Abstract
Abnormal activation of the nuclear factor‐kappa B (NF‐κB) signaling pathway is closely implicated in triple‐negative breast cancer growth, metastasis, and tumor immune escape. In this study, the anti‐cancer effects of icariin, a natural flavonol glycoside, toward breast cancer cells and the underlying mechanisms were investigated. This investigation showed that icariin selectively inhibited proliferation and triggered apoptosis in breast cancer cells in a concentration‐ and time‐dependent manner, but exhibited little cytotoxicity in normal breast cells. Moreover, icariin induced cell apoptosis via a mitochondria‐mediated pathway, as indicated by the upregulated ratio of Bax/Bcl‐2 and reactive oxygen species induction. Importantly, icariin impaired the activation of the NF‐κB/EMT pathway, as evidenced by upregulation of SIRT6, resulting in inhibition of migration and invasion of breast cancer cells. Additionally, oss‐128167, an inhibitor of SIRT6, dramatically attenuated anti‐migration and anti‐invasion effects of icariin. Transcriptomic analysis verified that impairment of NF‐κB led to the selective function of icariin in breast cancer cells. Notably, icariin exhibited a significant tumor growth inhibition and anti‐pulmonary metastasis effect in a tumor mouse model of MDA‐MB‐231 and 4T1 cells by regulating the tumor immunosuppressive microenvironment. Together, these results showed that icariin could effectively trigger apoptosis and inhibit the migration of breast cancer cells via the SIRT6/NF‐κB signaling pathway, suggesting that icariin might serve as a potential candidate drug for the treatment of breast cancer.
Keywords: anti‐tumor immunity, icariin, metastasis, NF‐κB, triple‐negative breast cancer
Icariin selectively induces redox‐dependent apoptosis and inhibits migration and invasion in breast cancer cells by impairing the activation of the NF‐κB signaling pathway. Icariin upregulated expression of SIRT6 to impair the NF‐κB/EMT pathway. Icariin exhibited a significant tumor growth inhibition and anti‐pulmonary metastasis effect in vivo by regulating the tumor immunosuppressive microenvironment.

1. INTRODUCTION
Breast cancer is the most common type of diagnosed cancer among women worldwide. Based on statistics, approximately 2 088 849 new breast cancer cases were detected and approximately 626 679 patients died from breast cancer in 2018 worldwide. 1 Even though there have been significant developments in the survival rates of patients with breast cancer, this disease remains a tremendous threat to women's health. This is particularly true for patients with triple‐negative breast cancer (TNBC), which is defined by the lack of expression of the estrogen receptor, progesterone (PR), and human epidermal growth factor receptor 2 (HER2). TNBC accounts for 11.2%‐16.3% of all breast cancers and is insensitive to hormonal therapy or HER2‐targeted drugs. 2 , 3 , 4 Furthermore, breast cancer metastasis is a predominant cause of clinical mortality, occurring when the tumor cells migrate from the primary tumor foci to the distant sites. 5 Commonly, one‐eighth of all diagnosed breast cancers are invasive/metastatic. 6 However, there are currently few effective drugs available to treat metastatic breast cancer and improve survival in these patients. 7 Hence, novel strategies are urgently needed to improve the anti‐tumor and anti‐metastasis therapies targeting TNBC.
Nuclear factor‐kappa B (NF‐κB) was discovered in 1986 as a B‐cell–specific transcription factor and is a key regulator that promotes cell proliferation, suppresses apoptosis, accelerates cell migration and invasion, and stimulates metastasis and angiogenesis. 8 , 9 Activation of NF‐κB is usually rapidly triggered by infection, DNA damage, or oxidative stress. 10 It has been reported that NF‐κB is constitutively active in both tumor cells and the tumor microenvironment, including in breast cancer, ovarian cancer, colorectal cancer, and others. 11 Activated NF‐κB plays a key role in cancer metastasis, as NF‐κB can directly induce metastatic dissemination via epithelial‐mesenchymal transition (EMT) and promote tumor cell escape from the primary tumor, which leads to dissemination via the blood or lymphatic vessels and colonization of distant organs, including the lungs, bone, brain, and lymph nodes. 12 , 13 In addition, NF‐κB activation can enhance cancer cell migration and invasion via the induction of matrix‐degrading enzymes such as MMP2 and MMP9. 14 Furthermore, NF‐κB is closely related to the tumor immunosuppressive microenvironment. Existing evidence revealed that activation of NF‐κB directly results in the proliferation of regulatory T cells (Treg) and transcription of PD‐L1. 15 , 16 Inhibition of NF‐κB can relieve the tumor immunosuppressive microenvironment and enhance tumor immune therapy. Therefore, targeting NF‐κB might be a promising therapeutic approach for the treatment of breast cancer. There are, however, few oral small molecular drugs that can effectively inhibit activation of NF‐κB and that have been approved for use in clinical application.
Plant‐derived natural drugs play an important role in the development of cancer chemotherapies. 17 It has been reported that over 60% of the presently used anti‐cancer drugs are directly or indirectly derived from natural sources, including plants, marine organisms, and micro‐organisms. 18 , 19 Small molecules, such as paclitaxel, vincristine, and camptothecin, which are derived from natural plants, have been used successfully as anti‐cancer drugs. 20 However, the demand for further discovery of novel therapeutic molecules derived from natural sources for cancer treatment is a never‐ending venture.
Icariin, a prenylated flavonol glycoside, which is extracted from the medical plant Herba Epimedii (Figure 1A), has demonstrated numerous pharmacological actions, including as an aphrodisiac, osteogenic, antidepressant, cardiovascular protective, and has immunomodulatory activities. 21 , 22 , 23 It has been reported that icariin can serve as an effective NF‐κB inhibitor to alleviated murine lupus nephritis and improved Fanconi anemia hematopoietic stem cell function. 24 , 25 In recent years, studies have demonstrated the anti‐cancer effect of icariin against osteosarcoma, prostate, lung, and gastric cancer cells. 26 , 27 , 28 However, the function of icariin in breast cancer and its related molecular mechanisms have not however been investigated. Therefore, considering the role of NF‐κB in breast cancer, we hypothesized that icariin might be a promising candidate for breast cancer treatment. In this study, we demonstrated that icariin acts as an effective agent against breast cancer cells both in vitro and in vivo, and the reactive oxygen species (ROS)‐dependent mitochondrial pathway and SIRT6/NF‐κB/EMT pathway are likely to be involved in icariin‐mediated apoptosis and migration. Our findings suggested that icariin could serve as a potential agent for the treatment of breast cancer.
FIGURE 1.
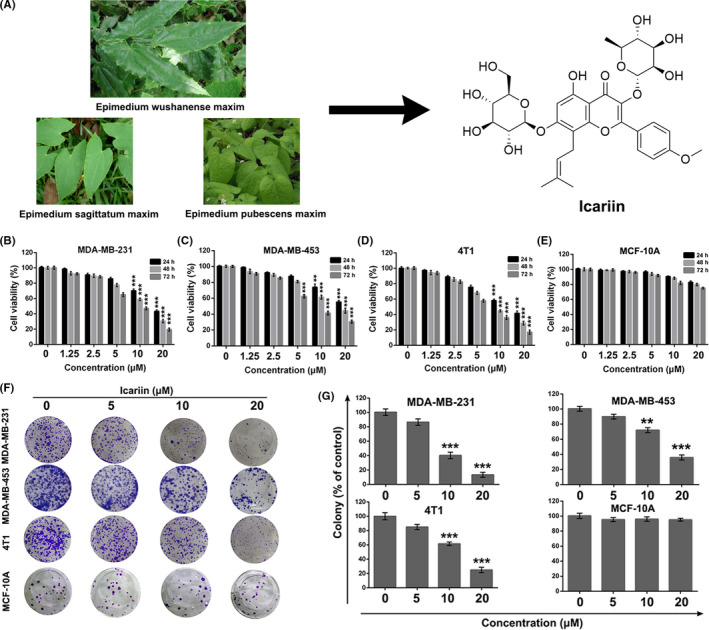
Icariin selectively inhibited breast cancer cell proliferation in vitro. A, Natural resources and chemical structure of icariin. B‐E, Cell viability analysis of MDA‐MB‐231, MDA‐MB‐453, 4T1, and MCF‐10A after treatment with indicated concentrations of icariin for 24, 48, or 72 h by MTT assay. F, G, Colony formation of MDA‐MB‐231, MDA‐MB‐453, 4T1, and MCF‐10A after treatment with different concentrations of icariin. Bars represent means ± SD of at least 3 independent experiments; *, P < .05, **, P < .01 and ***, P < .001 compared with the control group
2. MATERIALS AND METHODS
2.1. Drugs and reagents
Icariin (purity > 98%, as measured by high‐performance liquid chromatography analysis) was purchased from Energy Chemical and dissolved at a concentration of 20 mM in 100% dimethyl sulfoxide (DMSO) as a stock solution, stored at −20°C, and diluted with medium before use. Medium containing 0.1% DMSO served as the control. 3‐(4,5‐Dimethyl‐2‐thiazyl)‐2,5‐diphenyl‐2H‐tetrazolium bromide (MTT) Rh123, DCFH‐DA, and N‐acetyl cysteine (NAC) were bought from Sigma Chemical Co. Antibodies against Bcl‐2, Bax, cleaved caspase3, SIRT6, HSK9 Ac, H3, p‐IκBα, NF‐κB p65, fibrillarin, E‐cadherin, N‐cadherin, MMP‐2, and β‐actin were purchased from Cell Signaling Technology. An inhibitor of SIRT6 (oss‐128167) was purchased from Selleck Chemicals. Anti‐Ki‐67 mouse monoclonal antibody was brought from Merck Millipore. An Annexin V‐FITC/PI Apoptosis Detection Kit was obtained from KeyGen Biotech.
