This study characterized the function of antimicrobial-resistant phenotypes attributed to plasmid-encoded guanidinium-selective small multidrug resistance (Gdm/SugE) efflux pumps. These sequences are frequently monitored as biocide resistance markers in antimicrobial resistance surveillance studies. Our findings reveal that enterobacterial gdm sequences transmitted on plasmids possess a guanidine II riboswitch, which restricts transcript translation in the presence of guanidinium. Cloning and overexpression of this gdm sequence revealed that it confers higher resistance to quaternary ammonium compound (QAC) disinfectants (which possess guanidium moieties) when grown as biofilms. Since biofilms are commonly eradicated with QAC-containing compounds, the presence of this gene on plasmids and its biofilm-specific resistance are a growing concern for clinical and food safety prevention measures.
KEYWORDS: small multidrug resistance (SMR), efflux pump, biocide, disinfectant, antiseptic, quaternary ammonium compound (QAC), guanidinium, riboswitch, multidrug resistance plasmids, Gdx, SugE, biofilm, disinfectant resistance, plasmid, small multidrug resistance proteins
ABSTRACT
Members of the small multidrug resistance (SMR) efflux pump family known as SugE (recently renamed Gdx) are known for their narrow substrate selectivity to small guanidinium (Gdm+) compounds and disinfectant quaternary ammonium compounds (QACs). Gdx members have been identified on multidrug resistance plasmids in Gram-negative bacilli, but their functional role remains unclear, as few have been characterized. Here, we conducted a survey of sequenced proteobacterial plasmids that encoded one or more SugE/Gdx sequences in an effort to (i) identify the most frequently represented Gdx member(s) on these plasmids and their sequence diversity, (ii) verify if Gdx sequences possess a Gdm+ riboswitch that regulates their translation similarly to chromosomally encoded Gdx members, and (iii) determine the antimicrobial susceptibility profile of the most predominate Gdx member to various QACs and antibiotics in Escherichia coli strains BW25113 and KAM32. The results of this study determined 14 unique SugE sequences, but only one Gdx sequence, annotated as “SugE(p),” predominated among the >140 plasmids we surveyed. Enterobacterales plasmids carrying sugE(p) possessed a guanidine II riboswitch similar to the upstream region of E. coli gdx. Cloning and expression of sugE(p), gdx, and emrE sequences into a low-copy-number expression vector (pMS119EH) revealed significant increases in QAC resistance to a limited range of detergent-like QACs only when gdx and sugE(p) transformants were grown as biofilms. These findings suggest that sugE(p) presence on proteobacterial plasmids may be driven by species that frequently encounter Gdm+ and QAC exposure.
IMPORTANCE This study characterized the function of antimicrobial-resistant phenotypes attributed to plasmid-encoded guanidinium-selective small multidrug resistance (Gdm/SugE) efflux pumps. These sequences are frequently monitored as biocide resistance markers in antimicrobial resistance surveillance studies. Our findings reveal that enterobacterial gdm sequences transmitted on plasmids possess a guanidine II riboswitch, which restricts transcript translation in the presence of guanidinium. Cloning and overexpression of this gdm sequence revealed that it confers higher resistance to quaternary ammonium compound (QAC) disinfectants (which possess guanidium moieties) when grown as biofilms. Since biofilms are commonly eradicated with QAC-containing compounds, the presence of this gene on plasmids and its biofilm-specific resistance are a growing concern for clinical and food safety prevention measures.
INTRODUCTION
Small multidrug resistance (SMR) proteins are a family of multidrug-selective efflux pumps found in bacteria and archaea. They are small proteins (100 to 150 amino acids) composed of 4 transmembrane (TM) α-helices that dimerize to form a functional proton motive force-driven efflux pump in the plasma membrane (1). Depending on their subclass designation, SMR members can confer resistance to a variety of cation-containing chemical compounds, including antiseptic and surfactant quaternary ammonium compounds (QACs) (benzalkonium [BZ] and cetrimide [CET]), QAC interchelating dyes (acriflavine and ethidium), and small guanidinium (Gdm+) molecules (2–5). SMR proteins can be divided into three major subclasses based on their sequence homology and their substrate selectivity, as follows. (i) Members of the small multidrug protein (SMP) subclass confer resistance to a wide range of QACs and lipophilic dyes from the expression of a single gene and have homology to Escherichia coli EmrE (reviewed in references 2 to 4). (ii) The suppressor of groEL mutations (SUG) subclass was recently renamed to “riboswitch-regulated Gdm+ exporters” (GDM) (5). The GDM subclass was originally named “suppressor of the groEL mutations” based on an incorrectly identified groEL cloning artifact associated with the E. coli sugE (gdx) gene (2, 4). (iii) Paired small multidrug resistance proteins (PSMRs) are SMR members that require the expression of two separate proteins that dimerize in the membrane to form a heterologous dimeric efflux pump with substrate selectivity similar to that of either the SMP or GDM subclass based on their sequence homology (4, 5).
Studies characterizing GDM member structure and function are limited compared to those characterizing SMP members due to the narrow substrate selectivity of known GDM members. However, GDM members have recently gained renewed attention based on their association with riboswitches that regulate their translation (6). E. coli Gdx (formerly named Sug or SugE) is one of the most well characterized GDM subclass members and confers resistance to low-molecular-weight Gdm+ compounds (5) as well as a narrow range of QAC antiseptic detergents, which include cetyltrimethylammonium (CTAB) and cetylpyridinium (CTP) when overexpressed (2, 4, 7, 8). Recent studies of representative SMR protein family members have shown that chromosomally encoded GDM members are regulated exclusively by one of three Gdm+ binding riboswitch classes (guanidine classes I, II, and III) (5, 6). Gdm+ riboswitch sequences are located in the 5′ untranslated region (5′UTR) of the mRNA transcript and regulate mRNA translation when in the presence of increased Gdm+ concentrations. In the presence and absence of Gdm+, each riboswitch class adopts a distinct “P” stem-loop arrangement that results in different stem-loop configurations, regulating protein translation (reviewed in reference 6). All chromosomally encoded GDM members are believed to possess a Gdm+ riboswitch and have been shown to phylogenetically group apart from SMP members into separate clades based on their riboswitch class (2, 5). The close association between the 5′UTR and GDM sequence highlights the importance of monitoring both the riboswitch region and GDM gene sequence when assessing efflux pump function (5). gdx genes transmitted by plasmids are predicted to lack Gdm+ riboswitches based on limited phylogenetic and sequence surveys, where they are hypothesized to be expressed exclusively by plasmid promoters (5); however, experimental evidence in support of this has not been shown.
The aims of this study were to perform in-depth analysis of GDM sequence diversity on multidrug resistance plasmids in Gram-negative bacilli to address three primary knowledge gaps: (i) to identify frequently detected GDM sequences transmitted on plasmids; (ii) to determine if GDM sequences carried on plasmids possess any known Gdm+ riboswitch sequences; and (iii) to characterize the antiseptic substrate selectivity of the most frequently identified GDM genes from plasmids in E. coli K-12 strains. For the first aim, bioinformatic GDM sequence surveys of publicly available proteobacterial plasmid sequence databases were used to identify predominant GDM sequences. For the second aim, we performed sequence searches of collected plasmidic gdx sequences to identify if they possessed one of three known Gdm+ riboswitch classes within their 500-nucleotide (nt) upstream region. Lastly, for the third aim, we cloned two of the most frequently identified gdx sequences into the expression vector pMS119EH and transformed them into Escherichia coli K-12 strains BW25113 and KAM32 to determine their antiseptic substrate selectivity. Both vector-transformed strains were used for antimicrobial susceptibility testing (AST) against a library of 13 antimicrobials (9 QACs, 1 bisbiguanide, 1 aminoglycoside, and 1 macrolide) grown as planktonic cultures (broth), colonies (agar spots), and biofilm (minimal biofilm eradication concentration [MBEC] device; Innovotech Inc., Calgary, Canada). Different growth methods were used to determine if GDM-selective antimicrobial resistance phenotypes differed during various modes of growth. The outcome of this study identified that only 14 unique GDM sequences are transmitted by the proteobacterial plasmids we analyzed. Only one GDM member, SugE(p), was frequently detected in Enterobacterales and possessed a guanidine II riboswitch. Cloning and expression of Enterobacterales SugE(p) revealed that this gene significantly increased E. coli biofilm tolerance to a limited range of QAC and antibiotic compounds compared to other SMR members or GDM members grown planktonically or as colonies. Since many QACs are routinely used as antiseptics and disinfectants (9) and are frequently used to eradicate biofilms (10, 11), the outcome of this study reveals greater insights into how the acquisition of GDM members on plasmids may impact antiseptic tolerance mechanisms in Gram-negative species.
RESULTS AND DISCUSSION
Bioinformatic sequence surveys identify a predominant GDM sequence among gammaproteobacterial plasmids.
