Summary
Short sleep has been linked to adolescent obesity risk, but questions remain regarding the dietary mechanisms by which this occurs. We tested whether mildly shortening sleep influences how rewarding and appealing healthy adolescents find several kinds of foods. Eighty-eight healthy adolescents completed a within-subjects crossover sleep experiment comparing 5 days of Short Sleep (6.5 hour sleep opportunity) vs. 5 days of Healthy Sleep (9.5 hour sleep opportunity). Following each condition, adolescents completed measures of food appeal and reinforcing value of food across five food types: sweets/desserts, fruits/vegetables, lean meats/eggs, fast food entrees, and processed snacks. Adolescents averaged 2.2 hours/night longer sleep periods in Healthy Sleep vs. Short Sleep. We observed a significant interaction of experimental order with sleep condition on three of four primary outcomes related to the appeal and reinforcing value of foods (p’s<.005). When Short Sleep preceded Healthy Sleep, adolescents endorsed significantly greater appeal (p<.04) and rewarding value of food (p’s ranging from <.01 to .048) during Short Sleep (compared to Healthy Sleep). However, when Healthy Sleep preceded Short Sleep, we did not observe a main effect of sleep condition on the same outcomes (p’s>.05). This study provides evidence that restricting adolescents’ sleep opportunity to 6.5 hours (compared to sleeping a healthy amount) increases the appeal and reinforcing value of a variety of foods, but this may occur only under protracted short sleep. Increased food reward may be one mechanism linking chronically shortened sleep with obesity risk in adolescence.
Keywords: adolescent, sleep restriction, dietary decision making, food appeal, food value
Shortened sleep has emerged as a significant risk factor for adolescent obesity (Miller, Kruisbrink, Wallace, Ji, & Cappuccio, 2018). Restricting sleep leads to greater food consumption in school-aged youth (Hart et al., 2013) and greater adolescent consumption of foods that are high in glycemic index and load (Beebe et al., 2013), without compensatory increases in moderate-to-vigorous physical activity (Van Dyk et al., 2018). However, the mechanism driving the increase in food intake following short sleep remains unclear.
Adolescence is an important developmental time with respect to eating behavior. In addition to establishing long-term dietary patterns, adolescents are much more independent in their food choices than are younger children, so their internal motivational states about food may directly impact eating behavior. It is possible that adolescent sleep may influence food reward, an internal process that involves both determining the desirability of the food (perceived food appeal) and the degree to which the food motivates an individual to eat (perceived food value; Berridge, Ho, Richard, & Difeliceantonio, 2010). Higher levels of food appeal have been associated with overeating across development (Appelhans et al., 2011; Davis et al., 2007) and with excessive weight in children (De Decker et al., 2016; Verbeken, Braet, Lammertyn, Goossens, & Moens, 2012). Adolescents undergoing sleep restriction also rate pictures of sweet/dessert foods to be more appealing (Simon, Field, Miller, Difrancesco, & Beebe, 2015), show heightened neural activation in reward-related brain regions in the presence of food (Jensen et al., 2019), and report higher ratings of food reward (Duraccio, Zaugg, & Jensen, 2019) compared to when undergoing healthy sleep.
Perceived value of food may also be sensitive to sleep duration in adolescents. In children, reinforcing value of food differs by weight status (Kral, Remiker, Strutz, & Moore, 2014) and higher reinforcing value of food may predict BMI increases over time for children (Hill, Saxton, Webber, Blundell, & Wardle, 2009) and adolescents (Epstein, Yokum, Feda, & Stice, 2014). Interestingly, Hart and colleagues (2013) observed no differences in how much effort school-aged children were willing to put forth in exchange for foods after periods of experimentally shortened sleep (1.5 fewer hours in bed each night for 1 week) and enhanced sleep (1.5 hours more hours in bed per night for 1 week). However, this null finding may have been driven by the dose of the sleep restriction or the impact of sleep restriction on overall effort (how hard children were willing to work on tasks in general, independent of the nature of the reward).
There is promising evidence that changes in adolescent sleep might impact food reward processes, but findings are far from well-established. Outcome measures have been limited both in nature (e.g., assessment of appeal or value, but not both) and types of foods addressed. Additionally, prior studies’ samples have been small, limiting the ability to test for moderators (e.g., variations in food types, body mass, demographics). Compared to other types of foods (e.g., snacks, fast foods), short sleep may differentially affect adolescents’ consumption of sweet/dessert foods (Beebe et al., 2013), with adolescents consuming more under conditions of restricted (vs. healthy) sleep. Another recent study found differing effects of sleep restriction on self-reported food reward depending on adolescents’ weight (Duraccio, Zaugg, et al., 2019), with only those of normal-weight (without overweight/obesity) showing increases in food reward following sleep restriction (vs. healthy sleep). No other studies have tested food type or weight as moderators of appetitive responses to adolescent sleep restriction. Sex differences have been noted on food appeal ratings in adolescents (Jensen, Duraccio, Barnett, & Stevens, 2016) and food reward in younger children (Rollins, Loken, Savage, & Birch, 2014). However, no published study has explored the interaction of sleep with sex on food reward processes. Understanding whether restricted sleep differentially affects certain groups may offer insight into sleep-related disparities and guide targeted clinical treatment.
Given the limited examination of how sleep impacts food reward processes in adolescents, we examined indices of both perceived food appeal and food value for several types of foods following experimental shortened sleep. We hypothesized that adolescents undergoing short sleep would rate pictured foods as more appealing and would assign greater reinforcing value to foods, particularly when rating sweets/desserts, as compared to when they were well- rested. Secondarily, we conducted exploratory analyses to examine the moderating influence of weight status, sex, and experimental order effect.
Methods
The Institutional Review Board at Cincinnati Children’s Hospital Medical Center approved and oversaw all study procedures, which were undertaken after both verbal and written parent consent and adolescent assent. Data presented in this manuscript are part of a larger experimental trial investigating the impact of experimental changes in sleep duration on weight-related behaviors. All data were collected during participants’ summer breaks across 2015–2018, so as to avoid negative effects of short sleep on academics. The sleep manipulation procedures, outlined in the “Sleep Manipulation” section below, are identical to those reported by Beebe and colleagues (2018), who reported on unrelated cognitive test outcomes for ~1/4 of the current sample.
