Abstract
Copper is an indispensable trace metal element in the human body, which is mainly absorbed in the stomach and small intestine and excreted into the bile. Copper is an important component and catalytic agent of many enzymes and proteins in the body, so it can influence human health through multiple mechanisms. Based on the biological functions and benefits of copper, an increasing number of researchers in the field of biomaterials have focused on developing novel copper-containing biomaterials, which exhibit unique properties in protecting the cardiovascular system, promoting bone fracture healing, and exerting antibacterial effects. Copper can also be used in promoting incisional wounds healing, killing cancer cells, Positron Emission Tomography (PET) imaging, radioimmunological tracing and radiotherapy of cancer. In the present review, the biological functions of copper in the human body are presented, along with an overview of recent progress in our understanding of the biological applications and development of copper-containing materials. Furthermore, this review also provides the prospective on the challenges of those novel biomaterials for future clinical applications.
Keywords: Copper, Biomaterials, Angiogenesis, Osteogenesis, Antibacterial
Graphical abstract
Highlights
-
•
This paper overviews biological functions of copper in the human body.
-
•
This paper summerizes the recent research progress of the biological applications and development of copper-containing biomaterials.
-
•
This paper is emphasized on the beneficial effects and related mechanisms of Cu-containing biomaterials.
-
•
This review also provides the prospective on the challenges of these novel biomaterials for future clinical applications.
1. Introduction
Trace elements are elements present in the body but at levels less than one-thousandth of the body weight in humans. There are more than 70 different trace elements in the human body. Although their levels in the body are very low, they have important biological effects [[1], [2], [3]].
Copper (Cu), a trace element that is indispensable in organisms, cannot be produced and synthesized in the body and thus needs to be obtained in food. The World Health Organization (WHO) recommends an upper limit of 2–3 mg Cu for adults every day [4,5]. Cu plays important roles in the growth and development of the body, as well as in maturation of the nervous, hematopoietic, bone, and other systems [6,7]. Cu is also an important component of enzymes involved in the metabolism of glucose, amino acids, and cholesterol, and plays a unique role in various catalytic reactions [8,9]. As with other trace elements, it is necessary for Cu to be maintained in equilibrium in the body. Abnormal Cu metabolism or content can cause numerous diseases, such as those associated with deteriorated immune function, diabetes, coronary heart disease, and osteoporosis [10].
In recent years, based on the important roles of Cu in the body, an increasing number of researchers have attempted to use the advantageous effects of Cu to develop new biomedical materials to benefit the human body. More and more studies showed that Cu-containing metallic biomaterials showed excellent properties in protecting the cardiovascular system, exerting antibacterial effects, and promoting bone fracture healing [5,11,12].
In this review, we summarize the current biological functions of Cu in the human body and focus on the development and biological applications of Cu-containing biomaterials. We hope that this review can help researchers from different fields to know the attractive properties of copper-containing biomaterials in biological applications so as to further expand the applications of those novel biomaterials.
2. Cu, an essential trace element
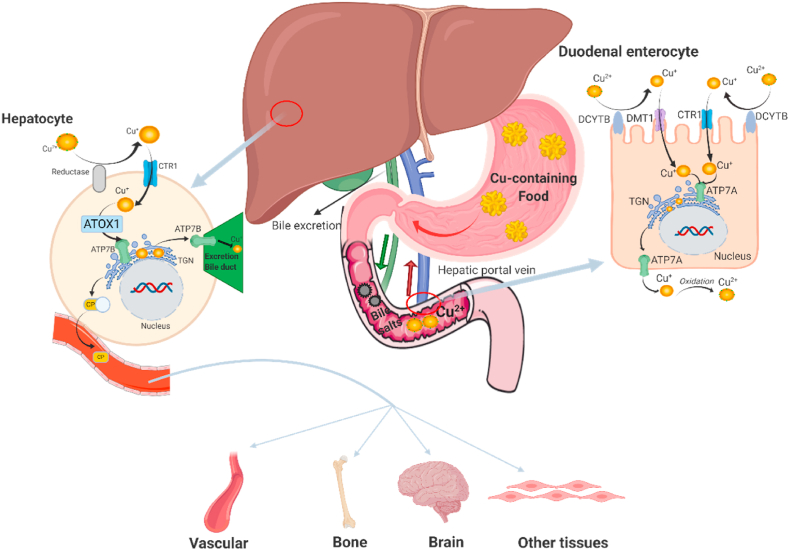
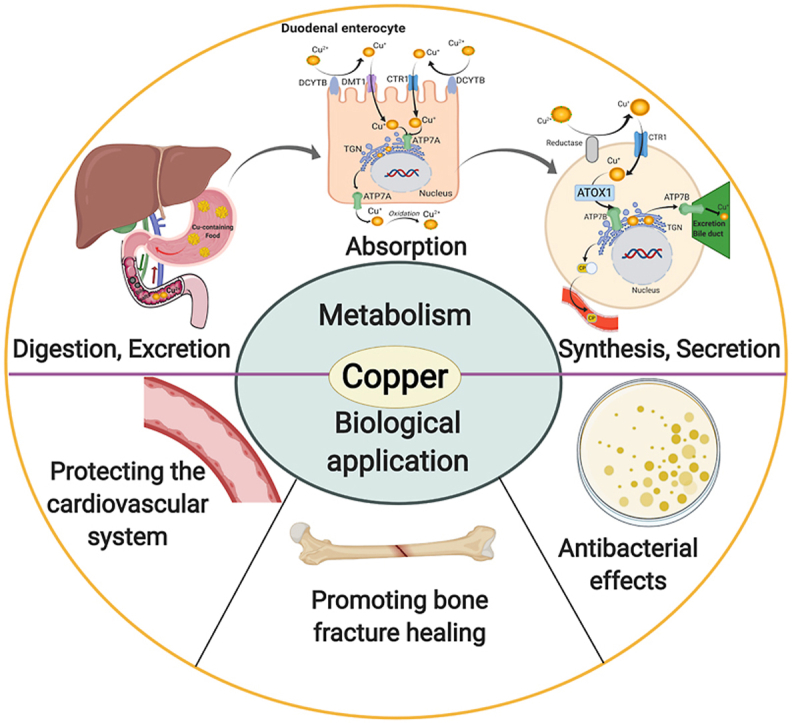
The total amount of Cu in normal adults is 50–150 mg, of which 50%–70% is distributed in muscle and bone, 20% in liver, and 5%–10% in blood. Cu mainly exists in the ceruloplasmin and cuprase in vivo, and there are few free Cu ions in the body [4,13]. Cu in the diet is mainly absorbed in the stomach and small intestine, especially the duodenum, which absorbs about 40% of the total [11,14]. After absorption, Cu is transported into the liver and throughout the body through the blood. Apart for some Cu-containing proteins are stored in the liver, and the rest are synthesized in liver or other human tissues, such as cytochrome oxygenase and monoamine enzyme [8,15]. More than 90 different enzymes and proteins have been found to contain Cu to various extents [16,17]. Cu metabolism is also regulated according to the physiological demand, but the mechanisms involved have yet to be elucidated [13,[17], [18], [19], [20]]. There is a complex system of Cu transport and regulation in the human body, the most important of which is to maintain the balance of Cu in the body by intestinal absorption and biliary excretion [21,22]. Especially in the biliary tract, the concentrations of Cu are controlled by excretion into the bile; the biliary Cu is complexed with bile salts and thus cannot be reabsorbed in the intestinal tract [23] (See Fig. 1).
Fig. 1.
Copper metabolism in human body. Copper is supplied entirely by food in the human body, which is mainly absorbed in stomach and small intestine, especially in duodenum. In the duodenal enterocyte, Cu2+ requires reduction prior to absorption, which may be mediated by DCYTB (duodenal cytochrome B). After reduction, Cu+ is transported into enterocytes by CTR1 (copper transporter 1) or DMT1 (divalent metal-ion transporter 1). Then copper is pumped into the TGN (trans Golgi network) by ATP7A, supporting cuproenzyme synthesis, or exported from the cell by ATP7A. When exported from enterocyte, Cu+ is spontaneously oxidized into Cu2+ by dissolved oxygen in blood, then mainly bounded to albumin and α2-macrogloubuoin in the portal blood and finally delivered to the liver. In the hepatocyte, firstly, Cu2+ requires a reduction, which may be mediated by reductase. After reduction, Cu+ is taken up into hepatocytes via CTR1. Then, ATOX1 (antioxidant protein 1) delivers Cu+ to ATP7B, which transports Cu+ into TGN for incorporation into CP (cuproenzymes). ATP7B also transports excessive Cu+ across the canalicular membrane into bile for excretion. CP, which is released into blood, is transported to blood vessels, brain, bone and other tissues for biological functions.
It is becoming increasingly understood that the homeostasis of Cu, which is regulated by complex mechanisms in the human body, is important in human health. Both the overload and the deficiency of Cu can influence human health through multiple mechanisms [24,25]. Disorders featuring overload of Cu mainly include two aspects: (1) acute Cu toxicity (the acute toxic reaction caused by exposure to a large number of copper-containing substances in a short period of time) and (2) chronic Cu toxicity (the chronic toxic reaction caused by long-term repeated entry of low-dose copper containing substances into the body). With regard to acute Cu toxicity, nausea, vomiting, headache, diarrhea, hemolytic anemia, gastrointestinal hemorrhage, and liver and kidney failure, as well as death, may occur with an increase of the Cu dose [26,27]. In the case of chronic Cu toxicity, this can cause Wilson disease [28]. This background underlines the fact that Cu is a trace element that is essential for life and good health. There are multiple clinical symptoms caused by Cu deficiency in humans [20,29]. Early and common signs of acquired Cu deficiency are hematological manifestations such as anemia, leukopenia, neutropenia, and pancytopenia [30]. Bone abnormalities including osteoporosis, bone fractures, and bone malformations are also often observed in Cu-deficient low-birthweight infants and young children [31].
As Cu is an indispensable trace element with key biological functions in the human body, there are various biological applications of Cu-containing metallic biomaterials, as presented below [19,21,32,33].
