Abstract
Four different tests showed the effectiveness of Azolla pinnata plant extracts against Aedes aegypti and Aedes albopictus mosquitoes. In the adulticidal test, there was a significant increase in mortality as test concentration increases and A. pinnata extracts showed LC50 and LC95 values of 2572.45 and 6100.74 ppm, respectively, against Ae. aegypti and LC50 and LC95 values of 2329.34 and 5315.86 ppm, respectively, against Ae. albopictus. The ovicidal test showed 100% eggs mortality for both species tested for all the concentrations tested at 1500 ppm, 1000 ppm, 500 ppm, 250 ppm and 125 ppm. Both tested samples of Ae. aegypti and Ae. albopictus did not lay any eggs in the plastic cups filled with the A. pinnata extract but instead opted to lay eggs in the plastic cups filled with water during the oviposition deterrence test. Similarly, the non-choice test of Ae. aegypti mosquitoes laid eggs on the sucrose solution meant for the nutrient source of the mosquitoes instead of in the plastic cup that was designed to facilitate oviposition filled with the extract. This clearly indicates the presence of bioactive compounds which are responsible in adulticidal and ovicidal activity in Aedes mosquitoes and at the same time inducing repellence towards the mosquitoes. The LC–MS results showed mainly three important chemical compounds from A. pinnata extracts such as 1-(O-alpha-D-glucopyranosyl)-(1,3R,25R)-hexacosanetriol, Pyridate and Nicotinamide N-oxide. All these chemicals have been used for various applications such as both emulsion and non-emulsion type of cosmetics, against mosquito vector such as Culex pipens and Anopheles spp. Finally, the overall view of these chemical components from A. pinnata extracts has shown the potential for developing natural product against dengue vectors.
Subject terms: Biochemistry, Chemical biology
Introduction
Dengue is transmitted by viral infected female mosquitoes known as Aedes albopictus and Aedes aegypti, primary vectors for vector-borne diseases which affect humans1. Dengue is known as one of the most common infections for humans, which is transmitted through bites of infected Aedes mosquitoes. It is considered to be a major health problem in tropical and subtropical countries2. Currently, Malaysia has recorded 80,000 dengue cases, with 113 deaths between January and August 20193.
Physical control method is always a daunting task as it provides temporary solutions and is currently used as equipment’s such as bed nets, clothes and mosquito rackets4. Meanwhile, the use of chemical method such as temephos and pyrethroids is more prominent but it has resistance challenges. Problems of resistance in mosquitoes towards the application of permethrin and temephos has been reported in two major cities in Malaysia, namely Kuala Lumpur and Penang5,6. The insecticide resistances in mosquitoes are caused by factors of alterations in its targeted sites and the increase in insecticide metabolisms rate7.
Current application of the synthetic chemical controls and its constant repetitive applications have resulted in resistant mosquitoes and environmental pollutions8. Thus, the limited success of biocontrol programs on Aedes has encouraged the necessity for new insecticide search. Plant products have produced positive outcomes as an alternative to chemicals in dengue vector control programs. With this concept in mind, the use of Azolla pinnata has been recently reported against Aedes vectors4,6,8–10. Most of A. pinnata studies were only conducted for Aedes larvicidal effects and for its chemical compositions analyzed with emitted compounds by gas chromatographic techniques. Our previous findings revealed that the most effective A. pinnata extraction technique used was soxhlet extraction using methanol solvent in showingAedes larvicidal activity. Thus, as a progression, this current study used the same extraction techniques4,6,8. Furthermore, previous findings of A. pinnata plant reiterates the killing mechanisms of Aedes larvae by direct contact applications or poison effects without being toxic to other organisms4. Thus, it is vital to investigate the applications of direct contact from A. pinnata plant extracts against adult Aedes mosquitoes and to its entire life cycle satges. Moreover, it is necessary to investigate the chemical constituent of A. pinnata in its liquid compositions, which may be responsible against Aedes mosquitoes’ interactions. Meanwhile, unlike conventional insecticides, the advantages of plant-derived insecticides composed by botanical blends of multiple chemical compounds is that it may act concertedly on both physiological and behavioural processes11.
