Abstract
The present study analyzed the antitumor effect of γδT cells transduced with the TCR of cancer‐specific CTLs to establish forceful cancer‐specific adoptive immunotherapy. We cloned the TCRαβ genes from CTLs showing HLA‐B15 restricted recognition of Kita‐Kyushu lung cancer antigen‐1 (KK‐LC‐1), a cancer/germline gene antigen, identified in a lung adenocarcinoma case (F1121). The TCRαβ and CD8 genes were transduced into γδT cells induced from PBLs of healthy volunteers stimulated with zoledronate and IL‐2. The KK‐LC‐1‐specific TCRαβ‐CD8 γδT cells showed cytotoxic activity against the KK‐LC‐1 positive lung cancer cell line F1121L and produced IFN‐γ against F1121L and KK‐LC‐1 peptide‐pulsed F1121 EBV‐B cells. These responses were blocked by HLA class I and HLA‐B/C antibodies. An in vivo assay using NOD/SCID mice with xenotransplantation of human lung cancer cells was performed, and the TCRαβ‐CD8 transduced γδT cells (TCRαβ‐CD8 γδT cells) were intravenously injected. Growth inhibition of KK‐LC‐1+, HLA‐B15+ lung cancer cells was confirmed in mice with injection of the TCRαβ‐CD8 γδT cells from 1 wk after xenotransplantation of cancer cells but not in those treated 2 wk after xenotransplantation. The resected specimens of the tumor, 2 wk after xenotransplantation, highly expressed FasL but not programmed death ligand‐1 (PD‐L1) by immunohistochemical staining. FasL highly expressed cancer cells xenotransplanted 2 wk ago were resistant to TCRαβ‐CD8 γδT cells injection. These results suggested that apoptosis of Fas‐positive TCRαβ‐CD8 γδT cells may be induced by a Fas‐mediated signal after interacting with FasL‐positive cancer cells.
Keywords: CTL, FasL, KK‐LC‐1, lung cancer, T‐cell receptor
(1) Kita‐Kyushu lung cancer antigen‐1 (KK‐LC‐1)‐specific cytotoxicity, which is the same as that observed in CTL clones, was obtained by transducing KK‐LC‐1‐specific TCRαβ and CD8 cells into γδT cells. (2) An in vivo assay using NOD/SCID mice with xenotransplantation of human lung cancer cells was performed, and the TCRαβ‐CD8 transduced γδT cells were intravenously injected. Growth inhibition of KK‐LC‐1+, HLA‐B15+ lung cancer cells was observed in mice with tumors 1 wk after xenotransplantation but not in those mice with tumors 2 wk after xenotransplantation. (3) Cancer cells expressed a high level of FasL at 2 wk after xenotransplantation and escaped adoptive treatment. We should be alert for Fas‐mediated apoptosis when applying such cellular immunotherapy.
Abbreviations
- B901L‐HLA‐B15
B901L transfected with HLA‐B15
- CM
culture medium
- EBV‐B
Epstein‐Barr virus‐transformed B cells
- FasL
Fas ligand
- HLA
human leukocyte antigen
- HPV
human papillomavirus
- IL‐2
interleukin 2
- KK‐LC‐1
Kita‐Kyushu lung cancer antigen‐1
- NK
natural killer
- NOD/SCID mouse
non‐obese diabetic/severe combined immunodeficiency mouse
- NSCLC
non–small‐cell lung carcinoma
- PBL
peripheral blood lymphocytes
- PD‐1
programmed cell death protein 1
- PD‐L1
programmed death ligand‐1
- TCR
T‐cell receptor
- TCR‐T cells
T‐cell‐receptor gene transduced T cells
- TCRαβ‐CD8 γδT cells
γδT cells co‐transfected with the TCRαβ and CD8 genes
- TGF‐β
transforming growth factor‐β
- TILs
tumor‐infiltrating lymphocytes
- TNF
tumor necrosis factor
1. INTRODUCTION
This paper is a revised version of a paper previously retracted after it was published by our group in Cancer Science. 1 Added points of this paper are data and considerations regarding the escape mechanism from adoptive immunotherapy.
Non–small‐cell lung carcinoma (NSCLC) is the leading cause of death from cancer worldwide. 2 , 3 The development of immunotherapy allows for therapies directed toward programmed cell death protein 1 (PD‐1)‐related target therapy, which has shown promising effects in patients with advanced NSCLC and superior survival outcomes compared with cytotoxic chemotherapies in patients with metastatic NSCLC. 2 , 3 , 4 , 5 , 6 , 7
Tumor‐specific TCR gene transduced T cells (TCR‐T cells) were developed as effective immune cell therapy for refractory tumors. 8 TCR‐T cells recognize peptide antigens that are presented by HLA molecules. Antigens that are specifically expressed in tumor cells, such as cancer/germline gene antigens, and mutated proteins are attractive targets of immunotherapy. Antigens that are abundantly expressed in tumor cells are also potential targets. Thus, the combination of ideal target antigens and the selection of immune receptors with an appropriate receptor‐antigen affinity is important for the establishment of effective immune cell therapy.
We previously identified a cancer/germline gene antigen from a lung cancer patient (F1121). A lung cancer cell line (F1121L) was then established, and the regional lymph node lymphocytes of patient F1121 were stimulated with the cell line F1121L and induced a specific CTL clone against F1121L. The CTL clone also recognized allogeneic HLA‐B*1501‐positive and HLA‐B*1507‐positive lung cancer cell lines in an HLA‐restricted manner. Using this clone, we identified the Kita‐Kyushu lung cancer antigen‐1 (KK‐LC‐1) as a new antigen‐coding gene using a cDNA expression cloning technique.