2.2. Cell culture
The human breast cancer cell line MDA‐MB‐231 (RRID:CVCL_0062), MDA‐MB‐453 (RRID:CVCL_0418), mouse mammary carcinoma cell line 4T1 (RRID:CVCL_0125), and human mammary epithelium cell line MCF‐10A (RRID:CVCL_0598), were bought from the American Type Culture Collection, and were cultured in Dulbecco’s modified Eagle's medium or RPMI 1640 medium containing 10% fetal bovine serum (FBS, HyClone) and 1% antibiotics in 5% CO2 in air at 37°C. All human cell lines used in this study has been authenticated using short tandem repeat profiling within the last 3 y. All experiments were performed with mycoplasma‐free cells.
2.3. Cell viability assay
MTT assays were used to assess the viability of icariin‐treated cancer cells. Cells were seeded into a 96‐well plate and treated with different concentrations of icariin for the indicated times. Then, 20 μL of MTT solution (5 mg/mL in cell culture medium) was added into each well, and the cells were incubated for another 4 h at 37°C. Then, 150 μL DMSO was added to dissolve the formazan precipitates, and the color absorbance was measured at 570 nm using a Spectra MAX M5 microplate spectrophotometer (Molecular Devices). All experiments were conducted in triplicate.
2.4. Colony formation assay
Cells were seeded in 6‐well plates (400‐600 cells/well) and treated with different concentrations of icariin. After incubation for an additional 6‐8 d, the cells were fixed with methanol for 5 min and stained with 0.5% crystal violet for 20 min. Colonies that contained more than 50 cells were counted using a microscope (Olympus), and the data presented were the average of 3 experiments.
2.5. Cell apoptosis assay
Cells treated with icariin were stained with Annexin V‐FITC/PI and measured using a FACSCalibur flow cytometer (BD). Annexin V+/PI+ cells were considered to be apoptotic cells.
2.6. Measurement of ROS levels
After treatment with different concentrations of icariin for 24 h, the cells were harvested and incubated with DCFH‐DA for 20 min at 37°C. ROS levels of treated cells were measured via flow cytometry (FCM). For inhibition experiments, cells were treated with an antioxidant (NAC, 2 mM) for 1 h prior to icariin exposure.
2.7. Mitochondrial membrane potential (ΔΨm) assay
Rh123 was exploited to measure the changes in the ΔΨm of cells via FCM. Cells were treated with different concentrations of icariin and then incubated with Rh123 (5 μg/mL) at 37°C for 30 min. Finally, the ΔΨm of the treated cells was measured by FCM.
2.8. Wound healing assay
Cells were seeded into 6‐well plates and scratched with a 10 μL pipette tip when the cells had grown to approximately 90% confluency. Next, the cells were treated with icariin and further cultured in medium with 1% FBS for 24 h. The motility of the treated cells was observed using a microscope (Olympus).
2.9. Transwell migration and invasion assay
For evaluation of cell migration, 8‐mm pore size culture inserts (transwell; Costar) were placed into the wells of 24‐well culture plates, separating the upper and the lower chambers. In the upper chamber, 5 × 104 cells suspended in 300 μL of serum‐free medium containing different concentrations of icariin were added, and 500 μL of culture medium containing 10% FBS were added to the lower chamber. For Matrigel invasion assays, 1 × 105 cells were added to the upper chamber pre‐coated with Matrigel and containing different concentrations of icariin. After incubation for 24 h at 37°C, the non‐migrated and non‐invasive cells located on the upper surface of the filter were removed with a cotton swab, and the migrated and invasive cells on the bottom surface of the membrane were fixed with methanol and stained with 0.1% crystal violet. Finally, the cells were imaged and counted using a microscope (Olympus).
2.10. Western blot analysis
The treated cells were harvested and lysed in RIPA buffer. For some experiments, NE‐PER™ Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher) were used to isolate the nuclear and cytosolic proteins in accordance with the manufacturer's instructions. Equal amounts of proteins from each group were loaded onto a 10% SDS‐PAGE gel. After electrophoresis, the proteins were transferred to membranes, and the membranes were blocked with 5% non‐fat dried milk and incubated with primary antibodies overnight at 4°C. Subsequently, the proteins were incubated with the corresponding HPR‐conjugated secondary antibodies for 30 min at 37°C and visualized and imaged using a chemiluminescence (ECL) detection system.
2.11. Immunofluorescence assay
MDA‐MB‐231 cells were cultured on glass circles in 24‐well plates and were exposed to different treatments. Next, cells were fixed with 4% paraformaldehyde for 10 min, incubated with permeabilization solution (1% Triton X‐100) for 15 min, washed with cold PBS 3 times and blocked with 5% BSA solution for 1 h. Cell were incubated with primary antibodies overnight at 4°C, and then washed with cold PBS and incubated with FITC‐conjugated goat anti‐rabbit IgG secondary antibody (dilution, 1:500, Beyotime) for 1 h at 37°C. Finally, the nuclei of MDA‐MB‐231 cells were stained with DAPI (dilution, 1:10 000) for 10 min, and cell images were obtained using a laser scanning confocal microscope (Leica).
2.12. Transcriptomic analysis
MDA‐MB‐231 cells and MCF‐10A cells were treated with icariin (0 or 20 μM) respectively. After 24 h of treatment, total RNA of MDA‐MB‐231 and MCF‐10A cells were extracted. RNA‐seq libraries were prepared using the Illumina HiSeq system in accordance with the manufacturer’s instructions. By screening comparison, H‐cluster analysis was used to analyze the levels of differentially expressed genes.
2.13. In vivo anti‐tumor evaluation
All animal experiments were approved by the Institutional Animal Care and Treatment Committee of Chengdu University of TCM (Chengdu, China). Female BALB/c nude mice and BALB/c mice (6‐8 wk old) were purchased from HFK Bioscience CO., Ltd. These BALB/c mice or BALB/c nude mice were inoculated with 100 μL of 4T1 cell suspension (1.0 × 106 cells) or MDA‐MB‐231 cell suspension (1.0 × 107 cells). At 5 d after inoculation, when the tumor volume had reached approximately 100 mm3, the mice were equally and randomly divided into 3 treatment groups (control, 20, and 40 mg/kg) (n = 6). Mice received icariin treatment by intraperitoneal injection once daily for 2 wk. The tumor volume (0.5 × L × W 2, where L is length and W is width) and body weight of the mice were recorded every 2 d. The mice were euthanized by cervical dislocation at the termination of the experiments. Tumors were isolated, imaged, weighed, and fixed with paraformaldehyde for further immunohistochemistry evaluation.
2.14. Tumor immune microenvironment
To investigate the effect of icariin on the tumor microenvironment, single‐cell suspensions of tumors from different treated groups were prepared and stained with various antibodies (CD3, CD4, CD8, CD11b, and Gr‐1) to analyze the populations of CD4+ T cells, CD8+ T cells and myeloid‐derived suppressor cells (MDSC) by FCM.
