Summary
Although the influence of sleep quality on the immune system is well documented, the mechanisms behind its impact on natural host immunity remain unclear. Meanwhile, it has been suggested that neuroimmune interactions play an important role in this phenomenon. To evaluate the impact of stress-induced sleep disturbance on host immunity, we used a murine model of rapid eye movement sleep deprivation (RSD) integrated with a model of malaria blood-stage infection. We demonstrate that sleep disturbance compromises the differentiation of T follicular helper cells, increasing host susceptibility to the parasite. Chemical inhibition of glucocorticoid (Glcs) synthesis showed that abnormal Glcs production compromised the transcription of Tfh-associated genes resulting in impaired germinal center formation and humoral immune response. Our data demonstrate that RSD-induced abnormal activation of the hypothalamic-pituitary-adrenal axis drives host susceptibility to infection. Understanding the impact of sleep quality in natural resistance to infection may provide insights for disease management.
Subject Areas: Behavioral Neuroscience, Immunology
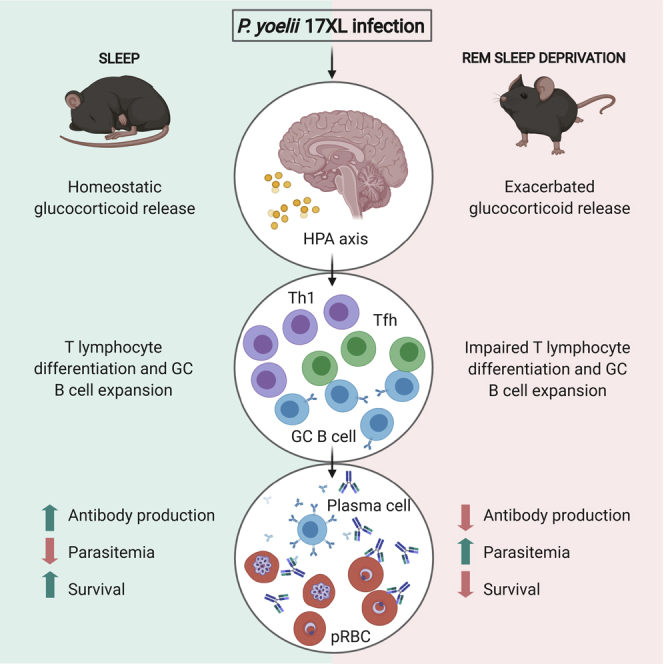
Graphical Abstract
Highlights
-
•
REM sleep deprivation (RSD) worsens malaria induced by Plasmodium yoelii infection
-
•
RSD decreases germinal center formation and impairs specific antibody production
-
•
Exacerbated glucocorticoid production impairs T lymphocyte differentiation
-
•
The relationship between sleep and immunity is a target for malaria management
Behavioral Neuroscience; Immunology
Introduction
Sleep is a behavioral and physiological phenomenon, fundamental for homeostasis. In modern 24-hr society, it is noteworthy a reduction in sleep duration (from nearly 9 hr in the 60s to less than 7 hr nowadays) and in sleep quality, due to extended work routines, stressful situations, and the presence of sleep disorders that alter sleep quality (Chattu et al., 2018). Several studies suggest that inadequate sleep duration is strongly associated with the development of hypertension (Grandner et al., 2018), obesity, cardiovascular diseases (Sabanayagam and Shankar, 2010), diabetes, and cancer (Haus and Smolensky, 2013; Hakim et al., 2014; Ma et al., 2016; Shi et al., 2020). In addition, several studies provided clues regarding the cross talk between sleep homeostasis and the immune system functions (Besedovsky et al., 2019). Thus, the comprehension of the relationship between sleep and the immune system is essential to design new approaches to improve sleep and immune response quality.
Sleep architecture comprises two very distinct phases: the rapid eye movement (REM) sleep (or paradoxical sleep) and non-REM sleep (NREM, which includes three different stages). Under normal circumstances, sleep regulatory substances (SRS), such as melatonin, regulate the circadian rhythm. However, other SRS, such as IL-1β and TNF-α, exert a significant influence in the sleep-awake cycle, acting as somnogenic molecules, modulating NREM sleep. Elevated IL-1β and TNF-α levels, detected during infectious diseases, increase the NREM sleep period (Krueger et al., 2007). Noteworthy, the NREM period is characterized by increased growth hormone (GH) secretion, diminished cortisol release, and a predominant activity of CD4+ T helper-1 (Th1) lymphocytes, which is essential for host immunity against intracellular parasites (Besedovsky et al., 2012; Lange et al., 2006). There is also growing evidence associating longer periods of sleep with a substantial reduction in parasitism levels (Opp, 2009) and reduced sleep quality with increased risk of infection and poor infection outcome (Patel et al., 2012; Prather et al., 2015; Besedovsky et al., 2019).
Considering that the majority of clinical and experimental models to study sleep disturbance involve total or partial sleep deprivation or sleep fragmentation, it is virtually impossible to distinguish between the effects of NREM or REM sleep periods. Thus, the relationship between REM sleep and immune system's activity remains poorly understood. Therefore, we used a murine model of REM sleep deprivation (RSD) integrated with a model of malaria blood-stage Plasmodium yoelii infection (Sanni et al., 2002) to determine the impact of REM sleep quality on host immunity against a parasitic infection.
Malaria is an infectious disease caused by different Plasmodium species, with an estimated 228 million cases and 405,000 deaths worldwide in 2018 (World Health Organization, 2019). In humans, inoculated sporozoites infect hepatocytes and gain circulation as blood-stage parasites (merozoites), invading and replicating in red blood cells (RBCs) (Kurup et al., 2019). The cyclical rupture of the infected erythrocyte is responsible for the clinical manifestations (mainly fever, chills, headache, malaise, and fatigue) of disease, which may lead to life-threatening complications if left untreated (Ashley et al., 2018). In malaria-endemic areas, repeated exposure to the parasite results in a protection against clinical manifestations, mediated by humoral immune response (Tran et al., 2014). Although specific antibodies do not provide sterile immunity, they are essential to control parasitemia levels by limiting parasite replication (Boyle et al., 2015). The key feature for antibody production is the maturation of B cells in the germinal center (GC) and further differentiation into memory or antibody-producing plasma cells (Mesin et al., 2016). This process is closely related to the interaction with a specialized subset of CD4+ T cells, known as T follicular helper (Tfh) cells, that provide differentiation signals to GC B cells (Crotty, 2019). From this perspective, chronic intermittent infection, or even certain types of treatment, has been associated with fatigue, irritability, and sleep disturbances, with loss of up to half of normal sleep (Nevin and Croft, 2016). Therefore, events that influence the development of humoral immunity may contribute to morbidity and mortality associated with mild and severe malaria (Ashley et al., 2018).
Sleep disturbances have been extensively associated with the activation of the hypothalamic-pituitary-adrenal (HPA) axis (Scheiermann et al., 2013), which is characterized by increased endogenous glucocorticoids levels that ultimately may exert immunosuppressive effects (Di Comite et al., 2007). Thus, we hypothesized that the stress induced by RSD during Plasmodium infection might impair the development of a protective humoral response. Herein, we show that RSD during the development of the immune response against malaria parasites impairs Tfh differentiation and GC formation, reducing the antibody response and ultimately host resistance to the parasite. The primary mechanism responsible for this phenomenon is the exacerbated production of glucocorticoids (Glcs) due to RSD during the acute phase of P. yoelii infection. Thus, our data highlight the impact of sleep quality on the development of protective humoral immunity against malaria. Furthermore, we suggest that Glc levels during the development of the acute phase of malaria infection may represent a useful marker to predict disease severity.
