Abstract
Background
Schizophrenia is known for their theory of mind (ToM) impairment. However, this impairment in schizotypy (schizotypal traits) lacks investigation.
Aims
The present study investigated: (1) whether ToM ability was impaired in schizotypy; (2) whether the ERP amplitudes in nine brain regions of interest associated with ToM (e.g., frontal region) in schizotypy and healthy controls differed; and (3) whether the relationship between ToM performances and ERP amplitudes in schizotypy differed from that in healthy controls.
Method
Forty eight adolescents and young adults (16 schizotypy) with the mean age of 18 years were tested. The Reading the Mind in the Eyes Test (RMET) was used to assess their ToM during which ERP amplitudes were recorded.
Results
The schizotypy group showed significantly lower ERP amplitudes in all conditions of RMET in frontal, frontal-central, central, occipital and temporal regions when compared to those in healthy controls. Also, schizotypy's ERP amplitudes in the frontal, frontal-central, central, occipital, and temporal regions were different from those in the healthy individuals in responding to different types of ToM stimuli (positive, negative and neutral). In schizotypy group, reaction time responding to emotional stimuli was negatively related to ERP amplitudes in the frontal, central-parietal, parietal, occipital, and occipito-temporal regions during RMET while no significant correlations were found in healthy controls.
Conclusion
The present findings inform us with the knowledge regarding the neural and behavioral abnormality of ToM in schizotypy, suggesting that brain activity can be an alternative to detect ToM impairment in schizotypy.
Keywords: Schizotypy, Theory of mind, Event-related potential, Brain activity
1. Introduction
Theory of mind (ToM) is important for facilitating social interactions and functioning (Couture et al., 2006; Frith, 2004). Previous studies have shown that ToM is impaired in schizophrenia, yet this deficit and corresponding neural activity in schizotypy warrants investigation. Schizotypy refers to both healthy individuals with schizotypal personality traits in the general population (Lenzenweger, 2018; Raine, 2006) and clinical patients with schizotypal personality disorder (SPD), which is an attenuated form of schizophrenia without psychosis (Kwapil and Barrantes-Vidal, 2012). As suggested by Kwapil and Barrantes-Vidal (2012), schizotypy is a multidimensional construct that is expressed across a broad range of personality, subclinical, and clinical psychosis phenomenology. They are characterized by negative affect and beliefs, perceptual distortions (positive schizotypy dimension), social anhedonia (negative schizotypy dimension) (Kwapil et al., 2012; Raine, 2006). According to Johnstone et al. (2005), 12.27% of the schizotypy developed schizophrenia within 30 months. Additionally, the overall transition rate in developing psychotic disorder for each schizotypy was 34.9% within 10 years (Nelson et al., 2013). It is noteworthy to investigate schizotypy which might help prevent the development of schizophrenia- spectrum disorder. The present study focused on the sub-clinical schizotypal individuals.
Previously, there has been a lack of research investigating ToM in schizotypy while most studies focused on schizophrenia (e.g., Fett and Maat, 2013; Guastella et al., 2013). For instance, Bora et al. (2009) conducted a meta-analysis that examined ToM using different tasks such as the Reading the Mind in the Eyes test (RMET) among healthy and schizophrenia individuals. Schizophrenia and individuals in the early stages of schizophrenia/schizoaffective disorder showed a significant ToM impairment as measured by RMET (Navarra-Ventura et al., 2018). In a Chinese sample, similar results were found in which schizophrenia individuals had poorer ToM than healthy individuals (Li et al., 2020). Specifically, the performance on negative words was poorer in schizophrenia and this might be due to the deficit in their higher-level processing which requires an integration of perceptual information (i.e. eye-region expressions) and evaluative components (i.e. emotional words). These findings suggest that ToM impairment might affect one's ability to evaluate social information promptly and accurately. Taken all these findings together, it is predicted that schizotypy which might pose the risk for developing schizophrenia also have ToM impairment.
Existing literature has mixed findings regarding ToM impairment in schizotypy. Using RMET to measure ToM, it was revealed that the accuracy in different conditions (i.e. positive-valence, negative-valence, and neutral) in schizotypal personality disorder (SPD) was not different from that in healthy controls (Ripoll et al., 2013). Similarly, no significant difference was found in ToM performance among three schizotypy groups (low schizotypy, positive schizotypy, and negative schizotypy group) (Gooding et al., 2010; Gooding and Pflum, 2011). Bedwell et al. (2014) also did not find significant relationship between ToM measured by RMET and any of the four factors of schizotypy (cognitive perceptual, negative, paranoid, and disorganized factor of schizotypy). In a recent study using RMET (Zhang et al., 2018), it was demonstrated that ToM worsened along with the development of psychosis. Specifically, both schizophrenia and schizotypy group reacted more slowly and less accurately than healthy control group. Apart from RMET, some other assessment tools, such as Hinting task and Empathic Accuracy Task, have been used to assess ToM in schizotypy (Gooding and Pflum, 2011; Ripoll et al., 2013). For instance, Ripoll et al. (2013) used Empathic Accuracy Task to measure ToM in SPD individuals and found that schizotypy might have the deficits in understanding others' negative affect but not the positive affect. However, a number of prior studies found that ToM deficits in various clinical or sub-clinical groups were not revealed in their behavioral performance but in the associated neural activity (e.g., Brune et al., 2011; Shu et al., 2014). In summary, prior literature revealed inconsistent findings regarding the relationship between ToM and schizotypy. Therefore, the present study was sought to investigate ToM ability as well as its underlying neural mechanism in schizotypy. In view of previous findings showing no differential ToM impairments across different schizotypal subtypes (Bedwell et al., 2014; Gooding et al., 2010; Gooding and Pflum, 2011) and unitary schizotypy has been studied previously (e.g., Lam et al., 2016), the present study examined schizotypy as a unitary construct.