Participants
Research staff recruited healthy adolescents (ages 14–17 years old) via community flyers, online advertisements, and e-mails in a large pediatric health network. Exclusion criteria included: ongoing psychiatric disorders that could be adversely affected by sleep deprivation (e.g., major depression, bipolar disorder, psychosis; Kaufman et al., 1997); a history of neurologic illness or injury (e.g. epilepsy, traumatic brain injury); intellectual disability or autism spectrum disorder; known/suspected recurrent substance use; use of medications known to impact sleep (e.g. stimulants); self- or parent-report symptoms of obstructive sleep apnea or periodic limb movements via validated screeners (Chervin, Hedger, Dillon, & Pituch, 2000; Chervin & Hedger, 2001); self- or parent-report symptoms of current eating disorder symptoms (Kaufman et al., 1997); atypical sleep patterns (sleeping <6 hours or >10 hours on school nights); daily caffeine consumption >1 coffee or energy drink or >2 caffeinated sodas; obligations that would not permit bed- or wake-times prescribed during the protocol; and BMI above 30.
Sleep Manipulation
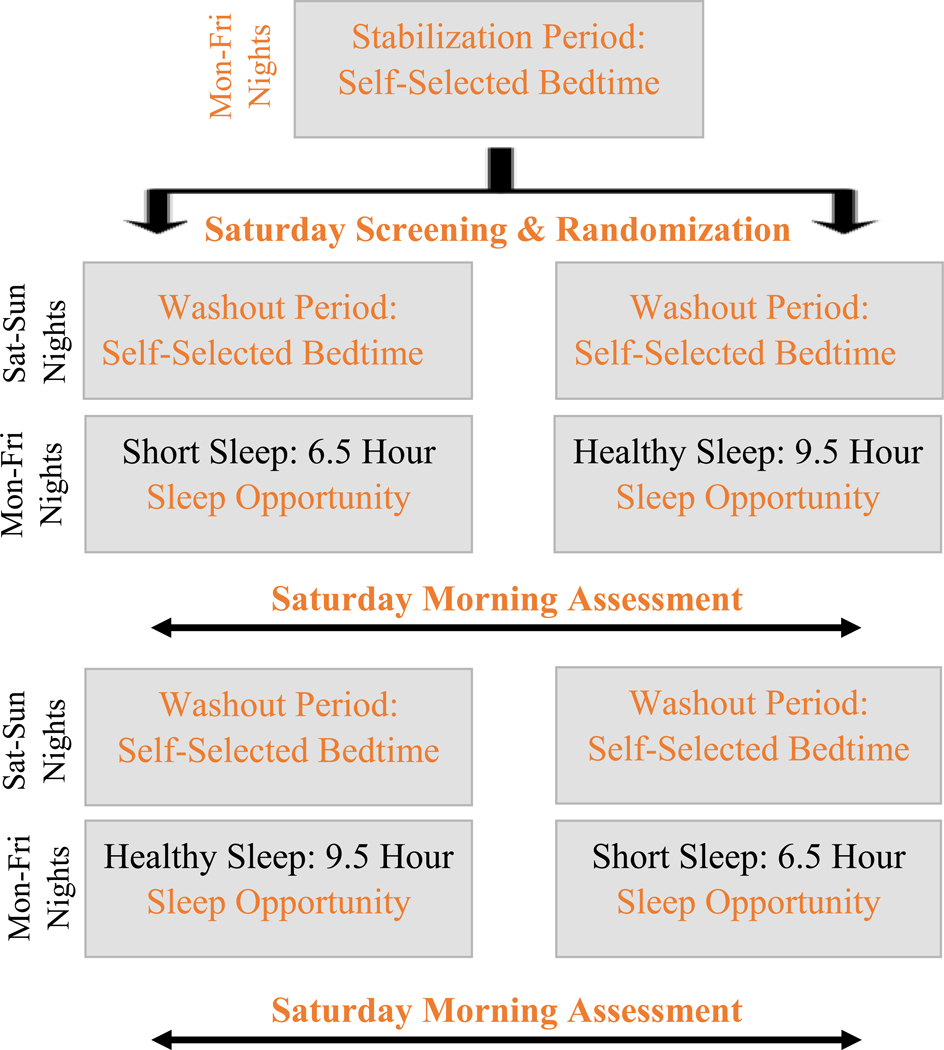
The 3-week within-subjects counterbalanced sleep manipulation protocol is summarized in Figure 1 (Beebe et al., 2018). Interested families were prescreened for potential eligibility first by phone, then verbally consented and mailed a sleep diary and actigraph along with study instructions. Throughout the study, prescribed wake times remained consistent, set at the time adolescents would need to rise to attend 8:00am research visits. Because ethics considerations led us to conduct data collection during the summer break from school, all participants underwent an initial 5-night phase stabilization period intended to establish a school-like waking pattern prior to randomization; adolescents self-selected their bedtimes but maintained the prescribed wake time. On the Saturday morning following the stabilization period, they attended an 8:00am office visit in which consent/assent and study eligibility were confirmed and actigraph data were reviewed for adherence to the prescribed rise time.
Figure 1.
Overview of the Sleep Manipulation Protocol
Eligible adolescents were then randomized to start the experimental protocol with either the Short Sleep (6.5 hour sleep opportunity) or Healthy Sleep (9.5 hour sleep opportunity) condition, which was achieved by manipulating bedtimes. Both experimental conditions (Short Sleep and Healthy Sleep) lasted 5 nights and began after a 2-day washout period (≥8 sleep opportunity). Adolescents were assessed the Saturday morning following each experimental condition. In that visit, they completed food reward tasks, reviewed their actigraph-measured sleep with study staff, and received instructions for another 2-night washout and the sleep condition that they had not already completed. Participants who completed the full study participated in both experimental sleep conditions, with order randomly counterbalanced. We chose this experimental approach due to our prior experience using a similar protocol that resulted in high rates of adherence, lack of moderating order effects, and sleep stabilization and washouts in which adolescents’ sleep patterns were similar to what has been reported on school nights (Beebe et al., 2013; Simon et al., 2015; Van Dyk et al., 2018). Throughout the study, adolescents were instructed not to nap, to maintain minimal caffeine intake, to wear their actigraphs, and to fill out sleep diaries daily.
Measures
Background information.
Adolescents and parents provided demographic information during the baseline assessment visit, including age, sex, race, ethnicity, and household yearly income. Adolescents’ height and weight were objectively measured in triplicate using research-calibrated equipment to calculate BMI, which was then converted to age- and sex-adjusted z-scores (zBMI) referenced against data from the US Centers for Disease Control and Prevention.