3. Beneficial effects of Cu on the cardiovascular system
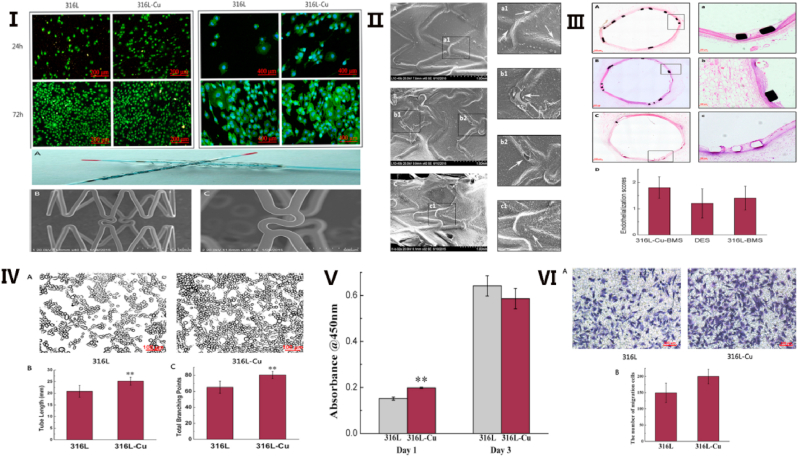
Early in 1980, McAuslan and Gole showed that Cu can stimulate blood vessel formation by introducing CuSO4 into anterior chamber implants at micromolar levels [34]. Later studies further suggested that Cu could not only stimulate the proliferation and migration of endothelial cells to promote the formation of new blood vessels, but also inhibit the proliferation of vascular smooth muscle cells and thrombosis [22,35]. These results prompted more intense research on Cu in vessels. Vascular endothelial growth factor (VEGF) is the most effective growth factor for promoting angiogenesis. The VEGF signaling pathway can significantly promote the proliferation, migration, and chemotaxis of endothelial cells in various tissues and organs. Rigiracciolo et al. [36] proposed that Cu ions could activate the EGFR/ERK/c-Fos pathway to increase the expression of VEGF in cancer cells. Fibroblast growth factor (FGF) can promote the growth of fibroblasts and is a strong growth factor of connective tissue cells. There are many isomers of FGF, and the active center of some isomers requires the participation of Cu [37]. In recent years, studies have shown that hypoxia can cause microvascular formation through promoting the expression of VEGF [38], especially in the tumor environment, and that hypoxia inducible factor-1 (HIF-1) is a regulatory factor of angiogenesis under hypoxia, which has a wide range of target gene profiles [39,40]. Cu can not only activate HIF, but also promote its expression, which increases vascularization [41,42]. In addition, Li et al. [43] found that 5Cu-BG/ESM films containing 5 mol% Cu can stimulate angiogenesis by improving VEGF and HIF-1a protein secretion as well as the expression of angiogenesis-related genes [VEGF, HIF-1a, VEGF receptor 2 (KDR), and endothelial nitric oxide (eNOS)] of human umbilical vein endothelial cells (HUVECs). Nitric oxide (NO) is an important vasorelaxant produced along with l-citrulline from l-arginine in a reaction catalyzed by endothelial nitric oxide synthase (eNOS) [44], and it can relax blood vessels, and promote the differentiation, proliferation, and migration of endothelial cells by activating NO synthetase and the MAPK signaling pathway [45]. Demura et al. found that only when the concentration of Cu2+ was higher than 10−6 M could it significantly increase the eNOS activity in human pulmonary artery endothelial cells [46] (See Fig. 2).
Fig. 2.
Structure of 316-Cu stent and it promotes vascular endothelialization. Ⅰ. The vWF staining and F-actin filament staining of HUVECs seeded on 316L and 316L-Cu, respectively. Ⅱ. Scanning electron micrographs of surface characterization of explanted endovascular stent. Ⅲ. Hematoxylin-eosin stain of explanted stents of 316L-Cu-BMS (A a), DES (B b), and 316L-BMS (C c). Ⅳ. Tube formation induced by the extracts of 316L and 316L-Cu, respectively. Ⅴ. CCK-8 proliferation assay of HUVECs cultured on different substrates. Ⅵ. Cell migration induced by the extracts of 316L and 316L-Cu, respectively. Reproduced with permission from Ref. [15], copyright 2017, Nature.
The above research results show that Cu, as an indispensable trace element in the human body, can promote the endothelialization of blood vessels and inhibit the proliferation of smooth muscle cells and thrombosis. In recent years, therefore, increasing research has focused on how to add Cu to medical materials to treat cardiovascular disease. Atherosclerotic occlusive diseases, the main forms of which include coronary disease, apoplexy, and peripheral artery disease, are the major causes of death in humans [47]. Stent implantation is one of the most important therapeutic modalities for these diseases, which can dredge blood vessels and restore blood flow through a minimally invasive approach [48]. Stent restenosis is the main complication after stent implantation, and its incidence has gradually increased in recent years [49]. The main factors causing stent restenosis include: (1) that the vascular endothelium is seriously damaged when the stenosis or occlusion is opened; and (2) after stent implantation, smooth muscle cells proliferate and a thrombus forms [50]. However, increasing research on Cu-containing stents has indicated that the addition of an appropriate amount of Cu in the stent material may be a promising approach for reducing the incidence of stent restenosis. Xu et al. found that Cu-bearing stainless steel (SS) could promote the adhesion and proliferation of vascular endothelial cells (VECs), and reduce the early apoptosis rate of these cells through Cu-bearing SS inoculated with VECs and incubated for 1, 2, or 3 days [51]. Subsequently, Jin et al. obtained almost the same results when HUVECs were incubated with an L605-Cu cobalt alloy [5]. Furthermore, they demonstrated that L605-Cu significantly increased the mRNA expression of VEGF in HUVECs. However, it had no effect on the secretion of NO or mRNA expression of eNOS, which may have been because the concentration of released Cu ions was relatively low. Besides promoting the proliferation of vascular endothelial cells [17,22], studies showed that Cu-bearing SS or Ti alloy (Ti–Cu) could inhibit the proliferation of vascular smooth muscle cells, thus reducing the formation of thrombosis to in turn lower the occurrence of in-stent restenosis [52,53]. Subsequent studies shifted the focus from in vitro to in vivo. For example, Liu et al. [54] suggested that the time to complete endothelialization was significantly shorter than that of bare stents after plating a copper film on the surface of metal stents. They further found that vascular smooth muscle cells and vascular endothelial cells tended to undergo apoptosis to some degree when the Cu concentration was over 7.5 μg/ml. Moreover, Jin et al. implanted 316L-Cu bare metal stents (BMS), drug-eluting stents (DES), and 316L BMS into swine to evaluate the re-endothelialization ability in vivo; their results demonstrated that 316L-Cu BMS showed the best effect on endothelialization along with good biosafety [15]. Previous studies have shown that copper containing metal materials with copper content of 3-5 wt% could promote the endothelialization and inhibit the growth of smooth muscle cells [5,48,49,51,52]. Consequently, Cu-containing metal stents are considered promising for treating atherosclerotic occlusive diseases. The main beneficial effects of Cu-containing materials on the cardiovascular system are summarized in Table 1.
Table 1.
Beneficial effects of Cu-containing materials on cardiovascular system.
| Effect | Materials | Functions | Targets |
|---|---|---|---|
| Protecting the cardiovascular system | Cu ions [36,46] | Increasing the expression of VEGF [36], increase the eNOS activity [46] | Activating EGFR/ERK/c-fos pathway [36] |
| 5Cu-BG/ESM [43] | Stimulating proangiogenesis [43] | Improving VEGF and HIF-1a protein secretion, activating angiogenesis-related gene expression (VEGF, HIF-1a and eNOS) [43] | |
| Cu-bearing SS [51,52] | Promoting the adhesion, proliferation of VEC, reducing the early apoptosis ratio of VEC [51], inhibiting the proliferation of VSMC [52] | – | |
| L605-Cu [5] | Promoting the adhesion, proliferation of HUVECs [5] | Increasing the mRNA expression of VEGF [5] | |
| Ti-Cu [53] | Inhibiting the proliferation of VSMC, reducing the formation of thrombosis [53] | – | |
| 316L-Cu stent [15] | Showing the best effect on endothelialization with good biosafety [15] | Improving the angiogenesis-related gene expression of VEGF and eNOS [15] |
4. Cu-containing materials promoting bone fracture healing
Besides the beneficial effects of Cu on the cardiovascular system, recently, research has indicated that a certain amount of Cu2+ could induce the differentiation of mesenchymal stem cells and osteoblastic cells [55,56], indicating that Cu-containing biomaterials may help to accelerate the bone fracture healing [14,57,58].
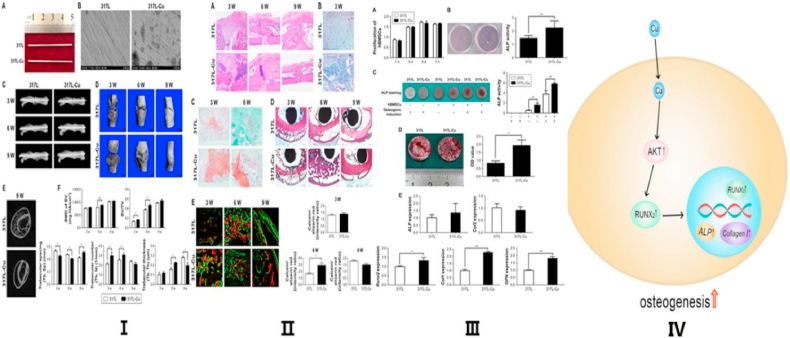
The widespread use of motorized transport, along with natural disasters and increases in industrial accidents, has led to a dramatic increase in trauma and bone fractures [59,60]. However, the development of surgical techniques and implant materials has greatly improved the prognosis of fractures [61,62]. Nonetheless, it was reported that 5%–10% of fractured bones ended in nonunion and/or incomplete healing, which could even lead to disability in some cases [63]. The principles for treating bone fractures mainly involve early treatment, anatomic reduction, and rigid fixation [64,65]. Open fractures, fractures with failed manual reduction, and obsolete fractures without ideal function are treated surgically. This always requires fixation devices, such as screws, intramedullary nails, and internal plates, to fix the fracture broken end or assist in functional recovery [66,67]. The implant materials should ideally create the optimal healing environment for the injured bones. Therefore, conferring osteogenicity to existing metal implants is crucial for improving the prognosis of bone fractures and reducing postoperative complications [[68], [69], [70]] (See Fig. 3).
Fig. 3.
Promoting osteogenesis and its mechanism of copper-containing implants. Ⅰ. General view and scanning electron microscope view of 317L SS and 317L-Cu SS (A and B); The fracture line is clearly observed in X-ray (C); Micro-CT showed the callus formation stage (D and E); Callus bone mineral density in the 317L-Cu SS group is higher than that of 317L SS group (F). Ⅱ. H&E staining displays the general evolution of 317L-Cu SS group calluses and 317L SS group, respectively (A); Masson staining showed the amount of fibrous tissue of 317L-Cu SS group calluses and 317L SS group, respectively (B); Safranin O/fast green staining (C); Van Gieson staining (D); Calcein/alizarin red double labeling (E). Ⅲ. 317L-Cu stainless steel significantly promoted the osteogenic differentiation of human bone mesenchymal stem cells in vitro. Reproduced with permission from Ref. [63], copyright 2017, Dove Press. Ⅳ. Schematic diagram for copper-containing implants stimulated osteogenesis. Reproduced with permission from Ref. [4], copyright 2019, Elsevier. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Based on research and development of metal-manufacturing process, there are three main methods of fabricating Cu-containing biomaterials: (1) melting metals with the addition of Cu; (2) depositing Cu coating on the surface of metals; and (3) incorporating Cu nanoparticles into metals. For the first method, Ren et al. developed a new Cu-bearing 317L SS (317L-Cu) by directly immobilizing an appropriate amount of Cu into medical 317L SS during the metallurgical process, which could enable the continuous release of trace amount of Cu2+ ions from the surface of the steel. The results demonstrated that Cu2+ ions could promote osteogenic differentiation by stimulating alkaline phosphatase enzyme activity and osteogenic gene expression (type I collagen, runt-related transcription factor 2, and osteopontin), and enhancing the adhesion and proliferation of osteoblasts cultured on the steel surface [11]. In a further study, Yuan et al. found that Cu2+ ions released from 316L-Cu stainless steel could enhance the gene expression of collagen I, ALP, and Runx2, and showed that the protein expression of Runx2 was stimulated by an Akt cell signaling pathway [4]. Moreover, Liu et al. indicated that a Ti–Cu alloy could upregulate the expression of osteogenesis-related genes including alkaline phosphatase, collagen I, osteopontin, and osteocalcin, but had no significant impact on the adhesion, proliferation, and apoptosis of osteoblasts cultured with Ti–Cu alloy [7]. The difference in experimental results may be related to the release of Cu ions. Large amount of copper should not be added to ensure the original properties of the material, therefore, the concentration of Cu ions released from Cu-containing alloy is usually finite due to the limited content in the alloy.