To the best of the authors' knowledge, little is known about the brain correlates associated with ToM in schizotypy when compared with schizophrenia. For instance, Hirao et al. (2008) found that schizophrenic individuals had poorer ToM and greater reduction in gray matter volumes in the DMPFC, left ventrolateral prefrontal cortex (VLPFC), ventromedial prefrontal cortex (VMPFC), anterior cingulate cortex (ACC), right superior temporal gyrus (STG), and right insula than healthy individuals. Moreover, an EPR study (Yan et al., 2017) suggested that patients with schizophrenia fail to coordinate neural activity among the central, frontal, parietal, and occipital brain regions to produce a fully integrated ToM perception. These findings suggested that central, frontal, parietal, and occipital brain regions were related to ToM impairment in schizophrenia. Yet, whether these findings in schizophrenia also apply to schizotypy remains unanswered. Among the very few studies in schizotypy, Batty et al. (2014) found reduced N170 amplitude when seeing inverted photographic faces in high schizotypy, compared to low schizotypy. This finding implied that the early visual processing of faces were intact in schizotypy, but they might have an impairment in processing facial configuration processing which is similar to the finding in schizophrenia (Akbarfahimi et al., 2013). Another ERP study (Wynn et al., 2008) found a smaller N250 amplitude in schizophrenia across three facial emotional recognition conditions (sex identification, emotion identification, and building identification), indicating that ToM were less efficient in schizophrenia. Additionally, Schwartzman et al. (2008) found reduced amplitude of the ERP components (P1 and P2, but not N170) in the parieto-temporal region for high visual hallucination-prone individuals. This finding suggested that individuals who were prone to experience visual hallucinations exhibited lower level of early visual processing while the face specific processing was intact. Also, a recent study (Davidson et al., 2018) examined three ERP components (P100, N170, and P300) during a face emotion task and found that N170 for face stimuli was not associated with schizotypal features. Moreover, a functional magnetic resonance imaging (fMRI) study in college students (Wang et al., 2015) found that negative schizotypy was positively correlated to the brain activations in the middle temporal gyrus, temporoparietal junction (TPJ), and medial frontal gyrus; while positive schizotypy was negatively correlated to the activation in the medial frontal gyrus. With the basis of previous ToM findings in schizophrenia and schizotypy, the present study aimed to examine seven frontal, central, parietal, occipital and temporal brain regions (frontal, frontal central, central, central-parietal, parietal, occipital, and occipito-temporal) as the regions of interest (ROIs) associated with ToM in schizotypy.
The present study aimed to examine whether there were differences in ToM between healthy controls and schizotypy in terms of the behavioral ToM performance and associated ERP amplitudes. Specifically, the study aimed to: (1) to examine if ToM ability was impaired in schizotypy in comparison to healthy controls using RMET. It was hypothesised that schizotypy would perform worse than healthy controls; (2) to compare between the two groups in terms of ERP amplitudes during RMET in nine ROIs (frontal, frontal central, central, central-parietal, parietal, occipital, and occipito-temporal). It was hypothesised that lower ERP amplitudes would be found in these regions in schizotypy; and (3) to test if the association between behavioral ToM performance (reaction time and accuracy) and the ERP amplitudes during RMET differed between these two groups. It was hypothesised that the association was negative and significant only in schizotypy group.
2. Methods
2.1. Participants
This study was approved by the Human Subjects Ethics Sub-committee (HSESC) of the Hong Kong Polytechnic University. A total of 474 participants were recruited from 10 primary and secondary schools on a voluntary basis and they had to meet the inclusion criteria for the participation in this study. Fourty-eight adolescents and young adults aged between 13 and 35 who met the inclusion criteria participated in this study: (a) not diagnosed with an Axis I psychotic diagnosis according to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (American Psychiatric Association, 2013); (b) without history or current presence of neurological diseases; and (c) without the presence of medical diseases. In order to categorize participants into schizotypy and healthy control groups, they were asked to fill in a set of questionnaires to assess their schizotypal levels. Participants were categorized as schizotypy if they (a) scored 9 or above in the Chinese version of the Prodromal Questionnaire (CPQ-16) (Chen et al., 2014; Chen et al., 2016), (b) scored above 8.18 in the Chinese version of the Community Assessment of Psychic Experiences (CAPE-C15) (Mark and Toulopoulou, 2017), or (c) scored 17 or above in the Chinese version of Schizotypal Personality Questionnaire-Brief (SPQ—B) (Hsiung et al., 2019; Ma et al., 2010). The cutoff scores of these three schizotypy scales were adopted from previous studies (Chen et al., 2014; Hsiung et al., 2019; Ma et al., 2010; Mark and Toulopoulou, 2017) and these three schizotypy scales were also adopted in Overton (2015) to examine the levels of schizotypy. All three schizotypy scales have been used in adolescents and adults with good reliabilities (Chen et al., 2014; Lam et al., 2016; Mark and Toulopoulou, 2017). Sixteen participants were identified as schizotypy and 32 participants, who did not meet the aforementioned criteria, were identified as healthy controls. It is worth noting that 15 out of those 16 schizotypy participants met the cut-off criteria for CPQ-16 and CAPE-15 which primarily assessed positive schizotypy experiences. Hence, the results might be specific to positive schizotypy in the present study. Moreover, it was aimed to characterise schizotypy as a categorical condition because the level of schizotypal characteristics were examined by three schizotypy scales (CAPE-15, SPQ-B, and CPQ-16) instead of one single scale in order to make the assessment of schizotypy more stringent. Since there is no standardised way to compute the total schizotypy continuous scores based on the three scales used in the present study, we adopted the cutoff scores for the three schizotypy scales used in previous studies to dichotimize the participants into either schizotypy or healthy controls. For instance, if they rated above the cutoff score in one of the three scales mentioned above, the participants would be categorized as schizotypy. Those who rated below the cutoff scores for all three scales would be considered as healthy controls. There was no significant demographics difference (e.g, sex) between the two groups (ps > 0.05) except that schizotypy were significantly younger than healthy controls (t(45.84) = 3.02, p < .01) (Table 1).
Table 1.
Demographics for the healthy control group and schizotypy group.
| Variables | Healthy control group | Schizotypy group | t/X2 | df | p |
|---|---|---|---|---|---|
| Cases (n) | 32 | 16 | |||
| Demographics | |||||
| Age (years) [mean (SD)] | 21.31 (4.98) | 18.00 (2.61) | 3.02 | 45.84a | 0.004⁎⁎ |
| Male [n (%)] | 21 (65.63) | 10 (62.50) | 0.05 | 1 | 0.831 |
| Household income $30,001 or above [n (%)] | 8 (25) | 4 (25) | 1.12 | 6 | 0.981 |
| Education level: Bachelor's degree or above [n (%)] | 18 (56.25) | 9 (56.25) | 0.71 | 4 | 0.950 |
Since Levene's test for equality was found to be violated for the analysis on age, F(1, 46) = 4.16, p < .05, t statistic not assuming homogeneity of variance was computed.
p < .01.
2.2. Procedures
Written informed consent was obtained from the participants. Also, parental consent was obtained for the participants aged below 18 years. Then, all participants were assessed by CPQ-16, CAPE-C15, SPQ—B, and demographics information were collected. To assess participants' ToM ability and the associated brain activity, participants were instructed to perform RMET on the computer while the EEG data were recorded. Behavioral performances of RMET (reaction time and accuracy) were recorded by STIM2 and EEG data were collected via a 64-channel scalp electrodes.
2.3. Measures
2.3.1. ToM ability
A modified Chinese version of Reading the Mind in the Eyes Test (RMET) was used to measure the ability to attribute others' mental states based on the eye expressions. The test was adapted from Baron-Cohen et al. (2001). There were 2 runs and each run consists of 12 blocks and each block consists of 6 stimuli. The block of trials was either an emotional identification condition or a sex identification control condition. Participant had to complete both conditions. In the emotional identification condition, participants were required to identify the emotional state expressed by the pairs of eyes in the photo (e.g., curious, amused, hateful). In the control condition, participants were asked to identify the sex of the person (male or female). In both emotional identification and sex identification control conditions, two choices of answers were presented along with each photo showing a pair of eyes and the participants were asked to choose the answer out of the two choices presented for each photo. In total, there were 72 trials, in which 36 trials were emotional stimuli (18 positive-valence stimuli and 18 negative-valence stimuli) and 36 trials were neutral stimuli. Behavioral RMET performances were measured in terms of reaction time (in ms) and accuracy (% correct) in responding to the three types of stimuli (positive, negative and neutral). RMET has been administered to adolescents and adults (Baron-Cohen et al., 2001; Holt et al., 2014).
