Abstract
Mucoepidermoid carcinoma (MEC) is rare, but the most common primary malignancy of the salivary gland and not infrequent in young individuals. CRTC1/3‐MAML2 fusions are frequently detected in MEC and are useful as a diagnostic biomarker. However, there has been debate as to whether the fusions have prognostic significance. In this study, we retrospectively collected 153 salivary gland MEC cases from 11 tertiary hospitals in Japan. As inclusion criteria, the MEC patients in this study had curative surgery as the initial treatment, received no preoperative treatment, and had no distant metastasis at the time of the initial surgery. The MEC diagnosis was validated by a central pathology review by five expert salivary gland pathologists. The CRTC1/3‐MAML2 fusions were detected using FISH and RT‐PCR. In 153 MEC cases, 90 (58.8%) were positive for CRTC1/3‐MAML2 fusions. During the follow‐up period, 28 (18.3%) patients showed tumor recurrence and 12 (7.8%) patients died. The presence of the fusions was associated with favorable tumor features. Of note, none of the fusion‐positive patients died during the follow‐up period. Statistical analysis showed that the presence of the fusions was a prognostic indicator of a better overall survival in the total and advanced‐stage MEC cohorts, but not in the early‐stage MEC cohort. In conclusion, CRTC1/3‐MAML2 fusions are an excellent biomarker for favorable overall survival of patients with salivary gland MEC.
Keywords: CRTC1/3‐MAML2, mucoepidermoid carcinoma, NCCN Guidelines, salivary gland, survival analysis
Debate exists as to whether CRTC1/3‐MAML2 fusions have a prognostic significance in salivary mucoepidermoid carcinoma (MEC). We undertook a multiinstitutional study including 153 MEC cases, and found that the fusions were an excellent biomarker for better overall survival in the total and advanced‐stage MEC cohorts.

1. INTRODUCTION
Mucoepidermoid carcinoma (MEC) is rare, accounting for less than 1% of all head and neck cancers. 1 However, this carcinoma is the most common of the salivary gland and not rare in young individuals. Patients with MEC often have a favorable outcome but some patients follow a poor clinical course. 1 , 2 The diagnosis of MEC is sometimes difficult, especially in intermediate/high‐grade MEC cases, which must be distinguished from carcinomas with focal mucous and squamous cell differentiation. 1 , 2 , 3 In the clinical setting, the National Comprehensive Cancer Network (NCCN) Guidelines (version 1.2020) 4 for Salivary Gland Tumors are often used for the treatment of MEC patients. In these guidelines, tumors are divided into pT1/2 and pN0 tumors and pN1‐3 or pT3/4 tumors. The guidelines recommend postoperative therapy, including radiotherapy and systemic therapy, for completely resected tumors when adverse features (tumor spillage, neural/perineural invasion, close/positive margins, intermediate/high‐grade histology, lymphatic/vascular invasion, T3/4, and lymph node metastasis) are present.
More than half of and approximately 5% of salivary gland MECs have been associated with the recurring fusion genes, CRTC1‐MAML2 and CRTC3‐MAML2, respectively. 5 , 6 , 7 These genes activate the CREB pathway, and initiate a critical mechanism for cell transformation and activation of cAMP/CREB target genes. 8 , 9 , 10 These fusions are highly specific to MEC and are a useful diagnostic marker, especially in problematic cases. 1 , 11 , 12 However, the absence of the fusions does not exclude a diagnosis of MEC, and histologically typical MEC cases are also frequently negative for these fusions.
Whether the presence of CRTC1/3‐MAML2 fusions is associated with a favorable clinical course has been a subject of debate. The results of initial studies on this subject were reported in 2006 by Behboudi et al and Okabe et al, and showed that patients with the CRTC1‐MAML2 fusion had a significantly better chance of survival than those without the fusion. 11 , 13 Subsequently, several other studies also reported a survival advantage for CRTC1/3‐MAML2 fusions in patient groups of comparable or smaller size. 7 , 14 , 15 , 16 , 17 , 18 A recent meta‐analysis for the prognostic significance of the fusions supports the above findings. 19 However, some research groups have reported an opposing conclusion as to the prognostic significance of CRTC1/3‐MAML2 fusions in salivary gland MEC in patient groups of comparable or smaller size. 20 , 21 , 22 , 23
In this retrospective study, we collected 153 MEC cases and sought to evaluate the clinicopathologic significance of the CRTC1/3‐MAML2 fusions. The number of advanced‐stage MEC cases exceeded that of early‐stage MEC cases. As early‐stage MEC tumors often show an excellent prognosis after surgery, we speculated that the clinicopathologic impact of fusions would be more significant in the advanced‐stage than in the early‐stage tumors.
