Abstract
Readministration of recombinant adeno-associated virus (rAAV) may be necessary to treat cystic fibrosis (CF) lung disease using gene therapy. However, little is known about rAAV-mediated immune responses in the lung. Here, we demonstrate the suitability of the ferret for testing AAV2.5T-mediated CFTR delivery to the lung and characterization of neutralizing-antibody (NAb) responses. AAV2.5T-SP183-hCFTRΔR efficiently transduced both human and ferret airway epithelial cultures and complemented CFTR Cl– currents in CF airway cultures. Delivery of AAV2.5T-hCFTRΔR to neonatal and juvenile ferret lungs produced hCFTR mRNA at 200%–300% greater levels than endogenous fCFTR. Single-dose (AAV2.5T-SP183-gLuc) or repeat dosing (AAV2.5T-SP183-fCFTRΔR followed by AAV2.5T-SP183-gLuc) of AAV2.5T was performed in neonatal and juvenile ferrets. Repeat dosing significantly reduced transgene expression (11-fold) and increased bronchoalveolar lavage fluid (BALF) NAbs only in juvenile, but not neonatal, ferrets, despite near-equivalent plasma NAb responses in both age groups. Notably, both age groups demonstrated a reduction in BALF anti-capsid binding immunoglobulin (Ig) G, IgM, and IgA antibodies after repeat dosing. Unique to juvenile ferrets was a suppression of plasma anti-capsid-binding IgM after the second vector administration. Thus, age-dependent immune system maturation and isotype switching may affect the development of high-affinity lung NAbs after repeat dosing of AAV2.5T and may provide a path to blunt AAV-neutralizing responses in the lung.
Keywords: lung, adeno-associated virus, humoral immunity, repeat dosing, neutralizing antibodies, ferret, cystic fibrosis, gene therapy, immunoglobulin, proteasome inhibitor
Graphical Abstract
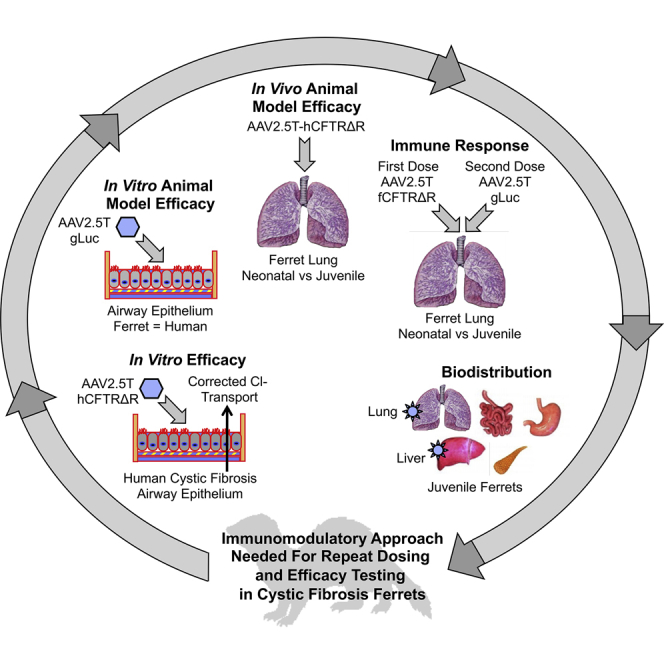
The AAV2.5T vector evolved to transduce human-airway epithelium, but a preclinical animal model to evaluate this virus had not yet been identified. We show that AAV2.5T also effectively transduces ferret airway epithelium in vitro and lungs in vivo, but neutralizing antibodies limited the efficacy of repeat dosing in juvenile, but not neonatal, ferrets.
Introduction
Cystic fibrosis (CF) is an autosomal recessive disease caused by mutations in the single gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR). CFTR is expressed in the apical membrane of certain airway epithelial cells where it functions as an anion channel to conduct chloride and bicarbonate across the epithelium. Although CF affects the epithelium in a number of organ systems, airway disease from chronic bacterial infections and inflammation in the lung is the major cause of morbidity and mortality in patients with CF.
More than 70,000 people are living with CF worldwide according to the Cystic Fibrosis Foundation patient registry. Approximately 2,000 variants in the CFTR gene have been identified, with disease-causing CFTR mutations classified into six categories that affect protein synthesis, protein maturation, channel gating, channel conductance, quantity of protein synthesized, and stability of the protein.1 The discovery of CFTR modulators, targeting mutant CFTR proteins with gating (class III), trafficking (class II), or conductance (class IV) defects, have shown tremendous promise for most patients with CF.2,3 The recent U.S. Food and Drug Administration (FDA)-approved Trikafta therapy (a triple combination of elexacaftor, ivacaftor, and tezacaftor) is now treating the CF patients 12 years and older who have at least one copy of ΔF508 mutation (class II), representing 90% of the CF population. Despite those successes, there remains an unmet need for CF patients who do not respond to CFTR modulators and/or have mutations that produce no or too-little CFTR protein (the class I, V, and VI mutations). A mutation-agnostic gene therapy has the potential to treat these subsets of CF patients who have no therapeutic options.
Adeno-associated virus (AAV) is a nonpathogenic parvovirus that requires a helper virus for permissive infection. Recombinant AAV (rAAV) has proven to be a powerful therapeutic platform for gene therapy to treat inherited and metabolic diseases, including inherited retinal blindness,4 muscular dystrophies,5 and hemophilia.6 Although the previous clinical trials for CF lung disease that used rAAV2 to deliver CFTR failed to demonstrate expression of vector-derived CFTR mRNA in CF lungs, a favorable safety profile of this vector was well documented.7, 8, 9 Subsequent studies on rAAV-transduction biology in human airway epithelia revealed several biologic reasons for the lack of clinical efficacy, including a low-vector tropism for the apical membrane10, 11, 12 and the weak promoter used for CFTR expression,13,14 the latter of which was limited by the packaging capacity of rAAV. Furthermore, many rAAV serotypes encounter a post-entry block in nuclear import after apical transduction of polarized airway epithelium both in vitro11, 12, 13,15 and in vivo.16 This rate-limiting step for effective rAAV-mediated transgene expression involves inefficient escape from the endosomal compartment10,11,17 and inefficient ubiquitination of the capsid,12 two events that are required for transport of the AAV virion into the nucleus. Importantly, these events that limit transduction can be overcome by transient application of proteasome inhibitors during or after the transduction period.11,12,15,18
In an effort to develop improved rAAV vectors for CF lung gene therapy, a new human airway tropic AAV capsid (AAV2.5T) was isolated through directed evolution of a library of AAV2 and AAV5 hybrid capsids after multiple rounds of apical transduction in human airway epithelium (HAE) cultured at an air-liquid interface (ALI).19 rAAV packaging limitations for CFTR delivery were also overcome with a short but strong 183-bp synthetic promoter and enhancer combination (F5tg83)14 and incorporation of a CFTR minigene (CFTRΔR) with a 156-bp deletion in the regulatory (R) domain.20 Similar to full-length CFTR, the CFTRΔR transgene produces CFTR-mediated Cl– currents and rescues the intestinal phenotype in CFTR-knockout mice.21 With these modifications, a new rAAV vector (AAV2.5T-SP183-CFTRΔR) was generated through the packaging of the AAV2.F5t83-CFTRΔR genome into the AAV2.5T capsid. Although the AAV2.5T capsid is effective at apically transducing HAE cultures,19 there has been no preclinical animal model for testing the efficacy of this vector system. In preclinical studies with a CF pig model, the AAV2.5T capsid was ineffective and required the directed evolution of a porcine tropic AAVH22 vector system that efficiently transduced the pig airways.22 AAVH22 also demonstrates species specificity and is inefficient in transducing HAE from the apical surface.
The most-abundant cell types accessible to a CFTR gene-transfer vector on the airway surfaces are terminally differentiated ciliated cells and secretory cells. Although the lifespan of these cell types in the human airway and other non-rodent models remains undefined, cellular turnover is expected and repeat dosing of a rAAV vector will likely be needed for gene-replacement therapy in CF. However, studies using rAAV vectors have raised concerns over the immunogenicity of AAV capsid in patients and in preclinical animal models, especially when the vector is systemically administered or subjects are repeatedly dosed. Such studies have shown that neutralizing antibodies (NAbs), even at relatively low titers, can block gene transfer when rAAV is delivered through the vasculature.23,24 Currently, little is known about rAAV-mediated immune responses in the lung of humans and CF animal models that are capable of testing the efficacy of CFTR gene replacement.
CFTR-knockout and CFTR-G551D CF ferrets25,26 closely reproduce the human CF-disease phenotypes in the lung,27, 28, 29, 30 pancreas,30, 31, 32, 33 gallbladder,32 liver,32 and intestine,32 and thus, the ferret may be a suitable species for preclinical studies with rAAV2.5T. In this study, we sought to evaluate the suitability of the ferret as a model for testing rAAV2.5T and humoral responses that could limit the efficacy of repeated dosing. To that end, we first compared the efficiencies of rAAV2.5T-mediated gene transfer to polarized human and ferret airway epithelial ALI cultures using AAV2.5T-SP183-gLuc (Gaussia luciferase reporter) and AAV2.5T-SP183-hCFTRΔR (hCFTRΔR denotes the human CFTR minigene) vectors. Subsequently, we evaluated AAV2.5T-SP183-hCFTRΔR transduction of hCFTR after intratracheal delivery to neonatal and juvenile ferret lungs. Lastly, we characterized the NAb responses and capsid-binding antibody isotypes in the bronchoalveolar lavage fluid (BALF) and plasma that were elicited by the AAV2.5T capsid and their effects on transgene expression in the lung after single and repeat dosing to neonatal and juvenile ferret lungs.
