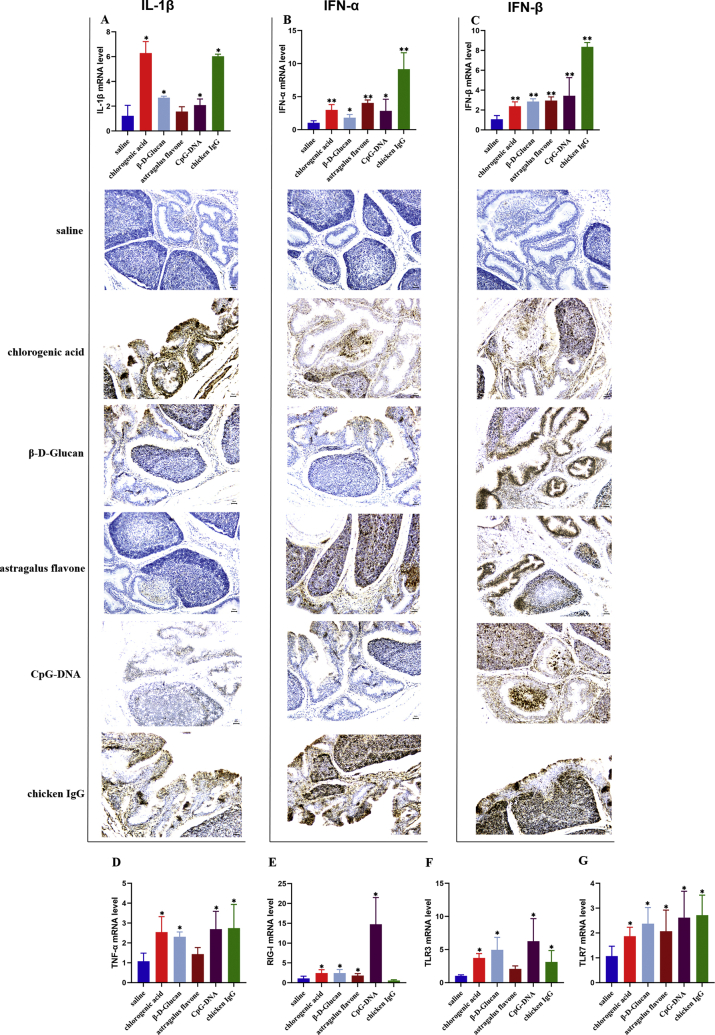
To determine the role of different immunopotentiators in the regulation of innate immune response by PRRs and inflammatory factor genes in Shaoxing ducklings, we detected the expression levels of IFN-α, IFN-β, IL-1β, TNF-α, RIG-I, and TLR 3/7 genes in the bursa tissues (Figure 5). The IL-1β level in bursa was significantly upregulated during chlorogenic acid, β-D-Glucan, astragalus flavone, CpG-DNA, and chicken IgG treatment (Figure 5A). In accordance with these data, the IFN-α and IFN-β levels were also enhanced significantly after all immunopotentiator treatments (Figures 5B, 5C). In addition, TNF-α mRNA level was significantly enhanced in all immunopotentiator groups, except for astragalus flavone (Figure 5D). The RIG-I expression level was significantly upregulated after injecting chlorogenic acid, β-D-Glucan, astragalus flavone, and CpG-DNA (Figure 5E). In addition, we analyzed the TLRs mRNA expression in response to 5 immunopotentiators treatment. The bursa TLR3 mRNA level was significantly high in groups of chlorogenic acid, β-D-Glucan, CpG-DNA, and chicken IgG. Meanwhile, chlorogenic acid, β-D-Glucan, astragalus flavone, CpG-DNA, and chicken IgG could also facilitate the production of TLR7 (Figures 5F, 5G).
Figure 5.
Expression of PRRs and inflammatory factors in bursa of ducklings injected with 5 different immunopotentiators. RT-qPCR and situ hybridization for IL-1β (A), IFN-α (B), and IFN-β (C), and RT-qPCR for TNF-α (D), RIG-I (E), TLR3 (F), and TLR7 (G). For the RT-qPCR data analysis, triplicate Ct values obtained from 5 different experimental mRNA samples were normalized to β-actin mRNA. Relative mRNA levels were calculated using the Ct method, and normalized Ct values were obtained from the control group. Vertical bars represent the mean ± SD (n = 5). Asterisks (∗) represent significant differences.
