Abstract
Circular RNA is a novel endogenous non‐coding RNA that can serve as a biomarker because of its stable loop structure. We investigated and examined the utility of plasma circERBB2 as a prognostic biomarker in 70 patients with gastric cancer who underwent gastrectomy. We investigated by real‐time quantitative PCR the circERBB2 concentrations in the preoperative and postoperative plasma and the circERBB2 expression in the resected tumors. The relationships between circERBB2 concentration in plasma and the clinicopathological features and prognosis were analyzed. circERBB2 was detected in the preoperative plasma samples of 37 patients. The presence of circERBB2 in preoperative plasma (high group) was significantly correlated with lymph node metastasis (P = .035) and tended to be correlated with men (P = .069). Both relapse‐free and overall survival were significantly poor in the high group (P = .001 and P = .009, respectively). The Cox proportional‐hazard model revealed that the high group was an independent prognostic factor of relapse‐free survival (P = .038). Among 16 patients of the high group, 13 patients did not show circERBB2 in the postoperative plasma. The concentration of circERBB2 in plasma was significantly higher in patients with recurrent cancer than those recurrence‐free patients (P < .001). In 2 patients with recurrent cancer, plasma circERBB2 concentrations were increased, whereas, in 2 recurrence‐free patients, these concentrations hardly changed during the treatment progress. The circERBB2 concentrations in preoperative plasma samples can be considered as a noninvasive prognostic biomarker for gastric cancer. Furthermore, monitoring the postoperative plasma circERBB2 concentrations may be useful for detecting gastric cancer recurrences.
Keywords: biomarker, circular ERBB2, circular RNA, gastric cancer, liquid biopsy
In patients with gastric cancer, high levels of preoperative plasma circERBB2 showed poor prognosis and the plasma level of circERBB2 increased at recurrence. Plasma circERBB2 can be a noninvasive prognostic biomarker and useful for the detection of gastric cancer recurrences.
Abbreviations
- CA19‐9
carbohydrate antigen 19‐9
- CEA
carcinoembryonic antigen
- PD
progressive disease
- PR
partial response
- PTX
paclitaxel
- RAM
ramucirumab
- RQ
relative quantification
- TS‐1
tegafur, gimeracil, and oteracil potassium combination
- XELOX
capecitabine and oxaliplatin
1. INTRODUCTION
Gastric cancer (GC) is the third leading cancer‐related cause of death globally. 1 Advances in surgical techniques, chemotherapy regimens, and perioperative management have decreased the mortality rates. However, patients with advanced GC frequently develop recurrences after radical resections and have poor survival rates. 2 , 3 Therefore, understanding the molecular mechanisms underlying GC progression would facilitate the development of effective diagnostic and therapeutic options for GC.
Several novel circulating biomarkers have been investigated for diagnosing GC. Clinically used tumor markers, including CEA and CA19‐9, are noninvasive and highly practical for diagnosing GC, but they show low sensitivity and specificity for GC. Therefore, to estimate the effectiveness of chemotherapy and to predict prognosis after radical resection, we need to investigate novel biological markers for GC. Circulating non‐coding RNAs that may be involved in various cancers 4 , 5 , 6 are detectable in the plasma or serum. 7 , 8
Circular RNA (circRNA), an endogenous non‐coding RNA, was identified by high‐throughput sequencing. 9 , 10 circRNAs are generated when precursor mRNAs (pre‐mRNA) back splice to join a downstream splice donor to an upstream splice acceptor and have a closed loop lacking a 5′ cap and a 3′ polyadenylation tail. 11 circRNAs sponge multiple miRNAs on their binding sites and play critical roles in cellular differentiation and disease occurrence. ciRS‐7, which is a highly represented circRNA in the human brain, acts as an inhibitor or as a sponge of miR‐7 and plays an important role in neurodegenerative diseases. 12 In cancer research, early studies have suggested that ciRS‐7 modulates miR‐7 expression, therefore elucidating the regulatory mechanisms of miR‐7 activity would likely advance the understanding of various cancer aetiologies. 13 At this time, several other circRNAs are also being investigated, and several endogenous circRNAs have been reported in cancer although the functional relevance of most of these circRNAs is still to be elucidated. 14
circRNAs are stable in the presence of RNase because of their closed‐loop structure. 15 Lin et al 16 reported that 3 plasma circRNAs, namely circ‐CCDC66, circ‐ABCC1, and circ‐STIL, could serve as a novel and independent diagnostic biomarkers for colorectal cancer. Liu et al 17 showed that in patients with lung adenocarcinoma plasma hsa_circ_0005962 expression was different before and after surgery. These studies suggested that these tumor‐derived circRNAs, which are detectable in the blood, could be ideal noninvasive biomarkers for cancer diagnosis and prognosis. 11 , 17 Furthermore, upregulation of circERBB2 derived from the ERBB2 gene locus in tumor tissue showed an unfavorable prognosis and facilitated GC progression. 18 Huang et al 19 suggested that circERBB2 promoted gallbladder cancer progression by regulating PA2G4‐dependent rDNA transcription and that circRNAs may have diverse functions, far more than only being miRNA sponges. However, no study has examined the dynamics of circulating circERBB2 in the clinical course of GC.
