Abstract
Considering the multifaceted protective and homeostatic roles of the complement system, many consequences arise when drug carriers, and particulate pharmaceutical formulations clash with complement proteins, and trigger complement cascade. Complement activation may induce formulation destabilization, promote opsonization, and affect biological and therapeutic performance of pharmaceutical nano- and micro-particles. In some cases, complement activation is beneficial, where complement may play a role in prophylactic protection, whereas uncontrolled complement activation is deleterious, and contributes to disease progression. Accordingly, design initiatives with particulate medicines should consider complement activation properties of the end formulation within the context of administration route, dosing, systems biology, and therapeutic perspective. Here we examine current progress in mechanistic processes underlying complement activation by pre-clinical and clinical particles, identify opportunities and challenges ahead, and suggest future directions in nanomedicine-complement interface research.
Keywords: Adjuvanticity, Adverse reactions, Complement inhibitors, Complement system, Complosome, Opsonization, Polymers, Protein adsorption
1. Introduction
The complement system, comprising >30 soluble and membrane-bound proteins, is an integral constituent of the innate immune system [1]. The lectin, alternative, and classical pathways are generally responsible for activation of the complement cascade (Figure 1). Complement orchestrates various protective, regenerative and inflammatory responses by bridging innate and adaptive arms of immune system, as well as cross talk with biological cascades such as the coagulation (contact) system (Figure 2) [1,2]. The two important defense functions of complement activation are opsonization, and the assembly of pore forming membrane-attack complex [1,3]. However, uncontrolled complement activation is deleterious to the host, causing cellular injury (e.g., through the assembly and insertion of the membrane attack complex into cell membranes), and contributing to inflammation, development and progression of disease (e.g., through liberation of excessive anaphylactic peptides) [2–6].
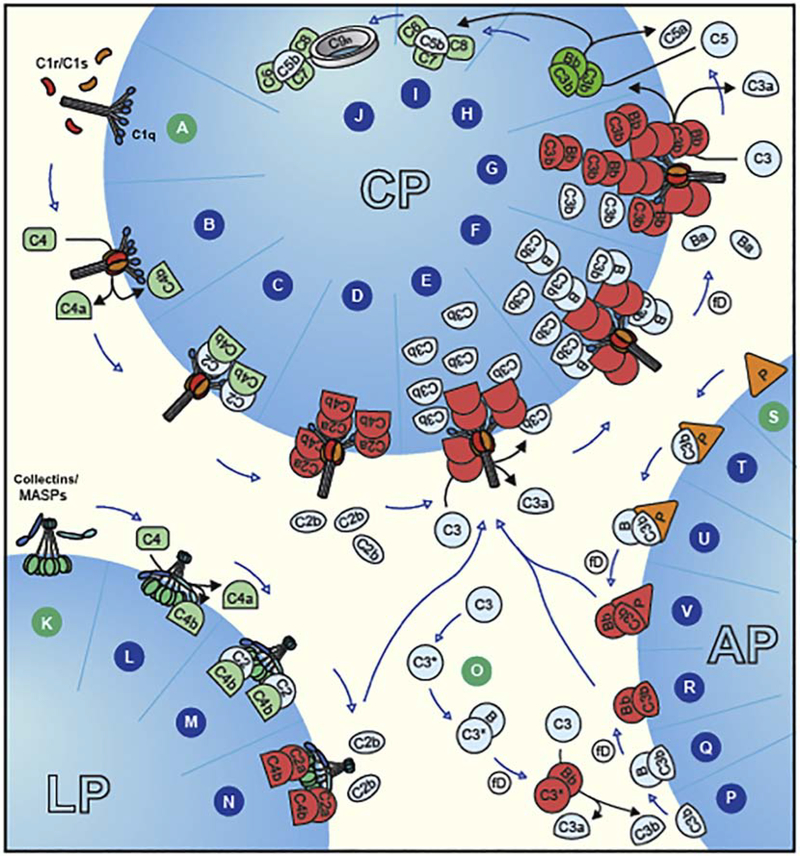
Figure 1.
Complement activation pathways. Steps A–E shows C1q-mediated classical pathway (CP) activation and formation of the C3 convertase (C4b2a) formation. Steps F & G depict merging with amplification route of the alternative pathway (AP) for more C3b deposition (opsonization) through C3bBb assembly (AP C3 convertase), and eventual formation of AP C5 convertase formation (C3bBb3b). Classical pathway activation can directly form a C5 convertase (C4b2a3b) as well. Steps H–J represent the terminal pathway of the complement system, regardless of initiation pathway, showing formation of the lytic complex C5b-9. Lectin pathway (LP) is triggered through binding of collectins/MASPs (K) to the particle surface, leading to the formation of C3 convertase (steps L–N). The scheme also shows AP tick-over, initiated by meta-stable C3(H2O) in the fluid phase (step O) eventually leading to the formation of AP C3 convertase (C3bBb) (steps P–R), and its stabilization on binding to properdin (triangle P) (step V). Steps S–U represent a proposed artificial situation, where properdin may act as a pattern-recognition molecule, and on binding may recruit C3b, and form AP C3 convertase (step V). Image credit: Peter Popp Wibroe.
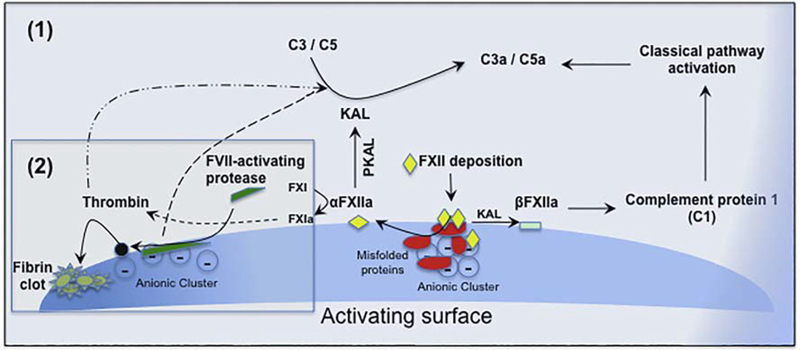
Figure 2.
Complement activation through contact (coagulation) system. The cascade is initiated on deposition of Hageman FXII of the contact pathway of coagulation on a negatively charged surface [Scheme (1)]. On binding, FXII is auto-activated to αFX11a. On adsorption, some misfolded proteins and protein aggregates may also auto-activate FXII. Then, αFX11a activates prekallikrein (PKAL) to form kallikrein (KAL), which cleaves C3 and C5 to generate their respective anaphylactic peptides C3a and C5a without involvement of canonical complement pathways. KAL could further generate βFX11a, which subsequently cleaves C1, resulting in activation of the classical pathway of the complement. In addition to these, αFX11a could also induce coagulation cascade with downstream generation of thrombin, and thrombin in turn is capable of cleaving C3 and C5, liberating C3a and C5a, respectively. Complement activation can also proceed on deposition and activation of FVII-activating protease on polyanionic domains [Scheme (2)] through thrombin activity. Modified and redrawn from [146].
The role of complement in drug delivery systems has roots in early liposome research where liposomes were used as model biological membranes to study how bilayer composition, fluidity, and curvature trigger complement activation, and induce lysis [7]. These studies not only advanced our mechanistic understanding of pore-forming membrane-attack complex (C5b-9 complex) insertion into biomembranes, but also led to the development of liposome-based (including haptenized vesicles) immunoassays for biomarker detection (e.g., antigens and antibodies), and evaluation of serum/plasma complement hemolytic levels [8–12]. Soon, it was realized that liposome-induced complement activation has many consequences in liposome application for targeted drug delivery. For instance, the insertion of the lytic C5b-9 complex into the liposomal bilayer could induce substantial leakage of encapsulated therapeutic cargo [9,13]. Moreover, complement activation was enriching liposomes with opsonic complement fragments C3b and iC3b, thereby priming vesicles for rapid interception by complement receptor-bearing macrophages of the reticuloendothelial system, as well as circulating monocytes, and neutrophils [14–16]. This defensive mechanism was limiting liposome targeting to sites outside the reticuloendothelial system. Other studies identified significant interspecies and interindividual variation in liposome-mediated complement activation pathways [16,17].
Since realization of the role of complement in liposome stability and biological performance, many studies began to examine the multifaceted roles of complement system in broader nanomedicine engineering, performance, and safety [18–20]. As such, the past two decades have witnessed many developments in mechanistic understanding of complement activation by preclinical, and clinical particulate formulations as well as serendipitous discovery of some complement-evading nanoparticles and drug carriers [18–20]. Collectively, these studies have identified physicochemical characteristics (e.g., particle curvature, size, shape and surface properties such as patterns generated by polymers and polymer configuration, and spatial distribution of functional groups) and integrated bio-interfacial parameters that modulate complement activation mechanisms and pathways, and a number of important findings have emerged. Here, we briefly outline lessons learned, and suggest future direction in particle-complement interface research in relation to complement activation mechanisms, formulation design, adjuvanticity, and combination approaches with complement inhibitors.
2. Current progress
2.1. Multi-parametric events in complement activation
Collective evidence from the past two decades has shown that nanoparticle-mediated complement activation is a multi-parametric event, where size, shape, and surface properties seem to cooperatively modulate enzymatic complement cascade [18,20]. For instance, curvature and surface stabilizers (e.g., dextran coating) affect IgM binding, and conformation. In the case of small (100 nm or below) dextran-coated nanoparticles, adsorbed anti-dextran IgM molecules are strained, and assume a staple conformation that triggers C1 binding activating the classical pathway [21]. On the other hand, while IgM binds to larger nanoparticles of similar surface properties equally well, it assumes a planar conformation [21]. This attracts C1 weakly, and hence low levels of complement activation [21]. Contrary to such observations, macrophage clearance tends to increase with particle size when normalizing surface area [21,22]. This may be a consequence of reduced local surface area available for the build up of the alternative pathway amplification loop (Figure 1) (where the size of the alternative pathway C3 convertase is 20–30 nm) on high curvature particles. On low curvature surfaces, the affinity of factor H (a regulatory complement protein) for the opsonic C3b (which is already deposited on the surface) decreases to a level, which is similar to that of factor B for C3b. This shifts the balance from regulation to amplification, thus ensuring covalent deposition of more C3b molecules, which is a signal for rapid phagocytic clearance through complement receptors [1]. Eventually, through many sequential steps, and with predominant involvement of factors H and I, surface-bound C3b molecules undergo inactivation, and cleavage, resulting in the release of many smaller fragments (e.g., C3f and C3c) leaving C3dg bound to the nanoparticles [1]. In addition to topological issues surrounding the surface assembly of C3 convertases, C3b binding is also rate limiting depending on reactivity of the activating surface for covalent binding as well as the available surface area [18,23]. It is estimated that each surface-bound C3b molecule could occupy an area of 40 nm2 [21,24]. Due to topological restraints, with high curvature particles the bulk of C3b molecules instead of being deposited on the surface are released into the fluid phase. Thus, many small particles (<40 nm) may escape efficient C3b opsonization; however, this does not mean that small particles are poor activators of the complement system, where anaphylactic peptides may still be liberated due to assembly of fluid phase C3 convertases. Accordingly, complement activation studies should monitor multiple activation products in the fluid phase in addition to C3b opsonization. It is also plausible that ultra small nanoparticles (e.g., particles < 10 nm) may trigger complement indirectly. For instance, this may occur when a few ultra small particles bind to large glycoproteins, where conformational changes in glycoprotein could expose complement-activating domains. Complement activation may even be related to nanoparticle-mediated protein aggregation in the fluid-phase, and their deposition on vascular endothelium.