Expression of iNOS and COX2 Genes
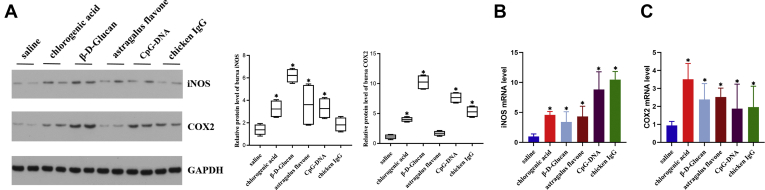
In addition, to analyze the activation of iNOS and COX2 in response to different immunopotentiators treatment, we detected their changes of expression levels in bursa tissues using qRT-PCR and Western blot (Figure 6). After injecting chlorogenic acid, β-D-Glucan, astragalus flavone, CpG-DNA, and chicken IgG, the bursa protein expression levels of iNOS and COX2 were significantly upregulated. The expression patterns of iNOS and COX2 mRNA were same as the protein.
Figure 6.
Effects of 5 immunopotentiators on the expression of inflammatory factor in ducklings' bursa tissues. The protein levels (A) and mRNA expressions (B, C) of iNOS and COX2 after injecting duckling with immunopotentiators. Vertical bars represent the mean ± SD (n = 5). Asterisks (∗) represent significant differences.
Expression of Apoptotic Relative Genes
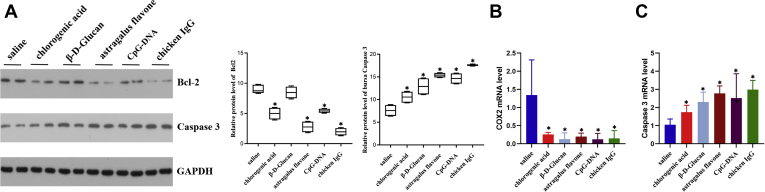
To investigate the effects of different immunopotentiators on apoptotic cell death, chlorogenic acid, β-D-Glucan, astragalus flavone, CpG-DNA, and chicken IgG were used to observe the mRNA and protein levels of Bcl2 and caspases 3 (Figure 7). It was found that the Bcl2 protein and mRNA levels in busra tissues were significantly lower in the chlorogenic acid, β-D-Glucan, astragalus flavone, CpG-DNA, and chicken IgG groups than those in the control group. Meanwhile, in the ducklings injected with chlorogenic acid, β-D-Glucan, astragalus flavone, CpG-DNA, and chicken IgG, the caspases 3 protein and mRNA levels showed a higher accumulation in busra tissues than those observed in the saline treatment.
Figure 7.
Effect of 5 immunopotentiators on the expression of apoptosis in ducklings' bursa tissues. The protein levels (A) and mRNA expressions (B, C) of Bcl2 and caspase 3 after injecting duckling with immunopotentiators. Vertical bars represent the mean ± SD (n = 5). Asterisks (∗) represent significant differences.
Discussion
Screening of Immunopotentiators on Apoptosis and Cytokines in DEFs
The aim of this study was to screen immunopotentiators and detect their mechanisms in triggering duck innate immune response that leads to the maturation of adaptive immune response together with powerful nonspecific immunomodulatory effects. As evidenced, the immunopotentiators were functional in the immunoenhancement of invertebrates, and some of them could improve the antiviral defense of hosts (Liu et al., 2009). However, the functional mechanism of the immunopotentiators in ducks was unknown. In some cases, these immunopotentiators do not show effects in ducks because of the lack of the information about the functional mechanism of immunopotentiators. Consequently, we screened the candidate immunopotentiators of different kinds and sources to determine their activities in driving the expression of apoptosis and proinflammatory cytokines. The main results showed that all groups treated with 22 immunopotentiators, respectively, induced a significant downregulation of Bcl2 and IL-2 levels and significantly upregulated the TNF-α and IFN-β levels, which was not consistent with the caspase 3 and IL-1β levels. The expression level of IL-1β mRNA showed significant increase after dihydromyricetin, chlorogenic acid, naringin, imiquimod, thymopentin, β-D-Glucan, astragalus polysacharin, astragalus saponin I, astragalus flavone, curcumin, CpG-DNA-2, and LPS treatment. And the level of caspase 3 was significantly upregulated while treated with chlorogenic acid, β-D-Glucan, astragalus polysacharin, astragalus flavone, curcumin, CpG-DNA-2, chicken IgG, LPS, and poly(I:C). These results indicate that DEFs were affected by these immunopotentiators in apoptosis and inflammatory progression, especially treated with chlorogenic acid, β-D-Glucan, astragalus flavone, CpG-DNA-2, and chicken IgG.
