Abstract
UDP‐glucuronosyltransferase (UGT) 1A enzymes detoxify a broad array of exogenous compounds including environmental toxins and carcinogens. Case‐control studies identified genetic variations in UGT1A genes leading to reduced glucuronidation activity, which were associated with hepatocellular carcinoma (HCC) formation and progression. The aim of the study was therefore to examine the direct effect of common UGT1A polymorphisms (SNPs) on HCC development and outcome in a diethylnitrosamine (DEN)‐induced mouse model. Therefore, a single intraperitoneal DEN injection (20 mg/kg) was administered to 15‐day‐old htgUGT1A‐WT and htgUGT1A‐SNP mice (containing a human haplotype of 10 common UGT1A SNPs) either receiving water or coffee cotreatment for the following 39 weeks. After this time, tumor incidence, size (>1 mm), histology, liver‐body ratio, serum aminotransferase activities, and UGT1A regulation and activity levels were determined. In DEN‐treated htgUGT1A‐SNP mice, a markedly higher number of tumors with a bigger cumulative diameter were detected. The relative liver weight and aminotransferase activity levels were also significantly higher in mice carrying UGT1A SNPs. After coffee + DEN cotreatment, susceptibility for tumor development and growth considerably decreased in both mouse lines, but was still higher in htgUGT1A‐SNP mice. In conclusion, our study provides experimental evidence for the protective role of UGT1A enzymes in neoplastic transformation. These data confirm case‐control studies implicating impaired UGT1A‐mediated carcinogen detoxification as a risk factor for individual cancer disposition. Coffee treatment, which is able to activate UGT1A expression and activity, reduced HCC development and provides an explanation for the protective properties of coffee on liver diseases including liver cancer.
Keywords: carcinogens, detoxification, diethylnitrosamine, glucuronidation, hepatocellular carcinoma
This study provides direct experimental evidence for case‐control studies reporting an association between the presence of low‐function UGT1A variants and an increased tumor incidence which can be protectively modified by coffee consumption.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- DEN
diethylnitrosamine
- H&E
hematoxylin‐eosin
- HCC
hepatocellular carcinoma
- NNAL
4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butatone
- PhIP
2‐amino‐1‐methyl‐6‐phenylimidazo(4,5‐b)pyridine
- SNP
single‐nucleotide polymorphism
- UGT
UDP‐glucuronosyltransferase
- WT
wild‐type
1. INTRODUCTION
The human body, and especially the liver, is continuously exposed to a broad array of potentially toxic compounds. Cancer initiation and progression have been causally linked to the exposure to environmental toxins and (pro)carcinogens, 1 which requires an effective detoxification system to prevent their accumulation and consecutive detrimental effects in the body. UDP‐glucuronosyltransferase 1A (UGT1A) enzymes constitute an essential system for the clearance of xenobiotic chemicals as well as potentially cancer‐promoting molecules. 2 , 3 By catalyzing the covalent addition of glucuronic acid to a broad array of endobiotic, xenobiotic, and carcinogenic compounds, UGT1As facilitate their renal or biliary elimination from the body, which contributes to cyto‐ and genoprotection. 4 The food‐borne carcinogen 2‐amino‐1‐methyl‐6‐phenylimidazo(4,5‐b)pyridine (PhIP), its major metabolite N‐hydroxy‐PhIP, hydroxylated benzo[a]pyrenes as well as cancerogenic primary amines and nitrosamines such as the nicotine‐derived tobacco‐specific 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butatone (NNAL) are among those compounds that have been characterized to undergo UGT1A‐mediated detoxification. 5 , 6 , 7 , 8 The capacity of UGT1A proteins to eliminate carcinogens has linked glucuronidation to the risk of cancer development and progression. 9 , 10 In addition, the identification of genetic factors influencing an individual cancer risk has been intensively studied in recent years. Case‐control studies identified the presence of genetic UGT1A polymorphisms, mainly single‐nucleotide polymorphisms (SNPs), as risk factors for individual cancer disposition. 11 , 12 Against this background, the hypothesis appears plausible that lower carcinogen‐metabolizing action as a result of either decreased UGT1A expression or lower enzymatic activity is likely to represent an important reason for the increased toxicological susceptibility and carcinogenic initiation in carriers of UGT1A SNPs. 13 , 14 , 15
With almost 800 000 new cases annually, hepatocellular carcinoma (HCC) is the fifth most common malignant tumor worldwide and the second most frequent cause of tumor‐related death. 16 , 17 Accounting for approximately 90% of all cases of primary liver cancer, the number of cases diagnosed with HCC is expected to further increase in industrialized countries. 18 The liver is a major organ of the human body involved in the metabolism of environmental xenobiotics. Due to high hepatic UGT1A mRNA levels, the liver is viewed as the major site of glucuronidation and subsequent excretion of mutagenic compounds. 19 , 20 A significant association between carriers of the low‐activity UGT1A7*3 variant and the incidence of HCC has been reported in case‐control studies among patients with hepatitis B or C. 10 , 21 , 22 , 23 The UGT1A7*3 polymorphism is characterized by the combination of a promoter polymorphism (−57T>G) and three amino acid substitutions (Asn129Lys, Arg131Lys, and Trp208Arg) in the first exon of the UGT1A7 gene. 24 These polymorphisms are part of a complex Gilbert syndrome–associated haplotype present in roughly 10% of the white population. 25 Although a plethora of case‐control studies points to the increased risk of cancer development in carriers of low‐activity UGT1A variants, direct experimental evidence is still missing. In addition, a growing number of epidemiological studies reported an inverse association of coffee consumption with hepatic fibrosis progression, liver cirrhosis, and HCC. 26 , 27 In line with this, a large meta‐analysis of epidemiological and case‐control studies discovered a 40% lower risk for HCC development in coffee drinkers versus non–coffee drinkers. 28 Interestingly, in vitro and in vivo studies performed by our own laboratory identified coffee as a potent activator of genoprotective UGT1A expression leading to hepatoprotective effects against the tobacco carcinogen benzo[a]pyrene. 29 , 30 Therefore, the aim of this study was to experimentally analyze the impact of multiple UGT1A polymorphisms, representing a haplotype of ten common occurring UGT1A SNPs, on HCC susceptibility and outcome in htgUGT1A mice after chemical induction (diethylnitrosamine, DEN) of hepatocarcinogenesis. Furthermore, the study was expanded by investigating the effects of coffee cotreatment on UGT1A induction and the associated hepatoprotection against DEN‐induced tumor development and progression.