2. MATERIALS AND METHODS
2.1. Case selection
We retrospectively collected salivary gland MEC cases from the following 11 tertiary hospitals in Japan: Nagoya City University Hospital, the International University of Health and Welfare Mita Hospital, Aichi‐Gakuin University Hospital, Kobe University Hospital, Hokkaido University Hospital, Tokyo Medical University Hospital, Tokai University Hospital, Aichi Cancer Center Hospital, Osaka Medical University Hospital, Kansai Medical College Hospital, and Kyushu University Hospital. The present study was approved by the Institutional Ethics Review Board of Nagoya City University Hospital and each of the 10 institutions that participated in this study. Considering that advanced‐stage MEC cases are usually rare, both early‐ and advanced‐stage tumors were collected from the former four hospitals, and advanced‐stage tumors were preferentially collected from the latter seven hospitals. The clinical data were retrieved from all patients. As inclusion criteria, MEC patients had curative surgery as the initial treatment, received no preoperative treatment, and had no distant metastasis at the time of the initial surgery (clinically M0). A central pathology review by five Japanese pathologists specializing in salivary gland tumors (Drs. T. Murase, K. Kusafuka, M. Urano, H. Yamamoto, and H. Inagaki) was aided by precise immunohistochemical and molecular evaluation. The presence of intracytoplasmic mucin was identified in all high‐grade MEC cases. The tumors were histologically evaluated by the Armed Forces Institute of Pathology grading systems for MEC, 24 and classified as low‐, intermediate‐, or high‐grade tumors. We also histologically evaluated surgical margins and neural/perineural tumor invasion in each case. After pathological assessment of the resected tumors, MEC cases were evaluated according to the UICC TNM classification and staging system (2017, 8th edition). 25 Subsequently, MEC cases were divided into pT1/2 and pN0 (designated here as early stage) tumors and pN1‐3 or pT3/4 (designated here as advanced stage) tumors according to the NCCN Guidelines (version 1.2020). 4
2.2. Detection of CRTC1/3‐MAML2 Fusions by FISH and RT‐PCR
We undertook FISH analysis for the MAML2 gene split as we previously described. 26 In brief, formalin‐fixed, paraffin‐embedded specimens, cut at 4 micrometers, were deparaffinized, and digested in a proteinase buffer at 37°C. Sections were subjected to denaturation and incubated with a MAML2 break‐apart probe (Zytovision) overnight at 37°C in a humid chamber. After posthybridization washes, sections were stained with diaminophenilindole and mounted. When two signals (a red and a green) were separately observed in a tumor cell, we considered the MAML2 gene to be split. The samples were considered positive if more than 10% (mean + 3SD, rounded up) of examined nuclei showed abnormal signals. The MEC cases known to possess CRTC1/3‐MAML2 fusions and normal parotid glands were used as positive and negative controls, respectively.
All MEC cases were also analyzed for the CRTC1‐MAML2 and CRTC3‐MAML2 fusion transcripts by RT‐PCR, as we described elsewhere. 7 , 26 Briefly, total RNA extracted from paraffin tumor sections was heated to 70°C. An RT‐PCR mixture containing outer primers was added. The thermocycler was programmed for 10 minutes at 42°C for an initial RT and then for 10 minutes at 95°C for inactivation of reverse transcriptase as well as for activation of the DNA polymerase. After 35 cycles of PCR, the products were subjected to a 35‐cycle nested PCR with inner primers. The MEC cases known to harbor the CRTC1‐MAML2 or CRTC3‐MAML2 fusion transcripts were used as positive controls. Representative images of the molecular analysis are shown in Figure S1.
2.3. Statistical analysis
The association of the presence of CRTC1/3‐MAML2 fusions and clinicopathologic factors was analyzed using Fisher’s exact test and the Mann‐Whitney U test. We analyzed overall survival (OS) and disease‐free survival (DFS). Overall survival was defined as the interval between the beginning of treatment and the date of death or the last follow‐up, and DFS was defined as the interval between the beginning of treatment to the date of relapse, as evaluated and recorded by the attending physician. The association of clinicopathologic factors with OS and DFS was analyzed by the Kaplan‐Meier method and univariate Cox proportional hazards model. The measure of association in this study was the hazard ratio with a 95% confidence interval. All statistical analyses were carried out using the statistical package JMP version 14.0 (SAS Institute). All tests were two‐sided, and a P‐value of <.05 was considered statistically significant.
3. RESULTS
3.1. Total MEC cohort (N = 153)
A total of 209 salivary tumor cases originally diagnosed as MEC were retrieved from 11 Japanese reference hospitals. After the central pathology review, the diagnosis of MEC was reconfirmed in 199 cases, but the original diagnosis was changed to non‐MEC in the remaining 10 (five cases were rediagnosed as squamous cell carcinoma, three as salivary duct carcinoma, and one each as clear cell carcinoma and adenocarcinoma‐not otherwise specified). Subsequently, 44 cases were excluded due to insufficient clinical data, one due to preoperative therapy, and one due to noncurative surgery. Finally, 153 MEC patients were included in this study. All patients had curative surgery as the initial treatment, received no preoperative treatment, and had no distant metastasis at the time of the initial surgery. The patient characteristics are shown in Table S1.
According to the NCCN Guidelines (version 1.2020), 4 MEC patients were divided into two groups, early stage (pT1/2 and pN0, 69/153) and advanced stage (pN1‐3 or pT3/4, 84/153). Adverse features were present in 107/153 patients, and 25 patients received postoperative therapy. The follow‐up period ranged from 2 to 320 months (median, 41). During the follow‐up period, 28 patients showed tumor recurrence (18 locoregional, 8 nodal, and 2 distant), and the 26 locoregional or nodal recurrences were surgically treated. At the last follow‐up, 141 patients were alive, 9 had died a tumor‐related death, and 3 had died of other causes. The OS rate was 89.3% at both 5 and 10 years of follow‐up, respectively. The DFS rates at 5 and 10 years of follow‐up were 79.5% and 71.0%, respectively (Figure S2).
Using the FISH technique, gene splits in MAML2 genes were detected in 90/153 (58.8%) MEC cases. The RT‐PCR assay carried out in all cases showed that the CRTC1‐MAML2 and CRTC3‐MAML2 fusion transcripts were present in 85/153 (55.6%) and 5/153 (3.3%), respectively. Consequently, 90/153 (58.8%) cases were positive for CRTC1/3‐MAML2 fusions. The remaining 63 cases were negative in both FISH and RT‐PCR assays. The presence of the fusions was associated with a lower age, a pT1/2 classification, a pN0 classification, an early clinical stage, a lower histological grade, not having undergone neck dissection, and not having postoperative therapy (Table S2).
