Highlights
-
•
MSlys is a choline binding protein from pneumococcal MS1 phage.
-
•
Planktonic and biofilm S. pneumoniae cells are affected by MSlys treatment.
-
•
MSlys is active against isolates from otitis media infections and works in the conditions commonly found in this environment.
Keywords: Endolysin, Streptococcus pneumoniae, MSlys, Antibacterial, Biofilm
Abstract
Despite the use of pneumococcal conjugate vaccines, the number of infections related to Streptococcus pneumoniae continues to be alarming.
Herein, we identified, characterized the MSlys endolysin encoded in the phage MS1. We further tested its antimicrobial efficacy against planktonic and biofilm cells, assessing the culturability of cells and biofilm structure by scanning electron microscopy, and confocal laser scanning microscopy.
The modular MSlys endolysin consists of an amidase catalytic domain and a choline-binding domain. MSlys is active against isolates of children with otitis media, and conditions close to those found in the middle ear. Treatment with MSlys (2 h, 4 μM) reduced planktonic cultures by 3.5 log10 CFU/mL, and 24- and 48-h-old biofilms by 1.5 and 1.8 log10 CFU/mL, respectively. Imaging of the biofilms showed thinner and damaged structures compared to control samples.
The recombinantly expressed MSlys may be a suitable candidate for treating pneumococcal infections, including otitis media.
1. Introduction
S. pneumoniae is a common colonizer of the nasopharynx of healthy humans since early infancy [1]. It is also responsible for several infectious diseases such as otitis media and sinusitis, as well as life-threatening invasive diseases, including pneumonia, sepsis, and meningitis [1,2]. Despite the implementation of pneumococcal conjugate vaccines into national immunization programs, S. pneumoniae continues to be a common cause of morbidity and mortality worldwide [2], resulting in 1.6 million deaths every year [3].
Biofilms are communities of microorganisms embedded in a self-produced extracellular polymeric matrix and attached to a surface [4], being a common cause of persistent bacterial infections [5]. Growth in biofilms benefits bacteria protecting against environmental stresses, host immune defenses, as well as antimicrobial agents [4]. S. pneumoniae tolerates high antibiotic concentrations and belongs to the World Health Organization priority list of pathogens in need for R&D of new antimicrobials [6].
S. pneumoniae phages, such as Dp-1 and Cp-1, have been isolated and thoroughly characterized. However, they do not infect encapsulated pneumococcal strains, which are highly prevalent in disease, limiting phage therapeutic use [7]. More recently, phage MS1, which is related to the Dp-1 phage (average nucleotide identity of 73.3 % on 62.3 % of aligned nucleotides), was isolated [8]. Unlike phages, phage endolysins can effectively kill this pathogen. Shortly after the isolation of Dp-1 and Cp-1 phages, the lytic enzymes Pal and Cpl-1 were identified. Their ability to kill S. pneumoniae when applied exogenously was, later on, demonstrated when used alone [9], together [10], and even combined with antibiotics [11]. Their antibacterial potential in animal models mimicking nasopharyngeal colonization [9], bacteremia [12], endocarditis [13], otitis media [14], among others, provided excellent results.
Endolysins identified in pneumococcal phages and also many proteins found in the pneumococcal cell wall (e.g., LytA autolysin) are characterized by a modular organization. The N-terminal of these is responsible for the catalytic activity and the C-terminal for the cell binding. This cell-binding domain is highly conserved (with the only natural exception being Cpl-7 from the Cp-7 phage), consisting of several choline-binding repeats that belong to the CW_binding_1 family and conferring specificity to these enzymes, which require the presence of choline in the cell wall teichoic acids for substrate recognition. Beyond the uniqueness, this characteristic makes the emergence of resistance negligible [15].
The work described herein focuses on the in silico identification, recombinant expression, and characterization of MSlys, a novel natural endolysin encoded in the pneumococcal phage MS1. Furthermore, its antibacterial activity against S. pneumoniae planktonic and biofilm cells, as well as its stability under different pH and temperature, was evaluated.