2.3.2. EEG acquisition and pre-processing
Continuous EEG data were recorded from 64 electrodes mounted in a scalp cap according to the international 10–20 system. EEG data were preprocessed and analysed using Curry 7 software (Compumedics Neuroscan, Charlotte, NC, United States). Electrooculogram (EOG) artifacts from eye blinking and horizontal movement, particularly electrode M1, M2, VEOG and HEOG were removed from the data analysis for all the trials of RMET (Wang et al., 2010). In addition, the contaminated EEG due to amplifier clipping, burst of electromyographic (EMG) activity, and threshold where epochs with greater than 33% contamination or peak-to-peak deflection exceeding ±400μv were excluded (Lomas et al., 2014; Wang et al., 2010). To extract EEG epochs from the continuous EEG, data were segmented from 200 ms pre-stimulus and 500 ms post-stimulus. Window for processing data rejection was set as [−200 μV, 200 μV]. Averaged ERP of negative emotion stimulus, positive emotion stimulus and sex stimulus were then generated by averaging epochs. The P3 component was indicated as the maximum positive peak at each electrode between 290 ms and 500 ms post-stimulus (Herbert et al., 2007). Peak amplitudes (microvolves) of the P3 evoked by negative emotion, positive emotion and sex (control) were used for data analysis.
2.4. Statistical analysis
IBM SPSS version 25.0 (IBM Corp., Armonk, NY, United States) was used to analyze the data. Two 2 × 3 between-within subject ANCOVAs were run with group (healthy controls, schizotypy) as between-subject variable, emotion (positive valence, negative valence, and neutral) as within-subject variable, and the demographics (age, sex, household income, and education level) as covariates. The dependent variable for each ANCOVA was either RMET accuracy or reaction time. Moreover, the main effect of group, emotion and interaction effect of the two on ERP amplitudes in each brain ROI (frontal, frontal-central, central, central-parietal, parietal, occipital, occipito-temporal region, middle temporal gyrus, and TPJ) was tested by ANCOVAs. Mean amplitudes were calculated by averaging two/three selected electrode sites (Table 2). The grouping of electrodes was adopted from previous studies (Akbarfahimi et al., 2013; Brune et al., 2011; Jiao et al., 2017; Shu et al., 2014; Song et al., 2019; Wang et al., 2015). By using the G*Power 3.1 statistical software to conduct the post-hoc power analysis, the sample size for the present study was shown to be adequate for the ANCOVA analyses to test the hypotheses (effect size = 0.5, alpha = 0.05, power = 0.8, and number of covariates = 4). Age was analysed as one of the covariates because it was found to be significantly correlated with RMET behavioral performances (reaction time and accuracy) (ps < 0.05) and the ERP amplitudes in a number of ROIs (frontal-central and central regions). Hence, age was included as one of the covariates in the present study although it might give rise to potential statistical problems because there was a age difference between the two groups (Miller and Chapman, 2001). Furthermore, other covariates (sex, household income, and education level) were included in the data analyses because these were important factors contributing to the brain correlates related to ToM, schizotypy and ToM ability in previous studies (e.g., Cohen et al., 2008; Gao et al., 2019; Miettunen et al., 2010). To examine if there was a relationship between behavioral RMET performance/elicited ERP amplitudes (aforementioned ROIs) in three ToM conditions (positive valence, negative valence, and neutral), bivariate correlations between these variables were computed. The significance thresholds for all analyses in the present study were set at p < .05.
Table 2.
The brain regions of interest and its corresponding EEG electrode names.
| Brain regions | EEG electrode names |
|---|---|
| Frontal | F3, FZ, F4 |
| Frontal-central | FC3, FCZ, FC4 |
| Central | C3, CZ, C4 |
| Central-parietal | CP3, CPZ, CP4 |
| Parietal | P3, PZ, P4 |
| Occipital | O3, OZ, O4 |
| Occipito-temporal | P7, P8 |
| Temporal (middle temporal gyrus) | T7, T8 |
| Temporal parietal junction (TPJ) | CP5, CP6 |
Note: Continuous EEG data were recorded from 64 electrodes mounted in a scalp cap according to the international 10–20 system. Eye-blinks and eye-movements were monitored by horizontal (i.e. HEOL, HEOR) and vertical (i.e. VEOU, VEOL) electrooculogram (EOG) electrodes. M1 and M2 electrodes were also used as reference electrodes.
3. Results
3.1. Behavioral performances
Regarding RMET reaction time, a significant main effect of emotion was found, F(2,41) = 7.26, p = .002. Specifically, participants responded significantly slower to the positive emotion stimuli (t(47) = 33.01, p < .01) and the negative valence stimuli (t(47) = 32.74, p < .01) when compared to the neutral stimuli. The main effect of group and the group × emotion interaction effect was not significant (p > .05) (Table 3).
Table 3.
ANCOVA analyses on behavioral RMET performance (reaction time).
| Independent variables | Descriptive statistics |
F-statistics |
|||||
|---|---|---|---|---|---|---|---|
| M | SE | F | p | b | ηp2 | ||
| Main effects | |||||||
| Emotion | Positive | 1679.64 | 30.41 | 7.26 | 0.002⁎⁎ | 1946.78 | 0.261 |
| Negative | 1675.76 | 30.64 | 1832.08 | ||||
| Neutral | 905.60 | 23.84 | 1367.23 | ||||
| Group | Healthy controlsa | 1390.75 | 30.77 | 1.05 | 0.311 | −57.54 | 0.024 |
| Schioztypy | 1449.92 | 45.33 | |||||
| Interaction effect (Group × Emotion) | |||||||
| Heathy | Positive | 1648.44 | 36.15 | 0.02 | 0.982 | −62.41 | 0.001 |
| Negative | 1644.53 | 36.43 | −62.45 | ||||
| Neutral | 879.28 | 28.34 | −52.64 | ||||
| Schizotypy | Positive | 1710.85 | 53.24 | ||||
| Negative | 1706.98 | 53.65 | |||||
| Neutral | 931.92 | 41.74 | |||||
Healthy controls (Group = 0) and schizotypy (Group = 1).
p < .01.
