Abstract
The purpose of this study was to evaluate the relationships of smoking, alcohol consumption, and obesity with thyroid cancer in Korean residents. The Korean National Health Insurance Service-Health Screening Cohort includes individuals ≥ 40 years who were assessed from 2002 to 2013. In total, 4977 thyroid cancer participants were matched with respect to age, sex, income, and region of residence with 19,908 controls at a ratio of 1:4. Crude and adjusted (for the Charlson comorbidity index, smoking status, frequency of alcohol consumption, and obesity) odds ratios (ORs) were analyzed using conditional logistic regression analyses. Additionally, 95% confidence intervals (CIs) were calculated. The adjusted OR of smoking for thyroid cancer was 0.62 (95% CI 0.54–0.72, P < 0.001), and that of alcohol consumption was 0.83 (95% CI 0.75–0.92, P < 0.001). The adjusted ORs of the BMI categories were 1.13 (95% CI 1.05–1.22, P = 0.002) for obese I, and 1.24 (95% CI 1.04–1.47, P = 0.014) for obese II. The ORs of smoking and alcohol consumption were lower, and those of overweight and obesity were higher in thyroid cancer patients than in individuals in the control group.
Subject terms: Cancer, Oncology
Introduction
The incidence of thyroid cancer is increasing in many countries1,2. Specifically, in South Korea, the incidence of thyroid cancer increased 15-fold between 1993 and 20113, and the age-standardized incidence of thyroid cancer per 100,000 people was 23.0 in men and 102.4 in women in 20124. A higher rate of overdiagnosis as a result of health screening with ultrasonography is apparently responsible for this3,5. However, it is not the only explanation of the increasing incidence of thyroid cancer6. Some etiologic factors, including obesity, might affect the increasing incidence of thyroid cancer7. Other conditions such as radiation exposure and iodine intake are also considered risk factors for thyroid cancer1. However, these contributions remain speculative.
Previously, several studies reported associations of obesity, smoking, and drinking alcohol with thyroid cancer7–9. However, the numbers of included participants were relatively few (< 1000 cases of thyroid cancer) in most studies. Few studies have analyzed obesity, smoking, and alcohol consumption in a single study after adjusting for the effects of these variables even though they are closely related.
The purpose of this study was to identify the factors that predispose individuals to thyroid cancer using a national health checkup cohort of South Korean residents. In this study, we analyzed the odds ratios (ORs) of obesity, smoking, and alcohol consumption in terms of thyroid cancer compared to the 1:4 matched control group. In this study, we focused on these factors because they could be modifiable and controlling them could affect the incidence of thyroid cancer.
Results
The rates of smoking and alcohol consumption were lower in the thyroid cancer group (smoking = 5.5% [273/4977]; alcohol consumption = 11.8% [598/4977]) than in the control group (smoking = 8.1% [1618/19,908]; alcohol consumption = 14.0% [2791/19,908]; each P = < 0.001, Table 1). The thyroid cancer group included more obese participants than the control group (P < 0.001). The general characteristics (age, sex, income, and region of residence) of the participants were the same due to the matching procedure (P = 1.000). The CCI score was different between the thyroid cancer and control groups (P < 0.001).
Table 1.
General participant characteristics.
| Characteristics | Total participants | ||
|---|---|---|---|
| Thyroid cancer (n, %) | Control (n, %) | P-value | |
| Age (years) | 1.000 | ||
| 40–44 | 104 (2.1) | 416 (2.1) | |
| 45–49 | 797 (16.0) | 3188 (16.0) | |
| 50–54 | 1427 (28.7) | 5708 (28.7) | |
| 55–59 | 1084 (21.8) | 4336 (21.8) | |
| 60–64 | 727 (14.6) | 2908 (14.6) | |
| 65–69 | 476 (9.6) | 1904 (9.6) | |
| 70–74 | 268 (5.4) | 1072 (5.4) | |
| 75–79 | 74 (1.5) | 296 (1.5) | |
| 80–84 | 19 (0.4) | 76 (0.4) | |
| 85 + | 1 (0.0) | 4 (0.0) | |
| Sex | 1.000 | ||
| Male | 1030 (20.7) | 4120 (20.7) | |
| Female | 3947 (79.3) | 15,788 (79.3) | |
| Income | 1.000 | ||
| 1 (lowest) | 609 (12.2) | 2436 (12.2) | |
| 2 | 562 (11.3) | 2248 (11.3) | |
| 3 | 762 (15.3) | 3048 (15.3) | |
| 4 | 984 (19.8) | 3936 (19.8) | |
| 5 (highest) | 2060 (41.4) | 8240 (41.4) | |
| Region of residence | 1.000 | ||
| Urban | 2418 (48.6) | 9672 (48.6) | |
| Rural | 2559 (51.4) | 10,236 (51.4) | |
| Charlson comorbidity index | < 0.001* | ||
| 0 | 4799 (96.4) | 19,617 (98.5) | |
| 1 | 0 (0.0) | 48 (0.2) | |
| 2 | 47 (0.9) | 59 (0.3) | |
| 3 | 14 (0.3) | 51 (0.3) | |
| ≥ 4 | 117 (2.4) | 133 (0.7) | |
| Obesity (BMI, kg/m2) | < 0.001* | ||
| < 18.5 (underweight) | 61 (1.2) | 350 (1.8) | |
| ≥ 18.5 to < 23 (normal) | 1662 (33.4) | 7097 (35.6) | |
| ≥ 23 to < 25 (overweight) | 1420 (28.5) | 5608 (28.2) | |
| ≥ 25 to < 30 (obese I) | 1643 (33.0) | 6196 (31.1) | |
| ≥ 30 (obese II) | 191 (3.8) | 657 (3.3) | |
| Smoking status | < 0.001* | ||
| Nonsmoker or past smoker | 4704 (94.5) | 18,290 (91.9) | |
| Current smoker | 273 (5.5) | 1618 (8.1) | |
| Alcohol consumption | < 0.001* | ||
| < 1 time a week | 4388 (88.2) | 17,117 (86.0) | |
| ≥ 1 time a week | 589 (11.8) | 2791 (14.0) | |
BMI body mass index, kg/m2.
