Abstract
Purpose of Review
This article reviews advances over the past 3 years in cardiac magnetic resonance (CMR) imaging in pulmonary hypertension (PH). We aim to bring the reader up-to-date with CMR applications in diagnosis, prognosis, 4D flow, strain analysis, T1 mapping, machine learning and ongoing research.
Recent Findings
CMR volumetric and functional metrics are now established as valuable prognostic markers in PH. This imaging modality is increasingly used to assess treatment response and improves risk stratification when incorporated into PH risk scores. Emerging techniques such as myocardial T1 mapping may play a role in the follow-up of selected patients. Myocardial strain may be used as an early marker for right and left ventricular dysfunction and a predictor for mortality. Machine learning has offered a glimpse into future possibilities. Ongoing research of new PH therapies is increasingly using CMR as a clinical endpoint.
Summary
The last 3 years have seen several large studies establishing CMR as a valuable diagnostic and prognostic tool in patients with PH, with CMR increasingly considered as an endpoint in clinical trials of PH therapies. Machine learning approaches to improve automation and accuracy of CMR metrics and identify imaging features of PH is an area of active research interest with promising clinical utility.
Keywords: Pulmonary hypertension, Cardiac MRI, CMR
Introduction
Pulmonary hypertension (PH) is a heterogeneous group of diseases that cause an elevated pulmonary artery pressure [1, 2]. Chronic heart and lung diseases are the underlying causes of most PH cases in the Western world [3] while living at high-altitude and schistosomiasis infection are more common in resource-limited countries [4]. Treatable causes include chronic thromboembolic disease (CTEPH) and pulmonary arterial hypertension (PAH) [5].
PH was historically considered as a rare, difficult to diagnose and untreatable disease [6]. However, our understanding of the epidemiology, diagnosis and treatment of PH has changed over the past two decades. Chronic comorbid cardiac and pulmonary diseases causing PH, such as systemic hypertension and COPD, are increasingly common in an ageing population [7–9]. Recent population-based studies suggest that the prevalence of PH has increased by almost 30% over the last 30 years [3]. PH is now estimated to have a prevalence of 1% of the world’s population and might be the fourth most prevalent cardiovascular disease [6, 8].
The management of PH relies on ascertaining the diagnosis, discerning the underlying cause of PH, assessing disease severity and monitoring response to treatment [1, 2]. The role of cardiac magnetic resonance (CMR) in each of these key aspects of PH management has been extensively studied and is increasingly established in clinical practice.
This article reviews significant advances over the past 3 years in CMR imaging in PH. We build on the previous articles by Zitzman et al. and Swift et al. to bring the reader up-to-date on the current developments in CMR applications in PH [10, 11]. We aim to cover the latest articles about diagnosis, prognosis, 4D flow, strain analysis, T1 mapping, machine learning and interesting ongoing research.
Diagnosis
PH is diagnosed by right heart catheter (RHC). The haemodynamic criteria for a diagnosis of PH from international guidelines are an elevated mean pulmonary artery pressure (mPAP) of ≥ 25 mmHg and pulmonary vascular resistance (PVR) of ≥ 3 Wood units [5]. A new mPAP threshold of > 20 mmHg has recently been proposed as a more accurate criterion, being two standard deviations above the normal threshold [5].
In a breathless patient, PH is increasingly suggested on common investigations such as chest radiography, echocardiogram or computer tomography [1, 12]. New advances in noninvasive imaging techniques have helped to establish the diagnosis of PH sooner in the course of the disease [13, 14]. Cardiac MRI has been used to estimate mPAP to provide diagnosis through non-invasive means. A recent study developed regression models to predict mPAP based on cardiac MRI [15]. A cohort of 600 patients who had both CMR and RHC was retrospectively included. The cohort was divided in half, the first half to derive the regression model and the second half to validate its performance. CMR parameters included in the linear regression model were the interventricular septum angle (IVS), ventricular mass index (VMI) and black blood slow flow. The model had a sensitivity of 93% and specificity 79% of detecting PH, allowing for an accurate non-invasive diagnosis. The model highly correlated with mPAP and had good interobserver reproducibility. The authors also recently updated their model in line with the new suggested mPAP threshold of > 20 mmHg [16]. The same group also developed a diagnostic and prognostic CMR model for patients with PH secondary to chronic obstructive pulmonary disease (COPD) [17]. Pulmonary artery (PA) indices of the diastolic area and relative area change in addition to the IVS and VMI were included in the COPD model. This model had a 92% sensitivity and 80% specificity in detecting PH in COPD patients. PA systolic and diastolic areas also had a high diagnostic accuracy in detecting PH in patients with interstitial lung disease [18]. CMR can be used as a one-stop study to provide functional, aetiological and prognostic information [19]. CMR showed a good correlation with RHC parameters and had high sensitivity and specificity in identifying the underlying causes of PH [19].
CMR-guided RHC is a recent technique that allows performing RHC in a CMR suite. It combines the benefits of radiation-free CMR information to haemodynamics in a single sitting. CMR-guided RHC has a rare failure rate and an acceptable procedure time that is comparable to a standard CMR study [20–22].
Prognosis and Therapy Response
PH is a chronic, progressive and mostly incurable disease with high morbidity and mortality. A new diagnosis of PH increases the risk of death at 1 year by sevenfold [3]. Mortality is attributed to right heart failure resulting from the increased afterload secondary to elevated pulmonary arterial pressures [2]. PAH prognosis, however, has significantly improved with the advancement of treatment, and the median survival has increased from 3 to 7 years over the last 20 years [23, 24].
The ESC/ERS guidelines describe the prognostic factors including imaging parameters in PAH [2]. Large right atrial size and the presence of pericardial effusion on echocardiogram or CMR imaging feature as prognostic markers in the ESC/ERS traffic light system representing low, intermediate and high risk. There is a wider range of prognostic factors, including the right ventricular metrics that are considered crucially important in prognostication. Further work to determine the most potent CMR prognostic factors and their incremental value in prognostic equations is required.
The most recent statement on imaging and PH from the Pulmonary Vascular Research Institute recommends cardiac MRI to monitor right ventricular (RV) function [1]. CMR can be used at follow-up to assess disease progression and treatment response and provide prognostic information.
