Abstract
Duchenne muscular dystrophy (DMD) is the most common and relentless form of muscular dystrophy. The pleiotropic effects of dystrophin deficiency include remarkable impacts on neuromuscular junction (NMJ) structure and function. Some of these alterations contribute to the severe muscle wasting and weakness that distinguish DMD, while others attempt to compensate for them. Experimental approaches that correct NMJ biology in pre-clinical models of DMD attenuate disease progression and improve functional outcomes, which suggests that targeting the NMJ may be an effective therapeutic strategy for DMD patients. The objectives of this review are to 1) survey the distinctions in NMJ structure, function, and gene expression in the dystrophic context as compared to the healthy condition, and 2) summarize the efforts, opportunities and challenges to correct NMJ biology in DMD. This information will expand our basic understanding of neuromuscular biology and may be useful for designing novel NMJ-targeted drug or behavioural strategies to mitigate the dystrophic pathology and other disorders of the neuromuscular system.
1. Introduction
Dystrophin is a highly abundant protein localized to the inner face of the plasma membrane of muscle fibres. The primary function of this molecule is to serve as a link between the sarcolemma and cytoskeletal ɣ–actin, which together provide strong structural support for the myofiber during mechanical stress. The absence of dystrophin protein, due to mutations in its gene, DMD, results in the most common and relentless form of muscular dystrophy, Duchenne muscular dystrophy (DMD). It has been known for over 30 years that dystrophic skeletal muscle is characterized by pathophysiological alterations in pre- and postsynaptic neuromuscular junction (NMJ) structure and function, which contribute to the hallmark muscle wasting and weakness in DMD [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]. Experimental approaches that impact skeletal muscle and affect NMJ biology in pre-clinical models of DMD attenuate disease progression and improve functional outcomes [14], [15], [16], [17], [18]. This evidence suggests that targeting the NMJ may be an effective therapeutic strategy for DMD patients. Thus, continued examination of the mechanisms that govern NMJ morphology and function in DMD will expand our comprehension of the basic biology of the disorder, as well as identify opportunities for innovative treatment approaches. In the current paper, we begin by reviewing recent progress in DMD research, with particular emphasis on novel salutary modalities. We then discuss NMJ biology in the healthy and dystrophic contexts and follow with a detailed analysis of the effects of normalizing the NMJ in muscular dystrophy. In closing, we highlight the most significant remaining knowledge gaps, as well as opportunities for future pursuit that will advance our understanding of, and therapeutic options for, the NMJ in DMD. This article expands on earlier, excellent reviews of similar themes [19], [20], [21], [22], [23].
2. Duchenne muscular dystrophy
DMD is the most common congenital neuromuscular disorder affecting approximately 1 in 6000 live male births [24,25]. The economic burden associated with DMD is significant for patients ($23,000–$54,000 USD) and their families ($58,000–$71,000 USD) all over the world [26]. Natural history studies demonstrate that boys with DMD experience progressive proximal muscle weakness and wasting at approximately 2–5 years of age, which is also accompanied by a delay in motor milestone achievements [25,27]. This gradual loss in limb function typically necessitates ambulatory supports by early adolescence. Other clinical hallmarks that manifest in these patients include the accumulation of intramuscular fatty and fibrotic tissue resulting in excessive enlargement (i.e., pseudohypertrophy), particularly of the gastrocnemius muscle, as well as a positive Gowers’ sign that presents as a predictable difficulty rising from a lying supine position. Respiratory and/or cardiac failure claim most DMD patients in their third or fourth decades.
DMD is caused by mutations in the DMD gene and the subsequent absence of its dystrophin protein product. Without dystrophin, its eponymous oligomeric structure termed the dystrophin-associated protein complex (DAPC) cannot be formed [27,28]. Highly localized along the sarcolemma, the DAPC is a critical signalling apparatus between the extracellular matrix and intracellular cytoskeleton that serves to maintain the structural integrity of muscle cells. The lack of dystrophin, and by extension the DAPC, ultimately lead to repetitive cycles of degeneration/regeneration, chronic inflammation, and muscle atrophy [25,29,30]. While dystrophin is certainly important, other members of the DAPC also play critical roles in health and disease. Although often overlooked when discussing the devastating effects of DAPC deficiency, NMJ structure and function are remarkably impacted in muscular dystrophies, DMD in particular (Fig. 1) [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]. Veritably, the biology of dystrophic NMJs is significantly compromised in several pre-clinical models, which strongly suggests that this phenomenon contributes to the muscle wasting and weakness apparent in DMD patients. This is not surprising considering the critically important role of the NMJ in regulating skeletal muscle phenotype and contractile activity [31].
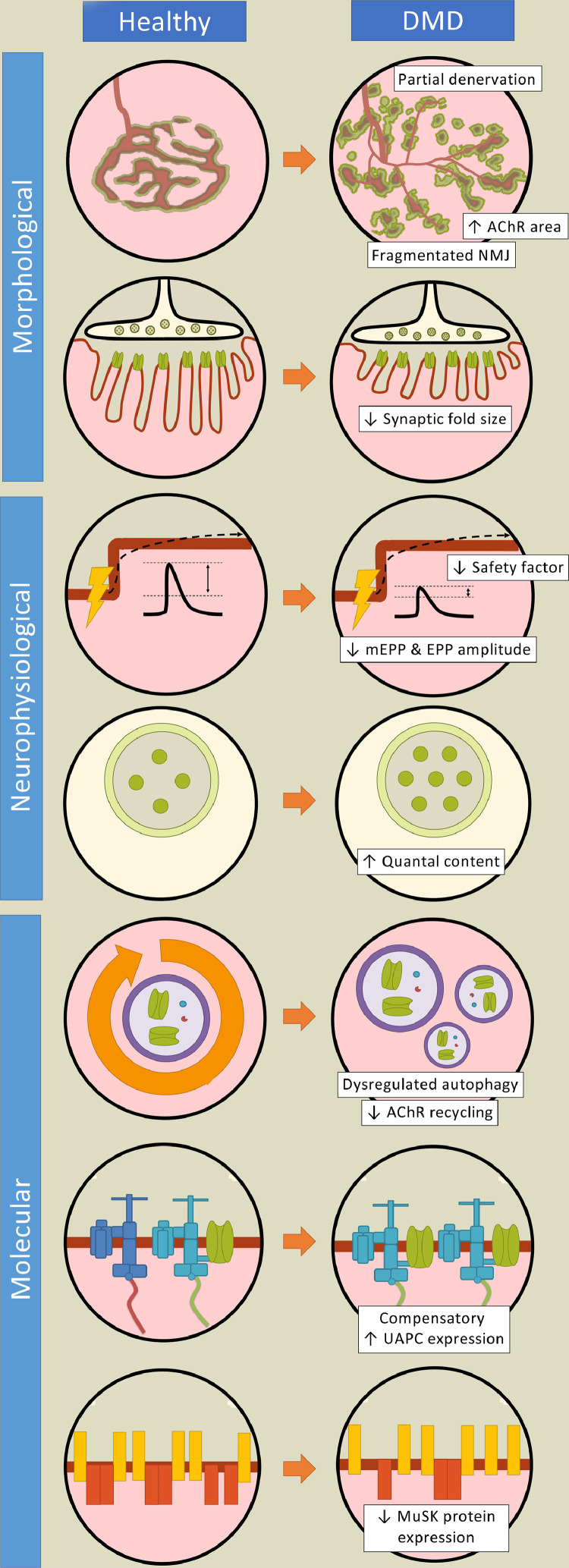
Fig. 1.
Neuromuscular junction biology in Duchenne muscular dystrophy. Evidence from Duchenne muscular dystrophy (DMD) patients and pre-clinical studies demonstrate numerous differences in neuromuscular junction (NMJ) structure, function, and gene expression between the healthy and dystrophic conditions. Morphological abnormalities of the synapse exhibited in DMD include denervation, fragmentation and denuded postsynaptic folds. Acetylcholine receptor (AChR) expression is also impacted. Neurophysiological properties such as impulse transmission also differ in dystrophic muscle. For instance, reduced endplate potential (EPP) and miniature EPP (mEPP) amplitudes, as well as a depressed safety factor, compromise the reliability of neurotransmission in dystrophic muscle. Furthermore, several disparities in the molecular biology of the NMJ between the healthy condition and DMD have been documented and include dysregulated autophagy, altered cyclic adenosine monophosphate (cAMP) signalling, as well as attenuated muscle specific kinase (MuSK) expression. Notably, a significant upregulation of synaptic and extrasynaptic utrophin content, as well as the larger utrophin-associated protein complex (UAPC), are hallmark characteristics of dystrophic muscle and are believed to compensate functionally for the lack of dystrophin.