Regarding RMET accuracy, the main effect of emotion was significant, F(2,41) = 6.46, p = .004. The effect size was 0.26 which is moderate. Participants were less accurate in responding to the positive valence stimuli than to the neutral stimuli, t(47) = −7.52, p < .01; and they were less accurate in responding to the negative valence stimuli than to the neutral stimuli, t(47) = −8.28, p < .01. The effect size was 0.24 which is moderate. There was no significant accuracy difference in responding to the positive valence stimuli and the negative valence stimuli when compared to the neutral one (p > .05). The main effect of group and the group × emotion interaction effect was not significant (p > .05) (Table 4). Similar moderate effect sizes were found for the main effect of emotion on the RMET performances (reaction time and accuracy) mentioned above suggested that the RMET performances were affected by the stimuli of different emotional valences in similar manner.
Table 4.
ANCOVA analyses on behavioral RMET performances (accuracy).a
| Independent variables | Descriptive statistics |
F-statistics |
|||||
|---|---|---|---|---|---|---|---|
| M | SE | F | p | b | ηp2 | ||
| Main effects | |||||||
| Emotion | Positive | 83.03 | 1.45 | 6.46 | 0.004⁎⁎ | 68.04 | 0.240 |
| Negative | 81.90 | 1.26 | 71.98 | ||||
| Neutral | 91.75 | 0.42 | 91.18 | ||||
| Group | Healthy controls | 85.75 | 0.94 | 0.05 | 0.829 | 0.38 | 0.001 |
| Schizotypy | 85.37 | 1.38 | |||||
| Interaction effect (Group × Emotion) | |||||||
| Heathy | Positive | 83.04 | 1.73 | 0.02 | 0.984 | 0.02 | 0.001 |
| Negative | 82.24 | 1.50 | 0.69 | ||||
| Neutral | 91.97 | 0.51 | 0.44 | ||||
| Schizotypy | Positive | 83.02 | 2.54 | ||||
| Negative | 81.55 | 2.21 | |||||
| Neutral | 91.54 | 0.74 | |||||
Healthy controls (Group = 0) and schizotypy (Group = 1).
p < .01.
3.2. ERP amplitudes
3.2.1. Main effect and group by emotion interaction effect
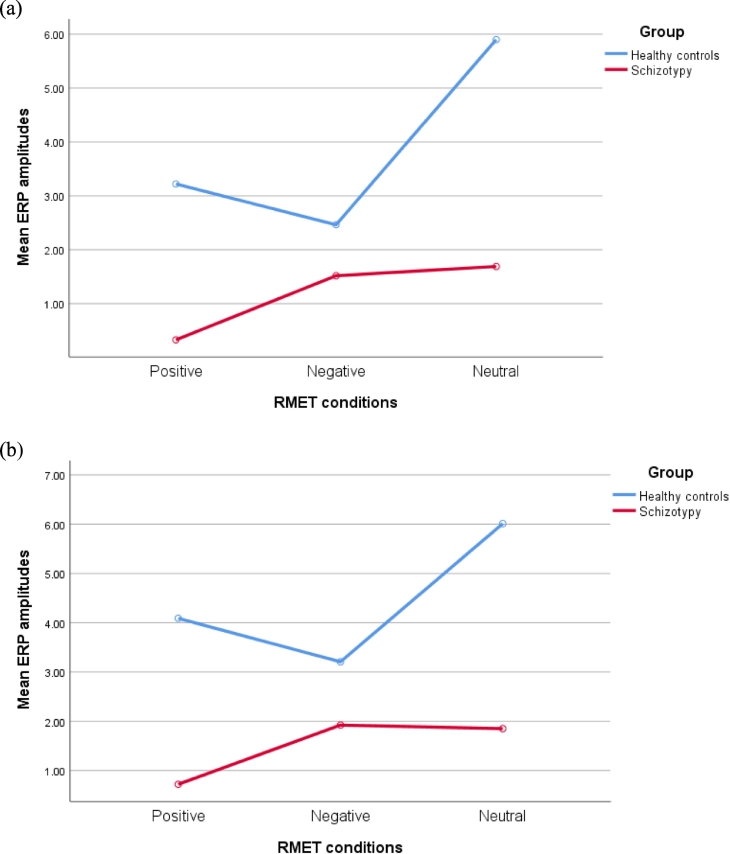
After controlling for the covariates, the main effect of group on the ERP amplitudes was significant in the frontal (F(1,42) = 5.21, p < .05), frontal-central (F(1,42) = 5.12, p < .05), central (F(1,42) = 5.12, p < .05), occipital (F(1,42) = 4.22, p < .05), and temporal regions (F(1,42) = 5.41, p < .05). The ERP amplitudes of the schizotypy group was significantly lower than that of the healthy control group in those regions. However, group difference was not significant in central-parietal region, parietal region, occipito-temporal region, and TPJ (ps > 0.05). No significant main effect of emotion (positive-valence, negative-valence, and neutral) on the ERP amplitudes was found in any ROIs (ps > 0.05) whereas the group by emotion interaction effect was significant in the parietal (F(2,41) = 3.49, p = .04) and occipital region (F(2,41) = 3.27, p = .05). Specifically, in both parietnal and occipital regions, healthy controls' ERP amplitudes were significantly higher than the ones in schizotypy when processing positive-valence and neutral stimuli (ps < 0.05) (See Fig. 1.). Although the difference was not significant, healthy controls had the lowest ERP amplitudes in both parietal and occipital regions when processing negative- valence stimuli when compared to other two conditions (ps > 0.05). The interaction effect was not significant in other ROIs (ps > 0.05) (Table 5). The effect sizes for the significant main effect of group on different ROIs mentioned above ranged from 0.09 to 0.12 which are small. Similar small effect sizes found for the main group effect on different ROIs suggested that schizotypy impacted on the ERP amplitudes in different ROIs in a similar manner.
Fig. 1.
(a) The mean ERP amplitudes in the parietal region. The significant group by emotion interaction effect on the elicited ERP amplitudes in parietal brain region was found. (b) The mean ERP amplitudes in the occipital region showed the significant group by emotion interaction effect on the elicited ERP amplitudes in occipital brain region was found. In both graphs, healthy controls' ERP amplitudes were significantly higher than the ones in schizotypy when processing positive-valence and neutral stimuli. Although the difference was not significant, healthy controls had the lowest ERP amplitudes in both parietal and occipital regions when processing negative- valence stimuli when compared to other two conditions.
Table 5.