A recent systematic review, including 1,938 patients, has shown that CMR is a powerful predictor of clinical worsening and mortality in PH [25••]. In particular, worse RV function and larger RV volume are associated with a worse outcome. A large study in 2017 assessed CMR prognostic features in 576 patients [26••]. The study has shown that RV end-systolic volume and pulmonary artery (PA) relative area change have incremental prognostic value over clinical parameters. Pulmonary artery stiffness assessed by a low relative area change and distensibility is associated with a more severe PH and a higher risk of mortality [26–28]. The prognostic features in patients with connective tissue disease (CTD) were shown to be different to other PAH subgroups. In CTD patients, metrics such as ventricular-vascular coupling (Ees/Ea) and RV mass appear to be more significant than function and volume [26••, 29–31]. New therapies have shown to improve RV contractility and reduce RV mass in CTD PAH, and CMR can play an important role in assessing their treatment response [32, 33].
Cardiac MRI assessment of the interventricular septal (IVS) angle helps to differentiate pre- and postcapillary PH from isolated postcapillary PH. An increased angle of ≥ 160° is associated with pre- and postcapillary PH and with a poorer prognosis [34]. In addition, increased trabeculation at the marginal IVS is associated with severe PH, reduced RV ejection fraction (RVEF) and exercise tolerance [35, 36].
A large study has set the prognostic thresholds for CMR indices [37••]. This study assessed the added value of CMR to the validated prognostic calculators such as the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) and the modified French Pulmonary Hypertension Registry (FPHR). The age- and sex-adjusted RV end-systolic volume index improved prognostication when combined with a risk score. The prognostic thresholds will serve as an important guide for cardiac MRI risk stratification in PAH. Notably, one of the most significant predictors for a worse outcome in IPAH was a background of even minor or mild parenchymal lung abnormality. A background of mild fibrosis or emphysema was associated with a 5-year survival of 22% compared to 78% in IPAH without any lung disease [17, 38]. IPAH with lung disease has, therefore, been suggested to be a separate phenotype of PAH [39].
Myocardial Strain Analysis
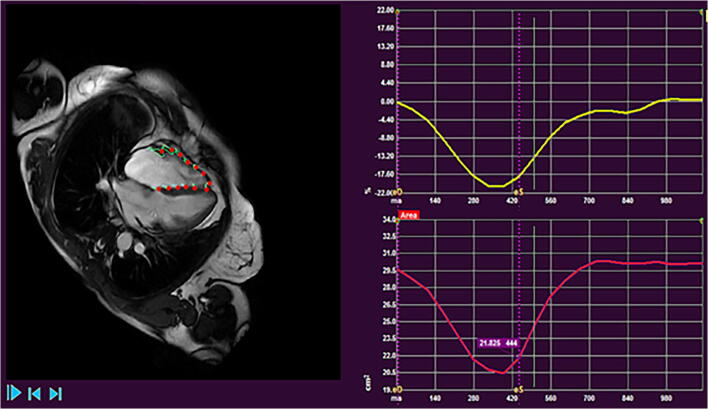
Strain analysis is an established CMR technique for the quantification of myocardial deformation and assessment of wall motion [40]. Feature tracking is one method of strain analysis which follows cardiac borders throughout the cardiac cycle on cine images (Fig. 1). Strain analysis in CMR uses similar assumptions to speckle tracking on echocardiogram with good agreement between the two modalities [41, 42].
Fig. 1.
Images from right ventricular strain analysis in a patient with PH
Biventricular strain is significantly impaired in PH and could assist in the early detection of right and left heart dysfunction [43–45]. Besides, feature tracking technology has been used to predict outcome in patients with PH. A reduced RV circumferential and longitudinal strain rates were associated with an impaired RVEF and a significant predictor of mortality [46]. The same holds true for the left ventricle (LV) where reduced LV circumferential and longitudinal strain rates in precapillary PH are associated with severely impaired RVEF and a higher risk of death [47]. Impaired right atrial (RA) strain and phasic function are a marker of disease severity. Reduced RA strain is associated with decompensated RV function and stiffness [48, 49]. The advancement of fully automated myocardial strain analysis is likely to push its role in future research in the diagnosis and prognosis of PH [50].
Myocardial Late Gadolinium Enhancement
Late gadolinium enhancement (LGE) is a CMR technique to identify the areas of myocardial fibrosis. Gadolinium has paramagnetic properties that shorten the myocardial T1 times. The T1 shortening is proportional to the concentration of gadolinium in the extracellular space. Gadolinium enhancement in the normal myocardium clears out early. However, its clearance is restricted in necrotic tissue due to the expansion of the extracellular space and damage to the cellular membranes of the myocytes [51].
LGE is associated with poor outcome and increased mortality in cardiomyopathies [52]. However, a study assessing LGE in 124 PH patients found that LGE did not predict mortality [53]. This finding confirms the results of two previous studies that suggested no added prognostic information from LGE in PH when added to other CMR parameters [54, 55]. Therefore, LGE in PH and particularly at the RV insertion points or IVS appears to be a consequence of increased mechanical stress and RV remodelling and not a sign of RV decompensation.
Myocardial T1 and Extracellular Volume Mapping
Native myocardial T1 and extracellular volume (ECV) mapping are novel biomarkers used in several cardiovascular disorders to aid diagnostic, prognostic and therapeutic decision-making [56]. Myocardial T1 mapping is a pixel-by-pixel representation of the longitudinal relaxation times (T1) within a tissue [57]. T1 values provide surrogate tissue characterisation data that are measured on a standardised scale [58]. Assessing T1 post-gadolinium can be used to estimate ECV [56, 59]. The ECV is calculated by subtracting the T1 values of the myocardium and blood pool pre- and post-contrast, corrected for the haematocrit level [56]. Elevated T1 mapping values and ECV can indicate areas of oedema and fibrosis in the myocardium [60, 61]. Several recent studies have looked into the clinical application of T1 mapping and ECV in PH [62–67]. T1 times are elevated in PH and in particular at the RV insertion points and are associated with an increased intraventricular septal angle and LV eccentricity [62•, 65]. Increased T1 values are therefore thought to be related to RV dilatation and the resultant shift of the septum towards the LV. The diagnostic application in PH, however, remains limited. Although T1 looked promising for differentiating between healthy volunteers and PH, the differences were much smaller in patients without a PH diagnosis in a clinical setting [62]. A raised T1 value in PH is weakly correlated to RVEF [62•, 64, 68]. However, T1 times did not predict mortality in a large cohort of PH patients [62]. An elevated ECV in PH patients with heart failure and preserved ejection fraction was associated with RV dilatation, stiffness and reduced RV strain and therefore might play a role as a marker for RV remodelling [67].