*Chi-square test. Significance at P < 0.05.
The adjusted OR (aOR) of smoking for thyroid cancer was 0.62 (95% CI 0.54–0.72, P < 0.001), and that of alcohol consumption was 0.83 (95% CI 0.75–0.92, P < 0.001, Table 2). The aORs of the BMI categories were 0.75 (95% CI 0.57–0.99, P = 0.042) for underweight, 1.08 (95% CI 1.00–1.17, P = 0.050) for overweight, 1.13 (95% CI 1.05–1.22, P = 0.002) for obese I, and 1.24 (95% CI 1.04–1.47, P = 0.014) for obese II compared to a normal BMI in the thyroid cancer group.
Table 2.
Crude and adjusted odd ratios (95% confidence intervals) of smoking, alcohol consumption, and obesity for thyroid cancer.
| Characteristics | N of thyroid cancer | N of control | ORs of thyroid cancer | |||
|---|---|---|---|---|---|---|
| (Exposure/total, %) | (Exposure/total, %) | Crude† | P-value | Adjusted†‡ | P-value | |
| Smoking status | 273/4977 (5.5%) | 1618/19,908 (8.1%) | 0.60 (0.52–0.69) | < 0.001* | 0.62 (0.54–0.72) | < 0.001* |
| Alcohol consumption | 589/4977 (11.8%) | 2791/19,908 (14.0%) | 0.79 (0.71–0.88) | < 0.001* | 0.83 (0.75–0.92) | < 0.001* |
| Obesity (BMI, kg/m2) | < 0.001* | < 0.001* | ||||
| < 18.5 (underweight) | 61/4977 (1.2%) | 350/19,908 (1.8%) | 0.74 (0.56–0.98) | 0.037* | 0.75 (0.57–0.99) | 0.042* |
| ≥ 18.5 to < 23 (normal) | 1662/4977 (33.4%) | 7097/19,908 (35.7%) | 1.00 | 1.00 | ||
| ≥ 23 to < 25 (overweight) | 1420/4977 (28.5%) | 5608/19,908 (28.2%) | 1.09 (1.00–1.17) | 0.045* | 1.08 (1.00–1.17) | 0.050* |
| ≥ 25 to < 30 (obese I) | 1643/4977 (33.0%) | 6196/19,908 (31.1%) | 1.14 (1.05–1.23) | 0.001* | 1.13 (1.05–1.22) | 0.002* |
| ≥ 30 (obese II) | 191/4977 (3.8%) | 657/19,908 (3.3%) | 1.25 (1.05–1.48) | 0.011* | 1.24 (1.04–1.47) | 0.014* |
*Conditional logistic regression analysis, Significance at P < 0.05.
†Stratified model for age, sex, income, and region of residence.
‡Adjusted model for Charlson comorbidity index, obesity, smoking state (current smoker compared to nonsmoker or past smoker) and frequency of alcohol consumption (≥ 1 time a week compared to < 1 time a week).
In subgroup analyses performed according to age and sex, the aOR of obesity was statistically significant in individuals ≥ 55 years and in men. The aORs of smoking reached statistical significance in all groups, while alcohol consumption was significant in individuals < 55 years and ≥ 55 years and in women (Table 3).
Table 3.
Crude and adjusted odd ratios (95% confidence interval) of smoking, alcohol consumption, and obesity for thyroid cancer in each stratified group according age and sex.
| Characteristics | N of thyroid cancer | N of tontrol | ORs of thyroid cancer | |||
|---|---|---|---|---|---|---|
| (Exposure/total, %) | (Exposure/total, %) | Crude† | P-value | Adjusted†‡ | P-value | |
| < 55 years (n = 11,640) | ||||||
| Smoking status | 164/2328 (7.0%) | 871/9312 (9.4%) | 0.68 (0.59–0.82) | < 0.001* | 0.71 (0.58–0.86) | < 0.001* |
| Alcohol consumption | 321/2328 (13.8%) | 1547/9312 (16.6%) | 0.77 (0.67–0.89) | < 0.001* | 0.80 (0.69–0.92) | 0.002* |
| Obesity (BMI, kg/m2) | 0.145 | 0.133 | ||||
| < 18.5 (underweight) | 32/2328 (1.4%) | 174/9312 (1.9%) | 0.77 (0.52–1.13) | 0.182 | 0.79 (0.54–1.16) | 0.235 |
| ≥ 18.5 to < 23 (normal) | 895/2328 (38.5%) | 3749/9312 (40.3%) | 1.00 | 1.00 | ||
| ≥ 23 to < 25 (overweight) | 660/2328 (28.4%) | 2574/9312 (27.6%) | 1.08 (0.96–1.21) | 0.199 | 1.08 (0.96–1.21) | 0.186 |
| ≥ 25 to < 30 (obese I) | 668/2328 (28.7%) | 2565/9312 (27.6%) | 1.10 (0.98–1.23) | 0.116 | 1.09 (0.97–1.22) | 0.136 |
| ≥ 30 (obese II) | 73/2328 (3.1%) | 250/9312 (2.7%) | 1.23 (0.94–1.61) | 0.138 | 1.21 (0.92–1.59) | 0.175 |
| ≥ 55 year (n = 13,245) | ||||||