The alternative uses of bio-insecticides will provide a more suitable and sustainable solution against Ae. aegypti and Ae. albopictus. In line with United Nations (UN) global sustainable goals and following a safer and greener alternative conception from A. pinnata plant, the purpose of this study is to evaluate the effectiveness of A. pinnata liquid extracts on Aedes adulticidal, ovicidal and ovipositional deterring activity and its liquid chemical compounds.
Results
Adulticidal activity
As presented in Fig. 1, the adulticidal bioassay test showed a significant increase in mortality with increasing A. pinnata plant extract concentration. The lethal concentrations, LC50 and LC95, for Ae. aegypti were recorded at 2572.45 ppm and 6100.74 ppm, respectively, while testing on Ae. albopictus recorded the LC50 and LC95 values at 2329.34 ppm and 5315.86 ppm, respectively (Table 1). In the control assay, there was no significant mortality.
Figure 1.
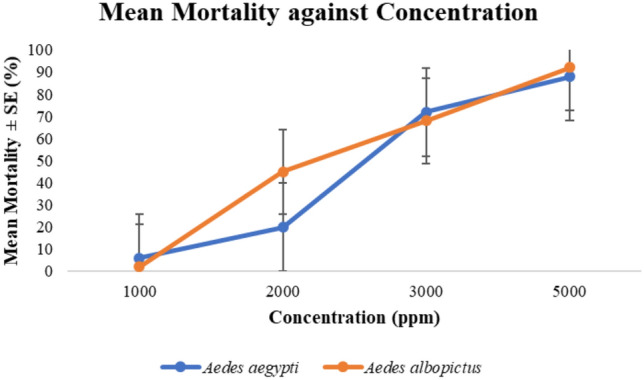
Dose response relationship of the different concentrations of A. pinnata extracts on the mortality on Ae. aegypti and Ae. albopictus.
Table 1.
Mean LC50 and LC95 (in ppm) adult efficacy of A. pinnata plant after 24 h of exposure on Ae. aegypti and Ae. albopictus (95% confidence limit).
| Species | LC50 | LC95 | Regression equation |
|---|---|---|---|
| Ae. aegypti | 2572.45 | 6100.74 | Y = − 14.96 + 4.39X |
| Ae. albopictus | 2329.34 | 5315.86 | Y = − 17.55 + 1.55X |
Ovicidal activity
The ovicidal test showed that all the eggs of Ae. aegypti and Ae. albopictus possess mortality during the immersion into the trays containing A. pinnata plant extracts of varying concentrations as shown in Fig. 2 and Table 2. The concentration of 30 ppm caused 81% mortality for Ae. aegypti and 83% for Ae. albopictus. Furthermore, the concentration of 75 ppm caused 90% mortality for Ae. aegypti and 91% for Ae. albopictus. All the other concentrations recorded 100% mortality. Meanwhile, the full hatchability of Aedes eggs can be seen in control treatments.
Figure 2.
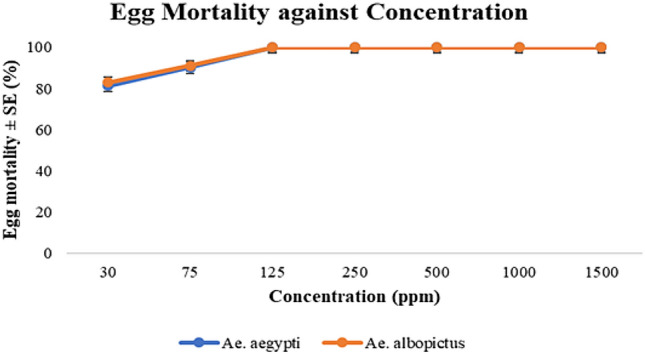
Graph of egg mortality (%) of Ae. aegypti and Ae. albopictus on different concentrations of Azolla extracts.
Table 2.