Contrary to the belief that stealth (PEGylated) nanoparticles can deter protein adsorption and fend off body’s defenses, PEGylated liposomes, including clinical formulations such as Doxil® (and follow-on products), trigger the human complement system [25–28]. The anionic phosphate-oxygen moiety of methoxyPEG-phospholipid moiety was found to be a key culprit in triggering complement, since methylation of phosphate-oxygen prevented complement activation, and opsonization processes with PEGylated vesicles [28]. More recently, C3 opsonization of nanoparticles was not only shown to be continuous and variable in vivo, but also a vital role for non-specific blood protein adsorption in complement activation, including PEGylated vesicles, was identified [23,29]. Apparently, some of these proteins generate necessary antigenic epitopes for docking of a few natural antibodies of IgG class on some, but not all nanoparticles, which subsequently serve as target for nascent C3b attack, and formation of alternative pathway C3bBb-properdin convertases [29]. Dynamics of non-specific blood protein binding might to some extent be responsible for interindividual disparities seen in complement activation kinetics, and opsonization as discussed above [30]. It is also plausible that misfolded or denatured protein aggregates on nanoparticles may also contribute to complement activation through contact system (Figure 2).
The ability of PEGylated nanoparticles to trigger complement, initiated further studies in mapping the effect of polymer density, and mobility on complement activation. Indeed, conformation and/or architectural arrangement of surface protected PEG chains (and other related polymers), which in turn is also modulated by curvature, were shown to shift complement activation from one pathway (e.g., classical) to another (e.g., lectin pathway) (Figure 3) [31], which has now been extended to other coating materials such as polysaccharides [32–34]. Although the mechanistic details pertaining these dynamics are still to be worked out, these observations indicate that such intricate patterning might transiently generate ‘pathogen-mimicking’ clusters for differential docking of complement sensing molecules either directly or through non-specific protein deposition. Intriguingly, the terminal regions of surface projected polymers such as poloxamers and poloxamines, which are used to sterically stabilize nanospheres, resemble structural motifs of N-acetyl-D-glucosamine/D-mannose recognizable by lectin pathway initiators mannose-binding lectin (MBL) and L-ficolin [18]. Thus, in close proximity, polymer dynamics might transiently generate water-coordinated end-terminal clusters of appropriate architecture for MBL and/or L-ficolin docking, thereby triggering complement activation through the lectin pathway [18,31].
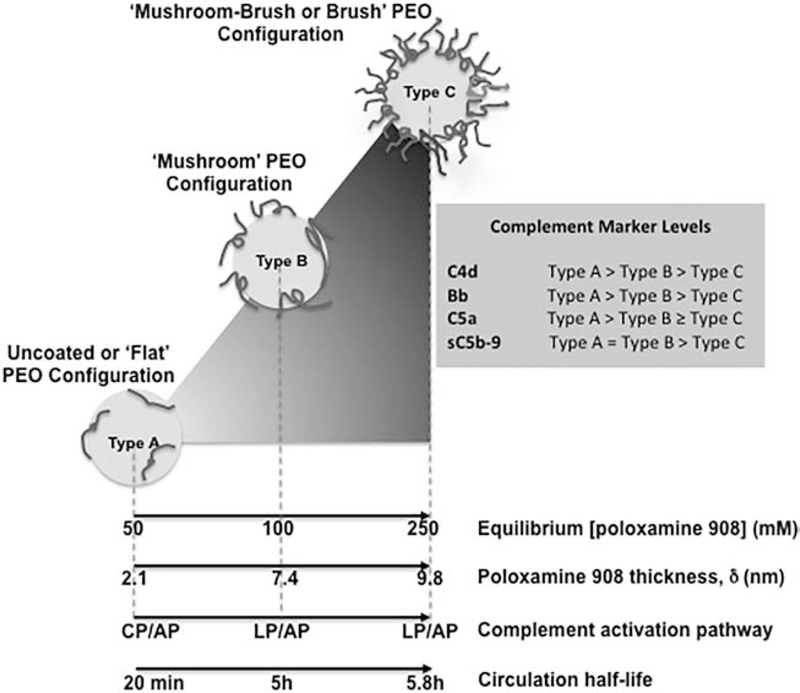
Figure 3.
Complement activation pattern of poloxamine 908-coated polystyrene nanospheres. The projected conformation of polyethylene oxide (PEO) chains of poloxamine 908 can shift complement activation from one pathway to another. With Type A nanospheres complement activation is exclusively due to C1q-mediated classical pathway (CP) and alternative pathway (AP). With Type B, complement activation still proceeds through AP, but instead of CP, lectin pathway (LP) is triggered. With Type C nanospheres, complement activation arises from LP, but contribution of AP only arises from the amplification loop. However, with Type C nanospheres, the extent of complement activation is considerably lower than Types B and A.
2.2. Species disparities in complement activation and dangers of extrapolation
There are reported disparities in nanomaterial-mediated complement activation processes among different species [35–39]. Indeed, this goes back to earlier work in liposome pharmacokinetic studies, where ganglioside GM1 liposomes showed long circulating properties in mice, but not in rats [35,40]. The short circulation half lives in rats was apparently due to presence of natural anti-GM1 antibodies and vesicular complement activation, which facilitated liposome recognition by the hepatic Kupffer cells [35]. More recent studies, however, have indicated that assessment of complement activation by various drug carriers and diagnostic nanoparticles in animals or in their sera/plasma may not necessarily represent human events. For instance, the so-called stealth poly(oxazoline)-grafted nanoparticles, which circulate for prolonged periods of time in rodents and resist complement activation, are potent activators of the human complement system, where complement fixation (C3b/iC3b opsonization) on nanoparticles promotes their uptake by primary human phagocytes (Figure 4) [39]. Another example is dextran-coated iron oxide nanocrystals, which trigger complement activation differently in human and mice sera [36–38]. While dextran cross-linking with epichlorohydrin prevented complement activation in mice, neither this step nor modifications of alcohol functionalities in dextran with alkylating and acetylating agent had any effect on complement activation in human sera [38]. Collectively, these studies suggest the importance of testing immune compatibility of particulate diagnostics and drug carriers with human materials, rather than extrapolating results from animal sources.
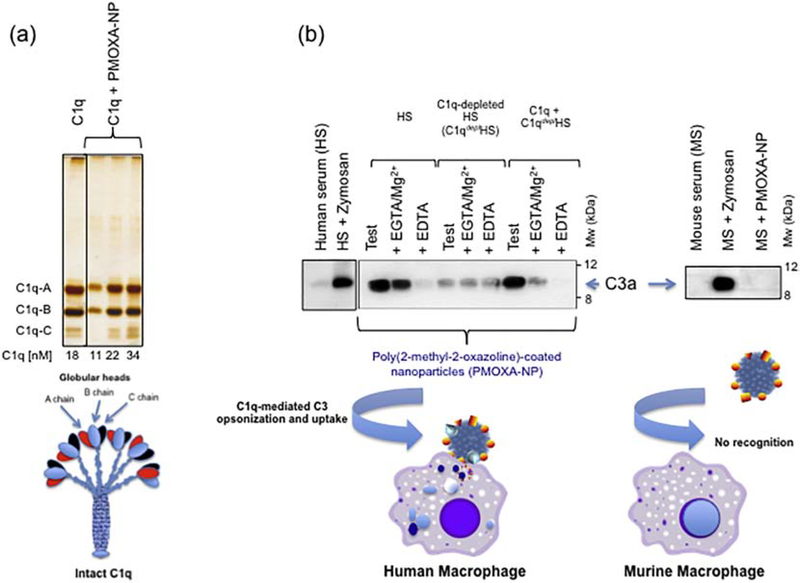
Figure 4.
Complement activation by poly(2-methyl-2-oxazoline)-coated nanoparticles (PMOXA-NP). Panel (a) SDS-PAGE of human C1q and its binding to PMOXA-NP in saline. C1q did not bind to uncoated NP. (b) In human serum PMOXA trigger complement activation through C1q-mediated classical pathway and promotes NP uptake through C3 opsonization by human macrophages. PMOXA-NP do not trigger mouse complement and remain stealth on challenge with murine macrophages. Modified with permission [39].
2.3. Uncontrolled complement activation and immunosuppression: pitfalls and benefits
Uncontrolled complement activation is deleterious to the host, causing cellular injury and contributing to broader development of inflammatory diseases [6,41]. For instance, intratumoral complement activation by nanoparticles, and particularly nanoparticles that trigger the alternative pathway, can promote tumor growth as shown in a syngeneic tumor model in immunocompetent mice [42,43]. The role of complement in tumor progression is complex and could involve many components and players. For instance, liberated C5a molecules promote influx of immunosuppressive Treg cells, and neutrophils [44–46]. Among its other roles, C5a can inhibit the production of IL-12 family cytokines in macrophages, and induce macrophage polarization by activation of the nuclear factor-κB [47,48]. Likewise, C3a—C3aR signaling also promote tumor proliferation by inhibiting neutrophil, and CD4+ T cell responses [49].
The expression of many complement proteins is also increased in many tumors [41], where some promote tumorigenesis independently of complement activation. One example is C1q, which is expressed in the stroma, and vascular endothelium of several human malignant tumors [50]. The mechanisms by which C1q promotes tumor growth is poorly understood, but C1q is capable of inducing macrophage polarization, and suppressing macrophage nucleotide-binding domain leucine-rich repeat and PYRIN domain containing receptor 3 (NLRP3) inflammasome activation [51]. Furthermore, C1q polarized macrophages express elevated levels of programmed death-ligand 1 and −2, and promote Treg proliferation [52]. It is therefore tempting to speculate that some extravasated polyanionic nanoparticles could bind intratumoral C1q, and modulate intratumoral macrophage (and other cells) inflammatory responses differently via its collagen-like domain. However, such responses may depend on C1q conformation, and the avidity of the bound particles for C1q receptors gC1q and cC1q (calreticulin).
It should be emphasized that the bulk of experimental anti-cancer nanomedicine research to date has been conducted in immunocompromised/inbred mouse strains, which often have complement deficiencies, and this may have contributed to over-interpretation of therapeutic efficacy [43]. Accordingly, by considering the integrated roles of complement proteins in tumor immunology, the extent of anti-cancer nanomedicine accumulation in tumors and their mode of local complement triggering could play an important role in therapeutic outcome in human subjects. Translational oncology research utilizing nanomedicine-based approaches should therefore consider, and re-evaluate these eventualities. Finally, with increasing application of nanoparticles as theranostics [22], the role of local nanoparticle-mediated complement activation in inflammatory conditions such as atherosclerosis, arthritis, and many skin diseases also requires re-evaluation. It is also tempting to speculate that some pathological conditions, and depending on the disease stage, may benefit through compartmental accumulation of complement activating nanoparticles, which through controlled complement activation could recruit immunosuppressive cells.