Results
The Ferret Is a Suitable Preclinical Species for Evaluation of AAV2.5T Gene Therapy to the Lung
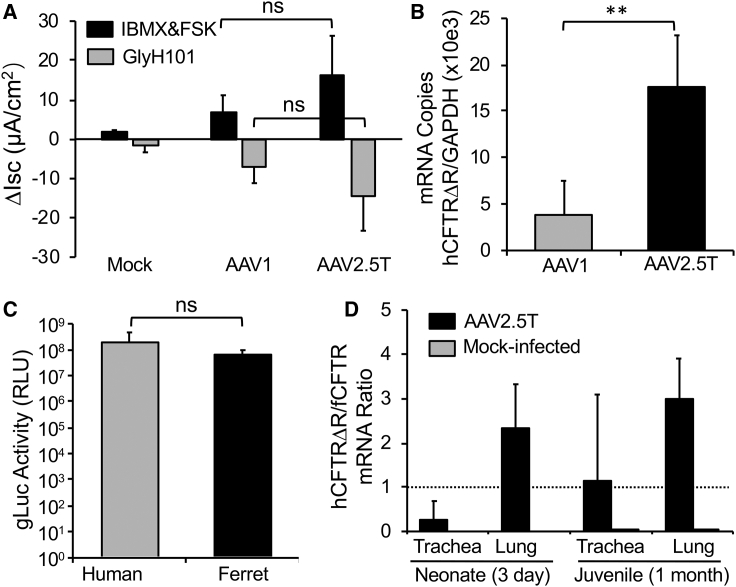
To evaluate whether the AAV2.5T capsid variant19 was capable of delivering functional CFTR to the CF airway, we tested the ability of AAV2.5T-SP183-hCFTRΔR to correct CFTR-mediated Cl– current in human CF ALI cultures after apical transduction. Because rAAV1 had been previously shown to be one of the best-performing serotypes for apically transduction of human ALI cultures,15 we also pseudopackaged the same AAV2-F5tg83-hCFTRΔR viral genome into the AAV1 capsid and performed a comparative analysis with AAV2.5T capsid. This comparison demonstrated that apical transduction with AAV2.5T-SP183-hCFTRΔR gave rise to slightly higher levels of CFTR-mediated Cl– current (Figure 1A) and significantly higher levels of hCFTR mRNA (Figure 1B) than did the AAV1.SP183-hCFTRΔR (the rAAV1 vector harboring the same genome).
Figure 1.
In Vitro and In Vivo Comparison of rAAV Vector Performance
(A) CF (F508del/F508del) human polarized ALI airway cultures were transduced apically with AAV1-SP183-hCFTRΔR or AAV2.5T-SP183-hCFTRΔR at a multiplicity of infection (MOI) of 100,000 DRP/cell in the presence of doxorubicin and N-acetyl-L-leucyl-L-leucyl-L-norleucinal (LLnL). Short-circuit current (Isc) measurements were then performed in a Ussing chamber at 12 days after transduction. Shown is the ΔIsc response to forskolin(FSK)/IBMX and GlyH101 (CFTR inhibitor). Data show the means ± SD for n = 4 transwells from two donors. Non-transduced ALI cultures treated with doxorubicin and LLnL (mock) served as baseline controls (n = 4 from two donors). (B) CF (F508del/F508del) ALI cultures were transduced apically as in (A) and harvested at 7 days after transduction. Transwell inserts were used to quantify the vector-derived hCFTRΔR mRNA copies by quantitative reverse transcriptase PCR (qRT-PCR), and normalized to human GAPDH mRNA copies. Values were then expressed as a ratio of hCFTRΔR to GAPDH. Data show means ± SD for n = 5 transwells in each group. (C) Human and ferret polarized tracheobronchial epithelia at ALI were transduced apically with AAV2.5T-SP183-gLuc at a MOI of 10,000 DNase-I-resistant particles (DRP)/cell in the presence of doxorubicin. Gaussia luciferase activity was measured at 14 days after transduction as relative luminescence units (RLUs). Data show the means ± SD for n = 6 transwells from two donors of each species. (D) Three-day-old ferrets or 1-month-old ferrets were intratracheally transduced with AAV2.5T-SP183-hCFTRΔR mixed with doxorubicin (4 × 1013 DRP/kg body weight). The mock-transduced group was inoculated with PBS and doxorubicin without the vector. The tracheae and lungs were then harvested at 11 days after transduction for quantification of vector-derived hCFTRΔR and endogenous fCFTR mRNA copies by qRT-PCR with GAPDH mRNA copy number normalization. The data represent the ratio (hCFTRΔR/fCFTR) of mRNA copies of hCFTRΔR and fCFTRΔR. The dotted line represents 100% endogenous fCFTR expression levels. Data show the means ± SD for n = 3 animals in each group. Statistical analysis used one-way ANOVA followed by Tukey’s post test in (A) and by two-tailed Student’s t test in (B) and (C). ∗∗p < 0.01; ns, not significantly different.
To evaluate whether the rAAV2.5T was also capable of transducing ferret airway epithelium, we first performed in vitro transduction assays in well-differentiated tracheobronchial ALI cultures derived from humans and ferrets with a vector harboring a secreted Gaussia luciferase (gLuc) reporter, AAV2.5T-SP183-gLuc (Figure 1C). Apical transduction of these cultures with AAV2.5T-SP183-gLuc demonstrated no significant difference in the levels of gLuc transgene expression between the two species. To confirm the tropism of rAAV2.5T for ferret lungs in vivo, we evaluated the transduction efficiency of AAV2.5T-SP183-hCFTRΔR in neonatal and juvenile ferret after intratracheal delivery. In these studies, expression of the transgene-derived hCFTRΔR mRNA was referenced to the endogenous ferret CFTR (fCFTR) mRNA as an index (i.e., the ratio of hCFTRΔR to fCFTR mRNA copies) for the efficiency of transduction. Using this metric, hCFTRΔR mRNA expression in the lungs was 2- to 3-fold greater than was the endogenous fCFTR mRNA in both neonates and juvenile ferrets (Figure 1D). By contrast, tracheal expression of hCFTRΔR mRNA was less than that of the endogenous fCFTR mRNA in neonates and near equivalent in juvenile animals. The low neonatal, as compared with juvenile, transduction of the trachea with rAAV2.5T was potentially due to the different intratracheal delivery method. Given the small size of the neonatal kits, a surgical approach was required to instill the virus into the middle of the trachea. By contrast, juvenile ferrets received virus through a laryngoscope-guided microsprayer in the proximal end of the trachea. Thus, the vector exposure to the trachea in the two age groups was likely different. Overall, these in vitro and in vivo studies suggest that the ferret is a suitable species to study immunologic responses in the lung to rAAV2.5T transduction.
Previous Exposure of rAAV2.5T Virus to Lungs of Juvenile, but Not Neonatal, Ferrets Impairs Transduction of a Second Vector Administration
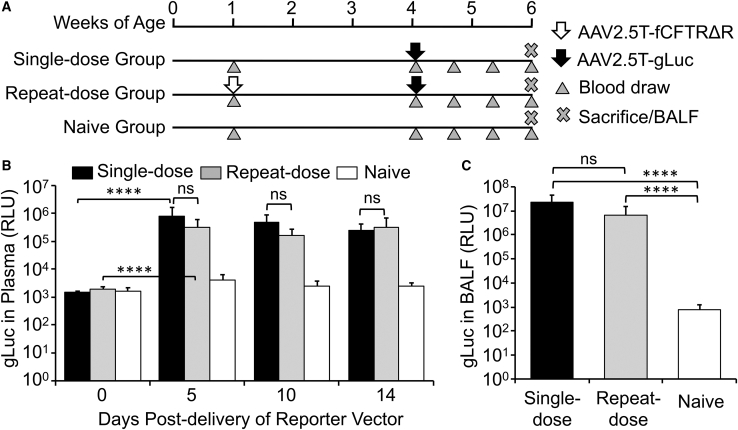
We used two rAAV vectors (AAV2.5T-SP183-fCFTRΔR and AAV2.5T-SP183-gLuc) to evaluate the feasibility of repeat dosing of rAAV2.5T to the ferret lung. AAV2.5T-SP183-fCFTRΔR was chosen for the first viral transduction because that vector should not mount an immune response to the transgene (fCFTRΔR denotes the ferret CFTR minigene). For the second viral transduction, we wanted a robust reporter that would allow for temporal and quantitative analysis of transgene expression and thus chose a secreted gLuc reporter vector (AAV2.5T-SP183-gLuc). gLuc has been previously used to study plasmid-mediated gene transfer in the lungs of sheep.34 The ferrets in the single-dose group were transduced with only the AAV2.5T-SP183-gLuc vector, and those of the repeat-dose group were transduced first with AAV2.5T-SP183-fCFTRΔR and then with AAV2.5T-SP183-gLuc. We first evaluated the repeat dosing in younger animals (Figure 2). We initiated these studies in neonatal ferrets, delivering AAV2.5T-SP183-fCFTRΔR virus to the repeat-dose group at 1 week of age and then, three weeks later, delivering AAV2.5T-SP183-gLuc virus to both the repeat-dose and single-dose groups (Figure 2A). Luciferase activity was monitored in blood samples during the 14 day period after transduction with AAV2.5T-SP183-gLuc and in BALF at the termination of the experiment. This study demonstrated that gLuc activity in plasma peaked by 5 days after transduction and remained stable to 14 days in both dosing groups (Figure 2B). There was also no significant difference in the level of plasma gLuc activity between the two dosing groups. Similarly, gLuc activity in the BALF at 14 days after transduction was also not significantly different between the two dosing groups (Figure 2C). In both the plasma and BALF, gLuc activity was significantly above the background levels of naive (non-transduced) controls (Figures 2B and 2C).
Figure 2.
Repeat Dosing of AAV2.5T in Neonatal Ferrets
(A) Study design involving three groups of neonatal ferrets receiving 0, 1, or 2 doses of virus at 1 × 1013 DRP/kg via intra-tracheal administration. The ferrets receiving one dose were administered the reporter vector AAV2.5T-SP183-gLuc at 4 weeks old, whereas the ferrets receiving two doses were administered AAV2.5T-SP183-fCFTRΔR at 1 week old and AAV2.5T-SP183-gLuc at 4 weeks old. Plasma or BALF samples were collected at the indicated ages. (B) Gaussia luciferase activity in the plasma at the indicated time points after delivery of AAV2.5T-SP183-gLuc. (C) Gaussia luciferase activity in BALF at 14 days after delivery of AAV2.5T-SP183-gLuc. Results show the means ± SD for n = 6 animals per group. The statistical significance was analyzed with one-way ANOVA, followed by Tukey’s multiple comparisons test on log2-transformed data. ∗∗∗∗p < 0.0001; ns, not significant.