2.15. Anti‐metastasis evaluation
The anti‐metastatic effect of icariin was evaluated in a lung metastatic 4T1 model. In total, 1 × 106 eT1‐Luciferase cells were injected into BALB/c mice via the tail vein to establish the pulmonary metastasis model. In total, 18 mice were equally and randomly divided into 3 groups (control, 5, and 10 mg/kg). The mice received icariin treatment 2 d after inoculation via intraperitoneal injection once daily for 2 wk. At the determined time (5, 10, or 15 d after treatment), the mice were anesthetized, intraperitoneally administered with 100 μL of d‐luciferin (30 mg/mL in PBS), and the bioluminescence from metastatic lung tumors was analyzed using an IVIS Lumina in vivo Imaging System (PerkinElmer). At the termination of the experiment, the lungs were isolated from the euthanized mice, and the number of metastatic nodules was counted; nodules were fixed with paraformaldehyde for further evaluation using hematoxylin and eosin (H&E) staining.
2.16. Statistical analysis
All data were presented as mean ± standard deviation (SD) of at least 3 independent experiments. One‐way analysis of variance (ANOVA) and Tukey's post‐hoc test were used for statistical analysis. Significant differences between the groups were labeled as follows: *, P < .05; **, P < .01; and ***, P < .001.
3. RESULTS
3.1. Icariin selectively inhibits breast cancer cell proliferation in vitro
To investigate the anti‐proliferation effect of icariin on breast cancer cells, the human TNBC cell lines MDA‐MB‐231, MDA‐MB‐453, murine breast cancer cell line 4T1 and human mammary epithelium cell line MCF‐10A were assessed via MTT assay. As shown in Figure 1B‐E, icariin exhibited a concentration‐dependent and time‐dependent cytotoxicity toward the MDA‐MB‐231, MDA‐MB‐453, and 4T1 cell lines, but demonstrated little cytotoxicity toward MCF‐10A cells. Furthermore, colony formation assays were used to confirm the anti‐proliferation effect of icariin. As shown in Figure 1F,G, the colony formation ability of the cells treated with 10 or 20 μM of icariin was significantly inhibited compared with the control group (P < .001). Moreover, the size of the colonies exposed to icariin was smaller than that of the control group. Colony formation of MCF‐10A cells was hardly inhibited by treatment with icariin.
3.2. Icariin selectively induces apoptosis in breast cancer cells via a mitochondria‐mediated apoptotic pathway
To examine whether the anti‐proliferation effect of icariin toward breast cancer cells was associated with apoptosis, Annexin V‐FITC/PI dual‐staining assays utilizing FCM were conducted after treatment with icariin. As shown in Figure 2A,B, compared with the control group, the apoptotic rate of the MDA‐MB‐231 cells treated with 10 or 20 μM icariin was 26.53 ± 2.3% (P < .001) and 45.17 ± 2.4% (P < .001), respectively. Treatment of 10 or 20 μM induced 13.1 ± 2.1% (P < .001) and 18.2 ± 1.6% (P < .001) apoptosis in MDA‐MB‐453 cells respectively in comparison with control group. For 4T1 cells, cell apoptosis was noticeably triggered following treatment with icariin (20 μM, P < .001) compared with the control group. However, little apoptosis was observed in MCF‐10A cells after treatment with icariin. From the results of western blot analysis (Figure 2C), the expression levels of cleaved caspase3 and Bax were increased in a concentration‐dependent manner, whereas that of Bcl‐2 was distinctly inhibited. In addition, the ratio of Bax/Bcl‐2 was significantly increased after treatment with icariin (Figure 2D), suggesting that a mitochondrial apoptotic pathway might participate in the observed icariin‐induced apoptosis.
FIGURE 2.
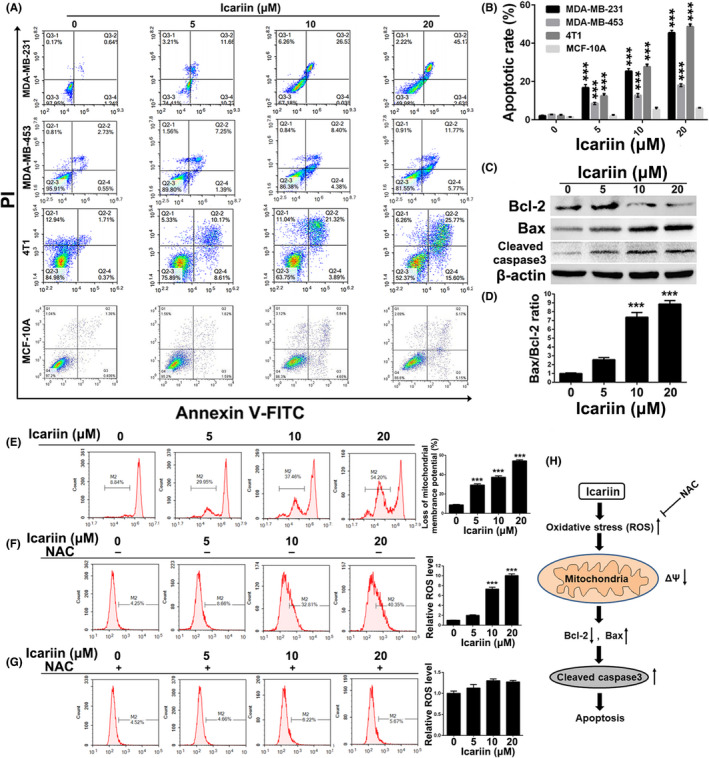
Effects of icariin on the intrinsic apoptosis mechanism in breast cancer cells. A, B, MDA‐MB‐231, MDA‐MB‐453, 4T1 and MCF‐10A cells were exposed to the indicated concentrations of icariin and then analyzed for apoptosis by FCM using the Annexin V/PI dual‐staining assay. C, Western blot analysis of apoptotic proteins in MDA‐MB‐231 cells after treatment with indicated concentrations of icariin. D, Protein expression ratio of Bax/Bcl‐2 in icariin‐treated MDA‐MB‐231 cells. E, Changes of mitochondrial membrane potential (ΔΨm) in MDA‐MB‐231 cells after treatment of icariin. F, G, The level of ROS was measured in MDA‐MB‐231 cells after exposure to icariin with or without pre‐treatment with NAC (10 μM). H, Overview of mitochondrial apoptosis triggered by icariin in breast cancer cells. Bars represent the means ± SD of at least 3 independent experiments; *, P < .05, **, P < .01 and ***, P < .001 compared with the control group
To verify the above hypothesis and assess the role of mitochondria in icariin‐induced cell apoptosis, we investigated whether icariin could induce a loss of ΔΨm in MDA‐MB‐231 cells. As shown in Figure 2E, icariin caused a significant loss of ΔΨm in a concentration‐dependent manner compared with the control group. Because a loss of ΔΨm participates in the generation of ROS, 29 we measured ROS levels in MDA‐MB‐231 cells. As displayed in Figure 2F, the level of ROS was dramatically increased in a concentration‐dependent manner after treatment with icariin, however pre‐treatment with NAC, a ROS inhibitor, significantly inhibited this increased ROS generation (Figure 2G). Together, these results suggested that icariin triggers breast cancer cell apoptosis via a mitochondrial‐mediated apoptotic pathway (Figure 2H).
3.3. Icariin suppresses migration and invasion of breast cancer cells via the SIRT6/NF‐κB/EMT pathway
Wound healing assays were used to assess the anti‐migration ability of icariin in 4T1 and MDA‐MB‐231 cells. As shown in Figure 3A,B, the mobilities of the MDA‐MB‐231 and 4T1 cells were inhibited in a concentration‐dependent manner following treatment with icariin. Furthermore, transwell migration assays were conducted to investigate the anti‐migration effect of icariin. The results of this analysis showed that icariin could significantly suppress migration of both MDA‐MB‐231 and 4T1 cells in a concentration‐dependent manner compared with the control group (P < .001). Moreover, results of transwell invasion analyses indicated that, compared with the control group, icariin exhibited an obvious anti‐invasion effect on both MDA‐MB‐231 and 4T1 cells in a concentration‐dependent manner (P < .001).
FIGURE 3.