In the 12 patients who died during the follow‐up period, the fusion‐positive and ‐negative cases numbered 0 and 12, respectively. In the prognostic analysis for OS, male gender, the absence of the fusions, a T3/4 classification, a pN1/2 classification, an advanced clinical stage, having undergone neck dissection, and having undergone postoperative therapy were associated with a worse prognosis (Table 1).
Table 1.
Prognostic analysis of the total case cohort of patients with mucoepidermoid carcinoma (N = 153)
| Factor | N | Overall survival | Disease‐free survival | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age (y) | |||||||
| <60 | 89 | 1.00 | 1.00 | ||||
| ≥60 | 64 | 2.07 | 0.65‐6.55 | .207 | 2.11 | 0.99‐4.47 | .048 |
| Sex | |||||||
| Male | 75 | 1.00 | 1.00 | ||||
| Female | 78 | 0.29 | 0.07‐1.08 | .046 | 0.91 | 0.43‐1.92 | .821 |
| Primary site | |||||||
| Major gland | 105 | 1.00 | 1.00 | ||||
| Minor gland | 48 | 2.12 | 0.68‐6.58 | .197 | 2.38 | 1.13‐5.01 | .024 |
| CRTC1/3‐MAML2 | |||||||
| Positive | 90 | 1.00 | 1.00 | ||||
| Negative | 63 | 7.60*109 | NA | <.001 | 1.86 | 0.88‐3.92 | .100 |
| pT classification | |||||||
| pT1/2 | 80 | 1.00 | 1.00 | ||||
| pT3/4 | 73 | 3.72 | 1.00‐13.79 | .032 | 2.31 | 1.06‐5.01 | .029 |
| pN classification | |||||||
| pN0 | 120 | 1.00 | 1.00 | ||||
| pN1‐3 | 33 | 8.84 | 2.65‐29.41 | <.001 | 3.74 | 1.77‐7.88 | <.001 |
| pStage (NCCN Guidelines) | |||||||
| Early | 69 | 1.00 | 1.00 | ||||
| Advanced | 84 | 9.65 | 1.24‐74.82 | .003 | 3.24 | 1.31‐8.01 | .005 |
| Tumor grade | |||||||
| Low | 115 | 1.00 | 1.00 | ||||
| Intermediate/high | 38 | 2.43 | 0.77‐7.66 | .144 | 1.92 | 0.88‐4.16 | .110 |
| Surgical margin | |||||||
| Negative | 111 | 1.00 | 1.00 | ||||
| Close/positive | 42 | 1.56 | 0.47‐5.21 | .476 | 1.70 | 0.78‐3.69 | .190 |
| Neck dissection | |||||||
| Yes | 57 | 1.00 | 1.00 | ||||
| No | 96 | 0.18 | 0.04‐0.66 | .004 | 0.60 | 0.28‐1.26 | .183 |
| Postoperative therapy | |||||||
| Not performed | 128 | 1.00 | 1.00 | ||||
| Performed | 25 | 4.25 | 1.35‐13.41 | .021 | 1.93 | 0.21‐1.21 | .155 |
Follow‐up median, 41 mo (range, 2‐320).
Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not available; NCCN, National Comprehensive Cancer Network.
In 28 patients who showed tumor recurrence, the fusion‐positive and ‐negative cases numbered 13 and 15, respectively. In prognostic analysis for DFS, an advanced age, a minor salivary gland tumor, a pT3/4 classification, pN1‐3, and an advanced clinical stage were risk factors for a worse prognosis. The OS and DFS survival curves according to the fusion status are shown in Figure 1C,D.
Figure 1.
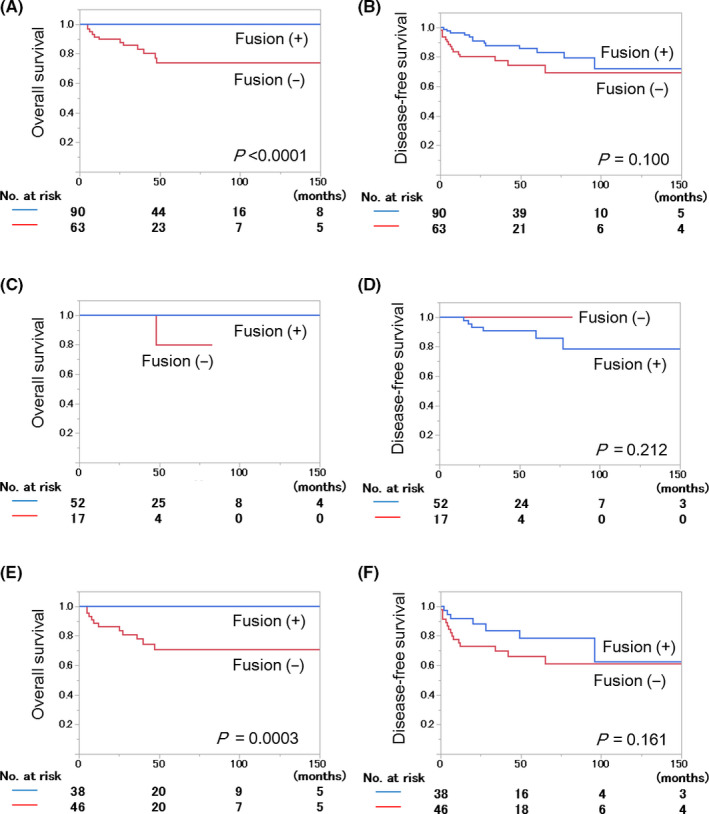
Survival of mucoepidermoid carcinoma (MEC) patients according to CRTC1/3‐MAML2 fusions. A, C, E, Overall survival in total (A, N = 153), early‐stage (C, N = 69), and advanced‐stage (E, N = 84) MEC cohorts. B, D, F, Disease‐free survival in total (B), early‐stage (D), and advanced‐stage (F) MEC cohorts. In the early‐stage cohort (C), prognostic analysis for overall survival was not carried out because only one patient died during the follow‐up period
3.2. Early‐stage MEC cohort (N = 69)
In the early‐stage MEC cohort, 23 of 69 cases had at least one adverse feature and 2 of 69 patients received postoperative therapy. The median of the follow‐up period was 45 months (range, 2‐263 months). The patients’ characteristics are shown in Table S3. In this cohort, six patients showed a tumor recurrence (five locoregional and one nodal). Recurrent tumors were surgically resected, and all six patients were alive at the last follow‐up. Another patient died of other causes (the cause of death was unknown) during the follow‐up period. The OS rate was 96.7% at both 5 and 10 years of follow‐up, respectively. The DFS rates at 5 and10 years of follow‐up were 88.0% and 81.2%, respectively.