To identify the most frequently detected GDM sequences transmitted on Gram-negative plasmids, we performed bioinformatic GDM sequence surveys of publicly available multidrug resistance proteobacterial plasmid sequence databases and identified a total of 137 proteobacterial plasmid sequences with one or more GDM sequences. From this analysis, a total of 14 unique GDM sequences were detected, where the majority (87%) of these sequences were annotated as “SugE” (Fig. 1A). Phylogenetic analysis the 14 plasmid-encoded GDM sequences revealed that the majority (10/14) separated into two distinct subclades: (i) a gammaproteobacterium-enriched clade with close homology to E. coli Gdx (accession no. NP_418572.4), with ≥79% pairwise identity, and (ii) a taxonomically diverse subclade that includes plasmids isolated from Rhizobiales, Bacteroides, and Morganella, all with lower pairwise sequence identity to each other (39 to 65% identity) and to E. coli Gdx (41 to 65% identity) (Fig. 1A). Interestingly, only one surveyed GDM sequence, SugE(p) (accession no. YP_002302254.1), was frequently identified in this survey (115/137 total GDM-containing plasmids) and from the most diverse proteobacterial orders (Fig. 1B). This indicates that a single GDM sequence is repeatedly detected in plasmids, as opposed to a variety of distinct SMR genes reported for “qac” SMP members, which have a number of plasmidic variants (qacED1, qacE, qacF, qacG, and qacH) (reviewed in reference 12). However, the majority of SugE(p) sequences were identified primarily from Enterobacterales E. coli and Salmonella enterica plasmids (Fig. 1B; see Table S1 in the supplemental material). Since SugE(p) was detected frequently (78%) on plasmids from species isolated from contaminated foods and retail animal (primarily poultry) sources (Table S1), this suggests that GDM sequence enrichment occurs on plasmids from these species, making SugE(p) one of the more relevant and noteworthy GDM efflux pump isoforms to functionally characterize.
FIG 1.
Analysis of the amino acid sequence variation and phylogenetic associations between identified GDM members encoded on Gram-negative plasmids. (A) A PhyML dendrogram of the 14 distinct GDM sequences identified from a tBLASTn survey of sequenced proteobacterial plasmids containing at least one GDM sequence. E. coli EmrE served as the outgroup for the analysis. Branching confidence is indicated beside each node and is based on 100 bootstrapped replicates. Subclades 1 and 2 within the dendrogram are indicated by blue and green, respectively. A summary of the different proteobacterial taxonomic orders with a plasmid-encoded GDM sequence is listed on the y axis of the bar chart (right) linked to the dendrogram (individual sequence details are listed in Table S1 in the supplemental material). (B) A COBALT multiple-protein sequence alignment of the 14 GDM amino acid sequences identified in this study. Yellow-highlighted regions indicate 100% amino acid sequence identity within the alignment generated via Jalview software v. 2.10.5 (51).
The second most frequently identified GDM sequence from plasmids was 100% identical to the archetypical chromosomally encoded E. coli Gdx (accession no. NP_418572.4) (Fig. 1B). This sequence was detected in ≤10 plasmids, indicating that the chromosomally carried gdx in E. coli is also mobilized on plasmids. Multiple-sequence alignments of the remaining 12 unique GDM protein sequences demonstrated closer sequence identity to SugE(p) or low sequence identity (25 to 30%) compared to the archetypical E. coli Gdx sequence and grouped outside subclades 1 and 2 or with subclade 2 (Fig. 1). These remaining GDM sequences were typically identified from a single plasmid (or <5 unique plasmids). It should be noted here that sequencing of multidrug resistance plasmids is highly biased toward gammaproteobacterial pathogens. Since plasmid sequences from nosocomial and nonpathogenic species are less abundant in these databases at the time of study, further interpretation of these surveyed sequence results cannot be confidently conducted.
The identification of a single predominate GDM sequence, SugE(p), on various plasmids (Fig. 1) was an important finding for a number of reasons. As we observed in our analysis, the SugE(p) sequence had high similarity (82.8% sequence identity) to the second-most-predominate GDM, Gdx, as well as to 4 other sequences located in the same subclade 1. Although SugE(p) was identified at 100% amino acid sequence identity in various proteobacterial orders, including Vibrionales and Aeromonadales, in our study, it predominated within Enterobacterales plasmids isolated from E. coli and Salmonella enterica. It is noteworthy that 1.2% of the sugE(p) nucleotide sequences we surveyed had synonymous nucleotide changes, suggesting that this gene did not encounter any mutations altering its amino acid translation. Furthermore, the sugE(p)-carrying plasmids we identified in this analysis were not identical in sequence, had different incompatibility groups, and possessed different antimicrobial resistance genes (Table S1), indicating that sugE(p) was conserved and mobilized compared to other plasmid regions. Nearly 2/3 of these plasmids were isolated from species found as contaminants in foods and meats (Table S1) sugE(p) identified in our survey was identical in sequence to gdx/sugE genes identified from previously sequenced plasmid and integron surveillance studies of Salmonella and E. coli (13, 14). In these studies, the sugE(p) sequence was frequently identified on different plasmids as well as mobile genetic elements as part of a four-gene cluster consisting of genes for (i) a Tn5 transposase (tnpA), (ii) the β-lactamase CMY-2 (blaCMY-2), and (iii) an outer membrane lipoprotein (blc). We also frequently observed this partial or complete four-gene cluster in our study [93% of sugE(p)-containing plasmids], where sugE(p) was detected on plasmids from other species, such as Vibrio and Aeromonas species (see Fig. S1 in the supplemental material). Altogether, this highlights the importance of sugE(p) due to its presence in this gene cluster and for its potential role in antimicrobial resistance and virulence.
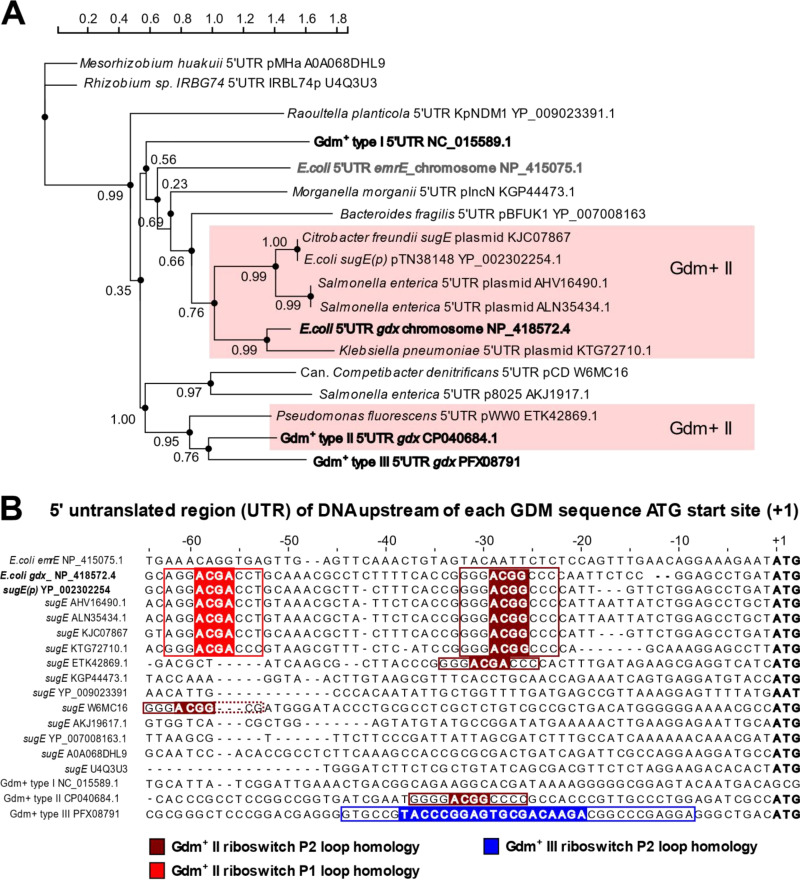
Many Enterobacterales plasmid-encoded GDM sequences possess a guanidine II riboswitch.