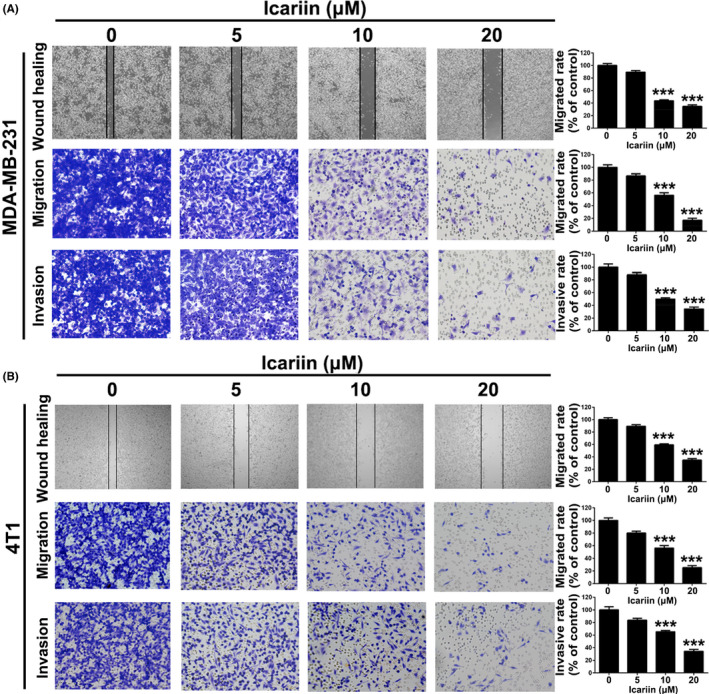
Icariin suppressed migration and invasion in breast cancer cells. A, After treatment with icariin, MDA‐MB‐231 cells were measured for wound healing, transwell migration, and transwell invasion. B, After treatment with icariin, 4T1 cells were measured for wound healing, transwell migration, and transwell invasion. Bars represent means ± SD of at least 3 independent experiments; *, P < .05, **, P < .01 and ***, P < .001 in comparison with control group
Accumulated evidence has shown that activation of the NF‐κB pathway plays an important role in cancer cell migration and invasion. 30 As such, we next examined whether icariin could impair breast cancer cell migration and invasion via the NF‐κB pathway. SIRT6 (sirtuin 6) is a specific histone H3 lysine 9 (H3K9) deacetylase that modulates chromatin structure, and deacetylation of H3K9 has been shown to play an important role in gene suppression. 31 , 32 Recent studies have indicated that SIRT6 deacetylates H3K9 at the promoters of NF‐κB target genes to destabilize NF‐κB. 33 Therefore, we attempted to detect the expression levels of SIRT6 and acetylated H3K9 in MDA‐MB‐231 cells after treatment with icariin. As displayed in Figure 4A,B, when the cells were exposed to 20 μM icariin the expression level of SIRT6 was significantly increased, whereas in acetylated H3K9 it was significantly reduced, indicating that icariin served to upregulate the expression level of SIRT6 and promote the deacetylation of H3K9. To investigate the possible inhibition of the NF‐κB pathway by icariin, both phosphorylated IκBα, which binds to the NF‐κB transcription complex and suppresses its nuclear translocation, and NF‐κB p65 subunit, which serves as a transcription factor and oncogene, were assessed via western blot analysis. 34 , 35 , 36 As shown in Figure 4C,D, the expression levels of p‐IκBα in the cytoplasm and NF‐κB p65 in the nucleus were both significantly inhibited following treatment with icariin. Importantly, abnormal activation of NF‐κB might function as a contributor to EMT, 37 as MDA‐MB‐231 cells exposed to icariin dramatically upregulated the expression levels of E‐cadherin and downregulated the expression levels of N‐cadherin and MMP‐2 (Figure 4E,F).
FIGURE 4.
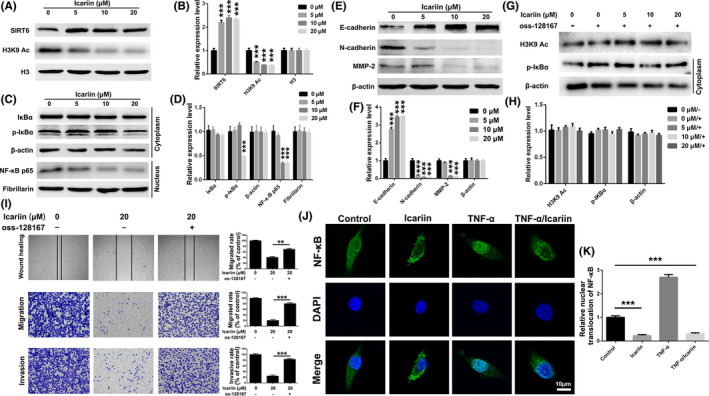
Icariin suppressed breast cancer cells (4T1) migration and invasion via the SIRT6/NF‐κB/EMT signaling pathway. A, B, Levels of SIRT6 and acylated H3K9 of icariin‐treated cells determined by western blot analysis. C, D, Expression levels of NF‐κB associated proteins of icariin‐treated cells determined by western blot analysis. E, F, Expression levels of EMT‐associated proteins of icariin‐treated cells determined by western blot analysis. G, H, Levels of acylated H3K9 and p‐IκBα of icariin‐treated cells that were pre‐treated with oss‐128167 (20 μM) and determined by western blot analysis. I, Wound healing, transwell migration, and transwell invasion assessment of icariin in MDA‐MB‐231 cells with or without pre‐treatment with oss‐128167. J, Immunofluorescence analysis of nuclear transportation of NF‐κB p65 protein in MDA‐MB‐231 cells. K, Statistical analysis of nuclear translocation of NF‐κB p65. Bars represent means ± SD of at least 3 independent experiments. *, P < .05, **, P < .01 and ***, P < .001 in comparison with the control group
Additionally, we used oss‐128167, an inhibitor of SIRT6, to investigate whether icariin targeted SIRT6 and subsequently affected the activation of the NF‐κB pathway. After pre‐treatment with 20 μM oss‐128167 for 2 h, icariin demonstrated little effect on the expression levels of acetylated H3K9 or p‐IκBα (Figure 4G,H). In addition, the anti‐migration and anti‐invasion effects of icariin in MDA‐MB‐231 cells were both significantly attenuated following pre‐treatment with oss‐128167 (Figure 4I, P < .001). Furthermore, we evaluated the translocation of NF‐κB after treatment with icariin in MDA‐MB‐231 cells. As illustrated by Figure 4J,K, the expression of NF‐κB p65 located in both cytoplasm and nucleus resulted from the constitutive activation of NF‐κB in MDA‐MB‐231 cells. When treated with icariin, the expression of NF‐κB p65 in the nucleus was significantly reduced. More importantly, icariin could significantly attenuate nuclear translocation of NF‐κB p65 protein which was induced by treatment with tumor necrosis factor (TNF)‐α. Taken together, these results suggested that icariin could upregulate the expression level of SIRT6 and deacetylate H3K9, followed by inhibition of the activation of NF‐κB and the EMT process. Ultimately, icariin treatment clearly impaired MDA‐MB‐231 cell migration and invasion in vitro.
3.4. Transcriptomic analysis of icariin in breast cancer cells
Icariin could significantly impair viability and induce apoptosis in 4T1 and MDA‐MB‐231 cells but had little effect on human mammary epithelium cell MCF‐10A. To gain insight into the molecular mechanism of the selective function of icariin, we utilized transcriptomics data to study the effects of icariin on MDA‐MB‐231 and MCF‐10A cells. As shown in Figure 5A, the expression profiles of treated MDA‐MB‐231 cells were different from that of untreated MDA‐MB‐231 cells. In MCF‐10A cells, there was no significant difference between treated and untreated groups. Moreover, after treatment with icariin, changes in the transcriptome with MDA‐MB‐231 cells were distinctly different with MCF‐10A cells. Subsequently, to determine the molecular pathways associated with icariin, we performed Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of the signaling pathways (Figure 5B,C). Results of KEGG analysis revealed significant enrichment for tumor cell apoptosis, migration, and invasion‐related signaling pathways, especially NF‐κB and TNF. Furthermore, well characterized genes that participated in NF‐κB and TNF pathways were significantly downregulated after treatment with icariin (Figure 5D). However, after treatment with icariin, NF‐κB‐related genes in MCF‐10A cells were almost unchanged. Transcriptomic analysis suggested that icariin impaired the activity of the NF‐κB signaling pathway by downregulating NF‐κB‐related genes, to selectively induce apoptosis and suppress migration and invasion in MDA‐MB‐231 cells.
FIGURE 5.