A nine‐mer peptide (KK‐LC‐1 (76‐84); RQKRILVNL) was identified as an epitope peptide. KK‐LC‐1 was not expressed in normal tissues, except for testis, and was located on the X chromosome (Xq22). Furthermore, KK‐LC‐1 was expressed in 40 of 100 NSCLC (40%) and 9 of 18 NSCLC cell lines established in our experiment. 9 KK‐LC‐1 was also expressed in 40% (12/30) of metastatic cervical cancer tissues. 10 The T‐cell reactivity against KK‐LC‐1 was detected in TILs from 2 of 8 additional KK‐LC‐1+ cervical cancer tissues, which suggests that it is naturally immunogenic. Furthermore, KK‐LC‐1 was expressed in 81% (40/49) of gastric cancers 11 and 53% (9/17) of triple‐negative breast cancers. 12 Of note, KK‐LC‐1 is not expressed in normal non‐germline tissues, 12 suggesting that it may be safely targeted by T cells.
However, the induction, maintenance, and culture of autologous cancer‐specific CTLs from individual patients is a serious obstacle for their application to adoptive immunotherapy. The present study therefore focused on creating promising TCR‐transduced long‐active effector cells with cancer‐specific characteristics after extracting TCRαβ genes from cancer‐specific CTL clones. The extracted TCRαβ genes were then transduced into γδT cells to establish forceful cancer‐specific adoptive immunotherapy.
2. MATERIALS AND METHODS
The study protocol was approved by the Human and Animal Ethics Review Committee of the University of Occupational and Environmental Health, Japan (05‐70) and a signed consent form was obtained from each subject before taking the tissue samples used in this study.
2.1. Culture medium
The CM we used consisted of RPMI 1640 (GIBCO‐BRL) supplemented with 10% heat‐inactivated fetal calf serum (FCS; Equitech‐Bio), 10 mmol/L hydroxyethylene‐piperazine ethanesulfonic (HEPES), 100 units/ml penicillin G, and 100 mg/ml streptomycin sulfate. The CM for γδT cells and γδT cells transduced with TCRαβ and CD8 genes was AlyS203‐700 (Cell Science and Technology Institute) supplemented with 10% heat‐inactivated FCS. The γδT cells were expanded with 1 μmol/L zoledronate (Novartis) followed by the addition of 100 units/ml interleukin 2 (IL‐2) (Figure 1).
FIGURE 1.
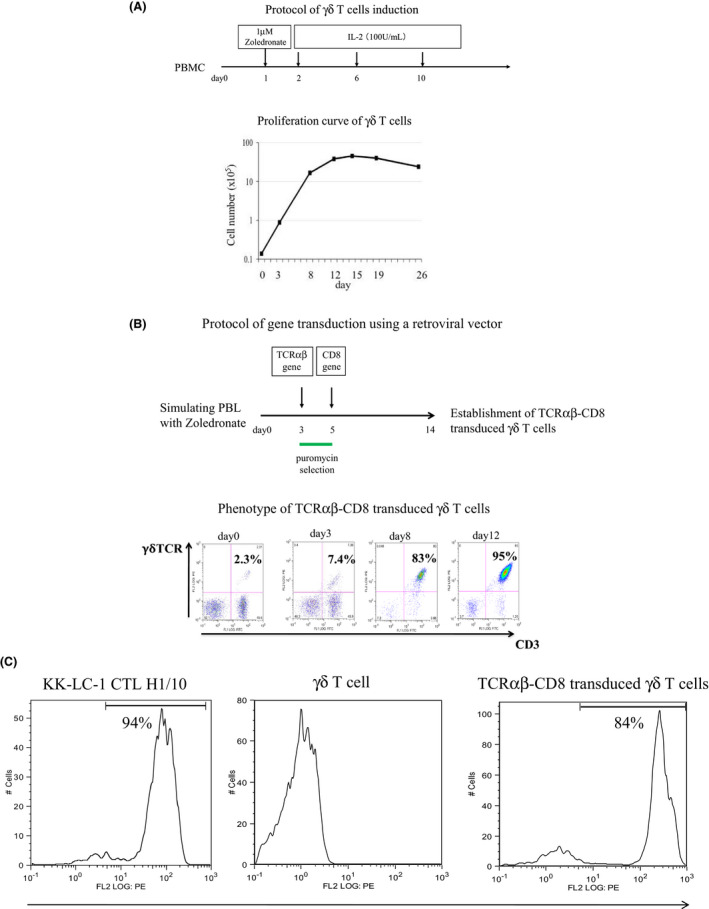
A, The γδT cells were expanded with 1 μmol/L zoledronate (Novartis) followed by the addition of 100 units/ml interleukin‐2 (IL‐2). The upper panel shows the induction protocol of the γδT cells. The lower panel shows the proliferation curve of the γδT cells. B, TCRαβ and CD8 were transduced 3 and 5 d after stimulation with zoledronate, respectively. TCRαβ transduced γδT cells were purified by puromycin selection. The upper panel shows the protocol of gene transduction using a retroviral vector. The lower panel shows the phenotype of TCRαβ‐CD8 transduced γδT cells by using flow cytometry. C, The transduction of TCRαβ was confirmed by flow cytometry staining using the KK‐LC‐176‐84/HLA‐B15 tetramer. TCRαβ transduced γδT cells were purified by puromycin selection
2.2. Cell lines
The lung adenocarcinoma cell lines F1121L, A110L, and B901L were established from surgical specimens in our laboratory with the following genotypes: HLA‐A*2402/0301, B*4006/1507, Cw*0303/0801; HLA‐A*2402/, B*5201/, Cw*1201; and HLA‐A*0206/2601, B*3901/4006, Cw*0702/0801, respectively. The 3 lung adenocarcinoma cell lines showed a positive expression for KK‐LC‐1. The method used to establish the lung cancer cell line has been described previously. 13
F1121 Epstein‐Barr virus‐transformed B cells (F1121 EBV‐B) were derived from PBLs of F1121 treated with 1 μg/ml cyclosporine A (Sandoz) and 20% of the supernatant of the Epstein‐Barr virus‐transduced marmoset monkey lymphocyte B95‐8. K562 is an erythroleukemia cell line that is sensitive to NK cell cytotoxicity. TNF‐sensitive WEHI 164cl3 cells were kindly donated by Dr. Coulie PG (Cellular Genetics Unit, Universite Catholic de Louvaine). WEHI‐164cl3 cells were maintained in CM with 5% FCS.
