Abstract
Introduction
Primary deficits in individuals with cerebellar degeneration include ataxia, unstable gait, and incoordination. Balance training is routinely recommended to improve function whereas little information is known regarding aerobic training.
Objective
To determine the feasibility of conducting a randomized trial comparing balance and aerobic training in individuals with cerebellar degeneration.
Design
Accessor blinded randomized control phase I trial
Setting
Assessments in medical center, home-training
Participants
20 participants with cerebellar degeneration were randomized to home balance or aerobic training.
Intervention
Aerobic training consisted of four-weeks of stationary bicycle training, five times per week for 30-minute sessions. Home balance training consisted of performing the same duration of easy, moderate, and/or hard exercises.
Outcome Measures
Scale for the Assessment and Rating of Ataxia (SARA), maximal oxygen consumption (VO2max), Dynamic Gait Index (DGI), Timed up and Go (TUG), gait speed
Results
All 20 participants completed assigned training with no major adverse events. Seven of each group attained target training duration, frequency, and intensity. Although both groups had significant improvements in ataxia severity, balance, and gait measures, there were greater improvements in individuals who performed aerobic training in ataxia severity and maximal oxygen consumption when compared to balance training. The effect size for these outcome measures was determined to be large, indicating a phase II trial comparing the benefits of aerobic and balance training was feasible and required 26 subjects per group.
Improvements in SARA score and VO2max remained in the aerobic training group at 3 months post-training, but these improvements were trending back to baseline. In contrast, all balance group measures for pre-training and 3 month post-training were statistically similar.
Conclusions
A phase II trial comparing balance and aerobic training in individuals with cerebellar degeneration is feasible. Benefits trended back toward baseline after training stopped, although benefits of longer duration exercise programs still need to be determined.
Keywords: Ataxia, rehabilitation, spinocerebellar degeneration, aerobic exercise, balance training, treatment outcome
Introduction
Degenerative cerebellar diseases are a group of rare, debilitating disorders that cause progressive decline in activities of daily living.1 Unfortunately, at present, there are no cures for these diseases.2 Current guidelines recommend performance of balance exercises to maintain function,3 although research regarding the benefit of balance training has shown inconsistent results.4–10
Upon examination of the literature, most home-training balance programs demonstrated limited improvement in ataxia severity4,5 whereas supervised training programs were more effective.7–10 One explanation for this discrepancy may be due to subjects not challenging themselves with the home-training. Indeed, even though significant improvements in ataxia severity were not seen, Keller et al found that individuals who reported the greatest challenge in their home balance-training improved the most.4
In contrast to balance training where multiple studies have been conducted, the benefits of aerobic training for individuals with degenerative cerebellar diseases are less studied. One investigation was conducted in 2014 with twenty individuals with spinocerebellar ataxia who were randomly assigned to either four weeks of aerobic training or control group, who performed upper extremity stretching exercises. Training duration was limited to fifteen minutes, and exercise intensity was not monitored. Results demonstrated only slight improvements in the aerobic training group when compared to control.12
We suspected that the minimal benefits of aerobic exercise seen in the 2014 article were due to the limited exercise performed. Thus, we designed a study to determine the feasibility of performing intensive aerobic training in individuals with cerebellar degeneration. We showed that our training program was safe and appeared to improve ataxia severity, balance, and gait speed when compared to a non-training control group. Results of this part of the study have been previously reported.13
This report is a continuation of the prior work where we compare the 4-week home intensive aerobic training program to a home balance program that the nontraining control group completed after waiting 4 weeks. We decided to divide the work into two manuscripts for clarity. The goal of the previously published article was to determine if intensive aerobic training was safe. We then compared the aerobic group to a non-training group, which required a four week wait period, to ensure we would see training effects with aerobic training before comparison with balance training.
The main goal of this work was to determine the effect size of outcome measures between aerobic and balance training to ensure a larger, phase II trial was feasible. This information was required because degenerative cerebellar diseases are rare, and if small effect sizes were determined, it would not be possible to recruit enough subjects for the phase II trial. In this paper, we also wanted to determine if training had longer term effects by monitoring outcome measure three months post-training.