| Smoking | 109/2649 (4.1%) | 747/10,596 (7.1%) | 0.52 (0.42–0.64) | < 0.001* | 0.53 (0.42–0.66) | < 0.001* |
| Alcohol consumption | 268/2649 (10.1%) | 1244/10,596 (11.7%) | 0.82 (0.70–0.95) | 0.009* | 0.86 (0.74–1.00) | 0.056 |
| Obesity (BMI, kg/m2) | 0.005* | 0.006* | ||||
| < 18.5 (underweight) | 29/2649 (1.1%) | 176/10,596 (1.7%) | 0.72 (0.48–1.07) | 0.107 | 0.72 (0.48–1.08) | 0.109 |
| ≥ 18.5 to < 23 (normal) | 767/2649 (29.0%) | 3348/10,596 (31.6%) | 1.00 | 1.00 | ||
| ≥ 23 to < 25 (overweight) | 760/2649 (28.7%) | 3034/10,596 (28.6%) | 1.10 (0.98–1.22) | 0.114 | 1.09 (0.97–1.22) | 0.141 |
| ≥ 25 to < 30 (obese I) | 975/2649 (36.8%) | 3631/10,596 (34.3%) | 1.17 (1.06–1.31) | 0.003* | 1.16 (1.05–1.29) | 0.005* |
| ≥ 30 (obese II) | 118/2649 (4.5%) | 407/10,596 (3.8%) | 1.27 (1.02–1.58) | 0.035* | 1.25 (1.01–1.56) | 0.045* |
| Men (n = 5150) | ||||||
| Smoking | 232/1030 (22.5%) | 1291/4120 (31.3%) | 0.63 (0.54–0.74) | < 0.001* | 0.65 (0.55–0.76) | < 0.001* |
| Alcohol consumption | 385/1030 (37.4%) | 1744/4120 (42.3%) | 0.82 (0.71–0.94) | 0.004* | 0.85 (0.73–0.98) | 0.024* |
| Obesity (BMI, kg/m2) | < 0.001* | < 0.001* | ||||
| < 18.5 (underweight) | 8/1030 (0.8%) | 59/4120 (1.4%) | 0.69 (0.32–1.46) | 0.330 | 0.67 (0.31–1.44) | 0.302 |
| ≥ 18.5 to < 23 (normal) | 232/1030 (22.5%) | 1188/4120 (28.8%) | 1.00 | 1.00 | ||
| ≥ 23 to < 25 (overweight) | 323/1030 (31.4%) | 1264/4120 (30.7%) | 1.32 (1.09–1.59) | 0.004* | 1.31 (1.08–1.58) | 0.006* |
| ≥ 25 to < 30 (obese I) | 429/1030 (41.7%) | 1,513/4,120 (36.7%) | 1.46 (1.22–1.74) | < 0.001* | 1.44 (1.20–1.72) | < 0.001* |
| ≥ 30 (obese II) | 38/1,030 (3.7%) | 96/4,120 (2.3%) | 2.05 (1.37–3.06) | < 0.001* | 2.01 (1.34–3.03) | < 0.001* |
| Women (n = 19,735) | ||||||
| Smoking | 41/3947 (1.0%) | 327/15,788 (2.1%) | 0.50 (0.36–0.69) | < 0.001* | 0.53 (0.38–0.74) | < 0.001* |
| Alcohol consumption | 204/3947 (5.2%) | 1047/15,788 (6.6%) | 0.77 (0.66–0.89) | < 0.001* | 0.80 (0.69–0.94) | 0.006* |
| Obesity (BMI, kg/m2) | 0.088 | 0.113 | ||||
| < 18.5 (underweight) | 53/3947 (1.3%) | 291/15,788 (1.8%) | 0.75 (0.56–1.02) | 0.062 | 0.76 (0.57–1.03) | 0.077 |
| ≥ 18.5 to < 23 (normal) | 1430/3947 (36.2%) | 5909/15,788 (37.4%) | 1.00 | 1.00 | ||
| ≥ 23 to < 25 (overweight) | 1097/3947 (27.8%) | 4344/15,788 (27.5%) | 1.05 (0.96–1.14) | 0.328 | 1.05 (0.96–1.14) | 0.329 |
| ≥ 25 to < 30 (obese I) | 1214/3947 (30.8%) | 4683/15,788 (29.7%) | 1.07 (0.99–1.17) | 0.107 | 1.07 (0.98–1.17) | 0.116 |
| ≥ 30 (obese II) | 153/3947 (3.9%) | 561/15,788 (3.6%) | 1.13 (0.94–1.37) | 0.202 | 1.12 (0.93–1.36) | 0.225 |
*Conditional logistic regression analysis, Significance at P < 0.05.
†Stratified model for age, sex, income, and region of residence.
‡Adjusted model for Charlson comorbidity index, obesity, smoking state (current smoker compared to nonsmoker or past smoker) and frequency of alcohol consumption (≥ 1 time a week compared to < 1 time a week).
We analyzed the ORs of thyroid cancer in the subgroups of nonsmokers or past smokers, current smokers, individuals who consume alcohol < 1 time a week, individuals who consume alcohol ≥ 1 time a week, patients who are underweight, patients who are normal weight, individuals who are overweight, individuals in the obese I group, and individuals in the obese II group (Table 4). Although these subgroup analyses did not follow the matched structure of this study, the results were consistent with the main results. These associations did not reach statistical significance in the underweight or obese II groups due to the relatively small number of participants.
Table 4.
Odd ratios (95% confidence interval) of smoking, alcohol consumption, and obesity for thyroid cancer in each group.