Therefore, we quantitatively detected circulating circERBB2 in preoperative plasma samples of GC patients who had undergone curative gastrectomy. Moreover, we examined the clinical potential of circERBB2 as a tool for real‐time evaluation of tumor progression by monitoring the dynamics of the plasma circERBB2 levels during treatment progress.
2. MATERIALS AND METHODS
2.1. Patients and samples
This study was approved by the local ethics committee (approval number: ERB‐C‐67‐3) and conducted following the Declaration of Helsinki principles. Before participating, all subjects gave written informed consent. Samples were blinded for analyses and the patients understood that the results would not be made available to them. The study cohort included 70 GC patients who underwent gastrectomy between January 2014 and June 2016 at the university hospital, Kyoto Prefectural University of Medicine. Patients with other cancers in the 5 y before surgery were excluded. Preoperative plasma samples and matched resected tumor samples were collected from all the patients. Clinicopathological data and the details regarding tumor recurrence and subsequent management were recorded.
Expert gastrointestinal endoscopists reviewed preoperative endoscopic images. Expert pathologists diagnosed the tumor tissue, and the pathological classifications of the tumors were determined following the 8th Union for International Cancer Control classification. 20 In patients with sufficient tumor tissues, the HER2 status was determined using formalin‐fixed paraffin‐embedded tissue samples and routine immunohistochemistry (IHC) methods to score from 0 to 3+. A score of IHC 0/1+ was considered to be HER2 negative, whereas IHC 2+/3+ was defined as HER2 positive. For prognostic analysis, only 60 patients were analyzed by the overall survival (OS) and relapse‐free survival (RFS) rates, excluding 2 and 8 patients who underwent R1 and R2 resection, respectively. After surgery, follow‐up procedures consisting of blood investigations and abdominal ultrasound and computed tomography scans were performed every 3‐6 mo.
All procedures followed were under the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Declaration of Helsinki 1964 and later versions. Informed consent or its substitute was obtained from all patients included in the study.
2.2. GC cell lines
Human GC cell lines MKN7 (RCB0999), MKN45 (RCB1001), MKN74 (RCB1002), HGC27 (RCB0500), and NUGC4 (RCB1939) were purchased from the RIKEN BioResource Center. NCI‐N87 (CRL‐5822) was purchased from the American Type Culture Collection. HGC27 was maintained in Dulbecco’s Modified Eagle Medium (Nacalai Tesque, Inc), and other cell lines were maintained in Roswell Park Memorial Institute 1640 medium (Nacalai Tesque, Inc) supplemented with 10% exosome‐depleted fetal bovine serum (System Biosciences). All cells were kept in a humidified incubator at 37°C with 5% CO2 in air.
2.3. Sample preparation and RNA isolation
Before surgery, a 7‐mL EDTA blood sample was obtained from each patient. Blood samples were also collected from patients at 1 mo after surgery, at time of recurrence, and at approximately 1 y after the end of adjuvant chemotherapy without tumor recurrence. After collection in sodium heparin tubes (BD Vacutainer), the plasma was immediately separated from the cellular fraction using a three‐spin protocol 21 and then stored at −80°C for further processing. Circulating RNA was isolated from 400 μL of the plasma sample using a miRNeasy® Serum/Plasma Advanced Kit (Qiagen) and following the manufacturer’s instructions.
Using a TLA100.3 Beckman fixed angle rotor, the exosomes were pelleted from 400 μL of the plasma sample by ultracentrifugation at 100 000 g for 23 min at 4°C. The pellets were diluted in PBS, and an additional washing step was performed following the same ultracentrifugation protocol. The pellets were solubilized in 700 μL of QIAzol Lysis Reagent (Qiagen), and exosomal RNA was extracted using a miRNeasy Mini Kit (Qiagen).
Tumor tissues were obtained from surgically resected specimens and promptly stored at −80°C until RNA extraction. Total RNA was extracted from frozen samples, and GC cell lines using the AllPrep® DNA/RNA/miRNA universal kit (Qiagen) following the manufacturer’s instructions. Tissue RNA concentration was determined using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific).
2.4. Microarray analysis
CircRNAs differentially expressed between GC and the adjacent normal tissues were examined using the Arraystar Human Circular RNA Array Service (Arraystar Inc). A single channel microarray (GEO platform ID: GPL21825) was used in this service. Details of the circRNA array data were registered in GSE141977.
2.5. Real‐time quantitative PCR for circERBB2 levels
To assess the circERBB2 levels in the plasma, tissue, and GC cell lines, we performed a real‐time quantitative PCR (rqPCR) analysis. Concentration in plasma was quantified by substituting the respective C t values into the obtained mathematical formula of the calibration curve. Gene expression in tissue and GC cell lines was normalized to the housekeeping gene glyceraldehyde 3‐phosphate dehydrogenase (GAPDH; Hs02786991_g1; Applied Biosystems). The designed primer sequences of circERBB2 and the corresponding mRNA ERBB2 (linERBB2) were as follows: circERBB2 (forward): 5′‐TGCTCCACACTGCCAAC‐3′, (probe): 5′‐TGCTCCACACTGCCAAC‐3′, (reverse): 5′‐GACAATCCTCAGAACTCTCTCC‐3′; linERBB2 (forward): 5′‐TACAGCGGTACAGTGAGGA‐3′, (probe): 5′‐CCTGCCCTCTGAGACTGATGGCTA‐3′, (reverse): 5′‐TGGCTGGTTCACATATTCAGG‐3′.