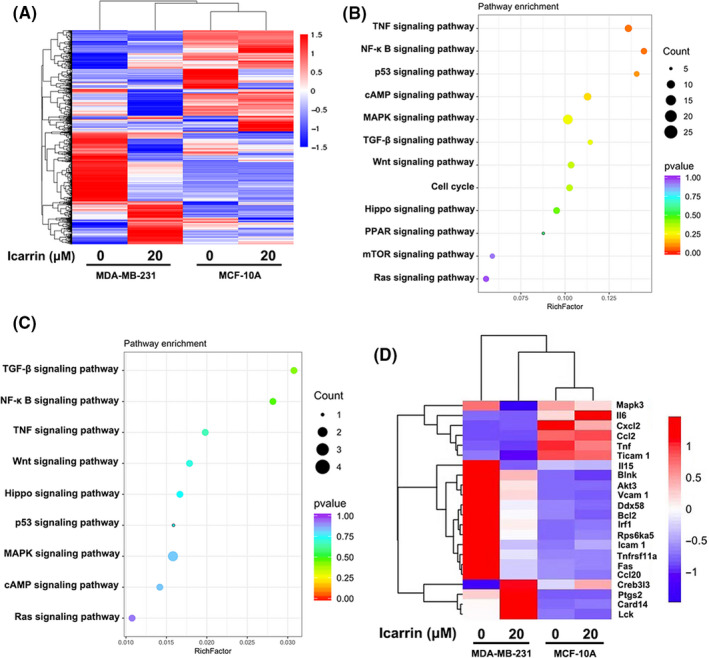
Transcriptomic analysis of icariin in MDA‐MB‐231 and MCF‐10A cells. A, Heatmap depiction of differentially expressed genes between different treated groups in MDA‐MB‐231 and MCF‐10A cells. B, KEGG analysis of representative signaling pathways enrichment between different treated groups in MDA‐MB‐231. C, KEGG analysis of representative signaling pathways enrichment between different treated groups in MCF‐10A cells. D, Heatmap depiction of differentially expressed genes of NF‐κB and TNF signaling pathways between different treated groups in MDA‐MB‐231 and MCF‐10A cells
3.5. Icariin suppresses tumor growth in vivo
To determine whether the in vivo anti‐tumor efficiency of icariin was consistent with its effects in vitro, MDA‐MB‐231 tumor‐bearing mice and 4T1 tumor‐bearing mice were treated with icariin at 20 and 40 mg/kg. As shown in Figure 6A‐C, treatment with icariin at 20 mg/kg (P < .01) or 40 mg/kg (P < .005) significantly inhibited MDA‐MB‐231 tumor growth and weight in a dose‐dependent manner compared with the control group. The body weight of the mice did not present any abnormal variation during the treatment process (Figure 6D). Moreover, the results of immunohistochemistry analysis of the tumors (Figure 6E) indicated that icariin inhibited the proliferation of nuclear Ki‐67‐positive cells, enhanced the expression of cleaved caspase3 to induce cell apoptosis, and downregulated the expression levels of NF‐κB in tumor sections. Moreover, western blot analysis of isolated tumors revealed that icariin enhanced expression levels of SIRT6, inhibited the nuclear expression level of NF‐κB p65, and downregulated the expression level of PD‐L1 (Figure 6F). In the 4T1 tumor‐bearing mouse model, icariin similarly retarded tumor growth significantly, and suppressed tumor cell proliferation and the expression level of NF‐κB (Figure 6G‐L). Overall, the above data suggested that icariin could suppress TNBC growth by inhibiting the NF‐κB signaling pathway, which was consistent with the in vitro data.
FIGURE 6.
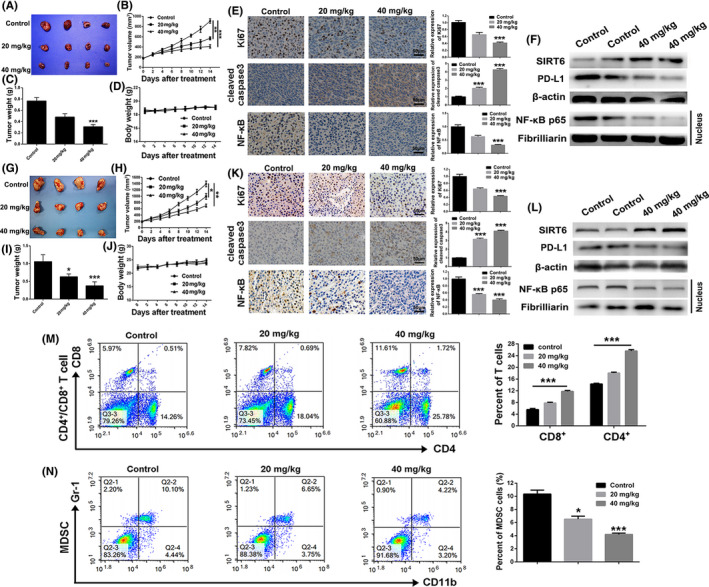
Icariin inhibited tumor growth and regulated the tumor immunosuppressive microenvironment. A, Representative image of MDA‐MB‐231 tumors of different groups at the termination of the experiment. B, The MDA‐MB‐231 tumor growth curves of different groups within the treatment process. C, MDA‐MB‐231 tumor weight of different treated groups at the termination of experiment. D, Variation of mice body weight of different groups within treatment progress. E, Immunohistochemistry analysis of Ki‐67 and cleaved caspase 3 of MDA‐MB‐231 tumor sections after different treatments. F, Expression level of NF‐κB associated proteins of different treated MDA‐MB‐231 tumor tissues was detected by western blot. G, Representative image of 4T1 tumors of different groups at the termination of the experiment. H, The 4T1 tumor growth curves of different groups within the treatment process. I, 4T1 tumor weight of different treated groups at the termination of experiment. J, Variation of mice body weight of different groups within the treatment progress. K, Immunohistochemistry analysis of Ki‐67 and cleaved caspase 3 of 4T1 tumor sections after different treatments. L, Expression levels of NF‐κB associated proteins of different treated 4T1 tumor tissues were detected by western blot. M, Changes of proportion of CD4+ and CD8+ T cells in 4T1 tumors after different treatments. N, Changes of proportion of MDSCs in 4T1 tumors after different treatments. Bars represent means ± SD of at least 3 independent experiments; *, P < .05, **, P < .01 and ***, P < .001 compared with the control group
3.6. Icariin regulates tumor immune microenvironment
Because of efficient inhibitory effect of icariin on the NF‐κB signaling pathway, we hypothesized that icariin probably had the ability to attenuate the tumor immunosuppressive microenvironment. MDSCs, which are usually accumulated in tumors, are responsible for the tumor immunosuppressive microenvironment. 38 , 39 So, we firstly investigated any changes in tumor infiltrating CD4+ and CD8+ T cells after treatment with icariin. As shown in Figure 6M, the proportion of tumor infiltrating CD4+ and CD8+ T cells was significantly increased after treatment with icariin. Additionally, icariin dramatically downregulated the proportion of MDSCs in tumors compared with the control group (Figure 6N). Overall, icariin could relieve the tumor immunosuppressive microenvironment, thus enhancing the anti‐tumor effect.
3.7. Icariin inhibits tumor lung metastasis in vivo
Because of the anti‐metastasis effect of icariin in vitro, we subsequently established a pulmonary metastasis model of 4T1‐luciferase cells in BALB/c mice to evaluate the anti‐metastatic efficacy of icariin in vivo. As shown in Figure 7A,B, the bioluminescence representing metastatic tumor cells was obviously enhanced over time compared with the icariin treatment group. At 15 d after tumor inoculation, the bioluminescence signals of the mice in the 20 mg/kg treatment group (3369 ± 223 p/s/cm2/sr, P < .005) and 40 mg/kg treatment group (5489 ± 358 p/s/cm2/sr, P < .001) were significantly lower than that of the control group (8374 ± 469 p/s/cm2/sr). In addition, the numbers of metastatic nodules at lung sites of the 20 mg/kg treatment group (34.2 ± 4.1, P < .005) and 40 mg/kg treatment group (57.5 ± 5.2, P < .001) were significantly reduced compared with the control group (81.6 ± 6.4) (Figure 7C,D). Moreover, collected lung tissues were sectioned and analyzed by H&E staining to determine the anti‐metastatic efficiency of icariin (Figure 7E). H&E staining of these tissue sections indicated that fewer tumor nodules were present in the icariin treatment group compared with the control group. These results implied that icariin could effectively inhibit 4T1 tumor pulmonary metastases.
FIGURE 7.