Methods
Participants
Twenty subjects with degenerative cerebellar disease aged 20 to 70 years volunteered to participate. Potential participants were recruited from the ataxia clinic at the Neurological Institute at New York Presbyterian-Columbia. All potential participants were screened in-person by a research team member for inclusion and exclusion criteria. Inclusion criteria included cerebellar atrophy on magnetic resonance imaging, at least mild ataxia on clinical exam (the Scale for the Assessment and Rating of Ataxia (SARA) >3)14, and the ability to sit safely and ride a stationary exercise bike (SARA sitting sub-score 0). Participants were excluded if they had other neurological diseases, cognitive impairment (i.e., Mini-Mental State exam15 score <22), heart disease, joint pain, inability to exercise, inability to walk without assistance from another individual, or if they were medically unstable. Genetic testing results were obtained if available. Individuals without a known genetic mutation (idiopathic cerebellar ataxia) were allowed to participate since this group represents about 20% of all cases.16 Individuals with Multiple System Atrophy-Cerebellar type were also allowed to participate as long as their predominant neurological deficit was ataxia.
The study was approved by the Columbia University Institutional Review Board (reference number AAAR5381) and registered at www.clinicaltrials.gov (study ID NCT03745248). It was conducted from November 14, 2018 to October 14, 2019. All participants gave written informed consent approved by the Institutional Review Board. The study consisted of one pre-training visit and two post-training visits (one conducted immediately after training and one three months post-training). The intervention was either 4-weeks of home aerobic training or home balance training.
Assessments
SARA
The primary clinical outcome measure was SARA, a 40-point scale that evaluates the degree of ataxia. Lower scores equate to less ataxia on exam. The scale has excellent test-retest reliability, inter-rater reliability, and internal consistency.14 Recent clinical trials have used a reduction of 1.0 point on the SARA scale as a clinically meaningful difference.17,18
Cardiopulmonary Exercise Testing
Cardiopulmonary exercise testing was used to determine maximal oxygen consumption by a previously described protocol.13 After a three-minute warm-up, participants used an electronic lower body cycle ergometer (CareFusion Corp, San Diego, CA) to undergo progressive ramped exercise. A Vmax Encore Metabolic System (CareFusion Corp, San Diego, CA) was used to measure oxygen consumption during the test. Maximal oxygen consumption criteria included respiratory exchange ratio 1.1, increases in ventilation without concomitant increases in oxygen consumption, and/or maximum age-predicted heart rate. The test was terminated if participants pedaled below 40 RPM for five seconds, or when the participant reached volitional exhaustion.19 A physician was available during all cardiopulmonary exercise tests to monitor cardiac response.
Balance and Gait Clinical Measures
Participants were asked to walk as fast as possible on a 10-meter runway three times. Gait speed was measured by averaging the three times. The Dynamic Gait Index20 and Timed Up and Go21 tests were performed according to established protocols to further monitor balance and gait.
Interventions
Aerobic Training
A ProGear 225 Folding Magnetic Upright Exercise Bike with Heart Pulse (Beverly Hills, CA) was provided to participants who did not have access to a bike with heart rate monitor. Individuals were asked to exercise 30 minutes a day, 5 days a week for a duration of 4 weeks. Exercise intensity started at 65% of Cardiopulmonary Exercise Testing (CPET) maximal heart rate and increased by 5% every week until participants maintained 80% of CPET maximal heart rate. Rate of perceived exertion (Borg scale)22 was also assessed on each training session with scores expected around 12 during initial training and 15 by the end of 4 weeks. After 4-weeks, participants were encouraged to continue exercising, but no longer required to as part of the study.
An exercise log was provided for entering dates and duration of exercise. Participants were also asked to record average heart rate, maximum heart rate, Borg score, and cycling distance. Weekly phone calls were performed to address questions, allow for the report of any adverse events, and encourage participants to continue exercise.
Balance Training
After 4 weeks of non-training, participants assigned to the balance training group were given three levels of exercises: easy, moderate, and hard. Within each level, exercises were labeled as: 1) sitting on stable surface, 2) sitting on unstable surface (exercise ball), 3) standing on a stable surface, 4) standing on unstable surface (pillow), or 5) walking. A list of all exercises can be found in [dummy_chk]Appendix 1.