Reverse transcription was performed in a 20‐μL reaction volume with RNA isolated from 400 μL of the plasma sample or 2000 ng of RNA from the tissue or cell line sample using Superscript® IV Reverse Transcriptase (Thermo Fisher Scientific) in accordance with the manufacturer’s instructions. The complementary DNA was subjected to rqPCR using the Eagle Taq Master Mix (ROX) (Roche Molecular Systems) and the above‐designed primers for circERBB2 or linERBB2. rqPCR was run using the Step One Plus Real‐Time PCR System (Applied Biosystems) under the following conditions: 10 min at 95°C followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. The circERBB2 levels in tissue and GC cell lines were calculated using the ΔΔC t method relative to GAPDH. For quantification of circERBB2 and linERBB2 concentration in plasma, synthesized gene fragments, gBlocks (Integrated DNA Technologies), were used as the rqPCR standards.
2.6. Direct sequencing analysis
To detect the circERBB2 sequence, we performed rt‐PCR using PrimeSTAR® HS DNA polymerase (TaKaRa Bio) in duplicates with 10 µg cDNA made from the cell line sample using the StepOne rt‐PCR systems (Thermo Fisher Scientific). The accurate length of the PCR products was confirmed using agarose gel electrophoresis. Images were obtained using the BLooK LED transilluminator (BIOHELIX) and a digital camera. PCR products were subjected to direct sequencing using the BigDye Terminator v1.1 Cycle Sequencing Kit (Thermo Fisher Scientific). Samples were run on a ABI 3500 genetic analyzer (Applied Biosystems).
2.7. Quantitative comparison of circERBB2 and linERBB2 expression
To construct the calibration curve for the PCR efficiency of circERBB2 and linERBB2, we used gBlocks Gene Fragment with stepwise 10‐fold dilution. The gene expression of circERBB2 and linERBB2 in each cell line was quantified by substituting the respective C t values into the obtained mathematical formula, and the expression ratio of circERBB2 and linERBB2 was calculated.
2.8. RNase R resistance assay
Total RNA (10 µg) was incubated with or without RNase R (Applied Biological Materials Inc). The RNase R mixture was made using 2 µL of RNase R (20 U), 2 µL of 10× RNase R buffer, and 1 µL of RNase inhibitor (Applied Biosystems); 5 µL of RNase‐free water (control) or RNase R mixture (test) was added to 10 µg of RNA and RNase‐free water in a total volume of 20 µL and then incubated at 37°C for 1.5 h. After the reaction, the products were immediately placed on ice and examined in an rt‐PCR assay.
2.9. Statistical analysis
To assess the relationships between circERBB2 expression in the plasma and the clinicopathological factors, we used the chi‐square or Fisher exact test. To assess the relationships between the concentration of circERBB2 in the plasma and expression of circERBB2 in the tumor tissue, we determined Spearman correlation coefficients. To compare the expression of circERBB2 and linERBB2 in preoperative plasma or the concentration of circERBB2 in preoperative and postoperative plasma in the same patients, we used a non‐parametric test (Wilcoxon signed‐rank test). Conversely, we used a parametric test (Mann‐Whitney U test) to compare the concentration of circERBB2 in plasma between patients with recurrent cancer and recurrence‐free patients. OS and RFS rates were calculated using Kaplan‐Meier analysis, with the date of gastrectomy designated as the starting point. Multivariate analysis was performed using the Cox proportional‐hazard model for factors that were significant prognostic factors in the univariate analysis. All statistical analyses were performed using JMP v.13 (ASA Institute). All statistical tests, except for paired tests, were two‐sided. Statistical significance was accepted at P < .05.
3. RESULTS
3.1. Biological structure and stability of circERBB2 in plasma
The circRNA array data (GSE141977) revealed that hsa_circRNA_405576 was the most highly expressed circRNA subtype. Table S1 shows a part of the microarray data. Hsa_circRNA_405576 is derived from exon 5‐12 regions of the ERBB2 locus and named as circERBB2 (Figure S1). Sanger sequencing showed that the 3′ end of exon 12 and the 5′ end of exon 5 formed a head‐to‐tail junction in GC cells (Figure 1A). circERBB2 expression was more stable against treatment with RNase R than linERBB2 expression in rqPCR (Figure 1B). Furthermore, circERBB2 concentrations were significantly higher than linERBB2 in the plasma (P < .001; Figure 1C). circERBB2 could be detected in 52.9% (37/70 patients) of the preoperative plasma samples, although linERBB2 could be detected only in 7.1% (5/70 patients). In the selected 6 patients whose circERBB2 was detectable in preoperative plasma, the amounts of circERBB2 in bulk plasma and exosome were compared. Exosomal circERBB2 could be detected in 4 patients, however the concentration was lower and the distributions were very different in each sample (Figure 1D).
FIGURE 1.