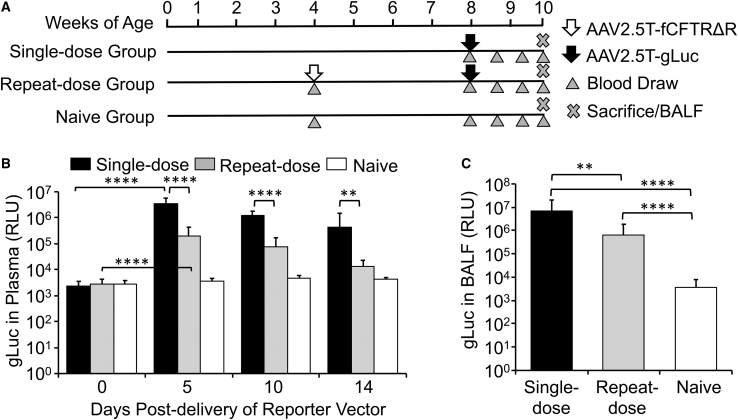
This study in neonatal ferrets demonstrated it was feasible to readminister AAV2.5T without a significant decline in transduction to the lung; however, the possibility remained that an underdeveloped immune system in neonatal ferrets could produce a tolerized immunologic state against the AAV capsid. For these reasons, we repeated experiments in juvenile ferrets by initiating the first delivery of AAV2.5T-SP183-fCFTRΔR in the repeat-dose group at 1 month of age, which approximately represents a 1- to 2-year-old toddler,33 after which the gLuc reporter vector (AAV2.5T-SP183-gLuc) was delivered to both the single-dose and repeat-dose groups 4 weeks later (Figure 3A). Findings from this second study demonstrated maximal plasma gLuc activity at 5 days after transduction in both groups; however, the repeat-dose group had lower (15-fold to 34-fold) plasma gLuc activity at all time points tested. In contrast to the stable plasma gLuc expression in single- and repeat-dose neonatal groups (Figure 2B), we observed a gradual decline in plasma gLuc activity in both juvenile groups, with a steeper trend in the repeat-dose animals (Figure 3B). Similarly, BALF gLuc activity was also significantly lower (11-fold) in the repeat-dose juvenile group (Figure 3C). Cumulatively, these studies suggested the potential for NAb responses against the AAV capsid in juvenile, but not neonatal, ferrets.
Figure 3.
Repeat Dosing of AAV2.5T in Juvenile Ferrets
(A) Study design involving three groups of juvenile ferrets receiving 0, 1, or 2 doses of virus at 1 × 1013 DRP/kg via intra-tracheal administration. The ferrets receiving one dose were administered the reporter vector AAV2.5T-SP183-gLuc at 8 weeks old, whereas the ferrets receiving two doses were administered AAV2.5T-SP183-fCFTRΔR at 4 weeks old and AAV2.5T-SP183-gLuc at 8 weeks old. Plasma or BALF samples were collected at the indicated ages. (B) Gaussia luciferase activity in the plasma at the indicated time points after delivery of AAV2.5T-SP183-gLuc. (C) Gaussia luciferase activity in BALF at 14 days after delivery of AAV2.5T-SP183-gLuc. Results show the means ± SD for n = 9–10 animals per group. The statistical significance was analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test on log2-transformed data. ∗∗p < 0.01, ∗∗∗∗p < 0.0001.
Repeat Dosing of rAAV2.5T Elicits a Greater NAb Response in BALF and Plasma
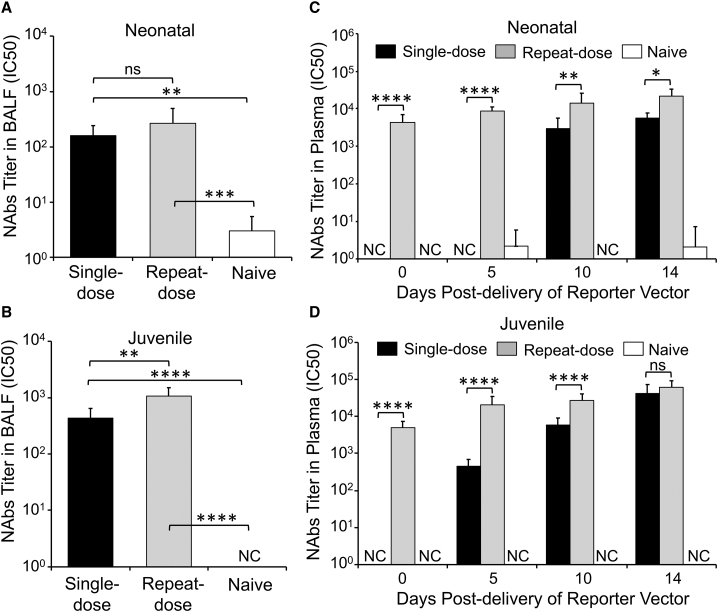
Given the reduced efficiency of rAAV2.5T transduction in the lungs of juvenile ferrets previously exposed to this virus, we sought to evaluate the NAbs in BALF and plasma of test animals. The titers of anti-AAV2.5T NAbs were determined as the half-maximal inhibitory concentration (IC50) for inhibition of AAV2.5T-SP183-fLuc transduction of A594 cells, a human airway cell line. Consistent with similar levels of transgene expression in single- and repeat-dosed neonatal ferrets, NAb titers in BALF were not significantly different between the two dosing conditions (Figure 4A). By contrast, NAb titers in the BALF of juvenile ferrets were significantly higher in the repeat-dose, as compared with the single-dose, group (Figure 4B). Furthermore, the absolute titers of NAbs in experiments with older animals of both single and repeat dose groups were 3-fold to 5-fold greater than those of the neonatal test groups, suggestive of a more fully developed immune response in the older ferrets.
Figure 4.
Titers of AAV2.5T Neutralizing Antibodies in the BALF and Plasma of Transduced Ferrets
(A–D) Neonatal and juvenile ferret samples, collected as outlined in Figures 2A and 3A, respectively, were evaluated or NAbs in the BALF (A and B) and plasma (C and D) using a transduction-inhibition assay. Serial dilutions of BALF or plasma were incubated with AAV2.5T-fLuc before transduction of A549 cells. The titer of NAbs was calculated as the concentration of BALF or plasma (dilution ratio) that resulted in 50% inhibition (half-maximal inhibitory concentration [IC50]) of transduction as assessed by firefly luciferase activity. AAV2.5T-fLuc-only transduced cells served as the baseline control, and mock-transduced cells served as a blank. The IC50 was calculated with Prism software; when there was no clear dose response, a value of zero was returned (those instances are marked as non-calculatable [NC]). Results show the means ± SD for n = 6 neonatal animals per group and n = 9–10 juvenile animals per group. Before statistical analysis, the data was log2(Y + 1) transformed, setting NC values equal to 1. The statistical significance was then analyzed with one-way ANOVA followed by Tukey’s multiple comparisons test. ∗p < 0.05,∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. ns, not significant.
Similar analyses on the plasma samples demonstrated no pre-existing NAbs in the control naive group (Figures 4C and 4D) and the test groups before rAAV2.5T transduction (data not shown). In both age groups, single- and repeat-dose animals demonstrated gradual time-dependent increases in plasma NAb titers after transduction, and repeat-dose juvenile ferrets produced slightly higher plasma NAb titers (2-fold to 2.8-fold) than produced in neonatal ferrets. Juvenile ferrets also produced NAbs more rapidly in the plasma of the single-dose group with an appearance at 5 days after transduction as compared with 10 days for neonatal ferrets. The level of plasma NAbs in the repeat-dose group was also significantly greater than that of single-dose group for both ages, with the exception of the 14-day post-transduction time point in the juvenile ferrets.