There is currently no effective treatment for DMD [32]. Symptomatic patients are prescribed glucocorticoids as a standard of care, which attenuates the decline in muscle strength and function while also delaying the onset of catastrophic respiratory and cardiac dysfunction [25,33]. However, long-term corticosteroid usage results in detrimental side effects such as abnormal behaviour and weight gain. Vamorolone (ReveraGen BioPharma) and Edaslonexent (Catabasis Pharmaceuticals), two anti-inflammatory medications currently in the latter phases of clinical trials, provide similar therapeutic effects of glucocorticoids without the adverse off-target effects [33]. Other emerging therapies include exon-skipping compounds, such as Viltepso (NS Pharma), Exondys 51 and Vyondys 53 (Sarepta Therapeutics), as well as the nonsense mutation suppressor, Translarna (PTC Therapeutics), which have been approved by the U.S. Food and Drug Administration and European Medicines Agency, respectively. However, these therapeutic strategies are not without their limitations. For example, Vyondys 53 might be effective only for those who have a DMD mutation that is germane to the skipping of exon 53, which represents approximately 8% of all DMD patients [34]. Moreover, premature termination codon readthrough capability could successfully address disease-causing mutations in a maximum of 15% of boys with DMD [35]. A common shortcoming of these genetic therapies is the restoration of a truncated and less functional dystrophin, which will ultimately translate, in a best case scenario, to a milder, Becker-like dystrophic phenotype [36]. Thus, there is a critical, unmet clinical need to identify effective curative approaches that apply to all DMD patients.
3. Development, maintenance and plasticity of the NMJ
The NMJ is an electrochemical signalling apparatus that lies at the interface between an α-motoneuron (αMN) and the skeletal muscle cells that it innervates. More specifically, it is comprised of an αMN nerve terminal, the endplate region of a muscle fibre, and perisynaptic Schwann cells that envelop the synapse [20,22,23,37]. Depolarization of the αMN terminal prompts the presynaptic exocytosis of acetylcholine (ACh)-containing vesicles. ACh from a single vesicle (i.e., quantal content) diffuses into the synaptic cleft and is either destroyed by acetylcholinesterase or binds to ACh receptors (AChR) to evoke a miniature endplate potential (mEPP) at the postsynaptic endplate. The summation of mEPPs elicited from a simultaneous release of multiple ACh transmitter quanta upon a single nerve impulse will cause a relatively larger, local postsynaptic depolarization known as an endplate potential (EPP). In the healthy condition, the EPP is almost always sufficient to surpass the threshold required for depolarization of the sarcolemma, which then causes a muscle action potential to ensue and leads to the contraction of all myofibers innervated by the αMN. The amplitude by which the EPP surpasses the depolarization threshold is known as the “safety factor”, and disruption to the morphology and/or function of the NMJ will lower this safety factor below depolarization threshold leading to transmission failure, muscle weakness and dysfunction [38].
The development and maturation of the NMJ is well characterized in rodents [22,23,39]. As early as embryonic day (E) 9.5, myofibers develop with broadly dispersed AChRs that subsequently accumulate to the central region of the fibre, a process known as pre-patterning. Shortly after at E11-12, motor axons are directed towards this central, pre-patterned domain [21]. The axons first branch, then extend to appose these AChRs to form nascent NMJs [22]. These newly formed, oval-like plaques are innervated by multiple nerve terminals that compete with each other in an activity-based manner to seize territory within an AChR cluster. Polyinnervation of AChR clusters is eliminated by approximately 14 days post-birth (P), when a dominant presynaptic terminal has been established [23]. Subsequently, these more mature synapses adopt a perforated, pretzel-like morphology. The maturation and stability of the NMJ is governed by multiple proteins including the canonical agrin/low-density lipoprotein receptor-related protein 4 (LRP4)/muscle-specific kinase (MuSK) signalling axis, downstream targets docking protein 7 (Dok7), and 43 kDa receptor-associated protein of the synapse (rapsyn), as well as additional synaptic gene regulators such as GA-binding protein (GABP; also known as nuclear respiratory factor 2, or NRF-2), and peroxisome proliferator-activated receptor ɣ coactivator-1α (PGC-1α). Other proteins, such as utrophin and protein kinase A (PKA) also modulate the morphology and function of the NMJ. While many additional signalling pathways have been identified that play a role in synapse specialization, including neuregulin/ErbB, Wnt/β-catenin, and Hippo/Yes-associated protein signalling cascades [40], the remainder of this section will emphasize molecules that have been examined in the DMD context and how they develop, maintain, and remodel synaptic structure and function. Readers interested in much more comprehensive surveys of NMJ maturation are enthusiastically referred to these recent, expert reviews [20,22,23,37,40,41].
3.1. Agrin/LRP4/MuSK signalling
Agrin was identified as the first neurotrophic factor to regulate NMJ biology [21,22]. The proteoglycan governs the expression and localization of AChRs and is therefore critical for the postnatal development and plasticity of the NMJ. Indeed, although rodents lacking agrin form AChR clusters, they are unstable and exhibit a dispersed organization in the broad middle region of myofibers, resembling an arrangement that is observed during development [22,39,41]. Additionally, recombinant agrin treatment of denervated muscles eliminates ectopic AChR cluster formation, further demonstrating the importance of this neural factor [39,40]. Agrin exerts its effects by initiating the LRP4/MuSK signalling cascade, which is essential for its downstream control of AChR expression. For instance, the agrin/LRP4/MuSK interaction promotes the expression of several synaptic proteins that encourage AChR transcription, stabilization, and turnover. These effector molecules include rapsyn, Dok7, Abl tyrosine kinase, geranylgeranyltransferase, Rho GTPases, and P21-activated kinase 1 [22,39]. While many of these factors exhibit functional redundancy and are therefore dispensable for the postnatal development of the NMJ, Dok7 and rapsyn are essential components of the postsynaptic apparatus [22]. Additional evidence for the importance of the agrin/LRP4/MuSK signalling axis is demonstrated by LRP4 and MuSK knockdown and knockout (KO) studies [20], [21], [22]. For example, LRP4 KO mice exhibit perinatal lethality due to severe impairments in NMJ postsynaptic endplate formation [42]. Thus, the agrin/LRP4/MuSK cascade serves as the regulatory centrepiece for the maturation and remodelling of the NMJ.
3.2. Transcriptional regulators of synaptic genes
The links between agrin/LRP4/MuSK and synaptic gene expression are still being identified. Established players include transcription factors that are part of the E26 transformation-specific (Ets) family of proteins, including GABP⍺/β and Ets variant 5 (Erm) [20]. These factors are also regulated at the synapse by mitogen activated protein kinase pathways, including c-Jun N-terminal kinases and extracellular signal-regulated kinase (ERK) signalling [40]. An abundance of subsynaptic genes contain a common, conserved sequence in their promoters known as the N-box motif (CCGGAA). Here, in subsynaptic myonuclei (also known as fundamental myonuclei), Ets transcription factors bind and facilitate the transcription of synaptic genes, including AChR subunits (Chrnδ and Chrnε), acetylcholinesterase (AChEst), Musk, Rapsn, and utrophin (Utrn). Although Ets factors are dispensable for synapse formation [43], [44], [45], these proteins play a remarkable role in NMJ remodelling. For example, rodents lacking GABP⍺/β exhibit impaired postsynaptic development as a result of attenuated agrin signalling and reduced subsynaptic gene transcription [43,44]. Furthermore, Hippenmeyer and colleagues demonstrate severe impairments in NMJ plasticity and function in animals lacking Erm [45]. This evidence underscores the importance of Ets transcription factors, specifically GABP⍺/β and Erm, in regulating the NMJ gene expression program and synaptic function.
The transcriptional coactivator PGC-1α is another important modulator of the NMJ [28,46,47]. PGC-1α is regulated through several post-translational modifications that are mediated by upstream molecules such as adenosine monophosphate-activated protein kinase (AMPK), which is critical protein that governs neuromuscular system biology [28]. Mechanistic links have been elegantly established between PGC-1α and synaptic gene regulation via GABP⍺/β/N-box signalling [15,48]. Further evidence for the role of PGC-1α in the NMJ gene program can be observed in vivo [17,18,49,50]. For instance, in addition to demonstrating slower, and more oxidative muscle characteristics such as mitochondrial biogenesis and type 1 and 2a myosin heavy chain expression, mice overexpressing PGC-1α specifically in skeletal muscle exhibit a strong postsynaptic gene expression signature, as well as a type 1 αMN phenotype [50,51]. Interestingly, the latter alteration indicates that the transcriptional coactivator mediates a retrograde signalling network from myofibers to their innervating αMN. Elaborating on this phenomenon, Mills and colleagues recently showed in cell culture experiments that a PGC-1α isoform directs axon recruitment and NMJ formation through the release of the myokine, neurturin [52]. Thus, interventions that stimulate PGC-1α in skeletal muscle, like exercise for example, may evoke adaptive plasticity throughout the peripheral neuromuscular system. This warrants further research into the therapeutic potential of this protein.
PGC-1α-mediated induction of utrophin via GABP⍺/β/N-box signalling has been demonstrated in numerous studies [15,48,53]. Utrophin is the key component of the utrophin-associated protein complex (UAPC), an oligomeric structure that is homologous to the DAPC. However, unlike the DAPC, which resides in the troughs of the postsynaptic folds at the NMJ and along the length of the sarcolemma, the UAPC is typically concentrated at the crests of the postsynaptic invaginations, but can also be found to a lesser extent extrasynaptically [54]. In fact, slow-twitch, oxidative muscles possess significantly greater amounts of extrasynaptic utrophin compared to faster, more glycolytic muscles, which is thought to be due, in part, to enhanced transcriptional and post-transcriptional processes in slow, oxidative myofibers [53]. Nonetheless, work from Deconinck et al. and Grady et al. demonstrated that the absence of utrophin only modestly affects NMJ biology [55,56]. The true impact of utrophin is revealed in the context of DMD, which will be discussed in greater detail later in our survey.