The induction of CTL from the regional lymph node lymphocytes in lung adenocarcinoma case F1121 and identification of antigenic peptide have been published elsewhere. 9
2.3. TCRαβ gene cloning from KK‐LC‐176‐84‐specific CTL clone
The TCRαβ gene was cloned from the HLA‐B15‐restricted CTL clone specific for KK‐LC‐1, which was identified from a patient with lung adenocarcinoma. 9 The cloned TCRα and TCRβ were then joined by a picornavirus‐line 2A “self‐cleaving” peptide by overlapped PCR. The short 18‐amino acid 2A sequence that separates TCRα and TCRβ‐results in equimolar expression of the TCRα and TCRβ via a “ribosomal skip” mechanism. 14 The TCRα and TCRβ combined with the 2A sequence were cloned into a pMX retroviral vector. The pMX vector harbors a 5′ LTR and the extended packaging signal derived from an MFG vector followed by a multi‐cloning site suitable for cDNA construction and the 3′ LTR of Moloney murine leukemia virus. The resulting vector pMX in combination with Plat‐A cells (Cell Biolabs Inc) produces an average of 1 × 107 IU/ml of virus. 15 The pMX vector had a conjugated puromycin‐resistant gene for the selection of the transductant.
2.4. TCRαβ gene transduction using a retroviral vector
An infectious, replication‐incompetent retrovirus was produced using the pMX retroviral vector system with Plat‐A cells and the Lipofectamine 2000 reagent (Invitrogen). In brief, Plat‐A cells were transfected with a TCR‐containing plasmid. After 1‐d culture following transfection, the supernatant of the Plat‐A cells was harvested, filtered using a 0.22 μm sterile filter, and concentrated by centrifugation at 5800 g overnight. The concentrated supernatant containing retrovirus was then used for gene transduction. The cloned TCRαβ as well as the CD8 gene (provided by TaKaRa Bio Inc) were transduced into γδT cells induced from PBL using a retroviral vector in recombinant human fibronectin fragment CH‐296 (Retronectin; Takara Bio)‐coated six‐well plates (Nunc). TCRαβ and CD8 were transduced 3 and 5 d after stimulation with zoledronate, respectively. TCRαβ transduced γδT cells were purified by puromycin selection. The CTL activity was examined using a cytotoxicity assay and a cytokine production assay.
2.5. Phenotypic analysis
The γδT cells co‐transduced with the TCRαβ and CD8 genes were doubly labeled with FITC‐ and PE‐conjugated monoclonal antibodies to CD3 and γδTCR.
2.6. KK‐LC‐1/HLA‐B15 tetramer staining
Transduction of TCRαβ was confirmed by staining of KK‐LC‐1‐specific tetramers. KK‐LC‐1‐specific tetramers (T‐Select MHC Tetramer) were purchased from Medical & Biological Laboratories Co., Ltd. TCRαβ‐transduced γδT cells were washed and resuspended in PBS with 1% human AB serum and then incubated for 30 min at 37°C with the KK‐LC‐176‐84/HLA‐B15 tetramer (20 nmol/L each) coupled with phycoerythrin. The cells were washed, fixed with 0.5% formaldehyde, and analyzed on a FACS Calibur flow cytometer (BD Biosciences) using the FlowJo software package (Tree Star Inc).
2.7. Monoclonal antibody (mAb) for the cytotoxicity assay and cytokine production
The culture supernatants of American Type Culture Collection (ATCC) HB‐145 (IVA12; anti‐HLA‐DR, ‐DP, ‐DQ Ab), HB‐95 (W6/32; anti‐HLA‐A, ‐B, ‐C Ab), C7709.A2.6 (anti‐HLA‐A24 Ab) and B1.23.2 (anti‐HLA‐B, ‐C Ab) were used for analyzing the HLA restriction of CTLs and antitumor effectors. The anti‐NKG2D Ab was purchased from BD Bioscience. Hybridomas (HB‐145, HB‐95) were purchased from the ATCC. Hybridomas (C7709.A2.6, B1.23.2) were kindly donated by Dr. Coulie PG (Cellular Genetics Unit, Universite Catholic de Louvaine).
2.8. Cytotoxicity assay and cytokine production of effector cells
The cytotoxicity of effector cells was assessed using a standard 4‐h 51Cr release assay, as described previously. 16 The supernatant was collected to measure the TNF production by a WEHI assay using TNF‐sensitive WEHI cells. 17 , 18 , 19
In brief, effector cells (6 × 104/ml) such as CTL clone or TCR‐transduced γδT cells, were incubated with tumor cells (6 × 105/ml) in CM with 10% FCS overnight, and the amount of IFN‐γ in the culture supernatant was measured in a triplicate assay using an IFN‐γ ELISA test kit (Life Technologies, Inc) in accordance with the manufacturer’s instructions. In the blocking assay using mAb, the four‐fold‐diluted culture supernatant of hybridomas such as HB‐95, C7709.A2.6, B1.23.2 and HB‐145 was used for the antibody inhibition assay.
2.9. Lung adenocarcinoma xenograft model
The γδT cells were expanded from PBMC of healthy volunteers with 100 units/ml rIL‐2 after stimulation with zoledronate. The number of γδT cells was calculated via a flow cytometry using anti‐TCR γδ Ab ((BD Biosciences). The γδT cells were transduced with TCRαβ gene derived from a KK‐LC‐1 specific CTL clone; the antitumor effect was then assessed against a lung adenocarcinoma (B901L) xenotransplanted NOD/SCID mouse model.