2. Materials and methods
2.1. Bacteria, phages, growth conditions, and plasmids
S. pneumoniae R6st was obtained from the Félix d'Hérelle Reference Center for Bacterial Viruses (Université Laval, Quebec, Canada) along with phages Dp-1 and MS1. Different S. pneumoniae isolates and Streptococcus and non-streptococcal species were tested to determine the lytic spectrum of MSlys (Table 1). S. pneumoniae were grown at 37 °C with 5% CO2 in Todd Hewitt Broth (THB) supplemented with 2 % (w/v) yeast extract (THBye) or in solid Tryptic Soy Broth (TSB) (TSB + 1.2 % (w/v) agar with 5% sheep blood) (TSAsb). H. influenzae was grown in Brain Heart Infusion Broth (BHI) supplemented with 10 μg/mL NAD (VWR) and 10 μg/mL Hemin (BHINH, 37 °C, 5% CO2). M. catarrhalis was grown in BHI (37 °C, 5% CO2), and S. aureus, P. aeruginosa, and E. faecalis were grown in TSB at 37 °C in aerobic conditions. Escherichia coli TOP10 and BL21(DE3) (Invitrogen, ThermoFisher Scientific) were grown in Lysogeny Broth (LB) in liquid or solid form (1.2 % (w/v) of agar) at 37 °C. The plasmid pET-28a(+) was purchased from Novagen (EMD Biosciences, Inc., Germany).
Table 1.
The spectrum of activity of MSlys.
| Species | Strain | Origin or Source | Growth conditions | Endolysin activity |
|---|---|---|---|---|
| Streptococcus pneumoniae | R6st | Félix d’Hérelle Reference Center for Bacterial Viruses | 37 °C, 5% CO2 | + |
| P046 | CIBER de Enfermedades Respiratorias, Madrid, Spain - Centro de Investigaciones Biológicas | + | ||
| MEF7_I1 (serotype 6A/B) | Middle ear fluid of children with otitis media, Hospital de Braga | + | ||
| MEF8_I2 | + | |||
| MEF12_I1 | + | |||
| MEF13_I1 | + | |||
| MEF14_I3 | + | |||
| MEF15_I3 (serotype 6A/B) | Middle ear fluid of children with otitis media, Trofa Saúde Hospital Braga Sul | + | ||
| MEF16_I1 | + | |||
| MEF18_I1 (serotype 19F) | + | |||
| MEF19_I1 | + | |||
| MEF26_I1 (serotype 6A/B) | + | |||
| MEF27_I1 | Middle ear fluid of children with otitis media, Trofa Saúde Hospital Braga Centro | + | ||
| MEF28_I1 | + | |||
| MEF29_I2 (serotype 6A/B) | + | |||
| MEF33_I2 (serotype 11A) | + | |||
| MEF35_I1 | + | |||
| C905005 (serotype 1) | Sputum, Hospital de Braga | + | ||
| I891301 (serotype 15B/C) | + | |||
| I895832 (serotype 1) | + | |||
| I903728 (serotype 19 F) | + | |||
| U944982 (serotype 4) | Blood culture, Hospital de Braga | + | ||
| Streptococcus mitis | I3124473 | Pus, Hospital de Braga | 37 °C, 5% CO2 | + |
| I310333 | Ocular, Hospital de Braga | + | ||
| U374030 | Pus, Hospital de Braga | + | ||
| Streptococcus agalactiae (Group B) | I302171 | Placenta, Hospital de Braga | 37 °C, 5% CO2 | – |
| I303139 | Perianal abscess, Hospital de Braga | – | ||
| Streptococcus anginosus (Anginosus group) | U365575 | Pus, Hospital de Braga | 37 °C, 5% CO2 | – |
| I298561 | Blood culture, Hospital de Braga | – | ||
| Streptococcus bovis (Group D) | U344929 | Urine, Hospital de Braga | 37 °C, 5% CO2 | – |
| U238779 | Pleural washout, Hospital de Braga | – | ||
| Streptococcus constellatus (Anginosus group) | I303868 | Periamydral abscess, Hospital de Braga | 37 °C, 5% CO2 | – |
| Streptococcus pyogenes (Group A) | C124992 | Ocular, Hospital de Braga | 37 °C, 5% CO2 | – |
| U164028 | Auricular, Hospital de Braga | – | ||
| Streptococcus salivarius | I299612 | Blood, Hospital de Braga | 37 °C, 5% CO2 | – |