CMR also plays a role in CTEPH treatment response assessment [69, 70]. Septal myocardial T1 mapping is elevated in CTEPH patients [63] and reduces after treatment with balloon pulmonary angioplasty [63, 71]. T1 mapping may, therefore, be utilised in CTEPH therapy monitoring.
Pulmonary MR Angiography and Perfusion
MR angiography (MRA) has a high spatial and low temporal resolution that allows for the assessment of the pulmonary vasculature. Perfusion MRI, on the other hand, has a low spatial and a high temporal resolution that enables evaluation of the capillary level tissue perfusion which makes it suitable in the clinical assessment of CTEPH [72].
The diagnostic accuracy of dynamic contrast-enhanced perfusion MRI in the diagnosis of CTEPH was shown to be comparable to computed tomography pulmonary angiography (CTPA) and perfusion single-photon emission tomography (SPECT) [73]. Perfusion MRI identified all cases of CTEPH and had comparable sensitivity and specificity to the other modalities. Recent studies have shown that ventilation and perfusion changes in CTEPH can be interrogated using phase-resolved functional lung MRI without the need for contrast agents [74]. Perfusion MRI is likely to play an essential role in the diagnostic pathway in centres that already perform CMR for CTEPH patients.
4D Flow
Four-dimensional flow (4D flow) is an emerging MRI technology that offers to circumvent issues with standard ultrasound imaging in PH. Not only 4D flow allows 3D visualisation of vascular flow, it also allows to make an accurate assessment of transvalvular or intra-cavity flow [75]. In the setting of PH, 4D flow has been used to assess the haemodynamic changes in the pulmonary circulation. Abnormal flow patterns in the main pulmonary artery (MPA), namely vortex formation, have been associated with PH [76, 77]. The presence and ‘persistence time’ of the vortex in the MPA are linearly associated with mPAP [78, 79] and can be used to estimate mPAP. Another physiological vascular parameter characterised with 4D flow is MPA wall shear stress (WSS), which has an impact on vascular remodelling [80]. 4D flow-derived MPA WSS appears to be reduced in patients with PH [80, 81]. Also, in patients with PH who have poor acoustic windows for echocardiography, 4D flow can provide reliable quantification of tricuspid regurgitation [82, 83]. 4D flow could also provide clinically relevant RV diastolic assessment in PH [84, 85]. To summarise, 4D flow MRI can provide several complementary diagnostic information in the assessment of patients presenting with suspected PH. Future studies need to evaluate the incremental role of 4D flow MRI assessment in patients with PH.
Machine Learning
Machine learning (ML) uses algorithms to recognise patterns in example data to make predictive decisions in unprecedented data [86]. ML can classify the data based on the differentiating patterns it has learnt [87, 88].
ML is likely to play an important role in PH [89]. Recent approaches include automated segmentation [90, 91], biventricular 3D model creation [92], computational models and decision tree analysis [93], diagnosis [94••] and prognostication [95••, 96].
ML has been used to analyse cardiac motion and predict mortality based on reduced ventricular contraction [95••]. The ML model was shown to improve outcome prediction compared to conventional CMR measurements alone.
ML was used to identify diagnostic features on CMR and classify them into PH or no PH [94••]. The discriminating features were mapped onto CMR voxel space and were shown as a visual overlay on the 4 chamber and short-axis images (Fig. 2). Interestingly, this approach does not require segmentation of the cardiac chambers, allowing for faster processing and reduced segmentation-induced error. This study gives a glimpse into the future of PH assessment that allows for rapid and accurate CMR diagnoses. An exciting development would be to ML methods utilised in predicting prognosis and treatment response in PH.
Fig. 2.
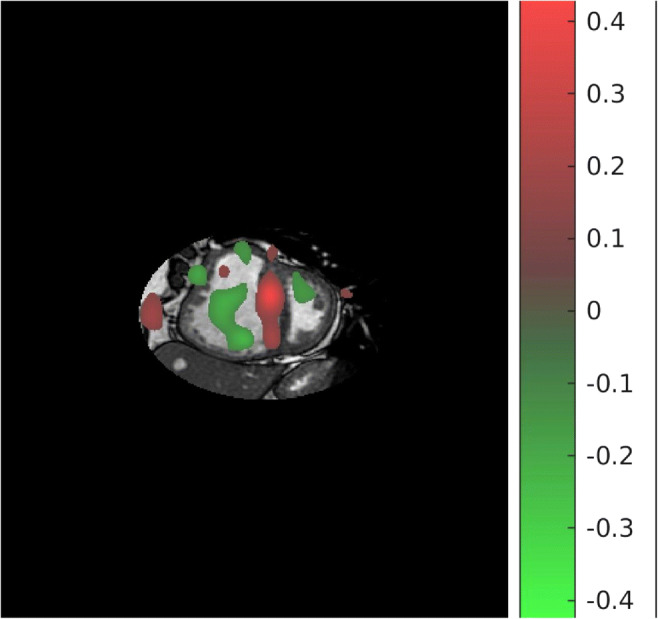
Machine learning feature map. Features compatible with PH are in red and non-PH features are in green
Ongoing Research
Repeatability of CMR Measurements
The RESPIRE study aims to assess the reproducibility of CMR measurements at follow-up. It will also compare the repeatability of CMR to other endpoints such as walking and blood tests. This study would help establish the evidence of the usefulness of CMR as a monitoring tool and its sensitivity to change [97].
CMR as Clinical Trial Endpoint
The REPAIR study is the first study to have MRI as a co-primary endpoint [98••]. Four ongoing randomised controlled trials, assessing beta-blockers, spironolactone, CXA-10 and dehydroepiandrosterone, have defined CMR parameters as an endpoint to evaluate treatment response [99–102]. A single-arm study of treprostinil in PH has defined the change in RV structure and function, on CMR compared to an echocardiogram, as the primary treatment response outcome [103].
Follow-up CMR Assessment
A prospective study is aiming to recruit 180 incident cases of PAH. Participants will have CMR and RHC at baseline and 6- and 24-month follow-up. The aim is to determine poor prognostic markers before decompensation occurs. This research would be valuable in early risk stratification as current studies include patients with more advanced stages of the disease [104].
CTEPH Diagnosis and Screening
CHANGE-MRI is a large European multicentre study that aims to compare dynamic contrast-enhanced MRI compared to VQ-SPECT in people with suspected CTEPH. This study is anticipated to set the standard for MRI in the diagnostic algorithm for CTEPH [105].