Results
RSD Impairs the Control of Malaria Infection and Increases Host Susceptibility to the Parasite
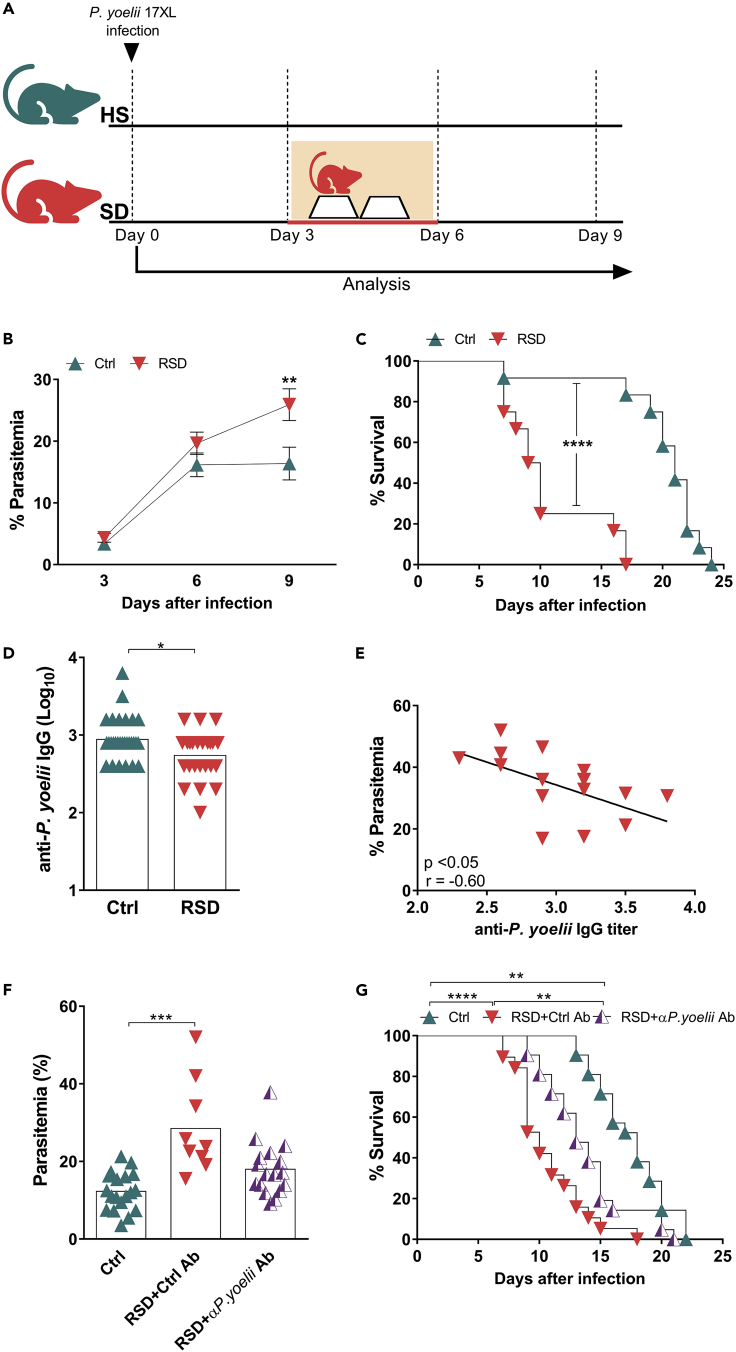
To determine whether sleep disturbance influences host immunity against malaria parasite, mice were injected with P. yoelii-infected erythrocytes before, at the same time, or after being subjected to RSD (Figures 1A and S1A). Our results showed that RSD initiated three days after infection (Figure 1A) exerted a critical effect on parasitemia and survival. Mice from this RSD group failed to control infection and, consequently, presented a lower survival rate. By contrast, the non-sleep deprived control group (Ctrl) controlled parasite growth (Figure 1B) and survived longer (Figure 1C), although blood parasitemia increased exponentially in the first six days post infection. RSD 3 days before, or at the same day of parasite inoculation (Figure S1B), did not impact disease progression to the same extent (Figures S1C and S1D).
Figure 1.
REM Sleep Deprivation Impairs the Control of Malaria Infection by Reducing Parasite-Specific Antibody Titers
(A) Experimental setup (see also Figure S1). Mice were submitted to REM sleep deprivation (RSD) 3 days after infection with Plasmodium yoelii, while the control group (Ctrl) was kept under regular sleep conditions.
(B) Parasitemia in Ctrl and RSD mice. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗∗p < 0.01) by two-way ANOVA.
(C) Mice survival in Ctrl and RSD groups. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗∗∗∗p < 0.0001) by log-rank (Mantel-Cox) test.
(D) Anti-P. yoelii IgG serum titers in Ctrl and RSD mice at day nine post infection. Data are from six independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by two-tailed unpaired t test.
(E) Correlation between parasitemia and anti-P. yoelii IgG titers. Data are from three independent experiments with at least five mice in each group.
(F) Six days after infection, RSD mice were transferred with purified IgGs obtained from control or P. yoelii-infected HS animals. Three days later, parasitemia levels were evaluated. Data are from two independent experiments with ten mice in each group. Mean ± SEM (∗∗∗p < 0.001) by one-way ANOVA.
(G) Mice survival after passive immunization with purified IgG from control or P. yoelii-infected animals. Data are from two independent experiments with ten mice in each group. Mean ± SEM (∗∗p < 0.01, ∗∗∗∗p < 0.0001) by log-rank (Mantel-Cox) test.
Based on these results, we sought to explore the effects of RSD 3 days after parasite inoculation. Of note, RSD itself did not impact weight loss in infected mice when compared to Ctrl mice (Figure S2A). However, the increased parasitemia found in the RSD-infected group impacted erythrocyte count (Figure S2B) and hematocrit (Figure S2C), but not hemoglobin levels (Figure S2D). By itself, RSD did not influence the same parameters in uninfected mice (Figures S2E–S2G, respectively).
Analysis of the humoral immune response showed that the susceptibility of the RSD group to P. yoelii infection correlated with a significant reduction in parasite-specific IgG titers (Figure 1D) that negatively correlated with parasitemia levels (Figure 1E), thus indicating that RSD impaired the production of protective antibodies. Besides the reduction in anti-parasite-specific IgG titers, the RSD group also presented lower levels of circulating IgG titers (Figure S3A) and antibody-secreting cells in the spleen (Figure S3B) against a 19-kDa major P. yoelii surface antigen, known as merozoite surface protein 1 (MSP-119).
To confirm the correlation between IgG titers and protection, we performed passive transfer experiments. Groups of RSD-infected animals received serum-purified IgGs taken from uninfected mice or from P. yoelii-infected mice 9–11 days post infection. No protection was conferred by IgGs from control mice, but IgGs from the infected mice reduced parasitemia (Figure 1F) and increased survival (Figure 1G). Thus, RSD impaired the production of protective antibodies.
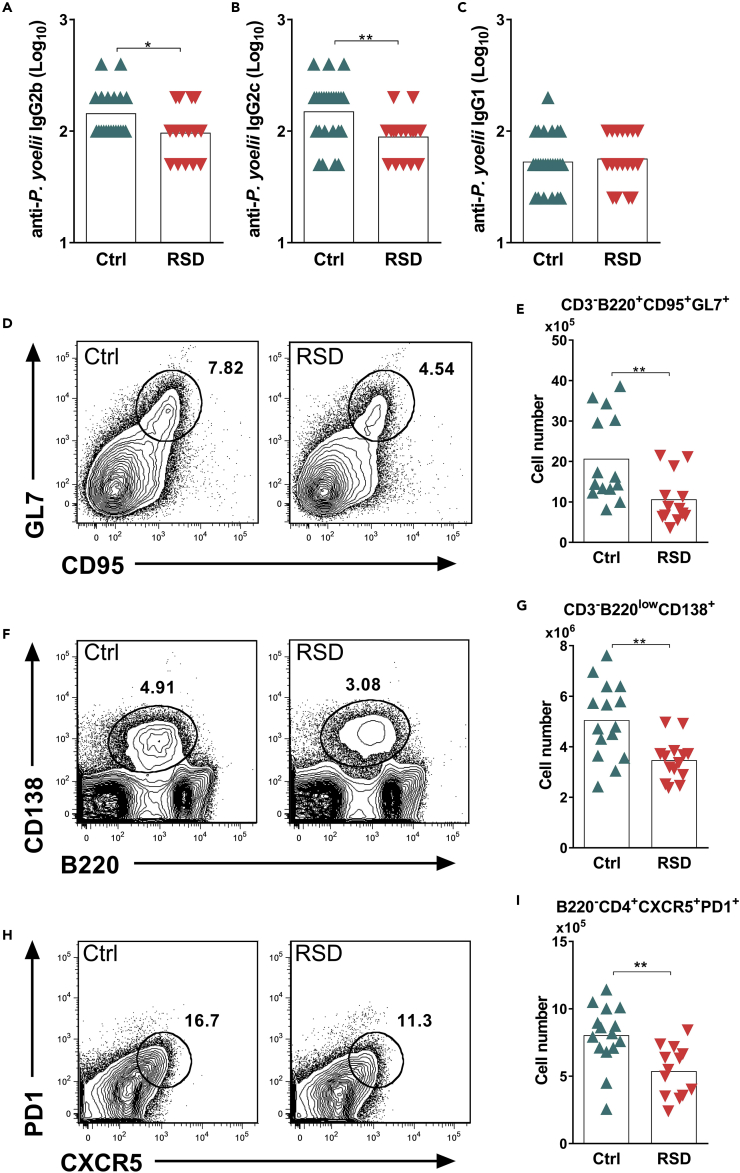
RSD Alters Antibody Class Switching and Reduces Germinal Center B Cells and Tfh Frequency during Infection
The analysis of parasite-specific antibody production revealed that RSD impaired the production of IgG2b and IgG2c isotypes without altering IgG1 titers (Figures 2A–2C, respectively). This result prompted us to analyze whether the reduction in IgG class switching was due to impaired GC and Tfh differentiation. Our results revealed that RSD impaired the differentiation of GC (CD3-B220+GL7+CD95+) (Figures 2D and 2E) and antibody-producing plasma (CD3-B220lowCD138+) B cells (Figures 2F and 2G). This phenomenon was closely related to a significant reduction in the frequency of Tfh cells (B220−CD3+PD-1+CXCR5+) (Figures 2H and 2I) since GC expansion and plasma B cell maturation are highly dependent on Tfh help (Victora et al., 2010). Thus, these data indicated that RSD compromised CD4+ T differentiation into Tfh cells and the development of specific B cells.