2.4. Complement activation and infusion-related adverse reactions: random acts of intervention?
Acute adverse reactions to nanomedicine injection/infusion in human subjects are idiosyncratic, non-IgE-dependent, and often result in cardiopulmonary distress with varying severity as well as other symptoms [53,54]. These responses are complex, but share similarities to immune responses seen with viral gene therapy vectors [55], and perhaps related to immune cell activation (e.g., macrophages, natural Killer cells, and dendritic cells) through crosstalk and cooperative effects between different receptors, such as Fcγ-receptors (FcγRs), and Toll-like receptors [54]. Some reports, however, have suggested a causal role for complement activation, and anaphylactic peptides C3a and C5a in adverse reactions to nanomedicines [reviewed in 56], but clear evidence linking complement to infusion reactions in human subjects (as well as preclinical models) is still missing [53,54]. At least, complement activation was not observed in nonhuman primates, and phase I patients on exposure to a PEGylated RNA aptamer (pegnivacogin) [57]. However, allergic-like reactions to pegnivacogin in a number of human subjects were antibody-mediated (due to high levels of pre-existing anti-PEG antibodies), where relatively large amounts of PEG (64 mg PEG for an 80 kg patient) were delivered in a single bolus dose of pegnivacogin [57]. This is presumably linked to rapid formation, and binding of pegnivacogin-immunoglobulin complexes to FcγRs, and signaling through them. The immunoglobulin-dependent responses in this trial [57] are in line with a study indicating that human sera contain low levels of anti-PEG antibodies, with nearly 7% and 1% of all specimens containing anti-PEG IgG and IgM in excess of 500 ng/mL [58]. However, not all patients with high levels of anti-PEG antibodies experienced allergic reactions to pegnivacogin [57]. This either suggests differences in susceptibility to antibody-triggered reactions among individuals (e.g., signaling threshold and/or desensitization through FcγRs) or differences in properties of anti-PEG antibodies in individual patients.
Since pigs instantly respond to nanomedicine injection with symptoms similar to responsive human subjects [59], a number of studies have tried to establish correlation between nanoparticle-mediated complement activation in human sera, and cardiopulmonary responses in pigs [25,26,56,60–62]. These empirical approaches, however, could not satisfactorily establish and mechanistically validate a role for the extent of complement activation in infusion reactions. For instance, a clinical study involving 29 cancer patients reported acute allergic reactions in 13 individuals on Doxil® infusion; however, Doxil®-induced complement activation was seen in 21 patients [26]. Among the sensitive patients, 12 had elevated plasma sC5b-9 levels (a marker of complement terminal pathway), but among the 16 non-responding individuals, 9 also had significant rises in plasma sC5b-9 level [26]. On the other hand, injection of minute quantities of Doxil® (0.1 mg lipid/kg body weight) induces reproducible cardiopulmonary distress in pigs, while doubling the dose causes cardiac arrest [63]. Doxil®, and many other clinical and preclinical nanomedicines, however, are poor activators of the porcine complement, and especially at such low doses [54,63–65]. The argument that the porcine model is sensitive to low levels of anaphylactic peptides does not hold, since injection of low doses of C5a shows no dramatic hemodynamic disturbances in pigs [63]. In another study, lack of complement activation by intravenous lipid emulsions in human sera was confirmed, whereas intravenous injection of lipid emulsions into pigs induced significant hemodynamic disturbances, which correlated with rapid rises in plasma thromboxane levels [62]. The study concluded this as an exceptional case, suggesting pigs are not ideal models for studying infusion responses to intravenous lipids [62]. Intriguingly, adverse responses to intravenous lipid emulsions despite its wide use are rarely reported. Our own earlier studies with liposomes have also questioned the ‘human-porcine’ analogy [66]. While liposome pre-incubation with HDL substantially reduced complement activation in human serum, injection of liposome-HDL into pigs was associated with a slow, but extended decline in mean arterial pressure with no recovery for the duration of the experiment (30 min) [66]. On the other hand, liposomes alone induced a sharp drop in mean arterial pressure (with a magnitude comparable to the lowest level reached with liposome-HDL) otherwise with full recovery within 5 min, whereas HDL injection alone was harmless [66].
In contrast to the above, a large body of evidence has indicated a direct role for some macrophage sub-populations such as the resident pulmonary intravascular macrophages (PIMs), in the aforementioned reactions in the porcine model irrespective of complement activation [20,54,64,67–69]. PIMs are an important component of innate immune system in pigs, and other species such as sheep, cat and horse (but not humans), contributing to robust clearance of particulate matters from the blood circulation, which is associated with immediate release of proinflammatory cytokines, metabolites (e.g., thromboxane), and other mediators (e.g., procoagulant factors) on ingestion [64,69–72]. Indeed, robust particle clearance by PIMs in pigs and sheep correlates with peak periods of cardiopulmonary distress [20,54,64,69,71], and on PIM destruction adverse responses to particulate matters, and even endotoxin and zymosan (two potent complement activating agents) injection disappears [64,73,74]. Slowing down particle infusion/injection rate [20,75], and thus reducing the rate of particle presentation to responsive immune cells, overcome infusion reactions in pigs. This phenomenon has also been observed in many sensitive human subjects [53,54]. Other approaches that slow the rate of particle recognition and ingestion by PIMs, such as particle ‘hitchhiking’ on erythrocytes, and particle shape modification (e.g., rods and discs), also slow down or overcome cardiopulmonary distress in the pigs [64]. Pre-dosing with minute quantities of drug-free PEG-liposomes has also overcome anaphylaxis on Doxil® injection in pigs [76]. The molecular basis of these desensitization phenomena is currently unknown, but may involve pre-existing antibodies, signaling through FcγRII/RIII, and macrophage polarization [20,54,77,78]. With non-PEGylated and PEGylated liposomes, binding of circulating anti-phospholipid antibodies, antibodies that recognize epitopes presented by proteins that are intercalated into the PEG cloud, and pre-existing anti-PEG antibodies might contribute to FcγRII/RIII desensitization processes.
The exact role of complement, if any, in infusion reactions to nanopharmaceuticals still remain to be deciphered. At the same time, by considering the oversensitivity of the porcine model (due to PIM physiology), it is very clear that nanomedicine safety assessment in pigs is problematic for predicting idiosyncratic human reactions. The porcine model might represent extreme situations as in the human adult respiratory distress syndrome, where ‘induced’ PIMs are abundant, but the model cannot be justified for use in preclinical development phases pertaining nanomedicines, since its over-sensitivity will exclude many promising nanopharmaceuticals from clinical trials [20,54]. Variable responses to nanomedicine infusion seen in human subjects are most likely related to a pre-existing subset of responsive and/or ‘induced’ immune cells (e.g., macrophages, natural killer cells) as discussed elsewhere [54].
3. Challenges and looking towards future
3.1. Protein adsorption
Considering the role of non-specific blood protein adsorption in complement activation, a better understanding of dynamic protein adsorption/desorption processes on pharmaceutical particles as well as improved approaches that could limit protein deposition are required. Conversely, promoting deposition of complement modulators on nanomedicines may minimize or halt complement activation.
3.1.1. Water structure and the role of specific water-mediated interactions in protein adsorption
Many qualitative and quantitative time-resolved proteomic efforts attest to the complexity of protein deposits on many nanoparticle types [23,29,39,79,80], but protein fingerprinting may not necessary reflect real-time orchestrated molecular build-up and proteolytic blood cascade events in complement activation, and in some cases complement proteins may non-specifically deposit without triggering the cascade. Another limitation is inadvertent contamination with lipoproteins on nanoparticle separation from plasma/serum. Notably, some sub-fractions of human HDL (e.g., HDL3) are enriched with many complement proteins, and regulators to include C3, C4a, C4b, C9, vitronectin, and clusterin (apolipoprotein J) [81], which may be picked on proteomic analysis, again leading to incorrect interpretation. Accordingly, controlled experiments with complement inhibitors seem necessary [39]. Similarly, proteomic approaches do not reveal conformational changes on protein adsorption and/or intercalation that trigger complement activation [29]. Consolidating functional studies are therefore necessary if protein fingerprinting on nanomedicines, and drug carriers is to be correlated with biological performance, and especially complement initiation, and cascade [29,39]. Notwithstanding, the majority of existing proteome interpretations are generic and/or inconclusive, and most likely a reflection of cumulative snapshots of non-specific surface events mediated by hydration- and structured-water at protein and nanoparticle domains. While specific water-mediated interactions in protein complexes have received considerable attention [82], such processes are yet to be translated to protein adsorption, and dynamics on pristine as well as polymer-projected surfaces. Not even two proteins of the same species may have identical hydration domains in a complex medium such as plasma. For instance, individual circulating albumin molecules may be different in terms of their bound fatty acids (or other hydrophobic companions), which ultimately generate a population of species with different hydration domains, and structured water. Therefore, protein binding/deposition may simply be ‘acts of random companionship’ controlled by hydration domains of the particle surface, and the nearby proteins, thus conforming to statistical, and non-specific binding. Interfacial waters may fill cavities, which in turn may mediate hydrogen bonding between a surface and a nearby protein, enhancing affinity without contributing to specificity. Furthermore, depending on surface free energy and nanoparticle surface characteristics such as physical defects, curvature, solvent-accessible surface area of functional groups, hydrophobic pockets and water retention times, biomolecule deposition may be inhomogeneous and patchy (Figure 5). On surface adsorption, solvation patterns of a typical protein may change (e.g., interior water molecules may escape) and this may promote non-specific interactions with the nearby proteins (resulting in protein build-up), or act as a nucleation site for fiber growth (which may generate patterns for antibody binding and complement sensing proteins), or alternatively causing protein destabilization and/or desorption. The ‘water-driven’ phenomenon might explain why so many different blood proteins simultaneously deposit on a typical particle surface, and why individual particles within a typical suspension may have different patterns of adsorbed proteins (or activate complement variably). From the complement point, the dynamics of hydration and structured water on a typical surface may play a modulatory role in spontaneous and ubiquitous formation of C3(H2O), a rearranged form of C3, which initiates antibody-independent activation of the complement system through C3(H2O)Bb convertase assembly [83]. Furthermore, pattern recognition by complement sensing proteins such as C1q, mannose binding lectin, collectins, and ficolins is, presumably, modulated by dynamics of hydration water. In support of this notion, structured water has already been indicated to regulate immune recognition [84]. For example, water molecules in the peptide-binding site of the major histocompatibility complex enhance binding energy, and provide mediating hydrogen bonds [84]. This non-specifically allows MHC to bind a large number of epitope sequences with high affinity [84]. Thus, studying the role of water-mediated interactions in surface protein association and folding, and particularly in relation to C3 and complement sensing molecules, may improve our understanding of complement activation kinetics on surfaces, thereby leading to better optimization of drug carrier engineering.
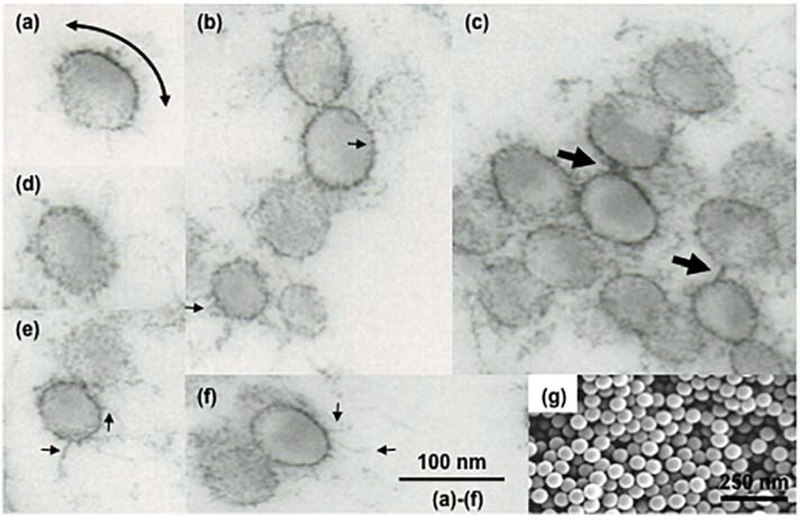
Figure 5.