Participants were told to perform 30 minutes of exercise, 5 times a week for 4-weeks. Specifically, they were asked to do six different exercises for a duration of 5 minutes each. They were to choose exercises from different sections and to constantly vary the exercise they performed to increase balance challenge.
An exercise log was provided for entering dates and duration of exercise. Participants were also asked to record level of balance challenge (1–10 scale with 10 being of highest difficulty) and confidence in maintaining balance (0–100% with 0 representing no loss of balance during training and 100% indicating a fall). Weekly phone calls were performed to address questions, allow for the report of adverse events, and encourage participants to continue exercise. Participants were also asked their average level of balance challenge for the week. If the number was less than 6, they were asked to perform more challenging exercises, such as going from easy level to moderate.
Group Assignment
A team member, who was not involved in outcome assessments, used stratified randomization, with a 1:1 allocation ratio and varying blocks of 2 and 4, to assign subjects. The stratum variable was disease severity (mild [SARA <10] and moderate [SARA ≥10]).23
Group allocation was done after baseline assessment to ensure blinding. At future assessment points, accessors were blinded to group allocation, and participants were reminded not to reveal their allocation. Due to the nature of the intervention, none of the participants were blinded to assignment.
Subjects in the aerobic group performed 4-weeks of cycling at home after one baseline assessment. Post-training assessments were performed immediately after completion of the exercise and three months post-training. Individuals in the balance group underwent two assessments, 4-weeks apart, with no intervention. After the second assessment, participants were given the balance training program to perform for 4-weeks. Post-training assessments were performed immediately and three months after balance training. At the 3-month post-training assessment for both groups, individuals were asked if they continued to train. If the participants continued to train, there were then asked about duration, frequency, and intensity of training.
Statistical Analysis
Participants baseline characteristics were described using means ± SDs for continuous variables. The unpaired t test, Wilcoxon rank-sum test, and the chi-squared were used to compare demographic and baseline outcome measures where applicable.
All analysis was done under intent-to-treat principles. Outcomes were analyzed using mixed effect model with group and time and their interaction. Within subject correlation was accounted by adding random intercept. All mixed effect models converged. Least square means procedure tested post-pre changes by group, and the results are equivalent to the contrast tests. Supplementary Table 1 and 2 provides the full parameter estimates of the mixed effect models.