2. MATERIAL AND METHODS
2.1. Induction of HCC, analysis of liver tumors, and tissue collection
Previously described male htgUGT1A‐wild‐type (WT) and SNP mice 30 , 31 were treated with a single intraperitoneal injection (20 mg/kg body weight) of saline‐dissolved DEN (Sigma‐Aldrich) at 15 days of age. 32 One week after DEN injection, mice of each genotype were further divided into two subgroups either receiving water or coffee as only available drinking source for the following 39 weeks. Vehicle‐injected, water‐drinking 42‐week‐old male htgUGT1A‐WT and SNP mice served as controls. All mice had ad libitum access to water/coffee and chow and were kept at 22°C with a 12‐hour day/night cycle in the Central Animal Facility of the University Hospital Bonn. Experiments were performed in accordance to the “German Animal‐Protection Law” and the “Guide for the Care and Use of Laboratory Animals” and authorized by the relevant North Rhine‐Westphalian State Agency for Nature, Environment, and Consumer Protection (LANUV).
Mice were sacrificed 39 weeks after DEN injection to macroscopically determine the number and size of neoplastic tumors visible on the surface of each liver lobe. All tumors larger than 1 mm in diameter were counted and dimensions were measured. The accumulated tumor measurement represents the sum of all diameters of all hepatic tumors. For determination of the relative liver weight, whole livers were removed and weighed, and the liver/body ratio was assessed. Tumors were carefully separated from normal (nontumorous) liver sections, and both were immediately snap‐frozen in liquid nitrogen and stored at −80°C until use. The right lateral lobe was separated before and fixed in 4% paraformaldehyde for 3 days. It was subsequently embedded in paraffin and used for histological examinations.
2.2. Histological analysis
Liver tumors were analyzed by immunofluorescence staining using Ki‐67 antibody (MA5‐14520, dilution 1:100, Thermo Scientific) and an appropriate secondary antibody (ab150077 Alexa Fluor® 488, dilution 1:200, Abcam). Deparaffinization, rehydration, and antigen retrieval of paraffin‐embedded tissue slides was accomplished by incubation of liver specimens in decreasing alcohol concentrations as described elsewhere. 33 Slides were then heated in sodium citrate buffer (pH 6.0) at 95‐100°C for 20 minutes and subsequently washed three times before being blocked with blocking buffer (1× PBS/5% goat serum) for 1 hour. Overnight incubation with the Ki‐67 antibody was carried out in TBS‐T containing 5% goat serum. The secondary antibody was added to the tissue sample area and incubated for 1 hour. A mounting medium with DAPI (Abcam) was applied according to manufacturer's instructions. The specimens were visualized under a microscope (Axio Scope.A1, Zeiss) on the same day to count the proportion of Ki‐67–positive cells. Hematoxylin‐eosin (H&E) staining was applied to visualize tumor histopathology following a standard protocol procedure. 34
2.3. Coffee preparation
For coffee administration, 500 mL water (Aqua Irrigation Solution, DeltaSelect) was boiled in a beaker and cooled for 10 seconds. Then, 10 g of ground coffee powder (Jacobs Krönung, Kraft Foods) was added and incubated for 1 minute, followed by filtration through a paper coffee filter (Melitta). 30
2.4. Measurement of serum aminotransferase activities
Serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured using a Fuji DRI‐CHEM NX500i (Fujifilm Cooperation) serum analyzer following the instructions of the manufacturer. For this analysis, blood was collected from DEN‐injected transgenic mice and centrifuged at 1900 g for 10 minutes. The obtained supernatant was stored at −20°C until further analysis.
2.5. Gene expression analysis
Total RNA was separately isolated from tumorous and nontumorous snap‐frozen liver tissue of sacrificed htgUGT1A‐WT and SNP mice by means of TRIzol Reagent (Invitrogen) according to the recommendations of the manufacturer. 5 μg of RNA was incubated with DNase I (Invitrogen) at room temperature for 15 minutes followed by a 10‐minute inactivation period at 65°C. SuperScript® III First‐Strand Synthesis System for RT‐PCR (Thermo Scientific) was subsequently used for synthesis of cDNA. Concentrations were determined by qPCR relative to mouse beta‐actin in a CFX96 real‐time PCR detection system (Bio‐Rad) with qPCR MasterMix (Eurogentec) and gene‐specific primers and probes. All reactions were performed in triplicates and were also repeated three times. Bio‐Rad CFX Manager 3.0 software was used to calculate the relative gene expression.
2.6. Western blot analysis
50 mg of snap‐frozen tumor or normal liver tissue was separately homogenized in 1 mL RIPA extraction buffer containing protease inhibitor cocktail (1:100) in a Qiagen TissueLyser and subsequently incubated for 1 hour on a shaking plate at 4°C. After 10 minutes centrifugation at 15 800 g at 4°C, supernatant was collected to determine protein concentration with Bradford reagent. For Western blot analysis, 60 µg of isolated protein was boiled at 95°C for 5 minutes in Laemmli sample buffer separated by SDS‐PAGE (10% polyacrylamide gel) and blotted onto a nitrocellulose membrane via electrotransfer by means of the Trans‐Blot®‐Turbo transfer system (Bio‐Rad). Incubation with primary antibodies (anti‐UGT1A, antibodies‐online.com ABIN2856950; and anti‐GAPDH, Santa Cruz sc‐32233) was carried out in 5% dry milk. After incubation with appropriate secondary antibodies (Santa Cruz sc‐516102 and sc‐2357), protein was visualized by chemiluminescence with the use of ChemiDOC™ MP imaging system (Bio‐Rad).