Figure 2.
REM Sleep Deprivation Impairs Antibody Production and Differentiation of Germinal Center B Cells, Plasma B Cells, and Tfh Cells
Infected Ctrl and RSD mice were analyzed at day nine post infection.
(A) Anti-P. yoelii IgG2b serum titers in Ctrl and RSD mice. Data are from six independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by two-tailed unpaired t test.
(B) Anti-P. yoelii IgG2c serum titers in Ctrl and RSD mice. Data are from six independent experiments with at least five mice in each group. Mean ± SEM (∗∗p < 0.01) by two-tailed unpaired t test.
(C) Anti-P. yoelii IgG1 serum titers in Ctrl and RSD mice. Data are from six independent experiments with at least five mice in each group. Mean ± SEM.
(D) Representative flow cytometry analysis of splenic GC B cells (CD3-B220+GL7+CD95+) in Ctrl and RSD mice.
(E) Absolute numbers of splenic GC B cells (CD3-B220+GL7+CD95+) in Ctrl and RSD mice. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗∗p < 0.01) by two-tailed unpaired t test.
(F) Representative flow cytometry analysis of splenic plasma B cells (CD3-B220lowCD138+).
(G) Absolute numbers of splenic plasma B cells (CD3-B220lowCD138+) in Ctrl and RSD mice. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗∗p < 0.01) by two-tailed unpaired t test.
(H) Representative flow cytometry analysis of splenic Tfh cells (B220−CD3+PD-1+CXCR5+).
(I) Absolute numbers of splenic Tfh cells (B220−CD3+PD-1+CXCR5+) in Ctrl and RSD mice. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗∗p < 0.01) by two-tailed unpaired t test.
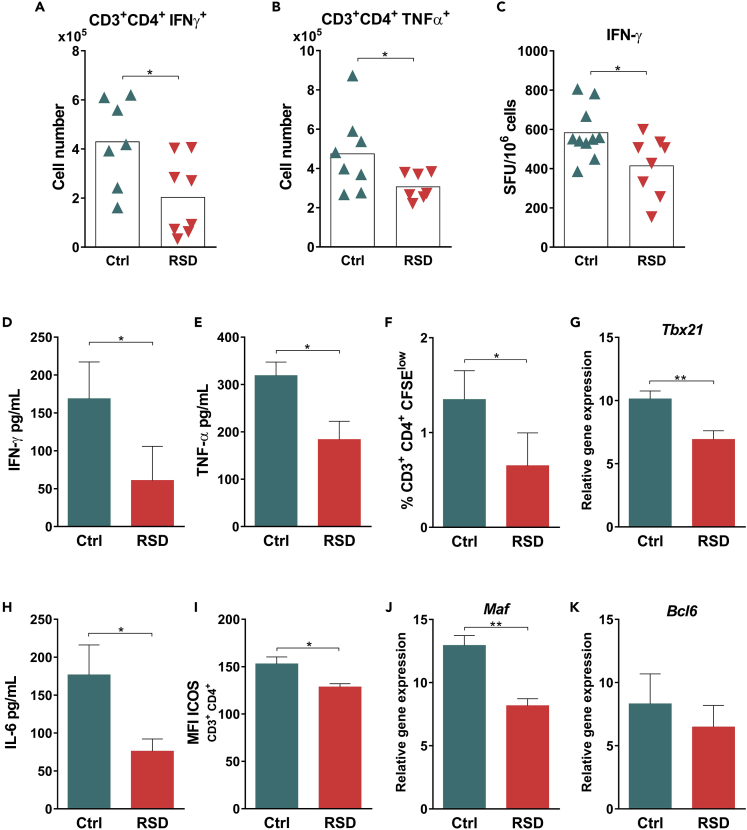
Sleep Disturbance Impairs T Cell Activity and Inhibits the Transcription of Genes Associated with Tfh Differentiation
In the blood-stage infection with the lethal strain of P. yoelii (17XL), IFN-γ production is essential to elicit parasite-specific IgG2a response and restrain parasite growth (Ishih et al., 2013). The ex vivo analysis of splenic cells by flow cytometry revealed that RSD mice presented lower numbers of IFN-γ- and TNF-α-producing CD4+ T cells (Figures 3A and 3B, respectively). Further analysis demonstrated that CD4+ T cells from the RSD group were not more susceptible to cell death when compared to those from Ctrl mice (Figures S4A and S4B); instead, these cells expressed lower levels of Ki67, indicating a lower proliferative capacity (Figure S4C). Therefore, these data indicate that RSD impaired T cell function. This idea was strengthened by the fact that splenocytes from RSD mice displayed lower numbers of IFN-γ-producing cells (Figure 3C), produced lower levels of the proinflammatory cytokines IFN-γ (Figure 3D) and TNF-α (Figure 3E), and decreased CD4+ T cell proliferation (Figure 3F) upon specific in vitro recall with P. yoelii MSP-119.
Figure 3.
REM Sleep Deprivation Impairs T Cell Activity and Inhibits the Transcription of Genes Associated with Tfh Differentiation
Infected Ctrl and RSD mice were analyzed at day nine post infection.
(A) Ex vivo analysis of splenic IFN-γ-producing CD3+CD4+ T cells from Ctrl and RSD mice. Data represent one experiment with at least 7 mice per group. Mean ± SD (∗p < 0.05) by two-tailed unpaired t test.
(B) Ex vivo analysis of splenic TNF-α-producing CD3+CD4+ T cells from Ctrl and RSD mice. Data represent one experiment with at least 7 mice per group. Mean ± SD (∗p < 0.05) by two-tailed unpaired t test.
(C) ELISPOT analysis of IFN-γ-producing cells from Ctrl and RSD mice, after culture with recombinant P. yoelii MSP119. Data are from two independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by two-tailed unpaired t test.
(D) Levels of IFN-γ in culture supernatants from Ctrl and RSD splenocytes after culture with recombinant P. yoelii MSP-119 for 48h. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by two-tailed unpaired t test.
(E) Levels of TNF-α in culture supernatants from Ctrl and RSD splenocytes after culture with recombinant P. yoelii MSP-119 for 48 hr. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by two-tailed unpaired t test.
(F) Frequency of specific proliferating (CFSElow) CD3+CD4+ T cells after culture in the presence of recombinant P. yoelii MSP-119. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by two-tailed unpaired t test.
(G) Tbx21 mRNA expression by sorted splenic CD4+ T cells from Ctrl and RSD mice. Data are from two independent experiments with at least four mice in each group. Mean ± SEM (∗∗p < 0.01) by two-tailed unpaired t test.