Conclusions
The last 3 years have seen several large studies examining the clinical utility of CMR in patients with PH. Evidence confirms the potential for CMR to provide diagnostic and prognostic information that can guide clinical practice. CMR has been utilised in clinical trials to detect the impact of PH therapies and is increasingly proposed as a trial endpoint. Machine learning approaches to improve automation and accuracy of CMR metrics and identify imaging features of PH shows potential and is likely to improve the clinical utility of CMR imaging.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Cardiac Magnetic
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.•• Kiely DG, Levin D, Hassoun P, Ivy DD, Jone P-N, Bwika J, et al. EXPRESS: statement on imaging and pulmonary hypertension from the Pulmonary Vascular Research Institute (PVRI). Pulm Circ. 2019;9(3):2045894019841990. This article provides guidance on the role of the different types of imaging in pulmonary hypertension including cardiac MRI. [DOI] [PMC free article] [PubMed]
- 2.Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, et al. ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2015;46(4):903–75. [DOI] [PubMed]
- 3.Wijeratne DT, Lajkosz K, Brogly SB, Lougheed MD, Jiang L, Housin A, et al. Increasing incidence and prevalence of World Health Organization groups 1 to 4 pulmonary hypertension: a population-based cohort study in Ontario, Canada. Circ Cardiovasc Qual Outcomes. 2018;11:e003973. doi: 10.1161/CIRCOUTCOMES.117.003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gidwani S, Nair A. The burden of pulmonary hypertension in resource-limited settings. Glob Heart. 2014;9:297–310. doi: 10.1016/j.gheart.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. [DOI] [PMC free article] [PubMed]
- 6.Rich S, Haworth SG, Hassoun PM, Yacoub MH. Pulmonary hypertension: the unaddressed global health burden. Lancet Respir Med. 2018;6:577–579. doi: 10.1016/S2213-2600(18)30268-6. [DOI] [PubMed] [Google Scholar]
- 7.Mathai SC, Ryan JJ. The Growing Burden of Pulmonary Hypertension in the Modern Era: A Zebra No More? Circ Cardiovasc Qual Outcomes. 2018;11(2):e004536. [DOI] [PubMed]
- 8.Hoeper MM, Humbert M, Souza R, Idrees M, Kawut SM, Sliwa-Hahnle K, et al. A global view of pulmonary hypertension. Lancet Respir Med. 2016;4:306–322. doi: 10.1016/S2213-2600(15)00543-3. [DOI] [PubMed] [Google Scholar]
- 9.Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JSR, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med. 2012;186:790–796. doi: 10.1164/rccm.201203-0383OC. [DOI] [PubMed] [Google Scholar]
- 10.Zitzmann S, Rolf A. The role of cardiac magnetic resonance imaging in pulmonary hypertension. Curr Cardiovasc Imaging Rep. 2016;9:18.
- 11.Swift AJ, Saunders LC, Sproson T, Hussain N, Collier GJ, Marshall H, et al. Multiparametric magnetic resonance imaging in pulmonary hypertension. Curr Cardiovasc Imaging Rep. 2015;8:45.
- 12.Kiely DG, Elliot CA, Sabroe I, Condliffe R. Pulmonary hypertension: diagnosis and management. BMJ. 2013;346:f2028. doi: 10.1136/bmj.f2028. [DOI] [PubMed] [Google Scholar]
- 13.Johns CS, Wild JM, Rajaram S, Swift AJ, Kiely DG. Current and emerging imaging techniques in the diagnosis and assessment of pulmonary hypertension. Expert Rev Respir Med. 2018;12:145–160. doi: 10.1080/17476348.2018.1420478. [DOI] [PubMed] [Google Scholar]
- 14.Hur DJ, Sugeng L. Non-invasive multimodality cardiovascular imaging of the right heart and pulmonary circulation in pulmonary hypertension. Front Cardiovasc Med. 2019;6:24. doi: 10.3389/fcvm.2019.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johns CS, Kiely DG, Rajaram S, Hill C, Thomas S, Karunasaagarar K, et al. Diagnosis of pulmonary hypertension with cardiac MRI: derivation and validation of regression models. Radiology. 2019;290:61–68. doi: 10.1148/radiol.2018180603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitfield AJ, Solanki R, Johns CS, Kiely D, Wild J, Swift AJ. MRI prediction of precapillary pulmonary hypertension according to the Sixth World Symposium on Pulmonary Hypertension. Radiology. 2020;294:482. doi: 10.1148/radiol.2019192078. [DOI] [PubMed] [Google Scholar]
- 17.Johns CS, Rajaram S, Capener DA, Oram C, Elliot C, Condliffe R, et al. Non-invasive methods for estimating mPAP in COPD using cardiovascular magnetic resonance imaging. Eur Radiol. 2018;28:1438–1448. doi: 10.1007/s00330-017-5143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chin M, Johns C, Currie BJ, Weatherley N, Hill C, Elliot C, et al. Pulmonary artery size in interstitial lung disease and pulmonary hypertension: association with interstitial lung disease severity and diagnostic utility. Front Cardiovasc Med. 2018;5:53. doi: 10.3389/fcvm.2018.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer GMB, Spilimbergo FB, Altmayer S, Pacini GS, Zanon M, Watte G, et al. Correction to: Multiparametric magnetic resonance imaging in the assessment of pulmonary hypertension: initial experience of a one-stop study. Lung. 2018;196:497. doi: 10.1007/s00408-018-0130-x. [DOI] [PubMed] [Google Scholar]
- 20.Knight DS, Kotecha T, Martinez-Naharro A, Brown JT, Bertelli M, Fontana M, et al. Cardiovascular magnetic resonance-guided right heart catheterisation in a conventional CMR environment – predictors of procedure success and duration in pulmonary artery hypertension. J Cardiovasc Magn Reson. 2019;21:57 [DOI] [PMC free article] [PubMed]
- 21.Rogers T, Ratnayaka K, Khan JM, Stine A, Schenke WH, Grant LP, et al. CMR fluoroscopy right heart catheterisation for cardiac output and pulmonary vascular resistance: results in 102 patients. J Cardiovasc Magn Reson. 2017;19:54. doi: 10.1186/s12968-017-0366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratnayaka K, Kanter JP, Faranesh AZ, Grant EK, Olivieri LJ, Cross RR, et al. Radiation-free CMR diagnostic heart catheterisation in children. J Cardiovasc Magn Reson. 2017;19:65. doi: 10.1186/s12968-017-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGoon MD, Humbert M. Pulmonary arterial hypertension: epidemiology and registries. Adv Pulm Hypertens. 2014;13(1):21–6.