In this early‐stage MEC cohort, 52 of 69 MEC cases (75%) were positive for CRTC1/3‐MAML2 fusions. The fusion status was not associated with any of the clinicopathologic factors examined (Table S4). Prognostic analysis for OS was not carried out because only one patient (fusion‐negative) died of other causes in this cohort during the follow‐up period.
In six patients who showed tumor recurrence, the fusion‐positive and ‐negative cases numbered six and zero, respectively. In the prognostic analysis for DFS, none of the factors examined was associated with the prognosis (Table 2). The OS and DFS survival curves according to the fusion status are shown in Figure 1B,C.
Table 2.
Prognostic analysis of the early‐stage cohort of patients with mucoepidermoid carcinoma (N = 69)
| Factor | N | Disease‐free survival | ||
|---|---|---|---|---|
| HR | 95% CI | P value | ||
| Age (y) | ||||
| <60 | 42 | 1.00 | ||
| ≥60 | 27 | 0.68 | 0.12‐3.76 | .654 |
| Sex | ||||
| Male | 32 | 1.00 | ||
| Female | 37 | 1.84 | 0.33‐10.09 | .468 |
| Primary site | ||||
| Major gland | 39 | 1.00 | ||
| Minor gland | 30 | 2.86 | 0.52‐15.79 | .209 |
| CRTC1/3‐MAML2 | ||||
| Positive | 52 | 1.00 | ||
| Negative | 17 | 3.88*10−9 | NA | .133 |
| Tumor grade | ||||
| Low | 59 | 1.00 | ||
| Intermediate/high | 10 | 1.38*10−9 | NA | .095 |
| Surgical margin | ||||
| Negative | 58 | 1.00 | ||
| Close/positive | 11 | 0.97 | 0.11‐8.46 | .981 |
| Neck dissection | ||||
| Yes | 3 | 1.00 | ||
| No | 66 | 2.11*108 | NA | .355 |
| Postoperative therapy | ||||
| Not performed | 67 | 1.00 | ||
| Performed | 2 | 4.02*10−9 | NA | .700 |
Follow‐up median, 45 mo (range, 2‐263).
Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not available.
3.3. Advanced‐stage MEC cohort (N = 84)
All advanced‐stage tumors had curative surgery and had one or more adverse features. In this cohort, 23 of 84 patients received postoperative therapy. Of 84 patients, 22 had tumor recurrence (13 locoregional, 7 nodal, and 2 distant) and 20 of these tumors, not including the 2 distant metastases, were surgically treated. The patients’ characteristics are shown in Table S5. During the follow‐up period (median, 39.5 months; range, 3‐320 months), nine patients died of disease and two died of other causes (the cause in one patient was prostatic cancer and that in the other was unknown). The OS rate was 82.9% at both 5 and 10 years of follow‐up, and the DFS rates at 5 and10 years of follow‐up were 71.7% and 62.7%, respectively.
In this advanced‐stage MEC cohort, 38 of 84 (45%) tumors were positive for CRTC1/3‐MAML2 fusions. The fusions were associated with a less advanced age, female gender, and a lower histological grade (Table S6).
In 11 patients who died during the follow‐up period, the fusion‐positive and ‐negative cases numbered 0 and 11, respectively. In the univariate analysis for OS, the tumor site (minor salivary gland), the absence of the fusions, and pN1‐3 were selected as risk factors (Table 3).
Table 3.