(H) Levels of IL-6 in culture supernatants from Ctrl and RSD splenocytes after culture with recombinant P. yoelii MSP-119 for 48hr. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by two-tailed unpaired t test.
(I) Median fluorescence intensity (MFI) of ICOS on splenic CD3+CD4+ T cells from Ctrl and RSD mice. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by two-tailed unpaired t test.
(J) Maf mRNA expression by sorted CD4+ T cells from Ctrl and RSD mice. Data are from two independent experiments with at least four mice in each group. Mean ± SEM (∗∗p < 0.01) by two-tailed unpaired t test.
(K) Bcl6 mRNA expression by sorted CD4+ T cells from Ctrl and RSD mice. Data are from two independent experiments with at least four mice in each group. Mean ± SEM.
To further characterize these CD4+ T cells, we analyzed the expression of the Th1 key transcription factor T-bet (Tbx21) (Szabo et al., 2000). Indeed, sorted CD4+ T cells from RSD mice displayed lower expression of the Tbx21 gene when compared to Ctrl mice (Figure 3G). Taken together, these data demonstrated that RSD impaired the development of fully functional CD4+ T cells.
We next evaluated the impact of RSD on CD4+ T cell differentiation into Tfh, a critical cellular population for GC formation. The differentiation of naive CD4+ T cells into the Tfh subpopulation is an intricate event coordinated by cytokines, such as IL-6 and IL-21 (Choi et al., 2013; Nurieva et al., 2008). CD4+ T cells, activated in the IL-6/21 context, start to express transcription factors, such as Bcl-6 and Maf (Nurieva et al., 2012; Kroenke et al., 2012). As a consequence, these cells increase the expression of cell surface molecules such as ICOS, PD-1, and CXCR5, which define the Tfh population (Vinuesa and Cyster, 2011). Although we did not detect a significant production of IL-21 by splenocytes upon in vitro specific recall (data not shown), IL-6 levels were lower in the RSD than in the Ctrl group (Figure 3H). Accordingly, flow cytometry analysis showed a reduction in the number of ICOS-expressing CD4+ T cells in RSD versus Ctrl splenocytes (Figure 3I). As a proof of concept, we used sorted CD4+ T cells to determine the expression of genes linked to Tfh differentiation such as Bcl-6, and Maf. Expression analysis showed that RSD downregulated Maf expression (Figure 3J), while we did not detect a significant difference in the levels of Bcl-6 transcripts between the Ctrl and RSD groups (Figure 3K). Overall, these data support the idea that RSD impaired factors associated with Tfh differentiation.
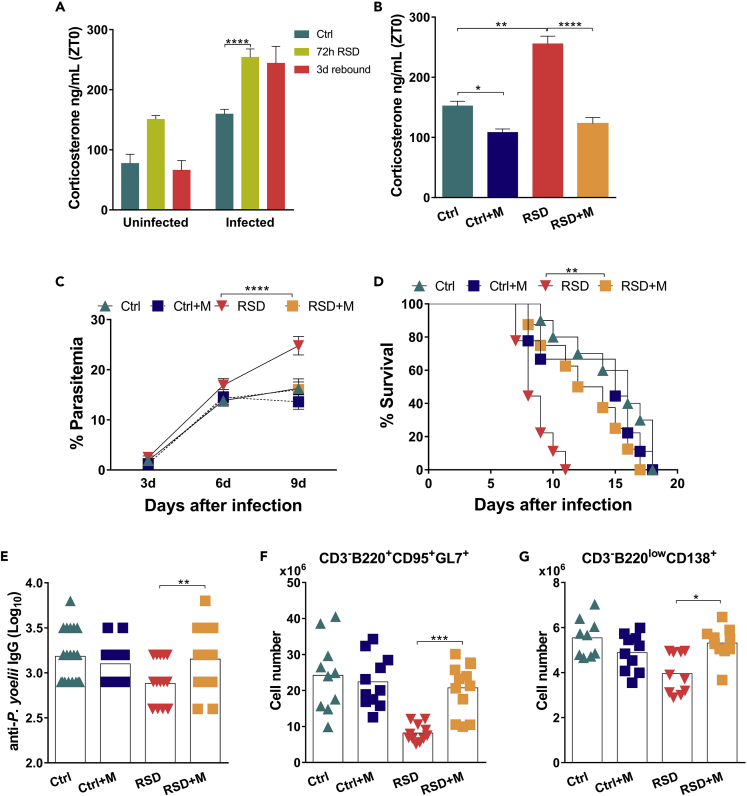
Exacerbated Synthesis of Glucocorticoids Triggered by RSD and P. yoelii Infection Inhibits Tfh Differentiation, GC Formation, and Protective Humoral Responses
The data outlined above indicated that sleep disturbance during the development of the immune response against malaria triggered an immunosuppressive response that impaired host resistance to infection. As we did not find a significant increase of suppressive cytokines, such as IL-10 (data not shown), we sought to explore other potential immunosuppressive mechanisms. A previous study demonstrated that the modified multiple platform method used for RSD activates the HPA axis, increasing Glc synthesis (Suchecki et al., 1998). In addition, infection itself is a Glc inducer. Physiological production of Glcs is required to contain immune-mediated tissue injury; however, it also compromises host immunity against pathogens (Jamieson et al., 2010).
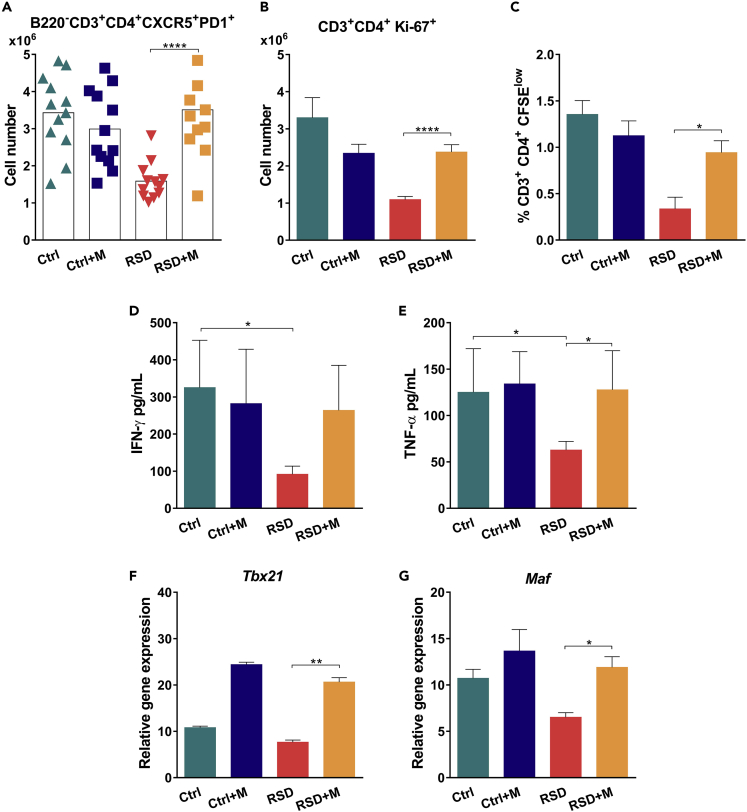
Although RSD and Plasmodium infection themselves resulted in Glc production, sleep disturbance during the early onset of infection exerts an additive effect, resulting in a systemic peak of Glc production (Figure 4A). Moreover, we observed that uninfected mice submitted to RSD restore normal levels of corticosterone in a few days; however, the higher corticosterone concentration in infected RSD mice persists even after 3 days of recovery (Figure 4A), suggesting a prolonged systemic exposure to Glcs. To explore the influence of the Glc peak on the development of host immunity against Plasmodium, we treated mice with metyrapone, an inhibitor of Glc synthesis (Igarashi et al., 2005; Macphee et al., 1989; Besedovsky et al., 2014). Metyrapone treatment reduced both infection and RSD-induced Glc levels (Figure 4B). Despite reducing infection-induced Glc synthesis in Ctrl mice, metyrapone treatment did not improve parasite burden control, indicating that the production of Glcs in response to infection did not impact immunity against malaria (Figure 4C). By contrast, in the infected-RSD group, inhibition of Glc synthesis increased resistance against infection, reducing parasitemia, and mice death (Figures 4C and 4D, respectively). This phenomenon was associated with an increase in specific antibody titers (Figure 4E) and a recovery in GC (Figure 4F) and plasma B cell numbers (Figure 4G). Also, following metyrapone treatment, the number of Tfh cells was restored (Figure 5A) and CD4+ T cell recovered their unspecific (Figure 5B) and parasite-specific (Figure 5C) proliferative responses. In line with these results, splenocytes from metyrapone-infected RSD (RSD + M) group produced higher levels of IFN-γ (Figure 5D) and TNF-α (Figure 5E). The Glc decay was associated with the recovery of Tbx21 and Maf transcript expression in sorted CD4+ T cells (Figures 5F and 5G, respectively). Overall, we conclude that inhibition of Glc synthesis recovered host functional immune response.