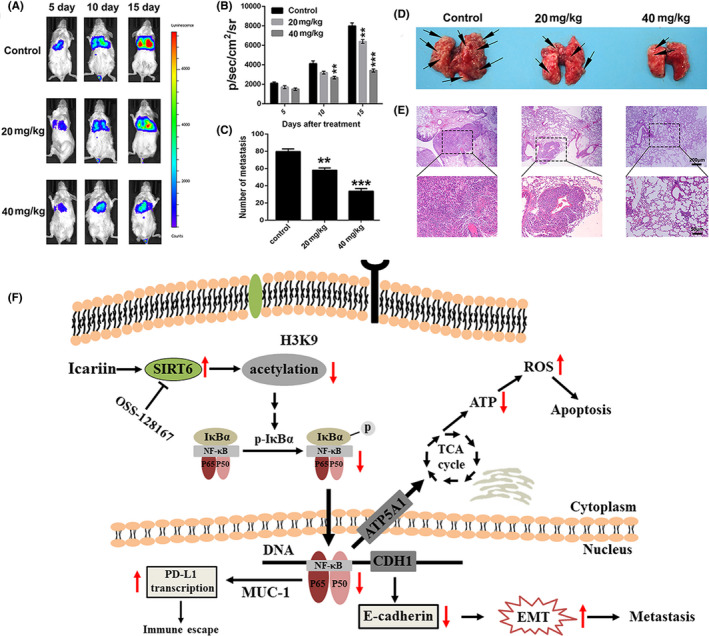
Icariin inhibited metastasis in the pulmonary metastatic tumor mouse model of 4T1. A, Bioluminescence images of mice bearing a pulmonary metastasis model of 4T1‐luciferase cells after different treatments at determined time points (7, 10, 13, or 16 d). B, Quantitation of the bioluminescence signal in mice after different treatments at determined time points. C, Number of nodules of lungs from different treated groups. D, Images in vitro of lungs from different treated groups. E, H&E staining analysis of lung tissues from different treated groups. F, Overview of pathways for icariin‐mediated apoptosis induction and anti‐metastasis in breast cancer cells. Icariin‐induced apoptosis, inhibited metastasis, and regulated immunosuppressive microenvironment by the SIRT6/NF‐κB signaling pathway in triple‐negative breast cancer cells. oss‐127167, inhibitor of SIRT6
4. DISCUSSION
Breast cancer is the most common type of diagnosed cancer among women worldwide and demonstrates considerable metastatic potential, multi‐drug resistance, and high mortality. 40 With advancements in medical technology, early diagnosis of breast cancer and treatment with chemoradiation therapy on its own or in combination with surgery can effectively retard the cancer’s progress. However, the treatment of breast cancer remains a significant challenge, and the need for more effective drug candidates is crucial. It has been reported that both abnormal activation and overexpression of NF‐κB are both closely associated with the progression and metastasis of breast cancer. 41 , 42 As such, we hypothesized that inhibition of NF‐κB might be a promising therapeutic target for the treatment of this cancer.
In this study, we reported that icariin, a natural prenylated flavonol glycoside, exhibited a promising anti‐cancer effect toward breast cancer. Our results from MTT and colony formation assays indicated that icariin could selectively inhibit the viability and proliferation of MDA‐MB‐231 and 4T1 cells in a concentration‐ and time‐dependent manner. Moreover, icariin significantly induced apoptosis in MDA‐MB‐231 and 4T1 cells. However, understanding the detailed mechanisms underlying how icariin kills breast cancer cells still requires further investigation.
Mitochondria are the major source of ROS generation, which plays an important role in cell apoptosis by altering the mitochondrial membrane potential (ΔΨm). 43 The anti‐apoptotic protein Bcl‐2 and pro‐apoptotic protein Bax clearly participate in the mitochondria‐mediated apoptotic pathways. 44 In this study, the activation of cleaved caspase 3 and an increase in Bax/Bcl‐2 were observed in 4T1 cells after treatment with icariin, as assessed by western blot analysis, and implying that icariin could induce cell apoptosis through the mitochondria‐mediated apoptotic pathway. Moreover, 4T1 cells treated with icariin induced a significant loss of ΔΨm and demonstrated increased ROS production. It was also observed that production of ROS in 4T1 cells induced by icariin could be attenuated by pre‐treatment with the antioxidant NAC, suggesting that the balance of ROS in the mitochondria was involved in the induction of cell apoptosis by icariin. Furthermore, icariin showed significant inhibition of tumor growth in a tumor mouse model of 4T1 cells. Similarity, Li et al demonstrated that icariin could induce apoptosis in human hepatoma cells via a ROS/JNK‐dependent mitochondrial pathway, 45 and Gu et al reported that icariin could exert an inhibitory effect on the growth of human esophageal carcinoma cells via ROS‐mediated alterations in ΔΨm. 46 Therefore, these results indicated that treatment with icariin was likely to trigger apoptosis in breast cancer cells via a ROS‐mediated mitochondrial apoptotic pathway.
One‐eighth of all breast cancers have already initiated cell migration and invasion at the time of diagnosis, having metastasized to the lymph nodes or distant organs. 6 Therefore, the anti‐metastasis effect of icariin was evaluated in MDA‐MB‐231 and 4T1 cells using transwell migration and invasion assays. Wound healing and transwell assays showed that icariin dramatically inhibited breast cancer cell migration and invasion, and our in vivo study showed that icariin distinctly inhibited tumor lung metastasis in a tumor mouse pulmonary metastatic model of 4T1 cells. Subsequently, we further investigated the mechanism by which icariin could effectively inhibit the migration and invasion of breast cancer cells. Aberrant NF‐κB pathway activation could function as an important contributor to the EMT process by directly or indirectly affecting SNAIL, SLUG, and ZEB1 expression levels. 47 Additionally, IκBα, which inhibits the activation of NF‐κB, could be phosphorylated by an activated Iκκ complex (NEMO, Iκκα, and Iκκβ) to induce self‐degradation. 48 Subsequently, the NF‐κB transcriptional complex is released into the nucleus where it binds negative regulatory DNA elements and promotes the transcription of pro‐metastatic genes. 35 , 36 Previous studies have shown that icariin can alleviate murine lupus nephritis and improve anemia hematopoietic stem cell function by inhibiting the activation of NF‐κB. 24 , 25 In this study, we found that the expression levels of p‐IκBα in the cytoplasm and NF‐κB p65 in the nucleus in 4T1 cells were both significantly inhibited following treatment with icariin. Moreover, 4T1 cells exposed to icariin dramatically upregulated the expression levels of E‐cadherin and downregulated the expression levels of N‐cadherin and MMP‐2, suggesting that icariin impaired the migration and invasion of breast cancer cells by inhibiting the NF‐κB/EMT pathway.
SIRT6 is a specific histone H3 lysine 9 (H3K9) deacetylase that modulates chromatin structure; deacetylation of H3K9 has been shown to play an important role in gene suppression. 31 , 32 Recent studies have indicated that SIRT6 deacetylates H3K9 on promoters of NF‐κB target genes to destabilize NF‐κB. 33 Western blot results indicated that in 4T1 cells icariin significantly upregulated the expression levels of SIRT6 and inhibited acylation of H3K9. When pre‐treated with 20 μM of the SIRT6 inhibitor, oss‐128167, icariin had almost no effect on H3K9 acylation or IκBα phosphorylation. Moreover, pre‐treatment with oss128167 clearly impaired the anti‐migration and anti‐invasion effects of icariin in 4T1 cells. Transcriptomic analysis verified that impairment of NF‐κB led to the selective function of icariin in breast cancer cells. How does icariin upregulate SIRT6 expression levels? It has been reported that lncRNAs closely participated in expression levels of SIRT6 in different types of cells. 49 , 50 , 51 , 52 Therefore, we needed to further explore if icariin upregulated the expression levels of SIRT6 by regulating the expression levels of lncRNAs. Furthermore, to further identify the activation mechanism of SIRT6 by icariin, the crystal structure of the icariin‐SIRT6 complex should be obtained to analyze the binding mode of icariin with SIRT6 and its structure‐activity relationship. Unfortunately, we did not have enough evidence to demonstrate how SIRT6 regulated p‐IκBα. It has been reported that SIRT6 may regulate phosphorylation and activation of TAK1 to inhibit NF‐κB signal‐related proteins. 53 Li et al demonstrated that SIRT6 interacted with the DNA binding domain of p65, and that this interaction decreased the occupancy of p65 at the NF‐κB target gene promoter and downregulated expression levels of p‐IκBα. 54 Furthermore, Santos‐Barriopedro and Vaquero revealed that SIRT6 prevented overactivation of the NF‐κB pathway, not only by deacetylating H3K9 at the promoter of NF‐κB target genes, but also by promoting suppressor of variegation 3‐9 homolog 1 (SUV39H1) cysteine monoubiquitination by recruiting and regulating the E3‐ubiquitin ligase S‐phase associate protein kinase 2 (SKP2) and inducing the dissociation of SUV39H1 from the promoter of Nfkb1a. 55 This in turn inhibited NF‐κB nuclear localization and promoted a general downregulation of the pathway. Together, the above results indicated that icariin could inhibit breast cancer cell migration and invasion by upregulating the expression of SIRT6, which was followed by impaired activation of the NF‐κB/EMT pathway (Figure 7F).