The parental B901L cell line expresses KK‐LC‐1 but does not possess the HLA‐B15 molecule. B901L‐parental and HLA‐B15 transduced B901L were inoculated subcutaneously (1 × 106 cells) into the lateral flank of a NOD/SCID mouse using 4 mice per group at day 0. TCRαβ‐ and CD8‐transduced γδT cells were injected via the tail vein of NOD/SCID mice weekly or twice‐weekly. Vehicle (PBS) was injected intravenously in the same manner as the control. The effects of treatment were evaluated by measuring the tumor size. The volume of the tumor was calculated using the formula “v = 0.4 × a × b2,” where a is the maximum diameter of the tumor, and b is the diameter at a right angle to a.
3. RESULTS
3.1. γδT cells expansion
Expansion of the γδT cells was obtained by adding 1 μmol/L of zoledronate in the presence of IL‐2 100 U/ml The population of γδT cells in the PBMCs was 2%‐3% initially and increased more than 95% at day 14 in accordance with a flow cytometry. Approximately 400‐fold expansion was therefore achieved over the 2 wk after stimulation with zoledronate (Figure 1A). The proliferation curve of the γδT cells is shown as the mean ± standard deviation on the basis of 3 independent experiments.
3.2. Transduction of tumor‐specific TCRαβ
The TCRαβ genes were extracted from the HLA‐B15‐restricted CTL clone specific for KK‐LC‐1 established from a patient with lung adenocarcinoma. Tetramer staining using the KK‐LC‐176‐84/HLA‐B15 tetramer was positive for the original KK‐LC‐1‐specific CTL clone but negative for the γδT cells. The cloned TCRαβ and CD8 genes were then co‐transduced into the γδT cells. After transduction of the TCRαβ genes, the expression of KK‐LC‐1‐specific TCR was confirmed by flow cytometry staining using mAbs of CD3‐FITC and γδ TCR‐PE, and the KK‐LC‐176‐84/HLA‐B15 tetramer. Here, 95% of double positive of CD3‐FITC and γδ TCR‐PE were detected (Figure 1B). More than 80% of tetramer‐positive cells were detected after puromycin selection (Figure 1C). The data are representative of 3 independent experiments. More than 80% of KK‐LC‐176‐84/HLA‐B15 tetramer‐positive cells were used for further experiments.
3.3. CTL activity of γδT cells co‐transduced with the TCRαβ and CD8 genes
Cytotoxicity and cytokine production assays were performed to evaluate the CTL activities against the KK‐LC‐1 epitope. The γδT cells showed strong NK activity and non‐specific cytotoxicity against the allogeneic lung cancer cell lines (Figure 2A). The γδT cells transduced with only the TCRαβ genes had neither CTL activity nor NK activity (Figure 2B). The NKG2D expression was examined to determine the inhibition mechanism of the NK activity of γδT cells after transduction with the TCRαβ genes. The downregulation of NKG2D was observed after TCRαβ transduction (Figure 2D). In contrast, the γδT cells co‐transfected with the TCRαβ and CD8 genes (TCRαβ‐CD8 γδT cells) showed tumor antigen‐specific cytotoxic activity that was similar to that of the original CTL clones (Figure 2C). The NK activity and non‐specific cytotoxicity against K562 and/or HLA‐B15 negative A110L were obviously inhibited after TCRαβ transduction (Figure 2C). TCRαβ‐CD8 γδT cells produced IFN‐γ in response to F1121L adenocarcinoma as well as EBV‐B cells pulsed with KK‐LC‐1 peptide (Figure 3). The production was blocked by anti‐HLA class I antibody, anti‐HLA‐B/C antibody and anti‐CD8 antibody.
FIGURE 2.
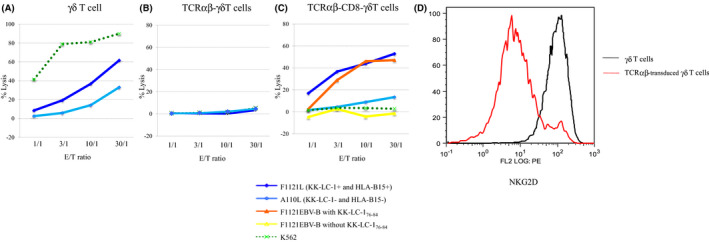
Cytotoxic activities of the γδT cells co‐transfected with the TCRαβ and CD8 genes. The cytotoxic activities of each effector cell, against autologous EBV‐B pulsed with KK‐LC‐176‐84 peptide, lung cancer cell lines (F1121L, A110L) and K562, were assessed by a standard 4‐h 51Cr release assay. The effector cells were γδT cells in (A), γδT cells transduced with only TCRαβ genes in (B), and γδT cells co‐transfected with the TCRαβ and CD8 genes in (C). E/T, effector/target. D, NKG2D expression was examined by flow cytometry staining
FIGURE 3.