Development of an ELISA-Based Assay for Quantifying Anti-AAV2.5T Capsid Antibody Isotypes
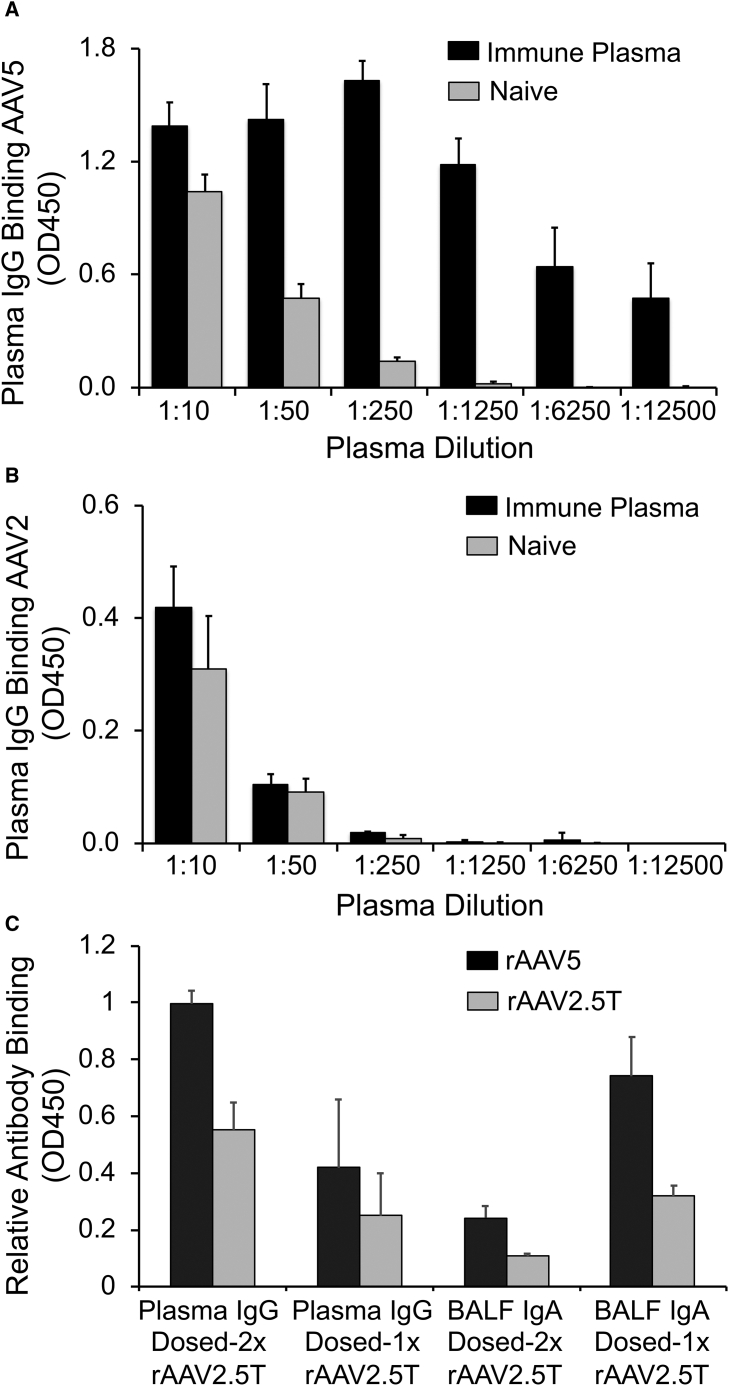
AAV2.5T capsid was evolved from an AAV2/AAV5 capsid-shuffled library. VP2 and the most abundant VP3 capsid proteins of AAV2.5T are derived from AAV5 with a single A581T mutation in VP1. VP1 of AAV2.5T is a hybrid of AAV2 and AAV5 capsids with the N-terminal unique sequence (VP1u) from the 1–128 aa of the AAV2 VP1, followed by the 129–725 aa of AAV5 capsid harboring the A581T mutation. The VP1u of AAV harbors a phospholipase A2 (PLA2) catalytic domain that is thought to be crucial to virion escape from the endosome.35,36 The AAV2-derived VP1u resides inside the intact virion, and thus, all surface-exposed fragment of capsids are AAV5 derived, with the exception of the A581T mutation. Thus, antibodies that bind to the intact virion of AAV2.5T would also be expected to bind to AAV5 virions. To evaluate AAV2.5T-capsid-specific immunoglobulins (Igs) in the plasma and BALF (IgG, IgM, and IgA) of rAAV2.5T-transduced ferrets, an ELISA assay with AAV viral particles as the coating antigen was developed. To validate the method, we used plasma collected from a ferret for which rAAV2.5T was delivered to the lung four times at 1–2-month intervals. Using rAAV5 as the coating antigen, differential IgG binding between naive and AAV2.5T-immune plasma was seen starting at a 1:50 dilution, and by a 1:1250 dilution, binding of naive plasma was absent, whereas the AAV2.5T-immune plasma-antibody binding remained high (Figure 5A). By contrast, when rAAV2 was used as the coating antigens, there was no difference in plasma IgG binding between the immune plasma and the naive plasma at all dilutions and the sensitivity of detecting IgG was much less than that for rAAV5 (Figure 5B). We also compared rAAV5 and rAAV2.5 as the coating antigen for IgG and IgA antibody binding in the ELISA assay with samples of plasma and BALF from rAAV2.5T single- and repeat-dosed ferrets. Across all groups, antibody-binding optical density 450 (OD450) values for rAAV5 were similar or greater than that for rAAV2.5T as the coating antigen, and a similar trend of immunoreaction was observed for both serotypes among the single- and repeat-dose samples (Figure 5C). For those reasons, we chose to use rAAV5 as the coating antigen for the classification of anti-AAV2.5T capsid-antibody isotypes in the BALF and plasma of test animals.
Figure 5.
Development of an ELISA-Based Assay for Quantifying Anti-capsid Antibody Isotypes
(A and B) Immune plasma was generated from a ferret transduced with AAV2.5T to the lung four times at 1- to 2-month intervals, starting at 1 month of age. The naive plasma was derived from a ferret of similar age. ELISA plates were coated with (A) AAV5 or (B) AAV2 and then evaluated for binding of immune and naive ferret plasma. The secondary detection antibody was against ferret IgG. Results show the means ± range for two technical replicates on each sample. (C) rAAV5 and rAAV2.5T were compared as the coating antigen for detection of anti-AAV2.5T IgG antibodies from the plasma and anti-AAV2.5T IgA antibodies in the BALF of single- and repeat-dose ferrets studied in Figure 4. Repeat-dose groups had n = 10 samples and single-dose groups had n = 7 samples tested. The secondary-detection antibodies were against ferret IgG (plasma) or IgA (BALF). Results show the means ± SD.
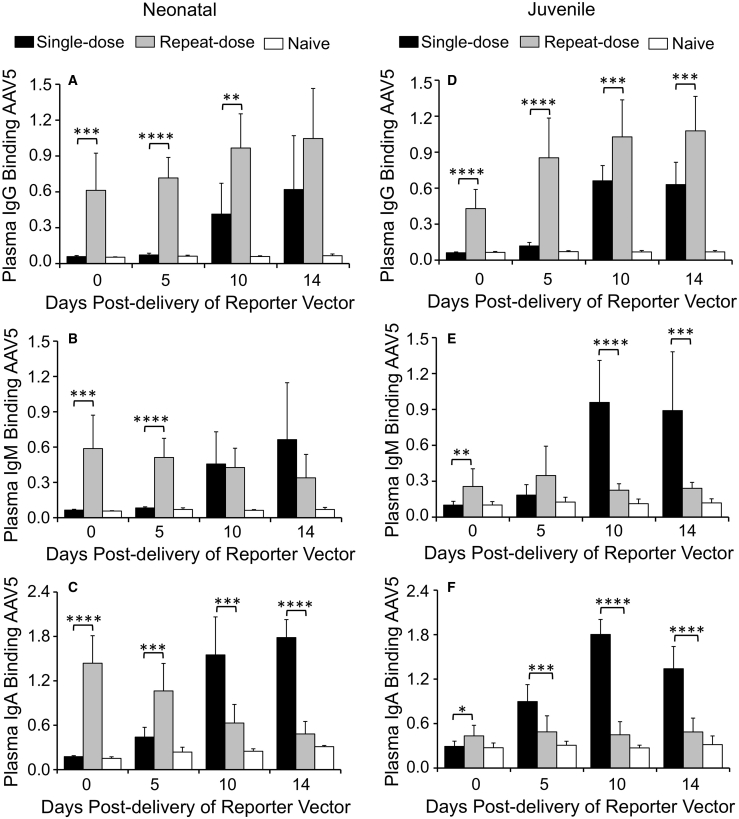
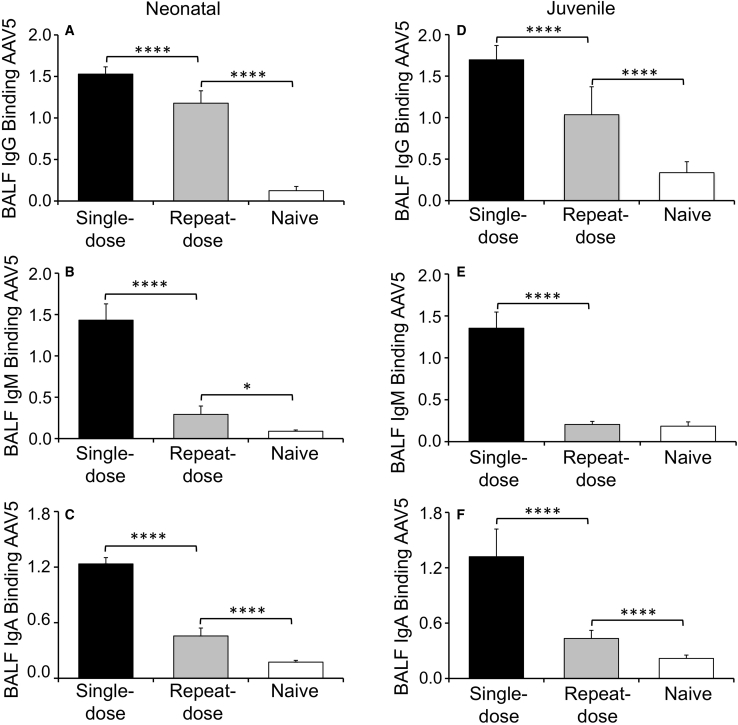
We next used that ELISA method for classification of anti-capsid antibody isotypes (IgG, IgM, and IgA) in the BALF and plasma of test animals (Figures 6 and 7). In general, neonatal and juvenile ferrets elicited similar AAV5-reactive IgG responses in the plasma of both single- and repeat-dosing groups, but titers were higher after repeat transduction (Figures 6A and 6D). By contrast, plasma AAV5-reactive IgM (Figures 6B and 6E) and IgA (Figures 6C and 6F) responses demonstrated differences from that of IgG with respect to the age of the animal and the dosing regimen. For example, capsid-binding plasma IgM levels were suppressed only in juvenile animals of the repeat-dose group (Figures 6B and 6E), whereas capsid-binding plasma IgA levels were suppressed in both age groups after repeat dosing. Furthermore, neonatal animals initially mounted a large anti-capsid IgA response after the second viral exposure, which subsided with time, whereas juvenile animals lacked that response (Figures 6C and 6F). These findings suggest that age-dependent differences in antibody-isotype switching may be affected by prior exposure to rAAV2.5T. Contrary to expectations, the AAV5-reactive IgG, IgM, and IgA in the BALF were significantly greater in the single-dose group, as compared with the repeat-dose group, for both neonatal and juvenile animals (Figure 7). Furthermore, the absolute level of capsid-binding IgG, IgM, and IgA was generally similar between both age groups and dosing conditions, despite higher levels of NAbs in the BALF of juvenile animals that were exposed twice to the virus (Figures 4A and 4B).
Figure 6.