We used the results obtained in this feasibility study to determine effect sizes (partial eta-squared, η2) and standard deviations. Pass Software (v19.0.3) was then used to estimate sample size for future studies setting power to 0.8 and α at 0.05.
Results
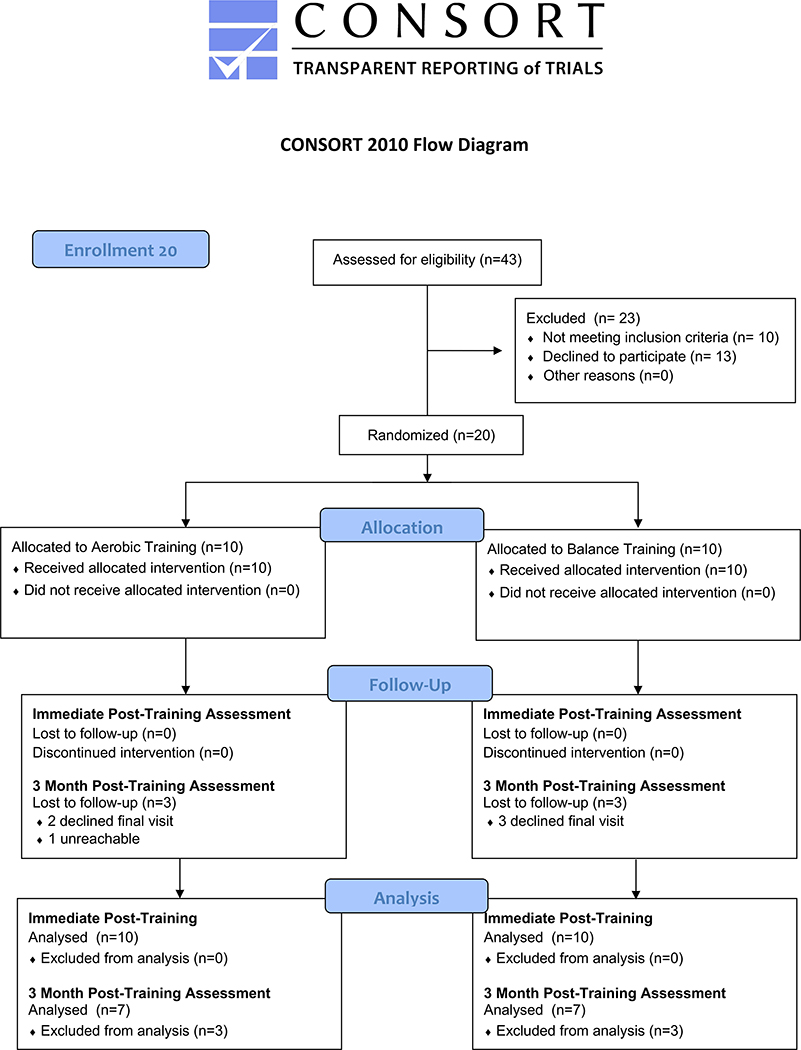
Twenty individuals with cerebellar degeneration enrolled in the study, and ten participants were randomized into each intervention group. There was no drop-out in either group at the time of the immediate post-training assessment. Six individuals, three in each group, declined to participate in the 3-month post-training assessment. Figure 1 shows the flow of participants through each stage of the randomized trial.
Figure 1.
Consort Flow Diagram
Adherence to the aerobic training program is published in a separate study, as is comparison of aerobic training to the non-training control waiting group.13 All participants exercised at least 30 minutes a day, 3 days a week. Seven of the ten aerobic training group participants achieved target training duration, frequency, and intensity.
Adherence to the balance training program is shown in Table 1. All participants in the balance group hit target training duration, seven of ten hit target training frequency, and eight of ten hit training intensity (at least a 6 on level of balance). All participants in the balance group trained at least 30 minutes a day, 3 days a week (Table 1).
Table 1.
Frequency, Duration, and Challenge of Balance Training
| ID | Training Frequency (days per week) | Training Duration (minutes) | Balance Challenge | Challenge Level | Achieved Training Targets |
|---|---|---|---|---|---|
| 2 | 4 | 30 | 69.6% | 7.3 | No |
| 3 | 5 | 30 | 62.5% | 6.25 | Yes |
| 5 | 5 | 30 | 78.5% | 7.2 | Yes |
| 6 | 5 | 30 | 72.5% | 7.25 | Yes |
| 9 | 5 | 30 | 67.5% | 7 | Yes |
| 11 | 5 | 30 | 68% | 6 | Yes |
| 15 | 5 | 30 | 75% | 8 | Yes |
| 17 | 5 | 30 | 55% | 6 | Yes |
| 19 | 4 | 30 | 50% | 5 | No |
| 20 | 3 | 30 | 50% | 5 | No |
Balance Challenge was determined by taking the average of individual’s ratings of maintaining balance during each session (0-100% with 0 representing no loss of balance during training and 100% indicating a fall). Challenge level was calculated by taking the average of individual’s ratings of balance difficulty on a scale of 1-10 with 10 being of highest difficulty. Achieved Training Target was determined if patient met training frequency of 5 days per week, 30 minutes per day, and challenge level of at least 6.
There were no major adverse events in either group. In the aerobic training group, one participant experienced an episode of back pain, which was relieved with medication and did not hinder further training. In the balance group, there were three reported falls. As the participants were instructed to perform exercises in a safe environment, (for example, next to the bed), there were no injuries associated with the falls, and the falls did not alter training.