Prognostic analysis of the advanced‐stage case cohort of patients with mucoepidermoid carcinoma (N = 84)
| Factor | N | Overall survival | Disease‐free survival | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age (y) | |||||||
| <60 | 47 | 1.00 | 1.00 | ||||
| ≥60 | 37 | 1.72 | 0.52‐5.65 | .370 | 2.77 | 1.15‐6.66 | .018 |
| Sex | |||||||
| Male | 43 | 1.00 | 1.00 | ||||
| Female | 38 | 0.34 | 0.09‐1.29 | .093 | 1.23 | 0.53‐2.86 | .622 |
| Primary site | |||||||
| Major gland | 66 | 1.00 | 1.00 | ||||
| Minor gland | 18 | 4.41 | 1.34‐14.49 | .016 | 3.44 | 1.48‐7.99 | .005 |
| CRTC1/3‐MAML2 | |||||||
| Positive | 38 | 1.00 | 1.00 | ||||
| Negative | 46 | 3.68*10^9 | NA | <.001 | 1.86 | 0.75‐4.57 | .161 |
| pT classification | |||||||
| pT1/2 | 11 | 1.00 | 1.00 | ||||
| pT3/4 | 73 | 0.84 | 0.18‐3.92 | .828 | 0.89 | 0.30‐2.68 | .851 |
| pN classification | |||||||
| pN0 | 51 | 1.00 | 1.00 | ||||
| pN1‐3 | 33 | 4.96 | 1.31‐18.74 | .010 | 2.58 | 1.10‐6.05 | .027 |
| Tumor grade | |||||||
| Low | 56 | 1.00 | 1.00 | ||||
| Intermediate/high | 28 | 2.08 | 0.63‐6.84 | .235 | 2.15 | 0.92‐4.99 | .081 |
| Surgical margin | |||||||
| Negative | 53 | 1.00 | 1.00 | ||||
| Close/positive | 31 | 1.29 | 0.37‐4.42 | .688 | 1.52 | 0.64‐3.58 | .341 |
| Neck dissection | |||||||
| Yes | 54 | 1.00 | 1.00 | ||||
| No | 30 | 0.36 | 0.07‐1.70 | .160 | 1.09 | 0.46‐2.62 | .832 |
| Postoperative therapy | |||||||
| Not performed | 61 | 1.00 | 1.00 | ||||
| Performed | 23 | 2.37 | 0.72‐7.78 | .164 | 1.32 | 0.53‐3.23 | .551 |
Follow‐up median, 39.5 mo (range, 3‐320).
Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not available.
In 22 patients who showed tumor recurrence, the fusion‐positive and ‐negative cases numbered 7 and 15, respectively. In the prognostic analysis for DFS, advanced age, a minor salivary gland tumor, and pN1‐3 were risk factors for a worse prognosis. The OS and DFS survival curves according to the fusion status are shown in Figure 1E,F.
3.4. Fusion‐negative MEC cohort (N = 63)
In this cohort, 51 cases had at least one adverse feature and 16 patients received postoperative therapy. Of 63 patients, 15 had tumor recurrence (10 locoregional, 3 nodal, and 2 distant) and 13 of these tumors, not including the 2 distant metastases, were surgically treated. The patients’ characteristics are shown in Table S7. During the follow‐up period (median, 36 months; range, 3‐208 months), nine patients died of disease and three died of other causes (the cause in one patient was prostatic cancer and that in the other two was unknown). The OS rate was 74.1% at both 5 and 10 years of follow‐up, and the DFS rates at 5 and 10 years of follow‐up were 74.3% and 69.3%, respectively.
In the prognostic analysis for OS, pN1‐3 was selected as a risk factor (Table 4). In the prognostic analysis for DFS, a minor salivary gland tumor, pN1‐3, and an advanced clinical stage were risk factors for a worse prognosis. The OS and DFS survival curves are shown in Figure 1A,B.
Table 4.
Prognostic analysis of the fusion‐negative case cohort of patients with mucoepidermoid carcinoma (N = 63)
| Factor | N | Overall survival | Disease‐free survival | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age (years) | |||||||
| <60 | 30 | 1.00 | 1.00 | ||||
| ≥60 | 33 | 1.49 | 0.47‐4.72 | .491 | 2.16 | 0.73‐6.35 | .148 |
| Sex | |||||||
| Male | 37 | 1.00 | 1.00 | ||||
| Female | 26 | 0.41 | 0.11‐1.51 | .155 | 1.49 | 0.50‐4.37 | .456 |
| Primary site | |||||||
| Major gland | 43 | 1.00 | 1.00 | ||||
| Minor gland | 20 | 2.49 | 0.80‐7.72 | .120 | 3.06 | 1.10‐8.53 | .033 |
| pT classification | |||||||
| pT1/2 | 22 | 1.00 | 1.00 | ||||
| pT3/4 | 41 | 1.63 | 0.44‐6.05 | .442 | 2.30 | 0.65‐8.19 | .163 |
| pN classification | |||||||
| pN0 | 41 | 1.00 | 1.00 | ||||
| pN1‐3 | 22 | 4.55 | 1.36‐15.15 | .010 | 4.36 | 1.48‐12.80 | .005 |
| pStage (NCCN Guidelines) | |||||||
| Early | 17 | 1.00 | 1.00 | ||||
| Advanced | 46 | 3.78 | 0.48‐29.31 | .126 | 2.26*10^9 | NA | .002 |
| Tumor grade | |||||||
| Low | 37 | 1.00 | 1.00 | ||||
| Intermediate/high | 26 | 1.13 | 0.36‐3.59 | .825 | 2.52 | 0.89‐7.09 | .075 |
| Surgical margin | |||||||
| Negative | 44 | 1.00 | 1.00 | ||||
| Close/positive | 19 | 1.50 | 0.45‐5.01 | .518 | 1.32 | 0.45‐3.88 | .614 |
| Neck dissection | |||||||
| Yes | 32 | 1.00 | 1.00 | ||||
| No | 31 | 0.31 | 0.08‐1.15 | .059 | 0.35 | 0.11‐1.12 | .061 |
| Postoperative therapy | |||||||
| Not performed | 47 | 1.00 | 1.00 | ||||
| Performed | 16 | 2.38 | 0.75‐7.50 | .153 | 2.08 | 0.74‐5.87 | .177 |
Follow‐up median, 36 mo (range, 3‐208).
Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not available; NCCN, National Comprehensive Cancer Network.
4. DISCUSSION
According to the inclusion criteria, the MEC patients included in this study had curative surgery as the initial treatment, received no preoperative treatment, and had no distant metastasis at the time of the initial treatment. The diagnosis of MEC was confirmed histopathologically by a central pathology review by five expert salivary gland pathologists. Statistical analysis showed that the presence of the fusions was a prognostic factor for a favorable OS in both total and advanced‐stage cohorts but not in the early‐stage cohort. Fusion‐positive tumors showed a tumor recurrence similar to fusion‐negative tumors, and the fusions were not a prognostic factor for DFS in any of the cohorts analyzed.