To determine if GDM sequences carried on plasmids possess any known Gdm+ riboswitch sequences, we conducted a phylogenetic analysis of the 500-nucleotide (nt) 5′UTRs corresponding to each plasmid-encoded GDM sequence. This analysis revealed a cluster of 6 unique Enterobacterales sugE sequences, including sugE(p), that possessed high overall sequence identity (≥85%) to the chromosomal E. coli gdx 5′UTR guanidine II riboswitch (Fig. 2A). These riboswitch sequences were located close to the ATG start site, within the 60-nt region upstream from the ATG site (Fig. 2B). A second guanidine II riboswitch was also identified on a plasmid from Pseudomonas fluorescens (accession no. ETK42869.1) with high confidence (E value of ≤1.0 × 10−79) to the characterized 5′UTR of P. aeruginosa gdx (6, 15) (Fig. 2A). However, the riboswitch region of the P. fluorescens sequence was located further upstream from the aligned ATG start site, similar to P. aeruginosa riboswitch regions; the P1 stem-loop was located at nt −73 to −65 and the P2 stem-loop at nt −35 to −25 within the 500-nt alignment. Hence, some of the plasmidic GDM sequences possessed a guanidine II riboswitch region. Other GDM sequences, which are not highlighted in Fig. 2A, were obtained from enterobacterial species (Morganella, Bacteroides, Raoultella, and Salmonella), alphaproteobacterial Rhizobiales species, and “Candidatus Competibacter denitrificans” plasmids. These sequences demonstrated poor homology to any other characterized Gdm+ riboswitch class using BLASTn searches or phylogenetic analyses (Fig. 2A), i.e., class I Desulfotomaculum ruminis Gdx (accession no. AEG60294.1) and class III Nocardia facinica EmrE (accession no. PFX08791) (15, 16). It is unclear if these GDM sequences are transcriptionally regulated by any guanidinium riboswitch sequence or if they possess an uncharacterized riboswitch region. As many GDM sequences were notably surrounded by putative transposon and integron elements under closer sequence inspection, it is possible that promoter regions associated with these regions play a role in the expression of these genes, similar to SMP genes.
FIG 2.
Phylogenetic and nucleotide sequence analysis of the 5′UTR region upstream of the 14 GDM sequences identified from various proteobacterial plasmids. (A) Maximum-likelihood dendrogram of aligned 500-nucleotide upstream sequences to determine their phylogenetic relationship to one of three Gdm+ riboswitch sequences (indicated in bold). Confidence intervals associated with each branched node are shown below nodes (ranging from 0.0 to 1.0). The 500-nucleotide regions with high confidence and sequence identity to the 5′UTR of the guanidine II riboswitch region of E. coli gdx (accession no. NP_418572.4) are highlighted in pink. The 500-nucleotide 5′UTR regions corresponding to other known Gdm+ riboswitch classes (I to III) are highlighted in bold and were included as riboswitch references. (B) Multiple-sequence alignment of the region upstream of nucleotide −65 of each GDM sequence identified in this study. Gdm+ riboswitch regions corresponding to various classes (I and II) are colored to show sequence identity to a respective riboswitch stem-loop region (P1 or P2) indicated below the alignment. Guanidine I riboswitch class Gdm+ P1 and P2 regions are not highlighted since their sequence motifs occurred more than −100 nucleotide positions in the upstream alignment and showed poor sequence alignment/identity to any GDM sequence in this study.
The fact that most GDM members had high sequence similarity to a characterized guanidine II riboswitch region, particularly SugE(p) as well as GDM members with close sequence homology to E. coli Gdx, is an important finding for a number of reasons (Fig. 1 and 2). First, this finding suggests that Gdx sequence diversification and recombination may be occurring within the GDM gene sequences carried by plasmids but not to the extent that they lose their riboswitch regions. Second, this finding suggests that Gdm+ molecules may also be recognized by and regulate plasmidic GDM efflux pumps, which has yet to be demonstrated. Lastly, with respect to QAC activation of riboswitches, QAC binding to any guanidinium II riboswitches has not been convincingly demonstrated by any experiment, specifically, in vitro RNA riboswitch binding assays. Only small Gdm+ molecules with the fewest chemical modifications, such amino-guanidinium and methyl-guanidinium, have demonstrated high-affinity binding to riboswitch RNA compared to Gdm+ (17). It is unclear if QAC exposure of proteobacteria in possession of riboswitch-regulated GDM sequences on plasmids could possibly trigger Gdm+ riboswitch activation. Since QACs are substituted amine cations [N+(CH3)4] compared to a guanidinium cation [C(NH2)3+] and only small guanidinium molecules have been shown to easily access and bind “P” stem-loops of the riboswitch (5, 15, 17), QAC degradation into smaller amine components may have an indirect impact on riboswitch activation. A recent study identified that a single P2 loop (ACGR→ACGG) transversion mutation to the riboswitch of E. coli gdx could increase tolerance to imidazolium ionic liquids which contain QAC salt solutions (18). It is important to note that QACs are anaerobically and aerobically degraded into to small substituted amine molecules by abiotic actions as well as bacteria that mediate the degradation of QACs in polluted environments over time (19). This process involves various cellular oxidative pathways that rely on the nucleophilic attack of either the alkyl chain or nitrogen moieties of the QAC, ultimately shortening, oxidizing, and hydroxylating the compounds into mixtures of smaller quaternary amine compounds (19). Hydroxylated substituted amines and nitrosamines derived from QAC breakdown may interfere in amino acid and guanidine biosynthesis pathways (20), potentially altering intracellular guanidinium and toxic amine metabolism and production levels that could impact riboswitches. Unfortunately, other studies of QAC degradation products and their impacts on cell physiology are not available. Hence, more metabolic studies of QACs, their breakdown products, and their role in guanidium riboswitch regulation are warranted. Therefore, the presence and maintenance of these Gdm+-sensing systems on plasmids may be beneficial for cells that inherit them, particularly among species that persist in QAC-exposed environments.
AST of overexpressed GDM genes in E. coli K-12 BW25113 shows increased QAC-resistant phenotypes when strains were grown as biofilms.
To determine how plasmid-acquired GDM sequences may influence antimicrobial susceptibility to QACs and antibiotics, sugE(p) and E. coli gdx and emrE sequences were cloned and overexpressed in a low-copy-number vector, pMS119EH. This vector was used in previous AST experiments to determine antimicrobial resistance phenotypes for archetypical E. coli SMR members, including emrE and gdx (3, 4). Both sugE(p) and gdx genes were cloned without their 5′UTR region in order to assess their antimicrobial substrate selection of the efflux pump without the need for additional Gdm+ supplementation. We deliberately chose to omit the riboswitch for two important reasons. First, antimicrobial cations with amine and polyamine moieties have the potential to nonspecifically interact with RNA (21) and interfere with Gdm+-regulated riboswitch SMR gene translation, leading to toxic efflux pump accumulation as added drug concentrations increase. Second, removal of the riboswitch allowed us to determine the substrate selectivity without the uncertain involvement or influence of the QACs and other antimicrobials we tested, based on the recent findings by Higgins et al. (18). This study examined guanidine II riboswitch activation of E. coli gdx by imidazole and QAC solutions. E. coli emrE and gdx sequences were included in our AST analysis to serve as SMR family member reference controls, in addition to the negative-control empty parental vector pMS119EH (Tables 1 and 2). Three different AST experiments involving the same library of 13 different antimicrobial compounds (Table 3) were performed in E. coli strains BW25113 and KAM32. Each AST was performed using broth microdilution, agar spot plating, and MBEC biofilm techniques to represented planktonic, colony, and biofilm growth physiologies, respectively (Tables 1 and 2). All MIC and MBEC values calculated from AST experiments are summarized in Table 3. Our rationale for comparing each AST growth technique is based on the current bias toward only colony and planktonic AST of QACs and antimicrobials, despite our knowledge that biofilms are a representative physiology outside laboratory environments (22, 23). Additionally, QACs are frequently used to inhibit and eradicate biofilms (10, 11). Hence, a comparison of growth physiologies is essential to evaluate how GDM-containing plasmids may alter bacterial growth.
TABLE 1.
Strains used in this study
| E. coli K-12 strain | Genotype | Resistance marker | Reference |
|---|---|---|---|
| BW25113 | F− Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) λ− rph-1 Δ(rhaD-rhaB)568 hsdR514 | 42 | |
| JW0451 | F− Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) ΔacrB747::kan λ− rph-1 Δ(rhaD-rhaB)568 hsdR514 | Kanamycin | 42 |
| KAM32 | F− Δ(lac-pro) supE thi hsdΔ5/F′ traΔ36 proA+B+ lacIq lacZΔM15 ΔacrAB ΔmdtK/ydhE | 43 | |
| TG1 | F− supE thi-1 Δ(lac-proAB) Δ(mcrB-hsdSM)5 (rK− mK−) | 43 |
TABLE 2.
Plasmids used in this study
| Plasmid | Gene or DNA region cloned | GenBank protein accession no. | Parental vector | Reference |
|---|---|---|---|---|
| pMS119EH | pJF119EH | 40 | ||
| pEmrE | emrEa | NP_415075.1 | pMS119EH | 58 |
| pSugE(p)b | sugE(p)b | YP_002302254.1 | pMS119EH | This study |
| pGdxb | gdx/sugEa | NP_418572.4 | pMS119EH | 8 |
Gene was directionally cloned in frame with the Ptac promoter into the multiple-cloning site of pMS119EH at 5′ XbaI and 3′ HindIII restriction sites.
Gene was directionally cloned in frame with the Ptac promoter into the multiple-cloning site of pMS119EH at 5′ XbaI and 3′ PstI restriction sites.
TABLE 3.
