Highlights
-
•
Anti-Leptospira IgG seroprevalence was estimated on cats from Lisbon, Portugal via ELISA.
-
•
A mathematical model was applied to raw data to establish the real cut-off value of seroprevalence.
-
•
Of the 243 samples, 59.3% tested positive for anti-Leptospira IgG.
-
•
A positive correlation between low anti-Leptospira IgG and FIV+ was detected (p = 0.02).
-
•
No correlation was detected between anti-Leptospira IgG values and outdoor lifestyle.
Keywords: Leptospirosis, IgG, FIV positive, Free-roaming cats, One Health
Abbreviations: IgG, Immunoglobulin G; ELISA, enzyme-linked immunosorbent assay; FIV, Feline Immunodeficiency Virus infection; FeLV, Feline Leukemia Virus; WHO, World Health organization; PAHO, Pan American Health organization; MAT, microscopic agglutination tests; IDIU, Infectious Diseases Isolation Unit; VTH, Veterinary Teaching Hospital; CBC, complete blood count; CKD, Chronic Kidney Disease; USG, Urine Specific Gravity; ALP, serum alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase
Abstract
Leptospirosis is a zoonosis of global importance caused by Leptospira species. Rodents are the main reservoirs, known to shed the bacteria in urine, thus contaminating water and soil and infecting other animals and people. Leptospirosis has been re-emerging in both developing and developed countries including Europe. It has been hypothesized that cats could be asymptomatic carriers of Leptospira. This study aims to evaluate cats’ exposure to Leptospira in Lisbon, Portugal, by measuring IgG titres and correlating them with possible factors that may increase the risk of exposure in urban cats. Two hundred and forty-three samples were collected from the biobank. An ELISA test followed by a seroprevalence analysis using a finite mixture model was performed to detect and measure anti-Leptospira IgG antibodies titres. In parallel, a survey was conducted to identify possible risk factors for seropositivity.
According to the ELISA test protocol, only twenty-three cats (9.5%; 95% CI =(6.1%;13.9%)) could be considered as seropositive to Leptospira antigens. However, when the same data were analysed by the best different mixture models, one hundred and forty-four cats (59.3%; 95%CI = (52.8%; 65.5%)) could be classified as intermediate and high antibody responders to Leptospira antigens. Seropositivity to Feline Immunodeficiency Virus infection (FIV) was found to be the only significant risk factor associated with anti-Leptospira IgG antibodies. In conclusion, the present studies raises the possibility of a higher exposure of cats to Leptospira than previously thought due to the identification of a subpopulation of cats with intermediate antibody levels.
Introduction
Leptospirosis is one of the leading zoonotic diseases in terms of morbidity and mortality worldwide, often in regions where the burden of leptospirosis is underestimated. Globally, the total number of leptospirosis cases has been estimated at 1.03 million and 58.900 deaths every year (Costa et al., 2015 ). It is also considered by many the most widespread bacterial zoonotic disease (Costa et al., 2015) and a silent epidemic disease by the World Health organization (WHO), Pan American Health organization (PAHO) and Health and Climate Foundation (Schneider et al., 2013).
Leptospirosis is caused by an infection with spirochete bacterium of the genus Leptospira and affects humans as well as a broad spectrum of animal hosts. There are currently 27 Leptospira species as delineated by DNA–DNA hybridization (Masuzawa et al., 2019). Phylogenetic analysis of these species using the 16S rRNA gene has resulted in the broad classification of the species into pathogenic, saprophytic and intermediate (Perolat et al., 1998). Within the species Leptospira interrogans over 500 serovars are recognized (Caimi & Ruybal, 2020).
Although the respective molecular classification is not problematic for clinicians and individual treatment, it poses a problem regarding public health and epidemiology as it does not have enough discriminatory power to determine the infecting serovar (Levett, Paul, 2001). However, methods are being developed to improve this situation (Bezerra da Silva, Carvalho, Hartskeerl & Ho, 2011).
Most mammalian species are natural carriers of pathogenic Leptospira (Hartskeerl, Collares-Pereira & Ellis, 2011). These include feral, semi-domestic, farm and pet animals. Leptospirosis is commonly diagnosed in livestock species such as cattle, sheep, goats, horses, pigs and dogs (Pal, Mahendra, 1996).
Leptospira infection is also commonly described and investigated in dogs, while in cats it is less well described. Recently, the role of cats as concurrent carriers for illness has been questioned. Cats are the main predators for rodents and can act as reservoir hosts, with some studies proving transmission of pathogenic Leptospira between cats and other animals (Ojeda, Salgado, Encina, Santamaria & Monti, 2018). There is also the premise that feral cats or cats living in shelters are more likely to have been infected with these bacteria. Considering the predator-prey relationship cats have with rodents and their close proximity to Humans, their role as a potential source for this agent needs to be better evaluated. These last options are more likely to happen with stray cats, cats who have outdoor access or even cats that live in rural environments. The direct contact with other cats, dogs or cattle is also considered to be a risk factor for this infection (Arbour, Blais, Carioto & Sylvestre, 2012; Hartmann et al., 2013).