The most important finding in this study was that the OS of the fusion‐positive MEC patients was excellent and superior to that of the fusion‐negative MEC patients. Of 12 patients who died during the follow‐up period, 12 had fusion‐negative tumors. The superior OS was evident in the total and advanced‐stage MEC cohorts, but was not seen in the early‐stage cohort. The patients with early‐stage tumors showed an excellent OS and only one of the 69 patients died of some other cause. In the prognostic analysis of this cohort, none of the factors emerged as a risk factor, suggesting that an early‐stage tumor could be the most important prognostic factor in this cohort. 2 , 27 Another important finding in this study was that the fusions were not useful for prediction of tumor recurrence. Recurrence was recorded in 28 of 153 (18.3%) patients in total, and 13 and 15 cases were CRTC1/3‐MAML2 fusion‐positive and ‐negative, respectively. Interestingly, none of the fusion‐positive tumors showed distant tumor metastasis. The DFS analysis did not reveal any remarkable difference in survival between fusion‐positive and ‐negative patients. Taken together with the OS and DFS data, fusion‐positive MEC could have the unique feature that patients with fusion‐positive tumors rarely die while locoregional or nodal tumor recurrence is not very rare.
The NCCN Guidelines recommend considering postoperative therapy, usually radiotherapy, for completely resected salivary gland tumors when they show adverse features. 4 Clinicians are strongly encouraged to avoid unnecessary postoperative therapy, and the indications for therapy should be considered carefully because it can sometimes result in significant complications, especially in young and old patients. 28 , 29 , 30 , 31 , 32 Whether postoperative therapy would improve the clinical outcomes of MEC patients has not been well clarified. 33 , 34 We found that postoperative therapy was associated with worse OS. This result should be interpreted as indicating that postoperative therapy was preferentially carried out in MEC patients with a poor prognosis, and as a result, the therapy was not very effective. When MEC is resected curatively, postoperative therapy might not be necessary, even if the tumor has adverse features.
There has been debate regarding the prognostic value of CRTC1/3‐MAML2 fusions. Many studies have reported that MEC patients with these fusions had a significantly better survival than those without the fusions. 7 , 11 , 13 , 14 , 15 , 16 , 17 , 18 , 19 In addition, it has become widely accepted that fusion‐positive MEC tumors are associated with favorable clinicopathologic and molecular features. 16 , 35 , 36 Despite the favorable features of the fusion‐positive MEC patients, some research groups have reported that the fusions are not a prognostic factor, often basing on their observations on the fact that the fusions failed to achieve statistical significance in their prognostic analyses. 20 , 21 , 22 , 23 One speculation regarding discrepancy often encountered in these studies is that the worse prognosis in fusion‐negative MEC tumors may be diluted by high‐grade non‐MEC cases, such as adenosquamous carcinomas. 15 , 20 , 23 , 37 In the present large cohort study (N = 153), we found that CRTC1/3‐MAML2 fusions are highly associated with a favorable OS (no death was recorded among the fusion‐positive cases) but have no impact on DFS. The source of the discrepancies between the above reports and ours is difficult to discern but the following concerns should be considered. A P‐value is not an absolute discriminant index in medical issues, and the more cases that are recruited in a study, the less the P‐value would be. Unfortunately, the numbers of the total MEC cases and the advanced‐stage MEC cases recruited in these previous studies have not been large enough (total, N = 36‐90 and advanced‐stage, N = 24‐34). As suggested in our study, the number of advanced‐stage cases could be more important than that of the total cases. The old argument regarding P values has recently been reconsidered with a warning that it is not appropriate to conclude that statistically nonsignificant results indicate no association. 38 The survival of fusion‐positive patients has always been and consistently better than that of fusion‐negative patients in any study conducted, including those reports that concluded that the fusions had no prognostic significance. 21 , 22 , 23 A recent meta‐analysis study supports the association of CRTC1/3‐MAML2 fusions with a favorable outcome for the patients. 19 In patients with fusion‐positive MEC tumors, tumor recurrence might not directly indicate a poor OS. In our study, 13 patients with fusion‐positive MECs had tumor recurrence (locoregional or nodal, but not distant), but none of these patients died during the follow‐up period. An accurate tumor diagnosis of the cases included in these studies is vital. We believe that the possibility of the inclusion of non‐MEC tumors in this study cohort was minimal as the diagnosis of MEC was validated by a central pathology review by five expert salivary gland pathologists. “Adenosquamous carcinoma” has been often listed as one of differential diagnoses of high‐grade MEC. 15 , 20 , 23 , 37 However, it should be noted that “adenosquamous carcinoma” is not included in the present WHO classification of salivary gland tumors, 1 and one should be careful in listing it as a differential diagnosis of high‐grade MEC. Considering the ambiguous pathological definition of MEC and the disease framework of the salivary gland tumors described in the present WHO classification, 1 some problematic cases are compelled to be diagnosed as MEC.
We should mention some limitations of this study. As MEC is a rare tumor and our study design was retrospective in nature, an inherent bias existed. In this study, we included 69 cases of early‐stage tumors and 84 cases of advanced‐stage tumors. As we had thought that the clinicopathologic significance of fusions was more important in advanced‐stage cases than in early‐stage cases, the number of our MEC cases was biased toward advanced‐stage cases. In the “real world,” the ratio of early‐stage to advanced‐stage MEC is estimated to be 1.6‐3.1. 11 , 17 , 21 , 22 The number of cases analyzed for CRTC1/3‐MAML2 fusions is, to our knowledge, the largest reported. However, partly owing to the rarity of MEC and the generally favorable prognosis of the patients, The number of deceased patients and those who had tumor recurrence was 12 and 28, respectively, which made multivariate prognostic analysis difficult.