Summary of AST MIC and MBEC values determined for E. coli BW25113 transformants grown as planktonic (broth), colony (agar), and biofilm (MBEC) cultures
| Growth type and transformed plasmid | Mean MIC or MBEC (μg/ml)a
of: |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTP | CET | BZ | DDAB | CDEB | CTAB | DOM | ET | AC | MV | CHX | ERY | TOB | |
| Agarb | |||||||||||||
| pMS119EH | 8 | 64 | 16 | 4 | 4 | 64 | 32 | 256 | 80 | 320 | 8 | 64 | 4 |
| pEmrE | 8 | 64 | 32 | 8 | 8 | 64 | 32 | >1,024 | 320 | 640 | 8 | 32 | 4 |
| pGdx | 8 | 64 | 32 | 4 | 4 | 128 | 32 | 512 | 160 | 640 | 8 | 64 | 4 |
| pSugE(p) | 8 | 64 | 32 | 4 | 4 | 128 | 32 | 512 | 160 | 320 | 8 | 64 | 8 |
| Brothb | |||||||||||||
| pMS119EH | 10 | 14 | 10 | 1.5 | 8 | 2 | 8 | NAd | NA | 512 | 1 | 38 | 4 |
| pEmrE | 14 | 14 | 10 | 2 | 8 | 4 | 8 | NA | NA | >1,024 | 2 | 38 | 4 |
| pGdx | 14 | 14 | 10 | 2 | 8 | 4 | 8 | NA | NA | 768 | 1 | 38 | 5 |
| pSugE(p) | 14 | 14 | 10 | 2 | 8 | 4 | 8 | NA | NA | 512 | 1 | 38 | 4 |
| MBECc | |||||||||||||
| pMS119EH | 64 | 32 | 64 | 13 | 16 | 128 | 32 | 3,750 | 64 | 4,096 | 128 | 1,024 | 4 |
| pEmrE | 256 | 64 | 256 | 32 | 106 | 32 | 48 | 7,500 | 512 | 16,384 | 256 | 2,048 | 8 |
| pGdx | 1,024 | 256 | 32 | 13 | 64 | 128 | 24 | 6,250 | 256 | 2048 | 128 | 2,048 | 4 |
| pSugE(p) | 256 | 256 | 32 | 13 | 64 | 512 | 24 | 6,250 | 171 | 16,384 | 128 | 2,048 | 8 |
For agar and broth, values are MIC (n = 3); for MBEC, values are MBEC (n = 6). Abbreviations: CTP, cetylpyridinium chloride; CET, cetrimide bromide; BZ, benzalkonium chloride; DDAB, didecyldimethylammonium bromide; CDEB, cetyldimethylethylammonium bromide; CTAB, cetyltrimethylammonium bromide; DOM, domiphen bromide; ET, ethidium bromide; MV, methyl viologen dichloride; CHX, chlorhexidine dichloride; AC, acriflavine; ERY, erythromycin; TOB, tobramycin. Boldface indicates MIC or MBEC values >2-fold from their respective pMS119EH control.
Plasmid-transformed cultures (10−4 dilutions) adjusted to an OD600 of 1.0 as a starting inoculum.
Twenty-four-hour biofilms that formed on MBEC device pegged lids were incubated with antimicrobials at increasing concentrations for 24 h.
NA, data not available because drug concentration absorbance values exceeded detection thresholds.
The results of our planktonic and agar colony AST analyses for BW25113 transformants of pSugE(p) or pGdx demonstrated insignificant MIC values (within a 2-fold MIC value of the parental vector) for all of the antimicrobials we tested, with the exception of the pEmrE transformant (Table 3). As emrE overexpression has been shown to confer resistance to QACs by planktonic and colony AST methods in previous studies (4, 24), we have greater confidence in our MIC results. In contrast to previous QAC agar colony AST studies of overexpressed gdx/sugE in E. coli DH5α (7), our colony AST results did not identify significant MIC values toward any QAC by either sugE(p) or gdx. This outcome would appear to agree with findings from Kermani et al. that would suggest that these proteins exclusively export Gdm+ (5). However, only BW25113 biofilm AST experiments showed significantly reduced (≥4-fold change) susceptibility to various QACs when transformed with pEmrE, pSugE(p), or pGdx vectors compared to the parental vector (Table 3). BW25113 transformants of pEmrE, pSugE(p), or pGdx grown as biofilms increased MBEC values for cetylpyridinium (CTP), cetrimide (CET), and cetyldimethylethylammonium bromide (CDEB) (Table 3), as previously observed in a colony AST study by Chung and Saier (7). GDM members also conferred tolerance to particular substrates tested, where pSugE(p) conferred enhanced resistance toward acriflavin (AC) and pGdx increased resistance to methyl viologen (MV), which are known to be associated with EmrE (3, 5). These MBEC values also reaffirmed previous findings that indicate that GDM members recognize and transport a narrower substrate range than EmrE (3, 5).
Taking the results together, we observed significantly increased QAC tolerance by BW25113 transformants expressing sugE(p) and gdx to the broadest range of QAC detergents and dyes when grown as biofilms. Since biofilms represent the most recalcitrant antimicrobial-resistant growth physiology and are also commonly eradicated with disinfectant QACs, our findings show that the acquisition and expression of plasmidic GDM members enhance QAC tolerance in “wild-type” E. coli. The fact that this enhanced tolerance is detected only in E. coli transformants grown as biofilms may reflect the many differences in transcriptional regulation of metabolic pathways (that may involve Gdm+ metabolism) between planktonic, colony, and biofilm growth, as observed in previous studies of Gram-negative Pseudomonas aeruginosa (25). It is important to note again that we removed the riboswitch regions from all of our GDM sequences to identify the substrate recognition profile of overexpressed sugE(p) and gdx. Future experiments involving the activation of guanidine II riboswitch regions in the presence of QACs commonly used as commercial and clinical disinfectants/antiseptics may be a valuable direction to explore in biofilm eradication research. In our study, GDM members conferred tolerance to many detergent-based QACs (CET, BZ, CTP, CDEB, and didecyldimethylammonium bromide [DDAB]) (Table 3), all of which are extensively used to disinfect food and meat preparation surfaces, particularly for poultry processing (26, 27). GDM members have been associated with a growing increase in QAC tolerance and resistance by clinically relevant proteobacteria (28–31). However, the role that Gdm+ compounds plays in antimicrobial resistance that is mediated by efflux pumps such as GDM is less clear. In the study by Kermani et al., transports of QAC and labeled 14C-Gdm+ were compared in vitro using radioactive liposome transport assays (5). Among the guanidinyl and quaternary amine compounds tested, only two multiple-aryl-ring-containing QACs, ethidium (ET) and tetraphenylphosphonium, were tested. Neither QAC could significantly compete with 14C-Gdm+ in these transport assays (5); it is possible that ET was transported during these assays but not recorded, as only labeled 14C-Gdm+ was monitored. It is worth noting that previous studies of GDM members E. coli and Citrobacter freundii Gdx/SugE in vitro have demonstrated ET binding to GDM members (32). AST studies of GDM members from Enterobacter cloacae and Aeromonas molluscorum identified that overexpressing both genes conferred low-level resistance to ET (2-fold increase), where A. molluscorum sugE conferred tolerance to the toxic tin-containing quaternary cation compounds di- and tri-butyltin (33, 34). Although ET is a commonly used efflux pump substrate, its mutagenic and genotoxic properties in cultures make it a less reliable QAC for in vivo AST experiments. Hence, small guanidinyl compounds, QACs, and their degradation products, as well as other quaternary cationic compounds to which proteobacteria are exposed, may be factors that favor the transmission of plasmid-encoded GDM members. This is worth experimentally pursuing further to improve our understanding of riboswitch regulation and efflux substrate selectivity related to antimicrobial resistance and environmental pollution.
AST of KAM32 transformant GDM strains identifies QACs with altered resistance and susceptibility profiles.
Since the activity of SMR efflux pumps may be masked by the dominant resistance-nodulation-division (RND) AcrAB efflux pump system, as observed in a previous study (24), we repeated all AST experiments in the E. coli strain KAM32. KAM32 is a commonly used E. coli strain for efflux pump characterization due to its lack of RND efflux pumps, acrB, and multidrug and toxin extruder (MATE) family member mdtK (Table 4). Similar to the case for previous studies of KAM32 (35), we observed reduced growth fitness in our transformants due to the loss of these efflux pumps; all KAM32 transformants we tested showed lower MIC and MBEC values for the same antimicrobials than the BW25113 strain (Table 4). Significantly increased MIC values were identified for KAM32 transformants pSugE(p) and pGdx grown as agar colonies, but not broth cultures, than for the parental vector (Table 4). Enhanced MIC values to both CTP and AC were detected by agar spot colony AST of KAM32 transformants pSugE(p) and pGdx compared to those for the parental vector (Table 4). All KAM32 transformants grown as biofilms demonstrated significantly increased MBEC values to interchelating QAC dyes (ET and AC) compared to those for the parental vector strain (Table 4), indicating that overexpression of all three SMR efflux pumps compensates for the loss of efflux pumps in the presence of QAC interchelating dyes. Unexpectedly, all three KAM32 SMR transformants grown as biofilms had significantly reduced MBEC values for CTP compared to those of the parental vector (Table 4). Additionally, KAM32 transformants pSugE(p) and pGdx grown as biofilms also exhibited a unique susceptibility to specific QACs; CET and DDAB susceptibility was observed with pGdx transformants, whereas BZ susceptibility was demonstrated with pSugE(p) (Table 4). The KAM32 AST results indicate that the loss of dominant efflux pumps produces different antimicrobial resistance phenotypes when expressed in cells growing under different physiologies.