Figure 4.
Exacerbated Synthesis of Glucocorticoids Triggered by REM Sleep Deprivation and P. Yoelii Infection Inhibits GC Formation and Host Humoral Immunity
Inhibition of corticosterone synthesis was accomplished by treating mice with metyrapone during the RSD period (RSD + M). As an internal control of the drug effect on disease outcome, infected Ctrl received the same treatment (Ctrl + M).
(A) Corticosterone concentration in uninfected and infected mice immediately after RSD and after 3 days of sleep recovery (3d rebound). Data of uninfected Ctrl and uninfected RSD mice represent one experiment with at least 5 mice in each group. Mean ± SD; data from Ctrl and RSD infected mice are from two independent experiments with at least five mice in each group. Mean ± SEM (∗∗∗∗p < 0.0001) by two-way ANOVA.
(B) Corticosterone plasma levels in infected Ctrl and RSD mice after metyrapone treatment. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05; ∗∗p < 0.01; ∗∗∗∗p < 0.0001) by one-way ANOVA.
(C) Parasitemia levels in Ctrl and RSD mice treated or not with metyrapone. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗∗∗∗p < 0.0001) by two-way ANOVA.
(D) Mice survival following treatment with metyrapone. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗∗p < 0.01) by log-rank (Mantel-Cox) test.
(E) Anti-P. yoelii IgG1 serum titers in Ctrl and RSD mice treated or not with metyrapone. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗∗p < 0.01) by one-way ANOVA.
(F) Absolute numbers of splenic GC B cells (CD3-B220+GL7+CD95+) in Ctrl and RSD mice treated or not with metyrapone. Data are from two independent experiments with at least five mice in each group. Mean ± SEM (∗∗∗p < 0.001) by one-way ANOVA.
(G) Absolute numbers of splenic plasma B cells (CD3-B220lowCD138+) in Ctrl and RSD mice treated or not with metyrapone. Data are from two independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by one-way ANOVA.
Figure 5.
Exacerbated Synthesis of Glucocorticoids Triggered by REM Sleep Deprivation and P. Yoelii Infection Inhibits Tfh Cell Differentiation
Inhibition of corticosterone synthesis was accomplished by treating mice with metyrapone during the RSD period (RSD + M). Analyses were performed at day nine post infection. As an internal control of the drug effect on malaria outcome, infected Ctrl received the same treatment (Ctrl + M).
(A) Absolute numbers of splenic Tfh cells (B220−CD3+PD-1+CXCR5+) in Ctrl and RSD mice treated or not with metyrapone. Data are from two independent experiments with at least five mice in each group. Mean ± SEM (∗∗∗∗p < 0.0001) by one-way ANOVA.
(B) Ex vivo analysis of proliferating CD4+ T cells stained with Ki67+. Data are from two independent experiments with at least five mice in each group. Mean ± SEM (∗∗∗∗p < 0.0001).
(C) Frequency of specific proliferating (CFSElow) CD3+CD4+ T cells after culture in the presence of recombinant P. yoelii MSP-119 for five days. Data are from two independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by one-way ANOVA.
(D) Levels of IFN-γ in cell supernatants from Ctrl and RSD splenocytes after culture with recombinant P. yoelii MSP-119 for 48 hr. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by one-way ANOVA.
(E) Levels of TNF-α in cell supernatants from Ctrl and RSD splenocytes after culture with recombinant P. yoelii MSP-119 for 48 hr. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by one-way ANOVA.
(F) Tbx21 mRNA expression by sorted CD4+ T cells from Ctrl and RSD mice. Data are from two independent experiments with at least five mice in each group. Mean ± SEM (∗∗p < 0.01) by one-way ANOVA.
(G) Maf mRNA expression by sorted CD4+ T cells from Ctrl and RSD mice. Data are from two independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by one-way ANOVA.
Discussion
The notion that sleep disturbance reduces natural resistance against pathogens is part of folk wisdom, and a fact well documented by several studies (Besedovsky et al., 2019). However, the underlying mechanisms of these events remain poorly understood, primarily due to the difficulty of addressing the influence of a specific sleep stage in physiological processes as immune responses. Thus, it is a considerable challenge to depict the REM sleep influence on the immune system's activity.
To assess the active influence of REM sleep on the development of immunity against P. yoelii, we used the modified multiple platform method to selectively deprive mice of REM sleep at pre-determined periods. Although RSD has been linked to increased susceptibility to another species of murine malaria parasite, Plasmodium chabaudi (Lungato et al., 2015), the mechanisms underlying this phenomenon were not evaluated.
First, we observed that a time window between RSD and parasite inoculation constituted a turning point for host susceptibility. Starting RSD three days after infection resulted in the worst disease outcome, indicating that the immune context at this time point was the most affected by sleep disturbance. Previous studies demonstrated that protection against clinical malaria is dependent on the humoral responses (Cohen et al., 1961; Egan et al., 1996). In agreement with this notion, specific IgG titers inversely correlated with parasitemia levels in RSD mice. The key role of IgG in protection against the parasite was further confirmed by transferring total IgG from previously infected mice to RSD-infected mice. Therefore, our data indicated that in this specific time window, RSD impaired immunological events involved in the development of IgG-producing B cells.
In germinal centers, mature B cells differentiate into memory and plasma cells in a T cell-dependent reaction (Victora et al., 2010; Schwickert et al., 2011). Although experimental Plasmodium infection has been described to induce specific CD4+ T cell depletion (Xu et al., 2002), our ex vivo analysis did not reveal any increase in CD4+ T cell death. Instead, we found that CD4+ T cells displayed a reduced proliferative capacity both ex vivo and in vitro, to unspecific and specific stimuli. In parallel, T cells also exhibited impaired effector activity, characterized by a reduced production of proinflammatory cytokines, such as IFN-γ and TNF-α. As a consequence, B cells exhibited a reduced capacity to differentiate into plasma B cells, as demonstrated by the reduced numbers of GC B cells, IgG-producing B cells, and lower titers of IgG2b/c in RSD mice. Also, we did not find a significant difference in the number of Ki67+ B cells between the Ctrl and RSD groups (Figure S4D), suggesting that the problem within the B cell compartment was associated with the maturation process.
B cell maturation is strictly linked with the help of Tfh cells (Crotty, 2014). In this sense, we found that in parallel with the decrease in the T cell effector activity, RSD mice presented a reduced number of Tfh cells. A recent study reported that during Plasmodium infection, a dominant Tfh population emerges as soon as four days after infection (Arroyo and Pepper, 2019). In our model, this phase represents the beginning of the sleep disturbance period; therefore, we hypothesized that RSD during the acute phase of the immune response to malaria compromised both Tfh differentiation and Th1 polarization. This idea was reinforced by the fact that at day seven post infection, both Maf, a Tfh differentiation factor (Andris et al., 2017; Nurieva and Chung, 2010), and T-bet, a pro-Th1 transcription factor (Szabo et al., 2000; Saravia et al., 2019), were reduced in the RSD group.