Egg mortality (%) of Ae. aegypti and Ae. albopictus on different concentrations of Azolla extracts.
| Species | Control | Concentration (ppm) | ||||||
|---|---|---|---|---|---|---|---|---|
| 30 | 75 | 125 | 250 | 500 | 1000 | 1500 | ||
| Ae. aegypti | 0 | 81 | 90 | 100 | 100 | 100 | 100 | 100 |
| Ae. albopictus | 0 | 83 | 91 | 100 | 100 | 100 | 100 | 100 |
Oviposition deterrence assay
Both Aedes mosquitoes preferred to lay eggs only in the untreated cups. This can be clearly seen in Fig. 3, 4, 5, 6 and further proven through the data on Table 3 and 4 where by, the effective repellency is at 100% for all five concentrations. However, for choice test, Ae. aegypti and Ae. albopictus mosquitoes at a concentration of 30 ppm caused 80% repellence for Ae. aegypti and 81% for Ae. albopictus. The 75 ppm concentration caused 89% repellence for Ae. aegypti and 90% for Ae. albopictus. The non-choice test recorded 82% repellence for both Ae. aegypti and Ae. albopictus for 30 ppm concentration and at concentration 75 ppm caused 91% repellence for Ae. aegypti and 90% for Ae. albopictus. Interestingly, Ae. aegypti in the non-choice test laid eggs on sucrose solution which was placed as diet enrichment of mosquitoes as seen in Fig. 6.
Figure 3.
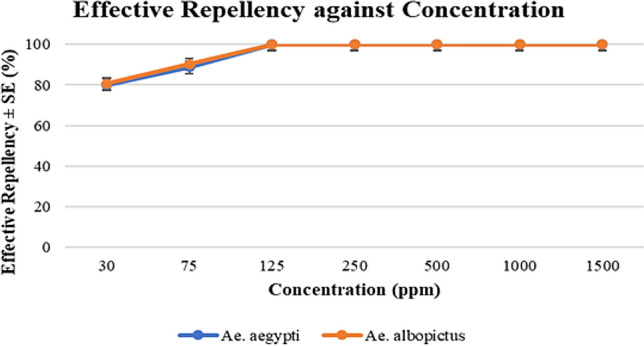
Graph of effective repellence of different concentrations of Azolla extracts on Ae. aegypti and Ae. Albopictus.
Figure 4.
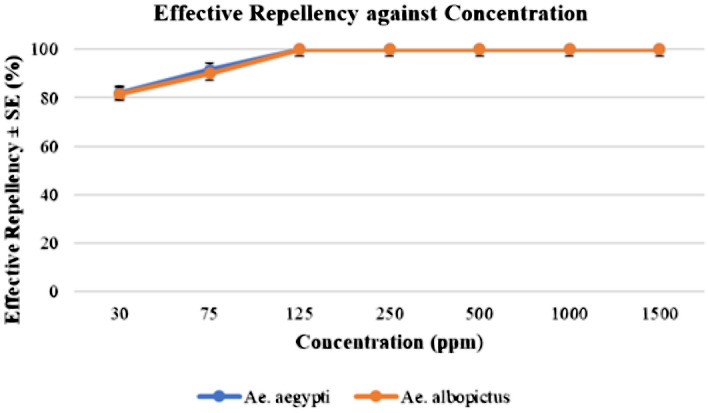
Graph of effective repellence of different concentrations of Azolla extracts on Ae. aegypti and Ae. albopictus (non-choice test).
Figure 5.