TABLE 4.
Summary of AST MIC and MBEC values determined for E. coli KAM32 transformants grown as planktonic (broth), colony (agar spot), and biofilm (MBEC) cultures
| Growth type and transformed plasmid | Mean MIC or MBEC (μg/ml)a
of: |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTP | CET | BZ | DDAB | CDEB | CTAB | DOM | ET | AC | MV | CHX | ERY | TOB | |
| Agarb | |||||||||||||
| pMS119EH | 2 | 1 | 1 | 0.5 | 2 | 4 | 2 | 12 | 1 | 80 | 4 | 2 | 4 |
| pEmrE | 8 | 4 | 2 | 2 | 8 | 8 | 4 | 256 | 8 | 160 | 4 | 2 | 2 |
| pGdx | 8 | 2 | 1 | 0.5 | 4 | 8 | 2 | 16 | 4 | 320 | 4 | 2 | 4 |
| pSugE(p) | 8 | 2 | 2 | 0.5 | 8 | 4 | 2 | 16 | 4 | 80 | 4 | 2 | 4 |
| Brothb | |||||||||||||
| pMS119EH | 1.2 | 2 | 2 | 0.5 | 2 | 1 | 1 | 16 | 8 | 80 | 1 | 1.5 | 2 |
| pEmrE | 2.4 | 4 | 2 | 1 | 2 | 1 | 1.5 | 64 | 64 | 320 | 1 | 1 | 2.5 |
| pGdx | 1.6 | 4 | 2 | 1 | 2 | 1 | 1.5 | 16 | 8 | 160 | 1 | 2 | 2 |
| pSugE(p) | 2.4 | 4 | 1 | 1 | 2 | 1 | 1.5 | 16 | 8 | 80 | 1 | 1 | 2 |
| MBECc | |||||||||||||
| pMS119EH | 128 | 16 | 32 | 16 | 16 | 8 | 8 | 64 | 32 | 4,096 | 32 | 64 | 3 |
| pEmrE | 32 | 32 | 16 | 16 | 64 | 8 | 26 | 512 | 256 | 16,384 | 64 | 128 | 10 |
| pGdx | 32 | <8 | 32 | 4 | 13 | 32 | 13 | 341 | 64 | 16,384 | 64 | 85 | 8 |
| pSugE(p) | 32 | 32 | 8 | 8 | 11 | 8 | 26 | 512 | 53 | 16,384 | 64 | 64 | 4 |
For agar and broth, values are MIC (n = 3); for MBEC, values are MBEC (n = 6). Abbreviations: CTP, cetylpyridinium chloride; CET, cetrimide bromide; BZ, benzalkonium chloride; DDAB, didecyldimethylammonium bromide; CDEB, cetyldimethylethylammonium bromide; CTAB, cetyltrimethylammonium bromide; DOM, domiphen bromide; ET, ethidium bromide; MV, methyl viologen dichloride; CHX, chlorhexidine dichloride; AC, acriflavine; ERY, erythromycin; TOB, tobramycin. Boldface indicates MIC or MBEC values >2-fold from their respective pMS119EH control.
Transformant cultures (10−4 dilutions) adjusted to an OD600 of 1.0 as a starting inoculum.
Twenty-four-hour biofilms grown on MBEC device pegged lids incubated with drug for 24 h in LB broth.
To ensure that a lack of SMR protein accumulation was not the cause of insignificant broth AST MIC values, we isolated E. coli membranes from broth cultures of BW25113 and KAM32 transformants for sodium dodecyl sulfate-Tricine-polyacrylamide gel electrophoresis (SDS-Tricine-PAGE) analysis (see Fig. S3 in the supplemental material). PAGE confirmed that 12-kDa bands corresponding to the estimated size of each overaccumulated SMR protein were detectable in both BW25113 and KAM32 transformant membrane extracts in comparison to control empty pMS119EH transformants. Furthermore, we also verified that the concentrations for IPTG (isopropyl-β-d-thiogalactopyranoside) induction selected for this study did not result in toxic efflux pump overexpression in BW25113 or KAM32 transformant broth cultures, based on 24-h growth curves (see Fig. S2 in the supplemental material). Optical densities at 600 nm (OD600) demonstrated that at final IPTG concentrations of ≤0.05 mM, E. coli transformant broth cultures of pGdx, SugE(p), and pEmrE did not significantly alter growth curves compared to those with the parental vector control (Fig. S2).
The main finding from the E. coli BW25113 and KAM32 AST results was that QAC-resistant phenotypes due to GDM member overexpression was most prominent when cultures were grown as biofilms (Tables 3 and 4). The insignificant MIC values corresponding to broth-based AST methods with BW25113 are not surprising considering how planktonic growth offers bacteria little physical surface area protection from membrane-disrupting QACs in comparison to agar-spotted colonies or surface-attached biofilms (36). Significantly increased MIC values (≤2-fold increase) to QACs were detected in GDM vector-transformed E. coli AST only when grown as agar-spotted colonies and only by the KAM32 strain, which lacks dominant efflux pumps (ΔacrB and ΔmdtK) (Table 4). Our AST findings support previous results of AST for GDM members in KAM32 strains, which typically uses agar plating methods due to the chemical solubility and absorbance properties of many QACs (8, 37, 38). Most antimicrobial compound AST methods favor microbroth-based AST for their preparation speed, but in our study, nearly all QAC-resistant phenotypes associated with GDMs were less likely to be observed under broth-based screening conditions.
E. coli KAM32 pSugE(p) transformants demonstrate significantly increased biofilm formation.
To determine if SMR gene overexpression altered biofilm formation, 24-h biofilms of BW25113 and KAM32 transformants were grown without antimicrobial exposure. Biofilm accumulation on the pegged lids of MBEC devices was used to quantify total biofilm biomass using a standard crystal violet (CV) staining assay. Both BW25113 and KAM32 transformants showed no significant reduction of biofilm biomass based on CV stain A550 values compared to their respective parental vector controls (Fig. 3A and B). However, BW25113 pGdx and the KAM32 pSugE(p) transformants demonstrated significantly increased biomass formation (1.5- to 2-fold increase; P < 0.05) compared to the pMS119EH control (Fig. 3B). This result suggests that GDM expression can promote biofilm biomass formation depending on which efflux pump is expressed. To verify that the deletion of the dominant RND efflux pump acrB was not an influential factor in biofilm biomass formation, BW25113 biofilm formation was compared to that of JW0451 (ΔacrB) and KAM32 was compared to its respective parental strain, TG1 (Fig. 3C); in this comparison, no significant differences in biomass were observed for any strain combination.
FIG 3.
(A and B) Determination of biofilm biomass of strain BW25113 (A) and KAM32 (B) SMR transformants based on CV staining absorbance at 550 nm. (C) Determination of biofilm biomass of E. coli strains BW25113, JW0451 (ΔacrB), TG1, and KAM32 (ΔacrB ΔmdtK). For all panels, biofilm biomass measurements were taken after 24 h of growth at 37°C from CV-stained MBEC pegged-lid devices. *, P < 0.05 compared to either the pMS119EH (parental vector) control or the respective parental strain controls within the chart.
Therefore, GDMs encoded on plasmids transmitted among enterobacterial species frequently exposed to QACs and Gdm+ molecules may drive their selection on plasmids. The gain of GDM efflux pumps may benefit proteobacterial species when grown as antimicrobial-resistant biofilms to persist under toxic cationic conditions. A correlation between sugE(p) identification and biofilm formation among E. coli isolates collected from retail chicken was recently shown by Sun et al. and highlights an association between GDMs, QACs, and biofilms (38). Based on that study, E. coli isolates that possessed sugE(p) and were grown as biofilms demonstrated eradication at significantly higher QAC concentrations than susceptible control strains (38). Previous studies showed that Salmonella and E. coli isolates with GDM sequences collected from food and clinical samples and grown as biofilms were also harder to eradicate with commonly used QACs (38, 39), suggesting a role for GDM members in QAC tolerance.
Conclusions.