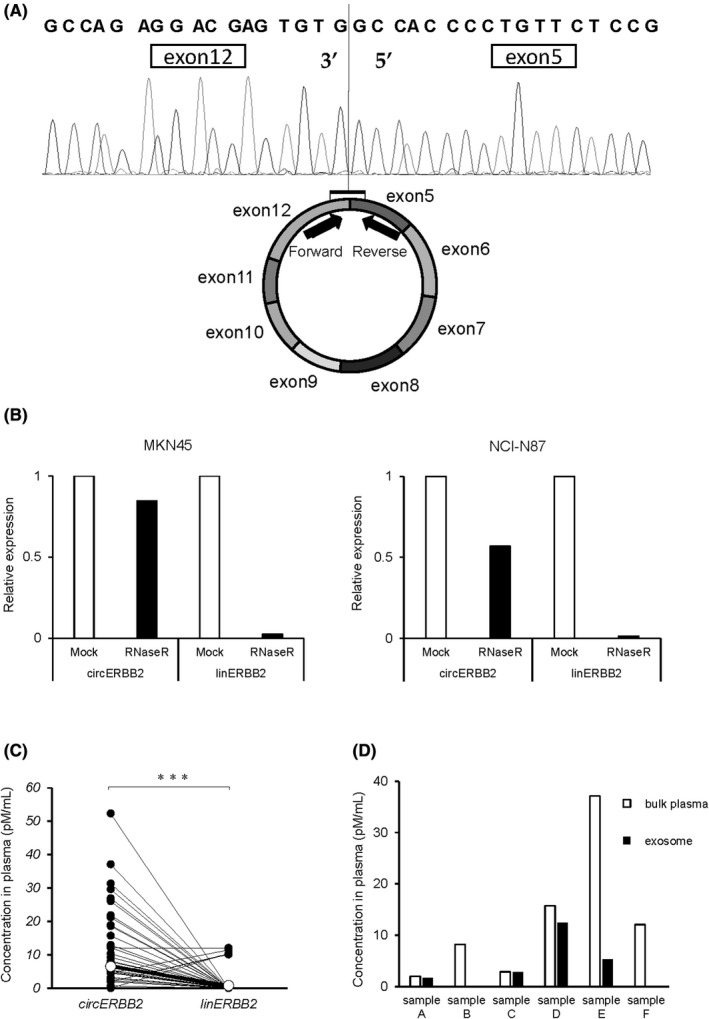
Biological characteristics of circERBB2. A, Schematic showing that circERBB2 was derived from ERBB2 exons 5–12. The fusion sequence was detected using a direct sequence. B, Levels of circERBB2 and linERBB2 treated with RNase R were shown. The RNAs were isolated from GC cells and examined by rqPCR. C, Comparison of the concentrations of circERBB2 and linERBB2 in the plasma samples of the patients with gastric cancer. The levels were determined and quantified by rqPCR and the calibration curve. The differences were calculated by paired t test. D, Comparison of concentration of circERBB2 in bulk plasma and exosome extracted from same volume of plasma
MKN7 and NCI‐N87, which are ERBB2‐amplified cell lines, showed higher expression of circERBB2 and linERBB2 than the other cell lines (Figure S2A). Quantitative comparison analysis using the standard curve revealed that the ratios of circERBB2 to linERBB2 expression in GC cell lines were 0.74%‐4.10% (Figure S2B). These results confirmed that circERBB2 was circular and was highly stable in the cell or plasma.
3.2. Preoperative plasma circERBB2 levels and clinical data
The 70 patients were divided into 2 groups based on the detection of preoperative plasma circERBB2: detected (high) and not detected (low) groups. In our assay, maximum C t and ΔC t cutoff values for positive determination were 38 and 10, respectively. The high group was significantly correlated with lymph node metastasis (P = .035) and tended to be correlated with men (P = .069; Table 1). The high group showed significantly poor prognosis in RFS and OS (P = .001 and P = .009, respectively; log‐rank test, Figure 2A,B). The Cox proportional‐hazard model revealed that high plasma circERBB2 levels were an independent prognostic factor of RFS (P = .038; Table 2). Recurrence patterns were peritoneal dissemination in 10 patients, lymph node metastasis in 5 patients, and hematogenous metastasis in 3 patients (Table S2). No correlations were observed between recurrence patterns and the plasma circERBB2 levels.
TABLE 1.
Correlations between clinicopathological characteristics and the amounts of preoperative plasma circERBB2
| Variables | High | Low | P‐value a |
|---|---|---|---|
| n = 37 | n = 33 | ||
| Age | |||
| <70 | 18 (48.6%) | 12 (36.4%) | .341 |
| 70≤ | 19 (51.4%) | 21 (63.6%) | |
| Sex | |||
| M | 30 (81.1%) | 20 (60.6%) | .069 |
| F | 7 (18.9%) | 13 (39.4%) | |
| BMI | |||
| 22.0≤ | 15 (40.5%) | 15 (45.5%) | 0.810 |
| <22.0 | 22 (59.5%) | 18 (54.5%) | |
| Macroscopic appearance | |||
| Localized | 15 (40.5%) | 11 (33.3%) | .623 |
| Diffuse | 22 (59.5%) | 22 (66.7%) | |
| Tumor size (mm) | |||
| <50 | 13 (35.1%) | 10 (30.3%) | .800 |
| 50≤ | 24 (64.9%) | 23 (69.7%) | |
| Histopathology b | |||
| Differentiated | 21 (56.8%) | 16 (48.5%) | .632 |
| Undifferentiated | 16 (43.2%) | 17 (51.5%) | |
| pT c | |||
| 1 | 6 (16.2%) | 7 (21.2%) | .760 |
| 2‐4 | 31 (83.8%) | 26 (78.8%) | |
| pN c | |||
| 0 | 6 (16.2%) | 13 (39.4%) | .035 |
| 1‐3 | 31 (83.8%) | 20 (60.6%) | |
| pM c | |||
| 0 | 34 (91.9%) | 30 (90.9%) | 1.000 |
| 1 | 3 (8.1%) | 3 (9.1%) | |
| ly d | |||
| 0 | 5 (13.5%) | 7 (21.2%) | .528 |
| 1‐3 | 32 (86.5%) | 26 (78.8%) | |
| v d | |||
| 0 | 7 (18.9%) | 9 (27.3%) | .570 |
| 1‐3 | 30 (81.1%) | 24 (72.7%) | |
| IHC score of ERBB2 e | |||
| 0‐1 | 25 (75.8%) | 22 (75.9%) | 1.000 |
| 2‐3 | 8 (24.2%) | 7 (24.1%) | |
| Unknown | 4 | 4 | |
Abbreviation: BMI, body mass index.