2.7. UGT‐Glo‐Assay
The activity of UGT1A enzymes was assessed using the UGT‐Glo™ Assay Kit (Promega). For microsome isolation, a pool of 100 mg snap‐frozen tumor or normal liver tissue was separately homogenized in 1 mL buffer (50 mmol/L Tris‐HCl with 10 mmol/L MgCl2 [pH 7.6]) and subsequently centrifuged at 10 000 g for 10 minutes at 4°C. The supernatant was collected, and the pellet was resuspended in 1 mL buffer and again centrifuged at 10 000 g for 10 minutes at 4°C to collect the supernatant once more. Afterwards, the combined supernatants were centrifuged at 100 000 g for 60 minutes at 4°C, and the pellet was resuspended in 0.4 mL buffer. Microsomal protein concentrations were determined by the method of Bradford and subsequently stored at −80°C until use. For UGT activity assay, 1 µg of microsomes isolated from mouse tissue was used per reaction, and enzyme activity was measured in triplicates after 35 minutes of incubation using 50 µmol/L proluciferin UGT multienzyme substrate according to manufacturer's instructions.
2.8. Statistical analysis
Data are expressed as mean ± standard deviation determined by Student’s t test to define significance. A pool of 9‐11 mice per group was analyzed (four mice per control group); P values below 0.05 were considered statistically significant. Given the high number of multiple comparisons for UGT1A expression levels, Bonferroni correction was applied. Here, the typical significance level of 0.05 was divided by 9, yielding a significance criterion of P < 0.006.
3. RESULTS
3.1. Incidence and size of HCC‐like tumor formations in htgUGT1A mice
In order to examine the effect of common UGT1A polymorphisms on the development and outcome of DEN‐induced hepatocarcinogenesis, tumor burden was recorded as the number and size of macroscopically evident tumor formations in htgUGT1A‐WT and SNP mice. A striking difference between the groups was observed, demonstrating a considerably higher tumor burden in the presence of SNPs (Figure 1A). Tumors were histologically analyzed by counting the proportion of Ki‐67–positive cells and evaluation of the histomorphological changes visible in H&E‐stained liver sections (Figure 1B). Even though the histological analysis revealed the presence of microvesicular fat incorporations, eosinophilic inclusions within the cytoplasm, cytoplasmic bile pigments, increased cell proliferation as well as elevated infiltration of inflammatory cells, no significant tumor‐specific differences and characteristics were observed between htgUGT1A‐WT and SNP mice and among the differently treated groups.
Figure 1.
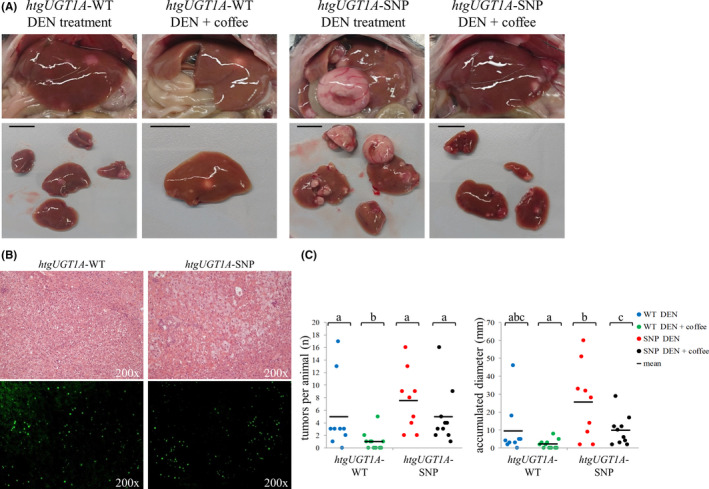
Manifestation of chemically induced hepatocarcinogenesis in htgUGT1A mice 40 wk after diethylnitrosamine (DEN) injection. A, Representative images of the liver of htgUGT1A–wild‐type (WT) and single‐nucleotide polymorphism (SNP) mice immediately after laparotomy (upper 4 panels) and the respective tumor‐affected liver lobes (lower 4 panels; black scale bar: 10 mm). B, Hematoxylin‐eosin (H&E) staining showing DEN‐induced liver tumors and Ki‐67 staining indicating increased cell proliferation, microvesicular fat incorporations, eosinophilic inclusions within the cytoplasm, and elevated infiltration of inflammatory cells. C, Assessment of tumor number and the cumulative diameter of each htgUGT1A mouse separately (each mouse is represented by one dot). Both, the total number of tumors and the accumulated diameter were highest in DEN‐injected htgUGT1A‐SNP and lowest in coffee‐cotreated htgUGT1A‐WT mice. Groups without the same letter are significantly different; P < 0.05 was considered statistically significant
All DEN‐treated htgUGT1A‐SNP mice (water‐ and coffee‐drinking) developed macroscopically detectable tumors within 40 weeks (Figure 1C, every mouse is represented by one spot), whereas no visible tumor formations were detected in one water‐drinking and five coffee‐cotreated htgUGT1A‐WT mice. This clearly shows a differential effect of both the presence of SNPs and the induction of UGT1As by coffee. Although the susceptibility to tumor growth differed considerably between individual mice within one group, the average number of tumors per mouse and the accumulated diameter of all tumors were higher in mice carrying the UGT1A SNP haplotype. Both number and size of tumors were decreased in coffee + DEN‐cotreated htgUGT1A‐WT and SNP mice. In total, 62 macroscopically visible tumors with a total cumulative diameter of 231 mm developed in DEN‐treated htgUGT1A‐SNP mice, compared with 44 tumors measuring 86 mm in diameter detected in htgUGT1A‐WT mice (Figure 2A + B), indicating a considerable difference of tumor burden between those groups.
Figure 2.