In terms of the second method of fabricating Cu-containing biomaterials, most researchers used micro-arc oxidation technology, which relies on the instantaneous high temperature and high pressure effect generated by discharge, to make copper ions to get into the surface of aluminum, magnesium, titanium and their alloys in the electrolyte solution, and the ceramic coating dominated by matrix metal oxides [[71], [72], [73]]. Zhao et al. demonstrated that magnesium–copper–fluorine-containing titanium coatings might promote osteogenesis through ERK1/2 signaling [72]. In addition, Huang et al. employed the micro-arc oxidation technique to fabricate Cu-containing ceramic coating on a titanium substrate, and then cultured macrophages on the surface. They found that the integration of Cu into the material's surface enhanced macrophage-mediated osteogenesis and bactericidal capacity [73]. Other studies employed this method to fabricate Cu-containing materials and obtained the same positive results for improving bone fracture healing [[74], [75], [76]]. Compared with the method of melting metals with the addition of Cu, this method does not change the hardness and toughness of the graft. However, the Cu containing layer might lead to the massive release of Cu ions or tiny Cu particles, which might cause a relatively severe and persistent foreign body reaction in vivo [77]. To overcome this limitation, some researchers innovatively used the sustained-release properties of nanomaterials to prepare copper-containing materials via screening and analysis of nanoparticle size [[78], [79], [80]]. For example, Wang et al. developed a novel nano-copper-bearing SS with nano-sized copper-precipitation (317L-Cu SS) to explore the effect of 317L-Cu SS on bone fracture healing in vivo. Their results demonstrated that 317L-Cu SS not only promoted osteogenic differentiation and the expression of osteogenesis-related genes, but also upregulated lysyl oxidase activity [63]. Subsequently, Huang et al. employed a Cu-containing micro/nano-topographical bioceramic surface (Cu-Hier-Ti surface) as a material model to explore the role played by Cu2+ release or the material surface in regulating macrophage-mediated osteogenic and bactericidal effects [6]. They found that M1 macrophages activated by Cu2+ exhibited a stimulatory effect on osteoblast maturation. Besides, focusing on Cu-containing alloy, Zhang et al. fabricated aqueous soluble graphene oxide-copper nanocomposites (GO-Cu) [80], which were used to coat porous calcium phosphate scaffolds for the regeneration of vascularized bone. The results demonstrated that the GO-Cu coating enhanced the adhesion and osteogenic differentiation of rat bone marrow stem cells and upregulated the expression of Hif-1α by activating the Erk1/2 signaling pathway in vitro. Furthermore, they found that the GO-Cu-coated calcium phosphate cement scaffolds significantly promoted angiogenesis and osteogenesis. Moreover, Wang et al. investigated a method based on the controlled delivery of Cu ions from borate bioactive glass scaffolds for stimulating angiogenesis and osteogenesis in a rodent calvarial defect model in vivo [81]. They found that the controlled delivery of Cu ions from borate bioactive glass implants is a promising approach for the healing of bone defects. More studies to utilize nanotechnology to fabricate Cu-containing materials indicated basically similar results that Cu2+ could promote osteoblast proliferation and accelerate bone fracture healing [[82], [83], [84], [85]]. Li et al. found that at a concentration of 10 μM lower, Cu2+ was safe for mouse osteoblastic cell line (MC3T3-E1) and beneficial for its proliferation in a dose-dependent manner. The half maximal inhibitory concentration (IC50) was about 134.6 μM and the inhibition ratio rapidly rose to 100% when concentration of Cu2+ ranged from 111 μM to 333 μM86. The above-mentioned results clearly show that different methods using Cu-containing materials can promote bone fracture healing. Some examples of Cu-containing materials promoting bone fracture healing are summarized in Table 2.
Table 2.
Cu-containing materials promoting bone fracture healing.
| Effect | Materials | Functions | Targets |
|---|---|---|---|
| Promoting bone fracture healing | 317L-Cu [11] | Promoting the osteogenic differentiation, enhancing the adhesion and proliferation of osteoblasts [11] | Stimulating the Alkaline phosphatase enzyme activity and osteogenic gene expressions [11] |
| 316L-Cu [4] | Enhancing the gene expression of collagen I, ALP and Runx2 [4] | Activating Akt signaling pathway [4] | |
| Ti-Cu [7] | Upregulate the expressions of alkaline phosphatase, Collagen I, osteopontin and osteocalcin [7] | – | |
| Magnesium-copper-fluorine containing titanium coatings [72] | Promoting osteogenesis [72] | Activating ERK1/2 signaling [72] | |
| Cu-containing ceramic coatings on titanium [73] | Enhancing macrophage-mediated osteogenesis [73] | – | |
| Nano-copper-bearing 317L SS63 | Promoting osteogenic differentiation and the expression of osteogenesis-related genes, upregulating the lysyl oxidase activity [63] | – | |
| Cu-Hier-Ti [6] | Regulating macrophage-mediated osteogenic [6] | Activating M1 macrophages [6] | |
| GO-Cu [80] | Enhancing the adhesion and osteogenic differentiation [80] | Activating the Erk1/2 signaling pathway [80] |
5. Antibacterial activity of Cu-containing alloys
Infection remains one of the most common postoperativce complications to be resolved in all kinds of surgical operations, even in minimally invasive interventional surgery, although modern medicine has advanced aseptic management and developed effective broad-spectrum antibiotics [[87], [88], [89], [90], [91]]. In addition, implant-related infection is a devastating complication following the application of a medical implant. For example, implant-related infection in orthopedics has become a common problem against the background of a markedly increasing number of implant surgeries being performed [[92], [93], [94]]. In the United States, implant-related infection is the main cause of total knee arthroplasty, accounting for more than 20% of all cases, and severe infection may lead to amputation [95,96]. The treatment of implant-related infection is complicated; preventing such infection from occurring at all is obviously the ideal solution, but complete prevention is typically not possible. Surgeons routinely perform a variety of strategies to avoid infection, such as applying strict aseptic techniques, wound dressing, and administering antibiotics [97,98]. However, these measures can only provide short-term protection and inhibition of infection. Some studies have shown that residual bacteria on implants or distant bacterial infection cause opportunistic infection of implants, which damages implant functions and can even lead to bacteremia [99,100]. With the advances of materials for biomedical applications, studies have demonstrated that biofilm formation on the surfaces of materials is the main cause of implant-related infections and has serious consequences [101,102]. Increasing research has now focused on developing new implant materials or modifying the surfaces of implants to inhibit persistent biofilm-related infections. To ensure biosafety, almost all studies in this field need to meet the following requirements: (1) the material for the implant must be biocompatible; (2) the stability and properties of the implant should not be weakened; and (3) the antibacterial effect of new implants should have a broad spectrum. Originally, some studies used antibiotic-coated metallic materials to prolong the prevention of infection through surface modification technology [103,104]. However, there are two major problems to this approach. On the one hand, the bonding between the coating and the metal surface is usually unstable. On the other hand, long-term antibiotic release increases the risk of drug resistance emerging [105,106]. Reddy et al. performed a novel microscopic physical surface modification method (Sharklet) for preventing bacterial colonization and migration [107]; their results showed that the Sharklet micropattern was effective at inhibiting bacterial colonization and migration. Unfortunately, manufacturing of the Sharklet micropattern is too complicated to apply it to metal implants.
Based on the strong antibacterial performance of Cu, which is one of the most essential trace elements present in the human body [108], researchers have increasingly focused on fabricating Cu-containing implantable materials to inhibit the occurrence of infections. Stainless steel is one of the most commonly used materials in the biomedical field [9,109]. Combined with the above conditions, Nisshin Steel (Tokyo, Japan) first developed antibacterial Cu-containing SS in the 1990s102. Related research demonstrated that the strong and broad-spectrum antibacterial ability of Cu-containing SS is mainly due to the Cu ions released from the steel's surface, which damage bacterial cell walls and cell membranes, adsorb electrons from bacteria, and generate reactive oxygen species (ROS), resulting in severe damage to and death of bacteria and fungi [24,110,111], such as Staphylococcus haemolyticus [112,113], Escherichia coli [114], and Candida albicans [111,115]. Subsequently, Yang et al. from the Institute of Metal Research, Chinese Academy of Sciences (Shenyang, China), proposed a new concept of a bio-functional design for metallic biomaterials, focusing on the development of new metallic biomaterials with the combination of excellent mechanical properties, corrosion resistance, and antibacterial ability [21]. 317L SS, which has a good balance of biocompatibility and corrosion resistance, has been applied in various implantable and non-implantable medical devices [102]. Therefore, Yang et al. added an appropriate amount of Cu in 317L SS in steelmaking to fabricate a novel Cu-bearing 317L SS (317L-Cu SS) [21]. Sun et al. showed that 317L-Cu SS (4.46% Cu) exhibited satisfactory antibacterial ability against Staphylococcus haemolyticus, and its antibacterial rate reached 98.3% after 24 h of treatment in vitro [116]. Similarly, Yang et al. indicated that 317L-Cu SS achieved a 99% inhibition rate of sessile Staphylococcus aureus cells after 5 days of incubation [102]. Guan et al. found that 317L-Cu SS could effectively kill Escherichia coli on the material surface without having an adverse effect on osteoblasts in vitro [117]. Moreover, in vitro and in vivo studies by Chai et al. demonstrated that 317L-Cu SS possessed a strong antibacterial effect to prevent implant-related infection [118].
Besides 317 L-Cu SS, other Cu-containing materials also exhibited satisfactory antibacterial abilities [24]. For example, Ren et al. fabricated a Cu-bearing 316L SS (316L-Cu SS) containing 3.77% Cu, with the aim of preventing implant-related infection (IRI) [12]. The results demonstrated that 316L-Cu SS exhibited a broad-spectrum antibacterial effect against Staphylococcus aureus, Escherichia coli, and Staphylococcus epidermidis, with bacteriostatic rates of 95.2%, 94.8%, and 94.1%, respectively, in vitro. Furthermore, 316L-Cu SS showed lower bone infection when 316L-Cu SS nails were used to treat a rat model. Compared with the conventional 304 SS, Cu-bearing 304 SS (304 SS-Cu) could effectively kill Porphyromonas gingivalis, indicating that it may inhibit the occurrence of peri-implantitis [119]. Ma et al. [120] showed that the Ti–5Cu alloy exhibited excellent antibacterial property and corrosion resistance, providing a great potential in clinical application for dental implants. Li et al. [121] demonstrated that 420-Cu SS presented excellent antibacterial performance against the mixed bacteria, including E. coli and S. aureus, with an approximately 99.4% of antibacterial rate. Yan et al. [122] and Liu et al. [123] also successfully prepared a Mg–Cu alloy, which showed excellent antibacterial effects both in vitro and in vivo.