Electron micrographs of model sulfated polystyrene nanospheres after dipping in fresh human plasma. (a–f) Transmission electron microscopy of polystyrene nanospheres (60 nm) dipped in neat human plasma for 2 min. The micrograph in (a) shows contrasting electron density on a nanosphere surface. This is a reflection of variable density of surface-bound proteins. The curved arrow depicts an electron dense region arising from excessive protein-protein interactions. Micrographs also show inhomogeneous and patchy protein deposition on many nanospheres as well as the presence of many electron dense spikes (small arrows). The spikes may represent nucleation sites arising from protein collapse into compact loops, strands, and turns leading to fibre formation. The arrows in (c) are example of protein deposits/fibers that have bridged adjacent nanospheres. (g) Scanning electron micrograph of untreated polystyrene nanospheres. Unpublished observations (S.M. Moghimi, Newcastle University).
3.1.2. Materials and surface engineering
Considering the role of blood protein binding in complement activation, design initiatives based on coating or grafting with super-hydrophobic low surface energy polymers and copolymers might overcome protein adsorption and resolve complement activation dilemma, but this approach is dependent on overall polymer biosafety, and fate in the body. Alternatively, a more conventional approach, and widely applicable to drug carriers such as polymeric nanospheres and liposomes, is surface fabrication through polymer pairing [85,86]. With conventional PEGylated surfaces, protein persistence at particle surfaces is modulated by polymer chain mobility and deformability [87]. Thus, surface modification with combinations of short and longer chain poly(ethylene glycols) and/or other polymers/copolymers might perturb energetic phases, and affect deformability of longer chain polymers through altered polymer configuration and structured-water arrangements, thereby negatively affecting protein adsorption and/or penetration into the polymer cloud. Indeed, a newly coarse-grained molecular dynamics simulations study has shown that surface pairing with appropriate combinations and proportions of long and short PEG chains (PEG2000 and PEG550) stretches PEG2000 brushes from the surface, and minimizes binding and/or intercalation of a model protein into the PEG cloud [88]. When translated into PEG-paired nanoparticles, PEG-pairing dramatically reduced complement activation in human sera, which was not a reflection of increasing surface PEG density [88]. It would be interesting to adopt this strategy through synthesis of copolymers with super-hydrophobic-polyethylene glycol composition to further affect ion pairing [89], and modulate the extent of protein deposition/intercalation further. Another alternative surface strategy is hierarchical double-PEG layering, where the second PEG layer is covalently attached to underlying chemically reactive PEG molecules [90]. Whether this approach could overcome complement activation in human blood remains to be tested, but such hierarchical approaches may not only introduce more complexity to formulation reproducibility and process manufacturing steps, but also compromise the stability of pharmaceutical nanoparticles in the blood circulation.
3.1.3. From serendipity to complement regulatory protein recruitment
By serendipity a number of complement benign drug carriers have been identified. For instance, recently, a brain-specific phage mimetic particle was shown to bypass complement activation [91]. Other examples include non-lamellar liquid crystalline nanodispersions based on citrem [92,93], an anionic citric acid ester of monoglycerides, and liposomes bearing lipids with carboxylic acid and sulfonate functionalities in their bilayer [94]. Although the underlying mechanisms are still to be unraveled, and presumably related to the mode of functional group presentation both individually and in clusters [88], lack of complement activation at least by citrem nanodispersions and the aforementioned vesicles may be related to surface recruitment of the complement glycoprotein factor H, which possesses cofactor activity for factor I mediated C3b cleavage, and hence minimization of C3b opsonization events. Indeed, factor H is known for its binding to glycerol side chain and carboxyl moiety of sialic acid and many virulent pathogens escape complement attack as they express sialylated glycans and polysialic acid, which recruits factor H [95,96]. Many studies are examining interactions between factor H and native heparin sulfate for understanding the underlying mechanisms by which surface-bound factor H interacts with C3b bound surfaces [97]. Understanding of these events could also lead to development of better materials and drug carrier libraries, which could inherently recruit factor H. Taking these into account, there are proven initiatives that at least show heparin-coated nanoparticles are poor activators of the complement system [98]. Again, nanoparticle surface engineering with a phage peptide, which efficiently recruits factor H, has also overcome complement activation [99]. Our own efforts with dextran-coated superparamagnetic iron oxide nanoworms have shown a direct correlation between the amounts of surface deposited C3 and factor H, but elevation of factor H levels in sera/plasma had no significant effect on C3 opsonization [29].
As a protective measure on different biological surfaces, complement activation is further controlled by multiple membrane-embedded proteins as well as circulating effectors that have redundant activity [2]. Although engineering of complement evading nanoparticles could be extended with such inhibitors, some natural vesicles such as exosomes (conserved membranous vesicular of 30–140 nm released from late endocytic compartments) and cell-derived microvesicles carry GPI-anchored CD55 (a regulator of C3 and C5 convertases) and CD59 (an inhibitor of complement membrane-attack complex assembly) that escape complement surveillance [100]. However, some exosomes still trigger complement activation [100], and our own efforts have shown that exosomes derived from some metastatic and nonmetastatic malignant cells are potent activators of the human complement system, activating complement through the calcium-sensitive pathways [101]. To date, increasing evidence suggest exosomes and microvesicles as important players in a multitude of pro- and anti-inflammatory events [101–104]. For instance, exosome levels are elevated in conditions where complement activation is dysregulated (e.g., polytrauma, hemorrhage, septic shock) and the crosstalk between exosomes and the complement system (as well as the coagulation system) seems important in regulating the extent of complement activity, and hence by modulating innate and adaptive immune responses [101–104]. Considering exosome stability and biological barrier penetration properties, and hence their potential applications in drug delivery [105], it is therefore necessary to screen exosomes for complement activation. Additionally, a deeper insight of exosome-complement crosstalk mechanisms might lead to identification of better immunomodulatory strategies to combat inflammatory diseases through exosome bioengineering.
3.1.4. Transformation factor and diametrical outcomes
Dynamic delivery systems such as some forms of micelles may undergo rapid transformation in the blood, and the extent of complement activation by such systems may be related to these transformational events. Examples include pharmaceutical nonionic solubilizers such as Cremophor EL (CEL) [106] and Pluronics [107,108]. CEL is manufactured by reacting the triglyceride rich castor oil with ethylene oxide yielding a complex mixture of hydrophilic and hydrophobic components that forms star-shaped micelles in aqueous solution at concentration >60 μg/mL, which triggers human complement [106]. CEL micelles affect the electrophoretic mobility of both HDL and LDL, and this is believed to occur from incorporation of some of the hydrophobic CEL components into lipoproteins [109,110]. This partial hydrophobic component depletion transforms the remaining components into forming droplets of 100–300 nm in size, and these are thought to trigger complement [106]. Pluronic micelles such as those based on poloxamer 188 and poloxamer 407 also activate complement, but the extent of activation of terminal complement pathway by these micelles is dramatically reduced in sera with elevated HDL and LDL levels [107,108]. This protective role of lipoproteins is partly due to micellar interaction with apolipoprotein AI, AII, and B-100 [108]. Indeed, apolipoproteins AI and AII are inhibitors of C9 polymerization, which minimizes the generation of complement lytic complexes [111]. Furthermore, Pluronic micelles directly interact with human lipoproteins and chylomicrons, yielding complex species. For instance poloxamer 407 on interaction with human LDL generates two different types of ‘remodeled LDL’ particles; a population of diffused round-shaped electron-dense species of 80–200 nm in diameter, and a second population of relatively less denser but irregularly shaped species [108]. In addition to these, some populations of ‘remodeled LDL’ entrapped clusters of smaller particles [108]. It is plausible that these transformations may have regulatory roles in complement activation. On the other hand, interactions with chylomicrons and VLDL seem to promote complement activation (unpublished observations). Collectively, these observations may have implications in future design, selection and patient-centric application of micellar solubilizers, such as selective administration to cohorts with appropriate lipoprotein composition and circulating levels to minimize complement activation.
3.2. Complement inhibitors, complement-deficient humans, and preclinical models
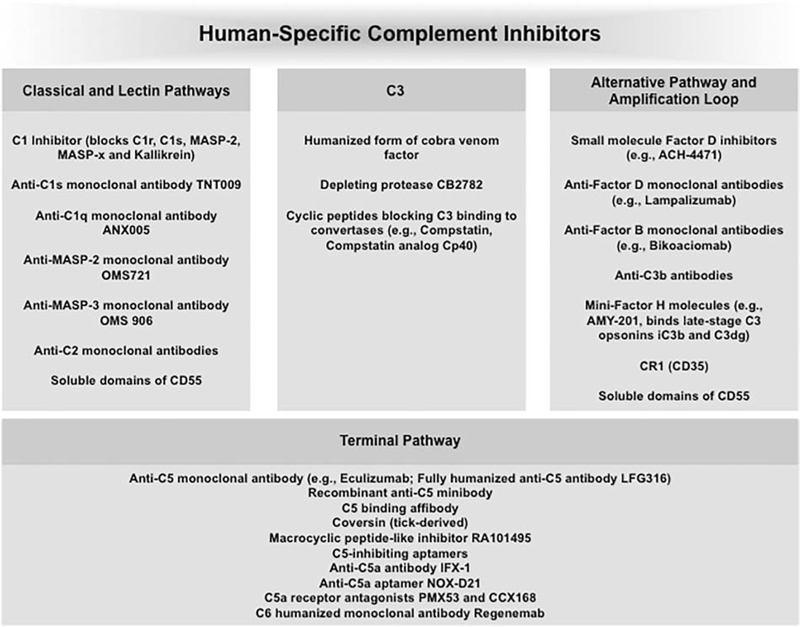
Availability of human pathway-specific and complement-receptor inhibitors are opening doors for functionalization of future generations of nanomedicines (and perhaps in combination with the ‘don’t eat me signal’ recombinant CD47 [112]) or as a combination strategy for addressing safety challenges surrounding complement activation (e.g., as in complement-mediated tumor growth) [113]. These inhibitors comprise monoclonal antibodies, nanobodies, cyclic and linear peptides, aptamers, and small molecules targeting different complement proteins, proteases, and receptors (Figure 6) [2,113–115]. As for combination strategies, the use of complement inhibitors must be considered cautiously, and particularly in relation to C3 inactivity, since this could promote susceptibility to and reactivation of many bacterial infections, for example in the lung, on repeated dosing of C3 inhibitors. However, a recent study has shown no susceptibility to infections in nonhuman primates on prolonged treatment with C3 inhibitor compstatin [116], thereby strengthening the notion for combination therapies with nanomedicines in conditions where local complement activation is beneficial to disease progression.
Figure 6.
A selection of human-specific complement inhibitors under investigation.