ANCOVA analyses on ERP amplitudes in nine brain regions of interest.
| Model | Brain region of interests | Descriptive statistics |
F-statisticsc |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive |
Negative |
Neutral |
F | p | ηp2 | ||||||
| M | SE | M | SE | M | SE | ||||||
| 1 | Frontal | Groupa | 5.21 | 0.028⁎ | 0.110 | ||||||
| Emotionb | 0.49 | 0.619 | 0.023 | ||||||||
| Healthy control | 0.53 | 0.88 | −1.02 | 1.06 | 2.32 | 0.70 | 0.97 | 0.389 | 0.045 | ||
| Schizotypy | −2.66 | 1.30 | −3.00 | 1.56 | −1.99 | 1.04 | |||||
| 2 | Frontal-central | Groupa | 5.12 | 0.029⁎ | 0.109 | ||||||
| Emotionb | 1.43 | 0.252 | 0.065 | ||||||||
| Healthy control | 0.56 | 1.01 | −0.28 | 1.00 | 3.38 | 0.85 | 2.18 | 0.126 | 0.096 | ||
| Schizotypy | −2.87 | 1.49 | −2.22 | 1.47 | −2.00 | 1.25 | |||||
| 3 | Central | Groupa | 5.77 | 0.021⁎ | 0.121 | ||||||
| Emotionb | 1.02 | 0.371 | 0.047 | ||||||||
| Healthy control | 1.07 | 1.00 | 0.26 | 0.98 | 4.06 | 0.80 | 2.67 | 0.081 | 0.115 | ||
| Schizotypy | −2.62 | 1.47 | −1.71 | 1.44 | −1.46 | 1.18 | |||||
| 4 | Central-parietal | Groupa | 1.58 | 0.215 | 0.036 | ||||||
| Emotionb | 1.56 | 0.223 | 0.071 | ||||||||
| Healthy control | 1.85 | 1.02 | 1.15 | 0.96 | 4.80 | 0.81 | 3.00 | 0.061 | 0.128 | ||
| Schizotypy | −0.27 | 1.50 | 0.76 | 1.45 | 1.18 | 1.19 | |||||
| 5 | Parietal | Groupa | 2.94 | 0.094 | 0.065 | ||||||
| Emotionb | 1.65 | 0.204 | 0.075 | ||||||||
| Healthy control | 3.22 | 0.99 | 2.46 | 0.97 | 5.90 | 0.74 | 3.49 | 0.040⁎ | 0.145 | ||
| Schizotypy | 0.33 | 1.45 | 1.52 | 1.43 | 1.69 | 1.09 | |||||
| 6 | Occipital | Groupa | 4.22 | 0.046⁎ | 0.091 | ||||||
| Emotionb | 1.36 | 0.268 | 0.062 | ||||||||
| Healthy control | 4.09 | 0.87 | 3.21 | 0.91 | 6.01 | 0.70 | 3.27 | 0.048⁎ | 0.138 | ||
| Schizotypy | 0.72 | 1.28 | 1.92 | 1.35 | 1.85 | 1.04 | |||||
| 7 | Occipito-temporal | Groupa | 2.38 | 0.130 | 0.054 | ||||||
| Emotionb | 1.67 | 0.201 | 0.075 | ||||||||
| Healthy control | 2.88 | 0.95 | 2.32 | 0.94 | 5.45 | 0.73 | 2.94 | 0.064 | 0.125 | ||
| Schizotypy | 0.44 | 1.40 | 1.56 | 1.38 | 1.68 | 1.07 | |||||
| 8 | Temporal | Groupa | 5.41 | 0.025⁎ | 0.114 | ||||||
| Emotionb | 0.69 | 0.509 | 0.032 | ||||||||
| Healthy control | 1.49 | 0.85 | 0.79 | 0.86 | 3.90 | 0.69 | 1.78 | 0.181 | 0.080 | ||
| Schizotypy | −1.70 | 1.26 | −0.86 | 1.27 | −0.50 | 1.02 | |||||
| 9 | TPJd | Groupa | 2.06 | 0.158 | 0.047 | ||||||
| Emotionb | 1.28 | 0.288 | 0.059 | ||||||||
| Healthy control | 2.15 | 0.98 | 1.52 | 0.93 | 4.85 | 0.78 | 2.89 | 0.067 | 0.124 | ||
| Schizotypy | −0.19 | 1.44 | 0.89 | 1.37 | 1.18 | 1.15 | |||||
Group: Healthy controls (Group = 0), schizotypy (Group = 1).
Positive, negative and neutral stimuli.
After controlling for the covariates.
TPJ refers to temporal parietal junction.
p < .05.
3.3. Relationship between behavioral ToM performance and ERP amplitudes
Bivariate correlations between behavioral performances and the elicited ERP amplitudes revealed several significant associations in the schizotypy group (ps < 0.05) but not in the healthy control group (ps > 0.05). Specifically, the relationships between RMET reaction time and ERP amplitudes elicited by positive emotion stimuli were significant in the frontal (r = −0.50), central parietal (r = −0.54), parietal region (r = −0.56), occipital (r = −0.57), occipitotemporal regions (r = −0.58), and TPJ (r = −0.53) (ps = 0.02 to 0.05). Relationships between the reaction time and ERP amplitudes elicited by negative emotion stimuli were significant in the occipital region (r = −0.50, p = .05). All other correlations were not significant (ps > 0.05). In addition, significant relationship between RMET accuracy and ERP amplitudes associated with neutral stimuli was found in the central parietal (r = 0.53), parietal (r = 0.57), occipital (r = 0.60), occipitotemporal regions (r = 0.59), and TPJ (r = 0.57) in schizotypy (ps = 0.01 to 0.03). All other correlations for schizotypy and all correlations for healthy controls were not significant (ps > 0.05).
4. Discussion
The present study investigated ToM and associated neural activity as well as the relationship between the two in schizotypy. Based on the current findings, hypothesis one was not supported, and hypotheses two and three were partially supported. Specifically, neural activity in frontal, frontal-central, central, occipital, and temporal regions were significantly reduced in schizotypy than in healthy controls. Additionally, schizotypy's neural activity in the frontal, frontal-central, central, occipital, and temporal regions were different from that in the healthy individuals in responding to different ToM stimuli. Poorer ToM performance in responding to emotional stimuli was related to reduced ERP amplitudes in the frontal, central parietal, parietal, occipital, occipitotemporal regions, and TPJ in schizotypy but these relationships were not found in healthy individuals. These differential findings in behavioral performance and brain activity of ToM inform us with the knowledge regarding the neural and behavioral abnormality in ToM in schizotypy which have potential clinical implications.
Regarding ToM behavioral performances, the present study found no group difference, indicating that schizotypy are not behaviorally impaired in decoding other people's mental state. This finding is in line with a number of previous studies using RMET to measure ToM ability (Gooding et al., 2010; Gooding and Pflum, 2011; Ripoll et al., 2013). In those studies, schizotypy and healthy controls did not differ in the total RMET accuracy score and the sub-scores for different ToM stimuli. However, a more recent study (Zhang et al., 2018) suggested that schizotypy performed poorer than healthy individuals. Their finding is different from our present finding which could possibly be explained by the difference in participants' psychotic level. In Zhang et al. (2018)'s study, schizotypy were clinically high risk of psychosis while in our study and several previous studies (Brune et al., 2011; Gooding and Pflum, 2011; Modinos et al., 2010), participants were nonclinical sample drawn from the general population. This indicates that the person's risk and severity in psychosis and whether or not he/she has been clinically identified could affect their ToM performance.