Sleep monitoring.
Adherence to the assigned sleep schedules was monitored throughout the protocol via wrist-worn actigraphy (Motionlogger Micro Watch; Ambulatory Monitoring, Inc., Ardsley, NY) and self-reported daily sleep diary that included nightly bed- and rise-times. Study staff reconciled the diary and actigraphic sleep data conjointly with adolescents and parents at each assessment point. This provided opportunity to clarify bed and wake-times, identify artifacts (e.g. removal of the watch), and promote adherence to subsequent conditions. Final determination of sleep-wake patterns for the present analyses was based upon actigraphy. Actigraphy data were scored using validated algorithms (Sadeh, Sharkey, & Carskadon, 1994) to obtain estimates of time of sleep onset and offset, duration of the sleep period (onset to offset) and sleep efficiency (percent of the sleep period spent asleep, recognizing the potential for periods of wakefulness between onset and offset). For this study, we adopted our previously-published definition of adherence (Beebe et al., 2013; Simon et al., 2015): at least one hour less nightly sleep on average during Short Sleep than Healthy Sleep.
Food Reward.
Food Appeal.
At each office visit, adolescents engaged in a computerized food appeal rating system that had sets of stock photos for five different types of foods, based upon the food groupings detailed in Beebe and colleagues (2013): sweet/dessert foods (e.g., cookies, cake); fruits and vegetables; lean meats and eggs; fast food entrees (e.g., pizza); and processed snacks (e.g., crackers, potato chips). Two matched sets of images were used across the two experimental conditions, with the order of the sets randomized. Within each set, there were 42 images for each type of food, with the exception of fruits/vegetables and meats/eggs; these were presented in a single 42-item “non-sweets” set in a prior study (Simon et al., 2015) and were split into 21-item sets for analysis here. The resulting 168 images in each set were interwoven with no two images of the same food type presented back-to-back, and adolescents were asked to rate the appeal of each food image on a 1–4 Likert scale, with higher responses indicating greater appeal. Previous pilot work using two types of foods – sweets/desserts and non-sweets – found that (a) the ratings had good reliability, (b) mean ratings were higher for sweets than non-sweets, and (c) sleep restriction significantly increased appeal ratings of sweets but not non-sweets (Simon et al., 2015). As in that prior work, a mean appeal rating was calculated for each food type in each condition. For each food type, internal consistencies within the current sample were strong (α = .86 – .95), and there was good stability in mean ratings across the two experimental weeks (r = .81 – .87).
Reinforcing Value of Food.
We also used the Food Purchasing Questionnaire Task (Epstein, Dearing, & Roba, 2010), which was initially designed to measure the reinforcing value of food in adults, and then was successfully used with adolescents (Feda, Roemmich, Roberts, & Epstein, 2015). During the phase stabilization visit, participants were asked to select their favorite food from an array of seven food images (taken from the 336 image database used in the food appeal task) in each of the five food categories (sweet/dessert foods; fruits and vegetables; lean meats and eggs; fast food entrees; and processed snacks). This ensured that all participants could identify a food they at least modestly enjoyed within each food group. Each participant’s set of preferred foods (one per food category) was then used to individualize the Food Purchasing Task Questionnaires that were administered following each experimental condition.
Following each experimental condition, individuals were presented with their selected preferred food in each category, and asked to indicate how many servings of that food they would purchase with their own money, with the intention of fully consuming whatever they purchased within that day (participants were instructed that they could not share or save the foods). The task began with each hypothetical serving being free (no charge) and then progressively increased in price (i.e., $0.01, $0.05, $0.13, $0.25, $0.50, $1.00, etc.) until the participant indicated that they would no longer purchase any food at that price point, up to a maximum of $1,120 per serving. Participants completed this individualized questionnaire for each of the five food categories following each experimental condition.
Three outcome variables were chosen for this task, given their previously-documented associations with BMI, dietary restraint, dietary disinhibition, and hunger ratings (Epstein et al., 2018): intensity (number of servings of food “purchased” if the food was free), Omax (maximum amount of money the person would spend on the food), and elasticity (a measure of the sensitivity of the relationship between amount of food purchased and price) for each food type across each experimental condition. Greater perceived value of a food is indicated by higher intensity (willing to consume greater quantities of the food when the food is free), higher Omax (willing to pay more money for a food), and lower elasticity (decreased sensitivity to the increases in price of foods, suggesting willingness to purchase more servings of foods at higher prices). Outcomes on this questionnaire approach have been validated against laboratory-based measures of relative reinforcing value of food that involve actual food consumption (Epstein et al., 2010). We elected a questionnaire format over such a laboratory-based measure to allow us to assess several different types of foods without participants having to eat all of them in a single session.
Response Plausibility:
Self-report reward measures are subject to non-plausible response patterns, which are commonly screened and excluded from analyses (Stein, Koffarnus, Snider, Quisenberry, & Bickel, 2015). For this study, we excluded on the following non-plausible patterns, which also reflected extreme outliers in our sample: (1) a sudden and marked reversal in responses to the purchase task, in which an adolescent indicated an intent to purchase markedly more of the same food as price increased; (2) report of intent to purchase ≥50 servings of a food at any price and ≥10 servings at >$1000 each; (3) markedly greater variability in appeal ratings across multiple food categories from one condition to the other, suggesting random or highly inattentive responses during a condition.
Analytic Plan
To examine differences between sleep variables across experimental sleep conditions, we conducted a series of paired-sample t-tests on sleep onset time, sleep offset time, sleep period, and sleep efficiency. For primary analyses, we conducted two condition (shortened sleep, healthy sleep) by five food group (sweets/desserts, fast food, snacks, meats, fruits and vegetables) generalized linear mixed-effect modeling to examine the within-subject factors of sleep condition and food type on food appeal, intensity, Omax, and elasticity outcomes. The significance threshold for primary analyses was set at p = .05. Exploratory moderator analyses then repeated these models, adding the between-subject potential moderating factors of sex, zBMI, and experimental order in separate analyses. As this resulted in 12 exploratory moderator analyses, we used the Bonferroni correction to set the significance value threshold to p = .0042 to reduce the likelihood of obtaining a type 1 error. To estimate the effect sizes in terms of Cohen’s d, the mean and SD of within-subject differences between shortened sleep and healthy sleep were calculated and used for effect size calculation. All statistical analyses were conducted using SAS Version 9.4 (SAS Institute, Inc, Cary, NC).