This study has identified and characterized a frequently identified GDM member transmitted on enterobacterial plasmids, referred to as sugE(p). By comparing this efflux pump’s features to other members of the SMR family, the sugE(p) sequence was found to share the closest similarities to E. coli gdx. Specifically, sugE(p) possesses a guanidine II riboswitch region and resists QAC eradication at higher concentrations than controls when overexpressed during biofilm growth. This study also identified a number of GDM members that were less frequently detected (<5 plasmids) from other proteobacterial phyla and orders, where each GDM member lacked confident riboswitch sequence identification and possessed low sequence identity and conservation compared to E. coli gdx. Given their presence on plasmids carrying multidrug resistance genes, these low-sequence-identity GDM members may be worth further experimental characterization to unravel their regulation and substrate selectivity on these plasmids.
MATERIALS AND METHODS
Strains and drugs used.
All E. coli strains examined in this study are listed in Table 1. Antimicrobial compounds tested in this study were obtained from either Tokyo Chemical Industry Co. (Portland, OR) or Millipore-Sigma (Burlington, MA). All antimicrobial stocks were prepared in sterile distilled water at 50-mg/ml stock concentrations (unless otherwise specified): cetylpyridinium chloride (CTP), cetrimide bromide (CET), benzalkonium chloride (BZ), didecyldimethylammonium bromide (DDAB), cetyldimethylethylammonium bromide (CDAB), cetyltrimethylammonium bromide (CTAB), domiphen bromide (DOM), ethidium bromide (ET), methyl viologen dichloride (MV), chlorhexidine dichloride in 95% ethanol (CHX) (0.25 mg/ml), acriflavine (ACR), erythromycin (ERY) (20 mg/ml), and tobramycin (TOB) (20 mg/ml).
SMR gene vector cloning and growth conditions used.
Plasmid-carried SMR nucleotide sequences corresponding to SugE(p) (accession no. YP_002302254.1), E. coli Gdx (NP_418572.4), and E. coli EmrE (NP_415075.1) were gene synthesized and cloned into pUC-57 by BioBasic Inc. Gene Synthesis Services (Markham, ON, Canada) (Table 2). Each gene was subcloned into the multiple-cloning region of 5′ XbaI and 3′ HindIII [or 3′ PstI for sugE(p)] restriction sites of the low-copy-number vector pMS119EH (40) for IPTG-inducible Ptac promoter expression using previously described cloning procedures (41). All vectors were constructed and manipulated in E. coli strain DH5α (E. coli Genetic Stock Center, Yale University). The sequence accuracy of each vector was verified using Sanger sequencing services from Eurofins Genomics (MWG Operon, CA) with forward and reverse sequencing primers used to subclone each gene. Plasmids were transformed into the E. coli K-12 wild-type BW25113 strain (42) as well as an efflux pump-deficient strain, KAM32 (acrAB mdtK) (43) (Table 1), for all AST experiments described below. All strains were grown in Luria-Bertani (LB) broth with a 100-μg/ml final concentration of ampicillin (LB-AMP) with 0.05 mM IPTG to maintain the transformed vector and induce expression of the cloned efflux pump gene, unless otherwise specified. Optimal IPTG induction to express SMR genes from the pMS119EH vector below a toxic threshold was determined using 24-h growth curve analysis in the presence of increasing IPTG concentrations in LB-AMP broth, as summarized in Fig. S2 in the supplemental material.
Determination of SMR protein accumulation.
SMR protein accumulation from extracted cell total membranes of each plasmid-transformed BW25113 and KAM32 strain was performed and confirmed using 16% acrylamide sodium dodecyl sulfate (SDS)-Tricine-PAGE analysis with the E. coli cell membrane extraction procedure described previously (35). Briefly, 500-ml cultures of each transformant strain were grown to mid-log phase (OD600 = 0.5) in LB-AMP with 0.05 mM IPTG, and cells were pelleted by centrifugation at 6,000 rpm in a JA-10 rotor in a Beckman Avanti-J-E centrifuge, washed with SMR-A buffer (50 mM morpholinepropanesulfonic acid [MOPS], 8% [vol/vol] glycerol, 5 mM EDTA, 1 mM dithiothreitol [DTT], pH 7), resuspended at 2 ml of SMR-A buffer per gram of cell mass, and then frozen at −80°C. Phenylmethylsulfonyl fluoride (10 mM) was added to thawed cell pellets at 1 μl per ml of cell slurry and then immediately passed through a French press (30,000-lb/in2 SimAminco cylinder) twice at 1,500 lb/in2 per pressing. The pressed slurry was centrifuged at 10,000 rpm in a JA-20 rotor to remove cell debris, and then the supernatant was ultracentrifuged at 40,000 rpm for 90 min in a type 70Ti rotor in a Beckman Optima XPN-100 centrifuge. The ultracentrifuged membrane pellets were washed with SMR-A buffer, resuspended in 2 ml of SMR-A buffer, and then frozen at −80°C at 10 mg/ml membrane protein, and protein concentrations were determined by a modified Lowry assay (44).
Each thawed total membrane protein extract was loaded into a 16% acrylamide SDS-Tricine-polyacrylamide gel at final total amount of 25 μg protein/well. Each loaded membrane protein extract was mixed with Tricine-PAGE sample loading dye (100 mM DTT, 150 mM Tris-HCl [pH 7], 12% [wt/vol] SDS, 30% [wt/vol] glycerol, 0.05% Coomassie brilliant blue G250 dye) at a sample/loading dye volume ratio of 3:1 prior to gel loading. Ten microliters of Bio-Rad low-range unstained protein standard was also loaded onto one lane of each gel to estimate sample protein band molecular weight in kilodaltons. Protein bands were visualized after 5 min of UV light irradiation (at a wavelength of 310 nm) using 0.5% (vol/vol) 2,2,2-trichloroethanol (TCE) staining, as TCE was added to the unpolymerized gel mixture during casting. Band intensity from gel images was quantified by densitometry using ImageJ (45). Polyacrylamide gel images and densitometry data are provided in Fig. S3 in the supplemental material.
Broth microdilution AST.
All AST analyses were performed using overnight (18-h) cultures of each transformant, which were inoculated in 96-well microplates for AST at 37°C with shaking at 155 rpm. Overnight cultures were standardized to an optical density at 600 nm (OD600) of 1.0 prior to their final dilutions for each method. Broth microdilution AST of plasmid-transformed E. coli strains (BW25113 and KAM32) was performed as described previously (46). Briefly, 20 μl of standardized overnight cultures was diluted 10−3 into 180 μl of fresh LB-AMP medium for a final in-well volume of 200 μl and final culture concentration of 10−4. Each row of a 96-well Nunc flat-bottom polystyrene microplate contained log2 dilution gradients of 1 of the 13 antimicrobial stock solutions listed above. Each microplate had wells containing uninoculated medium and antimicrobial drug as baseline controls. Growth was based on OD600 measurements using a Multiskan Spectrum UV/visible wavelength region microplate reader (Thermo Scientific, Waltham, MA). Three biological replicates of each strain were tested in duplicate (n = 6) and statistically assessed using the two-way Mann-Whitney test to determine significant P value differences compared to the parental vector. MIC values for each transformant were determined to be the lowest antimicrobial concentration that resulted in an OD600 value that was indistinguishable from that of the drug control well containing uninoculated medium.
Agar spot dilution AST.
Agar dilution AST of SMR plasmid transformants (BW25113 or KAM32) was performed as described previously (46) on LB-AMP agar petri plates to maintain expression vectors. Overnight cultures were standardized as described for broth microdilution AST and were diluted to 10−4 for spot plating. A sterilized Boekel Scientific 48-stainless-steel-pin replicator was used to spot 1 μl of diluted culture onto LB-AMP agar plates, with each plate containing a log2 serial dilution of the antimicrobial stock concentration and 0.05 mM IPTG. Agar dilution plates were incubated overnight for 24 h. Growth of each spot was scored by visual inspection, where the MIC for each transformant was determined to be the antimicrobial concentration that resulted in no visible colony growth. A minimum of three biological replicates were measured for each transformant and compared to the same strain transformed with the parental vector pMS119EH (Tables 3 and 4). A two-way Mann-Whitney test was used to determine significant P value differences compared to the parental vector.
Biofilm AST and biofilm biomass CV staining methods.