| Streptococcus Group C | U269790 | Hemolysis, Hospital de Braga | 37 °C, 5% CO2 | – |
| Streptococcus Group G | U284971 | Hemolysis, Hospital de Braga | 37 °C, 5% CO2 | – |
| I196480 | Oropharynx, Hospital de Braga | – | ||
| Haemophilus influenzae | C894248 | Sputum, Hospital de Braga | 37 °C, 5% CO2 | – |
| Moraxella catarrhalis | U225012 | Ocular, Hospital de Braga | 37 °C, 5% CO2 | – |
| Staphylococcus aureus | ATCC 6358 | Human lesion, American Type Culture Collection | 37 °C | – |
| Pseudomonas aeruginosa | PAO1 (DSM 22644) | Infected wound, DSMZ – German Collection of Microorganisms and Cell Cultures GmbH | 37 °C | – |
| Enterococcus faecalis | I809 | Urine, Hospital de Braga | 37 °C | – |
The activity of MSlys was considered positive (+) if it resulted in a reduction of the optical density greater than 30 % after 2 h of incubation at 37 °C, using PBS as a negative control.
2.2. In silico identification and characterization of MSlys
The genome of Streptococcus phage MS1 (Accession No. KY629621.2) was analyzed to identify putative endolysins, and similarities to other endolysins searched using BLASTP. The search for functional domains was completed using both Pfam [16] and PROSITE [17], the molecular weight and isoelectric point predicted using ExPASy ProtParam [18], and protein alignment with other streptococcal endolysins was done with ClustalW 2.1 [19].
2.3. Cloning
DNA was extracted from phage lysates using the phenol:chloroform extraction method [20]. Open reading frames (ORFs) encoding MSlys and Pal were amplified by PCR (see primers, Table 2) using Phusion DNA Polymerase (ThermoScientific, MA, USA) following the manufacturer’s instructions. PCR products were purified (D4005, ZYMO RESEARCH, CA, USA), double-digested with NdeI and HindIII-HF (NEB, MA, USA), and ligated to the pET-28a(+) vector using T4 DNA ligase. The insertion of the correct sequences into the plasmids was confirmed by Sanger sequencing. pET-28a_MSlys and pET-28a_Pal were propagated and maintained in E. coli TOP10.
Table 2.
List of primers used to clone the endolysins and their main characteristics. The restriction sites of the enzymes are underlined.
| Endolysin | Primer name | Primer sequence | Restriction enzyme | Tm (°C) | % GC | Product size (bp) |
|---|---|---|---|---|---|---|
| MSlys | MS_FW | GGTTTCATATGGGAGTAAATATTGATGAAGGCGTTGC | NdeI | 59.2 | 41.4 | 906 |
| MS_RV | CCCAAGCTTCTACTTAGTAGTAATGAGCCCGTCAGG | HindIII | 57.7 | 48.1 | ||
| Pal | Pal_FW | GGTTTCATATGGGAGTCGATATTGAAAAAGGCGTTGC | NdeI | 61.1 | 44.8 | 909 |
| Pal_RV | CCCAAGCTTTTAAACTTTAGCAGTAATGAGCCCGTCCGG | HindIII | 61.7 | 46.7 |
2.4. Recombinant protein expression and purification
E. coli BL21(DE3) cells with the recombinant plasmids were grown in 100 mL LB supplemented with 50 μg/mL of kanamycin (120 rpm, Orbital Shaker ES-20/60, BIOSAN, Latvia) to an optical density at 620 nm (OD620) of 0.5. Recombinant protein expression was induced with 0.5 mM IPTG, and cells incubated overnight (16 °C, 200 rpm, Orbital Shaker MIR-S100, PHCbi, Japan). Cells were harvested (9000 ×g, 30 min, 4 °C), resuspended in 5 mL of cold lysis buffer (20 mM NaH2PO4, 0.5 M NaCl, pH 7.4), and disrupted through 3 cycles of freeze-thawing (−80 °C to 30 °C). This was followed by 10 cycles of sonication on ice (30-sec pulse, 30-sec pause, 30 % amplitude, Cole-Parmer Ultrasonic