- 24.Lau EMT, Giannoulatou E, Celermajer DS, Humbert M. Epidemiology and treatment of pulmonary arterial hypertension. Nat Rev Cardiol. 2017;14:603–614. doi: 10.1038/nrcardio.2017.84. [DOI] [PubMed] [Google Scholar]
- 25.•• Alabed S, Shahin Y, Garg P, Alandejani F, Johns CS, Lewis RA, Condliffe R, Wild JM, Kiely DG, Swift AJ. Cardiac-MRI Predicts Clinical Worsening and Mortality in Pulmonary Arterial Hypertension: A Systematic Review and Meta-Analysis. JACC Cardiovasc Imaging. 2020;29:S1936-878X(20)30731–2. Epub ahead of print. This systematic review and meta-analysis summarizes all prognostic cardiac MRI studies in pulmonary arterial hypertension. The article concludes that cardiac MRI is a powerful tool to predict clinical worsening and mortality. [DOI] [PMC free article] [PubMed]
- 26.•• Swift AJ, Capener D, Johns C, Hamilton N, Rothman A, Elliot C, et al. Magnetic resonance imaging in the prognostic evaluation of patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2017;196:228–39. This study is the largest prognostic cardiac MRI study performed in pulmonary arterial hypertension. [DOI] [PMC free article] [PubMed]
- 27.Ray JC, Burger C, Mergo P, Safford R, Blackshear J, Austin C, et al. Pulmonary arterial stiffness assessed by cardiovascular magnetic resonance imaging is a predictor of mild pulmonary arterial hypertension. Int J Cardiovasc Imaging. 2019;35:1881–1892. doi: 10.1007/s10554-018-1397-y. [DOI] [PubMed] [Google Scholar]
- 28.Blyth KG, Bellofiore A, Jayasekera G, Foster JE, Steedman T, Chesler NC, et al. Dobutamine stress MRI in pulmonary hypertension: relationships between stress pulmonary artery relative area change, RV performance, and 10-year survival. Pulm Circ. 2017;7:465–475. doi: 10.1177/2045893217704838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abe N, Kato M, Kono M, Fujieda Y, Ohira H, Tsujino I, et al. Right ventricular dimension index by cardiac magnetic resonance for prognostication in connective tissue diseases and pulmonary hypertension. Rheumatology. 2020;59:622–633. doi: 10.1093/rheumatology/kez357. [DOI] [PubMed] [Google Scholar]
- 30.Simpson CE, Damico RL, Kolb TM, Mathai SC, Khair RM, Sato T, et al. Ventricular mass as a prognostic imaging biomarker in incident pulmonary arterial hypertension. Eur Respir J. 2019;53(4):1802067. [DOI] [PMC free article] [PubMed]
- 31.Brewis MJ, Bellofiore A, Vanderpool RR, Chesler NC, Johnson MK, Naeije R, et al. Imaging right ventricular function to predict outcome in pulmonary arterial hypertension. Int J Cardiol. 2016;218:206–211. doi: 10.1016/j.ijcard.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mercurio V, Mukherjee M, Tedford RJ, Zamanian RT, Khair RM, Sato T, et al. Improvement in right ventricular strain with ambrisentan and tadalafil upfront therapy in scleroderma-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2018;197:388–391. doi: 10.1164/rccm.201704-0789LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hassoun PM, Zamanian RT, Damico R, Lechtzin N, Khair R, Kolb TM, et al. Ambrisentan and tadalafil up-front combination therapy in scleroderma-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192:1102–1110. doi: 10.1164/rccm.201507-1398OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johns CS, Wild JM, Rajaram S, Tubman E, Capener D, Elliot C, et al. Identifying at-risk patients with combined pre- and postcapillary pulmonary hypertension using interventricular septal angle at cardiac MRI. Radiology. 2018;289:61–68. doi: 10.1148/radiol.2018180120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karakus G, Kammerlander AA, Aschauer S, Marzluf BA, Zotter-Tufaro C, Bachmann A, et al. Pulmonary artery to aorta ratio for the detection of pulmonary hypertension: cardiovascular magnetic resonance and invasive hemodynamics in heart failure with preserved ejection fraction. J Cardiovasc Magn Reson. 2015;17:79. doi: 10.1186/s12968-015-0184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong Y, Sun J, Yang D, He J, Cheng W, Wan K, et al. Right ventricular septomarginal trabeculation hypertrophy is associated with disease severity in patients with pulmonary arterial hypertension. Int J Cardiovasc Imaging. 2018;34:1439–1449. doi: 10.1007/s10554-018-1347-8. [DOI] [PubMed] [Google Scholar]
- 37.•• Lewis RA, Johns CS, Cogliano M, Capener D, Tubman E, Elliot CA, et al. Identification of cardiac magnetic resonance imaging thresholds for risk stratification in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2020;201:458–68. This study shows the additional prognostic impact of cardiac MRI to mortality risk scores and provides mortality risk thresholds for the different cardiac MRI volumetric and function measurements. [DOI] [PMC free article] [PubMed]
- 38.Lewis RA, Thompson AAR, Billings CG, Charalampopoulos A, Elliot CA, Hamilton N, et al. Mild parenchymal lung disease and/or low diffusion capacity impacts survival and treatment response in patients diagnosed with idiopathic pulmonary arterial hypertension. Eur Respir J. 2020;55(6):2000041.10.1183/13993003.00041-2020. [DOI] [PMC free article] [PubMed]
- 39.Peacock AJ, Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, et al. Idiopathic pulmonary arterial hypertension and co-existing lung disease: is this a new phenotype?. Pulm Circ. 2020;10(1):2045894020914851. 10.1177/2045894020914851. [DOI] [PMC free article] [PubMed]
- 40.Almutairi HM, Boubertakh R, Miquel ME, Petersen SE. Myocardial deformation assessment using cardiovascular magnetic resonance-feature tracking technique. Br J Radiol. 2017;90:20170072. doi: 10.1259/bjr.20170072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pedrizzetti G, Claus P, Kilner PJ, Nagel E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson. 2016;18:51. doi: 10.1186/s12968-016-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vo HQ, Marwick TH, Negishi K. MRI-derived myocardial strain measures in normal subjects. JACC Cardiovasc Imaging. 2018;11:196–205. doi: 10.1016/j.jcmg.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 43.Lin ACW, Seale H, Hamilton-Craig C, Morris NR, Strugnell W. Quantification of biventricular strain and assessment of ventriculo-ventricular interaction in pulmonary arterial hypertension using exercise cardiac magnetic resonance imaging and myocardial feature tracking. J Magn Reson Imaging. 2019;49:1427–1436. doi: 10.1002/jmri.26517. [DOI] [PubMed] [Google Scholar]