As we did not find a significant production of the immunosuppressive cytokine IL-10 in RSD mice (data not shown), we explored other potential mechanisms, and Glcs (cortisol in humans and corticosterone in rodents) appeared as candidates based on their known immunosuppressive effects (Di Comite et al., 2007). Sleep loss has multiple effects on the homeostasis of multiple hypothalamic areas (Fifel et al., 2018). Stress is inherent to sleep disorders, leading to a potent increase in HPA axis activation and subsequently Glc release by the adrenal gland cortex (Balbo et al., 2010). The Glc release is the primary and major response to stressful events, and several studies have demonstrated that RSD potently increases corticosterone production (Galvao Mde et al., 2009; Andersen et al., 2005; Nunes et al., 2018; Zager et al., 2009). Stressful events may also include infectious processes. Malaria patients infected with P. vivax and P. falciparum have been found to present increased levels of blood cortisol (van Zon et al., 1982; Dekker et al., 1997; Davis et al., 1997; van Thien et al., 2001; Wilson et al., 2001; Blumer et al., 2005; Muehlenbein et al., 2005). Moreover, elevated cortisol levels were also reported in P. falciparum-infected patients with cerebral malaria (Blumer et al., 2005), while other studies correlated cortisol levels with increased parasitemia in pregnant women (Vleugels et al., 1989; Bouyou-Akotet et al., 2004, 2005; Adam et al., 2007). Studies in murine models of malaria showed that an elevation in corticosterone levels upon infection correlated with increased parasitemia (Barthelemy et al., 2004) and disease severity (Van Zon et al., 1983).
In agreement with these findings, we observed that P. yoelii infection, as well as RSD, increased the release of endogenous Glcs, albeit to a lesser extent. However, the combination of RSD and P. yoelii infection resulted in an additive effect, leading to the exacerbated production of Glcs. The exacerbated Glc production induced higher parasitemia, a lower survival rate, and inhibition of Tfh, GC, and plasma B cell differentiation. These results are in line with those of previous studies using influenza infection and stress-inducing models that demonstrated that, together, these stimuli exert an additive effect on Glc production and impair specific IgG production and immune cell distribution in the periphery (Hermann et al., 1994). Therefore, our data indicated the HPA axis hyperactivation is responsible for impairing host immune response to the parasite.
Endogenous physiological concentration of Glcs is an important mechanism for immune regulation during Plasmodium infection (Vandermosten et al., 2018) and also for antibody production (Shimba et al., 2018). However, the long-term continuous exposure to exacerbated levels of Glcs may potentially suppress T lymphocyte-mediated immune responses (Van Laethem et al., 2001a, 2001b; Ashwell et al., 2000; Kovacs, 2014).
The inhibition of Glcs synthesis by metyrapone (Besedovsky et al., 2014; Machado et al., 2013) did not influence the outcome of malaria infection in Ctrl mice, corroborating the idea that induction of Glcs synthesis by is a physiological event, insufficient to hamper host immunity. By contrast, in the RSD-infected group, metyrapone treatment restored parasite control and survival, which was associated with higher specific antibody titers when compared to non-treated RSD mice. Additionally, metyrapone-treated RSD mice displayed higher numbers of Tfh, GC, and plasma B cells. In vitro assays revealed that T cells from these mice also produced higher levels of IFN-γ and TNF-α and restored proliferative capacity upon stimulation with cognate antigens. Furthermore, Glcs have been described as important modulators for transcription factors, such as T-bet (Liberman et al., 2007) and Maf (Mao et al., 2007). We found that metyrapone treatment restored the expression of Tbx21 and Maf, indicating that high levels of endogenous Glcs hindered host immunity through the inhibition of these transcription factors.
Collectively, our findings provide evidence that exacerbated endogenous Glcs production, during the development phase of the B cell response due to REM sleep disturbance and infection, was the mechanism responsible for hampering host resistance to the parasite. Thus, Glc levels may be a potential target for predicting disease severity or developing new therapeutic strategies for disease management in endemic areas.
Limitations of the Study
The comprehension of the bidirectional relationship between the nervous and immune systems is essential for the development of new strategies to potentiate the immune response and mitigate its effects on sleep homeostasis and vice versa. Herein we showed the effect of RSD on the host response against the blood stage of the malaria parasite. Thus, further experiments are important to extend these findings to the Plasmodium complex life cycle and determine the impact of host immunity on sleep homeostasis and how to manipulate this cycle to improve disease management.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Daniela Santoro Rosa (dsrosa@unifesp.br).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
There are no data sets and/or code associated with the paper.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This research was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil (FAPESP, grants number 2017/17471-7 and 2019/11490-5), Conselho Nacional de Desenvolvimento Científico e Tecnológico/Instituto de Investigação em Imunologia, Brazil (CNPq, grant number 465434/2014-2), Associação Fundo de Incentivo à Pesquisa (AFIP) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance Code 001). E.R.F., M.L.B., F.B.S., J.S.A., M.L.A., S.T, S.B.B., A.C.K., and D.S.R. received fellowships from CNPq/FAPESP. M.P.A. received a fellowship from CAPES.
We thank Mr. Geová Santos and Waldermaks Leite for their assistance at the animal facility, Claudio Romero Farias Marinho (University of São Paulo) for providing Plasmodium yoelii 17XL GFP, Daniela Teixeira for FACS Sorting, and Eduardo Kinio Sugawara and José da Rocha Joaquim for corticosterone quantification.
Graphical abstract was created with BioRender.com.
Author Contributions
E.R.F., M.L.B., M.P.A., F.B.S., and J.S.A. performed experiments; E.R.F., S.B.B., A.C.K., and D.S.R. designed the experiments; E.R.F., A.C.K., and D.S.R. analyzed the data; A.C.K. and D.S.R. wrote the paper; S.B.B., S.T., and M.L.A. edited the paper.
Declaration of Interests
The authors declare no competing interests.
Published: October 23, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101599.
Contributor Information
Alexandre Castro Keller, Email: ackeller.unifesp@gmail.com.
Daniela Santoro Rosa, Email: dsrosa@unifesp.br.
Supplemental Information
References
- Adam I., Nour B.Y., Almahi W.A., Omer E.S., Ali N.I. Malaria susceptibility and cortisol levels in pregnant women of eastern Sudan. Int. J. Gynaecol. Obstet. 2007;98:260–261. doi: 10.1016/j.ijgo.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Andersen M.L., Martins P.J., D'almeida V., Bignotto M., Tufik S. Endocrinological and catecholaminergic alterations during sleep deprivation and recovery in male rats. J. Sleep Res. 2005;14:83–90. doi: 10.1111/j.1365-2869.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- Andris F., Denanglaire S., Anciaux M., Hercor M., Hussein H., Leo O. The transcription factor c-maf promotes the differentiation of follicular helper T cells. Front. Immunol. 2017;8:480. doi: 10.3389/fimmu.2017.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo E.N., Pepper M. B cells are sufficient to prime the dominant CD4(+) Tfh response to Plasmodium infection. J. Exp. Med. 2019;217:e20190849. doi: 10.1084/jem.20190849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley E.A., Pyae Phyo A., Woodrow C.J. Malaria. Lancet. 2018;391:1608–1621. doi: 10.1016/S0140-6736(18)30324-6. [DOI] [PubMed] [Google Scholar]
- Ashwell J.D., Lu F.W., Vacchio M.S. Glucocorticoids in T cell development and function∗. Annu. Rev. Immunol. 2000;18:309–345. doi: 10.1146/annurev.immunol.18.1.309. [DOI] [PubMed] [Google Scholar]