Effect of Immunopotentiators on Serum Biochemical Markers
Serum biochemical characteristics, such as protein levels, enzymes, and electrolytes, are very useful in the assessment and management of animal health condition, which could provide information on internal organs, nutritional status, metabolic state, and so on (Peng et al., 2018). Serum TP is an index to evaluate the nutritional and metabolic status of animals, and TP measurements are based on dietary protein content, liver metabolism, and even protein loss caused by some lesions (Yang et al., 2017). Besides, serum ALB has important applications in many clinical indications, which is responsible for targeting and transporting the ligands form reversible bindings in varying degrees, exhibiting superb bioeffects such as free radicals scavenging, maintenance of blood colloid osmotic pressure, inhibition of platelet aggregation and anticoagulation, as well as influence in the transport of nutrients and drugs (Li et al., 2018). In this study, to further investigate the immunomodulatory function of the chlorogenic acid, β-D-Glucan, astragalus flavone, CpG-DNA-2, and chicken IgG as immunopotentiators in vivo, 6 groups of ducklings were injected with these candidate immunopotentiators and saline, and their serum biochemical parameters were determined. The results showed serum TP and ALB levels in duck were not significant between the chlorogenic acid, β-D-Glucan, astragalus flavone, CpG-DNA-2, and chicken IgG groups and control group. ALT and AST are one of liver-specific enzymes in humans and animals and widely exist in the liver and heart, but when liver or heart disease causes cell damage, a lot of ALT and AST enter the serum which leads to rapid increases of the 2 kinds of enzyme activity in the serum, so ALT and AST are important indicators of liver and heart function (Lv et al., 2017; Peng et al., 2018). The present study showed the levels of ALT were not significantly different between the experimental groups and the control group, and the AST in the chlorogenic acid, astragalus flavone, CpG-DNA, and chicken IgG groups were lower than those of the control group, which might mean that injecting the 5 different immunopotentiators have little damage of liver and heart tissues to some extent.
Effect of Immunopotentiators on Serum Cytokine Activities of Ducklings
Cytokines can regulate the physiological functions of leukocytes, mediate inflammatory responses, participate in immune responses, and repair tissue. When triggering the immune response, a proinflammatory signal arises from various components of the innate immune system that sense invading pathogens (Mogensen, 2009). IFN-α and IFN-β, as immunomodulatory pleiotropic factors, have been used as an indicator of cell-mediated immunity (Ottenhoff and Mutis, 1995). Besides, IL-12p40 and IL-1β, a major coordinator in immune response, can stimulate the immune responses by inducing the release of cytokines (Ottenhoff and Mutis, 1995). In order to evaluate the immune responses of duck after injecting immunopotentiators, the levels of cytokines in serum were tested in the present study. As shown in Figure 4, the chlorogenic acid, β-D-Glucan, astragalus flavone, CpG-DNA, and chicken IgG treatments increased the serum of IL-12p40 level. And the contents of IL-1β, IFN-α, and IFN-β were upregulated in serum and bursa tissues with chlorogenic acid, β-D-Glucan, CpG-DNA, and chicken IgG treatment, which indicated that the increased IL-1β, IFN-α, and IFN-β levels would activate cytokine IL-1β, IFN-α, and IFN-β production, respectively, and subsequently upregulated a number of inflammation-linked molecules (Haugland et al., 2005; Xing et al., 2018). These results revealed the cells of the immune system were sufficient to induce the secretion of several proinflammatory cytokines after immunopotentiators stimulation.