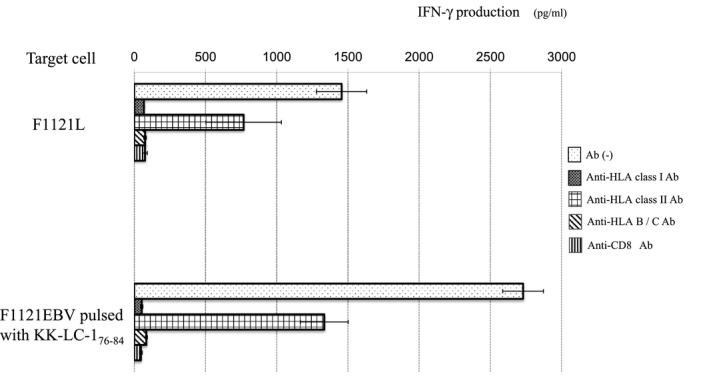
IFN‐γ production of the TCRαβ‐CD8 γδT cells was evaluated. The stimulators were lung cancer cell line (F1121L) in (A) and EBV‐B cells pulsed with KK‐LC‐1 peptide in (B). To analyze the restriction of the TCRαβ‐CD8 γδT cells, anti‐MHC class I Ab, anti‐MHC class‐II Ab, anti‐HLA, B, C Ab and anti‐CD8 Ab were used. The results are expressed as the mean ± SD
3.4. Effects of adoptive immunotherapy using TCRαβ‐CD8 γδT cells
HLA B15‐positive B901L (lung adenocarcinoma cell line) was established by stable transfection of the HLA‐B15 gene to evaluate the function of TCRαβ‐CD8 γδT cells in vivo. B901L‐parental was negative for HLA‐B15 but positive for KK‐LC‐1, so the TCRαβ‐CD8 γδT cells showed cytotoxic activity against HLA‐B15‐transfected B901L (B901L‐HLA‐B15), but not against B901L‐parental (Figure 4). Intravenous injection of TCRαβ‐CD8 γδT cells, started 1 wk after xenotransplantation of B901L‐HLA‐B15 induced growth inhibition of B901L‐HLA‐B15. B901L‐parental and B901L‐HLA‐B15 were xenotransplanted subcutaneously into the lateral flank of NOD/SCID mice on day 0. The growth of the susceptible cancer cell line (B901L‐HLA‐B15) was significantly inhibited by weekly intravenous injection of the TCRαβ‐CD8 γδT cells (1 × 106/injection) in comparison with the control cell line (B901L‐parental; Figure 5A). The growth rate of both cell lines was almost the same without the adoptive immunotherapy (data not shown). The growth of B901L‐HLA‐B15 was markedly decreased by twice‐weekly injection of TCRαβ‐CD8 γδT cells (Figure 5B). However, growth inhibition of xenotransplanted cancer cells was not observed in mice receiving intravenous injection of TCRαβ‐CD8 γδT cells starting 2 wk after xenotransplantation of cancer cells (Figure 5C,D).
FIGURE 4.
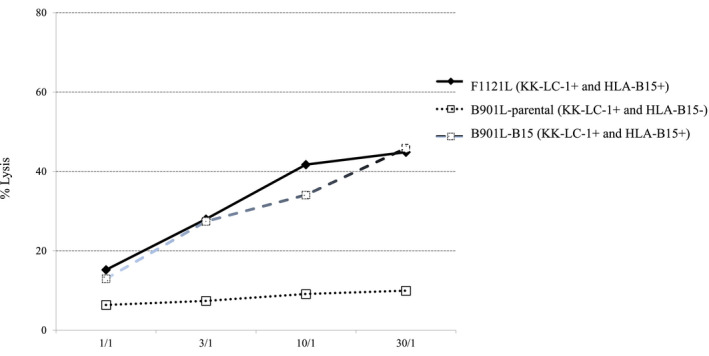
Cytotoxic activities of the γδT cells co‐transfected with the TCRαβ and CD8 genes against autologous lung cancer cell lines (F1121L, B901L‐parental, B901L‐HLA‐B15) were assessed by a standard 4‐h 51Cr release assay. E/T, effector/target
FIGURE 5.
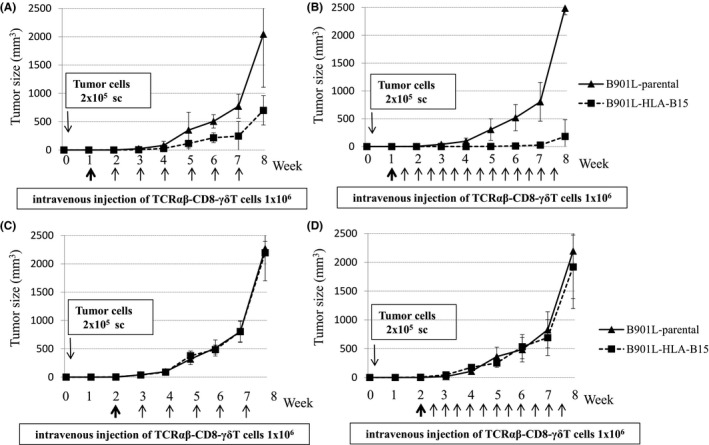
Tumor growth inhibition of B901L‐HLA‐B15 by adoptive transfer of TCRαβ‐CD8 γδT cells. B901L‐parental or B901L‐HLA‐B15 was xenotransplanted subcutaneously into the lateral flank of NOD/SCID mice on day 0. Intravenous injection of TCRαβ‐CD8 γδT cells was started 1 wk after xenotransplantation of B901L‐parental or B901L‐HLA‐B15 in (A, B) and 2 wk after xenotransplantation in (C, D). Weekly injections of TCRαβ‐CD8 γδT cells are shown in (A, C). The twice‐weekly injection of TCRαβ‐CD8 γδT cells are shown in (B, D). Results are expressed as the mean tumor volume ± SD. sc, subcutaneous injection
3.5. Infiltration of adoptively transferred TCRαβ‐CD8 γδT cells into the tumor
The histological examination and immunohistochemical staining revealed that the CD3‐positive human lymphocytes had infiltrated into the xenotransplanted tumor (B901L‐HLA‐B15). The infiltration of CD3‐positive human lymphocytes was observed more strongly in the B901L‐HLA‐B15 tumor than the control cell line (B901L‐parental) (Figure 6A). The central part of the tumor (B901L‐HLA‐B15) exhibited necrosis. The expression of the TCR Vα13 gene of the effector cells (TCRαβ‐CD8 γδT cells) was detected in the central part of the tumor tissue (B901L‐HLA‐B15) using reverse transcription (RT)‐PCR. The original CTL clone specific for KK‐LC‐1 also expressed TCR Vα13 (Figure 6B).
FIGURE 6.