Developments of species-specific complement inhibitors are equally important. This may allow for distinguishing and identifying similarities between human and animal responses (as well as variation between different preclinical models), and eventual validation of animal models for selected cases. An interesting example is Coversin, a 16.7 kDa recombinant protein originally discovered in the saliva of the Ornithodoros moubata tick, which prevents equally efficiently the cleavage of C5 in humans and pigs [117–119]. There are also indications that the human C1 esterase inhibitor is also effective in pigs and cats by acting on the classical pathway [120,121]. Nevertheless, such approaches may be linked with increasing availability of single/multiple complement gene knockout or spontaneous complement deficient animals [36,122–125] for mechanistic studies, and delineating suspect pathways. There are also spontaneous complement deficient large animal models such as hereditary C3 deficient dogs, where affected animals have less than 0.0003% of physiological amount of C3 in their plasma displaying reduced opsonic and chemotactic activities [124]. The CRISPER/Cas9-mediated gene targeting approach has also been used to generate C3-deficient pigs [125]. These C3 knockout pigs could be useful for reassessing the role of porcine C3 in PIM (and other immune cells)-mediated cardiopulmonary distress.
Finally, deficiencies of many complement proteins in human subjects are well known, and their role in inflammatory network is becoming clearer [53,126–129]. Perhaps, patient profiling for complement deficiencies should be considered in trials and treatments with nanomedicines.
3.3. Exploring complement adjuvanticity
Al(OH)3 (along with potassium aluminum sulfate or alum) is a complement activator, and commonly used as an adjuvant in human vaccines [130]. Studies in complement receptor-deficient mice have shown impaired immunization responses with antigen-bound alum, thus implying a modulatory role for complement activation in immunization [131]. This is in agreement with the role of C3 and its cleavage product C3d (in fluid phase and surface bound) in TH2 sensitization, B-cell activation and long-term memory [132–134]. Indeed, co-engagement of complement receptor 2 (CD21) through C3dg-tagged antigens/nanoparticles reduces the threshold for B cell receptor signaling, thus ensuring long-term B cell memory and optimal antibody production [132–135].
Polymeric nanoparticles, liposomes, hexosomes, cubosomes and many other particulate entities are gaining popularity in vaccination strategies both as an antigen depot systems and adjuvants [135,136], but show variable responses and effectiveness. The observed differences in the adjuvanticity of nanoparticles may in part be related to differences in the extent of complement activation. Thus, complement profiling per se might improve nanoparticle selection, and identify complement-activating platforms, and immunization routes (including sequential immunization through different routes) for favorable immune responses. Considering the role of complement in prophylactic protection, future studies should further consider and examine the role of complosome (the intracellular complement, which is present in all cells examined thus far), since complosome is apparently a critical regulator of basic cellular processes, where intracellular complement activation can trigger mammalian target of rapamycin (mTOR) activation, affect nutrient influx, augment glycolysis and oxidative phosphorylation, and regulate ROS production [137,138]. For example, resting CD4+ T cells have intracellular pools of C3 and C5, corresponding anaphylactic C3a and C5a receptors (C3aR and C5aR1), and the protease cathepsin L [139]. The latter continuously processes intracellular C3 as well C3(H2O), internalized through a recycling pathway [140], where liberated C3a engages with lysosomal C3aR to induce low level mTOR activation for cell survival. On T cell receptor stimulation, C3 and its cleavage products and cathepsin L are translocated to the cell surface resulting in autocrine activation through C3aR and CD46, which cumulates in the altered expression of LAMTOR5 (hepatitis B X-interacting protein), the glucose transporter GLUT1, the amino acid transporter LAT1, NLRP3 and IL-1β [reviewed in 137,138]. CD46 can also activate intracellular C5 and liberate anaphylactic peptide C5a, which engages with intracellular C5aR1 to amplify ROS production, which in turn drives NLRP3 inflammasome assembly, and subsequent IL-1β production for optimal TH1 induction [137,138].
4. Conclusions
The complement system plays multifaceted roles in nanomedicine performance; complement activation may not only improve therapeutic outcome, but might also compromise efficacy. During the past two decades we have learned much on complexities surrounding complement activation by different nano- and micro-particles, and unexpected processes have been discovered. In parallel, fundamental discoveries in this period have further improved our molecular understanding of complement activation processes and regulation, and identified crosstalk pathways by which complement regulates homeostasis, and disease pathogenesis. Collectively, these discoveries together with better understanding of systems immunology within the context of diseases will pave the way for future design of immunologically safer pharmaceutical colloids, viral vectors and sensors [55,141], and might introduce selective applicable combination of therapeutic strategies with complement inhibitors, including the newly described fusion protein inhibitors for dual targeting of lectin and alternative pathways [142]. Cancer serves as a good example. Intratumoral complement activation may depend on tumor type, location, developmental, and treatment stage as well as the host’s immune system responsiveness. Accordingly, experiments with large animals bearing spontaneous tumors (e.g., dogs) as well as immunocompetent animal models of human tumors, including ‘humanized’ species (mice) bearing elements of human immunity, should consider the role of complement functionality, variability, and crosstalk on testing the efficacy of anti-cancer nanomedicines [42,43,143]. Thus, in some cases complement activation may be beneficial. For instance, one study reported elevated tumor C3 levels between 1 and 24 h after photodynamic therapy, and increased alternative pathway activity in the serum between 1 and 3 days post therapy [144]. The efficacy of photodynamic therapy, however, was decreased on blocking anaphylactic peptide receptors, suggesting the importance of complement in photodynamic-mediated tumor destruction [144]. Finally, we foresee development of plasmonic, dynamic multi-component, and multi-parametric assay procedures, and platforms for simultaneous monitoring of nanomedicine-mediated complement activation, and crosstalk analysis with immune, and non-immune cells in the coming decade. Here, and by considering in vitro and in vivo differences seen in complement activation, C3 opsonization, and leukocyte responses [145], future efforts should also focus on developing species-specific in vitro complement assays that correlate with the in vivo dynamics of complement activation.
Acknowledgements
The authors gratefully acknowledge the input of many students and colleagues in their respective laboratories for advancing nanomedicine-complement research. We are also indebted to numerous organizations and funding bodies for their continuous support of complement research in our laboratories (S.M.M. acknowledges financial support by the Danish Agency for Science, Technology and Innovation, Lundbeckfonden, and the European Commission FP7, and E.P. acknowledges financial support from the University of Padova, Ex 60% and DOR 2014–2018, and the Strategic Project NAMECA). This study was also supported by the NIH grant R01 EB022040 to D.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Holers VM, Complement and its receptors: new insights into human disease, Annu. Rev. Immunol. 32 (2014) 433–459. [DOI] [PubMed] [Google Scholar]
- [2].Zipfel PF, Skerka C, Complement regulators and inhibitory proteins, Nat. Rev. Immunol. 9 (2009) 729–740. [DOI] [PubMed] [Google Scholar]
- [3].Davies A, Lachmann PJ, Membrane defence against complement lysis: the structure and biological properties of CD59, Immunol. Res. 12 (1993) 258–275. [DOI] [PubMed] [Google Scholar]
- [4].Amara U, Flieri MA, Rittirsch D, Klos A, Chen H, Acker B, Bruckner UB, Nilsson B, Gebhard F, Lambris JD, Huber-Lang M, Molecular intercommunication between the complement and coagulation systems, J. Immunol. 185 (2010) 5628–5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hovland A, Jonasson L, Garred P, Yindestad A, Aukrust P, Lappegard KT, Espevik T, Mollnes TE, The complement system and toll-like receptors as integrated players in the pathophysiology of atherosclerosis, Atherosclerosis 241 (2015) 480–494. [DOI] [PubMed] [Google Scholar]
- [6].Pio R, Corrales L, Lambris JD, The role of complement in tumor growth, Adv. Exp. Med. Biol. 772 (2014) 229–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Haxby JA, Kinsky CB, Kinsky SC, Immune response of a liposomal model membrane, Proc. Natl. Acad. Sci. U.S.A. 61 (1968) 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kinsky SC, Haxby JA, Zopf DA, Alving CR, Kinsky CB, Complement-dependent damage to liposomes prepared from pure lipids and Frossman hapten, Biochemistry 8 (1969) 4149–4158. [DOI] [PubMed] [Google Scholar]
- [9].Haxby JA, Gotze O, Muller-Eberhard HJ, Kinsky SC, Release of trapped marker from liposomes by the action of purified complement components, Proc. Natl. Acad. Sci. U.S.A. 64 (1969) 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Alving CR, Kinsky SC, Haxby JA, Kinsky CB, Antibody binding and complement fixation by a liposomal model membrane, Biochemistry 8 (1969) 1582–1587. [DOI] [PubMed] [Google Scholar]
- [11].Michalek MT, Bremer EG, Mold C, Effect of gangliosides on activation of the alternative pathways of human complement, J. Immunol. 140 (1988) 1581–1587. [PubMed] [Google Scholar]
- [12].Masaki T, Okada N, Yasuda R, Okada H, Assay of complement activity in human serum using large unilamellar liposomes, J. Immunol. Method. 123 (1989) 19–24. [DOI] [PubMed] [Google Scholar]
- [13].Moghimi SM, Hamad I, Liposome-mediated triggering of complement cascade, J. Liposome Res. 18 (2008) 195–209. [DOI] [PubMed] [Google Scholar]
- [14].Bradley DV, Wong K, Serrano K, Chonn A, Devine D, Liposome-complement interactions in rat serum: implications for liposome survival studies, Biochim. Biophys. Acta 1191 (1994) 43–51. [DOI] [PubMed] [Google Scholar]
- [15].Chonn A, Cullis P, Devine DA, The role of surface charge in the activation of the classical and alternative pathways of complement by liposomes, J. Immunol. 146 (1991) 4234–4241. [PubMed] [Google Scholar]