- Balbo M., Leproult R., Van Cauter E. Impact of sleep and its disturbances on hypothalamo-pituitary-adrenal axis activity. Int. J. Endocrinol. 2010;2010:759234. doi: 10.1155/2010/759234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthelemy M., Gabrion C., Petit G. Reduction in testosterone concentration and its effect on the reproductive output of chronic malaria-infected male mice. Parasitol. Res. 2004;93:475–481. doi: 10.1007/s00436-004-1160-2. [DOI] [PubMed] [Google Scholar]
- Besedovsky L., Lange T., Born J. Sleep and immune function. Pflugers Arch. 2012;463:121–137. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky L., Born J., Lange T. Endogenous glucocorticoid receptor signaling drives rhythmic changes in human T-cell subset numbers and the expression of the chemokine receptor CXCR4. FASEB J. 2014;28:67–75. doi: 10.1096/fj.13-237958. [DOI] [PubMed] [Google Scholar]
- Besedovsky L., Lange T., Haack M. The sleep-immune crosstalk in Health and disease. Physiol. Rev. 2019;99:1325–1380. doi: 10.1152/physrev.00010.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumer R.M., Van Thien H., Ruiter A.F., Weverling G.J., Vinh Thuan D., Endert E., Kager P.A., Sauerwein H.P. Adiponectin and glucose production in patients infected with Plasmodium falciparum. Metabolism. 2005;54:60–66. doi: 10.1016/j.metabol.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Bouyou-Akotet M.K., Issifou S., Meye J.F., Kombila M., Ngou-Milama E., Luty A.J., Kremsner P.G., Mavoungou E. Depressed natural killer cell cytotoxicity against Plasmodium falciparum-infected erythrocytes during first pregnancies. Clin. Infect. Dis. 2004;38:342–347. doi: 10.1086/380646. [DOI] [PubMed] [Google Scholar]
- Bouyou-Akotet M.K., Adegnika A.A., Agnandji S.T., Ngou-Milama E., Kombila M., Kremsner P.G., Mavoungou E. Cortisol and susceptibility to malaria during pregnancy. Microbes Infect. 2005;7:1217–1223. doi: 10.1016/j.micinf.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Boyle M.J., Reiling L., Feng G., Langer C., Osier F.H., Aspeling-Jones H., Cheng Y.S., Stubbs J., Tetteh K.K., Conway D.J. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity. 2015;42:580–590. doi: 10.1016/j.immuni.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattu V.K., Manzar M.D., Kumary S., Burman D., Spence D.W., Pandi-Perumal S.R. The global problem of insufficient sleep and its serious public Health implications. Healthcare (Basel) 2018;7:1. doi: 10.3390/healthcare7010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.S., Eto D., Yang J.A., Lao C., Crotty S. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J. Immunol. 2013;190:3049–3053. doi: 10.4049/jimmunol.1203032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Mc G.I., Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Di Comite G., Grazia Sabbadini M., Corti A., Rovere-Querini P., Manfredi A.A. Conversation galante: how the immune and the neuroendocrine systems talk to each other. Autoimmun. Rev. 2007;7:23–29. doi: 10.1016/j.autrev.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity. 2019;50:1132–1148. doi: 10.1016/j.immuni.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T.M., Li T.A., Tran Q.B., Robertson K., Dyer J.R., Phan T.D., Meyer D., Beaman M.H., Trinh K.A. The hypothalamic-pituitary-adrenocortical axis in severe falciparum malaria: effects of cytokines. J. Clin. Endocrinol. Metab. 1997;82:3029–3033. doi: 10.1210/jcem.82.9.4196. [DOI] [PubMed] [Google Scholar]
- Dekker E., Romijn J.A., Ekberg K., Wahren J., Van Thien H., Ackermans M.T., Thuy L.T., Chandramouli V., Kager P.A., Landau B.R., Sauerwein H.P. Glucose production and gluconeogenesis in adults with uncomplicated falciparum malaria. Am. J. Physiol. 1997;272:E1059–E1064. doi: 10.1152/ajpendo.1997.272.6.E1059. [DOI] [PubMed] [Google Scholar]
- Egan A.F., Morris J., Barnish G., Allen S., Greenwood B.M., Kaslow D.C., Holder A.A., Riley E.M. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J. Infect. Dis. 1996;173:765–769. doi: 10.1093/infdis/173.3.765. [DOI] [PubMed] [Google Scholar]
- Fifel K., Meijer J.H., Deboer T. Long-term effects of sleep deprivation on neuronal activity in four hypothalamic areas. Neurobiol. Dis. 2018;109:54–63. doi: 10.1016/j.nbd.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Galvao Mde O., Sinigaglia-Coimbra R., Kawakami S.E., Tufik S., Suchecki D. Paradoxical sleep deprivation activates hypothalamic nuclei that regulate food intake and stress response. Psychoneuroendocrinology. 2009;34:1176–1183. doi: 10.1016/j.psyneuen.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Grandner M., Mullington J.M., Hashmi S.D., Redeker N.S., Watson N.F., Morgenthaler T.I. Sleep duration and hypertension: analysis of > 700,000 adults by age and sex. J. Clin. Sleep Med. 2018;14:1031–1039. doi: 10.5664/jcsm.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim F., Wang Y., Zhang S.X., Zheng J., Yolcu E.S., Carreras A., Khalyfa A., Shirwan H., Almendros I., Gozal D. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Res. 2014;74:1329–1337. doi: 10.1158/0008-5472.CAN-13-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haus E.L., Smolensky M.H. Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med. Rev. 2013;17:273–284. doi: 10.1016/j.smrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Hermann G., Tovar C.A., Beck F.M., Sheridan J.F. Kinetics of glucocorticoid response to restraint stress and/or experimental influenza viral infection in two inbred strains of mice. J. Neuroimmunol. 1994;49:25–33. doi: 10.1016/0165-5728(94)90177-5. [DOI] [PubMed] [Google Scholar]
- Igarashi H., Medina K.L., Yokota T., Rossi M.I., Sakaguchi N., Comp P.C., Kincade P.W. Early lymphoid progenitors in mouse and man are highly sensitive to glucocorticoids. Int. Immunol. 2005;17:501–511. doi: 10.1093/intimm/dxh230. [DOI] [PubMed] [Google Scholar]
- Ishih A., Kawakami C., Todoroki A., Hirai H., Ohori K., Kobayashi F. Outcome of primary lethal and nonlethal Plasmodium yoelii malaria infection in BALB/c and IFN-gamma receptor-deficient mice following chloroquine treatment. Parasitol. Res. 2013;112:773–780. doi: 10.1007/s00436-012-3197-y. [DOI] [PubMed] [Google Scholar]
- Jamieson A.M., Yu S., Annicelli C.H., Medzhitov R. Influenza virus-induced glucocorticoids compromise innate host defense against a secondary bacterial infection. Cell Host Microbe. 2010;7:103–114. doi: 10.1016/j.chom.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs W.J. To B or not to B? Glucocorticoid impact on B lymphocyte fate and function. Endocrinology. 2014;155:339–342. doi: 10.1210/en.2013-2085. [DOI] [PubMed] [Google Scholar]
- Kroenke M.A., Eto D., Locci M., Cho M., Davidson T., Haddad E.K., Crotty S. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J. Immunol. 2012;188:3734–3744. doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J.M., Rector D.M., Churchill L. Sleep and cytokines. Sleep Med. Clin. 2007;2:161–169. doi: 10.1016/j.jsmc.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup S.P., Butler N.S., Harty J.T. T cell-mediated immunity to malaria. Nat. Rev. Immunol. 2019;19:457–471. doi: 10.1038/s41577-019-0158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laethem F., Baus E., Andris F., Urbain J., Leo O. A novel aspect of the anti-inflammatory actions of glucocorticoids: inhibition of proximal steps of signaling cascades in lymphocytes. Cell. Mol. Life Sci. 2001;58:1599–1606. doi: 10.1007/PL00000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laethem F., Baus E., Smyth L.A., Andris F., Bex F., Urbain J., Kioussis D., Leo O. Glucocorticoids attenuate T cell receptor signaling. J. Exp. Med. 2001;193:803–814. doi: 10.1084/jem.193.7.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange T., Dimitrov S., Fehm H.L., Born J. Sleep-like concentrations of growth hormone and cortisol modulate type1 and type2 in-vitro cytokine production in human T cells. Int. Immunopharmacol. 2006;6:216–225. doi: 10.1016/j.intimp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Liberman A.C., Refojo D., Druker J., Toscano M., Rein T., Holsboer F., Arzt E. The activated glucocorticoid receptor inhibits the transcription factor T-bet by direct protein-protein interaction. FASEB J. 2007;21:1177–1188. doi: 10.1096/fj.06-7452com. [DOI] [PubMed] [Google Scholar]