However, what is the relationship between NF‐κB and ROS level? It is well established that ROS production is closely associated with mitochondrial structure. 56 , 57 NF‐κB acts as an oxidative stress response transcription factor, and the NF‐κB pathway can affect mitochondria dynamics. 58 Vaisitti and colleagues reported that IT‐901, a new small‐molecule able to inhibit the NF‐κB subunit c‐Rel, exhibited a dose‐dependent mitochondrial ROS production and a marked decrease of ATP production in chronic lymphocytic leukemia cell lines, resulting from decreased expression levels of NF‐κB‐regulated gene (ATP5A1), involved in the tricarboxylic acid cycle or scavenging processes. 59 , 60 Therefore, icariin might suppress the tricarboxylic acid cycle to produce ROS and then trigger mitochondria‐dependent apoptosis in breast cancer cells by impairing NF‐κB pathway (Figure 7F). It has been reported that that activation of NF‐κB directly participates in the proliferation of regulatory T cells (Treg) and transcription of PD‐L1. 15 , 16 Yenkel demonstrated that NF‐kB c‐Rel ablation specifically impaired the generation and maintenance of the activated Treg (aTreg) subset, which is known to be enriched at the sites of tumors. 61 Treg cells specifically require c‐Rel to inhibit anti‐tumor effector responses. Yang et al revealed that Toll‐like receptor 4 (TLR4), which plays a vital role in innate immune response and cytokine production, closely participates in NF‐κB‐mediated immune regulation. 62 In our study, we found that icariin could significantly ameliorate the tumor immunosuppressive microenvironment by downregulating expression levels of PD‐L1, enhancing the proportion of infiltrating CD4+/CD8+ T cells and reducing the abundance of MDSCs in tumors.
In conclusion, our study showed that icariin could effectively trigger redox‐induced apoptosis, inhibit metastasis and regulate the immunosuppressive microenvironment of TNBC via the SIRT6/NF‐κB signaling pathway, indicating that icariin might serve as a potential candidate drug for the treatment of TNBC.
DISCLOSURE
The authors have no conflicts of interest.
ETHICAL APPROVAL
This article does not contain any studies with human participants performed by any of the authors. All the animal experiments in this study were performed in accordance with the National Institutes of Health (Bethesda, MD, USA) guidelines and were approved by the Institutional Animal Care and Treatment Committee of Chengdu University of Traditional Chinese Medicine.
ACKNOWLEDGMENTS
This work was supported by the Foundation of Science and Technology Department of Sichuan Province (2020YJ0147); Chinese Postdoctoral Science Foundation Program (2019M653833XB); the Foundation of Health Commission of Sichuan Province (19PJ033); Postdoctoral Science Foundation of Chengdu University of Traditional Chinese Medicine (030054080); Foundation of Science and Technology Department of Chengdu (2019‐YF05‐00218‐SN); and Foundation of Sichuan Administration of Traditional Chinese Medicine (2018JC010).
Song L, Chen X, Mi L, et al. Icariin‐induced inhibition of SIRT6/NF‐κB triggers redox mediated apoptosis and enhances anti‐tumor immunity in triple‐negative breast cancer. Cancer Sci. 2020;111:4242–4256. 10.1111/cas.14648
Linjiang Song and Xian Chen contributed equally to this article.
Funding information
Foundation of Health Commission of Sichuan Province (Grant/Award Number: '19PJ033'), Foundation of Sichuan Administration of Traditional Chinese Medicine (Grant/Award Number: '2018JC010'), Foundation of Science and Technology Department of Chengdu (Grant/Award Number: '2019‐YF05‐00218‐SN'), Postdoctoral Science Foundation of Chengdu University of Traditional Chinese Medicine (Grant/Award Number: '030054080'), Department of Science and Technology of Sichuan Province (Grant/Award Number: '2020YJ0147'), China Postdoctoral Science Foundation (Grant/Award Number: '2019M653833XB').
Contributor Information
Linjiang Song, Email: liangxin@cdutcm.edu.cn.
Xian Chen, Email: 646742880@qq.com.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Sharpe R, Pearson A, Herrera‐Abreu MT, et al. FGFR signaling promotes the growth of triple‐negative and basal‐like breast cancer cell lines both in vitro and in vivo. Clin Cancer Res. 2011;17:5275‐5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao Z, Lu P, Zhang H, et al. Nestin positively regulates the Wnt/beta‐catenin pathway and the proliferation, survival and invasiveness of breast cancer stem cells. Breast Cancer Res. 2014;16:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7‐30. [DOI] [PubMed] [Google Scholar]
- 5. Chen S, Han Q, Wang X, et al. IBP‐mediated suppression of autophagy promotes growth and metastasis of breast cancer cells via activating mTORC2/Akt/FOXO3a signaling pathway. Cell Death Dis. 2013;4:e842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563‐572. [DOI] [PubMed] [Google Scholar]
- 7. Steeg PS. Targeting metastasis. Nat Rev Cancer. 2016;16:201‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705‐716. [PubMed] [Google Scholar]
- 9. Zhang Q, Lenardo MJ, Baltimore D. 30 years of NF‐kappaB: a blossoming of relevance to human pathobiology. Cell. 2017;168:37‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karin M, Greten FR. NF‐kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749‐759. [DOI] [PubMed] [Google Scholar]
- 11. Staudt LM. Oncogenic activation of NF‐kappaB. Cold Spring Harb Perspect Biol. 2010;2:a000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fidler IJ, Poste G. The, "seed and soil" hypothesis revisited. Lancet Oncol. 2008;9:808. [DOI] [PubMed] [Google Scholar]
- 13. Karagiannis GS, Pastoriza JM, Wang Y, et al. Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM‐mediated mechanism. Sci Transl Med. 2017;9:eaan0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF‐kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188‐4197. [DOI] [PubMed] [Google Scholar]
- 15. Maeda T, Hiraki M, Jin C, et al. MUC1‐C induces PD‐L1 and immune evasion in triple‐negative breast cancer. Cancer Res. 2018;78:205‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vanamee ES, Faustman DL. TNFR2: a novel target for cancer immunotherapy. Trends Mol Med. 2017;23:1037‐1046. [DOI] [PubMed] [Google Scholar]
- 17. Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009;27:2712‐2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang X, Chen LX, Ouyang L, Cheng Y, Liu B. Plant natural compounds: targeting pathways of autophagy as anti‐cancer therapeutic agents. Cell Prolif. 2012;45:466‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shinde P, Banerjee P, Mandhare A. Marine natural products as source of new drugs: a patent review (2015–2018). Expert Opin Ther Pat. 2019;29:283‐309. [DOI] [PubMed] [Google Scholar]
- 20. Chen M, Wu J, Luo Q, et al. The anticancer properties of Herba Epimedii and its main bioactive Componentsicariin and Icariside II. Nutrients. 2016;8:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pan Y, Kong LD, Li YC, Xia X, Kung HF, Jiang FX. Icariin from Epimedium brevicornum attenuates chronic mild stress‐induced behavioral and neuroendocrinological alterations in male Wistar rats. Pharmacol Biochem Behav. 2007;87:130‐140. [DOI] [PubMed] [Google Scholar]
- 22. Xu HB, Huang ZQ. Icariin enhances endothelial nitric‐oxide synthase expression on human endothelial cells in vitro. Vascul Pharmacol. 2007;47:18‐24. [DOI] [PubMed] [Google Scholar]
- 23. He W, Sun H, Yang B, Zhang D, Kabelitz D. Immunoregulatory effects of the herba Epimediia glycoside icariin. Arzneimittelforschung. 1995;45:910‐913. [PubMed] [Google Scholar]
- 24. Su B, Ye H, You X, Ni H, Chen X, Li L. Icariin alleviates murine lupus nephritis via inhibiting NF‐kappaB activation pathway and NLRP3 inflammasome. Life Sci. 2018;208:26‐32. [DOI] [PubMed] [Google Scholar]