| Characteristics | N of thyroid cancer | N of control | ORs of thyroid cancer | |||
|---|---|---|---|---|---|---|
| (Exposure/total, %) | (Exposure/total, %) | Crude | P-value | Adjusted† | P-value | |
| Non or past smoker (n = 22,994) | ||||||
| Alcohol consumption | 456/4704 (9.7%) | 2003/18,290 (11.0%) | 0.87 (0.78–0.97) | 0.013* | 0.81 (0.72–0.91) | < 0.001* |
| Obesity (BMI, kg/m2) | 0.004* | 0.004* | ||||
| < 18.5 (underweight) | 58/4704 (1.2%) | 309/18,290 (1.7%) | 0.78 (0.58–1.03) | 0.084 | 0.77 (0.58–1.02) | 0.072 |
| ≥ 18.5 to < 23 (normal) | 1588/4704 (33.8%) | 6572/18,290 (35.9%) | 1.00 | 1.00 | ||
| ≥ 23 to < 25 (overweight) | 1344/4704 (28.6%) | 5123/18,290 (28.0%) | 1.09 (1.00–1.18) | 0.047* | 1.08 (1.00–1.18) | 0.054 |
| ≥ 25 to < 30 (obese I) | 1533/4704 (32.6%) | 5669/18,290 (31.0%) | 1.12 (1.03–1.21) | 0.005* | 1.11 (1.03–1.21) | 0.008* |
| ≥ 30 (obese II) | 181/4704 (3.9%) | 617/18,290 (3.4%) | 1.21 (1.02–1.45) | 0.029* | 1.22 (1.02–1.45) | 0.027* |
| Current smoker (n = 1891) | ||||||
| Alcohol consumption | 133/273 (48.7%) | 788/1618 (48.7%) | 1.00 (0.77–1.29) | 0.996 | 0.93 (0.71–1.21) | 0.565 |
| Obesity (BMI, kg/m2) | 0.046* | 0.080 | ||||
| < 18.5 (underweight) | 3/273 (1.1%) | 41/1618 (2.5%) | 0.52 (0.16–1.72) | 0.283 | 0.57 (0.17–1.90) | 0.360 |
| ≥ 18.5 to < 23 (normal) | 74/273 (27.1%) | 525/1618 (32.5%) | 1.00 | 1.00 | ||
| ≥ 23 to < 25 (overweight) | 76/273 (27.8%) | 485/1618 (30.0%) | 1.11 (0.79–1.57) | 0.545 | 1.05 (0.74–1.49) | 0.779 |
| ≥ 25 to < 30 (obese I) | 110/273 (40.3%) | 527/1618 (32.6%) | 1.48 (1.08–2.04) | 0.016* | 1.44 (1.05–2.00) | 0.026* |
| ≥ 30 (obese II) | 10/273 (3.7%) | 40/1618 (2.5%) | 1.77 (0.85–3.70) | 0.126 | 1.60 (0.75–3.42) | 0.222 |
| Consuming alcohol < 1 time a week (n = 21,505) | ||||||
| Smoking | 140/4388 (3.2%) | 830/17,117 (4.9%) | 0.65 (0.54–0.78) | < 0.001* | 0.58 (0.47–0.70) | < 0.001* |
| Obesity (BMI, kg/m2) | 0.003* | 0.004* | ||||
| < 18.5 (underweight) | 52/4388 (1.2%) | 303/17,117 (1.8%) | 0.71 (0.52–0.95) | 0.023* | 0.71 (0.52–0.95) | 0.024* |
| ≥ 18.5 to < 23 (normal) | 1522/4388 (34.7%) | 6256/17,117 (36.6%) | 1.00 | 1.00 | ||
| ≥ 23 to < 25 (overweight) | 1229/4388 (28.0%) | 4782/17,117 (27.9%) | 1.06 (0.97–1.15) | 0.201 | 1.06 (0.97–1.15) | 0.211 |
| ≥ 25 to < 30 (obese I) | 1427/4388 (32.5%) | 5212/17,117 (30.5%) | 1.13 (1.04–1.22) | 0.004* | 1.12 (1.03–1.22) | 0.006* |
| ≥ 30 (obese II) | 158/4388 (3.6%) | 564/17,117 (3.3%) | 1.15 (0.96–1.39) | 0.135 | 1.15 (0.96–1.39) | 0.136 |
| Consuming alcohol ≥ 1 time a week (n = 3380) | ||||||
| Smoking | 133/589 (22.6%) | 788/2791 (28.2%) | 0.74 (0.60–0.92) | 0.005* | 0.68 (0.54–0.85) | < 0.001* |
| Obesity (BMI, kg/m2) | 0.005* | 0.030* | ||||
| < 18.5 (underweight) | 9/589 (1.5%) | 47/2791 (1.7%) | 1.15 (0.55–2.40) | 0.709 | 1.17 (0.55–2.48) | 0.682 |
| ≥ 18.5 to < 23 (normal) | 140/589 (23.8%) | 841/2791 (30.1%) | 1.00 | 1.00 | ||
| ≥ 23 to < 25 (overweight) | 191/589 (32.4%) | 826/2791 (29.6%) | 1.39 (1.10–1.76) | 0.007* | 1.32 (1.03–1.68) | 0.026* |
| ≥ 25 to < 30 (obese I) | 216/589 (36.7%) | 984/2791 (35.3%) | 1.32 (1.05–1.66) | 0.019* | 1.24 (0.98–1.56) | 0.078 |
| ≥ 30 (obese II) | 33/589 (5.6%) | 93/2791 (3.3%) | 2.13 (1.38–3.30) | < 0.001* | 1.95 (1.25–3.05) | 0.003* |
| Underweight (BMI < 18.5, n = 411) | ||||||
| Smoking | 3/61 (4.9%) | 41/350 (11.7%) | 0.39 (0.12–1.30) | 0.126 | 0.34 (0.09–1.28) | 0.111 |
| Alcohol consumption | 9/61 (14.8%) | 47/350 (13.4%) | 1.12 (0.52–2.41) | 0.781 | 1.56 (0.64–3.77) | 0.327 |
| Normal weight (BMI ≥ 18.5 to < 23, n = 8759) | ||||||
| Smoking | 74/1662 (4.5%) | 525/7097 (7.4%) | 0.58 (0.46–0.75) | < 0.001* | 0.65 (0.49–0.85) | 0.002* |
| Alcohol consumption | 140/1662 (8.4%) | 841/7097 (11.9%) | 0.69 (0.57–0.83) | < 0.001* | 0.77 (0.63–0.94) | 0.012* |
| Overweight (BMI ≥ 23 to < 25, n = 7028) | ||||||
| Smoking | 76/1420 (5.4%) | 485/5608 (8.7%) | 0.60 (0.47–0.77) | < 0.001* | 0.52 (0.40–0.69) | < 0.001* |
| Alcohol consumption | 191/1420 (13.5%) | 826/5608 (14.7%) | 0.90 (0.76–1.07) | 0.222 | 0.93 (0.77–1.12) | 0.416 |
| Obese I (BMI ≥ 25 to < 30, n = 7839) | ||||||
| Smoking | 110/1643 (6.7%) | 527/6196 (8.5%) | 0.77 (0.62–0.96) | 0.017* | 0.71 (0.57–0.90) | 0.004* |
| Alcohol consumption | 216/1643 (13.2%) | 984/6196 (15.9%) | 0.80 (0.68–0.94) | 0.006* | 0.74 (0.62–0.89) | < 0.001* |
| Obese II (BMI ≥ 30, n = 848) | ||||||
| Smoking | 10/191 (5.2%) | 40/657 (6.1%) | 0.85 (0.42–1.74) | 0.660 | 0.57 (0.25–1.27) | 0.168 |
| Alcohol consumption | 33/191 (17.3%) | 93/657 (14.2%) | 1.27 (0.82–1.96) | 0.286 | 1.09 (0.66–1.80) | 0.749 |
*Unconditional logistic regression analysis, Significance at P < 0.05.