Analyzed by chi‐square or Fisher exact test.
Most undifferentiated histopathological finding.
Disease stage was defined in accordance with the International Union Against Cancer 8th tumor‐lymph node‐metastases classification using surgical‐pathologic findings.
Japanese classification of gastric carcinoma (15th).
Staining for ERBB2 protein.
FIGURE 2.
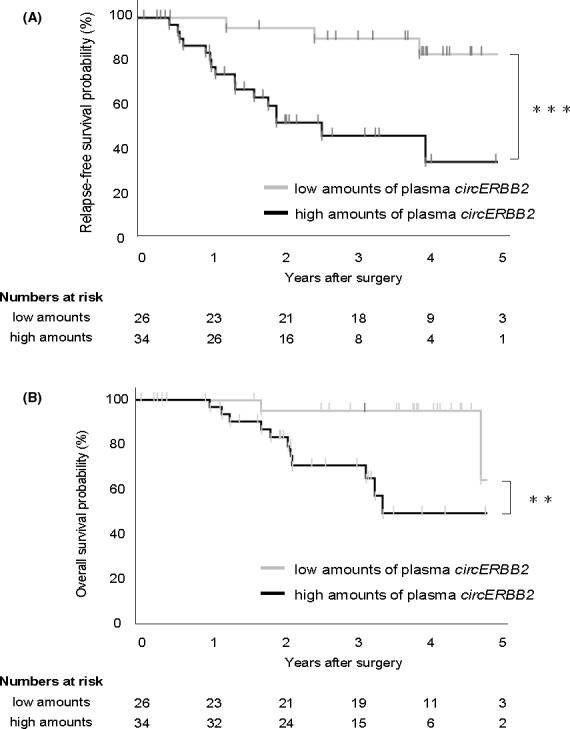
Prognostic impact of the plasma levels of circERBB2 in patients with GC. A, Kaplan‐Meier curves for relapse‐free survival rates of 60 patients with GC in accordance with the levels of plasma circERBB2. The log‐rank test was used for statistical analysis. B, Kaplan‐Meier curves for overall survival rates of 60 patients with GC in accordance with the levels of plasma circERBB2. The log‐rank test was used for statistical analysis
TABLE 2.
Univariate and multivariate analysis for clinicopathologic characteristics affecting prognosis
| Variables | RFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| P‐value a | Odds ratio | P‐value b | 95% CI | P‐value a | Odds ratio | P‐value b | 95% CI | |
| High amounts of cirERBB2 in plasma (vs low amounts) | .001 | 3.99 | .038 | 1.07‐25.90 | .009 | 4.96 | .066 | 0.92‐92.68 |
| Age 70 ≤ (vs < 70) | .761 | .713 | ||||||
| Male (vs female) | .539 | .409 | ||||||
| BMI < 22.0 (vs 22.0≤) | .783 | .763 | ||||||
| Localized appearance (vs Diffuse appearance) | .428 | .960 | ||||||
| Tumor size (mm) 50 ≤ (vs < 50) | .089 | .015 | 10.06 | .005 | 1.82‐189.77 | |||
| Differentiated type (vs undifferentiated type) | .420 | .878 | ||||||
| T stage pT2‐4 (vs pT1) | .062 | .352 | ||||||
| N stage pN1‐3 (vs pN0) | .001 | 1.7711e+9 | .015 | 1.66‐ | .014 | 4.022e+8 | .108 | 0.60‐15.23 |
| ly 1‐3 (vs 0) | .040 | 1.23 | 1.000 | 9.51e‐309‐0.04 | .140 | |||
| v 1‐3 (vs 0) | .058 | .367 | ||||||
| IHC score of ERBB2 2+/3+ (vs 0/1+) | .024 | 1.78 | .263 | 0.63‐4.75 | .048 | 2.95 | .103 | 0.80‐11.15 |
Analyzed by log‐rank test.
Analyzed using Cox proportional‐hazard model.