Laboratory diagnosis of leptospirosis is not straightforward and may involve tests to detect Leptospira (Musso & La Scola, 2013), leptospiral antigens, or leptospiral nucleic acid in animal tissues or body fluids and/or to detect anti-leptospiral antibodies. Serological testing includes microscopic agglutination tests (MAT), enzyme-linked immunosorbent assay (ELISA) and rapid immunomigration tests (Kodjo, Calleja, Loenser, Lin & Lizer, 2016; Lizer, Velineni, Weber, Krecic & Meeus, 2018).
Diagnosis of infection by antibody detection in cats is appealing since they are not currently vaccinated, and therefore, the chance of finding false positives is much smaller. Testing is not too expensive and it can be performed in veterinary hospitals with supporting diagnostic laboratories. However, the international market supply of Leptospira IgG ELISA kits applicable to cat samples is limited. For example, in Portugal, there is only one commercial kit available at the time of the study, developed by the Bioassay Technology Laboratory (BT Lab). Production of other Cat Leptospira IgG ELISA test kit had been discontinued, possibly due to lack of sales. The rationale for using antibody data is that the antibody concentrations in the serum could be an indicator of bacteria exposure, thus providing epidemiological information about cats which are currently or have been infected. Antibody quantification is usually done by means of traditional enzyme linked immunosorbent assays. Optical densities or titres in arbitrary units are then used for the subsequent data analysis. In this epidemiological scenario of extremely low frequency of disease, scarcity of ELISA tests to measure cat Leptospira IgG and weakness of these tests validation methods, it is timely to apply statistical approaches to determine in antibody data analysis for diseases like malaria to cat leptospirosis in an attempt to optimize an ELISA test result interpretation (Sepúlveda, Stresman, White & Drakeley, 2015).
The present study falls under the “One Health” scope, as cats are exposed to environmental risks and share a great proximity to their owners, consequently placing them at risk of contracting leptospirosis.
The objectives of this study were: (1) to determine the seroprevalence of Leptospira spp. antibodies in cats presented to the Veterinary Teaching Hospital (VTH) of the Veterinary Faculty (FMV) of the University of Lisbon (ULisboa) by analysing the data directly with a statistical modeling approach; (2) to investigate associated risk factors, namely indoor/outdoor lifestyle and presence of retroviral infection.
Materials and methods
Sample collection
Previously collected blood samples from 243 cats was used to assess the performance of an ELISA kit for the presence of anti-Leptospira IgG. Blood samples were collected from a biobank which was developed using cat's serum samples obtained from a well characterized population of cats. Biobank stored blood samples were previously collected by venipuncture of the jugular vein. To allow a better evaluation and simpler blood sample collections, cats were subjected to mild sedation with 0•2 to 0•5 mg/kg butorphanol solution sc (Dolorex, Intervet Portugal). Serum samples were collected after clotting of the sample had occurred by centrifugation (5000 g, 10 min), and were subsequently frozen at −80 °C until analysed. This population is composed of cats from three different locations: two different animal shelters in the Lisbon area; cats which went to a consultation at the Veterinary Teaching Hospital – University of Lisbon (VTH) and cats which were hospitalized at the Infectious Disease Unit at the hospital (IDIU). All samples were stored at −80 °C.
Data collection
Data was collected using clinical database software from 2014 to December 2018. Collected data included age, lifestyle (indoor or outdoor) and contact with other animals. Cat's lifestyle was considered unknown when this information was missing or it was stated that cats were indoor but had contact with other animals with unknown lifestyle. Cats were considered to have an indoor lifestyle if they had not been outdoors for more than 10 years.
Plasma samples and FIV/FeLV infection
A total of 243 samples were analysed. All blood samples were tested to confirm their viral infection status by means of commercially available ELISA kits (ViraCHEK/FIV and ViraCHEK/FeLV, Synbiotics). Therefore, two groups were set: one group of retroviral negative cats (Status FIV/Feline Leukemia Virus (FeLV) negative) and one group of positive-retroviral cats (which were positive for FIV, FeLV or both).
Leptospiral IGG screening
All samples were screened for the presence of anti- Leptospira IgG antibodies by using IgG ELISA test kit by Bioassay Technology Laboratory. The manufacturer's guidelines were followed for the making of this test and the OD values were read at 450 nm. The cut-off value to consider a sample positive was the sum of the value obtained for the negative control plus 0.15. For quality control purposes, both the OD value for a blank well (no solutions at all) and the OD for negative control had to be ≤ 1. For statistical purposes, data under analysis referred to the average antibody values from two independent replicates of ELISA performed in the same biological samples.