The ERP amplitudes in frontal, frontal-central, central, occipital, and temporal regions were lower in schizotypy than healthy individuals when they processed other's mental states. This group difference is in line with previous findings (Brune et al., 2011; Shu et al., 2014)'s findings on the abnormal brain activation in frontal and temporal regions, and cingulate cortex. These findings might be explained by the neuroanatomical functions. Specifically, frontal region is involved in affective empathy (Song et al., 2019) and the reasoning about others' mental state (Sabbagh et al., 2004) while the temporal region is involved in learning and recognition of others' intentional movements (Brune et al., 2011). Moreover, these findings indicate a generally weaker information processing in schizotypy. Previous findings reported that schizotypy were related to impaired executive functions (Kocsis-Bogar et al., 2017), which are important for inhibiting self-perspective and taking other-perspective (Decety and Jackson, 2004). The impairment in cognitive inhibition and flexibility contributed significantly to the differences in behavioral ToM performance between healthy controls and schizotypy (Kocsis-Bogar et al., 2017). Moreover, similar small effect sizes (0.09–0.12) found for the main group effect on different ROIs mentioned above suggested that schizotypy impacted on the ERP amplitudes in different ROIs in a similar manner. These findings suggest that neural activity in ROIs that are involved in emotional processing is abnormal in schizotypy. However, no significant group difference in the ERP amplitude in occipito-temporal region was found which is consistent with Akbarfahimi et al. (2013)'s observations of a delay in the N170 responses to facial expressions in schizophrenia individuals. This suggests that schizotypy processes facial expressions in a similar way as healthy individuals but the process is delayed.
Although no main emotion effect was found in ERP amplitudes in any ROIs across three stimuli (positive, negative and neutral), a significant group by emotion interaction effect in the ERP amplitudes in parietal and occipital regions was found. Specifically, in both parietal and occipital regions, healthy controls' ERP amplitudes were higher than the ones in schizotypy when processing positive-valence and neutral stimuli. Although the difference was not significant, healthy controls had the lowest ERP amplitudes in both parietal and occipital regions when processing negative- valence stimuli when compared to positive and neutral stimuli. These findings indicate that schizotypy and healthy controls are different in processing various types of emotional states in the others. Specifically, schizotypy showed reduced brain activity when processing positive emotional states of others when compared to healthy individuals. These findings might be because parietal and occipital regions are involved in spatial perception and attention (Cao et al., 2012), as well as emotional information processing (Sabbagh et al., 2004; Shu et al., 2014) which are important for the decoding of other's mental states (Wang et al., 2015). It is plausible that schizotypy requires a relatively more activated parietal and occipital regions so as to compensate for a deficit in processing positive emotional states of the others. This might explain why there is the brain activity abnormality in parietal and occipital regions while processing different emotional states of others in schizotypy.
The significant negative correlation between ToM performance and the ERP amplitudes while processing the mental states of others in schizotypy further indicates ToM impairment in schizotypy. Specifically, it was observed that longer time schizotypy spent on responding to the emotional stimuli was associated with lower ERP amplitudes in the frontal, central-parietal, parietal, occipital, occipitotemporal regions, and TPJ, which are involved in emotional information processing (e.g., Akbarfahimi et al., 2013), and the reasoning of others' mental states (Sabbagh et al., 2004). Although no significant differences were found in behavioral ToM performance between schizotypy and healthy individuals, schizotypy had a lower ERP amplitudes in processing and decoding other's mental states. This finding supports previous studies (Brune et al., 2011; Modinos et al., 2010), in which schizotypy did not show behavioral ToM impairment but greater activation in the prefrontal cortex, posterior cingulate cortex, and temporoparietal cortex. That is, schizotypy processes the mental states of others in a different manner neurally than the healthy individuals due to the compensatory over-activation of the brain regions so to compensate for their ToM deficits (Brune et al., 2011).
4.1. Limitations
The present study has a number of limitations. First, RMET that we used for measuring ToM ability only taps on the social-perceptive component of ToM. Since ToM is a multifaceted construct which includes both social-cognitive and social-perceptive components, future studies could include RMET and other tasks that measure the social-cognitive component of ToM (e.g. Hinting task). Also, it is suggested that different patterns of associations between different subtypes of schizotypy and social impairment (e.g., ToM). Moreover, it might be difficult to determine the extent to which the current schizotypy sample has positive, negative, or disorganized traits because there are more positive schizotypy items (in CAPE- 15 and CPQ-16) than other subtypes items included in the three schizotypy scales that were administered to the participants in the present study. Hence, it is possible that the sample is largely characterized by positive schizotypy, rather than negative or disorganized schizotypy. Future studies should address these concerns by investigating different schizotypal subtypes with a larger sample size. Moreover, the present study only considered the topographical distribution of the neural activities and elicited ERP amplitudes. Since a delay of the N170 responses to facial expressions was found in schizophrenia in previous study (Akbarfahimi et al., 2013), future studies should also investigate the latency of the ERP component (e.g., N270–400 and P300–500) which could facilitate the understanding of ToM processing in schizotypy. In addition, given the wide age range of the participants in the present study, although age had already been controlled for, the extent to which ToM measured by RMET would be expected to be comparable across participants of different ages in this study remains uncertain. Future studies should be conducted with a narrower age range in order to address this concern. Last but not least, the small sample size (16 schizotypy and 32 healthy controls) and dichotimizating participants into these two groups might affect the power of the statistical findings in the present study. For instance, dichotomizing participants into groups based on continuous schizotypy scores might lead to loss of information and affect the power of the findings (MacCallum et al., 2002). In order to achieve sufficient statistical power, future studies should replicate the present study with a larger sample size.
5. Conclusion
Reduced neural activity in frontal, frontal-central, central, occipital and temporal regions observed in schizotypy indicates insufficient ToM resources when compared with healthy individuals. Additionally, schizotypy and healthy individuals have different levels of ERP amplitudes in parietal and occipital regions in decoding different emotional states of others. Furthermore, poorer ToM increases with ERP amplitudes in frontal, central-parietal, parietal, occipital, and occipitotemporal regions only in schizotypy group which implies that they have abnormal neural activity during ToM processing. These differential findings in behavioral performance and brain activity of ToM inform us with the knowledge regarding the neural and behavioral abnormality of ToM in schizotypy. More importantly, the present findings suggest that brain activity can be an effective alternative to detect ToM impairments in schizotypy, thereby preventing the development of schizophrenia in these individuals.
CRediT authorship contribution statement
BYHL and SMW conceptualised and monitored all processes of this study. KSL collected and analysed the data. CL analysed the data and wrote the first draft. BYHL revised and finalised the manuscript. All authors have read and approved the content of this manuscript.
Declaration of competing interest
I declare no conflicts of interest.
Acknowledgements
Nil.