Results
Sample Characteristics
Demographics
Of the 149 adolescents recruited into the study, 22 failed to meet exclusion criteria or were non-adherent to the sleep stabilization instructions and were excluded prior to randomization. Of those randomized, 10 and 6 dropped out during the first and second experimental conditions, respectively. We excluded 18 adolescents for nonadherence to either of the sleep conditions and 5 for implausible response patterns on the study tasks. The final sample included 88 adolescents who completed the study, were adherent to sleep protocol, and provided plausible responses to outcome measures (69% of those randomized). Demographic and sleep characteristics for the final sample at the first office visit are presented in Table 1. Those retained in analyses did not significantly differ from those dropped or lost to follow-up post-randomization on sex, race, family income, zBMI, or in sleep characteristics for the phase stabilization week, p’s > .05.
Table 1.
Sample Characteristics at the Sleep Stabilization Office Visit (N = 88)
| Percent or Mean ± SD | Range | |
|---|---|---|
| Female (%) | 63 | |
| Race/Ethnicity (%) | ||
| White | 63 | |
| Black | 22 | |
| Hispanic (White) | 2 | |
| Asian | 3 | |
| Multiracial | 9 | |
| Age (years) | 15.77 ± 1.01 | 14.01 – 17.91 |
| Family income (%) | ||
| 60K or less | 25 | |
| >60K and <=125K | 44 | |
| >125K | 31 | |
| BMI-z | 0.37 ± 0.89 | -1.76 – 2.04 |
| Sleep Stabilization Sleep onset (time) | 00:06 ± 1:00 | 21:50 – 01:51 |
| Sleep Stabilization Sleep offset (time) | 06:58 ± 0:44 | 05:28 – 09:38 |
| Sleep Stabilization Sleep Period (hours) | 6.94 ± 1.07 | 3.67 – 11.35 |
| Sleep Stabilization Sleep efficiency | 90.89 ± 6.40 | 60.14 – 98.94 |
Sample features at time of randomization. Categorical variables are listed in percentages, while continuous variables are listed as Mean ± SD and Range.
Sleep Characteristics
As shown in Table 2, adolescents’ sleep period was 2.2 hours longer during the Healthy Sleep condition (M = 8.46, SD = 0.65) than the Short Sleep condition (M = 6.26, SD = 0.55), due to experimentally-induced earlier sleep onset during healthy sleep (p < .0001). As intended, differences in sleep offset were negligible across conditions. Sleep efficiency was significantly greater in Short Sleep (p < .0001), likely reflecting a homeostatic response to inadequate sleep (though sleep efficiency averages were well within a healthy range across both conditions).
Table 2.
Actigraph-Estimated Sleep Parameters across Experimental Conditions.
| Short Sleep (M ± SD) | Healthy Sleep (M ± SD) | p* | |
|---|---|---|---|
| Sleep onset (time) | 00:45 ± 0:32 | 22:30 ± 0:44 | <.0001 |
| Sleep offset (time) | 7:01 ± 0:22 | 6:58 ± 0:28 | .2745 |
| Sleep period (h) | 6.26 ± 0.55 | 8.46 ± 0.65 | <.0001 |
| Sleep efficiency | 92.41 ± 4.46 | 88.70 ± 6.08 | <.0001 |
p values were derived from paired-sample t-tests.
Food Reward Analyses
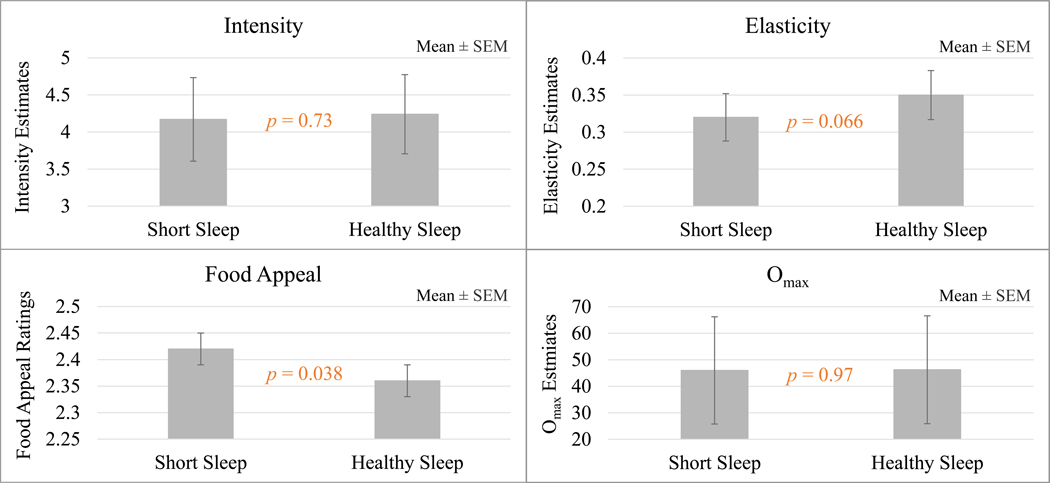
As shown in Table 3, Figure 2, and further detailed in Supplemental Table 1, sleep condition induced a significant main effect on food appeal (effect size = .19, p = .04) and a statistical trend towards an effect on elasticity (effect size = 0.10, p = .066); during Short Sleep, adolescents reported higher food appeal and lower elasticity, both suggesting heightened level of food reward. While the different food categories also appeared differentially rewarding (main effects of food type on intensity, elasticity, and food appeal; p < .01), of greater interest to the current study was the lack of significant interaction between the sleep condition and food category. That is, we found no evidence that the impact of sleep condition differed across the different food categories on any of the food reward measures.
Table 3.
Effects of Food Type and Condition on Primary Outcomes
| Main Effect of Condition | Main Effect of Food Type | Food x Condition Interaction Effect | ||||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Intensity | 0.12 | 0.7330 | 3.45 | 0.0083 | 0.66 | 0.6226 |
| Omax | 0.00 | 0.9660 | 0.40 | 0.8118 | 0.72 | 0.5750 |
| Elasticity | 3.38 | 0.0663 | 51.45 | <.0001 | 0.72 | 0.5793 |
| Appeal | 4.33 | 0.0379 | 6.38 | <.0001 | 0.18 | 0.9487 |
Figure 2.