†Adjusted model for age, sex, income, region of residence, Charlson comorbidity index, obesity, smoking state (current smoker compared to nonsmoker or past smoker) and frequency of alcohol consumption (≥ 1 time a week compared to < 1 time a week).
Discussion
In the present study, participants with thyroid cancer had higher ORs for overweight and obese and lower ORs for smoking and alcohol consumption than participants in the control group. These findings were consistent in the subgroups of age and sex.
Obesity could affect thyroid tumorigenesis through various mechanisms7. Obesity results in (i) hyperinsulinemia with an increase in IGF-1, (ii) an increase in leptin and a decrease in adiponectin, (iii) chronic inflammation via IL-6, TNF-α, PAI-1, and NF-κB, (iv) an increase in free fatty acids, and (v) oxidative stress including DNA damage10. Most previous studies have shown that obesity is associated with thyroid cancer, while a few studies exhibited inconsistent results. A previous large meta-analysis (n of thyroid cancer patients = 3587) reported that each 5 kg/m2 increase in BMI increased thyroid cancer risk 1.33 times (95% CI 1.04–1.70) in men and 1.14 times (95% CI 1.06–1.23) in women11. Another cohort study reported that the relative risk (RR) for thyroid cancer was 1.89 (95% CI 1.21–2.96) in men with a BMI ≥ 30.0 kg/m2, while the RR was 1.10 (95% CI 0.75–1.61) in women with a BMI ≥ 30.0 kg/m212. Another cohort study exhibited contrary results of a hazard ratio (HR) of 2.14 (95% CI 0.06–7.67) in men with a BMI ≥ 35.0 kg/m2 and an HR of 1.74 (95% CI 1.03–2.94) in women with a BMI ≥ 35.0 kg/m213. Some studies did not reveal any relationship between obesity and thyroid cancer (a cohort study in 432 participants)14. However, that study was based on a relatively small study population of 432 participants and analyzed the association of obesity with more aggressive features of differentiated thyroid cancer15. Another previous study even found a negative association in single hospital-based study16. However, that study also had small study population (253 patients) and investigated the relation of obesity with the risk of thyroid cancer in patients with indeterminate thyroid nodules16. We found that obesity was associated with thyroid cancer in the ≥ 55-year-old group and in men using the Asian BMI classification17. Although the P values of obesity in women and the < 55-year-old group did not reach statistical significance, their ORs showed an increasing trend as BMI increased. Differences in the effects of estrogen from adipose tissue with different aromatization might cause these differences with regard to age and sex18. In Korea, the rate of obesity (≥ 25 kg/m2) increased from 25.1% in 1998 to 42.3% in 2016 in men, while the rate was stable at approximately 26.2–26.4% during the same time period in women19. The abrupt increase in obesity in men might affect the difference between men and women. Unconsidered factors such as dietary and exercise habits, which are related to obesity, may be involved in this difference.
Relatively consistently, smoking has been reported as a factor that reduces the risk of thyroid cancer. A pooled analysis reported ORs of 0.6 (95% CI 0.6–0.7) in current smokers and 0.9 (95% CI 0.8–1.1) in former smokers8. A recent meta-analysis reported an OR of 0.79 (95% CI 0.70–0.88) in ever smokers20. The OR of thyroid cancer was 0.49 (95% CI 0.31–0.78) in current smokers and 0.75 (95% CI 0.48–1.17) in former smokers compared to nonsmokers in a group of Korea adults21. Smoking decreases thyroid-stimulating hormone (TSH) levels, which has major effects on thyroid cancer development22. Thiocyanate, a byproduct of smoking, has shown antithyroid activity23. Smoking enhances the formation of inactive catechol estrogens by altering the metabolism of estradiol24, and estrogen has growth-promoting effects in the thyroid25. The relatively lower body weights of current smokers were regarded as the connection resulting in a lower risk of thyroid cancer in smokers26; however, we found a consistent result after adjusting for BMI in this study. Although only a few women (1.9% [368/19,367]) were current smokers (Table S1), smoking was still significantly negatively associated with thyroid cancer in women.
In previous studies, alcohol consumption was negatively associated with thyroid cancer. A meta-analysis reported an RR of 0.74 (95% CI 0.67–0.83) for the highest use of alcohol compared to the lowest use9. Another meta-analysis displayed an RR of 0.80 (95% CI 0.71–0.90) for > one drink/day of alcohol compared to ≤ one drink/day27. The OR of consuming alcohol 1–5 times a week was 0.45 (95% CI 0.24–0.84) compared to nonconsumption28. However, this association was eliminated after adjusting for smoking in some studies8, and the relationship was different according to the kinds of alcoholic beverages29. Some plausible hypotheses can explain this finding. Alcohol intake may reduce TSH levels. Chronic alcohol consumption changes the hypothalamic-pituitary-thyroid (HPT) axis via the action of thyrotropin-releasing hormone (TRH) neurons of the paraventricular nucleus in animal studies30. The toxicity of alcohol might affect thyroid cells directly and reduce thyroid volume31. Moderate alcohol intake improves insulin sensitivity32 but is linked to thyroid cancer10. Consuming alcohol was related to decreased sex hormone-binding globulin33, which might influence thyroid cancer. In this study, consuming alcohol was negatively associated with thyroid cancer, with the exception of the ≥ 55-year-old group, but this difference was not statistically significant. We also analyzed the interaction between alcohol consumption and smoking, as they were closely related (Table S2). The interaction of smoking*consuming alcohol showed statistical significance with an OR of 0.80 (95% CI 0.75–0.84) in the crude model, while it was not significant in the adjusted model. Therefore, we could not find an interaction effect of smoking and alcohol consumption on thyroid cancer.