Quantification of IgG, IgM, and IgA Capsid-Binding Antibodies in the Plasma of AAV2.5T Transduced Ferrets
(A–F) Quantification of capsid-binding antibodies in the plasma of neonatal (A–C) and juvenile (D–F) ferrets for IgG (A and D), IgM (B and E), and IgA (C and F). Results show the means ± SD for n = 6 neonatal animals per group and n = 9–10 juvenile animals per group. The statistical significance was analyzed with one-way ANOVA followed by Tukey’s multiple comparisons test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. Unlabeled comparisons between single- and repeat-dose groups were not significantly different.
Figure 7.
Quantification of IgG, IgM, and IgA Capsid-Binding Antibodies in the BALF of AAV2.5T-Transduced Ferrets
(A–F) Quantification of capsid-binding antibodies in the BALF of neonatal (A–C) and juvenile (D–F) ferrets for IgG (A and D), IgM (B and E), and IgA (C and F). Results show the means ± SD for n = 6 neonatal animals per group and n = 9–10 juvenile animals per group. The statistical significance was analyzed with one-way ANOVA followed by Tukey’s multiple comparisons test. ∗p < 0.05, ∗∗∗∗p < 0.0001.
Biodistribution of rAAV2.5T after Lung Delivery
The magnitude of an immune response against rAAV2.5T after lung delivery could be affected by escape of the virus into the blood and uptake by peripheral organs. Additionally, because a gLuc reporter was used in our studies, there was a formal possibility that other peripheral organs were transduced and contributed to transgene detection in the blood. To that end, we delivered a non-secreted firefly luciferase (fLuc) AAV2.5T-SP183-fLuc vector to the lungs of 1-month-old ferrets and assessed the distribution of viral genomes and the level of transgene expression in 10 peripheral organs. Analysis of the vector shedding into the blood demonstrated a significant peak at 4 h after delivery, which declined to background levels by 5 days after delivery (Figure S1A). At 14 days after vector delivery, the only peripheral organs that had vector genomes that were significantly greater than the background signal observed in naive controls were the lung and trachea (92 copies per 100 ng of genomic DNA) and liver (14 copies per 100 ng of genomic DNA) (Figure S1B). In contrast to vector genome accumulation, only the lung and trachea cassette expressed fLuc significantly greater than the background signal observed in naive control samples (Figure S1C). Additionally, fLuc activity in the shorter-term blood sample (Figure S1A) was not greater than that of the naive control background levels (data not shown). These studies demonstrate that transgene expression after lung delivery of rAAV2.5T is limited to the lung. However, rAAV2.5T does shed into the blood and is taken up by the liver.
Discussion
Pre-existing37 and recall-response38 humoral immunity to AAV capsids are potential barriers for rAAV-mediated gene therapy, especially for re-administration of the same rAAV vector39,40 or systemic administration.41,42 Characterization of these immune-surveillance mechanisms will provide the basis for developing safe and effective gene therapies. We have developed CF ferret models28,30 and an efficient rAAV-based vector to deliver the CFTR gene to polarized human and ferret airway epithelial cultures in vitro, as well as ferret lungs in vivo. The co-development of an animal model with an effective vector system that performs well in both the test species and primary HAE provides a powerful preclinical approach toward developing gene therapies for CF lung disease. The main purpose of this study was to evaluate whether the ferret was an appropriate species to study humoral immune responses to rAAV2.5T and the feasibility of repeat administration.
Our studies demonstrated that rAAV2.5T is equally tropic for human and ferret polarized airway epithelium in vitro and also capable of complementing CFTR-mediated currents in CF airway epithelia (Figures 1A–1C). As predicted from these in vitro studies, rAAV2.5T was also tropic for neonatal and juvenile ferret lungs in vivo (Figure 1D). rAAV2.5T-mediated intralobar expression of human CFTRΔR mRNA appears to be within a therapeutic range at 2- to 3-fold greater than endogenous-level ferret CFTR. These findings supported dissection of the humoral immune response to rAAV2.5T after single and repeat dosing and how this affects the efficacy of transduction.
The transduction efficiency of rAAV2.5T in the lung of neonatal ferrets was not significantly affected by prior expose to the virus (Figure 2), despite the existence of NAbs in the BALF and plasma before the second virus exposure (Figures 4A and 4C). This was not the case for juvenile ferrets, where a significant 11-fold reduction in transgene expression was observed in the BALF of the repeat-dose group (Figure 3). Despite that reduction in transduction efficiency, transgene expression still remained 170-fold greater than background levels (Figure 3C). Given the more-rapid NAb response in the plasma for juvenile animals, we hypothesized that a less-mature mucosal immune system in neonatal ferrets facilitated the greater transduction efficacy after repeat dosing. Consistent with that hypothesis, flu vaccines are also less effective in generating a protective mucosal immune response in toddlers younger than 2 years,43,44 and two doses of flu vaccine are recommended for the protection of children younger than 8 years.44,45 Notably, however, the IC50 for the NAb response in the BALF was only marginally greater in the juvenile, as compared with the neonatal, group (2.8-fold for single-dose; 4.1-fold for repeat-dose groups; Figures 4A and 4B). For these reasons, we hypothesized that juvenile ferrets generate a more-effective local NAb response caused by isotype switching and memory B cells that expand Ig-producing plasma cells in the lung with greater efficacy for neutralization of viral transduction. However, it was not possible test the effectiveness of specific antibody isotypes for virus neutralizing, and thus, it remains unclear whether changes in the ratio of NAb isotypes contribute to the effectiveness of virus neutralizing in vivo.
Our results evaluating BALF and plasma anti-capsid IgG, IgM, and IgA are consistent with other known recall responses to influenza infection in the lung that produce isotype switching that propagate local IgG, IgA, and IgM immunity against the virus.46 These results support different capacities for isotype switching of IgA and IgM in neonates and juvenile animals, as reflected in the peripheral whole blood (Figure 6). In this regard, capsid-binding plasma IgM levels were significantly suppressed in the repeat-dose group only in juvenile ferrets (Figures 6B and 6E), whereas capsid-binding plasma-IgA responses were initially elevated only in repeat-dose neonatal ferrets (Figures 6C and 6F). That was not the case for IgG, in which neonatal and juvenile animals mounted similar capsid-binding IgG responses in plasma (Figures 6A and 6D). The similar BALF titers of capsid-binding IgG, IgM, and IgA between age groups and the only marginally more NAbs in the repeat-dose juvenile ferrets support the existence of non-neutralizing IgG, IgM, and IgA in the lung and the expansion of more-effective neutralizing Igs in the older animals. Furthermore, the reduction of capsid-binding Igs in the BALF after the second transduction in both age groups is consistent with the recall response involving isotype switching in the lung.47 Alternatively, the reduced capsid-binding IgG, IgM, and IgA in the lung after a second dose of virus could be due to vector neutralization of Igs and/or the expansion of Ig-secreting plasma cells that produce NAb of much greater affinity. The former scenario is consistent with findings describing the use of empty decoy AAV capsids to absorb antibodies, thus overcoming the inhibitory effect of pre-existing Nab.41
Similar repeat-dosing studies with rAAV vectors have been reported in other animal models, including the mouse,48, 49, 50 rabbit,51 and rhesus macaques.52 However, in most of these studies, foreign transgenic reporters were used, which could also mount immune responses. In mouse, rAAV2/9 was reported to be successfully readministered to the lung in the presence of NAbs as early as 1 month after the initial dose.50 Other work in mice has reported that transient immunosuppression at the time of primary AAV vector exposure allowed for successful repeat transduction and prevented production of neutralizing antibodies.49 Furthermore, studies evaluating cross-neutralization between AAV serotypes has demonstrated that transduction of mouse lung by rAAV6 is unaffected by the cross-reactive NAbs against AAV2 or AAV3 and that transduction by rAAV2 was unaffected by the NAbs against AAV6.48 However, the AAV2 virus elicited NAbs that completely blocked transduction of a second AAV2 vector, and the AAV6 capsid elicited NAbs that only partially inhibited transduction of a second AAV6 vector. Rabbits receiving two doses of rAAV2-CFTR into the airways generated high titers of serum anti-AAV NAbs; however, readministration of a third dose of virus did not inhibit rAAV2 or rAAV3 transduction with the GFP reporter gene. Collectively, these findings suggest that NAb responses to AAV serotypes can be quite diverse; however, these previous studies used histologic assessment of reporter gene expression that can be difficult to quantify accurately. In the present study, with a sensitive secreted reporter as the metric for whole-lung transduction, we observed that prior lung exposure to rAAV2.5T virus affects transduction by a second administration in an age-dependent fashion.
Our biodistribution studies on rAAV2.5T suggest that some of the viruses escape from the lung into the blood and are taken up by the liver, despite transgene expression being confined to the lung. At 4 h after vector delivery, ∼7 × 10−9% of the total vector load to the lung was detected in the blood. Additionally, based on the genomic DNA content of a single diploid cell, we estimate that the lung contained ∼1 vector genome per 100 cells at 14 days after vector delivery to the lung. The liver contained approximately 10-fold lower levels of vector genomes (∼1 vector genome per 1,000 cells). The mechanism of rAAV2.5T escape from the lung and the immunologic consequences of hepatic uptake on NAb responses remain unknown. However, these findings suggest that further investigation is warranted.
Although the use of a sensitive, secreted reporter gene was a strength for immunologic studies, it was also a limitation of the current study because the airway cell types transduced by rAAV2.5T could not be directly evaluated. The cellular targets for CF lung gene therapy are diverse, and the expression of the CFTR protein within these cell types is highly regulated, functioning in concert with other ion channels to regulate innate immunity and mucociliary clearance.53,54 Although the conducting airways are generally thought to be the primary targets for CF gene therapy, alveolar type-II cells express significant amounts of CFTR, and their contribution to CF lung disease still remains unknown. Thus, future studies with alternative, robust intracellular reporters are needed to determine whether the cellular distribution of rAAV2.5T transduction in the ferret lung is compatible with complementation of CF disease.
Although repeat administration of rAAV2.5T in juvenile ferret was partially effective, the significant decrement in transduction after a second dose of virus suggests that immunomodulatory strategies may be needed for repeat dosing in humans. However, the repeat-dosing window that maintains a robust NAb recall response remains unclear, and the studies described here were designed to maximize that response. In patients with CF, the interval between AAV dosing may be considerably longer than the 1-month dosing interval used in this study; thus, there is a need to determine how long NAbs persist in the lung. The discordance between titers of capsid-binding antibodies and NAbs in the BALF suggests the maturation of high-affinity NAbs in older animals. Age-dependent differences in isotype switching of capsid-binding NAbs in the periphery may affect the maturation of high-affinity NAbs in the lung and provides potential avenues for immunomodulation of these responses.