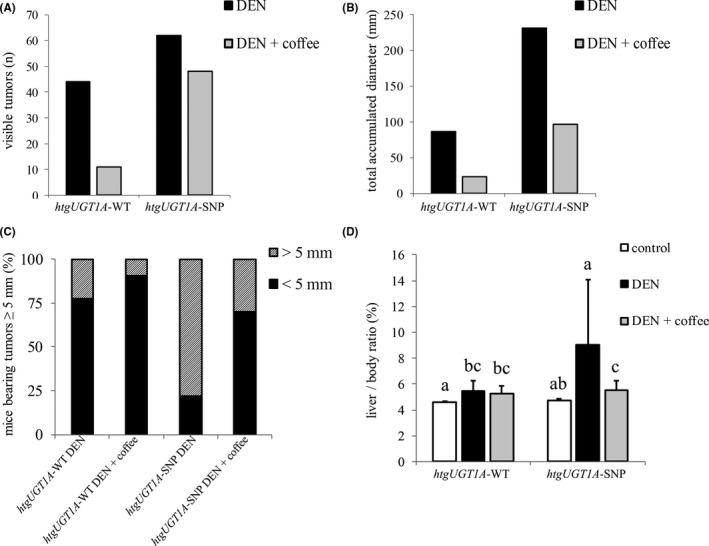
Tumor incidence and size of htgUGT1A mice 40 wk after chemical induction of hepatocarcinogenesis. Total number (A) and total accumulated diameter (B) of all visible tumors detected at the liver surface of htgUGT1A–wild‐type (WT) and single‐nucleotide polymorphism (SNP) mice. In the absence of UGT1A polymorphisms, less tumors with a reduced cumulative diameter were detected after diethylnitrosamine (DEN) and DEN + coffee cotreatment. C, Percentage proportion of mice bearing tumors bigger than 5 mm. The proportion of mice with especially large tumors was higher in htgUGT1A‐SNP mice. D, Liver/body ratio after DEN and DEN + coffee cotreatment. The relative liver weight was significantly higher in htgUGT1A‐SNP mice after DEN‐treatment, whereas a comparable ratio was observed in DEN + coffee‐cotreated mice. Groups without the same letter are significantly different; P < 0.05 was considered statistically significant
After coffee + DEN cotreatment, the number of visible tumors in both mouse lines was lower but was still shown to be higher in mice carrying the UGT1A SNP haplotype (SNP: 48 tumors/97 mm total diameter; WT: 11 tumors/23 mm total diameter). In addition, the percentage proportion of mice that developed especially large tumors (≥5 mm) reached 20% in DEN‐treated and 10% in coffee‐cotreated htgUGT1A‐WT mice (Figure 2C), compared with a considerably higher ratio detected in equally treated htgUGT1A‐SNP mice (water: 78%, coffee: 30%). In line with these results, the relative liver weight was significantly higher in DEN‐treated htgUGT1A‐SNP mice compared with htgUGT1A‐WT mice (Figure 2D). In coffee‐cotreated htgUGT1A‐SNP mice, the relative liver weight was lower and reached comparable levels to that measured in mice with regular UGT1A expression.
3.2. Serum aminotransferase activities in DEN‐treated htgUGT1A mice
Elevated aminotransferase activities are an indicator for hepatocellular damage and liver injury. 35 To assess their levels during mouse hepatocarcinogenesis, serum aminotransferase activities were determined in both experimental mouse lines (Figure 3). In DEN‐treated mice, significantly higher AST and ALT levels were measured in htgUGT1A‐SNP mice (AST: 107.4 U/L and ALT: 186.1 U/L) compared with minor elevations in htgUGT1A‐WT mice (AST: 62.2 U/L and ALT: 59.0 U/L). Coffee + DEN cotreatment lowered the AST and ALT values in both mouse lines, but the ALT levels remained slightly but significantly elevated in htgUGT1A‐WT (control: 31.0 U/L, coffee + DEN: 45.2 U/L) and htgUGT1A‐SNP mice (control: 36.2 U/L, coffee + DEN: 60.4 U/L) compared with their respective control group. Therefore, aminotransferase activities in serum appear to be higher in carriers of the SNP genotype and considerably decreased after induction treatment with coffee. Although significant (P < 0.05), the results did not meet the criteria for Bonferroni correction.
Figure 3.
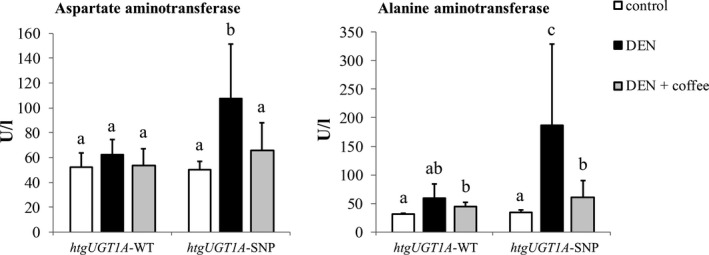
Aminotransferase activity level of htgUGT1A mice 40 wk after chemical induction of hepatocarcinogenesis. Liver injury was assessed by serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities. Mice carrying the Gilbert syndrome–associated single‐nucleotide polymorphism (SNP) haplotype had significantly higher AST and ALT levels. Each column represents the mean ± standard deviation. Groups without the same letter are significantly different; P < 0.05 was considered statistically significant. DEN, diethylnitrosamine; WT, wild‐type
3.3. Hepatic UGT1A expression in DEN‐treated htgUGT1A mice
To assess UGT1A regulation during tumorigenesis in the different groups, hepatic UGT1A gene expression was determined (Figure 4). In DEN‐treated htgUGT1A‐WT mice, the transcriptional activation of all investigated UGT1A genes was significantly higher in normal liver tissue compared with tumor tissue. As expected, lower UGT1A expression levels were determined in DEN‐treated htgUGT1A‐SNP mice. Interestingly, no transcriptional UGT1A induction was observed at the end of the 39‐week experiment after coffee cotreatment, although coffee had earlier been shown to increase UGT1A gene expression in vivo. 29 Of note, UGT1A expression within tumor tissue of coffee + DEN‐cotreated htgUGT1A‐WT mice was lower compared with that measured inside tumors of water‐receiving htgUGT1A‐WT mice. A comparable but lower expression level was observed in coffee + DEN‐cotreated htgUGT1A‐SNP mice. Only UGT1A1 and UGT1A6 expression showed a different pattern with elevated mRNA levels within tumors of coffee‐cotreated htgUGT1A‐SNP mice.
Figure 4.