Main Effects of Sleep Condition for Intensity, Omax, Elasticity, and Food Appeal
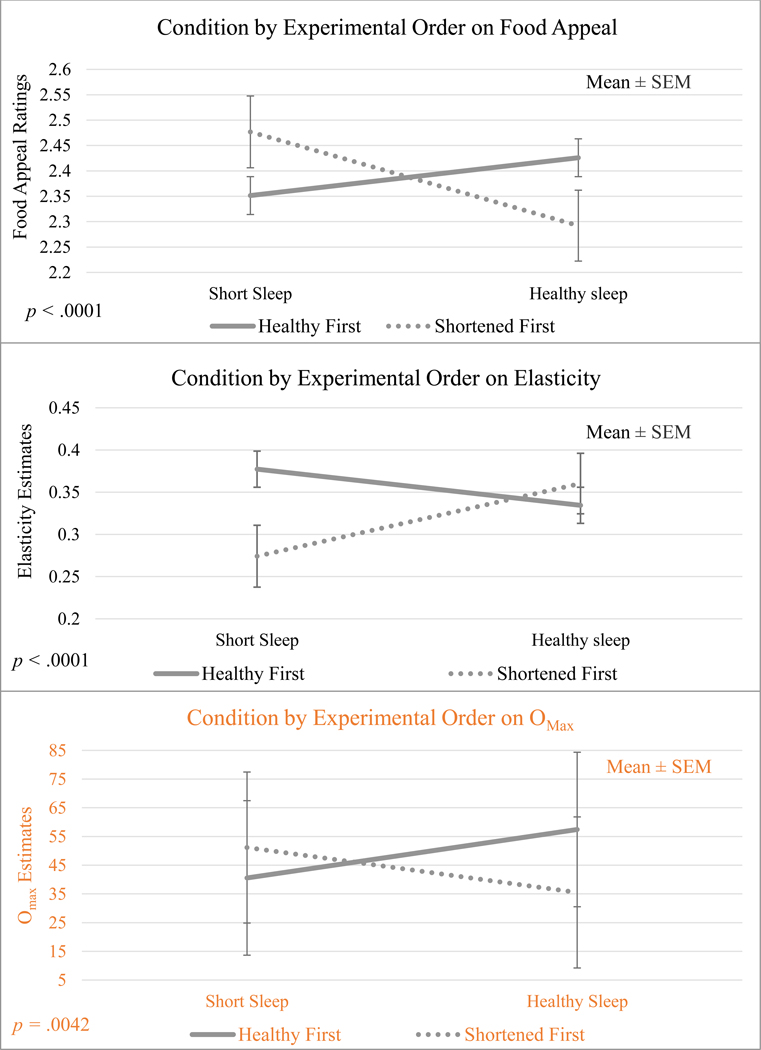
Importantly and unexpectedly, exploratory analyses suggested that the impact of the sleep manipulation on food reward differed depending on the order in which adolescents experienced the manipulation conditions. There were significant interactions of sleep condition with order on elasticity (p < .0001), Omax (p = .0042), and food appeal (p < .0001), where sleep condition effects were found only when the Short Sleep condition preceded the Healthy Sleep condition (effect sizes = 0.54, .08, and 0.38, respectively). When Healthy Sleep occurred before Short Sleep, the main effect of sleep condition on elasticity, Omax, and food appeal was absent (see Figure 3). We also observed a significant interaction of sleep condition with zBMI on elasticity (p = .0027), where the main effect of sleep condition on elasticity was significant only for adolescents of a normal weight (effect size = 0.13). For adolescents who were overweight, the main effect of sleep condition was absent. While the interaction of sex and sleep approached significance for Omax (p = .0057), no other interaction effects of sleep condition by sex or zBMI on primary outcomes met the corrected threshold for significance.
Figure 3.
Interaction of Sleep Condition by the Order in which Adolescents Experienced the Sleep Conditions
Discussion
The results of this experimental study partially support the notion that short sleep increases how appealing and rewarding adolescents find foods. Adolescents found a variety of both healthy and energy-dense/nutrient-poor foods more appealing and reported that they would be willing to pay increasingly higher prices for those foods when experiencing short sleep – but only when they began the experimental portion of the study in the Short Sleep condition.
While adolescents did rate certain food groups (e.g. sweets/desserts) as more appealing and rewarding across both sleep conditions, no food group showed a particularly greater shift in appeal or reward across sleep conditions. This finding was contrary to our hypothesis, given previous findings from a similar experimental cross-over study from our lab with a smaller sample (Simon et al., 2015). In that study, Simon and colleagues found that adolescents rated images of sweets/desserts (but not meats/eggs or fruits/vegetables) as more appealing in a 6.5-hour Short Sleep condition vs. a 10-hour Healthy Sleep condition. In the current study, we expanded on the variety of food types presented to adolescents by also including snack foods and fast foods. In doing so, we may have highlighted that food, in general, becomes more appealing when adolescents are sleep restricted. This sentiment is reflected in neuroimaging studies of adolescents (Jensen et al., 2019) and adults (Demos et al., 2017) which have similarly found no systematic differences in neural reward activation by specific food type in response to shortened sleep.