Materials and Methods
Production of Recombinant rAAV2.5T Viral Vectors
pAV2.5Trepcap18 was the AAV helper plasmid used to generate AAV2.5T capsid for the production of rAAV2.5T vectors: AAV2.5T-SP183-hCFTRΔR, AAV2.5T-SP183-fCFTRΔR, AAV2.5T-SP183-fLuc, and AAV2.5T-SP183-gLuc. rAAV2 proviral plasmids used for packaging were pAV2.F5tg83-hCFTRΔR and pAV2.F5tg83-fCFTRΔR,14 as well as the pAV2-F5tg83fLuc (firefly luciferase reporter) and pAV2-F5tg83 gLuc (Gaussia luciferase reporter).55 SP183 is a short synthetic promoter of 183 bp. It was generated by the fusion of a synthetic 100-bp enhancer (called F5)14 with a short 83-bp promoter (called tg83).13 SP183 is synonymous with F5tg83; however, for consistency with prior literature, the plasmid proviral vectors have retained the name F5tg83. AAV2.5T vectors were produced in the vector core of the Children’s Hospital of Philadelphia (CHOP) using a triple-plasmid transfection method. In brief, AAV capsid plasmid, pAV2.5Trepcap, and adenovirus helper pAd14 were transfected into HEK293 cells together with the AAV proviral vector with AAV2 inverted terminal repeat sequences (ITRs). rAAV vectors produced from the transfected HEK293 cells were purified on cesium chloride (CsCl)-density gradients. DNase-I-resistant particle (DRP) titers56,57 were determined by real-time quantitative polymerase chain reaction (qPCR) with primers and probes specific to the transgenes.14,55 The purities of the vector stocks were also evaluated by SDS-PAGE after silver staining.
In Vitro Evaluation of rAAV2.5T Vector in Human and Ferret Airway Epithelium
The tropism of rAAV2.5T virus for human and ferret airway epithelium was evaluated by in vitro transduction of well-differentiated tracheobronchial ALI cultures established with early passage primary cells. The reporter vector, AAV2.5T-SP183-gLuc, was inoculated apically onto primary human and ferret airway epithelial ALI cultures at an multiplicity of infection (MOI) of 10,000 DRP/cell. During the viral exposure period, the culture medium was supplemented with doxorubicin at the final concentration of 4 μM, and the relative luminescence units (RLUs) of the Gaussia luciferase activity was measured 14 days after transduction, according to the manufacturer’s instructions for the Renilla Luciferase Activity Assay kit (Promega),14 which was designed for the measurement of Gaussia luciferase and Renilla luciferase. Transwells that did not receive virus were used as controls for background luminescence.
In Vitro Comparison of CFTR-Mediated Currents after Transduction of CF HAE with AAV1-SP183-hCFTRΔR and AAV2.5T-SP183-hCFTRΔR Viruses
The effectiveness of AV2.5T-SP183-hCFTRΔR and AV1-SP183-hCFTRΔR for expressing hCFTRΔR and the complementation of the CFTR function were evaluated in polarized human ALI cultures derived from the proximal airway of patients with CF (F508del/F508del). Each vector was applied apically to the ALI cultures at a MOI of 100,000 DRP/cell in the presence of doxorubicin (2.5 μM; MilliporeSigma) and N-acetyl-L-leucyl-L-leucyl-L-norleucinal (LLnL) (20 μM; MilliporeSigma). These molecules have previously been shown to augment transduction by several AAV serotypes.14 At 12 day after transduction, CFTR-mediated Cl– currents were measured in Ussing chambers, as described previously,14,58 to determine the change in short-circuit current (ΔIsc) after cAMP stimulation (IBMX/Forskolin,100 μM/10 μM; MilliporeSigma) and CFTR inhibition (GlyH101, 50 μM; MilliporeSigma). ALI cultures treated with doxorubicin and LLnL, but not virus, were used as baseline controls. In a separate set of experiments, ALI cultures from patients with CF (F508del/F508del) were similarly transduced with AV2.5T-SP183-hCFTRΔR or AV1-SP183-hCFTRΔR at an MOI of 100,000 DRP/cell in the presence of doxorubicin (2.5 μM) and LLnL (20 μM), and cells were harvested at 7 day after transduction for analysis of vector-derived hCFTRΔR mRNA. ALI cultures were lysed for total RNA preparation with the RNeasy Plus Mini kit (QIAGEN). After conversion of mRNA to cDNA, the vector-derived hCFTRΔR mRNA was quantitated by TaqMan PCR and normalized to human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA.14
Analysis of rAAV2.5T Transduction in Neonatal and Juvenile Ferret Lungs
Three-day-old neonatal ferrets or 1-month-old juvenile ferrets intratracheally received 4 × 1013 DRP/kg body weight of the AAV2.5T-SP183-hCFTRΔR virus mixed with doxorubicin (final concentration, 250 μM). Because of the small size of neonatal kits, intratracheal vector instillation in kits was performed surgically by creating a small incision and inserting a small needle into the mid-region of the trachea. Intratracheal administration of the vector to juvenile ferrets was performed with a laryngoscope and insertion of a microsprayer-aerosolizing device sized for a rat (PennCentury, model IA-1B). Ferrets in the mock-transduction group were only inoculated with doxorubicin in PBS (250 μM). The animals were euthanized at 11 days after transduction, and the trachea and lung tissues were separately harvested, snap frozen, and pulverized for total RNA extraction. The vector-derived mRNA of the transgene hCFTRΔR and endogenous fCFTR were quantified by TaqMan, and the copy numbers of hCFTRΔR and fCFTRΔR were normalized to GAPDH and then expressed as the ratio of hCFTRΔR to fCFTR14.
Humoral Response Studies in Ferrets after Lung Administration of AAV2.5T-SP183-fCFTRΔR and/or AAV2.5T-SP183-gLuc
We evaluated repeat dosing of rAAV2.5T vectors to neonatal and juvenile ferrets using the following experimental design. Neonatal ferrets: AAV2.5T-SP183-gLuc reporter vector was intratracheally administered to 4-week-old ferrets that were either naive to AAV2.5T capsid or had been previously transduced with AAV2.5T-SP183-fCFTRΔR at 1 week old. Juvenile ferrets: AAV2.5T-SP183-gLuc reporter vector was intratracheally administered to 8-week-old ferrets that were either naive to AAV2.5T capsid or had been previously transduced with AAV2.5T-SP183-fCFTRΔR at 4 weeks old. For each dose, the animal received an inoculum containing AAV2.5T-SP183-gLuc (1 × 1013 DRP/kg) or AAV2.5T-SP183-fCFTRΔR vector (1 × 1013 DRP/kg) and doxorubicin (200 μM final concentration). Surgical intratracheal injection was performed in the 1-week-old, neonatal ferrets with a 150-μL inoculum administered to kits under anesthesia from a mixture of isoflurane and oxygen. For other ages, virus was administered intratracheally with a microsprayer aerosolizer (PennCentury, model IA-1B) under anesthesia via subcutaneous injection with a mixture of ketamine and xylazine. The volume of the vector and doxorubicin inoculum for aerosolization was normalized to ferret body weight (5 mL/kg).
Bleeding and BALF Collection for Measurement of gLuc Activity
The gLuc reporter has been previously used as a sensitive reporter to assess lung gene transfer in a number of species and is effectively secreted into the blood and BALF.34,55 Plasma was collected into heparinized tubes from anesthetized ferrets at 0, 5, 10, and 14 days after delivery of the AAV2.5T-SP183-gLuc reporter vector. Animals were euthanized with Euthasol (Virbac AH), and BALF was collected from the tracheal and lung cassette by instillation of 5 mL of PBS per 300 g of body weight. The gLuc activity in plasma and BALF were immediately measured after sample collection.
Antibody-Neutralization Assays with Plasma and BALF
Micro-neutralization assays were performed with modifications to a previously reported method.59 The titers of NAb in the plasma and BALF were quantified as the reduction in reporter gene expression after transduction of A549 cells with AAV2.5T-SP183-fLuc virus incubated with serially diluted plasma or BALF before transduction. Briefly, all plasma samples from ferrets were heat inactivated (56°C, 30 min). Five-fold serial dilutions of plasma (started at 1:50 and ended at 1:156,250) were incubated with AAV2.5T-SP183-fLuc in a total volume of 100 μL. For BALF, the same condition was applied, but the serial dilution started at 1:5 and ended at 1:3,125. These mixtures were incubated at 37°C for 1 h to facilitate antibody binding and neutralization and were then applied to a monolayer of A549 cells in 48-well plates (1 × 105 cells/well, MOI = 5,000 DRP/cell) in duplicate for each dilution. After incubating cells for 1 h at 37°C/5% CO2 with the virus mixture, the wells were supplemented with DMEM containing of 2% fetal bovine serum and incubated for an additional 24 h. Firefly luciferase activity in cell lysates was then measured with a Firefly Luciferase Assay Kit (Promega), according to manufacturer’s instruction. Each time this assay was performed, A549 cells transduced only with AAV2.5T-SP183-fLuc served as the reference control for 100% transduction. The neutralization titer of each plasma or BALF sample was calculated as the IC50 with Prism software (GraphPad).
ELISA Measurements of Capsid-Binding IgG, IgM, and IgA in Plasma and BALF
An ELISA procedure was used to capture and quantify the total capsid-binding IgG, IgM, and IgA in the plasma and BALF. In brief, rAAV5 in carbonate buffer was bound to 96-well ELISA plates overnight at 4°C (1 × 109 DRP/well). The plasma samples tested (diluted to 1:2,000 for IgG and IgM and to 1:20 for IgA) and undiluted BALF samples were applied to each well and incubated for 1 h at room temperature. After washing three times in PBS-T (0.05% Tween-20 in PBS), diluted horseradish peroxidase (HRP)-conjugated second antibodies were added and incubated for 1 h at room temperature. The HRP-conjugated second antibodies included chicken anti-ferret IgG (Gallus Immunotech) or goat anti-ferret IgG (Abcam) and goat anti-ferret IgM or IgA (LSBio). The HRP reaction product was then quantified by absorbance in a plate reader.