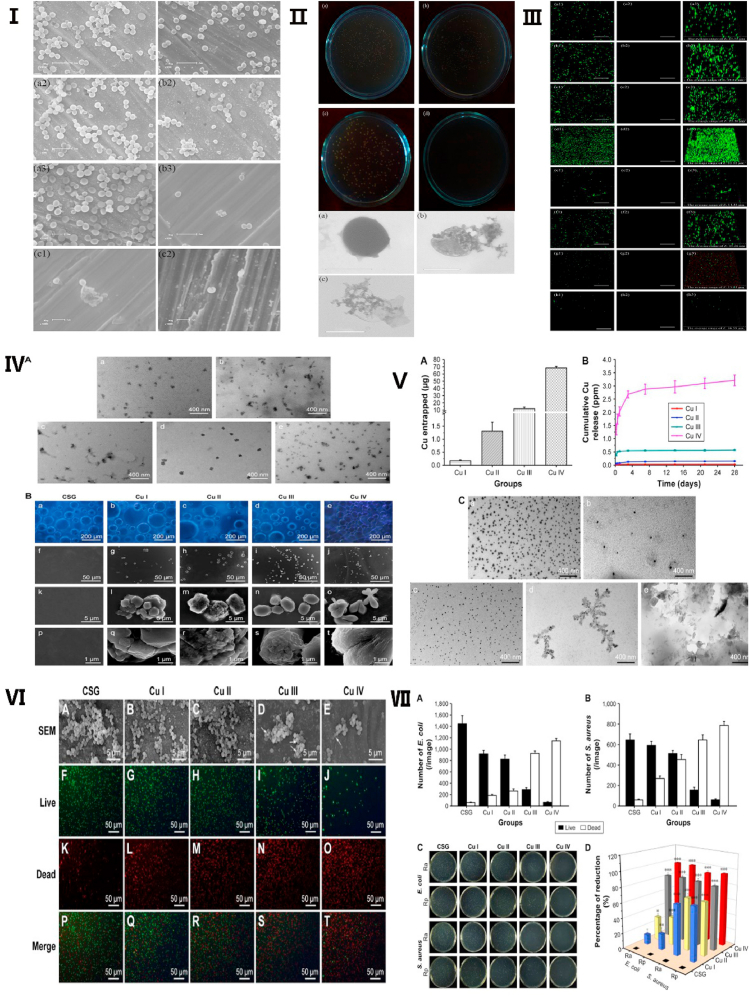
In recent years, Cu-containing titanium alloys have attracted increasing attention since titanium alloy is one of the most commonly used materials in orthopedics [[124], [125], [126]]. For example, Jin et al. demonstrated that 316L SS coated with a Ti–Cu layer (316L/Ti–Cu) showed an effective reduction of 99.9% of Escherichia coli within 12 h127. In addition, Ma et al. developed a Cu-containing antibacterial titanium alloy (Ti–5Cu), which possessed strong antibacterial activity against both Escherichia coli and Staphylococcus aureus [120]. Moreover, the research of Liu et al. revealed antimicrobial/antibiofilm activities of Ti–Cu alloy against the orally specific bacterial species Streptococcus mutans and Porphyromonas gingivalis [128]. Furthermore, Cu-bearing mesoporous bioglass could continuously release Cu ions, which effectively decreased bacterial viability and inhibited biofilm formation [41,129,130]. Gosau et al. also established a silicone material coated with Cu (silicone/Cu) and found that these new breast implants had anti-adherence and antibacterial effects against Staphylococcus epidermidis [131]. Lee et al. [132] found that it was possible to tune the antibacterial property of Ti-Cux alloys by changing the Cu concentration and aging, which could help to optimize the antibacterial properties of copper-containing materials. Li et al. found that when concentration of Cu2+ was over 37 μM, it showed inhibition ratio suddenly increased and approached to 100% at 111 μM on both E. coli and S. aureus. The in vitro study also showed that the proliferation of human umbilical vein endothelial cells (HUVECs) was promoted when concentration of Cu2+ was lower than 222 μM. At a concentration of 10 μM lower, Cu2+ was safe for MC3T3-E1 and beneficial for its proliferation. Therefore, Cu2+ performed on both cells and bacteria yields good osteogenic, angiogenic and antibacterial activity without cytotoxicity [86] (See Fig. 4).
Fig. 4.
Antibacterial activity and its mechanism of copper-containing materials. Ⅰ. Scanning electron microscope image of S. aureus cells on 317L-Cu SS surface. Ⅱ. Scanning electron microscope images showed the deformation process of S. aureus, which incubated with 317L-Cu SS. Ⅲ. Confocal laser scanning microscopy images of the S. aureus biofilm on 317L SS. Reproduced with permission from Ref. [116], copyright 2016, Nature Publishing Group. Ⅳ. Transmission electron microscopy images (A) and fluorescence images (B) of copper-containing coatings. Ⅴ. Cu entrapment and release of the copper-containing coatings. Ⅵ. Field emission scanning electron microscopy images and Live/Dead stain fluorescent images showed that copper-containing coatings inhibit proliferation of E. coli. Ⅶ. copper-containing coatings may inhibit proliferation of E. coli and S. aureus. Reproduced with permission from Refs. [82], copyright 2017, Dove Press.
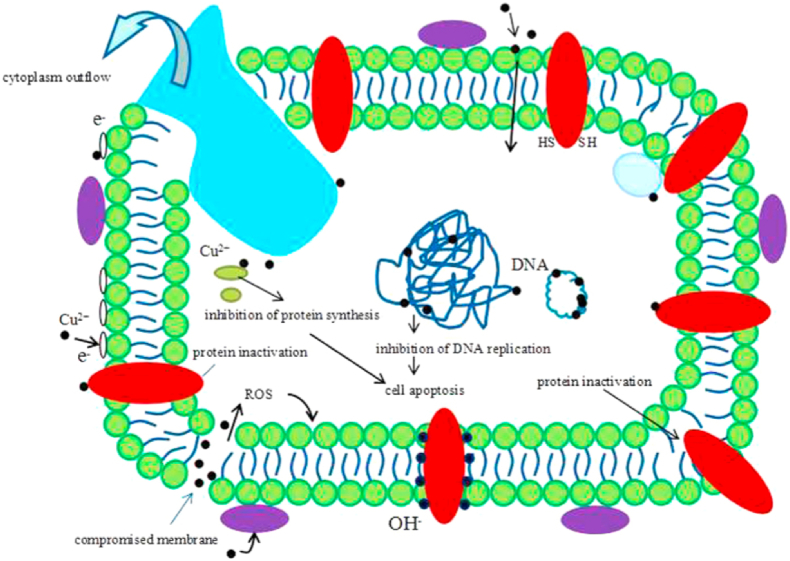
Although many studies have shown that Cu-containing materials exhibited strong antibacterial effects and good biocompatibility, the related antibacterial mechanisms of Cu2+ are still unclear [21]. Recent research showed that, as a result of the carboxyl group of lipoproteins, the surface of bacteria has a negative charge, which causes bacteria to attract Cu ions given their positive charge. Upon contacting Cu ions, the permeability of bacterial cell membranes changes and these cells are damaged. When Cu ions enter bacterial cells, the enzymes are out of activities, and DNA, RNA, and proteins along with cytoplasm leak out [133]. This could result in the bacteria dying (See Fig. 5, Fig. 6). Therefore, in contrast to the traditional antibacterial mechanism, Cu-containing materials possess long-lasting antibacterial effects. Some antibacterial effects of Cu-containing materials are summarized in Table 3.
Fig. 5.
The schematic image of hypothesis about the antibacterial mechanism of Cu2+. Reproduced with permission from Ref. [128], copyright 2016, Nature Publishing Group.
Fig. 6.
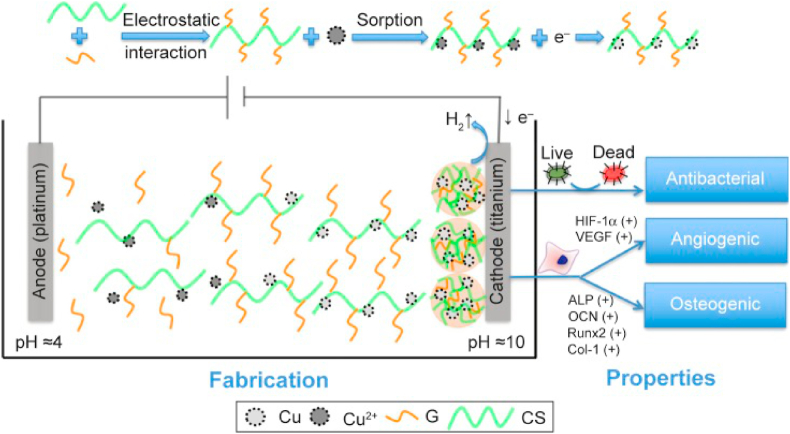
Schematic diagram for fabrication and properties of copper-containing coating materials. Reproduced with permission from Refs. [82], copyright 2017, Dove Press. Abbreviations: G, gelatin; CS, chitosan.
Table 3.
Antibacterial effects of Cu-containing materials.
| Effect | Materials | Functions | Targets |
|---|---|---|---|
| Antibacterial activity | Cu-containing SS [[110], [111], [112],114,115] | Resulting in the fatal damage and death of the bacteria and fungus [[110], [111], [112],114,115] | Generating the reactive oxygen species [110,111] |
| 317L-Cu [102,[116], [117], [118]] | Inhibiting and killing Staphylococcus haemolyticus, Staphylococcus aureus and Escherichia coli [102,[116], [117], [118]] | – | |
| 316L-Cu [12,119] | Exhibiting a broad-spectrum antibacterial effect [12,119] | – | |
| Mg-Cu [122,123] | Showing an excellent antibacterial effect both in vitro and in vivo [122,123] | – | |
| 316L/Ti-Cu [127] | Inhibiting Escherichia coli [127] | – | |
| Ti–5Cu120 | Strong antibacterial activity against both Escherichia coli and Staphylococcus aureus [120] | – | |
| Ti-Cu [128] | Inhibiting Streptococcus mutans and Porphyromonas gingivalis [128] | – | |
| Cu-bearing mesoporous bioglass [41,129,130] | Decreasing bacterial viability [41,129,130] | Inhibiting biofilm formation [41,129,130] | |
| Silicone/Cu [131] | Inhibiting Staphylococcus epidermidis [131] | – |
6. Other effect of copper
In addition to the above-mentioned functions of copper-containing biomaterials, Ghosh Deepanjan et al. found that delivery of ionic copper from sutures could promote incisional wounds healing, and copper-eluting fibers may have translational potential for accelerating repair on surgical and trauma wounds [134]. L. A. Volodina et al. developed ointment containing copper nano-particles (NPs), which could provide a high level of reparation of skin wounds [135].
Some studies suggest that copper-containing materials have a certain role in the treatment of cancer. Based on the previous studies, colloidal Cu NPs have been proven to be one of the effective inorganic materials against various cancer cell lines. As reported by Valodkar et al. [136,137], Cu NPs was toxic to human lung carcinoma cell line (A549)137, human liver hepatoma (HepG2), Chinese hamster ovary (CHO), human osteosarcoma (Saos), and mouse embryonic fibroblast (3T3L1) cells in a dose-dependent manner [136]. The study revealed that the capped Cu NPs by nontoxic aqueous extract of latex could be directly used for administration/in vivo delivery for cancer therapy [136]. In another study, Harne et al. [138] reported excellent viability against HeLa, A549 and BHK21 cells even at 120 μM concentration of Cu NPs. Anticancer studies demonstrated the in vitro cytotoxicity values of Cu NPs against the tested human colon cancer Caco-2 cells, human hepatic cancer HepG2 cells and human breast cancer Mcf-7 cells, which can be used as a photothermal treatment to kill cancer cells [139].
Copper has several radioisotopes, and 60Cu, 61Cu, 62Cu, 64Cu and 67Cu are particularly interesting for radiotherapy and imaging applications. Decay characteristics of copper radionuclides make them suitable for numerous medical applications, such as Positron Emission Tomography (PET) imaging, radioimmunological tracing and radiotherapy of cancer [140].