Estimation of IGG seroposivity to Leptospira antigens
Finite mixture models based on flexible Skew-Normal and Skew-t distributions were fitted to data. In theory, the basic assumption of these models is that the antibody distribution could represent different serological populations (e.g., seronegative population and different seropositive populations possibly describing different levels of exposure to Leptospira). Note that these mixture models were chosen, because they extend the classical mixture models based on normal and t distributions by introducing an additional parameter that controls the degree of asymmetry of the mixing distributions. The above models were estimated assuming one serological population (e.g., seronegative) to 5 different serological populations. In the case of models with more than one serological population, it was assumed that the serological population with the lowest average referred to hypothetical seronegative individuals, while the remaining serological populations referred to putative seropositive individuals with different degree of exposure to leptospira. Model estimation was performed based on the maximum likelihood method using the Expectation-Maximization algorithm. Akaike's Information Criterion (AIC), which is defined by the deviance minus twice the number of model parameters, was used to determine the best fitted model for the data. According to this criterion, the best model was the one showing with the lowest AIC estimate among all models tested (Supplementary Table 1).
After determining the best model for the data, the putative seronegative population was identified as the hypothetical serological population with the lowest antibody average. A cut-off for seropositivity was calculated using the estimated 99.9% quantile of the hypothetical seronegative population. Cats whose antibody values were above this cut-off, were considered seropositive and seronegative otherwise. After classifying each cat as either seropositive or seronegative, the seroprevalence of the sample was estimated by the proportion of the seropositive cats among all cats tested. Note that the choice of a cut-off value defining seropositivity was somehow arbitrary. Therefore, a more stringent cut-off value could have been used for estimating seroprevalence. Finally, univariate and multivariate logistic regression models were used to determine which factors were associated with seropositivity to leptospiral antigen.
All statistical analysis was conducted in the software R version 3.4.3. using the package mixsmsn to estimate finite mixture models (Prates, Cabral & Lachos, 2013) and glm function to fit regression to the corresponding seropositivity data. The significance level for statistical testing was specified at 5%.
Results
Characterization of the studied population: Retroviral status, lifestyle, other pets and concomitant diseases
One hundred and twenty two out of the 243 cats tested were seropositive for either FIV or FeLV (74 FIV positive, 37 FeLV positive and 11 FIV and FeLV co-infected) (Fig. 1A). Twelve and 159 cats (65.43%) had indoor and outdoor lifestyles, respectively, according to criteria defined in the section of data collection from Materials and Methods. The remaining 72 cats (29.63%) had an unknown lifestyle (Fig. 1B).
Fig. 1.
Sample distribution between different characteristics analysed. A – 50.21% (n = 122) were retroviral positive, of which 60.65% (n = 74) were FIV+, followed by 30.33% (n = 37) for FeLV+ and 9.02% (n = 11) had both retroviral infections; 49.79% (n = 121) were retroviral negative. B - 4.94% had an indoor lifestyle (n = 12), followed by 29.60% (n = 72) whose lifestyle could not be characterized, and 65.43% (n = 159) had access to the outdoor. C – 0.82% (n = 2) had close contact with other pets, followed by contact just dogs (2.42%, n = 6); 6.58% (n = 16) lived with other cats and dogs, followed by cats which lived alone (16.46%, n = 40); for 26.34% (n = 64), co-habitation with other animals could not be determined and 47.32% (n = 115) co-habited with other cats.
With respect to home contact with other animals, 115 cats had other cats in the same house, 64 cats were considered to have unknown lifestyles, 40 cats did not cohabit with other pets, 16 cats lived with other cats and dogs, 6 cats cohabited with dogs and 2 cats cohabited with other pets (birds, reptile, fish or others) (Fig. 1C).