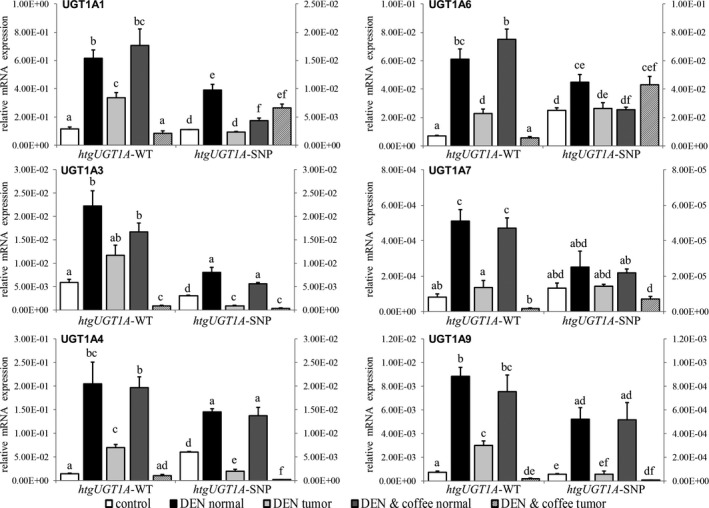
Hepatic UGT1A expression levels of htgUGT1A mice 40 wk after diethylnitrosamine (DEN)‐induced hepatocarcinogenesis. The graphs illustrate the mRNA expression of UGT1A genes relative to mouse β‐actin measured in normal or tumor tissue of DEN‐ and coffee + DEN‐treated htgUGT1A–wild‐type (WT) and single‐nucleotide polymorphism (SNP) mice. Values for htgUGT1A‐WT mice refer to the left y‐axis, those of htgUGT1A‐SNP mice refer to the right y‐axis. Significantly higher absolute expression levels were detected in htgUGT1A‐WT mice. No UGT1A induction was measured in normal liver tissue after DEN + coffee cotreatment. Except for UGT1A1 and UGT1A6 expression in htgUGT1A‐SNP mice, lower transcriptional activation was detected within tumor tissue. Each column represents the mean ± standard deviation. Groups without the same letter are significantly different; P < 0.006 was considered statistically significant
3.4. Hepatic UGT1A protein expression and enzyme activity in DEN‐treated htgUGT1A mice
With the intention to quantify hepatic UGT1A protein levels, Western blot analysis with an antibody detecting all human UGT1A isoforms was performed (Figure 5A). In line with the results of mRNA expression, hepatic protein expression in normal and tumor tissue of htgUGT1A‐WT mice was considerably higher compared with the levels measured in htgUGT1A‐SNP mice. Moreover, the downregulation of UGT1A protein in tumor tissue of DEN‐treated htgUGT1A‐WT and SNP mice was also detectable and is therefore in line with the mRNA results as well. After coffee + DEN cotreatment, an upregulation of UGT1A protein was measured in normal and especially in tumor tissue of htgUGT1A‐WT mice, whereas lower protein levels were observed in the presence of SNPs.
Figure 5.
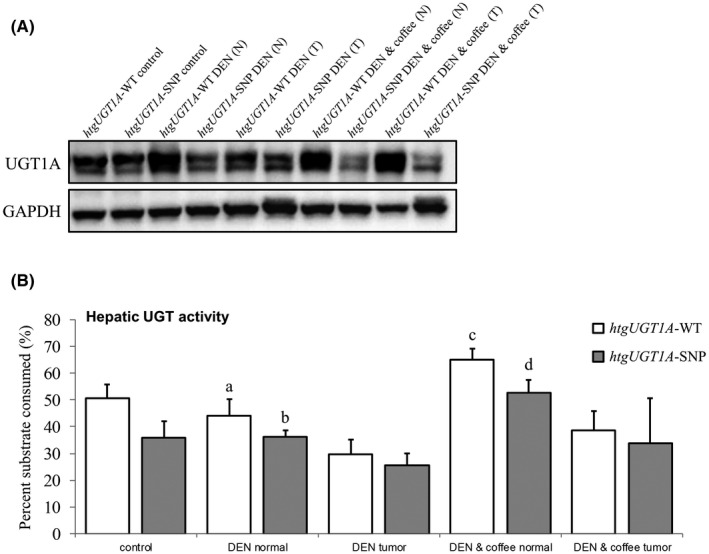
Hepatic UGT1A protein expression and enzyme activity in diethylnitrosamine (DEN)‐treated htgUGT1A mice 40 wk after DEN‐induced hepatocarcinogenesis. A, Western blot analysis of hepatic UGT1A protein quantity in normal (N) and tumor (T) tissue. Increased protein levels were detected in DEN‐treated htgUGT1A–wild‐type (WT) mice, and a considerable upregulation was observed after DEN + coffee cotreatment especially in tumor tissue. B, UDP‐glucuronosyltransferase (UGT) enzyme activity against a proluciferin substrate shown as percent of substrate consumed. Catalytic UGT activity was significantly induced after coffee cotreatment in both mouse lines with higher protein activity measured in htgUGT1A‐WT mice. Each column represents the mean ± standard deviation. Groups without the same letter are significantly different; P < 0.05 was considered statistically significant
In order to investigate a potential correlation of UGT1A enzyme activity with tumor burden and tumorigenesis, UGT activity levels were determined (Figure 5B). In general, significantly higher activity levels were measured in htgUGT1A‐WT mice compared with equivalently treated mice carrying the UGT1A SNP haplotype. In line with the results of UGT1A mRNA and protein expression, lower enzyme activity was measured within tumorous compared with nontumorous liver tissue. Of note, activity levels of UGT enzymes were significantly upregulated in normal liver tissue of both mouse lines after coffee + DEN cotreatment, with highest catalytic activity measured in coffee‐cotreated htgUGT1A‐WT mice (24.0% higher compared with htgUGT1A‐SNP mice). Moreover, the enzymatic activity also increased in tumor tissue after coffee + DEN cotreatment but did not reach the activity levels measured in normal tissue. In consequence, the UGT activity levels inversely correlate with the observed tumor burden of htgUGT1A‐WT and SNP mice, with higher activities reducing tumor incidence and growth. These data suggest that coffee‐mediated induction of UGT1A activity likely leads to an increased detoxification of carcinogenic compounds and consequently to an elevated degree of hepatoprotection, which in turn results in a decreased susceptibility for neoplastic transformation.