7. Conclusion
In the last few decades, considerable efforts have been expended in clarifying the functions of Cu2+ in the human body, as well as in exploring the biological applications of Cu-containing materials. As a trace element in the human body, Cu is an important component and catalytic agent of many enzymes and proteins. Therefore, Cu possesses multiple important bio-functions, such as protecting the cardiovascular system, promoting bone fracture healing, and exerting antibacterial effects. With the greater understanding of the biological effects and biosafety of Cu, more attention has been focused on developing Cu-containing materials in order to treat diseases and improve human health. In recent years, more and more substantial progress has been made in this field. For example, coronary stents made of Cu-containing stainless steel have been shown to prevent the occurrence of in-stent restenosis, while orthopedic grafts made of Cu-containing materials can promote osteoblast proliferation and inhibit bacterial adhesion so as to promote bone fracture healing and reduce the risk of infection. Despite the important progress that has been made, further investigation is needed to understand the mechanisms by which Cu-containing materials act in order to create a wide range of applications. With the increased focus on achieving further progress with Cu-containing materials, it is believed that the addition of an appropriate amount of Cu to biomaterials may become a novel and beneficial approach to improve current biomedical material applications.
8. Future prospective
Bioactivity is a future pursuit for development and application of biomaterials, making them to have better biocompatibility and therapeutic effect, and many efforts have been devoted to realize this target. Cu-containing biomaterials, including metals and ceramics, have been found to possess multiple bio-functions through continuous release of trace amount of Cu ions from the materials in the biological environment, which can be a prospective way for the development of bioactive materials. However, there still exist many challengers for future applications of those novel biomaterials, such as optimization of Cu content in materials to better present the bio-functions, optimization of comprehensive properties (bio-function, bio-safety, mechanical property, corrosion, processing ability, etc.), relevant mechanisms of bio-functional behaviors, and final clinical applications. More studies on these issues will help to have deep understanding on this novel class of biomaterials and to solve those technical problems for applications. It is much expected that the Cu-containing biomaterials will realize their clinical applications and benefit the majority of patients.
Authorship contribution statement
Ke Yang and Xun Qi supervised the whole work and revised the manuscript, Peng Wang and Yonghui Yuan made literature search and draft the manuscript of this review, Hongshan Zhong, Ke Xu, Yinghui Yang and Shiyu Jin edited the manuscript. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge financial support from National Natural Science Foundation of China (Nos. 81873918, 51631009), Construction Project of Liaoning Medical Imaging and Interventional Medical Engineering Research Center (Grant No. 18-006-9-01) and Key Research and Serving Local Area Projects of the Educational Department of Liaoning Province, China (Grant No. ZF2019005).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Ke Yang, Email: kyang@imr.ac.cn.
Xun Qi, Email: qixun716@hotmail.com.
References
- 1.Zhou X.-C., Yang F., Gong X.-Y., Zhao M., Zheng Y.-F., Sun Z.-L. New nitinol endovascular stent-graft system for abdominal aortic aneurysm with finite element analysis and experimental verification. Rare Met. 2019;38(6):495–502. [Google Scholar]
- 2.Huang Y.-S., Huang H.-H. Effects of clinical dental implant abutment materials and their surface characteristics on initial bacterial adhesion. Rare Met. 2019;38(6):512–519. [Google Scholar]
- 3.Li B.-Q., Li C.-L., Wang Z.-X., Lu X. Preparation of Ti–Nb–Ta–Zr alloys for load-bearing biomedical applications. Rare Met. 2019;38(6):571–576. [Google Scholar]
- 4.Yuan Y., Jin S., Qi X., Chen X., Zhang W., Yang K., Zhong H. Osteogenesis stimulation by copper-containing 316L stainless steel via activation of akt cell signaling pathway and Runx2 upregulation. J. Mater. Sci. Technol. 2019;35(11):2727–2733. [Google Scholar]
- 5.Jin S., Qi X., Wang T., Ren L., Yang K., Zhong H. In vitro study of stimulation effect on endothelialization by a copper bearing cobalt alloy. J. Biomed. Mater. Res. 2018;106(2):561–569. doi: 10.1002/jbm.a.36263. [DOI] [PubMed] [Google Scholar]
- 6.Huang Q., Ouyang Z., Tan Y., Wu H., Liu Y. Activating macrophages for enhanced osteogenic and bactericidal performance by Cu ion release from micro/nano-topographical coating on a titanium substrate. Acta Biomater. 2019;100:415–426. doi: 10.1016/j.actbio.2019.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Liu R., Ma Z., Kunle Kolawole S., Zeng L., Zhao Y., Ren L., Yang K. In vitro study on cytocompatibility and osteogenesis ability of Ti-Cu alloy. J. Mater. Sci. Mater. Med. 2019;30(7):75. doi: 10.1007/s10856-019-6277-z. [DOI] [PubMed] [Google Scholar]
- 8.Wapnir R.A. Copper absorption and bioavailability. Am. J. Clin. Nutr. 1998;67(5 Suppl):1054s–1060s. doi: 10.1093/ajcn/67.5.1054S. [DOI] [PubMed] [Google Scholar]
- 9.Xia C., Ma X., Zhang X., Li K., Tan J., Qiao Y., Liu X. Enhanced physicochemical and biological properties of C/Cu dual ions implanted medical titanium. Bioactive Materials. 2020;5(2):377–386. doi: 10.1016/j.bioactmat.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris E.D. Basic and clinical aspects of copper. Crit. Rev. Clin. Lab Sci. 2003;40(5):547–586. [PubMed] [Google Scholar]
- 11.Ren L., Wong H.M., Yan C.H., Yeung K.W.K., Yang K. Osteogenic ability of Cu-bearing stainless steel. J. Biomed. Mater. Res. B Appl. Biomater. 2015;103(7):1433–1444. doi: 10.1002/jbm.b.33318. [DOI] [PubMed] [Google Scholar]
- 12.Zhuang Y., Zhang S., Yang K., Ren L., Dai K. Antibacterial activity of copper-bearing 316L stainless steel for the prevention of implant-related infection. J. Biomed. Mater. Res. B Appl. Biomater. 2020;108(2):484–495. doi: 10.1002/jbm.b.34405. [DOI] [PubMed] [Google Scholar]
- 13.Doguer C., Ha J.-H., Collins J.F. Intersection of iron and copper metabolism in the mammalian intestine and liver. Comp. Physiol. 2018;8(4):1433–1461. doi: 10.1002/cphy.c170045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang E.-L., Fu S., Wang R.-X., Li H.-X., Liu Y., Ma Z.-Q., Liu G.-K., Zhu C.-S., Qin G.-W., Chen D.-F. Role of Cu element in biomedical metal alloy design. Rare Met. 2019;38(6):476–494. [Google Scholar]
- 15.Jin S., Qi X., Zhang B., Sun Z., Zhang B., Yang H., Wang T., Zheng B., Wang X., Shi Q., Chen M., Ren L., Yang K., Zhong H. Evaluation of promoting effect of a novel Cu-bearing metal stent on endothelialization process from in vitro and in vivo studies. Sci. Rep. 2017;7(1):17394. doi: 10.1038/s41598-017-17737-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertinato J., L'Abbé M.R. Maintaining copper homeostasis: regulation of copper-trafficking proteins in response to copper deficiency or overload. J. Nutr. Biochem. 2004;15(6):316–322. doi: 10.1016/j.jnutbio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Hu G.F. Copper stimulates proliferation of human endothelial cells under culture. J. Cell. Biochem. 1998;69(3):326–335. doi: 10.1002/(sici)1097-4644(19980601)69:3<326::aid-jcb10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 18.Kim B.-E., Turski M.L., Nose Y., Casad M., Rockman H.A., Thiele D.J. Cardiac copper deficiency activates a systemic signaling mechanism that communicates with the copper acquisition and storage organs. Cell Metabol. 2010;11(5):353–363. doi: 10.1016/j.cmet.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nan L., Cheng J., Yang K. Antibacterial behavior of a Cu-bearing type 200 stainless steel. J. Mater. Sci. Technol. 2012;28(11):1067–1070. [Google Scholar]
- 20.Scheiber I., Dringen R., Mercer J.F.B. Copper: effects of deficiency and overload. Met Ions Life Sci. 2013;13:359–387. doi: 10.1007/978-94-007-7500-8_11. [DOI] [PubMed] [Google Scholar]
- 21.Jin S., Ren L., Yang K. Bio-functional Cu containing biomaterials: a new way to enhance bio-adaption of biomaterials. J. Mater. Sci. Technol. 2016;32(9):835–839. [Google Scholar]
- 22.Klevay L.M. Cardiovascular disease from copper deficiency--a history. J. Nutr. 2000;130(2S Suppl):489S–492S. doi: 10.1093/jn/130.2.489S. [DOI] [PubMed] [Google Scholar]
- 23.Kelly E.J., Palmiter R.D. A murine model of Menkes disease reveals a physiological function of metallothionein. Nat. Genet. 1996;13(2):219–222. doi: 10.1038/ng0696-219. [DOI] [PubMed] [Google Scholar]
- 24.Liu R., Tang Y., Liu H., Zeng L., Ma Z., Li J., Zhao Y., Ren L., Yang K. Effects of combined chemical design (Cu addition) and topographical modification (SLA) of Ti-Cu/SLA for promoting osteogenic, angiogenic and antibacterial activities. J. Mater. Sci. Technol. 2020;47:202–215. [Google Scholar]
- 25.Li G.Y., Cao L.F., Zhang J.Y., Li X.G., Wang Y.Q., Wu K., Liu G., Sun J. An insight into Mg alloying effects on Cu thin films: microstructural evolution and mechanical behavior. J. Mater. Sci. Technol. 2020;57:101–112. [Google Scholar]
- 26.Franchitto N., Gandia-Mailly P., Georges B., Galinier A., Telmon N., Ducassé J.L., Rougé D. Acute copper sulphate poisoning: a case report and literature review. Resuscitation. 2008;78(1):92–96. doi: 10.1016/j.resuscitation.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Wang R., Qin G., Zhang E. Effect of Cu on Martensite Transformation of CoCrMo alloy for biomedical application. J. Mater. Sci. Technol. 2020;52:127–135. [Google Scholar]
- 28.Huster D. Wilson disease. Best Pract. Res. Clin. Gastroenterol. 2010;24(5):531–539. doi: 10.1016/j.bpg.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharjee A., Chakraborty K., Shukla A. Cellular copper homeostasis: current concepts on its interplay with glutathione homeostasis and its implication in physiology and human diseases. Metall. 2017;9(10):1376–1388. doi: 10.1039/c7mt00066a. [DOI] [PubMed] [Google Scholar]