This study has various merits including a large sample size. First, we used rigorous statistical analysis techniques such as the conditional logistics regression for the matched design. Additionally, 17 kinds of disease categories were adjusted using CCI scores. We also analyzed the interaction effects of obesity, smoking status, and alcohol consumption in a single study. Additionally, we analyzed the interactions in subgroups with respect to age, sex, obesity, smoking, and alcohol consumption using the large number of participants and maintaining statistical power. Using previous health checkup results before the diagnosis of thyroid cancer could have minimized the recall bias, which is very common in other surveyed cross-sectional studies using a questionnaire after the diagnosis of thyroid cancer. The mean time from the heath check-up to the index date was > 1 year in this study. A prior study demonstrated that the incidence of thyroid cancer in Korea was very high due to the increased incidence of diagnosis by ultrasonography, and this was more common in the higher income group3. Because we matched the participants according to income group, we could lessen this effect of detection bias. As obesity, smoking, and consuming alcohol could be different according to social classes and act as confounders, the matching of the subjects according to income group is an important strength of this study. Because we used the Asian-Pacific BMI classification, we calculated the OR of the relatively less obese BMI group (overweight from 23 to 25 kg/m2).
The limitations of this study were as follows. We used patient claim codes in the HIRA data to identify thyroid cancer. This method could lead to the underestimation of the incidence of thyroid cancer because thyroid cancer patients who did not visit clinics might be ignored. However, as stated above, the overdiagnosis of thyroid cancer is a major problem in Korea. We did not have information on the pathology of thyroid cancer, but 95% of thyroid cancer in Korea is papillary thyroid carcinoma34. We included only participants ≥ 40 years due to screening ages for health checkups. Despite the matching of income level, overdiagnosis in our cohort could influence to the current results. Radiation exposure, family history of thyroid cancer, dietary habits and physical activities were not surveyed in this study. Although we used survey data before the diagnosis of thyroid cancer, the chance of recall bias still exists. Because we only used health checkup data before the index date, this might be acting as a source of bias. We could not calculate the total smoking and alcohol consumption due to the categorical nature of the questionnaire. Because smoking and obesity were negatively associated, the positive association between obesity and thyroid cancer may have affected the inverse association between smoking and thyroid cancer, despite the adjustment. The relatively small fraction of current smokers and drinkers, especially women, could have affected the association of smoking or consuming alcohol with thyroid cancer in our cohort. The positive relation between and obesity was dominant in men but not in women. This might have been affected by the different contributions of obesity according to sex (Table S5). This study did not adjust for educational level or reproductive factors of women as covariates.
In conclusion, the odds of being overweight or obese were higher in the thyroid cancer group than in the control group. On the other hand, the odds of smoking and consuming alcohol were lower in the thyroid cancer group.
Materials and methods
Study population and data collection
The Ethics Committee of Hallym University (2017-I102) approved the use of these data. The study was exempted from the need for written informed consent by the Institutional Review Board of Hallym University. All methods were carried out in accordance with relevant guidelines and regulations.
This national cohort study relied on data from the Korean National Health Insurance Service-Health Screening Cohort (NHIS-HEALS). A detailed description of these data was described in our previous studies35.
Participant selection
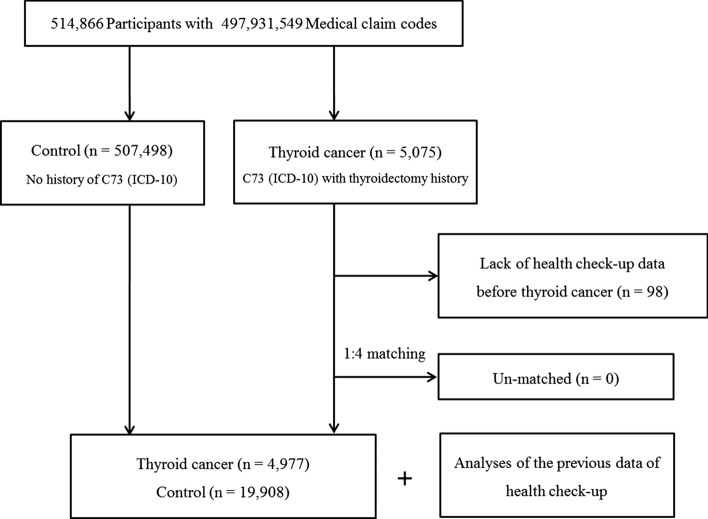
Out of 514,866 patients with 497,931,549 medical claim codes, we included participants who were defined as having thyroid cancer (n = 5075). The thyroid cancer participants were matched 1:4 with participants (the control group) who were never diagnosed with thyroid cancer from 2002 through 2013 among this cohort. The control group was selected from the original population (n = 507,498). Subjects were matched based on age group, sex, income group, and region of residence. Participants in the control group were sorted using a random number order and were selected from top to bottom to prevent selection bias when selecting the matched control participants. The index date was set as the date of diagnosis of thyroid cancer. It was assumed that the matched control participants were also involved in the cohort at the same time as each matched thyroid cancer participant (index date). Therefore, the control participant who died before the index date or who had no previous health check-up before the index date was replaced with another candidate. Thyroid cancer participants who had no previous health checkup before the index date were excluded (n = 98). In this study, we used the latest health checkup data before the index date. None of the thyroid cancer participants were excluded due to a lack of matched participants. The mean time from the heath checkup to the index date was 15.6 months (standard deviation [SD] = 18.3) in the thyroid cancer group and 19.5 months (SD = 20.8) in the control group. Finally, 1:4 matching resulted in the inclusion of 4977 thyroid cancer participants and 19,908 control participants. We analyzed the previous health checkup data in the thyroid cancer group and the control group after matching (Fig. 1).
Figure 1.
A schematic illustration of the participant selection process used in the present study. Of a total of 514,866 participants, 4977 thyroid cancer patients were matched with 19,908 control participants based on age, sex, income, and region of residence.
Variables
Independent variable
Tobacco smoking was surveyed and categorized based on the participant’s current smoking status (nonsmoker, past smoker, and current smoker), duration of smoking (nonsmoker, < 20 year, and ≥ 20 years), and current number of cigarettes smoked per day (0 cigarettes a day, < 20 cigarettes a day, and ≥ 20 cigarettes a day, Tables S3 and S4). Because every smoking category was different between the thyroid cancer group and the control group among smoking status, duration of smoking, or current number of cigarette (Table S3), we selected the smoking status of current smokers as the main variable for this study. Because the number of past smokers was small (5.9% [1462/24,885]) and we could not find a difference in thyroid cancer rates between nonsmokers and past smokers (Table S4), we merged past smokers with nonsmokers. Below, we used ‘smoking’ when referring to current smokers compared to nonsmokers or past smokers (Tables 2, 3, and 4).