Minimum biofilm eradication concentration (MBEC) determination for each SMR-transformed BW25113 and KAM32 E. coli strain was performed with 96-pegged-lid MBEC assay devices (Innovotech, AB, Canada) as described previously (47), with the following modifications. LB-AMP with 0.05 mM IPTG was used for all MBEC experiments, and inoculated MBEC device plates were always incubated at 37°C with shaking at 155 rpm for 24 h. The 24-h-biofilm-coated pegged lids were rinsed twice in sterile phosphate-buffered saline (PBS) and either stained with 0.5% (wt/vol) crystal violet (CV) for biomass determination as described previously (47) or aseptically transferred into a new 96-well microplate containing fresh sterile LB-AMP with log2 dilutions of antimicrobial stock solutions for MBEC determination. Incubation was at 37°C with shaking (155 rpm) for another 24 h, and any biofilms that remained after drug exposure were harvested by aseptically washing the lids in sterile PBS twice and transferred to a new 96-well plate containing 200 μl recovery medium. Sonication of the pegs in a new plate containing 200 μl recovery medium was performed with a Branson 3800 sonicating water bath, and sonicated biofilms recovered from the pegs in the 96-well plate were spot plated onto LB-AMP agar plates using the 48-steel-pin-replicator (1 μl transfer volume/pin) and incubated for 24 h at 37°C to determine cell viability per peg based on colony formation; the lowest antimicrobial concentration that prohibited colony growth was defined as the MBEC. A total of 6 biological replicates were measured for each plasmid-transformed strain. A 2-tailed Mann-Whitney test calculation was used to determine statistically significantly differences in MBEC values for the same E. coli strain transformed with each SMR vector compared to the strains with the empty parental vector pMS119EH.
GDM sequence surveys from accessible online databases.
Searches of sequenced proteobacterial plasmids carrying one or more GDM nucleotide sequences were conducted using the NCBI (https://www.ncbi.nlm.nih.gov/), UniProt (https://www.uniprot.org/), INTEGRALL (48), and BacMet (49) databases with query sequence E. coli Gdx (accession no. NP_418572.4) using tBLASTn sequence similarity searches (50). A total of 387 proteobacterial plasmids with identified GDM protein sequences were obtained, and only 137 were determined to be unique based on multiple-sequence alignments of the 387 plasmids using the Jalview software version 2.10.5 package (51). Protein sequences were aligned using the online server COBALT (52), and it was determined that only 14 GDM protein sequences had unique sequence identity (<99%) (see Table S1 and Fig. S2 in the supplemental material).
Sequence alignments of 500-nt upstream GDM regions.
The 500-nt upstream regions corresponding to each GDM protein sequence were aligned relative to each GDM gene +1 reading frame and their start site (ATG/GTG) within the alignment generated from EMBL-EBI Clustal Omega software (ClustalO; https://www.ebi.ac.uk/Tools/msa/clustalo). Riboswitch sequence identity to previously characterized Gdm+ class I-III riboswitch regions was determined using a downloaded BLAST+ software package (53) BLASTn program where each riboswitch class was used as a query sequence to search the 139-nucleotide data set (Gdm+ riboswitch accession numbers are listed Fig. 2). BLASTn E values of ≥1.0 × 10−5 were applied as cutoffs to confidently identify the presence of a riboswitch region within the 500-nucleotide range.
Phylogenetic analysis.
Phylogenetic analysis of the alignment of the final 14 GDM protein sequences was performed using the online Phylogenetic Maximum Likelihood (PhyML) v 3.0 program (54–56; http://www.atgc-montpellier.fr/phyml/), using the smart model selection (SMS) mode (57) with Bayesian information criterion (BIC) and 100 bootstrap replicates to determine confidence at specific nodes in the generated dendrogram. The 500-nucleotide upstream region alignment to identify riboswitch homology was also run using these conditions, but aBayes was selected to determine confidence intervals at dendrogram nodes. During PhyML estimations of protein and nucleotide sequences, both BIC and Akaike information criterion (AIC) SMS methods were used, and both resulted in identical clusters; BIC analyses are shown in all dendrograms.
Supplementary Material
ACKNOWLEDGMENTS
We thank Branden Gregorchuk for helpful comments on the manuscript, Nadine Lewis from BioBasic Inc. for her assistance with gene synthesis optimization, and Matthew Martin and Sherwin Santiano for their technical assistance with AST measurements.
Funding for this study was provided by a Manitoba Medical Service Foundation operating grant (8-2016-4) and an NSERC-Discovery grant (RGPIN-2016-05891) to D.C.B.
Carmine J. Slipski and Taylor R. Jamieson conducted all of the experimental data collection and analyses in this study. George G. Zhanel assisted in manuscript draft editing. Denice C. Bay assisted in the design, data analysis, writing for the manuscript, and research funding.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Slipski CJ, Zhanel GG, Bay DC. 2018. Biocide selective TolC-independent efflux pumps in Enterobacteriaceae. J Membr Biol 251:15–33. doi: 10.1007/s00232-017-9992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bay DC, Turner RJ. 2009. Diversity and evolution of the small multidrug resistance protein family. BMC Evol Biol 9:140. doi: 10.1186/1471-2148-9-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bay DC, Turner RJ. 2016. Small multidrug resistance efflux pumps, p 45–71. In Li X-Z, Elkins CA, Zgurskaya HI (ed), Efflux-mediated antimicrobial resistance in bacteria. Springer, Berlin, Germany. [Google Scholar]
- 4.Bay DC, Rommens KL, Turner RJ. 2008. Small multidrug resistance proteins: a multidrug transporter family that continues to grow. Biochim Biophys Acta 1778:1814–1838. doi: 10.1016/j.bbamem.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Kermani AA, Macdonald CB, Gundepudi R, Stockbridge RB. 2018. Guanidinium export is the primal function of SMR family transporters. Proc Natl Acad Sci U S A 115:3060–3065. doi: 10.1073/pnas.1719187115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battaglia RA, Ke A. 2018. Guanidine-sensing riboswitches: how do they work and what do they regulate? Wiley Interdiscip Rev RNA 9:e1482. doi: 10.1002/wrna.1482. [DOI] [PubMed] [Google Scholar]
- 7.Chung YJ, Saier MH. 2002. Overexpression of the Escherichia coli sugE gene confers resistance to a narrow range of quaternary ammonium compounds. J Bacteriol 184:2543–2545. doi: 10.1128/jb.184.9.2543-2545.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bay DC, Turner RJ. 2011. Spectroscopic analysis of the intrinsic chromophores within small multidrug resistance protein SugE. Biochim Biophys Acta 1808:2233–2244. doi: 10.1016/j.bbamem.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert P, Moore LE. 2005. Cationic antiseptics: diversity of action under a common epithet. J Appl Microbiol 99:703–715. doi: 10.1111/j.1365-2672.2005.02664.x. [DOI] [PubMed] [Google Scholar]
- 10.González‐Rivas F, Ripolles‐Avila C, Fontecha‐Umaña F, Ríos‐Castillo AG, Rodríguez‐Jerez JJ. 2018. Biofilms in the spotlight: detection, quantification, and removal methods. Compr Rev Food Sci Food Saf 17:1261–1276. doi: 10.1111/1541-4337.12378. [DOI] [PubMed] [Google Scholar]
- 11.Verderosa AD, Totsika M, Fairfull-Smith KE. 2019. Bacterial biofilm eradication agents: a current review. Front Chem 7:a824. doi: 10.3389/fchem.2019.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaglic Z, Cervinkova D. 2012. Genetic basis of resistance to quaternary ammonium compounds—the qac genes and their role: a review. Vet Med 57:275–281. doi: 10.17221/6013-VETMED. [DOI] [Google Scholar]
- 13.Fricke WF, McDermott PF, Mammel MK, Zhao S, Johnson TJ, Rasko DA, Fedorka-Cray PJ, Pedroso A, Whichard JM, LeClerc JE, White DG, Cebula TA, Ravel J. 2009. Antimicrobial resistance-conferring plasmids with similarity to virulence plasmids from avian pathogenic Escherichia coli strains in Salmonella enterica serovar Kentucky isolates from poultry. Appl Environ Microbiol 75:5963–5971. doi: 10.1128/AEM.00786-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noda T, Murakami K, Etoh Y, Okamoto F, Yatsuyanagi J, Sera N, Furuta M, Onozuka D, Oda T, Asai T, Fujimoto S. 2015. Increase in resistance to extended-spectrum cephalosporins in Salmonella isolated from retail chicken products in Japan. PLoS One 10:e0116927. doi: 10.1371/journal.pone.0116927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reiss CW, Strobel SA. 2017. Structural basis for ligand binding to the guanidine-II riboswitch. RNA 23:1338–1343. doi: 10.1261/rna.061804.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherlock ME, Breaker RR. 2017. Biochemical validation of a third guanidine riboswitch class in bacteria. Biochemistry 56:359–363. doi: 10.1021/acs.biochem.6b01271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherlock ME, Malkowski SN, Breaker RR. 2017. Biochemical validation of a second guanidine riboswitch class in bacteria. Biochemistry 56:352–358. doi: 10.1021/acs.biochem.6b01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins DA, Gladden JM, Kimbrel JA, Simmons BA, Singer SW, Thelen MP. 2019. Guanidine riboswitch-regulated efflux transporters protect bacteria against ionic liquid toxicity. J Bacteriol 201:e00069-19. doi: 10.1128/JB.00069-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tezel U, Pavlostathis SG. 2015. Quaternary ammonium disinfectants: microbial adaptation, degradation and ecology. Curr Opin Biotechnol 33:296–304. doi: 10.1016/j.copbio.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Berlinck RGS, Romminger S. 2016. The chemistry and biology of guanidine natural products. Nat Prod Rep 33:456–490. doi: 10.1039/c5np00108k. [DOI] [PubMed] [Google Scholar]