Estimation of Leptospiral seroprevalence
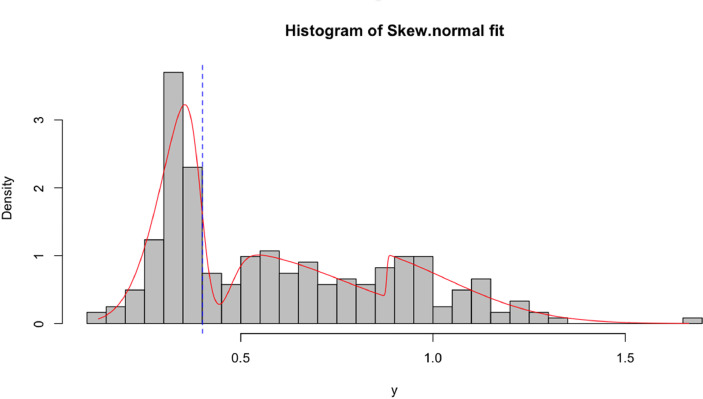
All samples were screened for the presence of anti- Leptospira IgG antibodies by using IgG ELISA test kit by Bioassay Technology Laboratory. Twenty-three out of the 243 cats (9.5%; 95% CI= (6.1%;13.9%)) analysed were tested positive using the seropositive cut-off value suggested by the manufacturer of the commercial ELISA used. An alternative seropositive cut-off value based on flexible finite mixture models was calculated for the same data. In brief, among all the ten mixture models fitted to the data (Supplementary Table 1), the lowest AIC estimate was obtained for the mixture model based on Skew-t distribution with three serological populations (Fig. 2). These serological populations were assumed to represent a seronegative population (with the lowest average) and two seropositive populations. Under this assumption, the cut-off for seropositivity was re-estimated at 0.40, which contrasts with the cut-off value of 1 as instructed by the manufacturer of the serological kits. Therefore, the intermediate serological population could be interpreted as putative seropositive cats on their way to sero-reversion. According to this new cut-off value, the seroprevalence to leptospirosis was re-estimated at 59.3% (n = 144/243; 95%CI = (52.8%; 65.5%)).
Fig. 2.
Positive samples distribution across population. The first peak represents samples positive for the presence of IgG anti-Leptospira. The dotted line represents the estimated cut-off value for the ELISA assay. The second and third peak represent samples with a low positivity result or false positives.
Analysis of potential factors contributing to Leptospiral seroprevalence
Regarding the analysis comparing retroviral infection and Leptospiral IgG seropositivity, 52.78% animals negative for retroviral infections tested positive for IgG anti-Leptospira (76/144) and 46.53% were positive for retroviral infections and IgG anti-Leptospira (67/144 - 38/67 for FIV+; 24/67 for FELV+ and 5/67 FIV+/ FELV+) (Fig. 3).
Fig. 3.
IgG Leptospira positive samples distribution within the retroviral status previously determined. Within positive IgG results, 3.47% (n = 5) had both retroviral infections, followed by FeLV+ individuals (16.67%, n = 24) and FIV+ (26.39%, n = 38). 52.78% (n = 76) were negative for retroviral infections.
From the risk assessment analysis, FIV is the only significant factor (p-value = 0.02 - (Table 1)). Four cats lived in animal refugees, with the other 140 positive cats being domestic. However, most of positive cats (n = 96) had an outdoor lifestyle (66.67%), with a small percentage being indoor cats (n = 5; 3.47%). 18.75% (27/144) of animals had some form of renal and/or hepatic laboratory parameter alteration and of these, 51.85% (14/27) were immunocompetent (data not shown).
Table 1.
Different variables analysed and respective significance values and intervals. Through multivariate logistic regression model, FIV is the only characteristic that can influence positiveness for IgG.
| Variable | Estimate (SE) | P | OR (CI 95%) |
|---|---|---|---|
| Intercept | −1.22 (1.87) | 0.52 | – |
| Gender (M) | 0.16 (0.43) | 0.72 | 1.17 (0.50–2.73) |
| FIV | −1.05 (0.45) | 0.02 | 0.35 (0.14–0.85) |
| FeLV | −0.15 (0.53) | 0.77 | 0.86 (0.30–2.42) |
| Lifestyle (Outdoor) | 0.73 (0.75) | 0.33 | 2.08 (0.48–9.07) |
| Dog | 1.01 (0.67) | 0.13 | 2.74 (0.73–10.22) |
| Cat | 0.98 (1.64) | 0.55 | 2.67 (0.11–66.29) |
| Other | 1.14 (1.99) | 0.57 | 3.13 (0.06–155.27) |
*CI – confidence interval; OR – odds-ratio; P – p-value; SE – Standard Error.
Discussion
This study comes under the perspective of One Health (where human and animal health are inexorably linked) with leptospirosis being an emerging infectious disease and zoonosis.
The frequency of clinical illness is low in cats, despite the presence of leptospiral antibodies in the feline free-roaming population indicating a high probability of leptospiral exposure. Serological surveys carried out in Europe found a serological prevalence of 10% in Glasgow (Agunloye & Nash, 1996), 18% in Munich (Weis et al., 2017) and 48% in France (Andre-Fontaine, Geneviève, 2006). Serovars Canicola, Grippotyphosa and Pomona have been isolated from cats (Adler & de la Peña Moctezuma, 2010). Clinical signs in cats are usually mild or not apparent, despite the presence of leptospiraemia and leptospiruria and histological evidence of renal and hepatic inflammation.
Several ELISAs have been developed and are primarily used for the detection of recent infections in dogs and livestock species. Problems with validation are a major constraint (OIE, 2018). A small number of ELISA tests for dogs (Hartman and Houten, 1984; Hartman, van den Ingh & Rothuizen, 1986) and cattle (Cousins, Robertson & Hustas, 1985) have been validated, using sequential serum samples from experimental animals but not beyond 6 months post-challenge. Laboratory variation and differences in host-specific humoral immune responses sometimes make correct assignment of antibody tests even more difficult. Many different serogroup antigens are tested in the assay, but false-negative results occur when the infecting serogroup is not included (Hartmann et al., 2013).