Another notable target of PGC-1α and GABP⍺/β/N-box transcriptional activation are the Chrn genes, including the δ and ε subunits [15,20,40]. The density of synaptic AChRs is regulated by processes that synthesize, stabilize, and dismantle the receptors. Innervated AChRs typically possess a half-life of ~14 days, after which they are endocytosed from the motor endplate and either stored in intracellular compartments, recycled back to the synapse, or degraded [19]. The fate of AChRs is heavily influenced by their phosphorylation status and stability, which are governed by the cAMP/protein kinase A (PKA) signalling pathway [19,37]. AChR elimination involves autophagic machinery such as autophagy related 7 (ATG7), muscle ring finger 1 (MuRF1), sequestosome 1, E3 ubiquitin-protein ligase TRIM63, and Rab5-GTPase [19,57]. Mechanisms that regulate AChR biology pose as a potential therapy for DMD and will be further discussed below.
4. Synaptic biology in DMD and therapies targeting the NMJ
Early studies in DMD patients and mdx mice revealed that the absence of dystrophin resulted in fragmented NMJs and denuded postsynaptic folds [4,6,7,12,13,58], as well as an accelerated degradation of AChRs [59] (Table 1). These alterations, which are also observed in ageing NMJs, may not necessarily translate into functional impairments in neuromuscular transmission [60], whereas a simplified endplate morphology can contribute to the loss of crucial postsynaptic membrane proteins [61]. These initial observations in postsynaptic alterations have since been reported numerous times in mdx mice [[9], [10], [11],[62], [63], [64]], golden retriever muscular dystrophy dogs [65], and other animal models of DMD [66,67]. Whether these changes occur independent from dystrophy-induced muscle degeneration/regeneration is debated [68]. The absence of dystrophin also alters the presynaptic apparatus of the NMJ likely due, in part, to fragmentation of the motor endplate [11]. Specifically, an increase in presynaptic nerve terminal branching, as well as partial denervation have been observed in mdx animals [11], while axonal sprouting occurs in DMD patients and mice [13,69].
Table 1.
NMJ morphology in DMD NMJ structure in pre-clinical models of DMD versus healthy littermates.
| Reference | DMD model | Age | Muscles studied | Presynapse | Postsynapse |
|---|---|---|---|---|---|
| Torres and Duchen 1987 | mdx | 4 wk 7 wk 4 mo |
Soleus | Not reported | Reduced synaptic fold size (all age groups) Fragmented (4 mo) |
| Nagel et al., 1990 | mdx | 2.5 wk 8.5 wk 37 wk |
Diaphragm | Not reported | Reduced synaptic fold size (all age groups) Increased AChR area (2.5 wk, 37 wk) Similar AChR area (8.5 wk) |
| Lyons and Slater 1991 | mdx | 8 wk | EPT | Not reported | Reduced synaptic fold size Fragmented |
| Grady et al., 1997 | mdx | 8 wk | Sternomastoid | Not reported | Reduced synaptic fold size |
| mdx:utrn−/− | 8 wk | Sternomastoid | Not reported | Reduced synaptic folds size | |
| Santa Neto et al., 2003 | mdx | 5–6 mo | Sternomastoid | Axon sprouting | Fragmented |
| Minatel et al., 2003 | mdx | P1 1 wk 2 wk 3 wk |
Sternomastoid | Greater monoinnervation (P1, 1 wk) Similar innervation (2 wk, 3 wk) |
Similar morphology (P1, 1 wk, 2 wk) Fragmented (3 wk) |
| Personius and Sawyer 2006 | mdx | 6–8 mo | Diaphragm | Not reported | Fragmented |
| Marques et al., 2008 | mdx | 1 mo 6 mo |
Sternomastoid | Not reported | Fragmented (both age groups) |
| Ferretti et al., 2011 | mdx | 2 mo | Intrinsic laryngeal, sternomastoid | Not reported | Fragmented |
| Pratt et al., 2013 | mdx | 2-3 mo | Quadriceps | Not reported | Fragmented (worsened with damage) |
| Pratt et al., 2014 | mdx | 3 mo | Quadriceps | Not reported | Fragmented (worsened with damage) |
| Pratt et al., 2015 | mdx | 3 mo | Quadriceps | Increased nerve branching Partial innervation |
Fragmented (worsened with damage) |
| Van der pijl et al., 2016 | mdx | 2–6 mo | EPT, diaphragm | Not reported | Fragmented |
| mdx:utrn−/− | 1–2 mo | EPT, diaphragm | Not reported | Fragmented | |
| Van der pijl et al., 2018 | mdx | 2–5 mo | EPT, diaphragm | Not reported | Fragmented |
| mdx-XistΔhs | 2–5 mo | EPT, diaphragm | Not reported | Fragmented | |
| Haddix et al., 2018 | mdx | P38 P66 P450 |
Sternomastoid | Similar nerve branching | Fragmented (all age groups) Fragmentation (worsened with age) |
| GRMD | 1–6 yr | Cranial tibial | Similar nerve branching | Fragmented Increased AChR area |
|
| NMJ structure in DMD patients compared to healthy participants | |||||
| Reference | Cohort size | Age | Muscles studied | Presynapse | Postsynapse |
| Jerusalem 1974 | 3 | 3–6 yr | Peroneus brevis | Similar terminal size Similar vesicle size |
Reduced synaptic fold size Similar AChR area |
| Harriman 1976 | 13 | 4–8 yr | Vastus internus, gastrocnemius, deltoid, palmaris longus, peroneus brevis | Increased axonal sprouting Partial innervation |
Reduced synaptic fold size |
| Sakakibara et al., 1977 | 3 | 5–11 yr | Intercostal | Not reported | Reduced synaptic fold size Similar AChR area |
DMD, Duchenne muscular dystrophy; AChR, acetylcholine receptor; EPT, epitrochleoanconeus; GRMD, golden retriever muscular dystrophy dog; NMJ, neuromuscular junction; wk weeks; mo, months; yr, years; P, postnatal day.
Initial electrophysiology experiments revealed modest declines in mEPP and EPP amplitudes and a compensatory increase in quantal content in the diaphragm of mdx mice, which is the muscle that most closely phenocopies the human dystrophic pathology (Table 2) [6,7]. More recently, Van der Pijl et al. demonstrated augmented quantal content, and reductions in mEPP amplitude and the safety factor for neurotransmission in mdx mice, as well as in the more severe dystrophin-utrophin double knockout animals [66]. Additionally, high-strain, low-repetition, eccentric muscle damage in mdx mice results in a loss of force production, as well as exacerbates pre-existing impairments in neuromuscular transmission and fragments the postsynaptic apparatus [9], [10], [11]. This drop in force may also be attributed to depressed muscle excitability secondary to the loss of sarcolemmal integrity [70], [71], [72]. Nonetheless, it is very likely that both neuronal and muscle impairments contribute to reduced muscle activity, and elevated wasting and weakness in the dystrophic condition. Consistent with rodent work, electrophysiological metrics such as impulse transmission, resting membrane potential, and the compound muscle action potential, are also affected in DMD patients (Table 2) [4,[73], [74], [75]]. Collectively, these data indicate that dystrophin is essential for the proper maturation of the synapse and is required for optimal neurotransmission at the NMJ.
Table 2.
NMJ electrophysiology in DMD Electrophysiology in pre-clinical models of DMD versus healthy littermates.