- 30.Halfdanarson T.R., Kumar N., Li C.-Y., Phyliky R.L., Hogan W.J. Hematological manifestations of copper deficiency: a retrospective review. Eur. J. Haematol. 2008;80(6):523–531. doi: 10.1111/j.1600-0609.2008.01050.x. [DOI] [PubMed] [Google Scholar]
- 31.Brewer G.J. Copper in medicine. Curr. Opin. Chem. Biol. 2003;7(2):207–212. doi: 10.1016/s1367-5931(03)00018-8. [DOI] [PubMed] [Google Scholar]
- 32.Zhao J., Ren L., Zhang B., Cao Z., Yang K. In vitro study on infectious ureteral encrustation resistance of Cu-bearing stainless steel. J. Mater. Sci. Technol. 2017;33(12):1604–1609. [Google Scholar]
- 33.Ma Z., Ren L., Shahzad M.B., Liu R., Zhao Y., Yang K. Hot deformation behavior of Cu-bearing antibacterial titanium alloy. J. Mater. Sci. Technol. 2018;34(10):1867–1875. [Google Scholar]
- 34.McAuslan B.R., Gole G.A. Cellular and molecular mechanisms in angiogenesis. Trans. Ophthalmol. Soc. U. K. 1980;100(3):354–358. [PubMed] [Google Scholar]
- 35.Harris E.D. A requirement for copper in angiogenesis. Nutr. Rev. 2004;62(2):60–64. doi: 10.1111/j.1753-4887.2004.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 36.Rigiracciolo D.C., Scarpelli A., Lappano R., Pisano A., Santolla M.F., De Marco P., Cirillo F., Cappello A.R., Dolce V., Belfiore A., Maggiolini M., De Francesco E.M. Copper activates HIF-1α/GPER/VEGF signalling in cancer cells. Oncotarget. 2015;6(33):34158–34177. doi: 10.18632/oncotarget.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajalingam D., Kumar T.K.S., Yu C. The C2A domain of synaptotagmin exhibits a high binding affinity for copper: implications in the formation of the multiprotein FGF release complex. Biochemistry. 2005;44(44):14431–14442. doi: 10.1021/bi051387r. [DOI] [PubMed] [Google Scholar]
- 38.Román C.L., Maiztegui B., Mencucci M.V., Ahrtz L., Algañarás M., Del Zotto H., Gagliardino J.J., Flores L.E. Effects of islet neogenesis associated protein depend on vascular endothelial growth factor gene expression modulated by hypoxia-inducible factor 1-alpha. Peptides. 2019;117:170090. doi: 10.1016/j.peptides.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Bao L., Chen Y., Lai H.-T., Wu S.-Y., Wang J.E., Hatanpaa K.J., Raisanen J.M., Fontenot M., Lega B., Chiang C.-M., Semenza G.L., Wang Y., Luo W. Methylation of hypoxia-inducible factor (HIF)-1α by G9a/GLP inhibits HIF-1 transcriptional activity and cell migration. Nucleic Acids Res. 2018;46(13):6576–6591. doi: 10.1093/nar/gky449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maybin J.A., Murray A.A., Saunders P.T.K., Hirani N., Carmeliet P., Critchley H.O.D. Hypoxia and hypoxia inducible factor-1α are required for normal endometrial repair during menstruation. Nat. Commun. 2018;9(1):295. doi: 10.1038/s41467-017-02375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu C., Zhou Y., Xu M., Han P., Chen L., Chang J., Xiao Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials. 2013;34(2):422–433. doi: 10.1016/j.biomaterials.2012.09.066. [DOI] [PubMed] [Google Scholar]
- 42.Feng W., Ye F., Xue W., Zhou Z., Kang Y.J. Copper regulation of hypoxia-inducible factor-1 activity. Mol. Pharmacol. 2009;75(1):174–182. doi: 10.1124/mol.108.051516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J., Zhai D., Lv F., Yu Q., Ma H., Yin J., Yi Z., Liu M., Chang J., Wu C. Preparation of copper-containing bioactive glass/eggshell membrane nanocomposites for improving angiogenesis, antibacterial activity and wound healing. Acta Biomater. 2016;36:254–266. doi: 10.1016/j.actbio.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 44.Cuzzocrea S., Persichini T., Dugo L., Colasanti M., Musci G. Copper induces type II nitric oxide synthase in vivo. Free Radic. Biol. Med. 2003;34(10):1253–1262. doi: 10.1016/s0891-5849(03)00110-2. [DOI] [PubMed] [Google Scholar]
- 45.Ziche M., Morbidelli L. Nitric oxide and angiogenesis. J. Neuro Oncol. 2000;50(1–2):139–148. doi: 10.1023/a:1006431309841. [DOI] [PubMed] [Google Scholar]
- 46.Demura Y., Ameshima S., Ishizaki T., Okamura S., Miyamori I., Matsukawa S. The activation of eNOS by copper ion (Cu2+) in human pulmonary arterial endothelial cells (HPAEC) Free Radic. Biol. Med. 1998;25(3):314–320. doi: 10.1016/s0891-5849(98)00056-2. [DOI] [PubMed] [Google Scholar]
- 47.Borén J., Chapman M.J., Krauss R.M., Packard C.J., Bentzon J.F., Binder C.J., Daemen M.J., Demer L.L., Hegele R.A., Nicholls S.J., Nordestgaard B.G., Watts G.F., Bruckert E., Fazio S., Ference B.A., Graham I., Horton J.D., Landmesser U., Laufs U., Masana L., Pasterkamp G., Raal F.J., Ray K.K., Schunkert H., Taskinen M.-R., van de Sluis B., Wiklund O., Tokgozoglu L., Catapano A.L., Ginsberg H.N. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehz962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eckstein H.H. European society for vascular surgery guidelines on the management of atherosclerotic carotid and vertebral artery disease. Eur. J. Vasc. Endovasc. Surg. 2018;55(1):1–2. doi: 10.1016/j.ejvs.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 49.Byrne R.A., Joner M., Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014. Eur. Heart J. 2015;36(47):3320–3331. doi: 10.1093/eurheartj/ehv511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goel S.A., Guo L.-W., Liu B., Kent K.C. Mechanisms of post-intervention arterial remodelling. Cardiovasc. Res. 2012;96(3):363–371. doi: 10.1093/cvr/cvs276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu L., Zhang Y., Yang K., Ren L., Wang Q. [Influence of copper-bearing stainless steel on adhesion, proliferation and apoptosis of vascular endothelial cell] Hua xi kou qiang yi xue za zhi. 2013;31(1) [PubMed] [Google Scholar]
- 52.Ren L., Xu L., Feng J., Zhang Y., Yang K. In vitro study of role of trace amount of Cu release from Cu-bearing stainless steel targeting for reduction of in-stent restenosis. J. Mater. Sci. Mater. Med. 2012;23(5):1235–1245. doi: 10.1007/s10856-012-4584-8. [DOI] [PubMed] [Google Scholar]
- 53.Huang B., Jing F., Akhavan B., Ji L., Leng Y., Xie D., Bilek M., Huang N. Multifunctional Ti-xCu coatings for cardiovascular interfaces: control of microstructure and surface chemistry. Mater Sci Eng C Mater Biol Appl. 2019;104:109969. doi: 10.1016/j.msec.2019.109969. [DOI] [PubMed] [Google Scholar]
- 54.Liu H., Pan C., Zhou S., Li J., Huang N., Dong L. Improving hemocompatibility and accelerating endothelialization of vascular stents by a copper-titanium film. Mater Sci Eng C Mater Biol Appl. 2016;69:1175–1182. doi: 10.1016/j.msec.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 55.Ewald A., Käppel C., Vorndran E., Moseke C., Gelinsky M., Gbureck U. The effect of Cu(II)-loaded brushite scaffolds on growth and activity of osteoblastic cells. J. Biomed. Mater. Res. 2012;100(9):2392–2400. doi: 10.1002/jbm.a.34184. [DOI] [PubMed] [Google Scholar]
- 56.Rodríguez J.P., Ríos S., González M. Modulation of the proliferation and differentiation of human mesenchymal stem cells by copper. J. Cell. Biochem. 2002;85(1) [PubMed] [Google Scholar]
- 57.Cai D.-G., Bao M.-M., Wang X.-Y., Yang L., Qin G.-W., Wang R.-X., Chen D.-F., Zhang E.-L. Biocorrosion properties of Ti–3Cu alloy in F ion-containing solution and acidic solution and biocompatibility. Rare Met. 2019;38(6):503–511. [Google Scholar]
- 58.Zhang J.-M., Sun Y.-H., Zhao Y., Liu Y.-L., Yao X.-H., Tang B., Hang R.-Q. Antibacterial ability and cytocompatibility of Cu-incorporated Ni–Ti–O nanopores on NiTi alloy. Rare Met. 2019;38(6):552–560. [Google Scholar]
- 59.Ghiasi M.S., Chen J., Vaziri A., Rodriguez E.K., Nazarian A. Bone fracture healing in mechanobiological modeling: a review of principles and methods. BoneKEy Rep. 2017;6 doi: 10.1016/j.bonr.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cottrell J., O'Connor J.P. Effect of non-steroidal anti-inflammatory drugs on bone healing. Pharmaceuticals. 2010;3(5):1668–1693. doi: 10.3390/ph3051668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pivonka P., Dunstan C.R. Role of mathematical modeling in bone fracture healing. BoneKEy Rep. 2012;1:221. doi: 10.1038/bonekey.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonafede M., Espindle D., Bower A.G. The direct and indirect costs of long bone fractures in a working age US population. J. Med. Econ. 2013;16(1):169–178. doi: 10.3111/13696998.2012.737391. [DOI] [PubMed] [Google Scholar]
- 63.Wang L., Li G., Ren L., Kong X., Wang Y., Han X., Jiang W., Dai K., Yang K., Hao Y. Nano-copper-bearing stainless steel promotes fracture healing by accelerating the callus evolution process. Int. J. Nanomed. 2017;12:8443–8457. doi: 10.2147/IJN.S146866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silverman S.L., Kupperman E.S., Bukata S.V. Fracture healing: a consensus report from the international osteoporosis foundation fracture working group. Osteoporos. Int. 2016;27(7):2197–2206. doi: 10.1007/s00198-016-3513-y. [DOI] [PubMed] [Google Scholar]
- 65.Einhorn T.A., Gerstenfeld L.C. Fracture healing: mechanisms and interventions. Nat. Rev. Rheumatol. 2015;11(1):45–54. doi: 10.1038/nrrheum.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Claes L., Recknagel S., Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012;8(3):133–143. doi: 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- 67.Foulke B.A., Kendal A.R., Murray D.W., Pandit H. Fracture healing in the elderly: a review. Maturitas. 2016;92:49–55. doi: 10.1016/j.maturitas.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 68.Tay W.-H., de Steiger R., Richardson M., Gruen R., Balogh Z.J. Health outcomes of delayed union and nonunion of femoral and tibial shaft fractures. Injury. 2014;45(10):1653–1658. doi: 10.1016/j.injury.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 69.Hak D.J., Fitzpatrick D., Bishop J.A., Marsh J.L., Tilp S., Schnettler R., Simpson H., Alt V. Delayed union and nonunions: epidemiology, clinical issues, and financial aspects. Injury. 2014;45(Suppl 2):S3–S7. doi: 10.1016/j.injury.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 70.Metsemakers W.J., Moriarty T.F., Nijs S., Pape H.C., Richards R.G. Influence of implant properties and local delivery systems on the outcome in operative fracture care. Injury. 2016;47(3):595–604. doi: 10.1016/j.injury.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 71.Huang Q., Liu X., Elkhooly T.A., Zhang R., Shen Z., Feng Q. A novel titania/calcium silicate hydrate hierarchical coating on titanium. Colloids Surf. B Biointerfaces. 2015;134:169–177. doi: 10.1016/j.colsurfb.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 72.Zhao Q., Yi L., Hu A., Jiang L., Hong L., Dong J. Antibacterial and osteogenic activity of a multifunctional microporous coating codoped with Mg, Cu and F on titanium. J. Mater. Chem. B. 2019;7(14):2284–2299. doi: 10.1039/c8tb03377c. [DOI] [PubMed] [Google Scholar]