Evidence for a high seroprevalence in cats suggests that exposure in these species and its role for transmitting the bacteria to humans could be of more clinical importance in this species than previously recognized (Azócar-Aedo, Monti & Jara, 2014), posing a non-negligible public health risk to cat owners. However, the role of cats in the epidemiology of this zoonosis has not received much attention and clinical reports of leptospirosis in cats are rare (Arbour, Blais, Carioto and Sylvestre, 2012; Hartmann et al., 2013). Cats are predators of rodents (Loss & Marra, 2017; Parsons, Banks, Deutsch & Munshi-South, 2018), so prey-predator transmission between cats and rodents is likely to occur, and adding the free access to the outside or leash-walking, the concern should be reinforced. Scarce number of available ELISA tests and their limited validation for cats are a problem when analysing the frequency of exposure to Leptospira in the cat population.
Antibody quantification regarding diseases with extremely low prevalence, for which few ELISA tests are available plus the weakness of these tests’ validation methods, advocate for a deeper analysis of the raw data in order to improve the accuracy of the serological classification of individuals/cats. This method was applied for other diseases with low rates of seropositive infected individuals such as malaria, where alternative measures based on antibodies have gained recent interest due to the possibility of estimating past disease exposure in absence of infected individuals. (Sepúlveda et al., 2015). This can be done using flexible mathematical models that could distinguish seronegative individuals from seropositive ones, thus, allowing the estimation of the seroprevalence, the proportion of seropositive individuals in the sample. The main advantage of using finite mixture models is allowing the raw data to determine the cut-off value for seroprevalence, rather than using the pre-established cut-off value. Such models also help determine specificity and sensitivity from the serologic classification obtained, which cannot be done when using a universal cut-off value.
Out of the 243 cats tested, (50% positive for retroviral infections and the rest immunocompetent), 23 samples tested positive for Leptospira IgG following the manufacturer's instruction and the indicated cut-off value for seropositivity. Applying the best model for quantitative antibody data used to determine the seropositivity of each individual to Leptospira antigen, 59.25% (n = 144) tested positive for the presence of IgG against Leptospira. This value is higher when compared to other studies, with positive results ranging from 18% to 43% (Dybing, Jacobson, Irwin, Algar & Adams, 2017; Lapointe, Plamondon & Dunn, 2013; Weis et al., 2017).
In these previous studies, the presence of Leptospira species was determined by IgM or PCR. However, it is valid to compare such results with the one obtained in this study when it comes to contact with the bacteria, because IgG remains after IgM and the bacteria have been cleared from the host. Nevertheless, the cut-off value used in these studies was pre-established by the manufacturer, which poses the question of whether or not one can trust such seroprevalence results as it is not known how the cut-off used was calculated. Seroprevalence comparison would be easier if cut-off was determined through the use of finite mixture models. Comparisons would also be more reliable since it would be known how the cut-off had been calculated.
The first risk factor analysed was retroviral infection status. This study showed that FIV infected cats have significantly lower anti-Leptospira IgG titres when compared to immunocompetent or FeLV infected cats. As immunocompromised cats often present a decreased capability on creating a memory immune response (Machado et al., 2019), a possible explanation relies on the fact that FIV infected cats have an impaired memory response towards Leptospira antigens used in this ELISA. Contrary to expectations, the same is not observed in FeLV cats. This can be due to a lower number of FeLV-infected cats comparing to FIV-infected ones as well as a non-standardization of lifestyle status between groups, which can reflect a different antigen-stimulus and, consequently, differences on memory immune response. Further studies are needed to clarify this finding.
This study analysed other risk factors that may eventually lead to cats’ contact with Leptospira species, despite no significant association was found. Some individuals had missing data, which reduced the statistical power to detect an association with IgG Leptospira seropositivity. Although only one of the proposed risk factors was significantly associated with the anti-Leptospira IgG titres observed (FIV+), careful examination of outdoor lifestyle and sharing the household with other animals should be further analysed using more indoor confined cats. Furthermore, the rough definition of “outdoor lifestyle” can also justify that no association was found – better parameters should have been determined in order to categorize into outdoor or indoor lifestyle.
The imbalanced frequency between indoor and outdoor distribution within the studied population, as well as the number of other animals sharing the household, may difficult the statistical analysis regarding these variables and their association to seropositivity. This aspect is a limitation of the study.