- [16].Moghimi SM, Hunter AC, Recognition by macrophages and liver cells of opsonized phospholipid vesicles and phospholipid headgroups, Pharm. Res. 18 (2001) 1–8. [DOI] [PubMed] [Google Scholar]
- [17].Alving CR, Swartz GM Jr., Antibodies to cholesterol, cholesterol conjugates and liposomes. Implications for atherosclerosis and autoimmunity, CRC Crit. Rev. Immunol. 10 91991) 441–453. [PubMed] [Google Scholar]
- [18].Moghimi SM, Andersen AJ, Ahmadvand D, Wibroe PP, Andresen TL, Hunter AC, Material properties in complement activation, Adv. Drug Deliv. Rev. 63 (2011) 1000–1007. [DOI] [PubMed] [Google Scholar]
- [19].Boraschi D, Italiani P, Palomba R, Decuzzi P, Duschl A, Fadeel B, Moghimi SM, Nanoparticles and innate immunity: new perspectives on host defence, Semin. Immunol. 34 (2017) 33–51. [DOI] [PubMed] [Google Scholar]
- [20].Moghimi SM, Simberg D, Skotland T, Yaghmur A, Hunter AC, The interplay between blood proteins, complement, and macrophages on nanomedicine performance and responses, J. Pharmacol. Exp. Ther. 370 (2019) 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pedersen MB, Zhou X, Larsen EK, Sorensen US, Kjems J, Nygaard JV, Nyengaard JR, Meyer RL, Boesen T, Vorup-Jensen T, Curvature of synthetic and natural surfaces is an important target feature in classical pathway complement activation, J. Immunol. 184 (2010) 1931–1945. [DOI] [PubMed] [Google Scholar]
- [22].Moghimi SM, Hunter AC, Andresen TL, Factors controlling nanoparticle pharmacokinetics: an integrated analysis and perspective, Annu. Rev. Pharmacol. Toxicol. 52 (2012) 481–503. [DOI] [PubMed] [Google Scholar]
- [23].Chen F, Wang G, Griffin JI, Brenneman B, Banda NK, Holers VM, Backos DS, Wu L, Moghimi SM, Simberg D, Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo, Nat. Nanotechnol. 12 (2017) 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Janssen BJ, Christodoulidou A, McCarthy A, Lambris JD, Gros P, Structure of C3b reveals conformational changes that underlie complement activity, Nature 444 (2006) 213–216. [DOI] [PubMed] [Google Scholar]
- [25].Szebeni J, Baranyi L, Savay S, Lutz H, Jelezarova E, Bunger R, Alving CR, The role of complement activation in hypersensitivity to pegylated liposomal doxorubicin (Doxil), J. Liposome Res. 10 (2000) 467–481. [Google Scholar]
- [26].Channan-Khan A, Szebeni J, Savay S, Liebes L, Rafique NM, Alving CR, Muggia FM, Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil): possible role in hypersensitivity reactions, Annal. Oncol. 14 (2003) 463–478. [DOI] [PubMed] [Google Scholar]
- [27].Wibroe PP, Ahmadvand D, Oghabian MA, Yaghmur A, Moghimi SM, An integrated assessment of morphology, size, and complement activation of the PEGylated liposomal doxorubicin products Doxil®, Caelyx®, DOXOrubicin, and SinaDoxosome, J. Control. Release 221 (2016) 1–8. [DOI] [PubMed] [Google Scholar]
- [28].Moghimi SM, Hamad I, Andresen TL, Jørgensen K, Szebeni J, Methylation of the phosphate oxygen moiety of phospholipid-methoxy(polyethylene glycol) conjugate prevents PEGylated liposome-mediated complement activation and anaphylatoxin production, FASEB J 20 (2006) 2591–2593. [DOI] [PubMed] [Google Scholar]
- [29].Vu VP, Gifford GB, Chen F, Benasutti H, Wang G, Groman EV, Scheinman R, Saba L, Moghimi SM, Simberg D, Immunoglobulin deposition on biomolecule corona determines complement opsonization efficiency of preclinical and clinical nanoparticles, Nat. Nanotechnol. 14 (2019) 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Benasutti H, Wang G, Vu VP, Scheinman R, Groman E, Saba L, Simberg D, Variability of complement response toward preclinical and clinical nanocarriers in the general population, Bioconjug. Chem. 28 (2017) 2747–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hamad I, Al-Hanbali O, Hunter AC, Rutt KJ, Andresen TL, Moghimi SM, Distinct polymer architecture mediates switching of complement activation pathways at the nanosphere-serum interface: implications for stealth nanoparticle engineering, ACS Nano 4 (2010) 6629–6638. [DOI] [PubMed] [Google Scholar]
- [32].Andersen AJ, Robinson JT, Dai H, Andresen TL, Moghimi SM, Single-walled carbon nanotube surface control of complement recognition and activation, ACS Nano 7 (2013) 1108–1119. [DOI] [PubMed] [Google Scholar]
- [33].Yu K, Lai BFL, Foley JH, Krisinger MJ, Conway EM, Kizhakkedathu JN, Modulation of complement activation and amplification on nanoparticle surfaces by glycopolymer conformation and chemistry, ACS Nano 8 (2014) 7687–7703. [DOI] [PubMed] [Google Scholar]
- [34].Coty JB, Eleamen Oliveira E, Vauthier C, Tuning complement activation and pathway through controlled molecular architecture of dextran chains in nanoparticle corona, Int. J. Pharm. 532 (2017) 769–778. [DOI] [PubMed] [Google Scholar]
- [35].Liu D, Song YK, Liu F, Antibody dependent, complement modified liver uptake of liposomes containing GM1, Pharm. Res. 12 (1995) 1775–1780. [DOI] [PubMed] [Google Scholar]
- [36].Banda NK, Mehta G, Chao Y, Inturi S, Fossati-Jimak L, Botto M, Wu L, Moghimi SM, Simberg D, Mechanisms of complement activation by dextran-coated superparamagnetic iron oxide (SPIO) nanoworms in mouse versus human serum, Part. Fibre. Toxicol. 11 (2014) 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Inturi S, Wang G, Chen F, Banda NK, Holers VM, Wu L, Moghimi SM, Simberg D, Modulatory role of surface coating of superparamagnetic iron oxide nanoworms in complement opsonisation and leukocyte uptake, ACS Nano 9 (2015) 10758–10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang G, Chen F, Banda NK, Holers VM, Wu L, Moghimi SM, Simberg D, Activation of human complement system by dextran-coated iron oxide nanoparticles is not affected by dextran/Fe ratio, hydroxyl modifications, and crosslinking, Front. Immunol. 7 (2016) 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tavano R, Gabrielli L, Lubian E, Fedeli C, Visentin S, De Laureto PP, Arrigoni G, Geffner-Smith A, Chen F, Simberg D, Morgese G, Benetti EM, Wu L, Moghimi SM, Mancin F, Papini E, C1q-mediated complement activation and C3 opsonization trigger recognition of stealth poly(2-methyl-2-oxazoline)-coated silica nanoparticles by human phagocytes, ACS Nano 12 (2018) 5839–5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gabizon A, Papahadjopoulos D, Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors, 85 (1988) Proc. Natl. Acad. Sci. U.S.A. 6949–6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang R, Liu O, Li T, Zhao QL,Y, Role of the complement system in the tumor microenvironment, Cancer Cell Int. 19 (2019) 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Moghimi SM, Cancer nanomedicine and the complement system activation paradigm: anaphylaxis and tumour growth, J. Control. Release 190 (2014) 556–562. [DOI] [PubMed] [Google Scholar]
- [43].Moghimi SM, Farhangrazi ZS, Just so stories: the random act of anti-cancer nanomedicine performance, Nanomedicine 10 (2014) 1661–1666. [DOI] [PubMed] [Google Scholar]
- [44].Markiewski MM, DeAngelis RA, Benecia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G, Lambris JD, Modulation of antitumor immune responses by complement, Nat. Immunol. 9 (2008) 1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ajona D, Ortiz-Espinosa S, Pio R, Complement anaphylatoxins C3a and C5a: emerging roles in cancer progression and treatment, Semin. Cell Dev. Biol. 85 (2019) 153–163. [DOI] [PubMed] [Google Scholar]
- [46].Vadrevu SK, Chintala NK, Sharma SK, Sharma P, Cleveland C, Riedliger L, Manne S, Fairlie DP, Gorczyca W, Almanza O, Karbowniczek M, Markiewski MM, Complement C5a receptor facilitates cancer metastasis by altering T-cell responses in the metastatic niche, Cancer Res. 74 (2014) 3454–3465. [DOI] [PubMed] [Google Scholar]
- [47].Hawlisch H, Belkaid Y, Baedler R, Hildeman D, Gerard C, Kohl J, C5a negatively regulates toll-like receptor 4-induced immune responses, Immunity 22 (2005) 415–426. [DOI] [PubMed] [Google Scholar]
- [48].Plao C, Zhang WM, Li TT,Zhang CC, Qiu S, Liu Y, Liu S, Jin M, Jia LX, Song WC, Du J, Complement 5a stimulates macrophage polarization and contributes to tumor metastases of colon cancer, Exp. Cell Res. 366 (2018) 127–138. [DOI] [PubMed] [Google Scholar]
- [49].Nabizadeh JA, Manthey HD, The complement C3a receptor contributes to melanoma tumorigenesis by inhibiting neutrophil and CD4 T cell responses, J. Immunol. 196 (2016) 4783–4792. [DOI] [PubMed] [Google Scholar]
- [50].Bulla R, Tripodo C, Rami D, Ling GS, Agostinis C, Guamotta C, Zorzet S, Durigutto P, Botto M, Tedesco F, C1q acts in the tumour microenvironment as a cancer-promoting factor independently of complement activation, Nat. Commun. 7 (2016) 10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Benoit ME, Clarke EV, Morgado P,Fraser DA, Tenner AJ, Complement protein C1q directs macrophage polarization and limits inflammasome activity during the uptake of apoptotic cells, J. Immunol. 188 (2012) 5682–5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Clarke EV, Weist BM, Walsh CM, Tenner AJ, Complement protein C1q bound to apoptotic cells suppresses human macrophages and dendritic cell-mediated Th17 and Th1 T cell subset proliferation, J. Leukoc. Biol. 97 (2015) 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Moghimi SM, Wibroe PP, Helvig SY, Farhangrazi ZS, Hunter AC, Genomic perspectives in inter-individual adverse responses following nanomedicine administration: the way forward, Adv. Drug Deliv. Rev. 64 (2014) 1385–1393. [DOI] [PubMed] [Google Scholar]
- [54].Moghimi SM, Nanomedicine safety in preclinical and clinical development: focus on idiosyncratic injection/infusion reactions, Drug Discov. Today 23 (2018) 1034–1042. [DOI] [PubMed] [Google Scholar]
- [55].Shirley JL, de Jong YP, Terhorst C, Herzog RW, Immune responses to viral gene therapy vectors, Mol. Ther. 28 (2020) 709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Szebeni J, Complement activation-related pseudoallergy: a stress reaction in blood triggered by nanomedicines and biological, Mol. Immunol. 61 (2014) 163–173. [DOI] [PubMed] [Google Scholar]
- [57].Ganson NJ, Povsic TJ, Sullenger BA, Alexander JH, Zelenkofske SL, Sailstad JM, Rusconi CP, Hershfield MS, Pre-existing anti-polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer, J. Allergy Clin. Immunol. 137 (2016) 1610–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jacobs TM, McCallen JD, Moore DT, Huckaby J, Edelstein JD, Lai S, Analysis of pre-existing IgG and IgM antibodies against polyethylene glycol (PEG) in the general population, Anal. Chem. 88 (2016) 11804–11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zamboni WC, Szebeni J, Kozlov SV, Lucas AT, Piscitelli JA, Dobrovolskaia MA, Animal models for analysis of immunological responses to nanomaterials: challenges and considerations, Adv. Drug Deliv. Rev. 136–137 (2018) 82–96. [DOI] [PubMed] [Google Scholar]