3.3. Dynamics of the plasma circERBB2
In 16 of 37 patients whose preoperative plasma circERBB2 was detectable by rqPCR testing, a comparative analysis between the preoperative and postoperative plasma samples showed a significant decrease of the plasma circERBB2 in postoperative plasma samples (P < .001). Plasma circERBB2 was not detectable in 13 postoperative plasma samples (Figure 3A). In the other 21 patients, because of refusal of blood collection or complications, the postoperative samples could not be collected.
FIGURE 3.
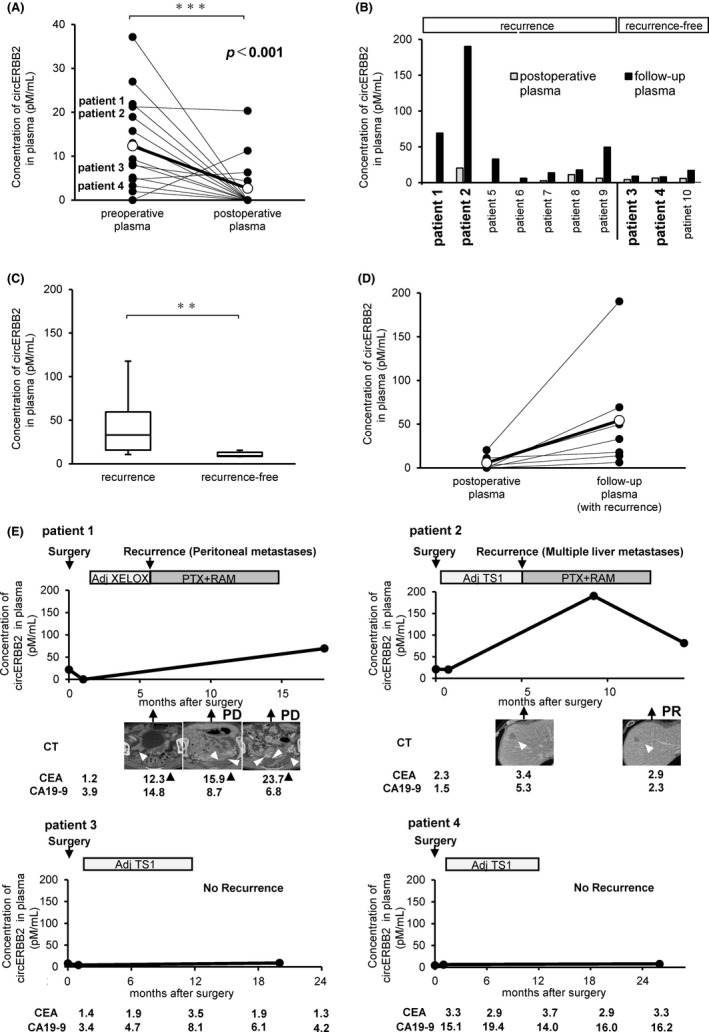
Alterations in the levels of plasma circERBB2 in the clinical courses of patients with GC. A, Comparison of the plasma levels of circERBB2 before and after the surgery in 16 patients with GC. Note that decreases in plasma circERBB2 concentrations were observed in most patients with plasma circERBB2 expression before the surgery. The levels were determined by rqPCR. The differences were calculated by paired t test. Postoperative: 1 mo after surgery. Follow‐up: at the time of recurrence or approximately 2 y after surgery in patients without recurrence. B, Concentration of circERBB2 in the postoperative and follow‐up plasma of 10 patients. C, Comparison of concentration of circERBB2 in the follow‐up plasma between 7 patients with recurrent cancer and 3 recurrence‐free patients. D, Comparison of concentration of circERBB2 between the postoperative and follow‐up plasma in the 7 patients with recurrent cancer. E, Alterations in the plasma concentration of circERBB2 and clinical information of the clinical courses of patients with GC. All cases were treated with postoperative adjuvant chemotherapy following curative resection
Postoperative and follow‐up plasma samples were obtained from 10 patients: 7 patients with recurrent cancer and 3 recurrence‐free patients (Figure 3B). At the time of recurrence, follow‐up plasma was collected in the patients with recurrent cancer and almost 2 y after surgery in recurrence‐free patients. The concentration of circERBB2 in the follow‐up plasma was significantly higher in patients with recurrent cancer than those recurrence‐free patients (P < .001, Figure 3C). In all 7 patients with recurrent cancer, the concentration of circERBB2 in follow‐up plasma increased compared with that in postoperative plasma (Figure 3D). However, they were not different in recurrence‐free patients.
We also assessed the clinical courses of 4 patients, who were followed up for a long time with repeated measurements of plasma circERBB2 concentrations (Figure 3E and Table 3). In 2 patients with tumor recurrence after adjuvant chemotherapy (patients 1 and 2), the plasma circERBB2 concentration increased markedly at recurrence, whereas in 2 patients (patients 3 and 4) without tumor recurrence, the plasma circERBB2 concentration hardly changed in the repeated measurements during the treatment progress. In these patients, plasma circERBB2 was detected before surgery, but the plasma circERBB2 levels decreased after the surgery, and CEA and CA19‐9 levels were found to be less than the cutoff values in all the clinical courses. Thus, plasma circERBB2 can be a sensitive and reproducible marker for detecting GC recurrence.
TABLE 3.