4. DISCUSSION
The presence of genetic polymorphisms leading to the impaired ability of an organism to detoxify genotoxic compounds and subsequently to an increased risk of tumorigenesis has been the hypotheses of case‐control studies investigating the individual genetic disposition for cancer. Low‐function UGT1A variants have been identified as an inheritable risk factor for cancer development. Thus far, direct experimental evidence corroborating this sequence of events has not been provided and was the aim of this study. By using htgUGT1A‐WT and SNP mice, combined with the well‐established model of DEN‐induced carcinogenic liver injury, we were able to confirm that expression and induction of UGT1A genes influence hepatocarcinogenesis, with higher activities exerting a protective function and reduced expression increasing tumorigenesis and tumor burden.
In agreement with the hypothesis of a protective effect of higher UGT1A detoxification function, mice carrying the UGT1A SNP haplotype, which is associated with lower carcinogen‐metabolizing gene expression and activity, were more susceptible to DEN‐induced hepatocarcinogenesis than htgUGT1A‐WT mice. This was clearly observable by a significantly higher number of liver tumors, accumulated tumor diameter, increased relative liver weight, and higher AST and ALT levels in htgUGT1A‐SNP mice compared with their WT counterparts. This corresponded to higher absolute expression and activity levels of UGT1A genes in the employed htgUGT1A‐WT mice compared with lower levels observed in htgUGT1A‐SNP mice. As glucuronidation of DEN has earlier been demonstrated in vivo, 36 the correlation between increased tumor incidences and the presence of UGT1A SNPs is likely attributable to the impaired clearance mechanism of cancerogenic DEN intermediates. Especially the reduced expression of UGT1A1, UGT1A4, and UGT1A7, which have been shown to play a crucial role in the detoxification of food‐borne primary amines, nitrosamines, and other cancerogenic compounds, 37 are a likely explanation for an increase of carcinogenesis in htgUGT1A‐SNP mice, in which they are expressed only at low levels. Interestingly, lower UGT1A expression and protein levels were measured inside liver tumors compared with normal surrounding non‐neoplastic liver tissue. Reduced UGT1A transcription in liver tumors may additionally decrease the ability of hepatocytes to eliminate mutagenic compounds and favor the conditions for the perpetuation of carcinogen‐induced cytotoxicity and genotoxicity potentially leading to additional mutational events. These data are in line with previously published results by Strassburg et al reporting lower UGT1A mRNA and protein levels in tumors of HCC patients compared with normal surrounding liver tissue of the same liver. 19 The consistency with such specific characteristics observed in humans suggests that the recreation of carcinogenesis in the DEN model shares important features with clinically encountered hepatocarcinogenesis in HCC in humans. Importantly, our data now provide direct experimental evidence that the regulation of UGT1A genes and their activity directly modulates the risk for neoplastic transformation and that this finding confirms case‐control association studies of UGT1A SNPs and HCC incidence.
The study further evaluated the effects of coffee in this context. In addition to its hepatoprotective properties and reduced cancer risk, coffee consumption is known for its capability to act as potent UGT1A inducer. 29 , 38 Coffee cotreatment was found to reduce tumor formation and progression in both mouse lines. Although coffee‐cotreated mice experienced a higher degree of protection than water‐drinking mice of the same genotype, coffee was not found to be associated with an increased UGT1A gene expression when samples were tested at the end of the 39‐week experimental period. Additionally, even lower mRNA expression levels were determined within tumors of coffee‐cotreated mice (except for UGT1A1 and UGT1A6 in htgUGT1A‐SNP mice). A likely explanation for this finding is the timing of the measurement. Most published reports show an induction of UGT1A gene regulation within days of exposure, which was different in our experimental setting. Data covering periods of 40 weeks have not yet been reported to our knowledge. However, coffee‐mediated induction of UGT1A enzymes especially during the initial phase of DEN administration and the molecular events prevalent at this time leading to neoplastic initiation are likely to occur in the same way as observed in previously published data. Due to the high cellular replication rate and the associated base mispairing as well as the error‐prone repair mechanism in the presence of carcinogen action, 39 young animals are more susceptible to DEN‐induced carcinogenesis. Therefore, the coffee‐mediated UGT1A activation immediately or shortly after DEN injection plays an essential role in the observed protection by coffee, and this may no longer be detectable after 40 weeks. Nevertheless, correlation between detoxification function of UGT1A enzymes and hepatoprotection by coffee was demonstrated, as increased UGT activity levels after coffee cotreatment were determined even at the end of the experimental period.
Besides the supposed role of UGT1As as effectors of the hepatoprotective properties of coffee in the prevention of carcinogen‐induced hepatocarcinogenesis, glucuronidation is also among the primary pathways for biotransformation of HCC chemotherapeutic agents such as sorafenib 40 and lenvatinib. 41 Accordingly, coffee consumption could also potentially influence the chemotherapeutic treatment of HCC. A coffee‐mediated induction of glucuronidation activity might increase the excretion of therapeutic agents, which would affect drug efficacy. On the other hand, protective effects of coffee consumption during irinotecan (a drug used in colorectal cancer therapy) treatment were shown in a recently published in vivo study. 42 In this study, a significant reduction of irinotecan toxicity–induced leukopenia, intestinal oxidative stress, and inflammation was demonstrated indicating potential clinical utility of coffee to reduce drug‐related side effects. However, to assess whether coffee‐mediated UGT1A activation influences drug efficacy during chemotherapy requires further study. Our study conclusively suggests that low‐function genetic UGT1A variants, observed in 10% of the white population, have a direct effect on the risk for neoplastic transformation and are a relevant risk factor for hepatocarcinogenesis and the development of HCC. High UGT1A expression and the induction of UGT1A transcription by coffee exposure were associated with protection against tumor development in a model of DEN‐induced hepatocarcinogenesis. As a consequence of our findings, UGT1A polymorphisms may play a role to predict HCC susceptibility and progression. In addition, chemoprevention by strategies aiming at inducing UGT1A expression, ie, by exposure to coffee constituents or other compounds may represent attractive preventive strategies in susceptible individuals for hepatocarcinogenesis.