Effect of Immunopotentiators on Immune-Related Genes Expression
TNF-α, as a stimulator of nuclear factor kappa-B, is a key factor in the inflammatory response and induces cell apoptosis in a variety of cells (Kruppa et al., 1992; Xing et al., 2018). PRRs play very important roles in acute inflammation to prevent invading microbes from entering host tissues (Akira, 2001). Moreover, the proinflammatory enzymes and cytokines, such as iNOS and COX2, play essential roles in tissues during inflammatory processes to regulate the cell survive (Baeuerle and Baltimore, 1996). In this study, as shown in Figure 5D, the expression level of TNF-α was upregulated with chlorogenic acid, β-D-Glucan, CpG-DNA, and chicken IgG treatments, and higher expression levels of RIG-I, TLR3, and TLR7 in the examined duck tissues were found in all immunopotentiators groups than in the control group. Furthermore, chlorogenic acid, β-D-Glucan, astragalus flavone, CpG-DNA, and chicken IgG could significantly upregulate the expression of the immune-related genes and PRRs after 18 d of injecting. The expression of both iNOS and COX2 in duck bursa tissues was facilitated. These results suggested that these immunopotentiators could modulate the immune-related genes.
Effects of Immunopotentiators on Expression of Apoptosis-Related Genes
Apoptosis is a form of cell death triggered during a variety of physiological conditions and is tightly regulated by a number of gene products that modulate immune responses (Ottenhoff and Mutis, 1995; Wang et al., 2017). The Bcl2 family, which includes antiapoptotic Bcl2, along with caspase 3, is the main regulatory protein involved in apoptosis (Reed et al., 1996). A previous study showed that chlorogenic acid decreased cell proliferation and regulated the expression of the apoptosis-related genes BCL2 and caspase 3 in A549 human lung cancer cells (Yamagata et al., 2017). Kim et al. have reported that the mRNA level of Bcl-2 significantly decreased, and that of caspase 3 was increased significantly with β-glucan treatment in human SNU-C4 cells (Kim et al., 2009). To determine the effects of immunopotentiators on apoptosis in ducks, we investigated the expression levels of apoptosis-related genes including Bcl2 and caspase 3 in the duck bursa tissues after injecting immunopotentiators and found that the expression of Bcl2 protein and mRNA in bursa tissues was reduced by chlorogenic acid, β-D-Glucan, astragalus flavone, CpG-DNA, and chicken IgG. In addition, the chlorogenic acid, β-D-Glucan, astragalus flavone, CpG-DNA, and chicken IgG increased bursa caspase 3 gene expression in protein and mRNA levels. Previous studies have also indicated that the activation of COX2 and iNOS gene transcription could contribute to apoptosis (Ayalasomayajula and Kompella, 2003; Zhang et al., 2014). These reports, together with our data, suggest that these immunopotentiators could mediate their proapoptotic effects via activating COX2 and iNOS in the nuclear factor kappa-B pathway.
Taken together, this study suggests that chlorogenic acid, β-D-Glucan, astragalus flavone, CpG-DNA, and chicken IgG can increase the immune capability by upregulation of functional expression of cytokines, proinflammatory factors, apoptosis, and PRRs in vitro and in vivo. As potential immunopotentiators, they may contribute to improve duck innate immune responses. Moreover, the study provides the first experimental evidence to demonstrate the functional mechanism of the 5 immunopotentiators, which may provide a novel strategy for reliable alternatives to antibiotics in duck as well as in other animals.
Acknowledgements
This study was supported by the Key R&D Program of Zhejiang Province (2018C04014), the Natural Science Foundation of Zhejiang Province (LQ17C170003), the National Natural Science Foundation of China (31702106, 31872982), and the Earmarked Fund for Modern Agro-industry Technology Research System (CARS-42-6).
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
Contributor Information
Guohong Chen, Email: ghchen2019@yzu.edu.cn.
Lizhi Lu, Email: lulizhibox@163.com.