- 73.Huang Q., Li X., Elkhooly T.A., Liu X., Zhang R., Wu H., Feng Q., Liu Y. The Cu-containing TiO coatings with modulatory effects on macrophage polarization and bactericidal capacity prepared by micro-arc oxidation on titanium substrates. Colloids Surf. B Biointerfaces. 2018;170:242–250. doi: 10.1016/j.colsurfb.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 74.Telgerd M.D., Sadeghinia M., Birhanu G., Daryasari M.P., Zandi-Karimi A., Sadeghinia A., Akbarijavar H., Karami M.H., Seyedjafari E. Enhanced osteogenic differentiation of mesenchymal stem cells on metal-organic framework based on copper, zinc, and imidazole coated poly-l-lactic acid nanofiber scaffolds. J. Biomed. Mater. Res. 2019;107(8):1841–1848. doi: 10.1002/jbm.a.36707. [DOI] [PubMed] [Google Scholar]
- 75.Yu L., Jin G., Ouyang L., Wang D., Qiao Y., Liu X. Antibacterial activity, osteogenic and angiogenic behaviors of copper-bearing titanium synthesized by PIII&D. J. Mater. Chem. B. 2016;4(7):1296–1309. doi: 10.1039/c5tb02300a. [DOI] [PubMed] [Google Scholar]
- 76.Wu Q., Li J., Zhang W., Qian H., She W., Pan H., Wen J., Zhang X., Liu X., Jiang X. Antibacterial property, angiogenic and osteogenic activity of Cu-incorporated TiO coating. J. Mater. Chem. B. 2014;2(39):6738–6748. doi: 10.1039/c4tb00923a. [DOI] [PubMed] [Google Scholar]
- 77.Bartsch I., Willbold E., Yarmolenko S., Witte F. In vivo fluorescence imaging of apoptosis during foreign body response. Biomaterials. 2012;33(29):6926–6932. doi: 10.1016/j.biomaterials.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 78.Singh M.R. Application of metallic nanomaterials in nanomedicine. Adv. Exp. Med. Biol. 2018:1052. doi: 10.1007/978-981-10-7572-8_8. [DOI] [PubMed] [Google Scholar]
- 79.Laux P., Tentschert J., Riebeling C., Braeuning A., Creutzenberg O., Epp A., Fessard V., Haas K.-H., Haase A., Hund-Rinke K., Jakubowski N., Kearns P., Lampen A., Rauscher H., Schoonjans R., Störmer A., Thielmann A., Mühle U., Luch A. Nanomaterials: certain aspects of application, risk assessment and risk communication. Arch. Toxicol. 2018;92(1):121–141. doi: 10.1007/s00204-017-2144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang W., Chang Q., Xu L., Li G., Yang G., Ding X., Wang X., Cui D., Jiang X. Graphene oxide-copper nanocomposite-coated porous CaP scaffold for vascularized bone regeneration via activation of hif-1α. Adv Healthc Mater. 2016;5(11):1299–1309. doi: 10.1002/adhm.201500824. [DOI] [PubMed] [Google Scholar]
- 81.Wang H., Zhao S., Zhou J., Shen Y., Huang W., Zhang C., Rahaman M.N., Wang D. Evaluation of borate bioactive glass scaffolds as a controlled delivery system for copper ions in stimulating osteogenesis and angiogenesis in bone healing. J. Mater. Chem. B. 2014;2(48):8547–8557. doi: 10.1039/c4tb01355g. [DOI] [PubMed] [Google Scholar]
- 82.Huang D., Ma K., Cai X., Yang X., Hu Y., Huang P., Wang F., Jiang T., Wang Y. Evaluation of antibacterial, angiogenic, and osteogenic activities of green synthesized gap-bridging copper-doped nanocomposite coatings. Int. J. Nanomed. 2017;12:7483–7500. doi: 10.2147/IJN.S141272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shi F., Liu Y., Zhi W., Xiao D., Li H., Duan K., Qu S., Weng J. The synergistic effect of micro/nano-structured and Cu-doped hydroxyapatite particles to promote osteoblast viability and antibacterial activity. Biomed. Mater. 2017;12(3) doi: 10.1088/1748-605X/aa6c8d. [DOI] [PubMed] [Google Scholar]
- 84.Shi M., Chen Z., Farnaghi S., Friis T., Mao X., Xiao Y., Wu C. Copper-doped mesoporous silica nanospheres, a promising immunomodulatory agent for inducing osteogenesis. Acta Biomater. 2016;30:334–344. doi: 10.1016/j.actbio.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 85.Cattalini J.P., Hoppe A., Pishbin F., Roether J., Boccaccini A.R., Lucangioli S., Mouriño V. Novel nanocomposite biomaterials with controlled copper/calcium release capability for bone tissue engineering multifunctional scaffolds. J. R. Soc. Interface. 2015;12(110) doi: 10.1098/rsif.2015.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li K., Xia C., Qiao Y., Liu X. Dose-response relationships between copper and its biocompatibility/antibacterial activities. J. Trace Elem. Med. Biol. 2019;55:127–135. doi: 10.1016/j.jtemb.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 87.Kjørholt K.E., Johnsen S.P., Kristensen N.R., Prieto-Alhambra D., Pedersen A.B. Increasing risk of hospital-treated infections and community-based antibiotic use after hip fracture surgery: a nationwide study 2005-2016. J. Bone Miner. Res. 2019;34(3):437–446. doi: 10.1002/jbmr.3620. [DOI] [PubMed] [Google Scholar]
- 88.Papadopoulos A., Ribera A., Mavrogenis A.F., Rodriguez-Pardo D., Bonnet E., Salles M.J., Dolores Del Toro M., Nguyen S., Blanco-García A., Skaliczki G., Soriano A., Benito N., Petersdorf S., Pasticci M.B., Tattevin P., Tufan Z.K., Chan M., O'Connell N., Pantazis N., Kyprianou A., Pigrau C., Megaloikonomos P.D., Senneville E., Ariza J., Papagelopoulos P.J., Giannitsioti E. Multidrug-resistant and extensively drug-resistant Gram-negative prosthetic joint infections: role of surgery and impact of colistin administration. Int. J. Antimicrob. Agents. 2019;53(3):294–301. doi: 10.1016/j.ijantimicag.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 89.Sun E., Mello M.M., Rishel C.A., Vaughn M.T., Kheterpal S., Saager L., Fleisher L.A., Damrose E.J., Kadry B., Jena A.B. Association of overlapping surgery with perioperative outcomes. J. Am. Med. Assoc. 2019;321(8):762–772. doi: 10.1001/jama.2019.0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arshad A.I., Ahmad P., Karobari M.I., Asif J.A., Alam M.K., Mahmood Z., Abd Rahman N., Mamat N., Kamal M.A. Antibiotics: a bibliometric analysis of top 100 classics. Antibiotics (Basel) 2020;9(5) doi: 10.3390/antibiotics9050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paterson T.E., Bari A., Bullock A.J., Turner R., Montalbano G., Fiorilli S., Vitale-Brovarone C., MacNeil S., Shepherd J. Multifunctional copper-containing mesoporous glass nanoparticles as antibacterial and proangiogenic agents for chronic wounds. Front Bioeng Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.00246. 246-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anguita-Alonso P., Hanssen A.D., Patel R. Prosthetic joint infection. Expert Rev. Anti Infect. Ther. 2005;3(5):797–804. doi: 10.1586/14787210.3.5.797. [DOI] [PubMed] [Google Scholar]
- 93.Zimmerli W., Sendi P. Orthopaedic biofilm infections. APMIS. 2017;125(4):353–364. doi: 10.1111/apm.12687. [DOI] [PubMed] [Google Scholar]
- 94.Zou F., Jiang J., Lv F., Xia X., Ma X. Preparation of antibacterial and osteoconductive 3D-printed PLGA/Cu(I)@ZIF-8 nanocomposite scaffolds for infected bone repair. J. Nanobiotechnol. 2020;18(1) doi: 10.1186/s12951-020-00594-6. 39-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Delanois R.E., Mistry J.B., Gwam C.U., Mohamed N.S., Choksi U.S., Mont M.A. Current epidemiology of revision total knee arthroplasty in the United States. J. Arthroplasty. 2017;32(9):2663–2668. doi: 10.1016/j.arth.2017.03.066. [DOI] [PubMed] [Google Scholar]
- 96.Thiele K., Perka C., Matziolis G., Mayr H.O., Sostheim M., Hube R. Current failure mechanisms after knee arthroplasty have changed: polyethylene wear is less common in revision surgery. J Bone Joint Surg Am. 2015;97(9):715–720. doi: 10.2106/JBJS.M.01534. [DOI] [PubMed] [Google Scholar]
- 97.Tschudin-Sutter S., Kuijper E.J., Durovic A., Vehreschild M.J.G.T., Barbut F., Eckert C., Fitzpatrick F., Hell M., Norèn T., O'Driscoll J., Coia J., Gastmeier P., von Müller L., Wilcox M.H., Widmer A.F. Guidance document for prevention of Clostridium difficile infection in acute healthcare settings. Clin. Microbiol. Infect. 2018;24(10):1051–1054. doi: 10.1016/j.cmi.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 98.Cho S.Y., Chung D.R. Infection prevention strategy in hospitals in the era of community-associated methicillin-resistant Staphylococcus aureus in the asia-pacific region: a review. Clin. Infect. Dis. 2017;64(suppl_2):S82–S90. doi: 10.1093/cid/cix133. [DOI] [PubMed] [Google Scholar]
- 99.Poultsides L.A., Papatheodorou L.K., Karachalios T.S., Khaldi L., Maniatis A., Petinaki E., Malizos K.N. Novel model for studying hematogenous infection in an experimental setting of implant-related infection by a community-acquired methicillin-resistant S. aureus strain. J. Orthop. Res. 2008;26(10):1355–1362. doi: 10.1002/jor.20608. [DOI] [PubMed] [Google Scholar]
- 100.Shiels S.M., Bedigrew K.M., Wenke J.C. Development of a hematogenous implant-related infection in a rat model. BMC Muscoskel. Disord. 2015;16:255. doi: 10.1186/s12891-015-0699-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 102.Sun D., Xu D., Yang C., Chen J., Shahzad M.B., Sun Z., Zhao J., Gu T., Yang K., Wang G. Inhibition of Staphylococcus aureus biofilm by a copper-bearing 317L-Cu stainless steel and its corrosion resistance. Mater Sci Eng C Mater Biol Appl. 2016;69:744–750. doi: 10.1016/j.msec.2016.07.050. [DOI] [PubMed] [Google Scholar]
- 103.Chen A.F., Parvizi J. Antibiotic-loaded bone cement and periprosthetic joint infection. J. Long Term Eff. Med. Implants. 2014;24(2–3):89–97. doi: 10.1615/jlongtermeffmedimplants.2013010238. [DOI] [PubMed] [Google Scholar]
- 104.Han J., Yang Y., Lu J., Wang C., Xie Y., Zheng X., Yao Z., Zhang C. Sustained release vancomycin-coated titanium alloy using a novel electrostatic dry powder coating technique may be a potential strategy to reduce implant-related infection. Biosci Trends. 2017;11(3):346–354. doi: 10.5582/bst.2017.01061. [DOI] [PubMed] [Google Scholar]
- 105.Liu J., Zhang D., Pan X., Wang L. Characterization of the complexation between Al3+ and extracellular polymeric substances prepared from alga-bacteria biofilm. Chin. J. Appl. Environ. Biol. 2009;15(3):347–350. [Google Scholar]