processor, CP-750, Illinois, USA). Insoluble cell debris was removed by centrifugation (9000 ×g, 20 min, 4 °C). The supernatant was collected and filtered through a 0.22 μm PES membrane. Purification was performed through 0.5 mL Ni2+-NTA agarose (HisPur™ Ni-NTA Resin, Thermo Scientific) stacked in a gravity flow column using imidazole concentrations (25−300 mM) [21]. Purified proteins were analyzed by SDS-PAGE, followed by BlueSafe staining (NZYTech, Lisbon, Portugal). Endotoxins of the first and second elutions were removed using ToxOut Rapid Endotoxin Removal Kit (BioVision Inc., Gentaur Molecular Products BVBS, Kampenhout, Belgium). The buffer was exchanged to PBS (8 g/L NaCl, 0.2 g/L, 1.44 g/L Na2HPO4, 0.24 g/L KH2PO4, pH 7.4) using Amicon Ultra® 0.5 mL (Merck). The protein concentration was determined using the Pierce™ BCA Protein Assay Kit (Thermo Scientific).
2.5. Circular dichroism (CD)
Secondary structures of MSlys and Pal were analyzed by circular dichroism (CD) spectroscopy in the far-UV region (190−260 nm), using a Jasco J-1500 CD spectrometer as performed previously [22]. Secondary structure estimation was made using CDSSTR [23] and CONTINLL [24] routine of the DICHROWEB [25], and the analysis complemented using PSIPRED [26].
2.6. Lytic spectra
The spectrum of MSlys was determined using different bacteria (Table 1). Bacteria were grown overnight, cells harvested [5000 ×g, 5 min, room temperature (RT)], and resuspended in PBS. MSlys (20 μL, final concentration of 2 μM) or PBS (20 μL, negative control) and 180 μL of the bacterial culture were added and incubated at 37 °C with or without 5 % CO2 (depending on the species tested). The endolysin was considered active if there was at least a 30 % decrease in OD620 within the 2 h assay.
For serotyping of S. pneumoniae strains, a multiplex PCR using sets of primers that target different serotypes (1, 2, 3, 4, 5, 6A/B, 6C/D, 7A/F, 7C, 9 V/9A, 9 N/L, 11A, 12 F/12A/12B/44/46, 14, 15B/C, 16 F, 17 F, 18A/B/C, 19A, 19 F, 20, 22 F/22A, 23 F, 23B, 23A and 33 F/33A/37) as well as the capsular polysaccharide (cps) locus was performed (Table S1) [27,28]. Bacterial colonies were resuspended in 200 μL of NZY bacterial cell lysis buffer (NZYTech, Lisbon, Portugal), followed by heating at 95 °C for 15 min. The Xpert Taq DNA Polymerase (Grisp, Porto, Portugal) was used for the multiplex PCR reaction following the manufacturer’s instructions.
2.7. Effect of pH, temperature, and choline on the activity of MSlys
S. pneumoniae R6st cells grown overnight were diluted 1:100 in fresh THBye and allowed to grow until the exponential phase. The thermostability of MSlys was assessed by heating the endolysin samples at several temperatures for 30 min. These samples were after cooled on ice (20 min), cells harvested (5000 ×g, 5 min, RT), and resuspended in PBS. The influence of pH on MSlys activity was tested on harvested cells resuspended in universal buffer (150 mM KCl, 10 mM KH2PO4, 10 mM Na3C6H5O7, and 10 mM H3BO3) adjusted to a range of pH (5–10). For the evaluation of choline supplementation, harvested cells were resuspended in PBS containing different choline chloride (VWR) concentrations. The antibacterial effect of MSlys (2 μM) was determined as described above (37 °C, 5% CO2), with the OD620 of MSlys-treated S. pneumoniae measured after 30 min, 1 h, and 2 h.