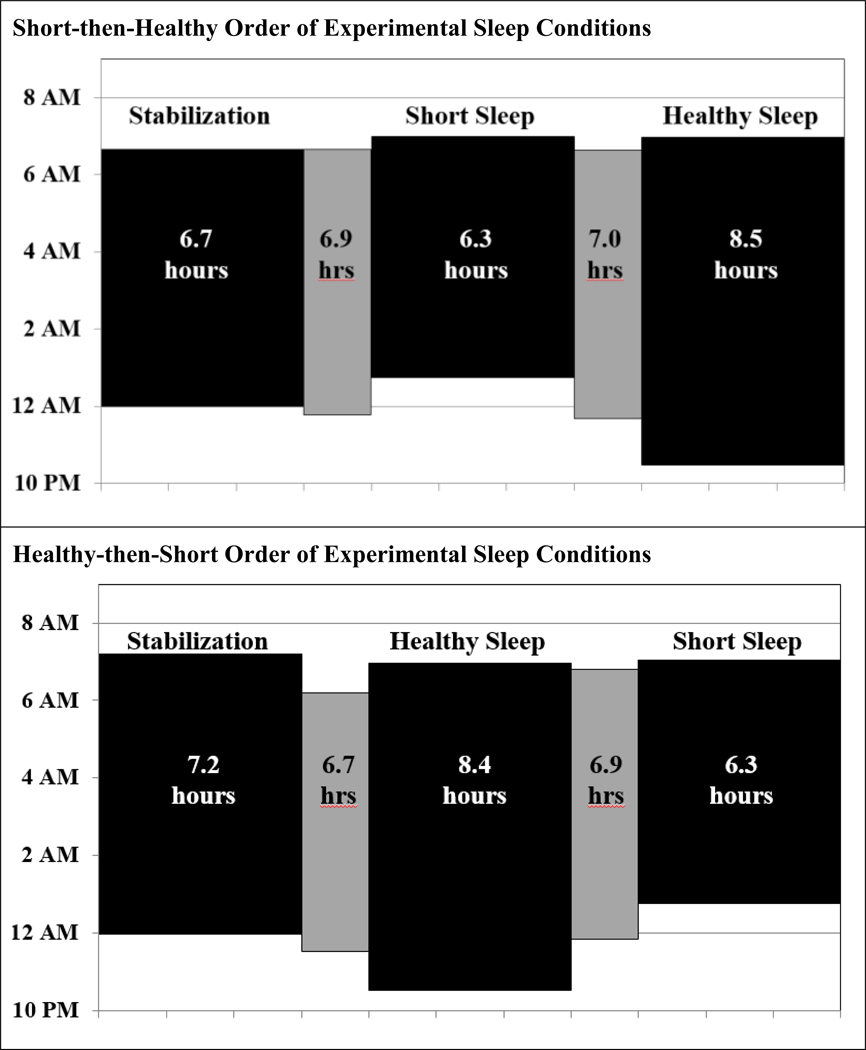
Even more surprising was the exploratory finding that the impact of sleep manipulation varied based upon the order in which it was experienced. Similar order effects have not been reported in prior experimental sleep manipulation studies with obesity-related outcomes (Beebe et al., 2013; Simon et al., 2015; Van Dyk et al., 2018), though that may have related in part to their smaller samples or other methodological differences. Although unanticipated, the effect appears important to consider, given highly significant interactions across three of four primary outcomes (p ≤ .0042). To better-understand the finding, we scrutinized the pattern of actigraphy-assessed sleep across the two order groups. As shown in Figure 4, order did not affect sleep onset, offset, or duration during either experimental condition; adolescents were equally effective at adhering to sleep directives regardless of the order of conditions. However, those who were randomized to Short Sleep before Healthy Sleep de facto experienced a more protracted period of moderately curtailed sleep. This is because, in this sample, the nightly sleep period was ~7 hours during both the sleep stabilization and weekend washout periods which, while similar to US adolescents’ typical sleep on school nights, is below consensus recommendations for nightly sleep duration (Emsellem et al., 2014; Hirshkowitz et al., 2015). As a result, for those who experienced Short Sleep before Healthy (top panel in Figure 4), the Short Sleep food reward assessment was obtained after nearly two weeks of chronic sleep restriction. In contrast, participants who were randomized into the Healthy Sleep condition first (but following the sleep stabilization week) experienced a shorter period of sleep restriction just prior to the Short Sleep food reward assessment (one week; bottom panel in Figure 4). The order effect may also have been compounded by somewhat shorter sleep during the sleep stabilization nights for adolescents who went on to experience Short Sleep before Healthy Sleep (M ± SD = 6.67 ± 1.07 hours) than those for whom Healthy Sleep preceded Short Sleep (7.24 ± 1.0 hours; p = .01).
Figure 4.
Sleep Periods across Order of Sleep Conditions (Short-then-Healthy vs. Healthy-thenShort)
Our finding that food became increasingly rewarding across protracted periods of shortened sleep is important because chronic mild sleep restriction is endemic on school nights in adolescents (Emsellem et al., 2014). It is also possible that the Healthy Sleep condition allowed for adolescents to “bank sleep” (Axelsson & Vyazovskiy, 2015), providing a protective factor against the deleterious effects of shortened sleep the subsequent week. Although current data suggest that five nights of recommended sleep duration may be sufficient to offset some effects of chronic mild sleep restriction on food reward processes, there is evidence from other measures that fewer nights (e.g., weekend “catch-up” sleep) may be insufficient (Lo, Ong, Leong, Gooley, & Chee, 2016). However, it is important to note that the current study was not designed to test the length of short sleep needed to induce changes in food reward, nor how much “recovery sleep” is needed to counteract this effect.
Given the unexpected order effects, we strongly recommend that future work systematically vary length/chronicity and dose of sleep restriction, as well as test how many nights of sufficient sleep are needed to counteract the effects of inadequate sleep. That work should also address other limitations of the current study, including our use of self-report proxies for food reward. We recommend replication using more objective metrics (e.g., functional neuroimaging of reward centers of the brain in response to food stimuli, laboratory-based “work for food” paradigms) or, conversely, more real-world measures (food choices in a naturalistic setting). The latter may also prove more sensitive to the effects of sleep changes on different food types, as the current study used attractive professional stock photos for all foods, which may have unrealistically homogenized their appeal. Similarly, because adolescents chose a preferred food within each food type for the food purchasing questionnaire (minimizing the chance that the stimuli would be a food they disliked), presenting only individually-favored foods may have obscured effects across different kinds of foods. Additionally, adult research has observed that shortened sleep impacts dietary processes most during the evening hours (Baron, Reid, Kern, & Zee, 2011; Markwald et al., 2013); future research should explore time-of-day effects on food reward processes following insufficient sleep in adolescents. Finally, future studies may consider standardizing the amount of sleep obtained during the stabilization period prior to randomization of experimental order, as we observed unintended differences across experimental order in sleep duration and sleep onset across the stabilization period.
To guard against Type I error, we applied a conservative Bonferroni correction to exploratory moderator analyses, which limited our statistical power. However, we did observe a significant BMI-by-sleep interaction, where normal-weight adolescents showed the hypothesized decrease in elasticity following Short Sleep (vs. Healthy Sleep), while those who were overweight showed unchanged elasticity estimates across the sleep conditions. This pattern is similar to that observed by Duraccio and colleagues (2019), in which they also observed that only normal-weight adolescents demonstrated increased food reward following sleep restriction (overweight adolescents had consistently high food reward in both sleep restriction and healthy sleep).We recommend that future work continue to examine BMI-by-sleep interactions on dietary outcomes, as well as sex-by-sleep interactions, which showed evidence of a “signal” that did not meet the Bonferroni significance correction. It is also important to better understand how food reward processes might relate to other dietary factors proposed to link sleep with adolescent intake, such as inhibitory control, emotional eating, and appetite-regulating hormones (Duraccio, Krietsch, Chardon, Van Dyk, & Beebe, 2019; Krietsch, Chardon, Beebe, & Janicke, 2019).