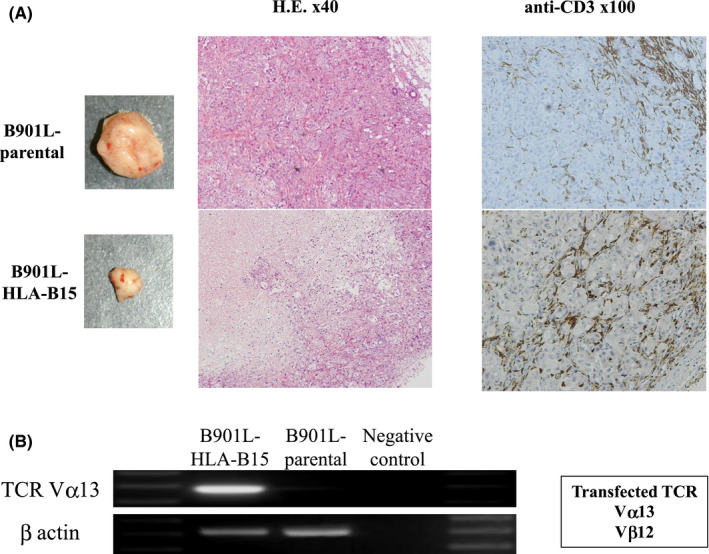
A, Histological examination and immunohistochemical staining using anti‐human CD3 antibody were performed to detect infiltration of TCRαβ‐CD8 γδT cells into the tumor. B, The original CTL clone specific for KK‐LC‐1 also possessed TCR Vα13. The expression of the TCR Vα13 gene of the TCRαβ‐CD8‐γδT cells in the tumor tissue (B901L‐HLA‐B15) was examined using RT‐PCR
3.6. An analysis of mechanisms underlying escape from adoptively transferred TCRαβ‐CD8 γδT cells
Given that the adoptive transfer of TCRαβ‐CD8 γδT cells 2 wk after xenotransplantation of cancer cells showed no marked effect, we analyzed the xenotransplanted cancer cells. Immunohistochemical staining of the xenotransplanted cancer tissues showed the stronger expression of FasL in mice receiving intravenous injection of the TCRαβ‐CD8 γδT cells starting 2 wk after xenotransplantation of cancer cells, compared with those treated at 1 wk after xenotransplantation. The expression of PD‐L1 was quite weak and roughly the same between the 2 conditions (Figure 7). There was also no significant difference in infiltration of T cells or expression of vascular endothelial growth factor (VEGF), transforming growth factor‐β (TGF‐β), and HLA class I of cancer cells (data not shown).
FIGURE 7.
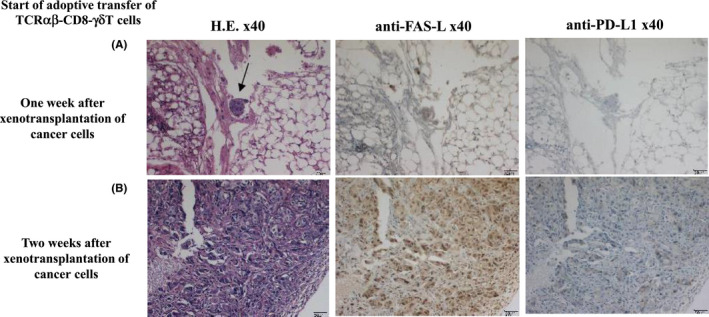
There was no effect of adoptively transferring TCRαβ‐CD8 γδT cells starting 2 wk after xenotransplantation of cancer cells (B901L‐HLA‐B15), so we analyzed the xenotransplanted cancer cells. Immunohistochemical staining of the xenotransplanted cancer cells using CD3, PD‐L1, FasL, VEGF, transforming growth factor‐β (TGF‐β) and HLA class I was performed. Intravenous injection of TCRαβ‐CD8 γδT cells was started 1 wk after xenotransplantation of B901L‐HLA‐B15 in (A) and 2 wk after xenotransplantation in (B). The difference between the 2 groups was seen in expression of FasL but not PD‐L1 expression
4. DISCUSSION
Adoptive immunotherapy requires a huge amount of tumor‐reactive T lymphocytes by ex vivo expansion to obtain a clinical response. Johnson et al reported that the average number of T cells infused in 6 of the 20 patients who experienced a partial tumor response using T cells transduced with a MART‐1‐specific TCR was 21.5 × 109 cells (range of 3.8 to 4.8 × 109 cells). 20 Robbins et al revealed that the average cell doses of NY‐ESO‐1‐specific TCR‐transduced T cells in patients who achieved at least a partial response were 66.4 × 109 cells for those with melanoma (range of 37‐130 × 109 cell; n = 5/11) and 62 × 109 cells for those with synovial cell sarcoma (range of 50‐83 × 109 cell; n = 4/6). 21
γδT cells, which account for 1%‐10% of peripheral blood T cells, 22 may be a candidate for killing cancer cells because of their direct recognition of cancer without restriction of HLA molecules. 23 After recognizing cancer cells, activated γδT cells can directly kill target cells by engaging death receptors on the surface of cancer cells and producing cytotoxic granules and cytokines. 24 Furthermore, the approximately 400‐fold expansion of γδT cells was able to be achieved with PBMCs within 2 wk after stimulation with zoledronate and IL‐2 in the present study. These data suggest that γδT cells may be useful for efficient TCR transfer.
Our data showed that KK‐LC‐1‐specific TCR‐transduced γδT cells lost NK activity compared with γδT cells without transduction. We confirmed the downregulation of NKG2D of γδT cells after TCRαβ transduction. Hiasa et al reported that γδTCR was also downregulated after transduction of TCRαβ. 25 The original γδTCRs were affected in accordance with the γδTCR signals by competition with the TCR mechanism with the transduced TCRαβ and the kinetics of activation through the transduced TCRαβ.
Cancer/germline gene antigens may be suitable targets for cancer immunotherapy. They are expressed in cancer cells but not in most somatic normal tissues, except for the testis, which does not express HLA genes. 26 The cancer‐restricted expression of antigen thus seems to be important for cancer immunotherapy, to prevent autoimmune disease. Stevanović et al reported that TILs derived from 2 human papillomavirus (HPV)‐induced cervical cancer patients recognized only KK‐LC‐1, but not other several cancer/germline gene antigens, and KK‐LC‐1 induced an effective immune response even in virally associated malignancies. 10 In addition, expression of KK‐LC‐1 has been frequently observed in previous studies, being noted in 40 of 100 non–small‐cell lung cancers (40%), 8 , 10 , 11 40 of 49 gastric cancers (81%) and 9 of 17 triple‐negative breast cancers (53%). KK‐LC‐1‐specific TCR may therefore be a promising target for adoptive immunotherapy.