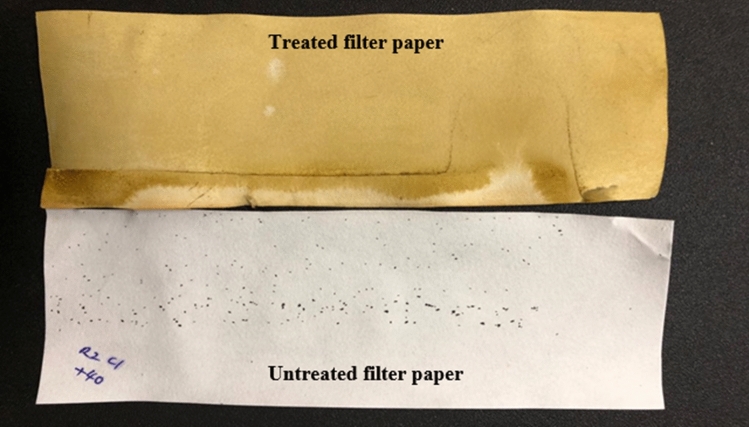
Eggs found on the untreated filter paper. No eggs found on the treated filter papers.
Figure 6.
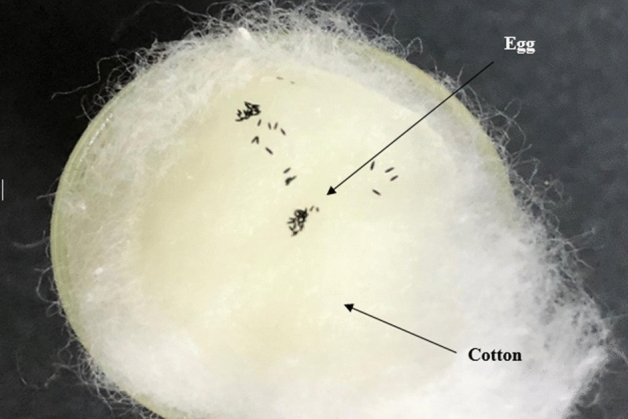
Eggs were found laid on the sucrose provided as a food sources in the non-choice oviposition test.
Table 3.
Effective repellence of different concentrations of Azolla extracts on Ae. aegypti and Ae. albopictus.
| Species | Control | 30 | 75 | 125 | 250 | Concentration (ppm) | ||
|---|---|---|---|---|---|---|---|---|
| 500 | 1000 | 1500 | ||||||
| Ae. aegypti | 0 | 80 | 89 | 100 | 100 | 100 | 100 | 100 |
| Ae. albopictus | 0 | 81 | 90 | 100 | 100 | 100 | 100 | 100 |
Table 4.
Effective repellence of different concentrations of Azolla extracts on Ae. aegypti and Ae. albopictus (Non-choice test).
| Species | Control | 30 | 75 | 125 | 250 | Concentration (ppm) | ||
|---|---|---|---|---|---|---|---|---|
| 500 | 1000 | 1500 | ||||||
| Ae. aegypti | 0 | 82 | 91 | 100 | 100 | 100 | 100 | 100 |
| Ae. albopictus | 0 | 82 | 90 | 100 | 100 | 100 | 100 | 100 |
Discussion
The experimental test showed that A. pinnata plant extracts can be used for Aedes adulticidal activities by impregnated paper method (method similarly used to test chemical insecticides). However, A. pinnata extract acted as relatively slow insecticidal chemical with moderate LC values when tested using Ae. aegypti and Ae. albopictus. During the adult bioassay tests, it can be observed that the tested mosquitoes tend to rest at the top of the bioassay kit instead of the impregnated papers. Thus, this suggests that the impregnated papers were repelling agent as well as killing agents for the mosquitoes. These findings can be confirmed by a study of Nsirim et al., (2013), which suggested that A. pinnata fresh plant possess repellent attributes which are commonly used in Southeast Nigeria for repelling mosquitoes. However, current study is the first study to test the crude extracts of A. pinnata plant instead of fresh plant applications.
Furthermore, findings of this current study have piqued our interest to further test on A. pinnata extract for ovicidal and oviposition deterrence. In current ovicidal test, there were no eggs hatched for higher than 125 ppm treated trays indicating, 100% egg mortality for all the concentrations of both Ae. aegypti and Ae. albopictus. Similarly results by Govindarajan et al., (2010), with the Ervatamia coronaria and Caesalpinia pulcherrima plant extracts caused 100% egg mortality on Culex quinquefasciatus, Ae. aegypti and Anopheles stephensi at higher than 300 ppm. Meanwhile, the eggs in the control treatment had 0% mortality when immersed in regular de-chlorinated water. Additionally, the eggs mortality was 80% till 90% for A. pinnata extract concentration of 30 ppm and 75 ppm. These proved that even at the lowest concentration,the efficacy was high in causing Aedes eggs mortality.