DISCLOSURE
The authors have no conflict of interest.
ACKNOWLEDGEMENTSACKNOWLEDGEMENTS
Open access funding enabled and organized by Projekt DEAL.
Landerer S, Kalthoff S, Paulusch S, Strassburg CP. UDP‐glucuronosyltransferase polymorphisms affect diethylnitrosamine‐induced carcinogenesis in humanized transgenic mice. Cancer Sci. 2020;111:4266–4275. 10.1111/cas.14635
[Correction added on 08 Oct 2020, after first online publication: Projekt Deal funding statement has been added.]
REFERENCES
- 1. Hu DG, Mackenzie PI, McKinnon RA, Meech R. Genetic polymorphisms of human UDP‐glucuronosyltransferase (UGT) genes and cancer risk. Drug Metab Rev. 2016;48(1):47‐69. [DOI] [PubMed] [Google Scholar]
- 2. Strassburg CP. Gilbert‐Meulengracht's syndrome and pharmacogenetics: is jaundice just the tip of the iceberg? Drug Metab Rev. 2010;42(1):168‐181. [DOI] [PubMed] [Google Scholar]
- 3. Bock KW. Functions and transcriptional regulation of adult human hepatic UDP‐glucuronosyl‐transferases (UGTs): mechanisms responsible for interindividual variation of UGT levels. Biochem Pharmacol. 2010;80(6):771‐777. [DOI] [PubMed] [Google Scholar]
- 4. Strassburg CP. Pharmacogenetics of Gilbert's syndrome. Pharmacogenomics. 2008;9(6):703‐715. [DOI] [PubMed] [Google Scholar]
- 5. Mojarrabi B, Butler R, Mackenzie PI. cDNA cloning and characterization of the human UDP glucuronosyltransferase, UGT1A3. Biochem Biophys Res Commun. 1996;225(3):785‐790. [DOI] [PubMed] [Google Scholar]
- 6. Malfatti MA, Felton JS. N‐glucuronidation of 2‐amino‐1‐methyl‐6‐phenylimidazo[4,5‐b]pyridine (PhIP) and N_hydroxy‐PhIP by specific human UDP‐glucuronosyltransferases. Carcinogenesis. 2001;22(7):1087‐1093. [DOI] [PubMed] [Google Scholar]
- 7. Wiener D, Doerge DR, Fang JL, Upadhyaya P, Lazarus P. Characterization of N‐glucuronidation of the lung carcinogen 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanol (NNAL) in human liver: importance of UDP‐glucuronosyltransferase 1A4. Drug Metab Dispos. 2004;32(1):72‐79. [DOI] [PubMed] [Google Scholar]
- 8. Dellinger RW, Chen G, Blevins‐Primeau AS, Krzeminski J, Amin S, Lazarus P. Glucuronidation of PhIP and N‐OH‐PhIP by UDP‐glucuronosyltransferase 1A10. Carcinogenesis. 2007;28(11):2412‐2418. [DOI] [PubMed] [Google Scholar]
- 9. Tukey RH, Strassburg CP. Genetic Multiplicity of the Human UDP‐Glucuronosyltransferases and Regulation in the Gastrointestinal Tract. Mol Pharmacol. 2001;59(3):405‐414. [DOI] [PubMed] [Google Scholar]
- 10. Vogel A, Kneip S, Barut A, et al. Genetic link of hepatocellular carcinoma with polymorphisms of the UDP‐Glucuronosyltransferase UGT1A7 gene. Gastroenterology. 2001;121:1136‐1144. [DOI] [PubMed] [Google Scholar]
- 11. Guillemette C, Ritter JK, Auyeung DJ, Kessler FK, Housman DE. Structural heterogeneity at the UDP‐glucuronosyltransferase 1 locus: functional consequences of three novel missense mutations in the human UGT1A7 gene. Pharmacogenetics. 2000;10(7):629‐644. [DOI] [PubMed] [Google Scholar]
- 12. Girard H, Thibaudeau J, Court MH, et al. UGT1A1 polymorphisms are important determinants of dietary carcinogen detoxification in the liver. Hepatology. 2005;42(2):448‐457. [DOI] [PubMed] [Google Scholar]
- 13. Strassburg CP, Vogel A, Kneip S, Tukey RH, Manns MP. Polymorphisms of the UDP‐glucuronosyltransferase (UGT) 1A7 gene in colorectal cancer. Gut. 2002;50:851‐856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guillemette C. Pharmacogenomics of human UDP‐glucuronosyltransferase enzymes. Pharmacogenomics J. 2003;3(3):136‐158. [DOI] [PubMed] [Google Scholar]
- 15. Strassburg CP, Kalthoff S, Ehmer U. Variability and function of family 1 uridine‐5'‐diphosphate glucuronosyltransferases (UGT1A). Crit Rev Clin Lab Sci. 2008;45(6):485‐530. [DOI] [PubMed] [Google Scholar]
- 16. Sia D, Villanueva A, Friedman SL, Llovet JM. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152(4):745‐761. [DOI] [PubMed] [Google Scholar]
- 17. Llovet JM, Zucman‐Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. [DOI] [PubMed] [Google Scholar]
- 18. Lin S, Hoffmann K, Schemmer P. Treatment of hepatocellular carcinoma: a systematic review. Liver Cancer. 2012;1(3–4):144‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strassburg CP, Manns MP, Tukey RH. Differential down‐regulation of the UDP‐glucuronosyltransferase 1A locus is an early event in human liver and biliary cancer. Cancer Res. 1997;57(14):2979‐2985. [PubMed] [Google Scholar]
- 20. Court MH, Zhang X, Ding X, Yee KK, Hesse LM, Finel M. Quantitative distribution of mRNAs encoding the 19 human UDP‐glucuronosyltransferase enzymes in 26 adult and 3 fetal tissues. Xenobiotica. 2012;42(3):266‐277. [DOI] [PubMed] [Google Scholar]
- 21. Wang Y, Kato N, Hoshida Y, et al. UDP‐glucuronosyltransferase 1A7 genetic polymorphisms are associated with hepatocellular carcinoma in Japanese patients with hepatitis C virus infection. Clin Cancer Res. 2004;10(7):2441‐2446. [DOI] [PubMed] [Google Scholar]