- 44.Kallianos K, Brooks GC, Mukai K, Seguro de Carvalho F, Liu J, Naeger DM, et al. Cardiac magnetic resonance evaluation of left ventricular myocardial strain in pulmonary hypertension. Acad Radiol. 2018;25:129–135. doi: 10.1016/j.acra.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Homsi R, Luetkens JA, Skowasch D, Pizarro C, Sprinkart AM, Gieseke J, et al. Left ventricular myocardial fibrosis, atrophy, and impaired contractility in patients with pulmonary arterial hypertension and a preserved left ventricular function: a cardiac magnetic resonance study. J Thorac Imaging. 2017;32:36–42. doi: 10.1097/RTI.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 46.de Siqueira MEM, Pozo E, Fernandes VR, Sengupta PP, Modesto K, Gupta SS, et al. Characterisation and clinical significance of right ventricular mechanics in pulmonary hypertension evaluated with cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson. 2016;18:39. doi: 10.1186/s12968-016-0258-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Padervinskienė L, Krivickienė A, Hoppenot D, Miliauskas S, Basevičius A, Nedzelskienė I, et al. Prognostic value of left ventricular function and mechanics in pulmonary hypertension: a pilot cardiovascular magnetic resonance feature tracking study. Medicina. 2019;55:73. doi: 10.3390/medicina55030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leng S, Dong Y, Wu Y, Zhao X, Ruan W, Zhang G, et al. Impaired cardiovascular magnetic resonance–derived rapid semiautomated right atrial longitudinal strain is associated with decompensated hemodynamics in pulmonary arterial hypertension. Circ Cardiovasc Imaging. 2019;12(5):e008582. [DOI] [PubMed]
- 49.Tello K, Dalmer A, Vanderpool R, Ghofrani HA, Naeije R, Roller F, et al. Right ventricular function correlates of right atrial strain in pulmonary hypertension: a combined cardiac magnetic resonance and conductance catheter study. Am J Physiol Heart Circ Physiol. 2020;318:H156–H164. doi: 10.1152/ajpheart.00485.2019. [DOI] [PubMed] [Google Scholar]
- 50.Ferdian E, Suinesiaputra A, Fung K, Aung N, Lukaschuk E, Barutcu A, et al. Fully automated myocardial strain estimation from cardiovascular MRI–tagged images using a deep learning framework in the UK Biobank. Radiology. 2020;2:e190032. doi: 10.1148/ryct.2020190032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Croisille P, Revel D, Saeed M. Contrast agents and cardiac MR imaging of myocardial ischemia: from bench to bedside. Eur Radiol. 2006;16:1951–1963. doi: 10.1007/s00330-006-0244-z. [DOI] [PubMed] [Google Scholar]
- 52.Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896–908. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 53.Abouelnour AE, Doyle M, Thompson DV, Yamrozik J, Williams RB, Shah MB, et al. Does late gadolinium enhancement still have value? Right ventricular internal mechanical work, E/E and late gadolinium enhancement as prognostic markers in patients with advanced pulmonary hypertension via cardiac MRI. Cardiol Res Cardiovasc Med. 2017;2017(1):CRCM–111. [PMC free article] [PubMed]
- 54.Swift AJ, Rajaram S, Capener D, Elliot C, Condliffe R, Wild JM, et al. LGE patterns in pulmonary hypertension do not impact overall mortality. JACC Cardiovasc Imaging. 2014;7:1209–1217. doi: 10.1016/j.jcmg.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 55.Freed BH, Gomberg-Maitland M, Chandra S, Mor-Avi V, Rich S, Archer SL, et al. Late gadolinium enhancement cardiovascular magnetic resonance predicts clinical worsening in patients with pulmonary hypertension. J Cardiovasc Magn Reson. 2012;14:11. doi: 10.1186/1532-429X-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson. 2016;18:89. doi: 10.1186/s12968-016-0308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puntmann VO, Peker E, Chandrashekhar Y, Nagel E. T1 Mapping in characterising myocardial disease: a comprehensive review. Circ Res. 2016;119:277–299. doi: 10.1161/CIRCRESAHA.116.307974. [DOI] [PubMed] [Google Scholar]
- 58.Messroghli DR, Plein S, Higgins DM, Walters K, Jones TR, Ridgway JP, et al. Human myocardium: single-breath-hold MR T1 mapping with high spatial resolution--reproducibility study. Radiology. 2006;238:1004–1012. doi: 10.1148/radiol.2382041903. [DOI] [PubMed] [Google Scholar]
- 59.Garg P. Role of cardiac T1 mapping and extracellular volume (ECV) in the assessment of myocardial infarction. Anatol J Cardiol. 2018;19:(6):404–411. [DOI] [PMC free article] [PubMed]
- 60.Bull S, White SK, Piechnik SK, Flett AS, Ferreira VM, Loudon M, et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart. 2013;99:932–937. doi: 10.1136/heartjnl-2012-303052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuruvilla S, Janardhanan R, Antkowiak P, Keeley EC, Adenaw N, Brooks J, et al. Increased extracellular volume and altered mechanics are associated with LVH in hypertensive heart disease, not hypertension alone. JACC: Cardiovasc Imaging. 2015;8(2):172–80. [DOI] [PMC free article] [PubMed]
- 62.• Saunders LC, Johns CS, Stewart NJ, Oram CJE, Capener DA, Puntmann VO, et al. Diagnostic and prognostic significance of cardiovascular magnetic resonance native myocardial T1 mapping in patients with pulmonary hypertension. J Cardiovasc Magn Reson. 2018;20:78. This large study assesses the diagnostic and prognostic role of myocardial T1 mapping in pulmonary hypertension. It concluded that while T1 mapping does not predict death it correlates with worse right ventricular function. [DOI] [PMC free article] [PubMed]
- 63.Roller FC, Wiedenroth C, Breithecker A, Liebetrau C, Mayer E, Schneider C, et al. Native T1 mapping and extracellular volume fraction measurement for assessment of right ventricular insertion point and septal fibrosis in chronic thromboembolic pulmonary hypertension. Eur Radiol. 2017;27:1980–1991. doi: 10.1007/s00330-016-4585-y. [DOI] [PubMed] [Google Scholar]
- 64.Chen YY, Yun H, Jin H, Kong DH, Long YL, Fu CX, et al. Association of native T1 times with biventricular function and hemodynamics in precapillary pulmonary hypertension. Int J Cardiovasc Imaging. 2017;33:1179–1189. doi: 10.1007/s10554-017-1095-1. [DOI] [PubMed] [Google Scholar]