| Reference | DMD model | Age | Muscles studied | Electrophysiological characteristics |
|---|---|---|---|---|
| Nagel et al., 1990 | mdx | 2.5 wk 8.5 wk 37 wk |
Diaphragm | Similar quantal content (2.5 wk) Increased quantal content (8.5 wk, 37 wk) Similar mEPP amplitude (2.5 wk, 8.5 wk) Reduced mEPP amplitude (37 wk) |
| Lyons and Slater 1991 | mdx | 8 wk | EPT | Similar quantal content Similar mEPP amplitude Similar mEPC amplitude Similar resting postsynaptic membrane potential |
| Grady et al., 1997 | mdx | 8 wk | Sternomastoid | Similar mEPP amplitude Similar neuromuscular transmission fatigue |
| mdx:utrn−/− | 8 wk | Sternomastoid | Similar mEPP amplitude Similar neuromuscular transmission fatigue Reduced twitch contraction force Increased twitch relaxation time |
|
| Deconinck et al., 1997 | utrn−/− | 8 wk | EDL and diaphragm | Similar EPP amplitude Similar EPC amplitude and delay time Reduced mEPP amplitude Similar mEPP frequency Similar quantal content |
| Carlson and Roshek 2001 | mdx | 5–7 wk 6–24 mo |
Diaphragm | Reduced mEPP amplitude (both age groups) Similar mEPP amplitude variance (5-7 wk) Increased mEPP amplitude variance (6-24 mo) |
| Personius and Sawyer 2006 | mdx | 6–8 mo | Diaphragm | Similar neuromuscular transmission fatigue Reduced peak twitch force Increased twitch relaxation time Increased twitch contraction force variability |
| Pratt et al., 2013 | mdx | 2–3 mo | Quadriceps | Increased neuromuscular transmission fatigue Reduced CMAP amplitude Reduced isometric force production |
| Pratt et al., 2014 | mdx | 3 mo | Quadriceps | Increased neuromuscular transmission fatigue Reduced isometric force production |
| Van der pijl et al., 2016 | mdx | 2–6 mo | Diaphragm (electrophysiology, force kinetics) EPT, and GSP complex (EMG) |
Increased quantal content Reduced mEPP amplitude Reduced safety factor Increased neuromuscular transmission fatigue Reduced CMAP amplitude Reduced tetanic and twitch contraction force |
| mdx:utrn−/− | 1–2 mo | Diaphragm (electrophysiology, force kinetics) EPT, and GSP complex (EMG) |
Increased quantal content Reduced mEPP amplitude Reduced safety factor Increased neuromuscular transmission fatigue Reduced CMAP amplitude Reduced tetanic and twitch contraction force |
|
| Van der pijl et al., 2018 | mdx | 2–5 mo | Diaphragm (electrophysiology, force kinetics, IHC) EPT, and GSP complex (EMG) |
Increased quantal content Increased EPP rise time, half width, and decay time Reduced EPP and mEPP amplitude Similar mEPP frequency Increased neuromuscular transmission fatigue Reduced CMAP amplitude |
| mdx-XistΔhs | 2–5 mo | Diaphragm (electrophysiology, force kinetics, IHC) EPT, and GSP complex (EMG) |
Increased quantal content Increased EPP rise time, half width, and decay time Reduced EPP and mEPP amplitude Similar mEPP frequency Increased neuromuscular transmission fatigue Reduced CMAP amplitude |
|
| Electrophysiology in DMD patients compared to healthy participants | ||||
| Reference | Cohort Size | Age | Muscles studied | Electrophysiological characteristics |
| Panayiotopoulos 1974 | 9 | 3–12 yr | Extensor digitorum brevis | Reduced motor unit action potential Similar peak terminal latency |
| Sakakibara 1977 | 3 | 5–11 yr | Intercostal | Similar quantal content Similar mEPP amplitude and frequency Reduced resting postsynaptic membrane potential |
| Hilton-Brown and Stalberg 1983 | 8 | 8–19 yr | Extensor digitorum communis | Increased jitter |
| Sharma et al., 1995 | 11 | 5–10 yr | Tibialis anterior | Similar neuromuscular transmission fatigue Reduced CMAP amplitude Reduced tetanic and twitch contraction force production Increased tetanic and twitch relaxation time |
CMAP, compound muscle action potential; DMD, Duchenne muscular dystrophy; EMG, electromyography; EPC, endplate potential current; EPP, endplate potential; EPT, epitrochleoanconeus; GSP, gastrocnemius-soleus-plantaris; mEPC, miniature EPC; mEPP, miniature EPP; hr, hours; mo, months; wk, weeks; yr, years.
4.1. Agrin/LRP4/MuSK signalling in DMD
The fragmented nature of the synapse in dystrophic muscle indicates that the mechanisms controlling NMJ morphology are dysregulated, specifically those that govern the post-synaptic apparatus. The absence of dystrophin dissociates the DAPC and its key agrin signalling machinery important for NMJ maturation [20,76]. For example, the loss of the DAPC component dystroglycan prevents agrin binding and disorganizes AChR clustering [76,77]. Furthermore, reduced MuSK mRNA and protein expression can be observed in the quadriceps muscles of animals lacking dystrophin [9,10]. Partial inactivation of the kinase, through transgenic ablation or MuSK autoantibodies, accelerates the loss of AChRs and impairs neurotransmission [78], [79], [80], which are characteristics of dystrophic synapses. This suggests that MuSK content and/or activity is attenuated in DMD, however firsthand data in patients are currently lacking.
Despite its lower expression and function levels in dystrophic muscle, the agrin/LRP4/MuSK signalling cascade may serve as valid candidates for muscular dystrophy therapies. Agrin-derived constructs, known as mini-agrins, ameliorate neuromuscular transmission and AChR clustering in murine models of congenital myasthenic syndrome (CMS) and limb gridle muscular dystrophy [41], as well as increase utrophin expression in cultured myotubes [81]. The use of agrin-based therapies has not yet been evaluated in the context of DMD. Augmenting MuSK expression in mdx animals via an adeno-associated virus (AAV) vector reduced neuromuscular failure from eccentric muscle damage and elevated DAPC/UAPC components [16]. Furthermore, AAV induction of downstream effector proteins rapsyn and Dok7 conferred unique neuromuscular alterations in several disparate models of NMDs, including DMD [16], CMS [82], SMA [83], and amyotrophic lateral sclerosis [84]. Thus, these pre-clinical data indicate that targeting the agrin/LRP4/MuSK cascade may eventually yield promising therapeutic effects in DMD patients.
4.2. Regulation of the NMJ gene expression program in DMD
Surprisingly, GABP⍺/β biology has not yet been directly examined in dystrophic muscle. However, we can infer the stimulation of this signalling pathway in DMD since a number of N-box-containing genes are elevated, such as Utrn and Chrn, which will be discussed in greater detail below. An alternative regulator of the N-box, ERK, may also be partly responsible for this selective increase of synaptic genes, as ERK signalling is upregulated in mdx mouse muscle [85]. The potential regulatory role of PGC-1α in GABP⍺/β/N-box signalling in dystrophic muscle [15,48], as well as the function of the transcriptional coactivator as a master regulator of neuromuscular phenotype [50,51], has led to an interest in PGC-1α as a possible therapeutic target in DMD. Indeed, several studies have shown that augmenting PGC-1α expression via genetic, pharmacologic, and physiological means, can reduce the dystrophic phenotype in pre-clinical models of DMD (see [28,53] for comprehensive review). The mechanisms by which PGC-1α exerts its beneficial effects in DMD include evoking the NMJ gene expression program, as demonstrated by increased Agrn, Musk, and Chrn expression [15,17,18]. Moreover, PGC-1α also drives the expression of slower, more oxidative characteristics, which endows muscle with a greater degree of protection against the dystrophic phenotype. A notable indicator of the slow, oxidative phenotype is high synaptic and extrasynaptic utrophin expression [53,86], however it is unclear whether utrophin is required for improved molecular and physiological outcomes in mdx mice elicited by, or associated with, PGC-1α induction [18,87].
Regardless of whether utrophin is necessary or not for the effects of PGC-1α, the dystrophin homologue is nevertheless a critically important NMJ molecule when considering potential therapeutic approaches for DMD. An endogenously expressed protein, utrophin content is consistently upregulated in the skeletal muscles of pre-clinical DMD animal models, as well as in DMD patients [88]. This is very likely a compensatory adaptation in an effort to account for the absence of dystrophin. In fact, some data indicate that a positive relationship exists in DMD patients between utrophin protein expression in skeletal muscle and the age of symptom onset [89], while other results indicate no correlation [90]. In mdx mice, further induction of utrophin significantly mitigates the dystrophic phenotype, as well as rescues NMJ morphology and improves synaptic function [27,29,53]. Conversely, the absence of utrophin significantly exacerbates the dystrophic pathology in mdx animals and results in a relatively more accurate phenocopy of the human DMD condition [28]. Therefore, utrophin is clearly an important molecule at the NMJ with substantial therapeutic potential in DMD. Successful translation of utrophin-mediated strategies in patients remains a high priority.
In dystrophic muscle, impaired cAMP/PKA signalling destabilizes and expedites the elimination of AChRs [37,59]. However, this loss is coincidentally offset by the upregulation of Chrn gene expression [11,91] and AChR incorporation at the synapse [59], which therefore maintains net AChR content at the motor endplate at a similar level as compared to the healthy, non-dystrophic condition. Interestingly, attenuating the accelerated loss of AChRs through cAMP signalling also decreases the rate of total protein degradation in skeletal muscle [59]. Several pre-clinical studies with mdx animals have demonstrated the therapeutic applicability of β2 adrenergic receptor agonists, which are potent stimulators of cAMP production and downstream signalling [92]. Specifically, chronic, low doses of β2 agonists, including formoterol and clenbuterol, reduce muscle degeneration and increase force production in mdx animals [92]. Clinical studies investigating the effects of cAMP agonists are limited in number, but have also demonstrated improvements in muscular health in Duchenne and Becker patients [93,94]. These β2 agonist-driven benefits are likely caused, at least in part, through cAMP/PKA signalling at the NMJ, and suggest that regulating AChR biology may provide some therapeutic utility for DMD in the future.