There was no statistical difference in baseline characteristics between groups, although there were chance imbalances observed (Table 2). Both balance training and aerobic training showed significant improvements in TUG, DGI, walking speed, and SARA scores. However, aerobic training improved SARA scores and VO2Max more than balance training. An average reduction of 2.1 points (95% CI [−1.32, −2.88]) in SARA was seen with aerobic training whereas balance training reduced SARA scores by an average of 1.0 point (95% CI [−0.38, −1.62]). VO2Max increased on average by 4.6ml/kg/min (95% CI [2.67, 6.59]) with aerobic training whereas balance training only increased VO2Max by an average of 1.5 ml/kg/min (95% CI [−0.49, 23.49]). The effect size, η2, between aerobic and balance training groups was 0.233 and 0.213 for these measures, respectively (Table 2). These effect sizes are considered large, and when used to calculate sample size for a phase II trial, they indicated that 26 participants per group were needed.
Table 2.
Comparison of Outcome Measures for Aerobic and Balance Training
| Aerobic Training | Balance Training | Group Difference Comparisons | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline Assessment* | Post-training Assessment** | Difference [95% CI] | Baseline Assessment* | Post-training Assessment** | Difference [95% CI] | η2 | P-Value | |
| SARA | 9.1 (2.9) | 7.0 (2.8) | −2.1 [−1.32, −2.88] | 10.6 (3.5) | 9.6 (3.6) | −1.0 [−0.38, −1.62] | 0.233 | 0.03 |
| Walking speed in m/s | 0.99 (0.28) | 1.14 (0.35) | 0.15 [0.09,0.21] | 0.77 (0.35) | 0.95 (0.33) | 0.18 [0.11, 0.25] | 0.064 | 0.28 |
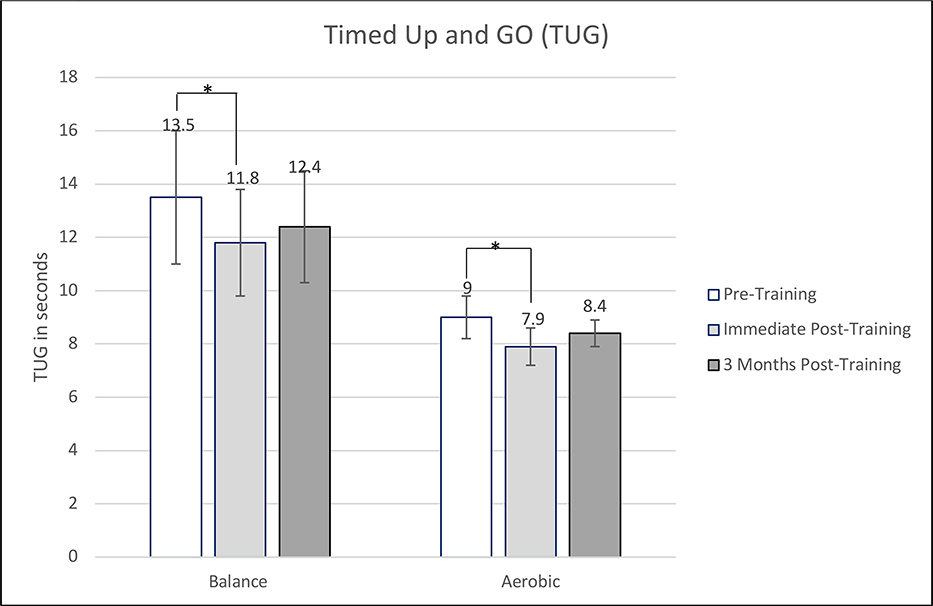
| TUG in seconds | 13.2 (8.0) | 11.5 (7.4) | −1.7 [−0.62, −2.78] | 16.3 (8.8) | 13.5 (6.5) | −2.9 [−1.60, −4.12] | 0.081 | 0.22 |
| VO2Max in ml/kg/min | 23.4 (10.0) | 28.1 (10.4) | 4.6 [2.67, 6.59] | 24.6 (7.2) | 26.1 (7.3) | 1.5 [−0.49, 3.49] | 0.213 | 0.04 |
| DGI | 17.5 (3.5) | 19.0 (3.3) | 1.5 [0.76, 2.24] | 14.3 (5.1) | 16.3 (4.7) | 2.0 [1.12, 2.88] | <0.01 | 0.44 |
Data shown is mean (SD). Effect size is reported by partial eta squared (η2).
No statistical difference (p>0.05) was seen between groups at baseline assessment.