2.8. Antibacterial activity against planktonic cells
Antibacterial assays were performed as previously [22] with slight modifications. S. pneumoniae R6st cells, grown in THBye overnight, were 1:100 diluted in fresh media and grown to the exponential phase. After, they were 100-fold diluted in PBS. Each culture (50 μL) was incubated at 37 °C with 5 % CO2, for 30 min, 1 h or 2 h with 50 μL of endolysin at 2 μM or 4 μM, or PBS (negative control). The effect was evaluated by 10-fold diluting in saline (0.9 % (w/v) NaCl) and plating on TSAsb to quantify the number of colony-forming units (CFU).
2.9. Colony biofilm formation and endolysin treatment
Overnight grown S. pneumoniae R6st cells were diluted to approximately 1 × 106 CFU/mL, and biofilms formed for 24, and 48 h on polycarbonate membranes (0.1 μm, 25 mm, UV-sterilised on both sides, Whatman, GE Healthcare Life Sciences, PA, USA) as previously described [29]. Membranes were placed with the shiny side up onto TSAsb plates and inoculated with 50 μL of culture, and incubated upright (37 °C, 5 % CO2), being transferred to a fresh plate every 24 h. For colony biofilm treatment with MSlys, membranes were transferred to 6-well plates, and 50 μL of MSlys (final concentration of 4 μM) or PBS (negative control) were applied on the whole surface and incubated for 30 min, 1 h or 2 h.
2.9.1. Viable biofilm cell quantification
After MSlys or PBS treatment, the membranes were transferred to PBS (1 mL), vortexed thoroughly, 10-fold diluted in saline, and plated on TSAsb to quantify the number of CFUs.
2.9.2. Scanning electron microscopy (SEM)
Biofilms formed on polycarbonate membranes treated with MSlys or PBS were fixed with 2.5 % (v/v) glutaraldehyde (4 °C, 1 h), and rinsed with PBS. Samples were dehydrated in ethanol series (30, 50, 70, 80, 90 % (v/v), and absolute), sputtered with gold, and analyzed by SEM (FEI Quanta 650 FEG, ThermoFisher Scientific).
2.9.3. Confocal laser scanning microscopy (CLSM)
Biofilms formed on polycarbonate membranes were fixed as above, rinsed with PBS, stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) according to the manufacturer’s instructions (Invitrogen), and analyzed by CLSM (LSM780, Zeiss, Jena, Germany).
2.10. Statistical analysis
Mean and standard deviations (SD) were determined for at least three independent experiments. Statistical comparison was performed using Two-Way ANOVA and Tukey’s multiple comparison statistical test, using GraphPad Prism 6. Differences were considered as statistically different if P ≤ 0.05 (95 % confidence interval).
3. Results
3.1. MSlys characterization
3.1.1. In silico and circular dichroism (CD) analysis
The genome of the lytic Streptococcus phage MS1 was checked for annotated endolysins. The gene ms1_61 annotated as a lysin was identified and named mslys. MSlys consists of 295 amino acids (AA) being a modular endolysin. Bioinformatics analysis shows that it comprises a catalytic domain with N-acetylmuramoyl-l-alanine amidase activity (Amidase_5; PF05382.13) and a cell-binding domain (or choline-binding domain) composed of 5 cell-wall binding repeats (CW; PS51170) (Fig. S1). MSlys has a theoretical MW of 34.3 kDa and a PI of 4.81. Although Pal is already well characterized, it was subjected to the same tools for analysis. Thus, Pal has 296 AAs encoding an N-terminal Amidase_5 domain and a choline-binding domain containing 6 CBRs (Fig. S1), having a MW of 34.5 kDa and a PI of 4.95. BLASTp of MSlys showed 81 % identity with Pal, and ClustalW showed a pairwise identity of 81.4 %, showing 241 identical and 55 different amino acids (Fig. S2).