4.3. Activity-induced plasticity of the dystrophic NMJ
Synaptic activity is essential for maintaining the structure and function of the NMJ [23]. Increases or decreases in neural stimulation drives remodelling of the neuromuscular synapse [19,95]. For example, advanced ageing is commonly associated with decreased peripheral nerve activity, NMJ fragmentation and neurotransmission impairments, which are all mitigated by chronic exercise [19,46]. The favourable alterations at the NMJ represent only a tiny fraction of the health benefits provided by habitual physical activity. The underlying molecular mechanisms responsible for exercise-induced NMJ adaptations remain undefined, but likely include MuSK upregulation, AMPK and PGC-1α signalling, and AChR stabilization [46]. To our knowledge, the data on exercise and the NMJ in DMD are limited. For instance, daily, volitional physical activity increased utrophin protein expression in mdx mice [96], and chronic, endurance exercise augmented Utrn in DMD patients [97]. In the absence of additional primary data, we can reasonably speculate as to what impacts exercise would have on neuromuscular synaptic morphology and function in DMD. For example, given that autophagy is essential for optimal NMJ function in the healthy condition, including AChR turnover [98], and exercise robustly stimulates autophagic processes in healthy and dystrophic skeletal muscle [99], then exercise-induced autophagy may act to rescue dystrophic NMJ morphology and function. Moreover, since low-intensity exercise preserves muscle health and physical function in DMD patients [100], it is likely that, as the interface between muscles and their motor nerves, the NMJ is also positively affected. Examining physical activity-evoked alterations in the structure and function of the NMJ in the DMD context, as well as the underlying molecular mechanisms driving phenotypic plasticity, will increase our understanding of the biology of the neuromuscular system and may lead to the identification and development of novel therapeutic strategies for this disorder.
4.4. Restoration of dystrophin
Perhaps the ideal resolution for the neuromuscular defects in DMD, including at the NMJ, is to restore dystrophin. Indeed, in mdx mice only 5–15% of normal dystrophin levels is sufficient to augment muscle function and prolong survival [101], whereas a higher requirement, estimated to be 20–50%, is needed to improve synaptic morphology and function [67]. However, translating this solution to the clinic is challenging. This is due, in part, to the exceptionally large size of the DMD gene, which limits the viability of some dystrophin-based gene therapies. On the other hand, delivery to the muscles of truncated variants of dystrophin, for example mini- or micro-dystrophin, leads to the reassembly of the DAPC and significantly attenuates the dystrophic pathology [29]. For instance, treatment of mdx animals with mini-dystrophin rescued postsynaptic fold complexity [2]. As small molecule, viral, and other dystrophin-based gene and cell therapies improve in their efficacy, safety and practicality, we anticipate correction of the NMJ and amelioration of the broader dystrophic pathology.
5. Outstanding questions
The accumulation of synaptic alterations and molecular dysregulation are common in pre-clinical models of DMD. Indeed, the absence of dystrophin significantly impacts NMJ structure, function, and gene expression. This is exemplified, in part, by increased NMJ fragmentation, reduced postsynaptic folding, and elevated neuromuscular transmission variability and fatigue that contribute to the progressive muscle atrophy and weakness that characterize pre-clinical DMD, and to a lesser extent due to the paucity of human studies in this area, DMD patients (Fig. 1, Tables 1 and 2). Molecular mechanisms underlying NMJ adaptations have been identified and include alterations in the agrin/LRP4/MuSK, GABP⍺/β/N-box, PGC-1⍺, and cAMP/PKA signalling pathways. Importantly, additional work is necessary to further confirm the importance of these cascades in DMD patients. Several pre-clinical, proof-of-principle studies employing genetic or pharmacological approaches have recently demonstrated that targeting these pathways attenuate NMJ morphological and functional abnormities, and by extension mitigate disease progression and severity in dystrophic muscle. A summary of these NMJ-modifying gene expression and signalling cascades are presented in Fig. 2. A current challenge is to translate these pre-clinical data into effective treatments for DMD patients. Continued evaluation of neuregulin/ErbB, Wnt/β-catenin, and Hippo/Yes-associated protein pathways may reveal novel mechanisms for therapeutic pursuit in muscular dystrophy. Additionally, low-intensity, rationally-prescribed (e.g., limiting stressful eccentric contractions) exercise-induced synaptic activity is a safe, accessible, and low-cost behavioural strategy well known to enhance NMJ biology in health conditions that mimic in some ways the dystrophic pathology, such as advanced ageing. However, further research is required to resolve the molecular mechanisms of exercise in the DMD context, particularly with respect to the NMJ. As more practical dystrophin-based gene, cell, and small molecule therapies with better efficacy continue to emerge, we anticipate their correction of NMJ biology as part of a broader improvement in the dystrophic pathology.
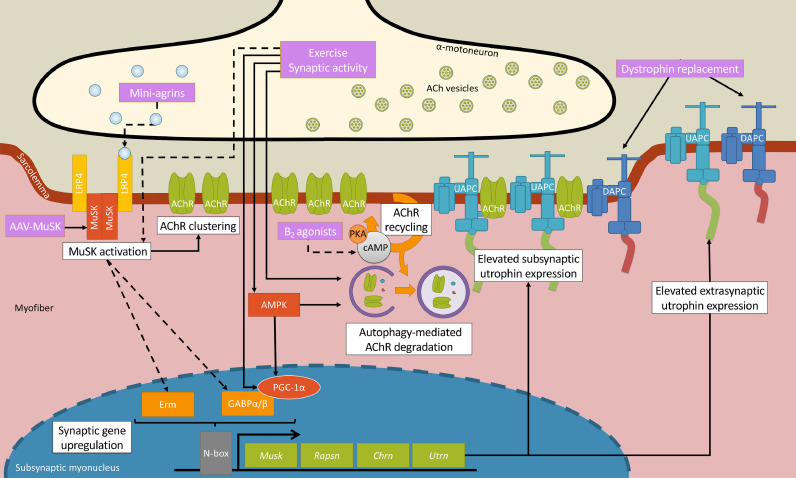
Fig. 2.
Therapeutic strategies targeting the NMJ in dystrophic muscle. Several recent pre-clinical studies have provided mechanistic insights into potential therapeutic approaches targeting the NMJ in DMD. Adeno-associated virus (AAV)-MuSK administration improves the dystrophic phenotype by stimulating low-density lipoprotein receptor-related protein 4 (LRP4)/MuSK activation, thereby enhancing AChR clustering and driving gene expression in subsynaptic (also known as fundamental) myonuclei. In particular, downstream of MuSK are the transcriptional activators Ets variant 5 (Erm) and GA-binding protein (GABP⍺/β; also known as nuclear respiratory factor 2, or NRF-2) that bind to the N-box response element found in numerous synaptic genes, including Musk, Rapsn, Utrn, and Chrn. Similarly, mini-agrin treatment likely improves dystrophic NMJ biology through LRP4/MuSK activation, however this has yet to be directly demonstrated. Elevating neural activity, for example via a prescribed chronic exercise program, evokes synaptic gene expression by stimulating AMP-activated protein kinase (AMPK)/peroxisome proliferator-activated receptor ɣ coactivator-1α (PGC-1⍺)/GABP⍺/β signalling. The chronic induction of Utrn expression results in augmented utrophin and UAPC content throughout the myofiber, most notably extrasynaptically along the sarcolemma where it serves to functionally compensate for the lack of dystrophin. Exercise and AMPK also elicit autophagy-mediated AChR recycling, a complementary molecular pathway that beneficially remodels the dystrophic NMJ. β2 adrenergic agonists increase cAMP/protein kinase A (PKA) signalling and normalize AChR recycling and may correct NMJ morphology in dystrophic animals. Lastly, dystrophin replacement strategies, such as mini-dystrophin, have also been shown to improve the stability and function of the NMJ, at least in part via rescue of dystrophin-associated protein complex (DAPC) expression. Solid lines indicate established connections between events and dashed lines refer to potential linkages between steps.
In conclusion, the continued examination of the NMJ in DMD will expand our basic understanding of neuromuscular biology. This information may be useful for designing NMJ-targeted drug or behavioural strategies to address the dystrophic pathology and other disorders of the neuromuscular system.
6. Search strategy and selection criteria
Data for this review were identified by searches of PubMed and Google scholar using the search terms (“DMD”) AND (“NMJ”, “neurotransmission”, “synaptic genes”, OR “therapies”). References from relevant articles were also manually sought after. All articles referenced are academic and peer reviewed. A preference was given to articles published recently.
Author contributions
SYN and VL conceptualized the paper. SYN wrote the manuscript, constructed the tables, and prepared figures. VL supervised the writing and editing of the manuscript. Both authors have read and approved the final version of the manuscript.
Declaration of Competing Interest
The authors have nothing to disclose.
Acknowledgements
We are grateful to members of the Integrative Neuromuscular Biology Laboratory and to colleagues in the Exercise Metabolism Research Group at McMaster University for rich discussion and culture. Work in the authors’ laboratory is funded by the Canadian Institutes of Health Research, the Canada Research Chairs program, the Natural Science and Engineering Research Council of Canada (NSERC), the Ontario Ministry of Economic Development, Job Creation and Trade (MEDJCT), and Muscular Dystrophy Canada. SYN is a recipient of an NSERC Postgraduate Graduate Scholarship. VL is the Canada Research Chair (Tier 2) in Neuromuscular Plasticity in Health and Disease and is a MEDJCT Early Researcher. All funding organizations did not have a role in the design, interpretation, or writing of this paper.