- Lungato L., Gazarini M.L., Paredes-Gamero E.J., Tufik S., D'almeida V. Paradoxical sleep deprivation impairs mouse survival after infection with malaria parasites. Malar. J. 2015;14:183. doi: 10.1186/s12936-015-0690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q.Q., Yao Q., Lin L., Chen G.C., Yu J.B. Sleep duration and total cancer mortality: a meta-analysis of prospective studies. Sleep Med. 2016;27-28:39–44. doi: 10.1016/j.sleep.2016.06.036. [DOI] [PubMed] [Google Scholar]
- Machado R.B., Tufik S., Suchecki D. Role of corticosterone on sleep homeostasis induced by REM sleep deprivation in rats. PLoS One. 2013;8:e63520. doi: 10.1371/journal.pone.0063520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macphee I.A., Antoni F.A., Mason D.W. Spontaneous recovery of rats from experimental allergic encephalomyelitis is dependent on regulation of the immune system by endogenous adrenal corticosteroids. J. Exp. Med. 1989;169:431–445. doi: 10.1084/jem.169.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X., Stewart A.K., Hurren R., Datti A., Zhu X., Zhu Y., Shi C., Lee K., Tiedemann R., Eberhard Y. A chemical biology screen identifies glucocorticoids that regulate c-maf expression by increasing its proteasomal degradation through up-regulation of ubiquitin. Blood. 2007;110:4047–4054. doi: 10.1182/blood-2007-05-088666. [DOI] [PubMed] [Google Scholar]
- Mesin L., Ersching J., Victora G.D. Germinal center B cell dynamics. Immunity. 2016;45:471–482. doi: 10.1016/j.immuni.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlenbein M.P., Alger J., Cogswell F., James M., Krogstad D. The reproductive endocrine response to Plasmodium vivax infection in Hondurans. Am. J. Trop. Med. Hyg. 2005;73:178–187. [PubMed] [Google Scholar]
- Nevin R.L., Croft A.M. Psychiatric effects of malaria and anti-malarial drugs: historical and modern perspectives. Malar. J. 2016;15:332. doi: 10.1186/s12936-016-1391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes J.O.F., Apostolico J.S., Andrade D.A.G., Ruiz F.S., Fernandes E.R., Andersen M.L., Keller A.C., Rosa D.S. Sleep deprivation predisposes allergic mice to neutrophilic lung inflammation. J. Allergy Clin. Immunol. 2018;141:1018–1027 e4. doi: 10.1016/j.jaci.2017.06.025. [DOI] [PubMed] [Google Scholar]
- Nurieva R.I., Chung Y. Understanding the development and function of T follicular helper cells. Cell Mol Immunol. 2010;7:190–197. doi: 10.1038/cmi.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R.I., Chung Y., Hwang D., Yang X.O., Kang H.S., Ma L., Wang Y.H., Watowich S.S., Jetten A.M., Tian Q., Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R.I., Podd A., Chen Y., Alekseev A.M., Yu M., Qi X., Huang H., Wen R., Wang J., Li H.S. STAT5 protein negatively regulates T follicular helper (Tfh) cell generation and function. J. Biol. Chem. 2012;287:11234–11239. doi: 10.1074/jbc.M111.324046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opp M.R. Sleeping to fuel the immune system: mammalian sleep and resistance to parasites. BMC Evol. Biol. 2009;9:8. doi: 10.1186/1471-2148-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.R., Malhotra A., Gao X., Hu F.B., Neuman M.I., Fawzi W.W. A prospective study of sleep duration and pneumonia risk in women. Sleep. 2012;35:97–101. doi: 10.5665/sleep.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather A.A., Janicki-Deverts D., Hall M.H., Cohen S. Behaviorally assessed sleep and susceptibility to the common cold. Sleep. 2015;38:1353–1359. doi: 10.5665/sleep.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabanayagam C., Shankar A. Sleep duration and cardiovascular disease: results from the national Health interview survey. Sleep. 2010;33:1037–1042. doi: 10.1093/sleep/33.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanni L.A., Fonseca L.F., Langhorne J. Mouse models for erythrocytic-stage malaria. Methods Mol. Med. 2002;72:57–76. doi: 10.1385/1-59259-271-6:57. [DOI] [PubMed] [Google Scholar]
- Saravia J., Chapman N.M., Chi H. Helper T cell differentiation. Cell. Mol. Immunol. 2019;16:634–643. doi: 10.1038/s41423-019-0220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann C., Kunisaki Y., Frenette P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013;13:190–198. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwickert T.A., Victora G.D., Fooksman D.R., Kamphorst A.O., Mugnier M.R., Gitlin A.D., Dustin M.L., Nussenzweig M.C. A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J. Exp. Med. 2011;208:1243–1252. doi: 10.1084/jem.20102477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T., Min M., Sun C., Zhang Y., Liang M., Sun Y. Does insomnia predict a high risk of cancer? A systematic review and meta-analysis of cohort studies. J. Sleep Res. 2020;29:e12876. doi: 10.1111/jsr.12876. [DOI] [PubMed] [Google Scholar]
- Shimba A., Cui G., Tani-Ichi S., Ogawa M., Abe S., Okazaki F., Kitano S., Miyachi H., Yamada H., Hara T. Glucocorticoids drive diurnal oscillations in T cell distribution and responses by inducing interleukin-7 receptor and CXCR4. Immunity. 2018;48:286–298 e6. doi: 10.1016/j.immuni.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Suchecki D., Lobo L.L., Hipolide D.C., Tufik S. Increased ACTH and corticosterone secretion induced by different methods of paradoxical sleep deprivation. J. Sleep Res. 1998;7:276–281. doi: 10.1046/j.1365-2869.1998.00122.x. [DOI] [PubMed] [Google Scholar]
- Szabo S.J., Kim S.T., Costa G.L., Zhang X., Fathman C.G., Glimcher L.H. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Van Thien H., Ackermans M.T., Dekker E., Thanh Chien V.O., Le T., Endert E., Kager P.A., Romijn J.A., Sauerwein H.P. Glucose production and gluconeogenesis in adults with cerebral malaria. QJM. 2001;94:709–715. doi: 10.1093/qjmed/94.12.709. [DOI] [PubMed] [Google Scholar]
- Tran T.M., Ongoiba A., Coursen J., Crosnier C., Diouf A., Huang C.Y., Li S., Doumbo S., Doumtabe D., Kone Y. Naturally acquired antibodies specific for Plasmodium falciparum reticulocyte-binding protein homologue 5 inhibit parasite growth and predict protection from malaria. J. Infect. Dis. 2014;209:789–798. doi: 10.1093/infdis/jit553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermosten L., Pham T.T., Knoops S., De Geest C., Lays N., Van Der Molen K., Kenyon C.J., Verma M., Chapman K.E., Schuit F. Adrenal hormones mediate disease tolerance in malaria. Nat. Commun. 2018;9:4525. doi: 10.1038/s41467-018-06986-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora G.D., Schwickert T.A., Fooksman D.R., Kamphorst A.O., Meyer-Hermann M., Dustin M.L., Nussenzweig M.C. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa C.G., Cyster J.G. How T cells earn the follicular rite of passage. Immunity. 2011;35:671–680. doi: 10.1016/j.immuni.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Vleugels M.P., Brabin B., Eling W.M., De Graaf R. Cortisol and Plasmodium falciparum infection in pregnant women in Kenya. Trans. R. Soc. Trop. Med. Hyg. 1989;83:173–177. doi: 10.1016/0035-9203(89)90632-9. [DOI] [PubMed] [Google Scholar]
- Wilson M., Davis T.M., Binh T.Q., Long T.T., Danh P.T., Robertson K. Pituitary-adrenal function in uncomplicated falciparum malaria. Southeast Asian J. Trop. Med. Public Health. 2001;32:689–695. [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2019. World Malaria Report. [Google Scholar]
- Xu H., Wipasa J., Yan H., Zeng M., Makobongo M.O., Finkelman F.D., Kelso A., Good M.F. The mechanism and significance of deletion of parasite-specific CD4(+) T cells in malaria infection. J. Exp. Med. 2002;195:881–892. doi: 10.1084/jem.20011174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zager A., Andersen M.L., Lima M.M., Reksidler A.B., Machado R.B., Tufik S. Modulation of sickness behavior by sleep: the role of neurochemical and neuroinflammatory pathways in mice. Eur. Neuropsychopharmacol. 2009;19:589–602. doi: 10.1016/j.euroneuro.2009.03.005. [DOI] [PubMed] [Google Scholar]
- van Zon A.A., Eling W.M., Hermsen C.C., Koekkoek A.A. Corticosterone regulation of the effector function of malarial immunity during pregnancy. Infect. Immun. 1982;36:484–491. doi: 10.1128/iai.36.2.484-491.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zon A.A., Eling W.M., Hermsen C.C., van de Wiel T.J., Duives M.E. Malarial immunity in pregnant mice, in relation to total and unbound plasma corticosterone. Bull. Soc. Pathol. Exot. Filiales. 1983;76:493–502. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
There are no data sets and/or code associated with the paper.