The oviposition deterrence test proved that the eggs can only be observed in the plain water medium and not in A. pinnata plant extracts. Additionally, the non-choice test shows that without any choice or options, the Ae. aegypti and Ae. albopictus laid their eggs on the cotton soaked with sucrose solution. As discussed earlier, it is obvious that the methanolic extract of A. pinnata had repellence properties. Thus, at 500 ppm concentration of plant extract, the Aedes mosquitoes will not lay their eggs in test cups.
In this current study, all the results of adulticidal, ovicidal, oviposition deterrence were between concentrations of 125 ppm till 6100 ppm. Meanwhile, previous larvicidal results by Ravi et al., (2018) on A. pinnata extract reported lethal concentration of 1000 ppm till 1500 ppm. In an overall view, the concentration of LC50 for adulticidal, 2572.45 ppm can be used for all the assays against Aedes mosquitoes. Thus, a single concentration of A. pinnata plant extracts will be effective for further field-based applications and it may serve as alternative for vector control strategies. In order to proceed with field-based applications, some contribution of knowledge is required on the chemical compositions from A. pinnata plant extracts.
The LC–MS results (Table 5) have shown the presence of various compounds in the A. pinnata extracts which can be difficult to determined. In this study, the three most likely active compounds with promising ovicidal and repellency activities, namely 1-(O-alpha-D-glucopyranosyl)-(1,3R,25R)-hexacosanetriol, pyridate and nicotinamide N-oxide were selectively identified. Other compounds were not likely to contribute to the ovicidal and repellency activities. According to Kawasaki et al., (2006), chemical compound of 1-(O-alpha-D-glucopyranosyl)-(1,3R,25R)-hexacosanetriol is used for antibacterial cosmetic composition which is easily applied to both emulsion and non-emulsion type of cosmetics. Thus,1-(O-alpha-D-glucopyranosyl)-(1,3R,25R)-hexacosanetriol compound has the potential to be used as a natural ingredient for formulations in developing mosquito repellence creams in near future. Furthermore, pyridate compound were also used in several studies against mosquito vector such as Culex pipens and has been proven effective12. Besides that, nicotinamide N-oxide is used as insecticides in commercialized products and tested to be effective against Anopheles13. Our previous studies on A. Pinnata have recorded several interesting chemical compounds with insecticidal properties such as n-hexadecanoic acid, diethyl phthalate, neophytadiene, 3,7,11,15-tetramethyl-2-hexadecen-1-ol4,8 . The chemical analysis conducted in previous studies of A. Pinnata were based on GC–MS analysis which differs compared to the current study due to the progressive experimental efforts in classifying all the volatile compounds, as LC–MS analysis is suitable for compounds of lower volatility. The overall view of these chemical components from A. pinnata extract has shown the potential for developing natural product against dengue vectors.
Table 5.
Liquid Chromatography-Mass Spectrometry (LCMS), chemical compounds, retention time, molecular weight and properties of A. pinnata extract.
Methodology
Plant extraction
Azolla pinnata plant were collected at Kuala Krai, Kelantan (50 31′ N 1020 12′ E), dried and blended. The fresh plant sample was then extracted using Soxhlet extraction technique (Favorit, Malaysia). Briefly, 40 g dried plant powder sample was weighed, and then placed on a paper thimble. Cotton wools were placed on the upper part of the thimble to prevent the sample from evaporating. Exactly 1 L, of methanol solvent was placed in the round-bottom flask with the heating mantle underneath. The extraction in soxhlet apparatus were at boiling point 70 °C for about 3 h until the solvent in the siphon arm becomes clear, indicating complete extraction process. The extracts were then evaporated in the vacuum evaporator in order. The crude extracts were kept at − 20 °C until further used.