- 65.Reiter U, Reiter G, Kovacs G, Adelsmayr G, Greiser A, Olschewski H, et al. Native myocardial T1 mapping in pulmonary hypertension: correlations with cardiac function and hemodynamics. Eur Radiol. 2017;27:157–166. doi: 10.1007/s00330-016-4360-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J, Zhao H, Wang Y, Herrmann HC, Witschey WRT, Han Y. Native T1 and T2 mapping by cardiovascular magnetic resonance imaging in pressure overloaded left and right heart diseases. J Thorac Dis. 2018;10:2968–2975. doi: 10.21037/jtd.2018.04.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel RB, Li E, Benefield BC, Swat SA, Polsinelli VB, Carr JC, et al. Diffuse right ventricular fibrosis in heart failure with preserved ejection fraction and pulmonary hypertension. ESC Heart Fail. 2020;7:254–264. [DOI] [PMC free article] [PubMed]
- 68.Mehta BB, Auger DA, Gonzalez JA, Workman V, Chen X, Chow K, et al. Detection of elevated right ventricular extracellular volume in pulmonary hypertension using accelerated and navigator-gated look-locker imaging for cardiac T1 estimation (ANGIE) cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2015;17:110. doi: 10.1186/s12968-015-0209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schoenfeld C, Hinrichs JB, Olsson KM, Kuettner M-A, Renne J, Kaireit T, et al. Cardio-pulmonary MRI for detection of treatment response after a single BPA treatment session in CTEPH patients. Eur Radiol. 2019;29:1693–1702. doi: 10.1007/s00330-018-5696-4. [DOI] [PubMed] [Google Scholar]
- 70.Maschke SK, Schoenfeld CO, Kaireit TF, Cebotari S, Olsson K, Hoeper M, et al. MRI-derived regional biventricular function in patients with chronic thromboembolic pulmonary hypertension before and after pulmonary endarterectomy. Acad Radiol. 2018;25:1540–1547. doi: 10.1016/j.acra.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 71.Roller FC, Kriechbaum S, Breithecker A, Liebetrau C, Haas M, Schneider C, et al. Correlation of native T1 mapping with right ventricular function and pulmonary haemodynamics in patients with chronic thromboembolic pulmonary hypertension before and after balloon pulmonary angioplasty. Eur Radiol. 2019;29:1565–1573. doi: 10.1007/s00330-018-5702-x. [DOI] [PubMed] [Google Scholar]
- 72.Johns CS, Swift AJ, Hughes PJC, Ohno Y, Schiebler M, Wild JM. Pulmonary MR Angiography and perfusion imaging—a review of methods and applications. Eur J Radiol. 2017;86:361–370. [DOI] [PubMed]
- 73.Johns CS, Swift AJ, Rajaram S, Hughes PJC, Capener DJ, Kiely DG, et al. Lung perfusion: MRI vs. SPECT for screening in suspected chronic thromboembolic pulmonary hypertension. J Magn Reson Imaging. 2017;46:1693–1697. doi: 10.1002/jmri.25714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pöhler GH, Klimes F, Voskrebenzev A, Behrendt L, Czerner C, Gutberlet M, et al. Chronic thromboembolic pulmonary hypertension perioperative monitoring using phase-resolved functional lung (PREFUL)-MRI. J Magn Reson Imaging. 2020;52(2):610–619. [DOI] [PubMed]
- 75.van der Geest RJ, Garg P. Advanced analysis techniques for intra-cardiac flow evaluation from 4D flow MRI. Curr Radiol Rep. 2016;4:38. doi: 10.1007/s40134-016-0167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reiter G, Reiter U, Kovacs G, Kainz B, Schmidt K, Maier R, et al. Magnetic resonance-derived 3-dimensional blood flow patterns in the main pulmonary artery as a marker of pulmonary hypertension and a measure of elevated mean pulmonary arterial pressure. Circ Cardiovasc Imaging. 2008;1:23–30. doi: 10.1161/CIRCIMAGING.108.780247. [DOI] [PubMed] [Google Scholar]
- 77.Sieren MM, Berlin C, Oechtering TH, Hunold P, Drömann D, Barkhausen J, et al. Comparison of 4D Flow MRI to 2D Flow MRI in the pulmonary arteries in healthy volunteers and patients with pulmonary hypertension. PLoS One. 2019;14:e0224121. doi: 10.1371/journal.pone.0224121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reiter U, Reiter G, Kovacs G, Stalder AF, Gulsun MA, Greiser A, et al. Evaluation of elevated mean pulmonary arterial pressure based on magnetic resonance 4D velocity mapping: comparison of visualisation techniques. PLoS One. 2013;8:e82212. doi: 10.1371/journal.pone.0082212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reiter G, Reiter U, Kovacs G, Olschewski H, Fuchsjäger M. Blood flow vortices along the main pulmonary artery measured with MR imaging for diagnosis of pulmonary hypertension. Radiology. 2015;275:71–79. doi: 10.1148/radiol.14140849. [DOI] [PubMed] [Google Scholar]
- 80.Wang Z, Lakes RS, Golob M, Eickhoff JC, Chesler NC. Changes in large pulmonary arterial viscoelasticity in chronic pulmonary hypertension. PLoS One. 2013;8:e78569. doi: 10.1371/journal.pone.0078569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barker AJ, Roldán-Alzate A, Entezari P, Shah SJ, Chesler NC, Wieben O, et al. Four-dimensional flow assessment of pulmonary artery flow and wall shear stress in adult pulmonary arterial hypertension: results from two institutions. Magn Reson Med. 2015;73:1904–1913. doi: 10.1002/mrm.25326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feneis JF, Kyubwa E, Atianzar K, Cheng JY, Alley MT, Vasanawala SS, et al. 4D flow MRI quantification of mitral and tricuspid regurgitation: reproducibility and consistency relative to conventional MRI. J Magn Reson Imaging. 2018;48:1147–1158. doi: 10.1002/jmri.26040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Driessen MMP, Schings MA, Sieswerda GT, Doevendans PA, Hulzebos EH, Post MC, et al. Tricuspid flow and regurgitation in congenital heart disease and pulmonary hypertension: comparison of 4D flow cardiovascular magnetic resonance and echocardiography. J Cardiovasc Magn Reson. 2018;20:5. doi: 10.1186/s12968-017-0426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fenster BE, Browning J, Schroeder JD, Schafer M, Podgorski CA, Smyser J, et al. Vorticity is a marker of right ventricular diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2015;309:H1087–H1093. doi: 10.1152/ajpheart.00278.2015. [DOI] [PubMed] [Google Scholar]
- 85.Barker N, Fidock B, Johns CS, Kaur H, Archer G, Rajaram S, et al. A systematic review of right ventricular diastolic assessment by 4D flow CMR. Biomed Res Int. 2019;2019:6074984. doi: 10.1155/2019/6074984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grossfeld B. Deep learning vs machine learning. Zendesk. Zendesk; 2020 [cited 2020 Apr 9]. Available from: https://www.zendesk.com/blog/machine-learning-and-deep-learning/.