Nineteen of the cats presented azotaemia (renal or post-renal). After careful record review, most of the azotaemic patients exhibit some form of post-renal azotaemia which, by itself, justifies renal tubular damage and impaired urine concentrating ability. However, there is evidence that Leptospira can lodge itself in the renal tissue of carrier species like the cat (Parsons et al., 2018). Anti-Leptospira IgG seropositivity show that these cats have had contact with the bacteria and, therefore, azotaemia due to renal colonization by the bacteria cannot be ruled out without doing further testing. Some studies confirm that seropositivity is significantly greater in Chronic Kidney Disease (CKD) diagnosed cats (Rodriguez et al., 2014). Although not conclusive, several clinical studies in human subjects also assert that there may be a strong association between Leptospira infection and the development of CKD, suggesting infection as a risk factor for CKD (Carrillo-Larco, Altez-Fernandez, Acevedo-Rodriguez, Ortiz-Acha & Ugarte-Gil, 2019; Yang, Chang & Yang, 2019).
Some of the sampled cats revealed changes in the complete blood count (CBC) (mainly leucocytosis, leukopenia and anemia). These changes can be explained by them presenting a suspicion of or diagnosed illness (urinary tract inflammation or infection, colangiohepatitis, among others). Many of these animals were FIV and/or FeLV infected, which can likely account for these CBC abnormalities in some part (Tvedten & Raskin, 2013). Overall, no consistent clinical signs pattern was associated with the IgG Leptospiral positive population.
The present work using a statistical analysis of the raw antibody data contributes to a better knowledge of feline leptospirosis exposure in the context of One Health. The more evidence is gathered by studies like this one, the easier it will be to raise public awareness for cats and their possible role in transmission of this zoonotic disease. In a future study, it would be interesting to evaluate the owner's Leptospira IgM/IgG seroprevalence, alongside their cats.
Conclusions
In conclusion, the direct analysis of antibody data provided evidence for an additional subpopulation of cats with intermediate antibody levels to Leptospira antigen. Since this putative subpopulation was considered as seronegative according to the commercial ELISA test, we hypothesize that the real exposure of cats to Leptospira bacteria might be in fact higher than previously reported and therefore, it might pose a health hazard for both animals and humans in the context of One Health. To assess the validity of this hypothesis, similar serological assessment should be conducted in the other cohorts of cats.
Funding
This work was supported by CIISA - Centro de Investigação Interdisciplinar em Sanidade Animal, Faculdade de Medicina Veterinária, Universidade de Lisboa, Lisboa, Portugal, Project UIDP/CVT/00,276/2020 (funded by FCT). The authors received funding from Boehringer Ingelheim Animal Health – Portugal for sponsoring the purchase of the ELISA kits.
Ethical approval
This work involved the use of non-experimental animals only (including owned or unowned animals and data from prospective or retrospective studies). Established internationally recognised high standards (‘best practice’) of individual veterinary clinical patient care were followed. Samples regarding shelters in the Lisbon area had previously been collected as part of a PhD project – this study was approved by the FMV's Ethics Committee CEBEA (CEBEA - CIISA502010). The remaining samples were collected as part of routine consultation at VTH or hospitalization at UIDI. In these situations, owners also signed a written consent, which allowed the use for their pet's clinical history and surplus of biological samples for research purposes.
Authors' contributions
JMS, SP, ROL and SG performed the experiments and analyzed the data. NS, TD and TN performed the statistical analysis and helped drafting and revising the manuscript. LT and VA contributed to the analysis, interpretation of data and revised the manuscript. SG and NS conceived the study, analyzed the data and participated in its coordination, helped to draft the manuscript and supervised throughout. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to acknowledge Dr. Pedro Fabrica, from Boehringer Ingelheim Animal Health – Portugal, for sponsoring the purchase of the ELISA kits.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.vas.2020.100144.
Contributor Information
Joana Moreira da Silva, Email: joanacmoreirasilva3@gmail.com.
Sara Prata, Email: saraprata@fmv.ulisboa.pt.
Tiago Dias Domingues, Email: tmdomingues@fc.ul.pt.
Rodolfo Oliveira Leal, Email: rleal@fmv.ulisboa.pt.
Telmo Nunes, Email: tnunes@fmv.ulisboa.pt.
Luís Tavares, Email: ltavares@fmv.ulisboa.pt.
Virgílio Almeida, Email: vsa@fmv.ulisboa.pt.
Nuno Sepúlveda, Email: Nuno.Sepulveda@lshtm.ac.uk.
Solange Gil, Email: solange@fmv.ulisboa.pt.