Clinicopathologic characteristics and treatment of 4 patients followed up
| Patient no. | Sex | Age (y) | cT a | cN a | cM a | Gastorectomy | pT a | pN a | pM a | Adjuvant chemotherapy | Recurrence | Treatment after recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 74 | 3 | 1 | 0 | Total | 2 | 3a | 0 | XELOX (6 mo) | Peritoneal metastasis | PTX and RAM |
| 2 | Male | 73 | 2 | 0 | 0 | Distal | 3 | 1 | 0 | TS‐1 (6 mo) | Multiple liver metastases | PTX and RAM |
| 3 | Male | 69 | 2 | 0 | 0 | Distal | 2 | 2 | 0 | TS‐1 (12 mo) | None | None |
| 4 | Male | 65 | 3 | 2 | 0 | Distal | 4a | 2 | 0 | TS‐1 (12 mo) | None | None |
Abbreviations: PTX, paclitaxel; RAM, ramucirumab; TS‐1, tegafur, gimeracil, and oteracil potassium combination; XELOX, capecitabine and oxaliplatin.
According to the International Union Against Cancer 8th tumor‐lymph node‐metastases classification.
4. DISCUSSION
Recently, several circRNAs have been identified because of the rapid development of high‐throughput sequencing, and bioinformatics. 9 , 10 circRNAs could be ideal biomarkers for cancer diagnosis due to their marked stability. 11 , 15 , 16 , 17 Plasma sample‐based liquid biopsies have significant advantages in reflecting gastrointestinal diseases and can be used for the noninvasive screening of biomarkers, thereby revolutionizing disease management. 16 To investigate the clinical significance of plasma circERBB2 in GC patients, we used rqPCR assays.
Plasma circERBB2 levels can be affected by multiple factors. Plasma levels of linERBB2 and circERBB2 increased depending on the pre‐mRNA levels of ERBB2, which is the raw material of linERBB2 and circERBB2. These may also depend on the stable formation of circERBB2 and transfer into the blood. Moreover, our study showed that high circERBB2 levels in the plasma were associated with poor prognosis, therefore plasma circERBB2 could be considered as a useful prognostic marker.
The presence of circERBB2 (high group) showed a significant correlation with lymph node metastasis, therefore circERBB2 may modulate the metastatic potential of the tumor. circERBB2 expression in GC tissues has been reported to promote GC progression. 18 However, in our study, circERBB2 knockdown, unfortunately, did not change the behavior of the GC cells (data not shown), therefore the function of circERBB2 could not be revealed in this study. Further studies, including the target miRNAs of circERBB2, may be necessary for the future. Plasma circERBB2 may sponge some miRNAs and regulate tumor growth or metastasis.
Expression levels of circERBB2 and linERBB2 were higher in MKN7 and NCI‐N87 cell lines, and the levels of circERBB2 (P = .016) and linERBB2 (P = .007) in tumor tissues were significantly correlated with the IHC score (Table S3). These results may support the idea that ERBB2 protein expression is correlated with linERBB2 and circERBB2 expression in tissues. There was no correlation, however, between plasma circERBB2 levels and IHC results in the tissues, although there was a significant correlation between the expression levels of circERBB2 in plasma and tumor tissue (Figure S3). Therefore, the exact reasons for the increase in plasma circERBB2 levels are unclear.
The expression ratio of circERBB2/linERBB2 for each cell line varied from 0.74% to 4.10%, and the formation of the circERBB2 structure may vary between each patient or tissue. The mechanism of circRNA formation is still unclear, although several studies have provided some information. A study showed that adenosine deaminase acting on RNA (ADAR1) is a regulator of circRNA formation, and the androgen receptor differentially regulated circRNA expression in hepatocellular carcinoma through ADAR1. 22 ADAR1 is commonly overexpressed in esophageal, liver, breast, and lung cancers or chronic myelogenous leukemia, and promotes cancer progression through the regulation of type I interferon. 23 Furthermore, exosomes may be involved in the stable secretion of circRNA into the plasma. 24 Various molecules are involved in exosome biosynthesis and release, and there will be individual differences in the release of exosomes and the contained non‐coding RNAs, including circRNAs. 25 In our study, the ratio of exosomal circERBB2 to plasma circERBB2 was relatively low and varied widely. The probability of circERBB2 formation or transfer into plasma could be increased by some mechanisms in patients with poor prognosis and therefore further analysis is needed to elucidate these mechanisms.
We showed that circERBB2 released into the plasma could be more stably detected than linERBB2. The circERBB2 level in the plasma was only a small percentage compared with linERBB2 levels. Thus, circRNAs have advantages as biomarkers because of their structural stability. ERBB2 protein expression is heterogeneous in tumor tissues, 26 , 27 and circERBB2 expression may also be heterogeneous in these tissues, therefore plasma circERBB2 concentrations may reflect the accurate expression status of circERBB2 in tumor tissues because plasma levels will be less susceptible to tissue heterogeneity.
This study had several limitations. The small patient population and the retrospective nature limited us from assertively stating any concrete conclusion regarding the effectiveness of plasma circERBB2 as a prognostic biomarker for GC. Further studies using large sample sizes and a prospective design are needed. In this study, the high or low group of plasma circERBB2 was defined depending on the detection of circERBB2 in the plasma by qPCR, but it is necessary to increase the sensitivity of plasma circERBB2 detection and judge the levels accurately. A droplet digital PCR may be useful for accurate quantification of plasma circRNA in clinical applications. Finally, we could not reveal how circERBB2 is involved in the pathology of GC, and further functional analysis is required to elucidate the roles of plasma circERBB2 in the prognosis. Prospective clinical trials of several plasma circRNAs may expand the possibility of finding useful plasma circRNA biomarkers.