- 22. Tseng CS, Tang KS, Lo HW, Ker CG, Teng HC, Huang CS. UDP‐glucuronosyltransferase 1A7 genetic polymorphisms are associated with hepatocellular carcinoma risk and onset age. Am J Gastroenterol. 2005;100(8):1758‐1763. [DOI] [PubMed] [Google Scholar]
- 23. Stücker I, Loriot MA, N'Koutchou G, et al. UDP‐glucuronosyltransferase UGT1A7 genetic polymorphisms in hepatocellular carcinoma: a differential impact according to seropositivity of HBV or HCV markers? BMC Cancer. 2007;7:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lankisch TO, Vogel A, Eilermann S, et al. Identification and characterization of a functional TATA box polymorphism of the UDP glucuronosyltransferase 1A7 gene. Mol Pharmacol. 2005;67(5):1732‐1739. [DOI] [PubMed] [Google Scholar]
- 25. Ehmer U, Kalthoff S, Fakundiny B, et al. Gilbert syndrome redefined: a complex genetic haplotype influences the regulation of glucuronidation. Hepatology. 2012;55(6):1912‐1921. [DOI] [PubMed] [Google Scholar]
- 26. Morisco F, Lembo V, Mazzone G, Camera S, Caporaso N. Coffee and liver health. J Clin Gastroenterol. 2014;48(Suppl 1):S87‐90. [DOI] [PubMed] [Google Scholar]
- 27. Marventano S, Salomone F, Godos J, et al. Coffee and tea consumption in relation with non‐alcoholic fatty liver and metabolic syndrome: A systematic review and meta‐analysis of observational studies. Clin Nutr. 2016;35(6):1269‐1281. [DOI] [PubMed] [Google Scholar]
- 28. Bravi F, Bosetti C, Tavani A, Gallus S, La Vecchia C. Coffee reduces risk for hepatocellular carcinoma: an updated meta‐analysis. Clin Gastroenterol Hepatol. 2013;11(11):1413‐1421.e1. [DOI] [PubMed] [Google Scholar]
- 29. Kalthoff S, Ehmer U, Freiberg N, Manns MP, Strassburg CP. Coffee induces expression of glucuronosyltransferases via the aryl hydrocarbon receptor and Nrf2 in liver and stomach. Gastroenterology. 2010;139(5):1699–1710. [DOI] [PubMed] [Google Scholar]
- 30. Kalthoff S, Landerer S, Reich J, Strassburg CP. Protective effects of coffee against oxidative stress induced by the tobacco carcinogen benzo[alpha]pyrene. Free Radic Biol Med. 2017;108:66‐76. [DOI] [PubMed] [Google Scholar]
- 31. Landerer S, Kalthoff S, Paulusch S, Strassburg CP. A Gilbert syndrome‐associated haplotype protects against fatty liver disease in humanized transgenic mice. Sci Rep. 2020;10(1):8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen B, Liu L, Castonguay A, Maronpot RR, Anderson MW, You M. Dose‐dependent ras mutation spectra in N‐nitrosodiethylamine induced mouse liver tumors and 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanone induced mouse lung tumors. Carcinogenesis. 1993;14(8):1603‐1608. [DOI] [PubMed] [Google Scholar]
- 33. Liou GY, Storz P. Detecting reactive oxygen species by immunohistochemistry. Methods Mol Biol. 2015;1292:97‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Itagaki H, Shimizu K, Morikawa S, Ogawa K, Ezaki T. Morphological and functional characterization of non‐alcoholic fatty liver disease induced by a methionine‐choline‐deficient diet in C57BL/6 mice. Int J Clin Exp Pathol. 2013;6(12):2683‐2696. [PMC free article] [PubMed] [Google Scholar]
- 35. Cheong JY, Kim DJ, Hwang SG, et al. Serum markers for necroinflammatory activity in patients with chronic viral hepatitis and normal or mildly elevated aminotransferase levels. Liver Int. 2011;31(9):1352‐1358. [DOI] [PubMed] [Google Scholar]
- 36. Wiench K, Frei E, Schroth P, Wiessler M. 1‐C‐glucuronidation of N‐nitrosodiethylamine and N‐nitrosomethyl‐n‐pentylamine in vivo and in primary hepatocytes from rats pretreated with inducers. Carcinogenesis. 1992;13(5):867‐872. [DOI] [PubMed] [Google Scholar]
- 37. Strassburg CP, Manns MP, Tukey RH. Expression of the UDP‐glucuronosyltransferase 1A locus in human colon. Identification and characterization of the novel extrahepatic UGT1A8. J Biol Chem. 1998;273(15):8719‐8726. [DOI] [PubMed] [Google Scholar]
- 38. Bravi F, Tavani A, Bosetti C, Boffetta P, La Vecchia C. Coffee and the risk of hepatocellular carcinoma and chronic liver disease: a systematic review and meta‐analysis of prospective studies. Eur J Cancer Prev. 2017;26(5):368‐377. [DOI] [PubMed] [Google Scholar]
- 39. Vesselinovitch SD, Mihailovich N. Kinetics of diethylnitrosamine hepatocarcinogenesis in the infant mouse. Cancer Res. 1983;43(9):4253‐4259. [PubMed] [Google Scholar]
- 40. Zimmerman EI, Roberts JL, Li L, et al. Ontogeny and sorafenib metabolism. Clin Cancer Res. 2012;18(20):5788‐5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dubbelman A‐C, Nijenhuis CM, Jansen RS, et al. Metabolite profiling of the multiple tyrosine kinase inhibitor lenvatinib: a cross‐species comparison. Invest New Drugs. 2016;34(3):300‐318. [DOI] [PubMed] [Google Scholar]
- 42. Kalthoff S, Paulusch S, Rupp A, Holdenrieder S, Hartmann G, Strassburg CP. The coffee ingredients caffeic acid and caffeic acid phenylethyl ester protect against irinotecan‐induced leukopenia and oxidative stress response. Br J Pharmacol. 2020;177:4193‐4208. [DOI] [PMC free article] [PubMed] [Google Scholar]