MSlys and Pal were overexpressed in E. coli and purified, showing single bands on SDS-PAGE (Fig. S3) at the expected molecular weight (35.1 and 35.3 kDa, respectively, for MSlys and Pal, considering the N-terminal His-tag).
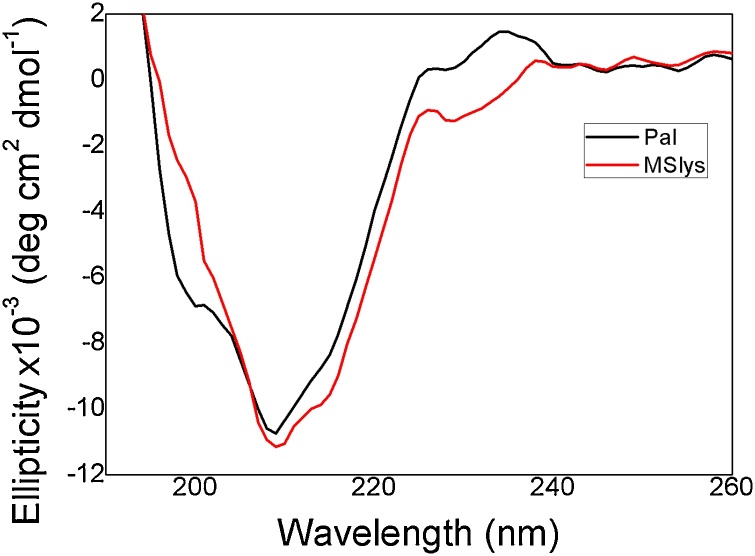
The CD spectrum of Pal showed two maximum peaks (at 220 and 240 nm), one minimum peak (at 209 nm) and one shoulder (at 200 nm), while in the MSlys spectrum, two maximum peaks (at 220 and 240 nm) and only one minimum peak (at 209 nm) were observed (Fig. 1). Estimates of secondary structures by the deconvolution of the CD spectra indicate that both MSlys and Pal endolysins fold predominantly in β-sheets (43 % as β-sheet, 4% as α-helices, 21 % as turns, and 32 % unordered). This matches with the β-sheet prevalence predicted by PSIPRED (Fig. S4).
Fig. 1.
Circular Dichroism spectra of pneumococcal endolysins. The spectrum of MSlys and Pal were analyzed in the far-UV (190-260 nm) using proteins dialyzed in PBS (pH 7.4).
3.1.2. Lytic spectra
The lytic spectra of MSlys (Table 1) showed high specificity towards S. pneumoniae, killing all strains recovered from otitis media infections. These clinical isolates were collected from male and female children aged 1–5 years. Moreover, MSlys was effective against encapsulated pneumococcal strains with different serotypes, and also lysed the unencapsulated R6st (R6 streptomycin resistant) strain and the P046 strain (double lytA lytC mutant descended from the strain R6) (Table 1 and Fig. S5). Besides S. pneumoniae, MSlys also lysed Streptococcus mitis while all other streptococci and non-streptococci species were not affected by MSlys.