References
- 1.Pilgram GSK, Potikanond S, Baines RA, Fradkin LG, Noordermeer JN. The roles of the dystrophin-associated glycoprotein complex at the synapse. Mol Neurobiol. 2010;41(1):1–21. doi: 10.1007/s12035-009-8089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks GB, Chamberlain JS, Froehner SC. Truncated dystrophins can influence neuromuscular synapse structure. Mol Cell Neurosci. 2009;40(4):433–441. doi: 10.1016/j.mcn.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panayiotopoulos CP, Scarpalezos S, Papapetropoulos T. Electrophysiological estimation of motor units in Duchenne muscular dystrophy. J Neurol Sci. 1974;23(1):89–98. doi: 10.1016/0022-510x(74)90145-2. [DOI] [PubMed] [Google Scholar]
- 4.Sakakibara H, Engel AG, Lambert EH. Duchenne dystrophy: Ultrastructural localization of the acetylcholine receptor and intracellular microelectrode studies of neuromuscular transmission. Neurology. 1977;27(8):741–745. doi: 10.1212/wnl.27.8.741. [DOI] [PubMed] [Google Scholar]
- 5.Minatel E, Santo Neto H, Marques MJ. Acetylcholine receptor distribution and synapse elimination at the developing neuromuscular junction of mdx mice. Muscle Nerve. 2003;28(5):561–569. doi: 10.1002/mus.10416. [DOI] [PubMed] [Google Scholar]
- 6.Nagel A, Lehmann‐Horn F, Engel AG. Neuromuscular transmission in the mdx mouse. Muscle Nerve. 1990;13(8):742–749. doi: 10.1002/mus.880130813. [DOI] [PubMed] [Google Scholar]
- 7.Lyons PR, Slater CR. Structure and function of the neuromuscular junction in young adult mdx mice. J Neurocytol. 1991;20 doi: 10.1007/BF01187915. [DOI] [PubMed] [Google Scholar]
- 8.Kong J, Anderson JE. Dystrophin is required for organizing large acetylcholine receptor aggregates. Brain Res. 1999;839(2):298–304. doi: 10.1016/s0006-8993(99)01737-0. [DOI] [PubMed] [Google Scholar]
- 9.Pratt SJP, Shah SB, Ward CW, Kerr JP, Stains JP, Lovering RM. Recovery of altered neuromuscular junction morphology and muscle function in mdx mice after injury. Cell Mol Life Sci. 2014;72(1):153–164. doi: 10.1007/s00018-014-1663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pratt SJP, Shah SB, Ward CW, Inacio MP, Stains JP, Lovering RM. Effects of in vivo injury on the neuromuscular junction in healthy and dystrophic muscles. J Physiol. 2013;591(2):559–570. doi: 10.1113/jphysiol.2012.241679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pratt SJP, Valencia AP, Le GK, Shah SB, Lovering RM. Pre- and postsynaptic changes in the neuromuscular junction in dystrophic mice. Front Physiol. 2015;6(SEP):252. doi: 10.3389/fphys.2015.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jerusalem F, Engel AG, Gomez MR. Duchenne dystrophy: II. Morphometric study of motor end-plate fine structure. Brain. 1974;97(1):123–130. doi: 10.1093/brain/97.1.123. [DOI] [PubMed] [Google Scholar]
- 13.Harriman DGF. A comparison of the fine structure of motor end-plates in duchenne dystrophy and in human neurogenic diseases. J Neurol Sci. 1976;28(2):233–247. doi: 10.1016/0022-510x(76)90106-4. [DOI] [PubMed] [Google Scholar]
- 14.Pisani C, Strimpakos G, Gabanella F, Di Certo MG, Onori A, Severini C. Utrophin up-regulation by artificial transcription factors induces muscle rescue and impacts the neuromuscular junction in mdx mice. Biochim Biophys Acta – Mol Basis Dis. 2018;1864(4):1172–1182. doi: 10.1016/j.bbadis.2018.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handschin C, Kobayashi YM, Chin S, Seale P, Campbell KP, Spiegelman BM. PGC-1α regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes Dev. 2007;21(7):770–783. doi: 10.1101/gad.1525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trajanovska S, Ban J, Huang J, Gregorevic P, Morsch M, Allen DG. Muscle Specific Kinase (MuSK) protects dystrophic mdx mouse muscles from eccentric contraction‐induced loss of force‐producing capacity. J Physiol. 2019 doi: 10.1113/JP277839. JP277839. [DOI] [PubMed] [Google Scholar]
- 17.Hollinger K, Gardan-Salmon D, Santana C, Rice D, Snella E, Selsby JT. Rescue of dystrophic skeletal muscle by PGC-1α involves restored expression of dystrophin-associated protein complex components and satellite cell signaling. Am J Physiol – Regul Integr Comp Physiol. 2013;305(1):13–23. doi: 10.1152/ajpregu.00221.2012. [DOI] [PubMed] [Google Scholar]
- 18.Chan MC, Rowe GC, Raghuram S, Patten IS, Farrell C, Arany Z. Post-natal induction of PGC-1α protects against severe muscle dystrophy independently of utrophin. Skelet Muscle. 2014;4(1) doi: 10.1186/2044-5040-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudolf R, Khan MM, Labeit S, Deschenes MR. Degeneration of neuromuscular junction in age and dystrophy. Front Aging Neurosci Front. 2014;6:99. doi: 10.3389/fnagi.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tintignac LA, Brenner HR, Rüegg MA. Mechanisms regulating neuromuscular junction development and function and causes of muscle wasting. Physiol Rev. 2015;95(3):809–852. doi: 10.1152/physrev.00033.2014. [DOI] [PubMed] [Google Scholar]
- 21.Burden SJ, Huijbers MG, Remedio L. Fundamental molecules and mechanisms for forming and maintaining neuromuscular synapses. Int J Mol Sci. 2018;19(2):1–19. doi: 10.3390/ijms19020490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Xiong W-C, Mei L. Neuromuscular junction formation, aging, and disorders. Annu Rev Physiol. 2018;80:159–188. doi: 10.1146/annurev-physiol-022516-034255. [DOI] [PubMed] [Google Scholar]
- 23.Darabid H, Perez-Gonzalez AP, Robitaille R. Neuromuscular synaptogenesis: coordinating partners with multiple functions. Nat Publ Gr. 2014;15:703. [PubMed] [Google Scholar]
- 24.Emery AEH. Population frequencies of inherited neuromuscular diseases-a world survey. Neuromuscul Disord. 1991;1(1):19–29. doi: 10.1016/0960-8966(91)90039-u. [DOI] [PubMed] [Google Scholar]
- 25.Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018;17:251–267. doi: 10.1016/S1474-4422(18)30024-3. Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landfeldt E, Lindgren P, Bell CF, Schmitt C, Guglieri M, Straub V, et al. The burden of Duchenne muscular dystrophy an international, cross-sectional study. 2014. [DOI] [PMC free article] [PubMed]
- 27.Guiraud S, Aartsma-Rus A, Vieira NM, Davies KE, van Ommen G-JB, Kunkel LM. The pathogenesis and therapy of muscular dystrophies. Annu Rev Genomics Hum Genet. 2015;16(1):281–308. doi: 10.1146/annurev-genom-090314-025003. [DOI] [PubMed] [Google Scholar]
- 28.Dial AG, Ng SY, Manta A, Ljubicic V. The role of AMPK in neuromuscular biology and disease. Trends Endocrinol Metab. 2018;29(5):300–312. doi: 10.1016/j.tem.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Guiraud S, Davies KE. Pharmacological advances for treatment in Duchenne muscular dystrophy. Curr Opin Pharmacol. 2017;34:36–48. doi: 10.1016/j.coph.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Allen DG, Whitehead NP, Froehner SC. Absence of dystrophin disrupts skeletal muscle signaling: roles of Ca2+, reactive oxygen species, and nitric oxide in the development of muscular dystrophy. Physiol Rev. 2015;96(1):253–305. doi: 10.1152/physrev.00007.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanes JR, Yamagata M. Many paths to synaptic specificity. Annu Rev Cell Dev Biol. 2009;25:161–195. doi: 10.1146/annurev.cellbio.24.110707.175402. [DOI] [PubMed] [Google Scholar]
- 32.Verhaart IEC, Aartsma-Rus A. Therapeutic developments for Duchenne muscular dystrophy. Nat Rev Neurol. 2019;15:373–386. doi: 10.1038/s41582-019-0203-3. Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman EP. Pharmacotherapy of Duchenne muscular dystrophy. Handb Exp Pharmacol. 2019:25–37. doi: 10.1007/164_2019_256. In: [DOI] [PubMed] [Google Scholar]
- 34.Frank DE, Schnell FJ, Akana C, El-Husayni SH, Desjardins CA, Morgan J. Increased dystrophin production with golodirsen in patients with Duchenne muscular dystrophy. Neurology. 2020;94(21):e2270–e2282. doi: 10.1212/WNL.0000000000009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haas M, Vlcek V, Balabanov P, Salmonson T, Bakchine S, Markey G. European medicines agency review of ataluren for the treatment of ambulant patients aged 5 years and older with Duchenne muscular dystrophy resulting from a nonsense mutation in the dystrophin gene. Neuromuscul Disord. 2015;25(1):5–13. doi: 10.1016/j.nmd.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Asher DR, Thapa K, Dharia SD, Khan N, Potter RA, Rodino-Klapac LR. Clinical development on the frontier: gene therapy for duchenne muscular dystrophy. Expert Opin Biol Ther. 2020;20:263–274. doi: 10.1080/14712598.2020.1725469. [DOI] [PubMed] [Google Scholar]