Despite these limitations, this study had several salient strengths. It is the first to examine both food appeal and food value – across several food type categories - within a single study, allowing us to evaluate the impact of sleep on various aspects of food reward. By considering several moderating variables, we found provocative areas for future examination (e.g., examining interaction effects of order, sex, and BMI). Further, given its within-subjects nature, this study had strong internal validity and increased power to detect effects. Further enhancing internal validity, we also established clear guidelines on adherence and plausible responding. Finally, both the dose and duration of sleep restriction in this study is similar to what many adolescents regularly experience during the school year, maximizing ecological validity.
In sum, findings from this study may suggest that prolonged shortened sleep increases certain aspects of food appeal and food value in adolescents. Results add to a small but growing literature implicating sleep-related changes in food reward and appeal as a mechanism to increased energy intake, with possible downstream effects on obesity. Interventions can improve adolescents’ sleep (Tan, Healey, Gray, & Galland, 2012); future studies should test whether such sleep interventions also impact food reward processes. There is some evidence that beginning an adolescent weight management program with a healthy sleep duration augments weight loss efforts (Sallinen et al., 2013; Valrie, Bond, Lutes, Carraway, & Collier, 2015), and that setting earlier bedtimes can reduce adolescents’ cravings for high glycemic foods and increase consumption of low glycemic foods (Asarnow, Greer, Walker, & Harvey, 2017). Although adult studies have found added weight loss benefit to incorporating targeted sleep intervention into behavioral weight management programs (Logue et al., 2012), no randomized control trials to date have examined this in adolescents.
Supplementary Material
Acknowledgements:
This project was supported by the United States National Institutes of Health (NIH; R01HL120879 and 5UL1TR001425). We would like to acknowledge the contributions of adolescent participants and their families. We also acknowledge the assistance of the many assistants who helped to run the study but are not authors on this paper, especially Shealan McAlister, Nathan Lutz, Juliana Rizzo, Caitlin Brammer, Perry Catlin, Angela Moore, and Tori Van Dyk.
Footnotes
Financial Disclosure: None to disclose
Non-Financial Disclosure: None to disclose
References
- Appelhans BM, Woolf K, Pagoto SL, Schneider KL, Whited MC, & Liebman R. (2011). Inhibiting food reward: Delay discounting, food reward sensitivity, and palatable food intake in overweight and obese women. Obesity, 19(11), 2175–2182. doi: 10.1038/oby.2011.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarnow LD, Greer SM, Walker MP, & Harvey AG (2017). The impact of sleep improvement on food choices in adolescents with late bedtimes. Journal of Adolescent Health, 60(5), 570–576. doi: 10.1016/j.jadohealth.2016.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson J, & Vyazovskiy VV (2015). Banking sleep and biological sleep need. Sleep, 38(12), 1843–1845. doi: 10.5665/sleep.5222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron KG, Reid KJ, Kern AS, & Zee PC (2011). Role of sleep timing in caloric intake and BMI. Obesity, 19(7), 1374–1381. doi: 10.1038/oby.2011.100 [DOI] [PubMed] [Google Scholar]
- Beebe DW, Powers SW, Slattery EW, & Gubanich PJ (2018). Short sleep and adolescents’ performance on a concussion assessment battery: An experimental sleep manipulation study. Clinical Journal of Sport Medicine, 28(4), 395–397. doi: 10.1097/jsm.0000000000000454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DW, Simon S, Summer S, Hemmer S, Strotman D, & Dolan LM (2013). Dietary intake following experimentally restricted sleep in adolescents. Sleep, 36(6), 827–834. doi: 10.5665/sleep.2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Ho C-Y, Richard JM, & Difeliceantonio AG (2010). The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Research, 1350, 43–64. doi: 10.1016/j.brainres.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervin RD, Hedger K, Dillon JE, & Pituch KJ (2000). Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Medicine, 1(1), 21–32. doi: 10.1016/s1389-9457(99)00009-x [DOI] [PubMed] [Google Scholar]
- Chervin RD, & Hedger KM (2001). Clinical prediction of periodic leg movements during sleep in children. Sleep Medicine, 2(6), 501–510. doi: 10.1016/s1389-9457(01)00069-7 [DOI] [PubMed] [Google Scholar]
- Davis C, Patte K, Levitan R, Reid C, Tweed S, & Curtis C. (2007). From motivation to behaviour: A model of reward sensitivity, overeating, and food preferences in the risk profile for obesity. Appetite, 48(1), 12–19. doi: 10.1016/j.appet.2006.05.016 [DOI] [PubMed] [Google Scholar]
- De Decker A, Sioen I, Verbeken S, Braet C, Michels N, & De Henauw S. (2016). Associations of reward sensitivity with food consumption, activity pattern, and BMI in children. Appetite, 100, 189–196. doi: 10.1016/j.appet.2016.02.028 [DOI] [PubMed] [Google Scholar]
- Demos KE, Sweet LH, Hart CN, McCaffery JM, Williams SE, Mailloux KA, . . . Wing RR (2017). The effects of experimental manipulation of sleep duration on neural response to food cues. Sleep, 40(11). doi: 10.1093/sleep/zsx125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraccio KM, Krietsch KN, Chardon ML, Van Dyk TR, & Beebe DW (2019). Poor sleep and adolescent obesity risk: a narrative review of potential mechanisms. Adolescent health, medicine, and therapeutics, 10, 117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraccio KM, Zaugg K, & Jensen CD (2019). Effects of sleep restriction on food-related inhibitory control and reward in adolescents. Journal of Pediatric Psychology, 44(6), 692–702. doi: 10.1093/jpepsy/jsz008 [DOI] [PubMed] [Google Scholar]
- Emsellem H, Knutson K, Hillygus D, Buxton O, Montgomery-Downs H, LeBourgeois M, & Spilsbury J. (2014). 2014 sleep in America poll: Sleep in the modern family. Retrieved from https://www.sleepfoundation.org/professionals/sleep-america-polls/2014-sleep-modern-family [Google Scholar]