References
- Akbarfahimi M., Tehrani-Doost M., Ghassemi F. Emotional face perception in patients with schizophrenia: an event-related potential study. Neuropsychology. 2013;45(3):279–287. [Google Scholar]
- Baron-Cohen S., Wheelwright S., Hill J., Raste Y., Plumb I. The “Reading the Mind in the Eyes” test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J. Child Psychol. Psychiatry. 2001;42(2) [PubMed] [Google Scholar]
- Batty R.A., Francis A.J.P., Innes-Brown H., Joshua N.R., Rossel S.L. Neurophysiological correlates of configural face processing in schizotypy. Front. Psychiatry. 2014;5(101):1–11. doi: 10.3389/fpsyt.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedwell J.S., Compton M.T., Jentsch F.G., Deptula A.E., Goulding S.M., Tone E.B. Latent factor modeling of four schizotypy dimensions with theory of mind and empathy. PLoS One. 2014;9(11):1–14. doi: 10.1371/journal.pone.0113853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E., Yucel M., Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr. Res. 2009;109(1–3):1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Brune M., Ozgurdal S., Ansorge N., von Reventlow H.G., Peters S., Nicolas V., Tegenthoff M., Juckel G., Lissek S. An fMRI study of “theory of mind” in at-risk states of psychosis: comparison with manifest schizophrenia and healthy controls. Neuroimage. 2011;55(1):329–337. doi: 10.1016/j.neuroimage.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Cao B., Li Y., Li F., Li H. Electrophysiological difference between mental state decoding and mental state reasoning. Brain Res. 2012;1464:53–60. doi: 10.1016/j.brainres.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Chen F., Wang L., Heeramun-Aubeeluck A., Wang J., Shi J., Yuan J., Zhao X. Identification and characterization of college students with Attentuated Psychosis Syndrome in China. Psychiatry Res. 2014;216:346–350. doi: 10.1016/j.psychres.2014.01.051. [DOI] [PubMed] [Google Scholar]
- Chen F., Wang L., Wang J., Heeramun-Aubeeluck A., Yuan J., Zhao X. Applicability of the Chinese version of the 16-item Prodromal Questionnaire (CPQ-16) for identifying attenuated psychosis syndrome in a college population. Early Interv. Psychiatry. 2016;10(4):308–315. doi: 10.1111/eip.12173. [DOI] [PubMed] [Google Scholar]
- Cohen P., Chen H., Gordon K., Johnson J., Brook J., Kasen S. Socioeconomic background and the developmental course of schizotypal and borderline personality disorder symptoms. Dev. Psychopathol. 2008;20(2) doi: 10.1017/S095457940800031X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture S.M., Penn D.L., Roberts D.L. The functional significance of social cognition in schizophrenia: a review. Schizophrenia. Bull. 2006;32(1):44–63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson C.A., Kiat J.E., Tarasenko M., Ritchie A.J., Molfese D., Spaulding W.D. Exploring electrophysiological correlates of social cognition in subclinical schizotypy. Personal. Ment. Health. 2018;12:179–191. doi: 10.1002/pmh.1413. [DOI] [PubMed] [Google Scholar]
- Decety J., Jackson P. The functional architecture of human empathy. Behav. Cogn. Neurosci. Rev. 2004;3(2):71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Fett A.K., Maat A. Social cognitive impairments and psychotic symptoms: what is the nature of their association? Schizophrenia. Bull. 2013;39(1):77–85. doi: 10.1093/schbul/sbr058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C. Schizophrenia and theory of mind. Psychol. Med. 2004;34(3):385–389. doi: 10.1017/s0033291703001326. [DOI] [PubMed] [Google Scholar]
- Gao Y., Rogers J.C., Pauli R., Clanton R., Baker R., Birch P., Ferreira L., Brown A., Freitag C.M., Fairchild G., Rotshtein P. Neural correlates of theory of mind in typically-developing youth: influence of sex, age and callous-unemotional traits. Sci. Rep. 2019;9(1):1–12. doi: 10.1038/s41598-019-52261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding D.C., Pflum M.J. Theory of mind and psychometric schizotypy. Psychiatry Res. 2011;188:217–223. doi: 10.1016/j.psychres.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Gooding D.C., Johnson M., Peterman J.S. Schizotypy and altered digit ratios: a second look. Psychiatry Res. 2010;178(1):73–78. doi: 10.1016/j.psychres.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Guastella A.J., Hermens D.F., Van Zwieten A., Naismith S.L., Lee R.S.C., Cacciotti-Saija C., Scott E.M., Hickie I.B. Social cognitive performance as a marker of positive psychotic symptoms in young people seeking help for mental health problems. Schizophr. Res. 2013;149:77–82. doi: 10.1016/j.schres.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Herbert B.M., Pollatos O., Schandry R. Interoceptive sensitivity and emotion processing: an EEG study. Int. J. Psychophysiol. 2007;65(3):214–227. doi: 10.1016/j.ijpsycho.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Hirao K., Miyata J., Fujiwara H., Yamada M., Namiki C., Shimizu M., Sawamoto N., Fukuyama H., Hayashi T., Murai T. Theory of mind and frontal lobe pathology in schizophrenia: a voxel-based morphometry study. Schizophr. Res. 2008;105:165–174. doi: 10.1016/j.schres.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Holt R.J., Chura L.R., Lai M.C., Suckling J., Von Dem Hagen E., Calder A.J., Bullmore E.T., Baron-Cohen S., Spencer M.D. ‘Reading the mind in the eyes’: an fMRI study of adolescents with autism and their siblings. Psychol. Med. 2014;44(15):3215. doi: 10.1017/S0033291714000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung D.Y., Tsai C.L., Chiang L.C., Ma W.F. Screening nursing students to identify those at high risk of poor mental health: a cross-sectional survey. BMJ Open. 2019;9(6) doi: 10.1136/bmjopen-2018-025912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao C., Wang T., Peng X., Cui F. Impaired empathy processing in individuals with Internet Addiction Disorder: an event-related potential study. Front. Hum. Neurosci. 2017;11:498. doi: 10.3389/fnhum.2017.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone E.V., Ebmeier K.P., Miller P., Owens D.G.C., Lawrie S.M. Predicting schizophrenia: findings from the Edinburgh High-Risk Study. Brit. J. Psychiat. 2005;186:18–25. doi: 10.1192/bjp.186.1.18. [DOI] [PubMed] [Google Scholar]
- Kocsis-Bogar K., Kotulla S., Maier S., Voracek M., Hennig-Fast K. Cognitive correlates of different mentalizing abilities in individuals with high and low trait schizotypy: findings from an extreme-group design. Front. Psychol. 2017;8(922):1–14. doi: 10.3389/fpsyg.2017.00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapil T.R., Barrantes-Vidal N. Oxford Library of Psychology. The Oxford Handbook of Personality Disorders. 2012. Schizotypal personality disorder: an integrative review; pp. 437–477. [Google Scholar]