Viral Biodistribution Studies after Lung Delivery of rAAV2.5T
To determine the extent to which rAAV2.5T exited the lung into the periphery, we performed viral genome and transduction analysis of other organs after rAAV2.5T lung delivery. For those studies, it was necessary to use a non-secreted form of luciferase (firefly luciferase, fLuc). One-month-old juvenile ferrets were intratracheally dosed with AAV2.5T-SP183-fLuc virus (1 × 1013 DRP/kg body weight) with Dox (final concentration, 200 μM). Age-matched ferrets that did not receive the virus or Dox were used as negative controls. Whole blood samples were collected before vector delivery (0 h) and at 4 h and 1, 5, 10, and 14 days after delivery for DNA extraction to access vector genomes. At 14 day after transduction, the following organs tissues were harvested and snap frozen in liquid nitrogen (lung and trachea, whole blood, stomach, heart, liver, spleen, thymus, intestine, kidney, pancreas, and muscle). With the exception of blood, the organs were then pulverized to a uniform powder for the extraction of DNA for vector genome quantification with TaqMan PCR and for assessment of fLuc activity. TaqMan PCR used 100 ng of genomic DNA, and the following primer and probe set against the fLuc cDNA (primers: 5′-TTTTTGAAGCGAAGGTTGTGG-3′ [forward] and 5′-CACACACAGTTCGCCTCTTTG-3′ [reverse]; probe: 5′-(6FAM)ATCTGGATACCGGGAAAACGC(BHQ1)-3′). The TaqMan PCR was performed and analyzed using the Bio-Rad MyiQ Real-Time PCR detection system and software. Viral genome copy number determination used a standard curve against the proviral plasmid. For fLuc activity assays, ∼100 mg of tissue powder was lysed in 500 μL of reporter lysis buffer (Promega, E3971), homogenized, and then centrifuged at 6,000 RPM for 3 min at 4°C. Protein concentrations of the supernatant were then measured with the BCA kit (Pierce) and normalized to 1 mg/mL. fLuc activity was then measured on 10 μg of protein with the Luciferase Assay System (Promega, E4550).
Statistical Analysis
Experimental data are presented as means ± SD and Prism 7 (GraphPad Software) was used for data analysis. The statistical significance between multiple groups was analyzed with one-way analysis of variance (ANOVA) followed by Tukey test (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001). In Figures 2 and 3, the data were log2 transformed before ANOVA and the Tukey test because of the large range in values. In Figure 4, log2(values + 1) transformed data were used before ANOVA and the Tukey test because certain samples returned an IC50 of zero because of the lack of a clear dose response. Similarly, Figure S1 used log2(values + 1) transformed data before ANOVA and the Tukey test because several values had zeros. Comparisons between only two datasets were made with a two-tailed Student’s t test.
Ethics Statement in Animal Care
All human cell studies were performed according to protocols approved by institutional review boards of the University of Iowa, and all animal experimentation was performed according to protocols approved by the institutional animal care and use committees of the University of Iowa.
Conflicts of Interest
J.F.E. was a co-founder of Talee Bio (now Spirovant Sciences), but he does not work for Spirovant Sciences. This study includes work of Y.T., who is funded by a sponsored research agreement from Spirovant Sciences to J.F.E. Z.Y. and J.F.E. are paid consultants for Spirovant Sciences. S.L. and E.Y. are employees of Spirovant Sciences. The other authors declare no competing interests.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL051670, DK047967, and HL123482 to J.F.E.) and sponsored research from Spirovant Sciences (formerly Talee Bio). We thank Dr. Kai Wang for statistical advice in the analysis of the data.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2020.09.008.
Supplemental Information
References
- 1.Cooney A.L., McCray P.B., Jr., Sinn P.L. Cystic fibrosis gene therapy: looking back, looking forward. Genes (Basel) 2018;9:538. doi: 10.3390/genes9110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Condren M.E., Bradshaw M.D. Ivacaftor: a novel gene-based therapeutic approach for cystic fibrosis. J. Pediatr. Pharmacol. Ther. 2013;18:8–13. doi: 10.5863/1551-6776-18.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Goor F., Hadida S., Grootenhuis P.D., Burton B., Stack J.H., Straley K.S., Decker C.J., Miller M., McCartney J., Olson E.R. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl. Acad. Sci. USA. 2011;108:18843–18848. doi: 10.1073/pnas.1105787108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trapani I., Auricchio A. Seeing the Light after 25 Years of Retinal Gene Therapy. Trends Mol. Med. 2018;24:669–681. doi: 10.1016/j.molmed.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Duan D. Systemic AAV micro-dystrophin gene therapy for Duchenne muscular dystrophy. Mol. Ther. 2018;26:2337–2356. doi: 10.1016/j.ymthe.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doshi B.S., Arruda V.R. Gene therapy for hemophilia: what does the future hold? Ther. Adv. Hematol. 2018;9:273–293. doi: 10.1177/2040620718791933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aitken M.L., Moss R.B., Waltz D.A., Dovey M.E., Tonelli M.R., McNamara S.C., Gibson R.L., Ramsey B.W., Carter B.J., Reynolds T.C. A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum. Gene Ther. 2001;12:1907–1916. doi: 10.1089/104303401753153956. [DOI] [PubMed] [Google Scholar]
- 8.Moss R.B., Milla C., Colombo J., Accurso F., Zeitlin P.L., Clancy J.P., Spencer L.T., Pilewski J., Waltz D.A., Dorkin H.L. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum. Gene Ther. 2007;18:726–732. doi: 10.1089/hum.2007.022. [DOI] [PubMed] [Google Scholar]
- 9.Moss R.B., Rodman D., Spencer L.T., Aitken M.L., Zeitlin P.L., Waltz D., Milla C., Brody A.S., Clancy J.P., Ramsey B. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest. 2004;125:509–521. doi: 10.1378/chest.125.2.509. [DOI] [PubMed] [Google Scholar]
- 10.Ding W., Zhang L., Yan Z., Engelhardt J.F. Intracellular trafficking of adeno-associated viral vectors. Gene Ther. 2005;12:873–880. doi: 10.1038/sj.gt.3302527. [DOI] [PubMed] [Google Scholar]
- 11.Duan D., Yue Y., Yan Z., Yang J., Engelhardt J.F. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J. Clin. Invest. 2000;105:1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan Z., Zak R., Luxton G.W., Ritchie T.C., Bantel-Schaal U., Engelhardt J.F. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J. Virol. 2002;76:2043–2053. doi: 10.1128/jvi.76.5.2043-2053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L.N., Karp P., Gerard C.J., Pastor E., Laux D., Munson K., Yan Z., Liu X., Godwin S., Thomas C.P. Dual therapeutic utility of proteasome modulating agents for pharmaco-gene therapy of the cystic fibrosis airway. Mol. Ther. 2004;10:990–1002. doi: 10.1016/j.ymthe.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Yan Z., Sun X., Feng Z., Li G., Fisher J.T., Stewart Z.A., Engelhardt J.F. Optimization of recombinant adeno-associated virus-mediated expression for large transgenes, using a synthetic promoter and tandem array enhancers. Hum. Gene Ther. 2015;26:334–346. doi: 10.1089/hum.2015.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan Z., Lei-Butters D.C., Liu X., Zhang Y., Zhang L., Luo M., Zak R., Engelhardt J.F. Unique biologic properties of recombinant AAV1 transduction in polarized human airway epithelia. J. Biol. Chem. 2006;281:29684–29692. doi: 10.1074/jbc.M604099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan Z., Sun X., Evans I.A., Tyler S.R., Song Y., Liu X., Sui H., Engelhardt J.F. Postentry processing of recombinant adeno-associated virus type 1 and transduction of the ferret lung are altered by a factor in airway secretions. Hum. Gene Ther. 2013;24:786–796. doi: 10.1089/hum.2013.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding W., Zhang L.N., Yeaman C., Engelhardt J.F. rAAV2 traffics through both the late and the recycling endosomes in a dose-dependent fashion. Mol. Ther. 2006;13:671–682. doi: 10.1016/j.ymthe.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan Z., Lei-Butters D.C., Keiser N.W., Engelhardt J.F. Distinct transduction difference between adeno-associated virus type 1 and type 6 vectors in human polarized airway epithelia. Gene Ther. 2013;20:328–337. doi: 10.1038/gt.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Excoffon K.J., Koerber J.T., Dickey D.D., Murtha M., Keshavjee S., Kaspar B.K., Zabner J., Schaffer D.V. Directed evolution of adeno-associated virus to an infectious respiratory virus. Proc. Natl. Acad. Sci. USA. 2009;106:3865–3870. doi: 10.1073/pnas.0813365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostedgaard L.S., Rokhlina T., Karp P.H., Lashmit P., Afione S., Schmidt M., Zabner J., Stinski M.F., Chiorini J.A., Welsh M.J. A shortened adeno-associated virus expression cassette for CFTR gene transfer to cystic fibrosis airway epithelia. Proc. Natl. Acad. Sci. USA. 2005;102:2952–2957. doi: 10.1073/pnas.0409845102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostedgaard L.S., Meyerholz D.K., Vermeer D.W., Karp P.H., Schneider L., Sigmund C.D., Welsh M.J. Cystic fibrosis transmembrane conductance regulator with a shortened R domain rescues the intestinal phenotype of CFTR−/− mice. Proc. Natl. Acad. Sci. USA. 2011;108:2921–2926. doi: 10.1073/pnas.1019752108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steines B., Dickey D.D., Bergen J., Excoffon K.J., Weinstein J.R., Li X., Yan Z., Abou Alaiwa M.H., Shah V.S., Bouzek D.C. CFTR gene transfer with AAV improves early cystic fibrosis pig phenotypes. JCI Insight. 