- [60].Meszaros T, Kozma GT, Shimizu T, Miyahara K, Turjeman K, Ishida T, Barenholz Y, Urbanics R, Szebeni J, Involvement of complement activation in the pulmonary vasoactivity of polystyrene nanoparticles in pigs: unique surface properties underlying alternative pathway activation and instant opsonization, Int. J. Nanomed. 13 (2018) 6345–6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].J Szebeni G Storm, Complement activation as a bioequivalence issue relevant to the development of generic liposomes and other nanoparticulate drugs, Biochem. Biophys. Res. Commun. 468 (2015) 490–497. [DOI] [PubMed] [Google Scholar]
- [62].Bedocs P, Capacchinoe J, Potts L, Chugani R, Weiszhar Z, Szebeni J, Buckenmaier CC, Hypersensitivity reactions to intravenous lipid emulsion un swine: relevance for lipid resuscitation studies, Anesth. Analg. 119 (2014) 1094–1101. [DOI] [PubMed] [Google Scholar]
- [63].Szebeni J, Baranyi L, Savay S, Bodo M, Milosevits J, Alving CR, Bunger R, Complement activation-related cardiac anaphylaxis in pigs: role of C5a anaphylatoxin and adenosine in liposome-induced abnormalities in ECG and heart function, Am. J. Physiol. Heart Cir. Physiol. 290 (2006) H1050–H1058. [DOI] [PubMed] [Google Scholar]
- [64].Wibroe PP, Anselmo AC, Nilsson PH, Sarode A, Gupta V, Urbanics R, Szebeni J, Hunter AC, Mitragotri S, Mollnes TE, Moghimi SM, Bypassing adverse injection reactions to nanoparticles through shape modification and attachment to erythrocytes, Nat. Nanotechnol. 12 (2017) 589–594. [DOI] [PubMed] [Google Scholar]
- [65].Jackman JA, Meszaros T, Fulop T, Urbanics R, Szebeni J, Cho NJ, Comparison of complement activation-related pseudoallergy in miniature and domestic pigs: foundation of a validatable immune toxicity model, Nanomedicine 12 (2016) 933–943. [DOI] [PubMed] [Google Scholar]
- [66].Moghimi SM, Hamad I, Bunger R, Andresen TL, Jorgensen K, Hunter AC, Baranji L, Rosivall L, Szebeni J, Activation of the human complement system by cholesterol-rich and PEGylated liposomes—Modulation of cholesterol-rich liposome-mediated complement activation by elevated serum HDL and LDL levels, J. Liposome Res. 16 (2006) 167–174. [DOI] [PubMed] [Google Scholar]
- [67].Moghimi SM, Simberg D, Translational gaps in animal models of human infusion reactions to nanomedicines, Nanomedicine (Lond.) 13 (2018) 973–975. [DOI] [PubMed] [Google Scholar]
- [68].Skotland T, Injection of nanoparticles into cloven-hoof animals: asking for trouble, Theransotics 7 (2017) 4877–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Schneberger D, Aharonson-Raz K, Singh B, Pulmonary intravascular macrophages and lung health: what are we missing?, Am. J. Physiol. Lung Cell Mol. Physiol 302 (2012) L498–L503. [DOI] [PubMed] [Google Scholar]
- [70].Brain JD, Molina RM, DeCamp MM, Warner AE, Pulmonary intravascular macrophages: their contribution to the mononuclear phagocyte system in 13 species, Am. J. Physiol. 276 (1999) L146–L154. [DOI] [PubMed] [Google Scholar]
- [71].Miyamoto K, Schultz E, Heath T, Mitchell MD, Albertine KH, Staub NC, Pulmonary intravascular macrophages and hemodynamic effects of liposomes in sheep, J. Appl. Physiol. 64 (1988) 1143–1152. [DOI] [PubMed] [Google Scholar]
- [72].Longworth KE, Westgate AM, Grady MK, Westcott JY, Staub NC, Development of pulmonary intravascular macrophage function in newborn lambs, J. Appl. Physiol. 73 (1992) 2608–2615. [DOI] [PubMed] [Google Scholar]
- [73].Gaca JG, Palestrant D, Lukes DJ, Olausson M, Parker W, Davis RD Jr., Prevention of acute lung injury in swine: depletion of pulmonary intravascular macrophages using liposomal clodronate, J. Surg. Res. 112 (2003) 19–25. [DOI] [PubMed] [Google Scholar]
- [74].Sone Y, Serikov VB, Staub NC Jr., Intravascular macrophage depletion attenuates endotoxin lung injury in anesthetized sheep, J. Appl. Physiol. 87 (1999) 1354–1359. [DOI] [PubMed] [Google Scholar]
- [75].Fulop T, Kozma GT, Vashegyi I, Meszaros T, Rosivall L, Urbanics R, Storm G, Metselaar JM, Szebeni J, Liposome-induced hypersensitivity reactions: risk reduction by design of safe infusion protocols in pigs, J. Control. Release 309 (2019) 333–338. [DOI] [PubMed] [Google Scholar]
- [76].Szebeni J, Bedocs P, Urbanics R, Bunger R, Rosivall L, Toth M, Barenholz Y, Prevention of infusion reactions to PEGylated liposomal doxorubicin via tachyphylaxis induction by placebo vesicles: a porcine model, J. Control. Release 106 (2012) 382–387. [DOI] [PubMed] [Google Scholar]
- [77].Escribese MM, Rosace D, Chivato T, Fernandez TD, Corbi AL, Barber D, Alternative anaphylactic routes: the potential role of macrophages, Front. Immunol. 8 (2017) 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Strait RT, Morris SC, Yang M, Qu XW, Finkelman FD, Pathways of anaphylaxis in the mouse, J. Allergy Clin. Immunol. 109 (2002) 658–668. [DOI] [PubMed] [Google Scholar]
- [79].Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA, Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts, Proc. Natl. Acad. Sci. U.S.A. 105 (2008) 14265–14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Tenzer S, Docter D, Kuharev J, Musyanovych A, Fetz V, Hecht R, Schlnek F, Fischer D, Kiouptsi K, Reinhardt C, Ladfester K, Schild H, Maskos M, Knauer SK, Stauber RH, Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology, Nat. Nanotechnol. 8 (2013) 772–781. [DOI] [PubMed] [Google Scholar]
- [81].Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AA, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW, Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL, J. Clin. Invest. 117 (2007) 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Raschke TM, Water structure and interactions with protein surfaces, Curr. Opin. Struct. Biol, 16 (2006) 152–159. [DOI] [PubMed] [Google Scholar]
- [83].Chen ZA, Pellarin R, Fischer L, Sali A, Nilges M, Barlow PN, Rappsilber J, Structure of complement C3(H2O) revealed by quantitative cross-linking/mass spectrometry and modeling, Mol. Cell Proteomics 15 (2016) 2730–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Petrone PM, Garcia AE, MHC-peptide binding is assisted by bound water molecules, J. Mol. Biol. 338 (2004) 419–435. [DOI] [PubMed] [Google Scholar]
- [85].Moghimi SM, Simberg D, complement activation turnover on surfaces of nanoparticles, Nano Today 15 (2017) 8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Moghimi SM, The effect of methoxy-PEG chain length and molecular architecture on lymph node targeting of immuno-PEG liposomes, Biomaterials 27 (2006) 136–144. [DOI] [PubMed] [Google Scholar]
- [87].Liu D, Guo J, Zhang J-H, Chain mobility and film softness mediated protein antifouling at the solid-liquid interface, J. Mater. Chem. B 4 (2016) 6134–6142. [DOI] [PubMed] [Google Scholar]
- [88].Pannuzzo M, Esposito S, Wu L-P, Key J, Aryal S, Celia C, di Marzio L, Moghimi SM, Decuzzi P, Overcoming nanoparticle-mediated complement activation by surface PEG-pairing, Nano Lett. (2020) doi: 10.1021/acs.nanolett.0c01011. [DOI] [PubMed] [Google Scholar]
- [89].Chorny I, Dill KA, Jacobson MP, Surfaces affect ion pairing, J. Phys. Chem. B 109 (2005) 24056–24060. [DOI] [PubMed] [Google Scholar]
- [90].Zhou H, Fan Z, Li PY, Deng J, Arhontoulis DC, Li CY, Bowne WB, Cheng H, Dense and dynamic polyethylene glycol shells cloak nanoparticles from uptake by liver endothelial cells for long blood circulation, ACS Nano 12 (2018) 10130–10141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wu L-P, Ahamdvand D, Su J, Hall A, Tan X, Farhangrazi ZS, Moghimi SM, Crossing the blood-brain-barrier with nanoligand drug carriers self-assembled from a phage display peptide, Nat. Commun. 10 (2019) 4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wibroe PP, Azmi IDM, Nilsson C, Yaghmur A, Moghimi SM, Citrem modulates internal nanostructure of glyceryl monooleate dispersions and bypasses complement activation: towards development of safe tunable intravenous lipid nanocarriers, Nanomedicine 11 (2015) 1909–1914. [DOI] [PubMed] [Google Scholar]
- [93].Azmi IDM, Wibroe PP, Wu L-P, Kazem AI, Amenitsch H, Moghimi SM, Yaghmur A, A structurally diverse library of safe-by-design citrem-phospholipid lamellar and non-lamellar liquid crystalline nano-assemblies, J. Control. Release 239 (2016) 1–9 [DOI] [PubMed] [Google Scholar]
- [94].Sou K, Tsuchida E, Electrostatic interactions and complement activation on the surface of phospholipid vesicle containing acidic lipids: effect of the structure of acidic groups, Biochim. Biophys. Acta Biomembr. 1778 (2008) 1035–1041. [DOI] [PubMed] [Google Scholar]
- [95].Langford-Smith A, Day AJ, Bishop PN, Clark SJ, Complementing the sugar code: role of GAGs and sialic acid in complement regulation, Front. Immunol. 6 (2015) 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Blaum BS, Hannan JP, Herbert AP, Kavanagh D, Uhrin D, Stehle T T, Structural basis for sialic acid-mediated self-recognition by complement factor H, Nat. Chem. Biol. 11 (2015) 77–82. [DOI] [PubMed] [Google Scholar]
- [97].Perkins SJ, Fung KW, Khan S, Molecular interactions between complement factor H and its heparin and heparin sulfate ligands, Front. Immunol 5 (2014) 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Chauvierre C, Marden MC, Vauthier C, Labarre D, Couvreur O, Leclerc L, Heparin coated poly(alkylcyanoacrylate) nanoparticles coupled to hemoglobin: a new oxygen carrier, Biomaterials 25 (2004) 3081–3086. [DOI] [PubMed] [Google Scholar]
- [99].Wu YQ, Qu HC, Sfyroera G, Tzekou A, Kay BR, Nilsson B, Ekdahl KN, Ricklin D, Lambris JD, Protection of nonself surfaces from complement attack by factor H-binding peptides: implications for therapeutic medicine, J. Immunol. 186 (2011) 4269–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Karasu E, Eisenhardt SU, Harant J, Huber-Lang A, Extracellular vesicles: packages sent with complement, Front. Immunol. 9 (2018) 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Whitehead B, Wu L, Hvam ML, Aslan H, Dong M, Dyrskjøt L, Ostenfeld MS, Moghimi SM, Howard KA, Tumour exosomes display differential mechanical and complement activation properties dependent on malignant state: implications in endothelial leakiness, J. Extracell. Vesicles 4 (2015) 29685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Robbins PD, Morelli AE, Regulation of immune responses by extracellular vesicles, Nat. Rev. Immunol. 14 (2014) 192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Sadallah S, Eken C, Schifferli JA, Ectosomes as modulators of inflammation and immunity, Clin. Exp. Immunol. 163 (2011) 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Sadallah S, Eken C, Schifferli JA, Ectosomes as immunomodulators, Semin. Immunopathol. 33 (2011) 487–495. [DOI] [PubMed] [Google Scholar]