- Epstein LH, Dearing KK, & Roba LG (2010). A questionnaire approach to measuring the relative reinforcing efficacy of snack foods. Eating Behaviors, 11(2), 67–73. doi: 10.1016/j.eatbeh.2009.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Paluch RA, Carr KA, Temple JL, Bickel WK, & Mackillop J. (2018). Reinforcing value and hypothetical behavioral economic demand for food and their relation to BMI. Eating Behaviors, 29, 120–127. doi: 10.1016/j.eatbeh.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Yokum S, Feda DM, & Stice E. (2014). Food reinforcement and parental obesity predict future weight gain in non-obese adolescents. Appetite, 82, 138–142. doi: 10.1016/j.appet.2014.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feda DM, Roemmich JN, Roberts A, & Epstein LH (2015). Food reinforcement and delay discounting in zBMI-discordant siblings. Appetite, 85, 185–189. doi: 10.1016/j.appet.2014.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CN, Carskadon MA, Considine RV, Fava JL, Lawton J, Raynor HA, . . . Wing R. (2013). Changes in children’s sleep duration on food intake, weight, and leptin. PEDIATRICS, 132(6), e1473-e1480. doi: 10.1542/peds.2013-1274 [DOI] [PubMed] [Google Scholar]
- Hill C, Saxton J, Webber L, Blundell J, & Wardle J. (2009). The relative reinforcing value of food predicts weight gain in a longitudinal study of 7-10-y-old children. The American Journal of Clinical Nutrition, 90(2), 276–281. doi: 10.3945/ajcn.2009.27479 [DOI] [PubMed] [Google Scholar]
- Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, Doncarlos L, . . . Ware JC (2015). National Sleep Foundation’s updated sleep duration recommendations: Final report. Sleep Health, 1(4), 233–243. doi: 10.1016/j.sleh.2015.10.004 [DOI] [PubMed] [Google Scholar]
- Jensen CD, Duraccio KM, Barnett KA, Carbine KA, Stevens KS, Muncy NM, & Kirwan CB (2019). Sleep duration differentially affects brain activation in response to food images in adolescents with overweight/obesity compared to adolescents with normal weight. Sleep, 42(4). doi: 10.1093/sleep/zsz001 [DOI] [PubMed] [Google Scholar]
- Jensen CD, Duraccio KM, Barnett KA, & Stevens KS (2016). Appropriateness of the food-pics image database for experimental eating and appetite research with adolescents. Eating Behaviors, 23, 195–199. doi: 10.1016/j.eatbeh.2016.10.007 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, . . . Ryan N. (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36(7), 980–988. [DOI] [PubMed] [Google Scholar]
- Kral TVE, Remiker AM, Strutz EM, & Moore RH (2014). Role of child weight status and the relative reinforcing value of food in children’s response to portion size increases. Obesity, 22(7), 1716–1722. doi: 10.1002/oby.20757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krietsch KN, Chardon ML, Beebe DW, & Janicke DM (2019). Sleep and weight-related factors in youth: A systematic review of recent studies. Sleep Medicine Reviews, 46, 87–96. doi: 10.1016/j.smrv.2019.04.010 [DOI] [PubMed] [Google Scholar]
- Lo JC, Ong JL, Leong RLF, Gooley JJ, & Chee MWL (2016). Cognitive performance, sleepiness, and mood in partially sleep deprived adolescents: The need for sleep study. Sleep, 39(03), 687–698. doi: 10.5665/sleep.5552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue EE, Bourguet CC, Palmieri PA, Scott ED, Matthews BA, Dudley P, & Chipman KJ (2012). The better weight-better sleep study: A pilot intervention in primary care. American Journal of Health Behavior, 36(3), 319–334. doi: 10.5993/ajhb.36.3.4 [DOI] [PubMed] [Google Scholar]
- Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, & Wright KP (2013). Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proceedings of the National Academy of Sciences, 110(14), 5695–5700. doi: 10.1073/pnas.1216951110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Kruisbrink M, Wallace J, Ji C, & Cappuccio FP (2018). Sleep duration and incidence of obesity in infants, children, and adolescents: a systematic review and meta-analysis of prospective studies. Sleep, 41(4), 1–19. doi: 10.1093/sleep/zsy018 [DOI] [PubMed] [Google Scholar]
- Rollins BY, Loken E, Savage JS, & Birch LL (2014). Measurement of food reinforcement in preschool children. Associations with food intake, BMI, and reward sensitivity. Appetite, 72, 21–27. doi: 10.1016/j.appet.2013.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Sharkey M, & Carskadon MA (1994). Activity-based sleep-wake identification: An empirical test of methodological issues. Sleep, 17(3), 201–207. doi: 10.1093/sleep/17.3.201 [DOI] [PubMed] [Google Scholar]
- Sallinen BJ, Hassan F, Olszewski A, Maupin A, Hoban TF, Chervin RD, & Woolford SJ (2013). Longer weekly sleep duration predicts greater 3-month BMI reduction among obese adolescents attending a clinical multidisciplinary weight management program. Obesity Facts, 6(3), 239–246. doi: 10.1159/000351819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Field J, Miller LE, Difrancesco M, & Beebe DW (2015). Sweet/dessert foods are more appealing to adolescents after sleep restriction. PLOS ONE, 10(2), 1–8. doi: 10.1371/journal.pone.0115434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Koffarnus MN, Snider SE, Quisenberry AJ, & Bickel WK (2015). Identification and management of nonsystematic purchase task data: Toward best practice. Experimental and Clinical Psychopharmacology, 23(5), 377–386. doi: 10.1037/pha0000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E, Healey D, Gray AR, & Galland BC (2012). Sleep hygiene intervention for youth aged 10 to 18 years with problematic sleep: A before-after pilot study. BMC Pediatrics, 12(1), 189. doi: 10.1186/1471-2431-12-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valrie CR, Bond K, Lutes LD, Carraway M, & Collier DN (2015). Relationship of sleep quality, baseline weight status, and weight-loss responsiveness in obese adolescents in an immersion treatment program. Sleep Medicine, 16(3), 432–434. doi: 10.1016/j.sleep.2014.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyk TR, Krietsch KN, Saelens BE, Whitacre C, McAlister S, & Beebe DW (2018). Inducing more sleep on school nights reduces sedentary behavior without affecting physical activity in short-sleeping adolescents. Sleep Medicine, 47, 7–10. doi: 10.1016/j.sleep.2018.03.007 [DOI] [PubMed] [Google Scholar]
- Verbeken S, Braet C, Lammertyn J, Goossens L, & Moens E. (2012). How is reward sensitivity related to bodyweight in children? Appetite, 58(2), 478–483. doi: 10.1016/j.appet.2011.11.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.