- 21.Hermann T, Westhof E. 1999. Docking of cationic antibiotics to negatively charged pockets in RNA folds. J Med Chem 42:1250–1261. doi: 10.1021/jm981108g. [DOI] [PubMed] [Google Scholar]
- 22.Dufour D, Leung V, Lévesque CM. 2010. Bacterial biofilm: structure, function, and antimicrobial resistance. Endod Topics 22:2–16. doi: 10.1111/j.1601-1546.2012.00277.x. [DOI] [Google Scholar]
- 23.Donlan RM. 2002. Biofilms: microbial life on surfaces. Emerg Infect Dis 8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tal N, Schuldiner S. 2009. A coordinated network of transporters with overlapping specificities provides a robust survival strategy. Proc Natl Acad Sci U S A 106:9051–9056. doi: 10.1073/pnas.0902400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikkelsen H, Duck Z, Lilley KS, Welch M. 2007. Interrelationships between colonies, biofilms, and planktonic cells of Pseudomonas aeruginosa. J Bacteriol 189:2411–2416. doi: 10.1128/JB.01687-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gradel KO, Randall L, Sayers AR, Davies RH. 2005. Possible associations between Salmonella persistence in poultry houses and resistance to commonly used disinfectants and a putative role of mar. Vet Microbiol 107:127–138. doi: 10.1016/j.vetmic.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Veluz GA, Pitchiah S, Brashears MM, Alvarado CZ. 2014. Efficacy of quaternary ammonium compounds on different conveyor chips contaminated with poultry rinsate. Food Prot Trends 14:15–19. [Google Scholar]
- 28.Sidhu MS, Sorum H, Holck A. 2002. Resistance to quaternary ammonium compounds in food-related bacteria. Microb Drug Resist 8:393–399. doi: 10.1089/10766290260469679. [DOI] [PubMed] [Google Scholar]
- 29.Zou L, Meng J, McDermott PF, Wang F, Yang Q, Cao G, Hoffmann M, Zhao S. 2014. Presence of disinfectant resistance genes in Escherichia coli isolated from retail meats in the USA. J Antimicrob Chemother 69:2644–2649. doi: 10.1093/jac/dku197. [DOI] [PubMed] [Google Scholar]
- 30.Zhang A, He X, Meng Y, Guo L, Long M, Yu H, Li B, Fan L, Liu S, Wang H, Zou L. 2016. Antibiotic and disinfectant resistance of Escherichia coli isolated from retail meats in Sichuan, China. Microb Drug Resist 22:80–87. doi: 10.1089/mdr.2015.0061. [DOI] [PubMed] [Google Scholar]
- 31.Soumet C, Meheust D, Pissavin C, Le Grandois P, Fremaux B, Feurer C, Le Roux A, Denis M, Maris P. 2016. Reduced susceptibilities to biocides and resistance to antibiotics in food-associated bacteria following exposure to quaternary ammonium compounds. J Appl Microbiol 121:1275–1281. doi: 10.1111/jam.13247. [DOI] [PubMed] [Google Scholar]
- 32.Son MS, Del Castilho C, Duncalf KA, Carney D, Weiner JH, Turner RJ. 2003. Mutagenesis of SugE, a small multidrug resistance protein. Biochem Biophys Res Commun 312:914–921. doi: 10.1016/j.bbrc.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Cruz A, Micaelo N, Felix V, Song JY, Kitamura S, Suzuki S, Mendo S. 2013. sugE: a gene involved in tributyltin (TBT) resistance of Aeromonas molluscorum Av27. J Gen Appl Microbiol 59:39–47. doi: 10.2323/jgam.59.47. [DOI] [PubMed] [Google Scholar]
- 34.He GX, Zhang C, Crow RR, Thorpe C, Chen H, Kumar S, Tsuchiya T, Varela MF. 2011. SugE, a new member of the SMR family of transporters, contributes to antimicrobial resistance in Enterobacter cloacae. Antimicrob Agents Chemother 55:3954–3957. doi: 10.1128/AAC.00094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bay DC, Stremick CA, Slipski CJ, Turner RJ. 2017. Secondary multidrug efflux pump mutants alter Escherichia coli biofilm growth in the presence of cationic antimicrobial compounds. Res Microbiol 168:208–221. doi: 10.1016/j.resmic.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Bridier A, Briandet R, Thomas V, Dubois-Brissonnet F. 2011. Resistance of bacterial biofilms to disinfectants: a review. Biofouling 27:1017–1032. doi: 10.1080/08927014.2011.626899. [DOI] [PubMed] [Google Scholar]
- 37.Chung YJ, Saier MH. 2002. Overexpression of the Escherichia coli sugE gene confers resistance to a narrow range of quaternary ammonium compound. Curr Opin Drug Discov Dev 4:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, Hu X, Guo D, Shi C, Zhang C, Peng X, Yang H, Xia X. 2019. Disinfectant resistance profiles and biofilm formation capacity of Escherichia coli isolated from retail chicken. Microb Drug Resist 25:703–711. doi: 10.1089/mdr.2018.0175. [DOI] [PubMed] [Google Scholar]
- 39.Corcoran M, Morris D, De Lappe N, O'Connor J, Lalor P, Dockery P, Cormican M. 2014. Commonly used disinfectants fail to eradicate Salmonella enterica biofilms from food contact surface materials. Appl Environ Microbiol 80:1507–1514. doi: 10.1128/AEM.03109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furste JP, Pansegrau W, Frank R, Blocker H, Scholz P, Bagdasarian M, Lanka E. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 41.Green MR, Sambrook J. 2012. Molecular cloning: a laboratory manual, 4th ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 42.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006–2008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J, Morita Y, Huda MN, Kuroda T, Mizushima T, Tsuchiya T. 2002. VmrA, a member of a novel class of Na(+)-coupled multidrug efflux pumps from Vibrio parahaemolyticus. J Bacteriol 184:572–576. doi: 10.1128/jb.184.2.572-576.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen YX, Xiao K, Liang P, Ma YW, Huang X. 2013. Improvement on the modified Lowry method against interference of divalent cations in soluble protein measurement. Appl Microbiol Biotechnol 97:4167–4178. doi: 10.1007/s00253-013-4783-3. [DOI] [PubMed] [Google Scholar]
- 45.Abràmoff MD, Magalhães PJ, Ram SJ. 2007. Image processing with ImageJ. Biophotonics Int 11:36–42. [Google Scholar]
- 46.Wiegand I, Hilpert K, Hancock REW. 2008. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 47.Harrison JJ, Stremick CA, Turner RJ, Allan ND, Olson ME, Ceri H. 2010. Microtiter susceptibility testing of microbes growing on peg lids: a miniaturized biofilm model for high-throughput screening. Nat Protoc 5:1236–1254. doi: 10.1038/nprot.2010.71. [DOI] [PubMed] [Google Scholar]
- 48.Moura A, Soares M, Pereira C, Leitão N, Henriques I, Correia A. 2009. INTEGRALL: a database and search engine for integrons, integrases and gene cassettes. Bioinformatics 25:1096–1098. doi: 10.1093/bioinformatics/btp105. [DOI] [PubMed] [Google Scholar]
- 49.Pal C, Bengtsson-Palme J, Rensing C, Kristiansson E, Larsson DGJ. 2014. BacMet: antibacterial biocide and metal resistance genes database. Nucleic Acids Res 42:D737–D743. doi: 10.1093/nar/gkt1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gertz EM, Yu YK, Agarwala R, Schaffer AA, Altschul SF. 2006. Composition-based statistics and translated nucleotide searches: improving the TBLASTN module of BLAST. BMC Biol 4:41. doi: 10.1186/1741-7007-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. 2009. Jalview version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Papadopoulos JS, Agarwala R. 2007. COBALT: constraint-based alignment tool for multiple protein sequences. Bioinformatics 23:1073–1079. doi: 10.1093/bioinformatics/btm076. [DOI] [PubMed] [Google Scholar]
- 53.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TLL. 2009. BLAST+: architecture and applications. BMC Bioinformatics 10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 55.Anisimova M, Gil M, Dufayard JF, Dessimoz C, Gascuel O. 2011. Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Syst Biol 60:685–699. doi: 10.1093/sysbio/syr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anisimova M, Gascuel O. 2006. Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Syst Biol 55:539–552. doi: 10.1080/10635150600755453. [DOI] [PubMed] [Google Scholar]
- 57.Lefort V, Longueville J-E, Gascuel O. 2017. SMS: smart model selection in PhyML. Mol Biol Evol 34:2422–2424. doi: 10.1093/molbev/msx149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Federkeil SL, Winstone TL, Jickling G, Turner RJ. 2003. Examination of EmrE conformational differences in various membrane mimetic environments. Biochem Cell Biol 81:61–70. doi: 10.1139/o03-031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.