- 106.Zhao W., Walker S.L., Huang Q., Cai P. Contrasting effects of extracellular polymeric substances on the surface characteristics of bacterial pathogens and cell attachment to soil particles. Chem. Geol. 2015;410:79–88. [Google Scholar]
- 107.Reddy S.T., Chung K.K., McDaniel C.J., Darouiche R.O., Landman J., Brennan A.B. Micropatterned surfaces for reducing the risk of catheter-associated urinary tract infection: an in vitro study on the effect of sharklet micropatterned surfaces to inhibit bacterial colonization and migration of uropathogenic Escherichia coli. J. Endourol. 2011;25(9):1547–1552. doi: 10.1089/end.2010.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ruparelia J.P., Chatterjee A.K., Duttagupta S.P., Mukherji S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008;4(3):707–716. doi: 10.1016/j.actbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 109.Xu W., Hou C., Mao Y., Yang L., Tamaddon M., Zhang J., Qu X., Liu C., Su B., Lu X. Characteristics of novel Ti–10Mo-xCu alloy by powder metallurgy for potential biomedical implant applications. Bioactive Materials. 2020;5(3):659–666. doi: 10.1016/j.bioactmat.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Espírito Santo C., Lam E.W., Elowsky C.G., Quaranta D., Domaille D.W., Chang C.J., Grass G. Bacterial killing by dry metallic copper surfaces. Appl. Environ. Microbiol. 2011;77(3):794–802. doi: 10.1128/AEM.01599-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Quaranta D., Krans T., Espírito Santo C., Elowsky C.G., Domaille D.W., Chang C.J., Grass G. Mechanisms of contact-mediated killing of yeast cells on dry metallic copper surfaces. Appl. Environ. Microbiol. 2011;77(2):416–426. doi: 10.1128/AEM.01704-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Santo C.E., Quaranta D., Grass G. Antimicrobial metallic copper surfaces kill Staphylococcus haemolyticus via membrane damage. MicrobiologyOpen. 2012;1(1):46–52. doi: 10.1002/mbo3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bao M., Liu Y., Wang X., Yang L., Li S., Ren J., Qin G., Zhang E. Optimization of mechanical properties, biocorrosion properties and antibacterial properties of wrought Ti-3Cu alloy by heat treatment. Bioactive Materials. 2018;3(1):28–38. doi: 10.1016/j.bioactmat.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hong R., Kang T.Y., Michels C.A., Gadura N. Membrane lipid peroxidation in copper alloy-mediated contact killing of Escherichia coli. Appl. Environ. Microbiol. 2012;78(6):1776–1784. doi: 10.1128/AEM.07068-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zheng S., Chang W., Li C., Lou H. Als1 and Als3 regulate the intracellular uptake of copper ions when Candida albicans biofilms are exposed to metallic copper surfaces. FEMS Yeast Res. 2016;16(3) doi: 10.1093/femsyr/fow029. [DOI] [PubMed] [Google Scholar]
- 116.Sun D., Xu D., Yang C., Shahzad M.B., Sun Z., Xia J., Zhao J., Gu T., Yang K., Wang G. An investigation of the antibacterial ability and cytotoxicity of a novel cu-bearing 317L stainless steel. Sci. Rep. 2016;6:29244. doi: 10.1038/srep29244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guan J., Guo L., Fu Y., Chai H. [In vitro evaluation of antibacterial activity and cytocompatibility of antibacterial stainless steel containing copper] Sheng wu yi xue gong cheng xue za zhi = Journal of biomedical engineering = Shengwu yixue gongchengxue zazhi. 2013;30(2):333–337. [PubMed] [Google Scholar]
- 118.Chai H., Guo L., Wang X., Fu Y., Guan J., Tan L., Ren L., Yang K. Antibacterial effect of 317L stainless steel contained copper in prevention of implant-related infection in vitro and in vivo. J. Mater. Sci. Mater. Med. 2011;22(11):2525–2535. doi: 10.1007/s10856-011-4427-z. [DOI] [PubMed] [Google Scholar]
- 119.Zhang D., Ren L., Zhang Y., Xue N., Yang K., Zhong M. Antibacterial activity against Porphyromonas gingivalis and biological characteristics of antibacterial stainless steel. Colloids Surf. B Biointerfaces. 2013;105:51–57. doi: 10.1016/j.colsurfb.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 120.Ma Z., Li M., Liu R., Ren L., Zhang Y., Pan H., Zhao Y., Yang K. In vitro study on an antibacterial Ti-5Cu alloy for medical application. J. Mater. Sci. Mater. Med. 2016;27(5):91. doi: 10.1007/s10856-016-5698-1. [DOI] [PubMed] [Google Scholar]
- 121.Li M., Nan L., Liang C., Sun Z., Yang L., Yang K. Antibacterial behavior and related mechanisms of martensitic Cu-bearing stainless steel evaluated by a mixed infection model of Escherichia coli and Staphylococcus aureus in vitro. J. Mater. Sci. Technol. 2021;62:139–147. [Google Scholar]
- 122.Yan X., Wan P., Tan L., Zhao M., Qin L., Yang K. Corrosion and biological performance of biodegradable magnesium alloys mediated by low copper addition and processing. Mater Sci Eng C Mater Biol Appl. 2018;93:565–581. doi: 10.1016/j.msec.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 123.Liu C., Fu X., Pan H., Wan P., Wang L., Tan L., Wang K., Zhao Y., Yang K., Chu P.K. Biodegradable Mg-Cu alloys with enhanced osteogenesis, angiogenesis, and long-lasting antibacterial effects. Sci. Rep. 2016;6:27374. doi: 10.1038/srep27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bai B., Zhang E., Liu J., Zhu J. The anti-bacterial activity of titanium-copper sintered alloy against Porphyromonas gingivalis in vitro. Dent. Mater. J. 2016;35(4):659–667. doi: 10.4012/dmj.2016-001. [DOI] [PubMed] [Google Scholar]
- 125.Guo S., Lu Y., Wu S., Liu L., He M., Zhao C., Gan Y., Lin J., Luo J., Xu X., Lin J. Preliminary study on the corrosion resistance, antibacterial activity and cytotoxicity of selective-laser-melted Ti6Al4V-xCu alloys. Mater Sci Eng C Mater Biol Appl. 2017;72:631–640. doi: 10.1016/j.msec.2016.11.126. [DOI] [PubMed] [Google Scholar]
- 126.Li M., Ma Z., Zhu Y., Xia H., Yao M., Chu X., Wang X., Yang K., Yang M., Zhang Y., Mao C. Toward a molecular understanding of the antibacterial mechanism of copper-bearing titanium alloys against Staphylococcus aureus. Adv Healthc Mater. 2016;5(5):557–566. doi: 10.1002/adhm.201500712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jin X., Gao L., Liu E., Yu F., Shu X., Wang H. Microstructure, corrosion and tribological and antibacterial properties of Ti-Cu coated stainless steel. Journal of the mechanical behavior of biomedical materials. 2015;50:23–32. doi: 10.1016/j.jmbbm.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 128.Liu R., Memarzadeh K., Chang B., Zhang Y., Ma Z., Allaker R.P., Ren L., Yang K. Antibacterial effect of copper-bearing titanium alloy (Ti-Cu) against Streptococcus mutans and Porphyromonas gingivalis. Sci. Rep. 2016;6:29985. doi: 10.1038/srep29985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bari A., Bloise N., Fiorilli S., Novajra G., Vallet-Regí M., Bruni G., Torres-Pardo A., González-Calbet J.M., Visai L., Vitale-Brovarone C. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 2017;55:493–504. doi: 10.1016/j.actbio.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 130.Koohkan R., Hooshmand T., Tahriri M., Mohebbi-Kalhori D. Synthesis, characterization and in vitro bioactivity of mesoporous copper silicate bioactive glasses. Ceram. Int. 2018;44(2) doi: 10.1021/acsbiomaterials.7b01030. [DOI] [PubMed] [Google Scholar]
- 131.Gosau M., Bürgers R., Vollkommer T., Holzmann T., Prantl L. Effectiveness of antibacterial copper additives in silicone implants. J. Biomater. Appl. 2013;28(2):187–198. doi: 10.1177/0885328212441957. [DOI] [PubMed] [Google Scholar]
- 132.Fowler L., Masia N., Cornish L.A., Chown L.H., Engqvist H., Norgren S., Öhman-Mägi C. Development of antibacterial Ti-Cu(x) alloys for dental applications: effects of ageing for alloys with up to 10 wt% Cu. Materials. 2019;12(23):4017. doi: 10.3390/ma12234017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cai X., Zhang B., Liang Y., Zhang J., Yan Y., Chen X., Wu Z., Liu H., Wen S., Tan S., Wu T. Study on the antibacterial mechanism of copper ion- and neodymium ion-modified α-zirconium phosphate with better antibacterial activity and lower cytotoxicity. Colloids Surf. B Biointerfaces. 2015;132:281–289. doi: 10.1016/j.colsurfb.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 134.Ghosh D., Godeshala S., Nitiyanandan R., Islam M.S., Yaron J.R., DiCaudo D., Kilbourne J., Rege K. Copper-eluting fibers for enhanced tissue sealing and repair. ACS Appl. Mater. Interfaces. 2020;12(25):27951–27960. doi: 10.1021/acsami.0c04755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Volodina L.A., Bayder L.M., Rakhmetova A.A., Bogoslovskaja O.A., Olkhovskaya I.P., Gluschenko N.N. Copper-induced change in the ESR signal of hemoglobin nitrosyl complexes in wound by the action of copper nanoparticles. Biofizika. 2013;58(5):876–881. [PubMed] [Google Scholar]
- 136.Lu F., Wang J., Yang L., Zhu J.J. A facile one-pot synthesis of colloidal stable, monodisperse, highly PEGylated CuS@mSiO2 nanocomposites for the combination of photothermal therapy and chemotherapy. Chem. Commun. 2015;51(46):9447–9450. doi: 10.1039/c5cc01725d. [DOI] [PubMed] [Google Scholar]
- 137.Meng Z., Wei F., Wang R., Xia M., Chen Z., Wang H., Zhu M. NIR-Laser-Switched in vivo smart nanocapsules for synergic photothermal and chemotherapy of tumors. Adv. Mater. 2016;28(2):245–253. doi: 10.1002/adma.201502669. [DOI] [PubMed] [Google Scholar]
- 138.Atkinson R.L., Zhang M., Diagaradjane P., Peddibhotla S., Contreras A., Hilsenbeck S.G., Woodward W.A., Krishnan S., Chang J.C., Rosen J.M. Thermal enhancement with optically activated gold nanoshells sensitizes breast cancer stem cells to radiation therapy. Sci. Transl. Med. 2010;2(55):55ra79. doi: 10.1126/scitranslmed.3001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu K., Liu K., Liu J., Ren Q., Zhao Z., Wu X., Li D., Yuan F., Ye K., Li B. Copper chalcogenide materials as photothermal agents for cancer treatment. Nanoscale. 2020;12(5):2902–2913. doi: 10.1039/c9nr08737k. [DOI] [PubMed] [Google Scholar]
- 140.Szymanski P., Fraczek T., Markowicz M., Mikiciuk-Olasik E. Development of copper based drugs, radiopharmaceuticals and medical materials. Biometals. 2012;25(6):1089–1112. doi: 10.1007/s10534-012-9578-y. [DOI] [PMC free article] [PubMed] [Google Scholar]