- [105].Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Douroux M, A comprehensive overview of exosomes as drug delivery vehicles – endogenous nanocarriers for targeted cancer therapy, Biochim Biophys Acta Rev. Cancer 1846 (2014) 75–87. [DOI] [PubMed] [Google Scholar]
- [106].Szebeni J, Alving CR, Savay S, Barenholz Y, Priev A, Danino D, Talmon Y Y, Formation of complement-activating particles in aqueous solutions of Taxol: possible role in hypersensitivity reactions, Int. Immunopharmacol. 1 (2001) 721–735. [DOI] [PubMed] [Google Scholar]
- [107].Moghimi SM, Hunter AC, Dadswell CM, Savay S, Alving CR, Szebeni J, Causative factors behind poloxamine 188 (Pluronic F68, Flocor™)-induced complement activation in human sera. A protective role against poloxamer-mediated complement activation by elevated serum lipoprotein levels, Biochim. Biophys. Acta 1689 (2004) 103–113. [DOI] [PubMed] [Google Scholar]
- [108].Hamad I, Hunter AC, Moghimi SM, Complement monitoring of Pluronic 127 gel and micelles: suppression of copolymer-mediated complement activation by elevated serum levels of HDL, LDL, and apolipoproteins A1 and B-100, J. Control. Release 170 (2013) 167–174. [DOI] [PubMed] [Google Scholar]
- [109].Kessel D, Woodburn K, Decker D, Sykes E, Fractionation of Cremophor EL delineates components responsible for plasma lipoprotein alterations and multidrug resistance reversal, Oncol. Res. 7 (1995) 207–212. [PubMed] [Google Scholar]
- [110].Woodburn K, Sykes E, Kessel D, Interactions of Solutol HS 15 and Cremophor EL with plasma lipoproteins, Int. J. Biochem. Cell Biol. 27 (1995) 693–699. [DOI] [PubMed] [Google Scholar]
- [111].Hamilton KK, Zhao J, Sims PJ, Interaction between apolipoproteins A-I and A-II and the membrane attack complex of complement. Affinity of the apolipoproteins for polymeric C9, J. Biol. Chem. 268 (1993) 3632–3638. [PubMed] [Google Scholar]
- [112].Tengood JE, Levy RJ, Stachelek SJ, The use of CD47-modified biomaterials to mitigate the immune response, Exp. Biol. Med. 241 (2016) 1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Gifford G, Vu VP, Banda NK, Holers VM, Wang G, Groman EV, Backos D, Scheinman R, Moghimi SM, Simberg D, Complement therapeutics meets nanomedicine: overcoming human complement activation and leukocyte uptake of nanomedicines with soluble domains of CD55, J. Control. Release 302 (2019) 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Ricklin D, Mastellos DC, Lambris JD, Therapeutic targeting of the complement system, Nat. Rev. Drug Discov. (2019) 10.1038/s41573-019-0055-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Jendza K, Kato A, Salcius M, Srinivas H, De Erkenez A, Nguyen A, McLaughlin D, Be C, Wiesmann C, Murphy J, Bolduc P, Mogi M, Duca J, Namil A, Capparelli M, Darsigny V, Meredith E, Tichkule R, Ferrara L, Heyder J, liu F, Horton PA, Romanowski MJ, Schirle M, Mainolfi N, Anderson K, Michaud GA, A small-molecule inhibitor of C5 complement protein, Nat. Chem. Biol. 15 (2019) 666–668. [DOI] [PubMed] [Google Scholar]
- [116].Reis ES, Berger N, Wang X, Koutsogiannaki S, Doot RK, Gumas JT, Foukas PG, Resuello RRG, Tuplano JV, Kukis D, Tarantal AF, Young AJ, Kajikawa T, Soulika AM, Mastellos DC, Yancopoulou D, Biglamia AR, Huber-Lang M, Hajishengallis G, Nilsson B, Lambris JD, Safety profile after prolonged C3 inhibition, Clin. Immunol. 197 (2018) 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Nunn MA, Sharma A, Paesen GC, Adamson S, Lissina O, Willis AC, Nuttall PA, Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata, J. Immunol. 174 (2005) 2084–2091. [DOI] [PubMed] [Google Scholar]
- [118].Fredslund P, Laursen NS, Roversi P, Jenner L, Oliveira CL, Pedersen JS, Nunn MA, Lea SM, Discipio R, Sottrup-Jensen L, Andersen GR, Structure and influence of a tick complement inhibitor on human complement component 5, Nat. Immunol. 9 (2008) 753–760. [DOI] [PubMed] [Google Scholar]
- [119].Pischke SE, Gustavsen A, Orrem HL, Egge KH, Courivaud F, Fontenelle H, Despont A, Bongoni AK, Rieben R, Tonnessen TI, Nunn MA, Scott H, Skulstad H, Barratt-Due A, Mollnes TE, Complement factor 5 blockade reduces porcine myocardial infarction size and improves immediate cardiac function, Basic Res. Cardiol. 112 (2017) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Horstick G, Heimann A, Gotze O, Hafner G, Berg O, Bohmer P, Becker P, Darius H, Rupprecht HJ, Loos M, Bhakdi S, Meyer J, Kempski O, Intracoronary application of C1 esterase inhibitor improves cardiac function and reduces myocardial necrosis in an experimental model of ischemia and reperfusion, Circulation 95 (1997) 701–708. [DOI] [PubMed] [Google Scholar]
- [121].Buerke M, Murohara T, Lefer AM, Cardioprotective effects of a C1 esterase inhibitor in myocardial ischemia and reperfusion, Circulation 91 (1995) 393–402. [DOI] [PubMed] [Google Scholar]
- [122].Holers VM, Phenotypes of complement knockouts, Immunopharmacology 49 (2000) 125–131. [DOI] [PubMed] [Google Scholar]
- [123].Bottger EC, Hoffmann T, Hadding U, Bitter-Suermann D, Guinea pigs with inherited deficiencies of complement C2 or C4 have characteristics of immune complex disease, J. Clin. Invest. 78 (1986) 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Ameratunga R, Winkelstein JA, Brody L, Binns M, Cork LC, Colombani P, Valle D, Molecular analysis of the thord component of canine complement (C3) and identification of the mutation responsible for hereditary canine C3 deficiency, J. Immunol. 160 (1998) 2824–2830. [PubMed] [Google Scholar]
- [125].Zhang W, Wang G, Wang Y, Jin Y, Zhao L, Xiong Q, Zhang L, Mou L, Li R, Yang H, Dai Y, Generation of complement protein C3 deficient pigs by CRISPER/Cas9-mediated gene targeting, Sci. Rep. 7 (2017) 5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Lachmann PJ, Rosen FS, Genetic defects of complement in man, Springer Semin. Immunopathol. 1 (1978) 339–353. [Google Scholar]
- [127].Schejbel L, Skattum L, Hagelberg S, Ahlin A, Schiller B, Berg S, Genel F, Truedsson L, Garred P, Molecular basis of hereditary C1q deficiency—revisited: identification of several novel disease-causing mutations, Gene. Immun. 12 (2011) 626–634. [DOI] [PubMed] [Google Scholar]
- [128].Mayilyan KR, Complement genetics, deficiencies, and disease associations, Protein Cell 3 (2012) 487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Lappegard KT, Christiansen D, Pharo A, Thorgersen EB, Hellerud BC, Lindstad J, Nielsen EW, Bergseth G, Fadnes D, Abrahamsen TG, Hoiby EA, Schejbel L, Garred P, Lambris JD, Harboe M, Mollnes TE, Human genetic deficiencies reveal the roles of complement in the inflammatory network: lessons from nature, Proc. Natl. Acad. Sci. U.S.A. 106 (2009) 15861–15866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Guven E, Duus K, Laursen I, Hojrup P, Houen G, Aluminum hydroxide adjuvant differentially activates the three complement pathways with major involvement of the alternative pathway, PLoS One 8 (2013) e74445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Chen Z, Koralov SB, Gendelman M, Carroll MC, Kelsoe G, Humoral immune responses in Cr2−/− mice: enhanced affinity maturation but impaired antibody persistence, J. Immunol. 164 (2000) 4522–4532. [DOI] [PubMed] [Google Scholar]
- [132].Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT, C3d of complement as a molecular adjuvant: bridging innate and acquired immunity, Science 271 (1996) 348–350. [DOI] [PubMed] [Google Scholar]
- [133].Rickert RC, Regulation of B lymphocyte activation by complement C3 and the B cell coreceptor complex, Curr. Opin. Immunol. 17 (2005) 237–243. [DOI] [PubMed] [Google Scholar]
- [134].Yalcindag A, He R, Laouini D, Alnius H, Carroll M, Oettgen HC, Geha RS, The complement component C3 plays a critical role in both TH1 and TH2 responses to antigen, J. Allergy Clin. Immunol. 117 (2006) 1455–1462. [DOI] [PubMed] [Google Scholar]
- [135].Irvine DJ, Hanson MC, Rakhra K, Tokatlian T, Synthetic nanoparticles for vaccines and immunotherapy, Chem. Rev. 115 (2015) 11109–11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Smith DM, Simon JK, Baker JR Jr., Applications of nanotechnology for immunology, Nat. Rev. Immunol. 13 (2013) 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Arbore G, Kemper C, Kolev M, Intracellular complement — the complosome — in immune cell regulation, Mol. Immunol. 89 (2017) 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].West EE, Kolev M, Kemper C, Complement and the regulation of T cell responses, Annu. Rev. Immunol. 36 (2018) 309–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Liszewski MK, Kolev M, Le Friec G, Leung M, Bertram PG, Fara AF, Subias M, Pickering MC, Drouet C, Meri S, Arstila TP, Pekkarinen PT, Ma M, Cope A, Reinheckel T, Rodriguez de Cordoba S, Afzali B, Atkinson JP, Kemper C, Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation, Immunity 39 (2013) 1143–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Elvington M, Liszewski MK, Bertram P, Kulkarni HS, Atkinson JP, A C3(H2O) recycling pathway is a component of the intracellular complement system, J. Clin. Invest. 127 (2017) 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Wu L, Uldahl KB, Chen F, Benasutti H, Logvinski D, Vu V, Banda NK, Peng X, Simberg D, Moghimi SM, Interaction of extremophilic archaeal viruses with human and mouse complement system and viral biodistribution in mice, Mol. Immunol. 90 (2017) 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Takasumi M, Omori T, Machida T, Ishida Y, Hayashi M, Suzuki T, Homma Y, Endo Y, Takahashi M, Ohira H, Fujita T, Sekine H, A novel complement inhibitor sMAP-FH targeting both the lectin and alternative complement pathways, FASEB J. (2020) doi: 10.1096/fj.201902475R [DOI] [PubMed] [Google Scholar]
- [143].Korangath P, Barnett JD, Sharma A, Henderson ET, Stewart J, Yu S-H, Kandala SK, Yang C-T, Caserto JS, Hedayati M, Armstrong TD, Jaffee E, Gruettner C, Zhou XC, Fu W, Hu C, Sukumar S, Simons BW, Ivkov R, Nanoparticle interactions with immune cells dominate tumor retention and induce T cell-mediated tumor suppression in models of breast cancer, Sci. Adv. 6 (2020) eaay1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Cecic I, Serrano K, Gyongyossy-Issa M, Korbelik M, Characteristics of complement activation in mice bearing Lewis lung carcinomas treated by photodynamic therapy, Cancer Lett. 225 (2005) 215–223. [DOI] [PubMed] [Google Scholar]
- [145].Wang G, Griffin JI, Inturi S, Brenneman B, Banda NK, Holers VM, Moghimi SM, Simberg D, In vitro and in vivo differences in murine third complement component (C3) opsonization and macrophage/leukocyte responses to antibody-functionalized iron oxide nanoworms, Front. Immunol. 8 (2017) 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Wiegner R, Chakraborty S, Huber-Lang M, Complement-coagulation crosstalk on cellular and artificial surfaces, Immunobiol. 221 (2016) 1073–1079. [DOI] [PubMed] [Google Scholar]