In conclusion, based on a well‐characterized MEC cohort, we showed that the presence of CRTC1/3‐MAML2 fusions was a prognostic factor for better OS in the total and advanced‐stage MEC cohorts. The incidence of fusion‐positive tumor recurrence was similar to that of fusion‐negative tumors, but with surgical resection of the recurrent tumor, the fusion‐positive patients showed an excellent prognosis. These findings are expected to contribute to the clinical management of MEC, especially in young MEC patients. When the carcinoma is positive for CRTC1/3‐MAML2 fusions, the indications for postoperative therapy should be considered carefully. It should be stressed that the MEC cohorts studied here do not represent all clinical forms of MEC. Those MEC cases with distant metastasis at the time of surgery and those in which curative surgery was not carried out were excluded from the present study, and are expected to become the subject of future investigations.
CONFLICT OF INTEREST
None to declare.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This study was supported in part by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (15K08351 to H. Inagaki and 17K08746 to T. Murase). The authors thank the following collaborators: Masato Nakaguro (Nagoya University Hospital, Nagoya, Japan), Kenichi Taguchi (National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan), Tetsuro Onitsuka (Shizuoka Cancer Center, Mishima, Japan), Yasushi Fujimoto (Nagoya University Graduate School of Medicine, Nagoya, Japan), Kazuo Sakurai (Fujita Health University, Toyoake, Japan), and Naohito Hato (Ehime University School of Medicine, Matsuyama, Japan).
Okumura Y, Nakano S, Murase T, et al. Prognostic impact of CRTC1/3‐MAML2 fusions in salivary gland mucoepidermoid carcinoma: A multiinstitutional retrospective study. Cancer Sci. 2020;111:4195–4204. 10.1111/cas.14632
Yoshihide Okumura and Satsuki Nakano contributed equally to the study.
Funding information
Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan, Grant/Award Numbers 15K08351 and 17K08746.
REFERENCES
- 1. Brandwein‐Gensler M, Bell D, Inagaki H, et al. Mucoepidermoid carcinoma In: El‐Naggar AK, Chan JKC, Grandis JR, eds. World Health Organization Classification of Head and Neck Tumours. Lyon: IARC Press; 2017:163‐164. [Google Scholar]
- 2. McHugh CH, Roberts DB, El‐Naggar AK, et al. Prognostic factors in mucoepidermoid carcinoma of the salivary glands. Cancer. 2012;118:3928‐3936. [DOI] [PubMed] [Google Scholar]
- 3. Chenevert J, Barnes LE, Chiosea SI. Mucoepidermoid carcinoma: a five‐decade journey. Virchows Arch. 2011;458:133‐140. [DOI] [PubMed] [Google Scholar]
- 4. NCCN Clinical Practice Guidelines in Oncology Head and Neck Cancers, version 1.2020; accessed April 18. Available from: https://www.nccn.org/professionals/physician_gls/PDF/head‐and‐neck.pdf [DOI] [PubMed]
- 5. Tonon G, Modi S, Wu L, et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003;33:208‐213. [DOI] [PubMed] [Google Scholar]
- 6. Enlund F, Behboudi A, Andrén Y, et al. Altered Notch signaling resulting from expression of a WAMTP1‐MAML2 gene fusion in mucoepidermoid carcinomas and benign Warthin's tumors. Exp Cell Res. 2004;292:21‐28. [DOI] [PubMed] [Google Scholar]
- 7. Nakayama T, Miyabe S, Okabe M, et al. Clinicopathological significance of the CRTC3‐MAML2 fusion transcript in mucoepidermoid carcinoma. Mod Pathol. 2009;22:1575‐1581. [DOI] [PubMed] [Google Scholar]
- 8. Wu L, Liu J, Gao P, et al. Transforming activity of MECT1‐MAML2 fusion oncoprotein is mediated by constitutive CREB activation. EMBO J. 2005;24:2391‐2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coxon A, Rozenblum E, Park Y‐S, et al. Mect1‐Maml2 fusion oncogene linked to the aberrant activation of Cyclic AMP/CREB regulated genes. Cancer Res. 2005;65:7137‐7144. [DOI] [PubMed] [Google Scholar]
- 10. Komiya T, Park Y, Modi S, et al. Sustained expression of Mect1‐Maml2 is essential for tumor cell growth in salivary gland cancers carrying the t(11;19) translocation. Oncogene. 2006;25:6128‐6132. [DOI] [PubMed] [Google Scholar]
- 11. Okabe M, Miyabe S, Nagatsuka H, et al. MECT1‐MAML2 fusion transcript defines a favorable subset of mucoepidermoid carcinoma. Clin Cancer Res. 2006;12:3902‐3907. [DOI] [PubMed] [Google Scholar]
- 12. Martins C, Cavaco B, Tonon G, et al. A Study of MECT1‐MAML2 in mucoepidermoid carcinoma and Warthin's tumor of salivary glands. J Mol Diagn. 2004;6:205‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Behboudi A, Enlund F, Winnes M, et al. Molecular classification of mucoepidermoid carcinomas‐prognostic significance of the MECT1‐MAML2 fusion oncogene. Genes Chromosomes Cancer. 2006;45:470‐481. [DOI] [PubMed] [Google Scholar]
- 14. Tirado Y, Williams MD, Hanna EY, et al. CRTC1/MAML2 fusion transcript in high grade mucoepidermoid carcinomas of salivary and thyroid glands and Warthin's tumors: Implications for histogenesis and biologic behavior. Genes Chromosomes Cancer. 2007;46:708‐715. [DOI] [PubMed] [Google Scholar]
- 15. Seethala RR, Dacic S, Cieply K, et al. A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol. 2010;34:1106‐1121. [DOI] [PubMed] [Google Scholar]