- 37.Rudolf R, Khan MM, Witzemann V. Motor endplate—anatomical, functional, and molecular concepts in the historical perspective. Cells. 2019;8(5):387. doi: 10.3390/cells8050387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wood SJ, R, Slater C. Safety factor at the neuromuscular junction. Prog Neurobiol. 2001;64:393–429. doi: 10.1016/s0301-0082(00)00055-1. [DOI] [PubMed] [Google Scholar]
- 39.Wu H, Xiong WC, Mei L. To build a synapse: Signaling pathways in neuromuscular junction assembly. Development. 2010;137:1017–1033. doi: 10.1242/dev.038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belotti E, Schaeffer L. Regulation of Gene expression at the neuromuscular Junction. Neurosci Lett. 2020;735 doi: 10.1016/j.neulet.2020.135163. Elsevier Ireland Ltd. [DOI] [PubMed] [Google Scholar]
- 41.Guarino SR, Canciani A, Forneris F. Dissecting the extracellular complexity of neuromuscular junction organizers. Front Mol Biosci. 2020;6(156) doi: 10.3389/fmolb.2019.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weatherbee SD, Anderson K V. Niswander LA. LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development. 2006 Dec;133(24):4993–5000. doi: 10.1242/dev.02696. [DOI] [PubMed] [Google Scholar]
- 43.Briguet A, Ruegg MA. The Ets transcription factor GABP is required for postsynaptic differentiation in vivo. J Neurosci. 2000;20(16):5989–5996. doi: 10.1523/JNEUROSCI.20-16-05989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaworski A, Smith CL, Burden SJ. GA-binding protein is dispensable for neuromuscular synapse formation and synapse-specific gene expression. Mol Cell Biol. 2007;27(13):5040–5046. doi: 10.1128/MCB.02228-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hippenmeyer S, Huber RM, Ladle DR, Murphy K, Arber S. ETS transcription factor erm controls subsynaptic gene expression in skeletal muscles. Neuron. 2007;55(5):726–740. doi: 10.1016/j.neuron.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 46.Taetzsch T, Valdez G. NMJ maintenance and repair in aging. Curr Opin Physiol. 2018;4:57–64. doi: 10.1016/j.cophys.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kupr B, Handschin C. Complex coordination of cell plasticity by a PGC-1α-controlled transcriptional network in skeletal muscle. Front Physiol. 2015;6(NOV):325. doi: 10.3389/fphys.2015.00325. http://journal.frontiersin.org/Article/10.3389/fphys.2015.00325/abstract [cited 2020 Aug 28]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Angus LM, Chakkalakal JV, Méjat A, Eibl JK, Bélanger G, Megeney LA. Calcineurin-NFAT signaling, together with GABP and peroxisome PGC-1α, drives utrophin gene expression at the neuromuscular junction. Am J Physiol – Cell Physiol. 2005;289:C908–C917. doi: 10.1152/ajpcell.00196.2005. [DOI] [PubMed] [Google Scholar]
- 49.Khan MM, Lustrino D, Silveira WA, Wild F, Straka T, Issop Y. Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease. Proc Natl Acad Sci USA. 2016;113(3):746–750. doi: 10.1073/pnas.1524272113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chakkalakal J V., Nishimune H, Ruas JL, Spiegelman BM, Sanes JR. Retrograde influence of muscle fibers on their innervation revealed by a novel marker for slow motoneurons. Development. 2010;137(20):3489–3499. doi: 10.1242/dev.053348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnold AS, Gill J, Christe M, Ruiz R, McGuirk S, St-Pierre J. Morphological and functional remodelling of the neuromuscular junction by skeletal muscle PGC-1a. Nat Commun. 2014;5(3569) doi: 10.1038/ncomms4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mills R, Taylor-Weiner H, Correia JC, Agudelo LZ, Allodi I, Kolonelou C. Neurturin is a PGC-1α1-controlled myokine that promotes motor neuron recruitment and neuromuscular junction formation. Mol Metab. 2018;7(November 2017):12–22. doi: 10.1016/j.molmet.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ljubicic V, Burt M, Jasmin BJ. The therapeutic potential of skeletal muscle plasticity in Duchenne muscular dystrophy: Phenotypic modifiers as pharmacologic targets. FASEB J. 2014;28(2):548–568. doi: 10.1096/fj.13-238071. [DOI] [PubMed] [Google Scholar]
- 54.Banks GB, Fuhrer C, Adams ME, Froehner SC. The postsynaptic submembrane machinery at the neuromuscular junction: Requirement for rapsyn and the utrophin/dystrophin-associated complex. J Neurocytol. 2003;32:709–726. doi: 10.1023/B:NEUR.0000020619.24681.2b. [DOI] [PubMed] [Google Scholar]
- 55.Grady RM, Merlie JP, Sanes JR. Subtle neuromuscular defects in utrophin-deficient mice. J Cell Biol. 1997;136(4):871–882. doi: 10.1083/jcb.136.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deconinck AE, Potter AC, Tinsley JM, Wood SJ, Vater R, Young C. Postsynaptic abnormalities at the neuromuscular junctions of utrophin- deficient mice. J Cell Biol. 1997;136(4):883–894. doi: 10.1083/jcb.136.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khan MM, Strack S, Wild F, Hanashima A, Gasch A, Brohm K. Role of autophagy, SQSTM1, SH3GLB1, and TRIM63 in the turnover of nicotinic acetylcholine receptors. Autophagy. 2014;10(1):123–136. doi: 10.4161/auto.26841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Torres LFB, Duchen LW. The mutant mdx: Inherited myopathy in the mouse: Morphological studies of nerves, muscles and end-plates. Brain. 1987;110(2):269–299. doi: 10.1093/brain/110.2.269. [DOI] [PubMed] [Google Scholar]
- 59.Rudolf R, Khan MM, Lustrino D, Labeit S, Kettelhut ÍC, Navegantes LCC. Alterations of cAMP-dependent signaling in dystrophic skeletal muscle. Front Physiol. 2013;4:290. doi: 10.3389/fphys.2013.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willadt S, Nash M, Slater CR. Age-related fragmentation of the motor endplate is not associated with impaired neuromuscular transmission in the mouse diaphragm. Sci Rep. 2016;6 doi: 10.1038/srep24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adams ME, Anderson KNE, Froehner SC. The α-syntrophin PH and PDZ domains scaffold acetylcholine receptors, utrophin, and neuronal nitric oxide synthase at the neuromuscular junction. J Neurosci. 2010;30(33):11004–11010. doi: 10.1523/JNEUROSCI.1930-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marques MJ, Taniguti A, Minatel E, Neto HS. Nerve terminal contributes to acetylcholine receptor organization at the dystrophic neuromuscular junction of mdx mice. Anat Rec Adv Integr Anat Evol Biol. 2007;290 doi: 10.1002/ar.20421. 1811–187. [DOI] [PubMed] [Google Scholar]
- 63.Ferretti R, Neto HS, Marques MJ. Expression of utrophin at dystrophin-deficient neuromuscular synapses of mdx mice: a study of protected and affected muscles. Anat Rec. 2011;294(2):283–286. doi: 10.1002/ar.21297. [DOI] [PubMed] [Google Scholar]
- 64.Personius KE, Sawyer RP. Variability and failure of neurotransmission in the diaphragm of mdx mice. Neuromuscul Disord. 2006;16(3):168–177. doi: 10.1016/j.nmd.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 65.Haddix SG, Lee Y il, Kornegay JN, Thompson WJ. Cycles of myofiber degeneration and regeneration lead to remodeling of the neuromuscular junction in two mammalian models of Duchenne muscular dystrophy. PLoS One. 2018;13(10) doi: 10.1371/journal.pone.0205926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Pijl EM, van Putten M, Niks EH, Verschuuren JJGM, Aartsma-Rus A, Plomp JJ. Characterization of neuromuscular synapse function abnormalities in multiple Duchenne muscular dystrophy mouse models. Eur J Neurosci. 2016;43(12):1623–1635. doi: 10.1111/ejn.13249. [DOI] [PubMed] [Google Scholar]
- 67.van der Pijl EM, van Putten M, Niks EH, Verschuuren JJGM, Aartsma-Rus A, Plomp JJ. Low dystrophin levels are insufficient to normalize the neuromuscular synaptic abnormalities of mdx mice. Neuromuscul Disord. 2018;28(5):427–442. doi: 10.1016/j.nmd.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 68.Lovering RM, Iyer SR, Edwards B. Alterations of neuromuscular junctions in Duchenne muscular dystrophy. Neurosci Lett. 2020 doi: 10.1016/j.neulet.2020.135304. [Internet][cited 2020 Aug 26]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neto HS, Martins AJ, Minatel E, Marques MJ. Axonal sprouting in mdx mice and its relevance to cell and gene mediated therapies for Duchenne muscular dystrophy. Neurosci Lett. 2003;343(1):67–69. doi: 10.1016/s0304-3940(03)00220-9. [DOI] [PubMed] [Google Scholar]