γδT cells co‐transduced with KK‐LC‐1‐specific TCRαβ and CD8 genes showed KK‐LC‐1‐specific activities not only in vitro but also in vivo using xenotransplanted model. The antigen specificity was also confirmed by KK‐LC‐176‐84/HLA‐B15 tetramer. Furthermore, KK‐LC‐1‐specific TCRαβ‐CD8 γδT cells infiltrated the tumor tissue and contributed to tumor regression. The gene transduction of KK‐LC‐1‐specific TCRαβ and CD8 into γδT cells is a promising strategy for developing a new adoptive immunotherapy against malignant diseases.
However, we were unable to confirm any effects of adoptively transferring TCRαβ‐CD8 γδT cells starting more than 2 wk after xenotransplantation of cancer cells, so we analyzed the xenoplanted cancer cells using immunohistochemical staining with anti‐CD3, VEGF, TGF‐β, PD‐L1, and FasL antibodies. The expression of FasL was stronger in tumors treated 2 wk after xenotransplantation of cancer cells than in those cells treated 1 wk after xenotransplantation. The expression of other molecules showed no significant differences. The high expression of FasL on cancer cells might induce the apoptosis signal in Fas‐positive effector cells after interaction.
Fas (DR2/CD95/Apo‐1) is a type I cell membrane protein with an extracellular domain that binds FasL and a cytoplasmic domain that induces the death signal. 27 , 28 FasL (CD95L/CD178/Apo‐1 L) is a type II cell membrane protein belonging to the TNF family that is inducibly expressed in lymphocytes and constitutively expressed in cells present in immune‐privileged tissues. 29 , 30 FasL interacts with its receptor, Fas, triggering a cascade of subcellular events culminating in apoptotic cell death. 31 Hirata et al reported that Fas‐induced apoptotic cell death process is an integration of multiple, parallel biochemical events that occur downstream of the activation of particular capases. 32 Fas/FasL interactions play an important role in inducing cell apoptosis. It was believed that cancer cells 2 wk after xenotransplantation could avoid being affected by the cellular immune response by upregulating FasL which may cause effector cells to undergo apoptosis. The role of FasL is mediating tumoral immune resistance has been discussed for many years. Several reports have illustrated that FasL expression by melanoma cells triggered TIL apoptosis 33 and high expression of FasL in tumor cells induced inflammation associated with the recruitment and activation of neutrophils resulting in tumor rejection. 34 , 35 Motz et al 36 reported that the FasL‐mediated apoptosis of antitumor T cells in ovarian carcinoma prevented access of CD8 T cells to tumor nests. Zhu et al 37 revealed that blocking FasL might augment the antitumor efficacy of adoptive T‐cell therapy. The escape mechanism is another important issue to achieve greater efficacy with adoptive immunotherapy.
We confirmed that: (i) KK‐LC‐1‐specific cytotoxicity, which is the same as that observed in CTL clones, was obtained by transducing KK‐LC‐1‐specific TCRαβ and CD8 cells into γδT cells; (ii) growth inhibition of KK‐LC‐1+, HLA‐B15+ lung cancer cells was observed in mice with tumors 1 wk after xenotransplantation but not in those mice with tumors 2 wk after xenotransplantation; (iii) cancer cells expressed a high level of FasL at 2 wk after xenotransplantation and escaped adoptive treatment. We should be alert for Fas‐mediated apoptosis when applying such cellular immunotherapy.
DISCLOSURE
No potential conflicts of interest were disclosed.
ACKNOWLEDGMENTS
This study was supported in part by Cancer Translational Research Project; Ministry of Health, Labour and Welfare of Japan; and Cancer Research Institute, UOEH Research Grant for Promotion of Occupational Health and Grant‐in‐Aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (JP20390375, JP21659327, JP18K08806, JP19K09294).
Ichiki Y, Shigematsu Y, Baba T, et al. Development of adoptive immunotherapy with KK‐LC‐1‐specific TCR‐transduced γδT cells against lung cancer cells. Cancer Sci. 2020;111:4021–4030. 10.1111/cas.14612
This paper is a revised version of a paper previously retracted after we published it in Cancer Science.