- 16. Jee KJ, Persson M, Heikinheimo K, et al. Genomic profiles and CRTC1‐MAML2 fusion distinguish different subtypes of mucoepidermoid carcinoma. Mod Pathol. 2013;26:213‐222. [DOI] [PubMed] [Google Scholar]
- 17. Luk PP, Wykes J, Selinger CI, et al. Diagnostic and prognostic utility of Mastermind‐like 2 (MAML2) gene rearrangement detection by fluorescent in situ hybridization (FISH) in mucoepidermoid carcinoma of the salivary glands. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121:530‐541. [DOI] [PubMed] [Google Scholar]
- 18. Techavichit P, Hicks MJ, López‐Terrada DH, et al. Mucoepidermoid carcinoma in children: A single institutional experience. Pediatr Blood Cancer. 2016;63:27‐31. [DOI] [PubMed] [Google Scholar]
- 19. Pérez‐de‐Oliveira ME, Wagner VP, Araújo ALD, et al. Prognostic value of CRTC1‐MAML2 translocation in salivary mucoepidermoid carcinoma: Systematic review and meta‐analysis. J Oral Pathol Med. 2020;49:386‐394. [DOI] [PubMed] [Google Scholar]
- 20. Seethala RR, Chiosea SI. MAML2 Status in mucoepidermoid carcinoma can no longer be considered a prognostic marker. Am J Surg Pathol. 2016;40:1151‐1153. [DOI] [PubMed] [Google Scholar]
- 21. Saade RE, Bell D, Garcia J, et al. Role of CRTC1/MAML2 translocation in the prognosis and clinical outcomes of mucoepidermoid carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142:234‐240. [DOI] [PubMed] [Google Scholar]
- 22. Birkeland AC, Foltin SK, Michmerhuizen NL, et al. Correlation of Crtc1/3‐Maml2 fusion status, grade and survival in mucoepidermoid carcinoma. Oral Oncol. 2017;68:5‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cipriani NA, Lusardi JJ, McElherne J, et al. Mucoepidermoid carcinoma: A comparison of histologic grading systems and relationship to MAML2 rearrangement and prognosis. Am J Surg Pathol. 2019;43:885‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ellis GL, Auclair PL, eds. AFIP Atras of Tumor Pathology, Series 4, Tumors of the Salivary Glands. Washington, DC: American Registry of Pathology, 2007:173‐193. [Google Scholar]
- 25. Brierley JD, Gospodarowicz MK, Wittekind C, eds. TNM Classification of Malignant Tumours , 8th edn. Hoboken: John Wiley & Sons; 2017. [Google Scholar]
- 26. Noda H, Okumura Y, Nakayama T, et al. Clinicopathological significance of MAML2 gene split in mucoepidermoid carcinoma. Cancer Sci. 2013;104:85‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Plambeck K, Friedrich RE, Schmelzle R. Mucoepidermoid carcinoma of salivary gland origin: classification, clinical‐pathological correlation, treatment results and long‐term follow‐up in 55 patients. J Craniomaxillofac Surg. 1996;24:133‐139. [DOI] [PubMed] [Google Scholar]
- 28. Walker MP, Wichman B, Cheng A‐L, et al. Impact of radiotherapy dose on dentition breakdown in head and neck cancer patients. Pract Radiat Oncol. 2011;1:142‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jensen SB, Pedersen AML, Vissink A, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support Care Cancer. 2010;18:1039‐1060. [DOI] [PubMed] [Google Scholar]
- 30. Eisbruch A, Ten Haken RK, Kim HM, et al. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity‐modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45:577‐587. [DOI] [PubMed] [Google Scholar]
- 31. Murdoch‐Kinch C‐A, Kim HM, Vineberg KA, et al. Dose‐effect relationships for the submandibular salivary glands and implications for their sparing by intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:373‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Little M, Schipper M, Feng FY, et al. Reducing xerostomia after chemo‐IMRT for head‐and‐neck cancer: beyond sparing the parotid glands. Int J Radiat Oncol Biol Phys. 2012;83:1007‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaur J, Goyal S, Muzumder S, et al. Outcome of surgery and post‐operative radiotherapy for major salivary gland carcinoma: Ten year experience from a single institute. Asian Pac J Cancer Prev. 2014;15:8259‐8263. [DOI] [PubMed] [Google Scholar]
- 34. Okumura Y, Murase T, Saida K, et al. Postoperative radiotherapy for T1/2N0M0 mucoepidermoid carcinoma positive for CRTC1/3‐MAML2 fusions. Head Neck. 2018;40:2565‐2573. [DOI] [PubMed] [Google Scholar]
- 35. Kang H, Tan M, Bishop JA, et al. Whole‐exome sequencing of salivary gland mucoepidermoid carcinoma. Clin Cancer Res. 2017;23:283‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matse JH, Veerman ECI, Bolscher JGM, et al. High number of chromosomal copy number aberrations inversely relates to t(11;19)(q21;p13) translocation status in mucoepidermoid carcinoma of the salivary glands. Oncotarget. 2017;8:69456‐69464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chiosea SI, Dacic S, Nikiforova M, et al. Prospective testing of mucoepidermoid carcinoma for the MAML2 translocation: Clinical Implications. Laryngoscope. 2012;122:1690‐1694. [DOI] [PubMed] [Google Scholar]
- 38. Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature. 2019;567:305‐307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