- Kwapil T.R., Brown L.H., Silvia P.J., Myin-Germeys I., Barrantes-Vidal N. The expression of positive and negative schizotypy in daily life: an experience sampling study. Psychol. Med. 2012;42(12):2555–2566. doi: 10.1017/S0033291712000827. [DOI] [PubMed] [Google Scholar]
- Lam B.Y., Raine A., Lee T.M. Effect of theory of mind and peer victimization on the schizotypy–aggression relationship. NPJ Schizophr. 2016;2(1):1–6. doi: 10.1038/npjschz.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzenweger M.F. Schizotypy, schizotypic psychopathology and schizophrenia. World Psychiatry. 2018;17(1):25–26. doi: 10.1002/wps.20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T.S., Liu C.M., Liu C.C., Hsieh M.H., Lin Y.T., Wang E.N., Hwang T.J., Chou T.L. Social cognition in schizophrenia: a network-based approach to a Taiwanese version of the Reading the Mind in the Eyes test. J. Formos. Med. Assoc. 2020;119:439–448. doi: 10.1016/j.jfma.2019.08.008. [DOI] [PubMed] [Google Scholar]
- Lomas T., Edginton T., Cartwright T., Ridge D. Men developing emotional intelligence through meditation? Integrating narrative, cognitive and electroencephalography (EEG) evidence. Psychol. Men. Masc. 2014;15(2):213–224. [Google Scholar]
- Ma W.-F., Wu P.-L., Yang S.-J., Cheng K.-F., Chiu H.-T., Lane H.-Y. Sensitivity and specificity of the Chinese version of the Schizotypal Personality Questionnaire-Brief for identifying undergraduate students susceptible to psychosis. Int. J. Nurs. Stud. 2010;47:1535–1544. doi: 10.1016/j.ijnurstu.2010.05.010. [DOI] [PubMed] [Google Scholar]
- MacCallum R.C., Zhang S., Preacher K.J., Rucker D.D. On the practice of dichotomization of quantitative variables. Psychol. Methods. 2002;7(1):19. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- Mark W., Toulopoulou T. Validation of the Chinese version of Community Assessment of Psychic Experiences (CAPE) in an adolescent general population. Asian J. Psychiatr. 2017;26:58–65. doi: 10.1016/j.ajp.2017.01.012. [DOI] [PubMed] [Google Scholar]
- Miettunen J., Veijola J., Freimer N., Lichtermann D., Peltonen L., Paunio T., Isohanni M., Joukamaa M., Ekelund J. Data on schizotypy and affective scales are gender and education dependent—study in the Northern Finland 1966 Birth Cohort. Psychiatry Res. 2010;178(2):408–413. doi: 10.1016/j.psychres.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Miller G.A., Chapman J.P. Misunderstanding analysis of covariance. J. Abnorm. Psychol. 2001;110(1):40. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Modinos G., Renken R., Shamay-Tsoory S.G., Ormel J., Aleman A. Neurobiological correlates of theory of mind in psychosis proneness. Neuropsychologia. 2010;48(13):3715–3724. doi: 10.1016/j.neuropsychologia.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Navarra-Ventura G., Fernandez-Gonzalo S., Turon M., Pousa E., Palao D., Cardoner N., Jodar M. Gender differences in social cognition: a cross-sectional pilot study of recently diagnosed patients with schizophrenia and healthy subjects. Can. J. Psychiatr. 2018;63(8):538–546. doi: 10.1177/0706743717746661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B., Yuen H.P., Wood S.J., Lin A., Spiliotacopoulous D., Bruxner A., Broussard C., Simmons M., Foley D.L., Brewer W.J., Francey S.M., Amminger G.P., Thompson A., McGorry P.D., Yung A.R. Long-term follow-up of a group at ultra high risk (“prodromal”) for psychosis: the PACE 400 study. JAMA Psychiat. 2013;70(8):793–802. doi: 10.1001/jamapsychiatry.2013.1270. [DOI] [PubMed] [Google Scholar]
- Overton D.J. 2015. Audiovisual Integration Deficits in Schizotypal Personality and Implications for Populations Diagnosed With Schizophrenia. (Doctoral dissertation) [Google Scholar]
- Raine A. Schizotypal personality: neurodevelopmental and psychosocial trajectories. Annu. Rev. Clin. Psychol. 2006;2:291–326. doi: 10.1146/annurev.clinpsy.2.022305.095318. [DOI] [PubMed] [Google Scholar]
- Ripoll L.H., Zaki J., Perez-Rodriguez M.M., Snyder R., Strike K.S., Boussi A., Bartz J.A., Ochsner K.N., Siever L.J., New A.S. Empathic accuracy and cognition in schizotypal personality disorder. Psychiatry Res. 2013;210(1):232–241. doi: 10.1016/j.psychres.2013.05.025. [DOI] [PubMed] [Google Scholar]
- Sabbagh M.A., Moulson M.C., Harkness K.L. Neural correlates of mental state decoding in human adults: an event-related potential study. J. Cognitive Neurosci. 2004;163:415–426. doi: 10.1162/089892904322926755. [DOI] [PubMed] [Google Scholar]
- Schwartzman D., Maravic K., Kranczioch C., Barnes J. Altered early visual processing components in hallucination-prone individuals. NeuroReport. 2008;19(9):933–937. doi: 10.1097/WNR.0b013e328301a640. [DOI] [PubMed] [Google Scholar]
- Shu I.W., Onton J.A., Prabhakar N., O’Connell R.M., Simmons A.N., Matthews S.C. Combat veterans with PTSD after mild TBI exhibit greater ERPs from posterior-medial cortical areas while appraising facial features. J. Affect. Disord. 2014;155:234–240. doi: 10.1016/j.jad.2013.06.057. [DOI] [PubMed] [Google Scholar]
- Song J., Wei Y., Ke H. The effect of emotional information from eyes on empathy for pain: a subliminal ERP study. PLoS One. 2019;14(12) doi: 10.1371/journal.pone.0226211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.W., Lin C.D., Yuan B., Huang L., Zhang W.X., Shen D.L. Person perception precedes theory of mind: an event related potential analysis. Neuroscience. 2010;170(1):238–246. doi: 10.1016/j.neuroscience.2010.06.055. [DOI] [PubMed] [Google Scholar]
- Wang Y., Liu W.-H., Li Z., Wei X.-H., Jiang X.-Q., Neumann D.L., Shum D.H.K., Cheung E.F.C., Chan R.C.K. Dimensional schizotypy and social cognition: an fMRI imaging study. Front. Behav. Neurosci. 2015;9(133) doi: 10.3389/fnbeh.2015.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn J.K., Lee J., Horan W.P., Green M.F. Using event related potentials to explore stages of facial affect recognition deficits in schizophrenia. Schizophrenia. Bull. 2008;34(4):679–687. doi: 10.1093/schbul/sbn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T., Wang W., Liu T., Chen D., Wang C., Li Y., Ma X., Tang X., Wu J., Deng Y., Zhao L. Increased local connectivity of brain functional networks during facial processing in schizophrenia: evidence from EEG data. Oncotarget. 2017;8(63) doi: 10.18632/oncotarget.20598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.H., Xu L.H., Cui H.R., Tang Y.Y., Wei Y.Y., Tang X.C., Liu X.H., Cao X.M., Li C.B., Wang J.J. Changes in correlation characteristics of time consumption and mind-reading performance in pre-onset and post-onset psychosis. Psychiatry Res. 2018;262:168–174. doi: 10.1016/j.psychres.2018.02.008. [DOI] [PubMed] [Google Scholar]