There was a statistical difference (p<0.05) between baseline assessment and post-training assessment for all outcome measures for both groups except for VO2Max in the balance group
Results of the 3-month post-training assessments for the fourteen individuals that participated are shown in Figure 2. Only two of seven participants in the aerobic group continued to perform training. The training, however, was on average twice a week and limited in intensity. In the balance group, only one person continued to train. This person continued to train on average 4 days a week, 30 minutes a day, with a level of balance challenge around 6. Results indicated that there was still a mean improvement in SARA scores (1.2 points) and VO2Max (2.4 ml/kg/min) after three months post-training in the aerobic group when compared to baseline measurements. However, all parameters were trending back toward baseline. None of the outcome measures were significantly different than baseline measures in the balance training group at 3-months post-training (Figure 2a,b,c,d,e).
Figure 2.
Comparison of outcome measure scores for pre-training, immediately post-training, and 3 months post-training. A) Scale for the Assessment and Rating of Ataxia (SARA). B) Walking speed. C) Timed Up and Go (TUG). D) Maximal Oxygen Consumption (VO2max). E) Dynamic Gait Index (DGI). *Statistically significant (p<0.05).
Discussion
In this study, twenty individuals with degenerative cerebellar disease were randomized to either home balance or home aerobic training. As expected, there was a significant improvement in fitness level as measured by VO2Max, which was not seen in the balance training group. Interestingly, individuals who performed aerobic training had a significantly larger reduction in ataxia severity than those who performed balance training. Although SARA scores and VO2Max remained significantly improved 3-months post-training in the aerobic group, ataxia severity, balance, and gait measures all trended back to baseline. There was no difference between pre-training and 3-month post-training balance, gait, and ataxia severity measures in the balance group.
Previous unsupervised home balance training exercises have not shown significant improvements in ataxia severity, and a possible reason was the lack of balance challenge.4,5 In this study, we focused on challenging the balance training group by giving exercises of varying balance challenge levels, asking participants to switch exercises regularly, and increasing challenge level of exercises if participants reported low balance challenge. As a result, our balance training program reduced ataxia severity by 1.0 points on the SARA scale. Although lower than the 1.4 to 2.8 point reduction in SARA score seen in some studies with supervised balance training,7–10 our results support that balance challenge is critical for improvements in ataxia severity for individuals with degenerative cerebellar disease. Of note, with increased challenge in our balance training group, three falls were seen. Most of these falls were at the beginning of the training program, possibly due to selecting overly challenging exercises. In a future study, it may be advisable to have one supervised balance training session to determine ability and to only give exercises for the level most appropriate.
Our study also indicates that home aerobic training may be more beneficial for individuals with degenerative cerebellar disease than home balance training. A statistical larger improvement in SARA and VO2max was seen in the aerobic training group than the balance training group whereas improvements in gait and balance were similar between groups. It is unclear the reason why this is the case. Most exercise requires at least 8-weeks to cause physiologic effects, so the benefits may not be a result of changes at the cellular level.25 Thus, the greater benefits may be due to higher ability to compensate for deficits with aerobic training versus balance training. For example, improved fitness may allow individuals to combat fatigue-induced worsening of ataxia. Moreover, leg strengthening from the cycling may have improved stability. Future studies should examine the mechanism in which both balance training and aerobic training improves individuals with degenerative cerebellar disease.
One of the major implications of this study was it demonstrates a larger, phase II trial comparing home aerobic training to home balance training is technically feasible. First, adherence to both the aerobic training and the balance training was high with 7 out of 10 participants hitting training frequency, duration, and intensity goals. Furthermore, all participants exercised, at a minimum, 30 minutes a day, 3 days per week. There was also no dropout from the study during the training phase, and only minor adverse events were reported with training. Using the effect sizes and standard deviations from this study, we calculated that 26 individuals would be required in each group to have 80% power to detect changes in ataxia severity between balance and aerobic training. Although there was no dropout in this study, we will conservatively estimate a 20% dropout rate in a larger trial. Thus, 32 individuals would be required per group in a more definitive, future trial.