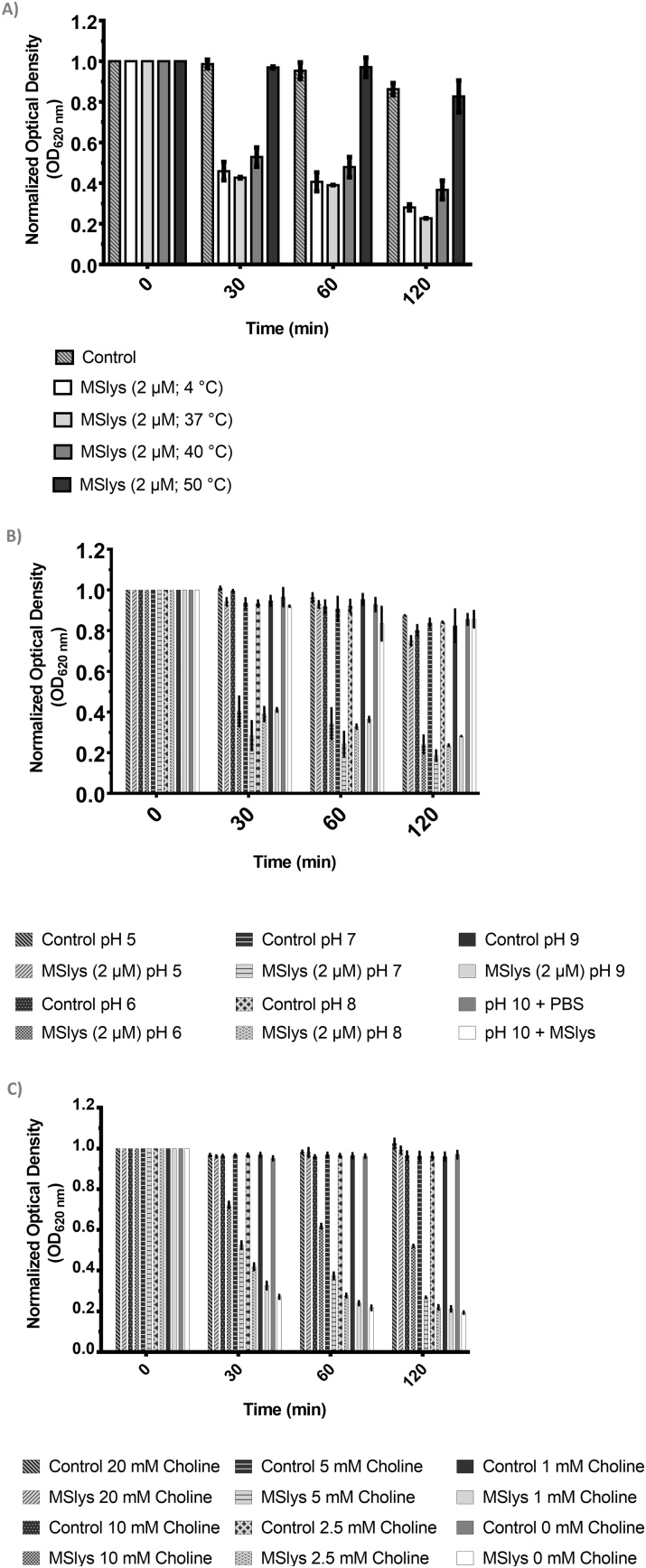
3.1.3. Effect of pH, temperature, and choline on the activity of MSlys
MSlys remained active after 30 min of incubation at 37 °C, slightly decreased activity after incubation at 40 °C, and became complete inactive after incubation at 50 °C (Fig. 2a). Also, MSlys remained active between a pH of 6.0–9.0 (Fig. 2b). Supplementation of the lysis reaction with 2.5 mM or less of choline did not significantly affect MSlys activity against S. pneumoniae after 2 h of treatment. However, 5 mM and, more clearly, 10 mM of choline reduced the action of MSlys against pneumococcal cells, with 20 mM of choline completely inhibiting the activity of the endolysin.
Fig. 2.
Influence of the temperature, pH and choline on the activity of MSlys. a) The endolysin was incubated at different temperatures for 30 min, cooled on ice for about 20 min and then used to treat S. pneumoniae cells suspended in PBS. b) The endolysin was used to treat S. pneumoniae cells suspended in universal buffer at different pH. c) The endolysin was used to treat S. pneumoniae cells suspended in PBS containing different concentrations of choline. The reduction in the optical density reduction of S. pneumoniae R6st cells after treatment with MSlys (2 μM) for 2 h at 37 °C was monitored. Normalized data are shown as mean ± SD.