2016;1:e88728. doi: 10.1172/jci.insight.88728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vandamme C., Adjali O., Mingozzi F. Unraveling the complex story of immune responses to AAV vectors trial after trial. Hum. Gene Ther. 2017;28:1061–1074. doi: 10.1089/hum.2017.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scallan C.D., Jiang H., Liu T., Patarroyo-White S., Sommer J.M., Zhou S., Couto L.B., Pierce G.F. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107:1810–1817. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- 25.Fisher J.T., Zhang Y., Engelhardt J.F. Comparative biology of cystic fibrosis animal models. Methods Mol. Biol. 2011;742:311–334. doi: 10.1007/978-1-61779-120-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keiser N.W., Engelhardt J.F. New animal models of cystic fibrosis: what are they teaching us? Curr. Opin. Pulm. Med. 2011;17:478–483. doi: 10.1097/MCP.0b013e32834b14c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen B.H., Evans T.I.A., Moll S.R., Gray J.S., Liang B., Sun X., Zhang Y., Jensen-Cody C.W., Swatek A.M., Zhou W. Infection is not required for mucoinflammatory lung disease in CFTR-knockout ferrets. Am. J. Respir. Crit. Care Med. 2018;197:1308–1318. doi: 10.1164/rccm.201708-1616OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun X., Olivier A.K., Liang B., Yi Y., Sui H., Evans T.I., Zhang Y., Zhou W., Tyler S.R., Fisher J.T. Lung phenotype of juvenile and adult cystic fibrosis transmembrane conductance regulator-knockout ferrets. Am. J. Respir. Cell Mol. Biol. 2014;50:502–512. doi: 10.1165/rcmb.2013-0261OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun X., Sui H., Fisher J.T., Yan Z., Liu X., Cho H.J., Joo N.S., Zhang Y., Zhou W., Yi Y. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J. Clin. Invest. 2010;120:3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun X., Yi Y., Yan Z., Rosen B.H., Liang B., Winter M.C., Evans T.I.A., Rotti P.G., Yang Y., Gray J.S. In utero and postnatal VX-770 administration rescues multiorgan disease in a ferret model of cystic fibrosis. Sci. Transl. Med. 2019;11:eeau7531. doi: 10.1126/scitranslmed.aau7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olivier A.K., Yi Y., Sun X., Sui H., Liang B., Hu S., Xie W., Fisher J.T., Keiser N.W., Lei D. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J. Clin. Invest. 2012;122:3755–3768. doi: 10.1172/JCI60610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun X., Olivier A.K., Yi Y., Pope C.E., Hayden H.S., Liang B., Sui H., Zhou W., Hager K.R., Zhang Y. Gastrointestinal pathology in juvenile and adult CFTR-knockout ferrets. Am. J. Pathol. 2014;184:1309–1322. doi: 10.1016/j.ajpath.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi Y., Sun X., Gibson-Corley K., Xie W., Liang B., He N., Tyler S.R., Uc A., Philipson L.H., Wang K. A transient metabolic recovery from early life glucose intolerance in cystic fibrosis ferrets occurs during pancreatic remodeling. Endocrinology. 2016;157:1852–1865. doi: 10.1210/en.2015-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griesenbach U., Vicente C.C., Roberts M.J., Meng C., Soussi S., Xenariou S., Tennant P., Baker A., Baker E., Gordon C. Secreted Gaussia luciferase as a sensitive reporter gene for in vivo and ex vivo studies of airway gene transfer. Biomaterials. 2011;32:2614–2624. doi: 10.1016/j.biomaterials.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Zádori Z., Szelei J., Lacoste M.C., Li Y., Gariépy S., Raymond P., Allaire M., Nabi I.R., Tijssen P. A viral phospholipase A2 is required for parvovirus infectivity. Dev. Cell. 2001;1:291–302. doi: 10.1016/s1534-5807(01)00031-4. [DOI] [PubMed] [Google Scholar]
- 36.Girod A., Wobus C.E., Zádori Z., Ried M., Leike K., Tijssen P., Kleinschmidt J.A., Hallek M. The VP1 capsid protein of adeno-associated virus type 2 is carrying a phospholipase A2 domain required for virus infectivity. J. Gen. Virol. 2002;83:973–978. doi: 10.1099/0022-1317-83-5-973. [DOI] [PubMed] [Google Scholar]
- 37.Shin J.H., Yue Y., Smith B., Duan D. Humoral immunity to AAV-6, 8, and 9 in normal and dystrophic dogs. Hum. Gene Ther. 2012;23:287–294. doi: 10.1089/hum.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dörner T., Radbruch A. Antibodies and B cell memory in viral immunity. Immunity. 2007;27:384–392. doi: 10.1016/j.immuni.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Louis Jeune V., Joergensen J.A., Hajjar R.J., Weber T. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum. Gene Ther. Methods. 2013;24:59–67. doi: 10.1089/hgtb.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amado D., Mingozzi F., Hui D., Bennicelli J.L., Wei Z., Chen Y., Bote E., Grant R.L., Golden J.A., Narfstrom K. Safety and efficacy of subretinal readministration of a viral vector in large animals to treat congenital blindness. Sci. Transl. Med. 2010;2:21ra16. doi: 10.1126/scitranslmed.3000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mingozzi F., Anguela X.M., Pavani G., Chen Y., Davidson R.J., Hui D.J., Yazicioglu M., Elkouby L., Hinderer C.J., Faella A. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci. Transl. Med. 2013;5:194ra92. doi: 10.1126/scitranslmed.3005795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mingozzi F., High K.A. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li-Kim-Moy J.P., Yin J.K., Heron L., Leask J., Lambert S.B., Nissen M., Sloots T., Booy R. Influenza vaccine efficacy in young children attending childcare: a randomised controlled trial. J. Paediatr. Child Health. 2017;53:47–54. doi: 10.1111/jpc.13313. [DOI] [PubMed] [Google Scholar]
- 44.Mameli C., Cocchi I., Fumagalli M., Zuccotti G. Influenza vaccination: effectiveness, indications, and limits in the pediatric population. Front Pediatr. 2019;7:317. doi: 10.3389/fped.2019.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lafond K.E., Englund J.A., Tam J.S., Bresee J.S. Overview of influenza vaccines in children. J. Pediatric Infect. Dis. Soc. 2013;2:368–378. doi: 10.1093/jpids/pit053. [DOI] [PubMed] [Google Scholar]
- 46.Onodera T., Takahashi Y., Yokoi Y., Ato M., Kodama Y., Hachimura S., Kurosaki T., Kobayashi K. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. Proc. Natl. Acad. Sci. USA. 2012;109:2485–2490. doi: 10.1073/pnas.1115369109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blutt S.E., Conner M.E. The gastrointestinal frontier: IgA and viruses. Front. Immunol. 2013;4:402. doi: 10.3389/fimmu.2013.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halbert C.L., Rutledge E.A., Allen J.M., Russell D.W., Miller A.D. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J. Virol. 2000;74:1524–1532. doi: 10.1128/jvi.74.3.1524-1532.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halbert C.L., Standaert T.A., Wilson C.B., Miller A.D. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J. Virol. 1998;72:9795–9805. doi: 10.1128/jvi.72.12.9795-9805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Limberis M.P., Wilson J.M. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc. Natl. Acad. Sci. USA. 2006;103:12993–12998. doi: 10.1073/pnas.0601433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beck S.E., Jones L.A., Chesnut K., Walsh S.M., Reynolds T.C., Carter B.J., Askin F.B., Flotte T.R., Guggino W.B. Repeated delivery of adeno-associated virus vectors to the rabbit airway. J. Virol. 1999;73:9446–9455. doi: 10.1128/jvi.73.11.9446-9455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fischer A.C., Beck S.E., Smith C.I., Laube B.L., Askin F.B., Guggino S.E., Adams R.J., Flotte T.R., Guggino W.B. Successful transgene expression with serial doses of aerosolized rAAV2 vectors in rhesus macaques. Mol. Ther. 2003;8:918–926. doi: 10.1016/j.ymthe.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 53.Jiang Q., Engelhardt J.F. Cellular heterogeneity of CFTR expression and function in the lung: implications for gene therapy of cystic fibrosis. Eur. J. Hum. Genet. 1998;6:12–31. doi: 10.1038/sj.ejhg.5200158. [DOI] [PubMed] [Google Scholar]
- 54.Tang Y., Yan Z., Engelhardt J.F. Viral vectors, animal models, and cellular targets for gene therapy of cystic fibrosis lung disease. Hum. Gene Ther. 2020;31:524–537. doi: 10.1089/hum.2020.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan Z., Feng Z., Sun X., Zhang Y., Zou W., Wang Z., Jensen-Cody C., Liang B., Park S.Y., Qiu J., Engelhardt J.F. Human bocavirus type-1 capsid facilitates the transduction of ferret airways by adeno-associated virus genomes. Hum. Gene Ther. 2017;28:612–625. doi: 10.1089/hum.2017.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clark K.R., Liu X., McGrath J.P., Johnson P.R. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum. Gene Ther. 1999;10:1031–1039. doi: 10.1089/10430349950018427. [DOI] [PubMed] [Google Scholar]
- 57.Clark K.R., Voulgaropoulou F., Fraley D.M., Johnson P.R. Cell lines for the production of recombinant adeno-associated virus. Hum. Gene Ther. 1995;6:1329–1341. doi: 10.1089/hum.1995.6.10-1329. [DOI] [PubMed] [Google Scholar]
- 58.Fisher J.T., Tyler S.R., Zhang Y., Lee B.J., Liu X., Sun X., Sui H., Liang B., Luo M., Xie W. Bioelectric characterization of epithelia from neonatal CFTR knockout ferrets. Am. J. Respir. Cell Mol. Biol. 2013;49:837–844. doi: 10.1165/rcmb.2012-0433OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu P., Lu J., Zhang X., Mei M., Feng L., Peng D., Hou J., Kang S.M., Liu X., Tang Y. Single Dose of Consensus Hemagglutinin-Based Virus-Like Particles Vaccine Protects Chickens against Divergent H5 Subtype Influenza Viruses. Front. Immunol. 2017;8:1649. doi: 10.3389/fimmu.2017.01649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.