- 25. Li Y, Li X, Cole A, McLaughlin S, Du W. Icariin improves Fanconi anemia hematopoietic stem cell function through SIRT6‐mediated NF‐kappa B inhibition. Cell Cycle. 2018;17:367‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geng YD, Yang L, Zhang C, Kong LY. Blockade of epidermal growth factor receptor/mammalian target of rapamycin pathway by Icariside II results in reduced cell proliferation of osteosarcoma cells. Food Chem Toxicol. 2014;73:7‐16. [DOI] [PubMed] [Google Scholar]
- 27. Lee KS, Lee HJ, Ahn KS, et al. Cyclooxygenase‐2/prostaglandin E2 pathway mediates icariside II induced apoptosis in human PC‐3 prostate cancer cells. Cancer Lett. 2009;280:93‐100. [DOI] [PubMed] [Google Scholar]
- 28. Wang Y, Dong H, Zhu M, et al. Icariin exterts negative effects on human gastric cancer cell invasion and migration by vasodilator‐stimulated phosphoprotein via Rac1 pathway. Eur J Pharmacol. 2010;635:40‐48. [DOI] [PubMed] [Google Scholar]
- 29. Chauhan D, Li G, Sattler M, et al. Superoxide‐dependent and ‐independent mitochondrial signaling during apoptosis in multiple myeloma cells. Oncogene. 2003;22:6296‐6300. [DOI] [PubMed] [Google Scholar]
- 30. Wu Y, Zhou BP. Inflammation: a driving force speeds cancer metastasis. Cell Cycle. 2009;8:3267‐3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Michishita E, McCord RA, Berber E, et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452:492‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693‐705. [DOI] [PubMed] [Google Scholar]
- 33. Kawahara TL, Michishita E, Adler AS, et al. SIRT6 links histone H3 lysine 9 deacetylation to NF‐kappaB‐dependent gene expression and organismal life span. Cell. 2009;136:62‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xiao X, Yang G, Bai P, et al. Inhibition of nuclear factor‐kappa B enhances the tumor growth of ovarian cancer cell line derived from a low‐grade papillary serous carcinoma in p53‐independent pathway. BMC Cancer. 2016;16:582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hayden MS, Ghosh S. Shared principles in NF‐kappaB signaling. Cell. 2008;132:344‐362. [DOI] [PubMed] [Google Scholar]
- 36. Kim HJ, Hawke N, Baldwin AS. NF‐kappaB and IKK as therapeutic targets in cancer. Cell Death Differ. 2006;13:738‐747. [DOI] [PubMed] [Google Scholar]
- 37. Chua HL, Bhat‐Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H. NF‐kappaB represses E‐cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB‐1 and ZEB‐2. Oncogene. 2007;26:711‐724. [DOI] [PubMed] [Google Scholar]
- 38. Wu C, Tan X, Hu X, Zhou M, Yan J, Ding C. Tumor microenvironment following gemcitabine treatment favors differentiation of immunosuppressive Ly6C(high) myeloid cells. J Immunol. 2019;204:212‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Inigo‐Marco I, Alonso MM. Destress and do not suppress: targeting adrenergic signaling in tumor immunosuppression. J Clin Invest. 2019;129:5086‐5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peart O. Metastatic Breast Cancer. Radiol Technol. 2017;88:519M‐539M. [PubMed] [Google Scholar]
- 41. Fusella F, Secli L, Busso E, et al. The IKK/NF‐kappaB signaling pathway requires Morgana to drive breast cancer metastasis. Nat Commun. 2017;8:1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang L, Tian Y, Leong WS, et al. Efficient and tumor‐specific knockdown of MTDH gene attenuates paclitaxel resistance of breast cancer cells both in vivo and in vitro. Breast Cancer Res. 2018;20:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Loo G, Saelens X, van Gurp M, MacFarlane M, Martin SJ, Vandenabeele P. The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death Differ. 2002;9:1031‐1042. [DOI] [PubMed] [Google Scholar]
- 44. Rogers C, Erkes DA, Nardone A, Aplin AE, Fernandes‐Alnemri T, Alnemri ES. Gasdermin pores permeabilize mitochondria to augment caspase‐3 activation during apoptosis and inflammasome activation. Nat Commun. 2019;10:1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li S, Dong P, Wang J, et al. Icariin, a natural flavonol glycoside, induces apoptosis in human hepatoma SMMC‐7721 cells via a ROS/JNK‐dependent mitochondrial pathway. Cancer Lett. 2010;298:222‐230. [DOI] [PubMed] [Google Scholar]
- 46. Gu ZF, Zhang ZT, Wang JY, Xu BB. Icariin exerts inhibitory effects on the growth and metastasis of KYSE70 human esophageal carcinoma cells via PI3K/AKT and STAT3 pathways. Environ Toxicol Pharmacol. 2017;54:7‐13. [DOI] [PubMed] [Google Scholar]
- 47. Menezes SV, Fouani L, Huang MLH, et al. The metastasis suppressor, NDRG1, attenuates oncogenic TGF‐beta and NF‐kB signaling to enhance membrane E‐cadherin expression in pancreatic cancer cells. Carcinogenesis. 2018;40:805‐818. [DOI] [PubMed] [Google Scholar]
- 48. Brady G, Haas DA, Farrell PJ, Pichlmair A, Bowie AG. Molluscum contagiosum virus protein MC005 inhibits NF‐kappaB activation by targeting NEMO‐regulated IkappaB kinase activation. J Virol. 2017;91:545‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lin H, Zhao Z, Hao Y, He J, He J. Long noncoding RNA HIF1A‐AS2 facilitates cell survival and migration by sponging miR‐33b‐5p to modulate SIRT6 expression in osteosarcoma. Biochem Cell Biol. 2020;98:284‐292. [DOI] [PubMed] [Google Scholar]
- 50. Cai Y, Sheng Z, Chen Y, Wang J. LncRNA HMMR‐AS1 promotes proliferation and metastasis of lung adenocarcinoma by regulating MiR‐138/sirt6 axis. Aging (Albany NY). 2019;11:3041‐3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dong Z, Li C, Yin C, Xu M, Liu S, Gao M. LncRNA PU.1 AS regulates arsenic‐induced lipid metabolism through EZH2/Sirt6/SREBP‐1c pathway. J Environ Sci (China). 2019;85:138‐146. [DOI] [PubMed] [Google Scholar]
- 52. Jain AK, Xi Y, McCarthy R, et al. LncPRESS1 is a p53‐regulated LncRNA that safeguards pluripotency by disrupting SIRT6‐mediated de‐acetylation of histone H3K56. Mol Cell. 2016;64:967‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xu K, Guo Y, Ping L, et al. Protective effects of SIRT6 overexpression against DSS‐induced colitis in mice. Cells. 2020;9:1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li P, Jin Y, Qi F, et al. SIRT6 acts as a negative regulator in dengue virus‐induced inflammatory response by targeting the DNA binding domain of NF‐κB p65. Front Cell Infect Microbiol. 2018;8:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Santos‐Barriopedro I, Vaquero A. Complex role of SIRT6 in NF‐κB pathway regulation. Mol Cell Oncol. 2018;5:e1445942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim B, Song YS. Mitochondrial dynamics altered by oxidative stress in cancer. Free Radic Res. 2016;50:1065‐1070. [DOI] [PubMed] [Google Scholar]
- 57. Jezek J, Cooper KF, Strich R. Reactive oxygen species and mitochondrial dynamics: the Yin and Yang of mitochondrial dysfunction and cancer progression. Antioxidants. 2018;7(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen L, Li S, Guo X, Xie P, Chen J. The role of GSH in microcystin‐induced apoptosis in rat liver: Involvement of oxidative stress and NF‐kappaB. Environ Toxicol. 2016;31:552‐560. [DOI] [PubMed] [Google Scholar]
- 59. Vaisitti T, Gaudino F, Ouk S, et al. Targeting metabolism and survival in chronic lymphocytic leukemia and Richter syndrome cells by a novel NF‐kappaB inhibitor. Haematologica. 2017;102:1878‐1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Capece D, Verzella D, Di Francesco B, Alesse E, Franzoso G, Zazzeroni F. NF‐kappaB and mitochondria cross paths in cancer: mitochondrial metabolism and beyond. Semin Cell Dev Biol. 2019;98:118‐128. [DOI] [PubMed] [Google Scholar]
- 61. Grinberg‐Bleyer Y, Oh H, Desrichard A, et al. NF‐κB c‐Rel is crucial for the regulatory T cell immune checkpoint in cancer. Cell. 2017;170:1096‐1108.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yang A, Fan H, Zhao Y, et al. An immune‐stimulating proteoglycan from the medicinal mushroom Huaier up‐regulates NF‐κB and MAPK signaling via Toll‐like receptor 4. J Biol Chem. 2019;294:2628‐2641. [DOI] [PMC free article] [PubMed] [Google Scholar]