Aedes rearing
Eggs of Aedes aegypti and Aedes albopictus were obtained from Vector Control Research Unit (VCRU) at Universiti Sains Malaysia (USM), Penang, Malaysia. We follow the method used by Ahbirami et al., (2014) in larvae rearing. The eggs were hatched in de-chlorinated water for 24 h and maintained at 25–30 °C, pH 6.95 to 7.03, and relative humidity of 80 ± 10% and dissolved oxygen of 5.5–6.1 mg/L in the laboratory. The larvae were then reared until they pupated. The pupae were then transferred into mosquito cages to allow the mosquitoes to emerge. Sucrose solution was provided in the mosquito cage to provide food source for the mosquitoes.
Adulticidal activity
The paper impregnation method was adopted from Kovendan et al., (2013). The Azolla pinnata plant extracts were diluted with methanol to make the different concentrations (30, 75, 125, 250, 500, 1000, 1500, 2000, 3000, 5000, 6000 ppm) prepared. Next, as shown in Fig. 7, the diluted plant extracts were impregnated on filter papers (140 × 120 mm)14. A blank paper consisting of only methanol was used as control14. All the papers were left to dry at room temperature to evaporate off the methanol overnight14. The methods for the adulticidal activity were adapted from WHO, 1997. Female, Ae. aegypti and Ae. albopictus mosquitoes aging 2–5 days old of each strain were used in the separate test as they are fully developed and have reached sexual maturity at this age. A total of 25 female mosquitoes were collected with an aspirator and were placed in each holding tubes of the bioassay kits. The holding tubes were left for an hour acclimation time to check on any weak or unhealthy mosquitoes that might die in the tube. Dead mosquitoes were replaced with new ones and the test was continued. Strips of impregnated papers with different concentrations of methanolic A. pinnata extracts were rolled into the exposure tubes of the bioassay kits. The mosquitoes in the holding tubes were then blown into the exposure tubes and left in the exposure tube for an hour (Fig. 2).
Figure 7.
Plant extracts were impregnated on filter papers (140 × 120 mm).
The numbers of the mosquitoes knocked-down were recorded at an interval of 5 min throughout the 60 min. The knockdown of mosquitoes was evaluated with the inability of the mosquitoes to stand up or fly (Jahangir et al., 2008). After 1 h, all 25 mosquitoes were then collected and placed in a paper cup with cotton bud soaked in sucrose solution containing 10% of vitamin B complex and was left for 24 h. The mortality of among the 25 post-treated mosquitoes was obtained. A negative control test was done with impregnated paper containing oil where no mortality was recorded. After the bioassay test, the remaining surviving mosquitoes after 24 h were taken out and were used for the subsequent test. A total of four replicates were done for each species and solvent used to obtain a better data set.
Ovicidal activity
The methodology for the ovicidal activity was followed by Su and Mulla (1998) with slight modification. Total of 100 eggs of Ae. aegypti and Ae. albopictus were immersed in 6 trays. 5 trays filled with methanolic A. pinnata extracts at different concentrations; 1500 ppm, 1000 ppm, 500 ppm, 250 ppm and 125 ppm. Control cup was filled with distilled water. The test was conducted in 5 replicates for each concentration. After treatment, the eggs from each treatment were counted under microscope and transferred into distilled water cup for hatching assessments. The percentage of eggs mortality was calculated based on the non-hatchability of eggs with unopened opercula. The hatch rates were assessed by 48 h post-treatment using formula by Govindarajan (2011);
Oviposition deterrence activities
The oviposition deterrent activity was conducted in a laboratory using the method of Reegan et al., (2013)15 and a dual-choice oviposition bioassay was performed on gravid females of Ae. aegypti. Fifteen, gravid females (5 days old) of Ae. aegypti were introduced into an insect cage (30 cm × 30 cm × 30 cm) under room conditions. The adults were provided with 10% glucose solution which was available at all time. Two 50 mL plastic cups were filled with dechlorinated water and two 50 mL plastic cups were filled with methanolic A. pinnata extracts for oviposition. Different concentrations of 1500 ppm, 1000 ppm and 500 ppm of a plant extract solutions were used in different cages. A support for oviposition was provided by placing a piece of filter paper (Whatman No.1) on the inner surface of each plastic cup so that the lower half of it was submerged in the treated solution or untreated solution for the whole paper to get moistened while the upper half of it was above the solution where the mosquitoes would lay their eggs on. The untreated and treated cups were placed at alternate diagonally opposite locations for each replicate to nullify any effect of their locations on oviposition. After 3 days, the numbers of eggs laid in the treated and untreated cups were counted under a stereomicroscope. A non-choice test, was also conducted by placing one 50 mL plastic cup filled with A. pinnata and placed with filter paper into the mosquito cage with 15 gravid female Aedes mosquitoes. The test was conducted with different concentrations as well. Percent effective repellency (ER percentage) for each oviposition repellent concentration was calculated using the following formula from Xue et al., (2001):
where ER = percent effective repellency; NC = number of eggs in control; and NT = number of eggs in treatment.