Alcohol consumption was surveyed and categorized based on the frequency of alcohol consumption (< 1 time a week and ≥ 1 time a week) and the amount of alcohol consumed at a time (< soju 1 bottle, 1 soju bottle, and > 1 soju bottle, Tables S3 and S4). Because every alcohol-related category was different between the thyroid cancer group and the control group (Table S1), we selected the frequency of alcohol consumption in this study. Because the rate of thyroid cancer was not different in the nondrinkers and < 1-time-a-week group (Table S2), we merged them. Below, we used ‘alcohol consumption’ to refer to consuming alcohol ≥ 1 time a week compared to consuming alcohol < 1 time a week.
To measure obesity, height and weight were measured using the scale and transformed BMI (body mass index, kg/m2). It was categorized as < 18.5 (underweight), ≥ 18.5 to < 23 (normal), ≥ 23 to < 25 (overweight), ≥ 25 to < 30 (obese I), and ≥ 30 (obese II) based on the Asia–Pacific criteria following the Western Pacific Regional Office (WPRO) 200017.
Covariates
The variables of age group, sex, income, and region of residence were categorized following the methods used in our prior study36. Age groups were classified as 40–44, 45–49, 50–54…, and 85 + years. The Charlson comorbidity index (CCI) was used for 17 comorbidities as a continuous variable (0 [no comorbidity] through 29 [multiple comorbidities])37.
Dependent variable
Thyroid cancer was defined using the ICD-10 code (C73). Among the patients, we included only the participants who were treated with any thyroidectomy (Claim code: P4551, P4552, P4553, P4554, or P4561) following the method used in our past study38.
Statistical analyses
Chi-square tests were used to compare the general characteristics between the thyroid cancer group and the control group.
To analyze the odds ratios (ORs) of smoking, alcohol consumption, and obesity in the context of thyroid cancer, conditional logistic regression analysis was used in the matched groups for age, sex, income, and region of residence. In this analysis, crude (simple) and adjusted models (CCI, obesity, smoking status, and frequency of alcohol consumption) were used. In addition, 95% confidence intervals (CIs) were calculated. In these analyses, age, sex, income, and region of residence were stratified. The interaction model between smoking and alcohol consumption is described in Table S3.
For the subgroup analyses, we divided the participants by age and sex (< 55 years and ≥ 55 years, men and women) to confirm these associations according to age and sex. The division of the age groups was determined by the median value of the total number of participants. We used unconditional logistic regression analysis in the subgroup analyses according to smoking status, alcohol consumption, and obesity status. These analyses did not meet the matched structure of the primary design.
Two-tailed analyses were conducted, and P values < 0.05 were considered to indicate significance. The results were statistically analyzed using SPSS version 22.0 (IBM, Armonk, NY, USA) and SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Supplementary information
Author contributions
H.G.C. designed the study, participated in data collection and data interpretation, and revised the manuscript. S.Y.A, S.Y.K., D.J.O., and H.G.C. participated in the interpretation of the data and drafted and revised the manuscript. C.M. and S.S. participated in data analysis, interpretation of data, and revised the manuscript. All authors approved the final version of the manuscript.
Funding
This work was supported in part by a research grant (NRF-2018-R1D1A1A02085328) from the National Research Foundation (NRF) of Korea, Hallym University Research Fund (HURF-2019-31), and the Dongnam Institute of Radiological & Medical Sciences (DIRAMS) grant funded by the Korea government (MSIT) (No. 50604-2019). This manuscript was edited for proper English language, grammar, punctuation, spelling, and overall style by the highly qualified native English-speaking editors at American Journal Experts (1AFB-6AF4-609C-7535-1CBP).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Soo-Youn An and So Young Kim.
Supplementary information
is available for this paper at 10.1038/s41598-020-76357-y.
References
- 1.Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat. Rev. Endocrinol. 2016;12:646–653. doi: 10.1038/nrendo.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vigneri R, Malandrino P, Vigneri P. The changing epidemiology of thyroid cancer: why is incidence increasing? Curr. Opin. Oncol. 2015;27:1–7. doi: 10.1097/CCO.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 3.Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”—screening and overdiagnosis. N. Engl. J. Med. 2014;371:1765–1767. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 4.Jung KW, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res. Treat. 2015;47:127–141. doi: 10.4143/crt.2015.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaccarella S, et al. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N. Engl. J. Med. 2016;375:614–617. doi: 10.1056/NEJMp1604412. [DOI] [PubMed] [Google Scholar]
- 6.Zhu C, et al. A birth cohort analysis of the incidence of papillary thyroid cancer in the United States, 1973–2004. Thyroid. 2009;19:1061–1066. doi: 10.1089/thy.2008.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pappa T, Alevizaki M. Obesity and thyroid cancer: a clinical update. Thyroid. 2014;24:190–199. doi: 10.1089/thy.2013.0232. [DOI] [PubMed] [Google Scholar]
- 8.Mack WJ, et al. A pooled analysis of case-control studies of thyroid cancer: cigarette smoking and consumption of alcohol, coffee, and tea. Cancer Causes Control. 2003;14:773–785. doi: 10.1023/A:1026349702909. [DOI] [PubMed] [Google Scholar]
- 9.Hong SH, Myung SK, Kim HS, Korean Meta-Analysis Study, G Alcohol intake and risk of thyroid cancer: a meta-analysis of observational studies. Cancer Res. Treat. 2017;49:534–547. doi: 10.4143/crt.2016.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim WG, Cheng SY. Mechanisms linking obesity and thyroid cancer development and progression in mouse models. Horm Cancer. 2018;9:108–116. doi: 10.1007/s12672-017-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 12.Leitzmann MF, et al. Prospective study of body mass index, physical activity and thyroid cancer. Int. J. Cancer. 2010;126:2947–2956. doi: 10.1002/ijc.24913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meinhold CL, et al. Nonradiation risk factors for thyroid cancer in the US Radiologic Technologists Study. Am. J. Epidemiol. 2010;171:242–252. doi: 10.1093/aje/kwp354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grani G, et al. Lack of association between obesity and aggressiveness of differentiated thyroid cancer. J. Endocrinol. Invest. 2019;42:85–90. doi: 10.1007/s40618-018-0889-x. [DOI] [PubMed] [Google Scholar]
- 15.Campenni A, Trimarchi F, Baldari S. Comment on: Lack of association between obesity and aggressiveness of differentiated thyroid cancer. J. Endocrinol. Invest. 2019;42:107–108. doi: 10.1007/s40618-018-0970-5. [DOI] [PubMed] [Google Scholar]
- 16.Mijovic T, et al. Body mass index in the evaluation of thyroid cancer risk. Thyroid. 2009;19:467–472. doi: 10.1089/thy.2008.0386. [DOI] [PubMed] [Google Scholar]
- 17.WHO, IASO, IOTR . The Asia-Pacific Perespective: Redefining Obesity and its Treatment. Haymarket: Health Communications Australia Pty Ltd; 2000. [Google Scholar]
- 18.Manole D, Schildknecht B, Gosnell B, Adams E, Derwahl M. Estrogen promotes growth of human thyroid tumor cells by different molecular mechanisms. J. Clin. Endocrinol. Metab. 2001;86:1072–1077. doi: 10.1210/jcem.86.3.7283. [DOI] [PubMed] [Google Scholar]
- 19.Survey, K. N. H. N. E., https://knhanes.cdc.go.kr/ (2018).