REFERENCES
- 1. Hanagiri T, Shigematsu Y, Kuroda K, et al. Antitumor activity of human γδ T cells transduced with CD8 and with T‐cell receptors of tumor‐specific cytotoxic T lymphocytes. Cancer Sci. 2012;103(8):1414‐1419. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2. Siegel RI, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7‐30. [DOI] [PubMed] [Google Scholar]
- 3. Didkowska J, Wojciechowska U, Mańczuk M, Łobaszewski J. Lung cancer epidermiology: contemporary and futures challenges worldwide. Ann Trans Med. 2016;4:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non‐small cell lung cancer. N Engl J Med. 2015;372:2018‐2028. [DOI] [PubMed] [Google Scholar]
- 5. Reck M, Rodriguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375:1823‐1833. [DOI] [PubMed] [Google Scholar]
- 6. Gettinger SN, Horn L, Gandhi I, et al. Overall survival and long‐term safety of nivolumab (anti‐programmed death 1 antibody, bms‐936558, ono‐4538) in patients with previously treated advanced non‐small‐cell lung cancer. J Clin Oncol. 2015;33(18):2004‐2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet. 2016;387:1540‐1550. [DOI] [PubMed] [Google Scholar]
- 8. Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fukuyama T, Hanagiri T, Takenoyama M, et al. Identification of a new can‐ cer/germline gene, KK‐LC‐1, encoding an antigen recognized by autologous CTL induced on human lung adenocarcinoma. Cancer Res. 2006;66:4922‐4928. [DOI] [PubMed] [Google Scholar]
- 10. Stevanović S, Pasetto A, Helman SR, et al. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science. 2017;356:200‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shida A, Futawatari N, Fukuyama T, et al. Frequent high expression of Kita‐kyushu lung cancer‐1 (KK‐LC‐1) in gastric cancer. Anticancer Res. 2015;35:3575‐3579. [PubMed] [Google Scholar]
- 12. Paret C, Simon P, Vormbrock K, et al. CXorf61 is a target for T cell based immunotherapy of triple negative breast cancer. Oncotarget. 2015;6:25356‐25367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sugaya M, Takenoyama M, Osaki T, et al. Establishment of 15 cancer cell lines from patients with lung cancer and the potential tools for immunotherpy. Chest. 2002;122:282‐288. [DOI] [PubMed] [Google Scholar]
- 14. Symczak AL, Workman CJ, Wang Y, et al. Correction of multi‐gene deficiency in vivo using a single ‘self‐cleaving’ 2A peptide‐based retroviral vector. Nat Biotechnol. 2004;22:589‐594. [DOI] [PubMed] [Google Scholar]
- 15. Kitamura T, Koshino Y, Shibata F, et al. Retrovirus‐mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp Hematol. 2003;11:1007‐1014. [PubMed] [Google Scholar]
- 16. Ichiki Y, Takenoyama M, Mizukami M, et al. Simultaneously cellular and humoral immune response against mutated p53 in a patient with lung cancer. J Immunol. 2004;172:4844‐4850. [DOI] [PubMed] [Google Scholar]
- 17. Sugaya M, Takenoyama M, Shigematsu Y, et al. Identification of HLA‐A24 restricted shared antigen recognized by autologous cytotoxic T lymphocytes from a patient with large cell carcinoma of the lung. Int J Cancer. 2007;120:1055‐1062. [DOI] [PubMed] [Google Scholar]
- 18. Nagata Y, Hanagiri T, Takenoyama M, et al. Identification of the HLA‐Cw*0702‐restricted tumor‐associated antigen recognized by a CTL clone from a lung cancer patient. Clin Cancer Res. 2005;15:5265‐5272. [DOI] [PubMed] [Google Scholar]
- 19. So T, Takenoyama M, Mizukami M, et al. Haplotype loss of HLA class‐I antigen as an escape mechanism from immune attack in lung cancer. Cancer Res. 2005;65:5945‐5952. [DOI] [PubMed] [Google Scholar]
- 20. Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T‐cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;11:535‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robbins RF, Morgan RA, Feldman SA, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY‐ESO‐1. J Clin Oncol. 2011;29:917‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Norell H, Moretta A, Silva‐Santos B, Morretta L. At the bench: preclinical rationale for exploiting NK cells and gammadelta T lymphocytes for the treatment of high‐risk leucemias. J Leukoc Biol. 2013;94(6):1123‐1139. [DOI] [PubMed] [Google Scholar]
- 23. Morita CT, Beckman EM, Bukowski JF, et al. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity. 1995;3(4):495‐507. [DOI] [PubMed] [Google Scholar]
- 24. Hayday AC. γδ cells: a right time and a right place for a conserved third way of protection. Ann Rev Immunol. 2000;18:975‐1026. [DOI] [PubMed] [Google Scholar]
- 25. Hiasa A, Nishikawa H, Hirayama M, et al. Rapid αβ TCR‐mediated responses in γδ T cells transduced with cancer‐specific TCR genes. Gene Ther. 2009;16:620‐628. [DOI] [PubMed] [Google Scholar]
- 26. Jassim A, Ollier W, Payne A, Biro A, Oliver RT, Festenstein H. Analysis of HLA antigens on germ cells in human semen. Eur J Immunol. 1989;19:1215‐1220. [DOI] [PubMed] [Google Scholar]
- 27. Peter ME, Budd RC, Desbarats J, et al. The CD95 receptor: apoptosis revisited. Cell. 2007;129:447‐450. [DOI] [PubMed] [Google Scholar]
- 28. Strasser A, Jost PJ, Nagata S. The many roles of Fas receptor signalling in the immune system. Immunity. 2009;30:180‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169‐1178. [DOI] [PubMed] [Google Scholar]
- 30. Lettau M, Paulsen M, Kabelitz D, Janssen O. Storage, expression and function of Fas ligand, the key death factor of immune cells. Curr Med Chem. 2008;15:1684‐1696. [DOI] [PubMed] [Google Scholar]
- 31. Mazar J, Thomas M, Bezrukov L, et al. Cytotoxicity mediated by the Fas ligand (FasL)‐activated apoptotic pathway in stem cells. J Biol Chem. 2009;284:22022‐22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hirata H, Takahashi A, Kobayashi S, et al. Caspases are activated in a branched protease cascade and control distinct downstream processes in Fas‐induced apoptosis. J Exp Med. 1998;187(4):587‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hahne M, Rimoldi D, Schröter M, et al. Melanoma cell expression of Fas (Apo‐1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363‐1366. [DOI] [PubMed] [Google Scholar]
- 34. Seino K, Kayagaki N, Okumura K, Yagita H. Antitumor effect of locally produced CD95 ligand. Nat. Med. 1997;3:165‐170. [DOI] [PubMed] [Google Scholar]
- 35. Arai H, Gordon D, Nabel EG, Nabel GJ. Gene transfer of Fas ligand induces tumor regression in vivo. Proc Natl Acad Sci USA. 1997;94:13862‐13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Motz GT, Santoro SP, Wang LP, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20:607‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu J, Tenbossche P, Cané S, et al. Resistance to cancer immunotherapy mediated by apoptosis of tumor‐infiltrating lymphocytes. Nat Commun. 2017;8:1404. [DOI] [PMC free article] [PubMed] [Google Scholar]