Liquid chromatography–mass spectrometry (LCMS)
LC–MS was performed with Shimadzu LCMS systems using Agilent C18 column (I.D. 2.0 × 150 mm,), at a flow-rate of 0.25 ml min−1 , 250 °C. The eluent was MeCN/H2O/HCOOH (3 : 92 : 5). Expected Empirical Formula: Polar Compounds (40–1300amu) mass range.
Conclusion
In conclusion, the A. pinnata plant extracts showed promising results in adulticidal and ovicidal test against Ae. aegypti and Ae. albopictus. The oviposition deterrence test showed that there are repellent properties present from the A. pinnata extract. With the identification of chemical compounds in the A. pinnata extract, it can be developed as bio-insecticides for Aedes mosquito vector control. This study also suggests that future research work can be conducted on the field applications of A. pinnata extracts for its long-term effects on other non-target organisms, including on human health.
Supplementary information
Author contributions
R.R.—wrote and edit the main manuscript; D.R.—wrote the manuscript W.-D.O—chemical analysis; M.S.M.R—data Collection; Z.H.—manuscript editor and proofreader; I.H.I.—project supervision; M.F.M.A— project supervision and funding provider.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Intan H. Ishak, Email: intanishak@usm.my
Mohamad Faiz Mohd Amin, Email: mohamadfaiz@umk.edu.my.
Supplementary information
is available for this paper at 10.1038/s41598-020-75054-0.
References
- 1.Peng Z, et al. Immune responses to mosquito saliva in 14 individuals with acute systemic allergic reactions to mosquito bites. J Allergy Clin Immunol. 2004;114:1189–1194. doi: 10.1016/j.jaci.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 2.McKerr C, Lo Y-C, Edeghere O, Bracebridge S. Evaluation of the national notifiable diseases surveillance system for dengue fever in Taiwan, 2010–2012. PLoS Negl. Trop Dis. 2015;9:e0003639. doi: 10.1371/journal.pntd.0003639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaki R, et al. Public perception and attitude towards dengue prevention activity and response to dengue early warning in Malaysia. PLoS ONE. 2019;14:e0212497. doi: 10.1371/journal.pone.0212497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravi R, et al. Chemical composition and larvicidal activities of Azolla pinnata extracts against Aedes (Diptera: Culicidae) PLoS ONE. 2018;13:e0206982. doi: 10.1371/journal.pone.0206982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranson, H., Burhani, J., Lumjuan, N. & Black IV, W. C. Insecticide resistance in dengue vectors. TropIKA 1. http://journal.tropika.net/pdf/tropika/v1n1/a03v1n1.pdf (2010).