- 20.Cho YA, Kim J. Thyroid cancer risk and smoking status: a meta-analysis. Cancer Causes Control. 2014;25:1187–1195. doi: 10.1007/s10552-014-0422-2. [DOI] [PubMed] [Google Scholar]
- 21.Myung SK, Lee CW, Lee J, Kim J, Kim HS. Risk factors for thyroid cancer: a hospital-based case-control study in Korean adults. Cancer Res Treat. 2017;49:70–78. doi: 10.4143/crt.2015.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorde R, Sundsfjord J. Serum TSH levels in smokers and non-smokers. The 5th Tromsø study. Exp. Clin. Endocrinol. Diabetes. 2006;114:343–347. doi: 10.1055/s-2006-924264. [DOI] [PubMed] [Google Scholar]
- 23.Fukayama H, Nasu M, Murakami S, Sugawara M. Examination of antithyroid effects of smoking products in cultured thyroid follicles: only thiocyanate is a potent antithyroid agent. Acta Endocrinol (Copenh) 1992;127:520–525. doi: 10.1530/acta.0.1270520. [DOI] [PubMed] [Google Scholar]
- 24.Baron JA, La Vecchia C, Levi F. The antiestrogenic effect of cigarette smoking in women. Am. J. Obstet. Gynecol. 1990;162:502–514. doi: 10.1016/0002-9378(90)90420-C. [DOI] [PubMed] [Google Scholar]
- 25.Derwahl M, Nicula D. Estrogen and its role in thyroid cancer. Endocr. Relat. Cancer. 2014;21:T273–283. doi: 10.1530/ERC-14-0053. [DOI] [PubMed] [Google Scholar]
- 26.Dal Maso L, et al. A pooled analysis of thyroid cancer studies. V. Anthropometric factors. Cancer Causes Control. 2000;11:137–144. doi: 10.1023/A:1008938520101. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Cheng W, Li J, Zhu J. A meta-analysis of alcohol consumption and thyroid cancer risk. Oncotarget. 2016;7:55912–55923. doi: 10.18632/oncotarget.10352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guignard R, Truong T, Rougier Y, Baron-Dubourdieu D, Guénel P. Alcohol drinking, tobacco smoking, and anthropometric characteristics as risk factors for thyroid cancer: a countrywide case-control study in New Caledonia. Am. J. Epidemiol. 2007;166:1140–1149. doi: 10.1093/aje/kwm204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang H, et al. Alcohol and Cancer. New York: Springer; 2018. pp. 1–14. [Google Scholar]
- 30.Zoeller RT, Fletcher DL, Simonyi A, Rudeen PK. Chronic ethanol treatment reduces the responsiveness of the hypothalamic-pituitary-thyroid axis to central stimulation. Alcoholism. 1996;20:954–960. doi: 10.1111/j.1530-0277.1996.tb05277.x. [DOI] [PubMed] [Google Scholar]
- 31.Knudsen N, et al. Alcohol consumption is associated with reduced prevalence of goitre and solitary thyroid nodules. Clin. Endocrinol. 2001;55:41–46. doi: 10.1046/j.1365-2265.2001.01325.x. [DOI] [PubMed] [Google Scholar]
- 32.Davies MJ, et al. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. JAMA. 2002;287:2559–2562. doi: 10.1001/jama.287.19.2559. [DOI] [PubMed] [Google Scholar]
- 33.Shiels MS, et al. Association of cigarette smoking, alcohol consumption, and physical activity with sex steroid hormone levels in US men. Cancer Causes Control. 2009;20:877–886. doi: 10.1007/s10552-009-9318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park S, et al. Association between screening and the thyroid cancer “epidemic” in South Korea: evidence from a nationwide study. BMJ. 2016;355:i5745. doi: 10.1136/bmj.i5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SY, Min C, Oh DJ, Choi HG. tobacco smoking and alcohol consumption are related to benign parotid tumor: a nested case-control study using a national health screening cohort. Clin. Exp. Otorhinolaryngol. 2019;12:412. doi: 10.21053/ceo.2018.01774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SY, et al. Gastroesophageal reflux disease increases the risk of chronic rhinosinusitis: a nested case-control study using a national sample cohort. Int. Forum Allergy Rhinol. 2019;9:357–362. doi: 10.1002/alr.22259. [DOI] [PubMed] [Google Scholar]
- 37.Quan H, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 38.Sung MW, et al. Increasing thyroid cancer rate and the extent of thyroid surgery in Korea. PLoS ONE. 2014;9:e113464. doi: 10.1371/journal.pone.0113464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.