Thus, plasma circERBB2 can be a useful noninvasive biomarker for the prognosis and monitoring of tumor dynamics in GC patients.
CONFLICT OF INTEREST
The authors have no conflicts of interest.
Supporting information
Fig S1
Fig S2
Fig S3
Table S1
Table S2
Table S3
ACKNOWLEDGMENTS
All authors contributed to the final manuscript.
Nanishi K, Konishi H, Shoda K, et al. Circulating circERBB2 as a potential prognostic biomarker for gastric cancer: An investigative study. Cancer Sci. 2020;111:4177–4186. 10.1111/cas.14645
REFERENCES
- 1. World Health Organization . 2018. https://www.who.int/news‐room/fact‐sheets/detail/cancer. Accessed April 8, 2020
- 2. Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362:305‐315. [DOI] [PubMed] [Google Scholar]
- 3. Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834‐838. [DOI] [PubMed] [Google Scholar]
- 5. Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857‐866. [DOI] [PubMed] [Google Scholar]
- 6. Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997‐1006. [DOI] [PubMed] [Google Scholar]
- 8. Pardini B, Sabo AA, Birolo G, Calin GA. Noncoding RNAs in extracellular fluids as cancer biomarkers: the new frontier of liquid biopsies. Cancers (Basel) 2019;11(8):1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hsiao KY, Sun HS, Tsai SJ. Circular RNA – new member of noncoding RNA with novel functions. Exp Biol Med. 2017;242(11):1136‐1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li X, Yang L, Chen LL. The biogenesis, functions, and challenges of circular RNAs. Mol Cell. 2018;71:428‐442. [DOI] [PubMed] [Google Scholar]
- 11. Li L, Han L. Circular RNAs as promising biomarkers in cancer: detection, function, and beyond. Genome Med. 2019;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akhter R. Circular RNA and Alzheimer's disease. Adv Exp Med Biol. 2018;1087:239‐243. [DOI] [PubMed] [Google Scholar]
- 13. Hansen TB, Kjems J, Damgaard CK. Circular RNA and miR‐7 in cancer. Cancer Res. 2013;73:5609‐5612. [DOI] [PubMed] [Google Scholar]
- 14. Kristensen LS, Hansen TB, Venø MT, Kjems J. Circular RNAs in cancer: opportunities and challenges in the field. Oncogene. 2018;37:555‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meng X, Li X, Zhang P, Wang J, Zhou Y, Chen M. Circular RNA: an emerging key player in RNA world. Brief Bioinform. 2017;18:547‐557. [DOI] [PubMed] [Google Scholar]
- 16. Lin J, Cai D, Li W, et al. Plasma circular RNA panel acts as a novel diagnostic biomarker for colorectal cancer. J Transl Med. 2019;74:60‐68. [DOI] [PubMed] [Google Scholar]
- 17. Liu XX, Yang YE, Liu X, et al. A two‐circular RNA signature as a noninvasive diagnostic biomarker for lung adenocarcinoma. J Transl Med. 2019;17:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li X, He M, Guo J, Cao T. Upregulation of circular RNA circERBB2 predicts unfavorable prognosis and facilitates the progression of gastric cancer via miR‐503/CACUL1 and miR‐637/MMP‐19 signaling. Biochem Biophys Res Commun. 2019;511:926‐930. [DOI] [PubMed] [Google Scholar]
- 19. Huang X, He M, Huang S, et al. Circular RNA circERBB2 promotes gallbladder cancer progression by regulating PA2G4‐dependent rDNA transcription. Mol Cancer. 2019;18:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brierley JD, Gospodarowicz MK, Wittekind C, editors. Union for International Cancer Control. TNM Classification of Malignant Tumors, 8th edn New York, NY: Wiley, 2017. [Google Scholar]
- 21. Tomita H, Ichikawa D, Ikoma D, et al. Quantification of circulating plasma DNA fragments as tumor markers in patients with esophageal cancer. Anticancer Res. 2007;27:2737‐2742. [PubMed] [Google Scholar]
- 22. Shi L, Yan P, Liang Y, et al. Circular RNA expression is suppressed by androgen receptor (AR)‐regulated adenosine deaminase that acts on RNA (ADAR1) in human hepatocellular carcinoma. Cell Death Dis. 2017;8:e3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu LD, Öhman M. ADAR1 editing and its role in cancer. Genes. 2018;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Y, Zheng Q, Bao C, et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25:981‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kurokawa Y, Matsuura N, Kimura Y, et al. Multicenter large‐scale study of prognostic impact of HER2 expression in patients with resectable gastric cancer. Gastric Cancer. 2015;18:691‐697. [DOI] [PubMed] [Google Scholar]
- 27. Nishida Y, Kuwata T, Nitta H, et al. A novel gene‐protein assay for evaluating HER2 status in gastric cancer: simultaneous analyses of HER2 protein overexpression and gene amplification reveal intratumoral heterogeneity. Gastric Cancer. 2015;18:458‐466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Table S1
Table S2
Table S3