References
- Akira S. Toll-like receptors in innate immunity. Int. Immunol. 2001;78:1–56. doi: 10.1016/s0065-2776(01)78001-7. [DOI] [PubMed] [Google Scholar]
- Ali M. Rasayana therapy in classical literature of Ayurveda: a review. Bull Indian Inst. Hist. Med. Hyderabad. 1998;28:95–110. [PubMed] [Google Scholar]
- Ayalasomayajula S., Kompella U.B. Celecoxib, a selective cyclooxygenase-2 inhibitor, inhibits retinal vascular endothelial growth factor expression and vascular leakage in a streptozotocin-induced diabetic rat model. Eur. J. Pharmacol. 2003;458:283–289. doi: 10.1016/s0014-2999(02)02793-0. [DOI] [PubMed] [Google Scholar]
- Baeuerle P.A., Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- Casewell M., Friis C., Marco E., Mcmullin P., Phillips I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003;52:159–161. doi: 10.1093/jac/dkg313. [DOI] [PubMed] [Google Scholar]
- Chen D., Ainsworth A. Glucan administration potentiates immune defence mechanisms of channel catfish, Ictalurus punctatus Rafinesque. J. Fish Dis. 2006;15:295–304. [Google Scholar]
- Dahiya J.P., Wilkie D.C., Kessel A.G.V., Drew M.D. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Anim. Feed Sci. Technol. 2006;129:0–88. [Google Scholar]
- Ganeshpurkar A., Saluja A.K. Experimental animal models used for evaluation of potential immunomodulators: a mini review. Bulletin of Faculty of Pharmacy Cairo University. 2017;55:211–216. [Google Scholar]
- Haugland y., Torgersen J., Syed M., Evensen y. Expression profiles of inflammatory and immune-related genes in Atlantic salmon (Salmo salar L.) at early time post vaccination. Vaccine. 2005;23:0–5499. doi: 10.1016/j.vaccine.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Hiss S., Sauerwein H. Influence of dietary ß-glucan on growth performance, lymphocyte proliferation, specific immune response and haptoglobin plasma concentrations in pigs. J. Anim. Physiol. Anim. Nutr. 2003;87:2–11. doi: 10.1046/j.1439-0396.2003.00376.x. [DOI] [PubMed] [Google Scholar]
- Jang S.I., Marsden M.J., Kim Y.G., Choi M.S., Secombes C.J. The effect of glycyrrhizin on rainbow trout, Oncorhynchus mykiss (Walbaum), leucocyte responses. J. Fish Dis. 2006;18:307–315. [Google Scholar]
- Jucker E. Antiviral agents: advances and problems. Prog. Drug Res. Mol. 2001;6:1061–1062. [Google Scholar]
- Kim M.J., Hong S.Y., Kim S.K., Cheong C., Park H.J., Chun H.K., Jang K.H., Yoon B.D., Kim C.H., Kang S.A. β-Glucan enhanced apoptosis in human colon cancer cells SNU-C4. Nutr. Res. Pract. 2009;3:180–184. doi: 10.4162/nrp.2009.3.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruppa G., Thoma B., Machleidt T., Wiegmann K., Krönke M. Inhibition of tumor necrosis factor (TNF)-mediated NF-κB activation by selective blockade of the human 55-kDa TNF receptor. J. Immunol. 1992;148:3152–3157. [PubMed] [Google Scholar]
- Li S., Li L., Chen Z., Xue G., Jiang L., Zheng K., Chen J., Li R., Yuan C., Huang M. A novel purification procedure for recombinant human serum albumin expressed in Pichia pastoris. Protein Expr. Purif. 2018;149:37–42. doi: 10.1016/j.pep.2018.04.012. [DOI] [PubMed] [Google Scholar]
- Liu W., Han F., Zhang X. Ran GTPase regulates hemocytic phagocytosis of shrimp by interaction with myosin. J. Proteome Res. 2009;8:1198–1206. doi: 10.1021/pr800840x. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D.J.M. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lv J., Xiao Q., Chen Y., Fan X., Liu X., Liu F., Luo G., Zhang B., Wang S. Effects of magnesium isoglycyrrhizinate on AST, ALT, and serum levels of Th1 cytokines in patients with allo-HSCT. Int. Immunopharmacol. 2017;46:56–61. doi: 10.1016/j.intimp.2017.02.022. [DOI] [PubMed] [Google Scholar]