Appendix. Supplementary materials
References
- Adler B., de la Peña Moctezuma A. Leptospira and leptospirosis. Veterinary Microbiology. 2010;140:287–296. doi: 10.1016/j.vetmic.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Agunloye C.A.&, Nash A.S. Investigation of possible leptospiral infection in cats in Scotland. The Journal of Small Animal Practice. 1996;37:126–129. doi: 10.1111/j.1748-5827.1996.tb02360.x. [DOI] [PubMed] [Google Scholar]
- André-Fontaine G. Canine leptospirosis-Do we have a problem? Veterinary Microbiology. 2006;117:19–24. doi: 10.1016/j.vetmic.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Arbour J., Blais M.-.C., Carioto L., Sylvestre D. Clinical Leptospirosis in Three Cats (2001–2009) Journal of the American Animal Hospital Association. 2012;48:256–260. doi: 10.5326/JAAHA-MS-5748. [DOI] [PubMed] [Google Scholar]
- Azócar-Aedo L., Monti G., Jara R. Leptospira spp. in domestic cats from different environments: Prevalence of antibodies and risk factors associated with the seropositivity. Animals. 2014;4:612–626. doi: 10.3390/ani4040612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra da Silva J., Carvalho E., Hartskeerl R.& ., Ho P. Evaluation of the Use of Selective PCR Amplification of LPS Biosynthesis Genes for Molecular Typing of Leptospira at the Serovar Level. Current Microbiology. 2011;62:518–524. doi: 10.1007/s00284-010-9738-7. [DOI] [PubMed] [Google Scholar]
- Caimi K., Ruybal P. Leptospira spp., a genus in the stage of diversity and genomic data expansion. Infection, Genetics and Evolution: Journal of Molecular Epidemiology and Evolutionary Genetics in Infectious Diseases. 2020;81 doi: 10.1016/j.meegid.2020.104241. [DOI] [PubMed] [Google Scholar]
- Carrillo-Larco R.M., Altez-Fernandez C., Acevedo-Rodriguez J.G., Ortiz-Acha K., Ugarte-Gil C. Leptospirosis as a risk factor for chronic kidney disease: A systematic review of observational studies. PLOS Neglected Tropical Diseases. 2019;13:1–10. doi: 10.1371/journal.pntd.0007458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa F., Hagan J.E., Calcagno J., Kane M., Torgerson P., Martinez-Silveira M.S. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLOS Neglected Tropical Diseases. 2015;9:0–1. doi: 10.1371/journal.pntd.0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins D.V., Robertson G.M., Hustas L. The use of the enzyme-linked immunosorbent assay (ELISA) to detect the IgM and IgG antibody response to Leptospira interrogans serovars hardjo, pomona and tarassovi in cattle. Veterinary Microbiology. 1985;10:439–450. doi: 10.1016/0378-1135(85)90026-4. [DOI] [PubMed] [Google Scholar]
- Dybing N.A., Jacobson C., Irwin P., Algar D., Adams P.J. Leptospira species in feral cats and black rats from Western Australia and Christmas Island. Vector-Borne Zoonotic Dis. 2017;17:319–324. doi: 10.1089/vbz.2016.1992. [DOI] [PubMed] [Google Scholar]
- Hartman E.G., Houten M.V.A.N. Veterinary Immunology and Immunopathology. 1984;7:245–254. doi: 10.1016/0165-2427(84)90083-7. 1984. 7, 245–254. [DOI] [PubMed] [Google Scholar]
- Hartman E.G., van den Ingh T.S.G.A.M., Rothuizen J. Clinical, pathological and serological features of spontaneous canine leptospirosis. An evaluation of the IgM- and IgG-specific ELISA. Veterinary Immunology and Immunopathology. 1986;13:261–271. doi: 10.1016/0165-2427(86)90078-4. [DOI] [PubMed] [Google Scholar]
- Hartmann K., Egberink H., Pennisi M.G., Lloret A., Addie D., Belák S., Boucraut-Baralon, C., Frymus, T., Gruffydd-Jones, T., Hosie, M.J., Lutz, H., Marsilio, F., Möstl, K., Radford, A.D., Thiry, E., Truyen, U., & Leptospira Species Infection in Cats: ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery. 2013;15:576–581. doi: 10.1177/1098612X13489217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartskeerl R.A., Collares-Pereira M., Ellis W.A. Emergence, control and re-emerging leptospirosis: Dynamics of infection in the changing world. Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 2011;17:494–501. doi: 10.1111/j.1469-0691.2011.03474.x. [DOI] [PubMed] [Google Scholar]
- Kodjo A., Calleja C., Loenser M., Lin D., Lizer J. A rapid in-clinic test detects acute leptospirosis in dogs with high sensitivity and specificity. BioMed Research International. 2016;2016:1–3. doi: 10.1155/2016/3760191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe C., Plamondon I., Dunn M. Feline leptospirosis serosurvey from a Quebec referral hospital. The Canadian Veterinary Journal. La Revue Veterinaire Canadienne. 2013;54:497–499. [PMC free article] [PubMed] [Google Scholar]