- 70.Call JA, Mckeehen JN, Novotny SA, Lowe DA. Progressive resistance voluntary wheel running in the mdx mouse. Muscle Nerve. 2010;42(6):871–880. doi: 10.1002/mus.21764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roy P, Rau F, Ochala J, Messéant J, Fraysse B, Lainé J. Dystrophin restoration therapy improves both the reduced excitability and the force drop induced by lengthening contractions in dystrophic mdx skeletal muscle. Skelet Muscle. 2016;6(1) doi: 10.1186/s13395-016-0096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baumann CW, Warren GL, Lowe DA. Plasmalemma function is rapidly restored in Mdx muscle after eccentric contractions. Med Sci Sports Exerc. 2020;52(2):354–361. doi: 10.1249/MSS.0000000000002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hilton-Brown P, Stålberg E. Motor unit size in muscular dystrophy, a macro EMG and scanning EMG study. J Neurol Neurosurg Psychiatry. 1983;46(11):996–1005. doi: 10.1136/jnnp.46.11.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharma RR, Mynhier MA, Miller RG. Muscular fatigue in duchenne muscular dystrophy. Neurology. 1995;45(2):306–310. doi: 10.1212/wnl.45.2.306. [DOI] [PubMed] [Google Scholar]
- 75.Carlson CG, Roshek DM. Adult dystrophic (mdx) endplates exhibit reduced quantal size and enhanced quantal variation. Pflugers Arch Eur J Physiol. 2001;442(3):369–375. doi: 10.1007/s004240100561. [DOI] [PubMed] [Google Scholar]
- 76.Belhasan DC, Akaaboune M. The role of the dystrophin glycoprotein complex on the neuromuscular system. Neurosci Lett. 2020;722(February) doi: 10.1016/j.neulet.2020.134833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Côté PD, Moukhles H, Lindenbaum M, Carbonetto S. Chimaeric mice deficient in dystroglycans develop muscular dystrophy and have disrupted myoneural synapses. Nat Genet. 1999;23(3):338–342. doi: 10.1038/15519. [DOI] [PubMed] [Google Scholar]
- 78.Hesser BA, Henschel O, Witzemann V. Synapse disassembly and formation of new synapses in postnatal muscle upon conditional inactivation of MuSK. Mol Cell Neurosci. 2006;31(3):470–480. doi: 10.1016/j.mcn.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 79.Morsch M, Reddel SW, Ghazanfari N, Toyka K V., Phillips WD. Muscle specific kinase autoantibodies cause synaptic failure through progressive wastage of postsynaptic acetylcholine receptors. Exp Neurol. 2012;237(2):286–295. doi: 10.1016/j.expneurol.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 80.Ghazanfari N, Morsch M, Reddel SW, Liang SX, Phillips WD. Muscle-specific kinase (MuSK) autoantibodies suppress the MuSK pathway and ACh receptor retention at the mouse neuromuscular junction. J Physiol. 2014;592(13):2881–2897. doi: 10.1113/jphysiol.2013.270207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gramolini AO, Burton EA, Tinsley JM, Ferns MJ, Cartaud A, Cartaud J. Muscle and neural isoforms of agrin increase utrophin expression in cultured myotubes via a transcriptional regulatory mechanism. J Biol Chem. 1998;273(2):736–743. doi: 10.1074/jbc.273.2.736. [DOI] [PubMed] [Google Scholar]
- 82.Arimura S, Okada T, Tezuka T, Chiyo T, Kasahara Y, Yoshimura T. DOK7 gene therapy benefits mouse models of diseases characterized by defects in the neuromuscular junction. Science. 2014;345(6203):1505–1508. doi: 10.1126/science.1250744. [DOI] [PubMed] [Google Scholar]
- 83.Kaifer KA, Villalón E, Smith CE, Simon ME, Marquez J, Hopkins AE. AAV9-DOK7 gene therapy reduces disease severity in Smn2B/- SMA model mice. Biochem Biophys Res Commun. 2020;530(1):107–114. doi: 10.1016/j.bbrc.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miyoshi S, Tezuka T, Arimura S, Tomono T, Okada T, Yamanashi Y. DOK7 gene therapy enhances motor activity and life span in ALS model mice. EMBO Mol Med. 2017;9(7):880–889. doi: 10.15252/emmm.201607298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lang JM, Esser KA, Dupont-Versteegden EE. Altered activity of signaling pathways in diaphragm and tibialis anterior muscle of dystrophic mice. Exp Biol Med. 2004;229(6):503–511. doi: 10.1177/153537020422900608. [DOI] [PubMed] [Google Scholar]
- 86.Gramolini AO, Bélanger G, Thompson JM, Chakkalakal J V., Jasmin BJ. Increased expression of utrophin in a slow vs. a fast muscle involves posttranscriptional events. Am J Physiol – Cell Physiol. 2001;281(4 50-4) doi: 10.1152/ajpcell.2001.281.4.C1300. [DOI] [PubMed] [Google Scholar]
- 87.Al-Rewashdy H, Ljubicic V, Lin W, Renaud JM, Jasmin BJ. Utrophin A is essential in mediating the functional adaptations of mdx mouse muscle following chronic AMPK activation. Hum Mol Genet. 2015;24(5):1243–1255. doi: 10.1093/hmg/ddu535. [DOI] [PubMed] [Google Scholar]
- 88.Guiraud S, Roblin D, Kay DE. The potential of utrophin modulators for the treatment of Duchenne muscular dystrophy. Expert Opin Orphan Drugs. 2018;6:179–192. [Google Scholar]
- 89.Kleopa KA, Drousiotou A, Mavrikiou E, Ormiston A, Kyriakides T. Naturally occurring utrophin correlates with disease severity in Duchenne muscular dystrophy. Hum Mol Genet. 2006;15(10):1623–1628. doi: 10.1093/hmg/ddl083. [DOI] [PubMed] [Google Scholar]
- 90.Vainzof M, Passos-Bueno MR, Man NT, Zatz M. Absence of correlation between utrophin localization and quantity and the clinical severity in Duchenne/Becker dystrophies. Am J Med Genet. 1995;58(4):305–309. doi: 10.1002/ajmg.1320580403. [DOI] [PubMed] [Google Scholar]
- 91.Capogrosso RF, Mantuano P, Cozzoli A, Sanarica F, Massari AM, Conte E. Contractile efficiency of dystrophic mdx mouse muscle: in vivo and ex vivo assessment of adaptation to exercise of functional end points. J Appl Physiol. 2017;122(4):828–843. doi: 10.1152/japplphysiol.00776.2015. [DOI] [PubMed] [Google Scholar]
- 92.Lynch GS, Ryall JG. Role of β-adrenoceptor signaling in skeletal muscle: Implications for muscle wasting and disease. Physiol Rev. 2008;88:729–767. doi: 10.1152/physrev.00028.2007. [DOI] [PubMed] [Google Scholar]
- 93.Oya Y, Masafumi O, Mitsuru K. Therapeutic trial of β2-adrenergic agonist clenbuterol in muscular dystrophies. Clin Neurol. 2001;41(10):698–700. [PubMed] [Google Scholar]
- 94.Skura CL, Fowler EG, Wetzel GT, Graves M, Spencer MJ. Albuterol increases lean body mass in ambulatory boys with Duchenne or Becker muscular dystrophy. Neurology. 2008;70(2):137–143. doi: 10.1212/01.WNL.0000287070.00149.a9. [DOI] [PubMed] [Google Scholar]
- 95.Deschenes MR. Adaptations of the neuromuscular junction to exercise training. Curr Opin Physiol. 2019;10:10–16. [Google Scholar]
- 96.Gordon BS, Lowe DA, Kostek MC. Exercise increases utrophin protein expression in the mdx mouse model of Duchenne muscular dystrophy. Muscle Nerve. 2014;49(6):915–918. doi: 10.1002/mus.24151. [DOI] [PubMed] [Google Scholar]
- 97.Timmons JA, Larsson O, Jansson E, Fischer H, Gustafsson T, Greenhaff PL. Human muscle gene expression responses to endurance training provide a novel perspective on Duchenne muscular dystrophy. FASEB J. 2005;19(7):750–760. doi: 10.1096/fj.04-1980com. [DOI] [PubMed] [Google Scholar]
- 98.Carnio S, LoVerso F, Baraibar MA, Longa E, Khan MM, Maffei M. Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging. Cell Rep. 2014;8(5):1509–1521. doi: 10.1016/j.celrep.2014.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hood DA, Memme JM, Oliveira AN, Triolo M. Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annu Rev Physiol. 2019;81(1):19–41. doi: 10.1146/annurev-physiol-020518-114310. [DOI] [PubMed] [Google Scholar]
- 100.Ng SY, Manta A, Ljubicic V. Exercise biology of neuromuscular disorders. Appl Physiol Nutr Metab. 2018:1–44. doi: 10.1139/apnm-2018-0229. [DOI] [PubMed] [Google Scholar]
- 101.van Putten M, Hulsker M, Nadarajah VD, van Heiningen SH, van Huizen E, van Iterson M. The effects of low levels of dystrophin on mouse muscle function and pathology. PLoS One. 2012;7(2):31937. doi: 10.1371/journal.pone.0031937. [DOI] [PMC free article] [PubMed] [Google Scholar]