- Mathew A.G., Cissell R., Liamthong S. Antibiotic resistance in bacteria associated with food animals: a United States perspective of livestock production. Foodborne Pathog. Dis. 2007;4:115–133. doi: 10.1089/fpd.2006.0066. [DOI] [PubMed] [Google Scholar]
- Millet S., Maertens L. The European ban on antibiotic growth promoters in animal feed: from challenges to opportunities. Vet. J. 2011;187:143–144. doi: 10.1016/j.tvjl.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Mogensen T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenhoff T.H.M., Mutis T. Role of cytotoxic cells in the protective immunity against and immunopathology of intracellular infections. Eur. J. Clin. Invest. 1995;25:371–377. doi: 10.1111/j.1365-2362.1995.tb01716.x. [DOI] [PubMed] [Google Scholar]
- Peng F., Chen X., Meng T., Li E., Zhou Y., Zhang S. Hematology and serum biochemistry parameters of captive Chinese alligators (Alligator sinensis ) during the active and hibernating periods. Tissue Cell. 2018;51:8–13. doi: 10.1016/j.tice.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Reed J.C., Miyashita T., Takayama S., Wang H., Sato T., Krajewski S., Aimé㏒Empé C., Bodrug S., Kitada S., Hanada M. BCL-2 family proteins: regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J. Cell Biochem. 1996;60:23–32. doi: 10.1002/(SICI)1097-4644(19960101)60:1%3C23::AID-JCB5%3E3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Saito H., Nagashima K., Tomioka H. Effects of bacterial immunopotentiators, LC 9018 and OK-432, on the resistance against Mycobacterium intracellulare infection in mice. Hiroshima J. Med. Sci. 1983;32:145–148. [PubMed] [Google Scholar]
- Sakai M. Current research status of fish immunostimulants. Aquaculture. 1999;172:63–92. [Google Scholar]
- Sakai M., Otubo T., Atsuta S., Kobayashi M. Enhancement of resistance to bacterial infection in rainbow trout, Oncorhynchus mykiss (Walbaum), by oral administration of bovine lactoferrin. J. Fish Dis. 1993;16:239–247. [Google Scholar]
- Ueno H., Hawrylowicz C.M., Banchereau J. Immunological intervention in human diseases. J. Transl Med. 2007;5:59–66. doi: 10.1186/1479-5876-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Zhang C., Song Y., Wang Z., Wang Y., Luo F., Xu Y., Zhao Y., Wu Z., Xu Y. Mechanism of immune evasion in breast cancer. Onco Targets Ther. 2017;10:1561–1573. doi: 10.2147/OTT.S126424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L., Xiangxian Y., Yanli L., Hua Y., Wanshan H., Meilan Y. Identification differential behavior of Gd@C 82 (OH) 22 upon interaction with serum albumin using spectroscopic analysis. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2018;203:383–396. doi: 10.1016/j.saa.2018.05.125. [DOI] [PubMed] [Google Scholar]
- Yamagata K., Izawa Y., Onodera D., Tagami M. Chlorogenic acid regulates apoptosis and stem cell marker-related gene expression in A549 human lung cancer cells. Mol. Cell Biochem. 2017;441:9–19. doi: 10.1007/s11010-017-3171-1. [DOI] [PubMed] [Google Scholar]
- Yang Z., Wang Z.Y., Yang H.M., Xu L., Gong D.Q. Effects of dietary methionine and betaine on slaughter performance, biochemical and enzymatic parameters in goose liver and hepatic composition. Anim. Feed Sci. Technol. 2017;228:48–58. [Google Scholar]
- Zhang X., Fu Y., Xu X., Li M., Du L., Han Y., Ge Y. PERK pathway are involved in NO-induced apoptosis in endothelial cells cocultured with RPE under high glucose conditions. Nitric Oxide. 2014;40:10–16. doi: 10.1016/j.niox.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Zhong T.Y., Arancibia S., Born R., Tampe R., Villar J., Del Campo M., Manubens A., Becker M.I. Hemocyanins stimulate innate immunity by inducing different Temporal patterns of proinflammatory cytokine expression in macrophages. J. Immunol. 2016;196:4650–4662. doi: 10.4049/jimmunol.1501156. [DOI] [PMC free article] [PubMed] [Google Scholar]