- Levett P.N. Leptospirosis. Clinical Microbiology. 2001;14:296–326. doi: 10.1128/CMR.14.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizer J., Velineni S., Weber A., Krecic M., Meeus P. Evaluation of 3 serological tests for early detection of leptospira-specific antibodies in experimentally infected Dogs. Journal of veterinary internal medicine / American College of Veterinary Internal Medicine. 2018;32:201–207. doi: 10.1111/jvim.14865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loss S.R., Marra P.P. Population impacts of free-ranging domestic cats on mainland vertebrates. Frontiers in Ecology and the Environment. 2017;15:1633. [Google Scholar]
- Machado I., Carvalho A., Gomes J., Cunha E., Tavares L., Almeida V.& .; Gil, S. 2019. Feline retrovirus-infected hospitalised cats – aetiology, co-infections and survival rates. Clinical / research abstracts accepted for presentation at ISFM Congress 2019; Cavtat, Croatia; 2019. pp. 843–852. 26-30 June 2019. [Google Scholar]
- Masuzawa T., Saito M., Nakao R., Nikaido Y., Matsumoto M., Ogawa M. Molecular and phenotypic characterization of Leptospira johnsonii sp. nov., Leptospira ellinghausenii sp. nov. and Leptospira ryugenii sp. nov. isolated from soil and water in Japan. Micro and Immuno. 2019;63(3–4):89–99. doi: 10.1111/1348-0421.12671. [DOI] [PubMed] [Google Scholar]
- Musso D., La Scola B. Laboratory diagnosis of leptospirosis: A challenge. Journal of Microbiology, Immunology and Infection. 2013;46:245–252. doi: 10.1016/j.jmii.2013.03.001. [DOI] [PubMed] [Google Scholar]
- O.I.E. World Organization for Animal Health; Paris, France: 2018. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2019; pp. 503–551. Chapter 3.1.12. [Google Scholar]
- Ojeda J., Salgado M., Encina C., Santamaria C., Monti G. Evidence of interspecies transmission of pathogenic leptospira between livestock and a domestic cat dwelling in a dairy cattle farm. The Journal of Veterinary Medical Science / the Japanese Society of Veterinary Science. 2018;80:1305–1308. doi: 10.1292/jvms.16-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal M. Leptospirosis: A contemporary zoonosis. The Veterinarian. 1996;20:11–12. [Google Scholar]
- Parsons M.H., Banks P.B., Deutsch M.A., Munshi-South J. Temporal and space-use changes by rats in response to predation by feral cats in an urban ecosystem. Frontiers in Ecology and Evolution. 2018;6:1–8. doi: 10.3389/fevo.2018.00146. [DOI] [Google Scholar]
- Perolat P., Chappel R.J., Adler B., Baranton G., Bulach D.M., Billinghurst M.L. Leptospira fainei sp. nov., isolated from pigs in Australia. International Journal of Systematic Bacteriology. 1998;48:851–858. doi: 10.1099/00207713-48-3-851. [DOI] [PubMed] [Google Scholar]
- Prates M.O., Cabral C.R.B., Lachos V.H. Mixsmsn: Fitting finite mixture of scale mixture of skew-normal distributions. Journal of Statistical Software. 2013;54:1–20. doi: 10.18637/jss.v054.i12. [DOI] [Google Scholar]
- Rodriguez J., Blais M.-.C., Lapointe C., Arsenault J., Carioto L., Harel J. Serologic and urinary PCR survey of leptospirosis in healthy cats and in cats with kidney disease. Journal of Veterinary Internal Medicine / American College of Veterinary Internal Medicine. 2014;28:284–293. doi: 10.1111/jvim.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M.C., Jancloes M., Buss D.F., Aldighieri S., Bertherat E., Najera P., Galan, D.I., Durski, K., &. Leptospirosis: A silent epidemic disease. International Journal of Environmental Research and Public Health. 2013;10:7229–7234. doi: 10.3390/ijerph10127229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepúlveda N., Stresman G., White M.T., Drakeley C.J. Current mathematical models for analyzing anti-malarial antibody data with an eye to malaria elimination and eradication. Journal of Immunology Research. 2015 doi: 10.1155/2015/738030. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tvedten H., Raskin R.E. Leukocyte Disorders. In: Willard M., Tvedten H., editors. Small Animal Clinical Diagnosis by Laboratory Methods. 5th ed. Elsevier Saunders; St. Louis, Missouri: 2013. pp. 126–155. eds. [Google Scholar]
- Weis S., Rettinger A., Bergmann M., Llewellyn J.R., Pantchev N., Straubinger R.K. Detection of Leptospira DNA in urine and presence of specific antibodies in outdoor cats in Germany. Journal of Feline Medicine and Surgery. 2017;19:470–476. doi: 10.1177/1098612X16634389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.Y., Chang C.H., Yang C.W. Leptospirosis and chronic kidney disease. Translational Research in Biomedicine. 2019;7:27–36. doi: 10.1159/000500380. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.