This future trial should also examine the long-term effects of training. The preliminary findings in this study show that improvements in training decline back to baseline if training is halted. The benefits of aerobic training were slightly longer lasting than the balance training benefits as improvements in SARA score and VO2max remained significant in the aerobic group, but not in the balance group. However, these benefits in SARA score and VO2max were significantly less than the benefits seen immediately post-aerobic training. Future studies should examine training of longer duration to see if this causes changes that are more long-lasting.
Limitations
One of the main limitations of this study is the small sample size. Even though there were no statistical differences between aerobic and balance groups, the small sample size allowed for discrepancies in baseline measures. For example, the mean aerobic training group baseline SARA score was 1.5 points lower than the mean balance training group baseline SARA score. These chance imbalances impact the interpretation of results and highlights the importance of a future, larger study. Another limitation is that a heterogenous group of degenerative cerebellar disorders was studied to ensure adequate enrollment. Thus, it is possible aerobic training may be of more benefit in certain degenerative cerebellar disorders than others. With only a few individuals with each specific disease, this study is unable to answer this question. Finally, as the role of rigorous aerobic training in cerebellar degeneration has not been previously studied, a power analysis was not conducted prior to this study.
Conclusions
A larger, phase II trial comparing aerobic and balance training is technically feasible. Although improvements in gait and balance may be similar, improvements in ataxia severity and fitness may be greater with aerobic training than balance training. Statistical improvements in SARA score and VO2max remained in the aerobic training group at 3-months post-training, but improvements were trending back to baseline. There was no difference between pre-training and 3 month post-training measures of ataxia severity, gait and balance for individuals who performed balance training.
Supplementary Material
Acknowledgements
The authors thank all people with cerebellar degeneration who participated in this study. The study was conceived, organized, and managed by S.B., S.A., S.K., M.O., and J.S. Data collection were by N.L, D.M., and I.O. L.B. designed the balance program. Statistic analysis was done by S.L. Recruitment was conducted by S.K. and S.B. Writing of the first draft of the paper was done by S.B. All listed authors were involved in preparation, review, and critique of the manuscript.
Financial Support
The study was funded by the department of physical medicine and rehabilitation at Columbia Medical Center. Additional funding was provided by the KL2 Career Development Grant, TR001874.
Footnotes
Declaration of Conflicting Interests
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Clinical Trial Registration Number htpp://www.clinicaltrials.gov. Unique identifier: NCT03745248
Study Location
Columbia Medical Center, 180 Fort Washington, New York, NY 10032
Supplemental Material
Supplemental material for this article is available online.
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/pmrj.12401
References
- 1.Salman MS. “Epidemiology of cerebellar disease and therapeutic approaches.” The Cerebellum. 2018; 17: 4–11. [DOI] [PubMed] [Google Scholar]
- 2.Parodi L, Coarelli G, Stevanin G, Brice A, Durr A. “Hereditary ataxias and paraparesias: clinical and genetic update.” Curr Opin Neurol. 2018; 31: 462–471. [DOI] [PubMed] [Google Scholar]
- 3. https://ataxia.org.