- 6.Zulkrnin H, et al. Larvicidal effectiveness of Azolla pinnata against Aedes aegypti (Diptera: Culicidae) with its effects on larval morphology and visualization of behavioural response. J. Parasitol. Res. 2018;2018:1383186. doi: 10.1155/2018/1383186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishak IH, Jaal Z, Ranson H, Wondji CS. Contrasting patterns of insecticide resistance and knockdown resistance (kdr) in the dengue vectors Aedes aegypti and Aedes albopictus from Malaysia. Parasites Vectors. 2015;8:181. doi: 10.1186/s13071-015-0797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravi R, et al. Evaluation of two different solvents for Azolla pinnata extracts on chemical compositions and larvicidal activity against Aedes albopictus (Diptera: Culicidae) J. Chem. 2018 doi: 10.1155/2018/7453816. [DOI] [Google Scholar]
- 9.Kusumawathie P, Wickremasinghe A, Karunaweera N, Wijeyaratne M. Larvivorous potential of fish species found in river bed pools below the major dams in Sri Lanka. J. Med. Entomol. 2006;43:79–82. doi: 10.1093/jmedent/43.1.79. [DOI] [PubMed] [Google Scholar]
- 10.Ravi R, Rasat MSM, Ishak IH, Amin MFM. Larvicidal effects of nano-synthesized silver particles from Azolla Pinnata extract against Aedes Aegypti (Diptera: Culicidae) Int. J. Innov. Technol. Explor. Eng. 2019;8:2278–3075. [Google Scholar]
- 11.Ghosh A, Chowdhury N, Chandra G. Plant extracts as potential mosquito larvicides. Indian J. Med. Res. 2012;135:581. [PMC free article] [PubMed] [Google Scholar]
- 12.Saba RM, Mohamed IA, Ahmed MA. Toxicological and Biochemical Investigation of certain Herbicides on Culex pipiens L. Adv. Nat. Appl. Sci. 2018;12:6–13. [Google Scholar]
- 13.Prapanthadara L, Promtet N, Koottathep S, Somboon P, Ketterman A. Isoenzymes of glutathione S-transferase from the mosquito Anopheles dirus species B: the purification, partial characterization and interaction with various insecticides. Insect Biochem. Mol. Biol. 2000;30:395–403. doi: 10.1016/S0965-1748(00)00013-8. [DOI] [PubMed] [Google Scholar]
- 14.Kovendan K, Murugan K, Kumar PM, Thiyagarajan P, William SJ. Ovicidal, repellent, adulticidal and field evaluations of plant extract against dengue, malaria and filarial vectors. Parasitol. Res. 2013;3:1205–1219. doi: 10.1007/s00436-012-3252-8. [DOI] [PubMed] [Google Scholar]
- 15.Reegan AD, Gandhi MR, Paulraj MG, Ignacimuthu S. Ovicidal and oviposition deterrent activities of medicinal plant extracts against Aedes aegypti L and Culex quinquefasciatusSay mosquitoes (Diptera: Culicidae) Osong Public Health Res. Perspect. 2015;1:64–69. doi: 10.1016/j.phrp.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boisard, S., Le Ray, A. M., Landreau, A., Kempf, M., Cassisa, V., Flurin, C., & Richomme, P. Antifungal and antibacterial metabolites from a French poplar type propolis. Evid-Based Complement. Altern. Med. (2015). [DOI] [PMC free article] [PubMed]
- 17.Saleem M, Nazir M, Ali MS, Hussain H, Lee YS, Riaz N, Jabbar A. Antimicrobial natural products: an update on future antibiotic drug candidates. Nat. Prod. Rep. 2010;2:238–254. doi: 10.1039/B916096E. [DOI] [PubMed] [Google Scholar]
- 18.Okubo T, Yokoyama Y, Kano K, Soya Y, Kano I. Estimation of estrogenic and antiestrogenic activities of selected pesticides by MCF-7 cell proliferation assay. Arch. Environ. Contam. Toxicol. 2004;4:445–453. doi: 10.1007/s00244-003-3017-6. [DOI] [PubMed] [Google Scholar]
- 19.Cage, P., Furber, M., Luckhurst, C., Perry, M., & Springthorpe, B. (Google Patents, 2009).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.