- 87.O’Regan DP. Putting machine learning into motion: applications in cardiovascular imaging. Clin Radiol. 2020;75:33–37. doi: 10.1016/j.crad.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 88.Leiner T, Rueckert D, Suinesiaputra A, Baeßler B, Nezafat R, Išgum I, et al. Machine learning in cardiovascular magnetic resonance: basic concepts and applications. J Cardiovasc Magn Reson. 2019;21:61. doi: 10.1186/s12968-019-0575-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johns CS, Kiely DG, Swift AJ. Novel imaging techniques in pulmonary hypertension. Curr Opin Cardiol. 2018;33(6):587–593. [DOI] [PubMed]
- 90.Avendi MR, Kheradvar A, Jafarkhani H. Automatic segmentation of the right ventricle from cardiac MRI using a learning-based approach. Magn Reson Med. 2017;78:2439–2448. doi: 10.1002/mrm.26631. [DOI] [PubMed] [Google Scholar]
- 91.Bai W, Sinclair M, Tarroni G, Oktay O, Rajchl M, Vaillant G, et al. Automated cardiovascular magnetic resonance image analysis with fully convolutional networks. J Cardiovasc Magn Reson. 2018;20:65. doi: 10.1186/s12968-018-0471-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duan J, Bello G, Schlemper J, Bai W, Dawes TJW, Biffi C, et al. Automatic 3D bi-ventricular segmentation of cardiac images by a shape-refined multi-task deep learning approach. IEEE Trans Med Imaging. 2019;38:2151–2164. doi: 10.1109/TMI.2019.2894322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lungu A, Swift AJ, Capener D, Kiely D, Hose R, Wild JM. Diagnosis of pulmonary hypertension from magnetic resonance imaging–based computational models and decision tree analysis. Pulm Circ. 2016;6(2):181–90. [DOI] [PMC free article] [PubMed]
- 94.•• Swift AJ, Lu H, Uthoff J, Garg P, Cogliano M, Taylor J, et al. A machine learning cardiac magnetic resonance approach to extract disease features and automate pulmonary arterial hypertension diagnosis. Eur Heart J Cardiovasc Imaging. 2020;30:jeaa001. This study is the first to use machine learning to extract pulmonary hypertension disease features using machine learning. [DOI] [PMC free article] [PubMed]
- 95.•• Dawes TJW, de Marvao A, Shi W, Fletcher T, Watson GMJ, Wharton J, et al. Machine learning of three-dimensional right ventricular motion enables outcome prediction in pulmonary hypertension: a cardiac MR imaging study. Radiology. 2017;283:381–90. This is the first study to assess the impact of machine learning on prognostic assessment in a mixed cohort of pulmonary hypertension. [DOI] [PMC free article] [PubMed]
- 96.Dawes TJW, Cai J, Quinlan M, de Marvao A, Ostrowski PJ, Tokarczuk PF, et al. Fractal analysis of right ventricular trabeculae in pulmonary hypertension. Radiology. 2018;288:386–395. doi: 10.1148/radiol.2018172821. [DOI] [PubMed] [Google Scholar]
- 97.Swift A, Cogliano M, Oram C, Kendall L, Capener D, Garg P, et al. Repeatability and sensitivity to change of right ventricular analysis methods using cardiac magnetic resonance imaging in PAH: results from the RESPIRE Study. Eur Respir J. 2019;54(supple 63):PA3164
- 98.• Noordegraaf AV, Vonk Noordegraaf A, Channick R, Cottreel E, Kiely D, Martin N, et al. Results from the REPAIR Study final analysis: effects of macitentan on right ventricular (RV) remodelling in pulmonary arterial hypertension (PAH). J Heart Lung Transplant 2020;39:S16–S17. The REPAIR study is the first study to have cardiac MRI as a co-primary endpoint in pulmonary hypertension.
- 99.Thenappan T. Beta-blockers in pulmonary arterial hypertension. Available from: Available from: https://clinicaltrials.gov/ct2/show/NCT02507011. Accessed 20 April 2020.
- 100.Solomon MA. Spironolactone for pulmonary arterial hypertension. Available from: Available from: https://clinicaltrials.gov/ct2/show/NCT01712620. Accessed 20 April 2020
- 101.Danoff T. PRIMEx - a study of 2 doses of oral CXA-10 in pulmonary arterial hypertension. Available from: Available from: https://clinicaltrials.gov/ct2/show/NCT03449524. Accessed 20 April 2020
- 102.Ventetuolo C. Effects of DHEA in pulmonary hypertension (EDIPHY). Available from: https://ClinicalTrials.gov/show/NCT03648385. Accessed 20 April 2020
- 103.Yacoub MH. Effects of treprostinil on right ventricular structure and function in patients with pulmonary arterial hypertension. Available from: Available from: https://clinicaltrials.gov/ct2/show/NCT03835676. Accessed 20 April 2020
- 104.Chaouat A, Cherifi A, Sitbon O, Girerd N, Zysman M, Faure M, et al. Evaluation of cardiac MRI in the follow up assessment of patients with pulmonary arterial hypertension. Rev Mal Respir. 2018;35:749–758. doi: 10.1016/j.rmr.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 105.Vogel-Claussen J. CTEPH DIAGNOSIS Europe - MRI. Available from: Available from: https://clinicaltrials.gov/ct2/show/NCT02791282. Accessed 20 April 2020