- 4.Bastian A and Keller J. “A home balance exercise program improves walking in people with cerebellar ataxia.” Neurorehabil Neural Repair. 2014; 28: 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunn LM, Marsden JF, Giunti P, Day BL. “Training balance with opto-kinetic stimuli in the home: a randomized controlled feasibility study in people with pure cerebellar disease.” Clinical Rehabilitation. 2015; 29: 143–153. [DOI] [PubMed] [Google Scholar]
- 6.Fonten EM, Heeren A, Engels JC, Den Boer JJ, van de Warrenburg BP, Weerdesteyn V. “Gait adaptability training improves obstacle avoidance and dynamic stability in patients with cerebellar degeneration.” Gait & Posture. 2014; 40:247–251. [DOI] [PubMed] [Google Scholar]
- 7.Miyai I, Mizuki I, Hattori N, Mihara M, et al. “Cerebellar ataxia rehabilitation trial in degenerative cerebellar diseases.” Neurorehabil Neural Repair. 2012; 26: 515–522. [DOI] [PubMed] [Google Scholar]
- 8.Ilg W, Synofzik M, Brotz D, Burkard S, et al. ‘Intensive coordinative training improves motor performance in degenerative cerebellar disease.” Neurology. 2009; 73: 1823–1830. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Diax JC, Velazquez-Perez L, Labrada RR, Rodriguez RA, Perez DL, et al. “Neurorehabilitation therapy in spinocerebellar ataxia type 2: a 24-week, rater-blinded, randomized, controlled trial.” Movement Disorders. 2018; 33: 1481–1487. [DOI] [PubMed] [Google Scholar]
- 10.Tercero-Perez K, Cortes H, Torres-Ramos Y, Rodriguez-Labrada R, et al. “Effects of physical rehabilitation in patients with spinocerebellar ataxia type 7.” Cerebellum. 2019. 10.1007/s12311-019-1006-1. [DOI] [PubMed] [Google Scholar]
- 11.Ilg W, Schatton C, Schicks J, Giese M, Schols L, Synofzik M. “Video game-based coordinative training improves ataxia in children with degenerative ataxia.” Neurology. 2012; 79:2056–2060. [DOI] [PubMed] [Google Scholar]
- 12.Chang Y, Chou C, Huang W, Song C, et al. Cycling regimen induces spinal circuitry plasticity and improves leg muscle coordination in individuals with spinocerebellar ataxia. Archives of physical medicine and rehabilitation. 2015; 96: 1006–1013. [DOI] [PubMed] [Google Scholar]
- 13.Barbuto S, Martelli DM, et al. “Phase I single blinded randomized trial investigating the role of aerobic training in degenerative cerebellar disease.” Clinical Rehabil. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yabe I, Matsushima M, Soma H, Basri R, Sasaki H. “Usefulness of the Scale for Assessment and Rating of Ataxia (SARA).” J Neurol Sci. 2008; 266: 164–166. [DOI] [PubMed] [Google Scholar]
- 15.Pangman VC, Sloan J, Guse L (2000). “An Examination of Psychometric Properties of the Mini-Mental Status Examination and the Standardized Mini-Mental Status Examination: Implications for Clinical Practice.” Applied Nursing Research. 2000; 13;4: 209–213. [DOI] [PubMed] [Google Scholar]
- 16.Rossi M, Perez-Lloret S, Doldan L, et al. “Autosomal dominant cerebellar ataxias: a systemic review of clinical features.” Eur J Neurol 2014;21:607–615. [DOI] [PubMed] [Google Scholar]
- 17.Romano S, Coarelli G, Marcotulli C, et al. “Riluzole in patients with hereditary cerebellar ataxia: a randomized, double blind, placebo-controlled trial.” Lancet Neurology. 2015; 14: 985–991. [DOI] [PubMed] [Google Scholar]
- 18. https://clinicaltrials.gov/ct2/show/NCT03347344?term=riluzole&cond=ataxia&draw=2&rank=2.
- 19.American College of Sports Medicine Guidelines for Exercise Testing and Prescription. 9th ed. Baltimore: Lippincott Williams and Wilkins, 2013. [Google Scholar]
- 20.Shumway-Cook A, Woollacott MH. “Motor control: theory and practical applications.” Williams & Wilkins Baltimore; 1995. [Google Scholar]
- 21.Podsiadlo D, Richardson S. “The timed “Up & Go”: a test of basic functional mobility for frail elderly persons.” J Am Geriatr Soc. 1991; 39: 142–148. [DOI] [PubMed] [Google Scholar]
- 22.Borg GA. “Psychophysical bases of perceived exertion”. Med Sci Sports Exerc. 1982; 14:377–81 [PubMed] [Google Scholar]
- 23.Lai RY, Tomishon D, Figueroa KP, Pulst SM, Perlman S, et al. “Tremor in the degenerative cerebellum: towards the understanding of brain circuitry for tremor.” Cerebellum. 2019. doi: 10.1007/s12311-019-01016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamini Y, Hochberg Y. “Controlling the false discovery rate: a practical and powerful approach to multiple testing.” Journal of the Royal Statistical Society. 1995; 57: 289–300. [Google Scholar]
- 25.Voet NB, Van der Kooi EL, et al. “Strength training and aerobic exercise training for muscular disease.” Cochrane Database Syst Rev. 2019; December 6;12:CD003907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.