3.2. Antibacterial activity against planktonic cells
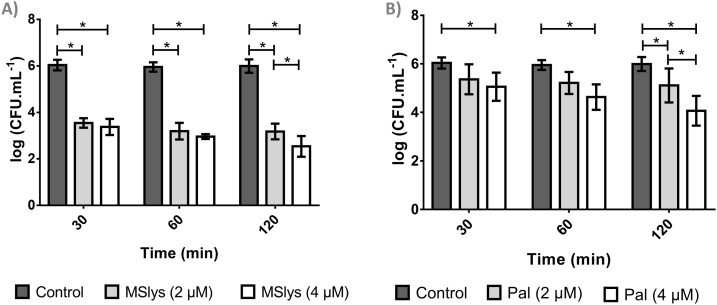
The activity of MSlys and Pal against S. pneumoniae R6st cells was assessed at two different concentrations (2 μM≃70 μg/mL and 4 μM≃140 μg/mL) (Fig. 3).
Fig. 3.
The logarithmic number of S. pneumoniae R6st cells after 30, 60, or 120 min of treatment with a) MSlys (2 or 4 μM) or b) Pal (2 or 4 μM) in comparison with control (PBS). Data are shown as mean ± SD. Differences were considered statistically significant if P ≤ 0.05 (*).
MSlys at 2 μM reduced the number of cells significantly (2.5 log10 CFU/mL) in 30 min (P ≤ 0.05) compared to the control. The decrease continued until 2 h (2.9 log10 CFU/mL). No significant differences were obtained between 2 and 4 μM of MSlys after 1 h post-treatment (P > 0.05). However, after 2 h, MSlys at a final concentration of 4 μM was significantly (P ≤ 0.05) more pronounced, reducing the viable cells counts by 3.5 log10 CFU/mL.
Pal treatment at 2 μM during 30 min or 1 h resulted in no significant differences compared with the control (P > 0.05), and led, after 2 h, to a reduction of 0.88 log10 CFU/mL compared with the controls. Pal at 4 μM had enhanced antibacterial effect at all time points assessed (P ≤ 0.05), resulting in an average reduction of 1.93 log10 CFU/mL after the 2 h treatment. Nonetheless, MSlys showed significantly higher antibacterial activity compared to Pal (P ≤ 0.05).
3.3. Antibacterial activity of MSlys against biofilm cells
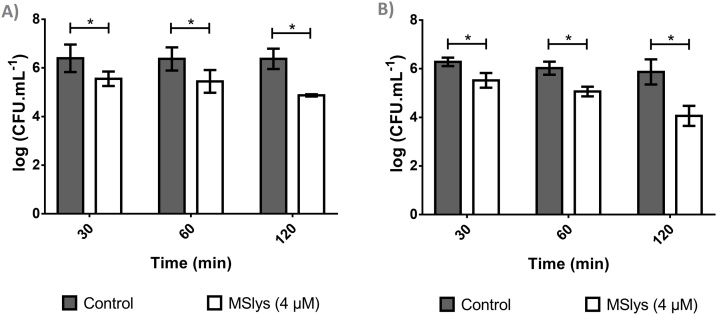
Control biofilms reached 6.39 ± 0.57 log10 CFU/mL after 24 h and 6.28 ± 0.17 log10 CFU/mL after 48 h (Fig. 4). MSlys significantly reduced the number of 24-h-old biofilms in all the time points tested (P ≤ 0.05), reducing 0.84, 0.92, and 1.50 log10 CFU/mL after 30 min, 1 h, and 2 h, respectively. MSlys also reduced 48-h-old biofilms, achieving approximately the same reductions (0.76, 0.80, and 1.80 log10 CFU/mL after 30 min, 1 h, and 2 h, respectively). S. pneumoniae usually undergo autolysis to escape phagocytosis, but if autolysis had occurred, a CFU/mL decrease in the controls would be observed.
Fig. 4.
The logarithmic number of S. pneumoniae R6st cells from a) 24-h or b) 48-h biofilms after 30, 60, or 120 min of treatment with MSlys (4 μM) in comparison with control (PBS). Data are shown as mean ± SD. Differences were considered statistically significant if P ≤ 0.05 (*).
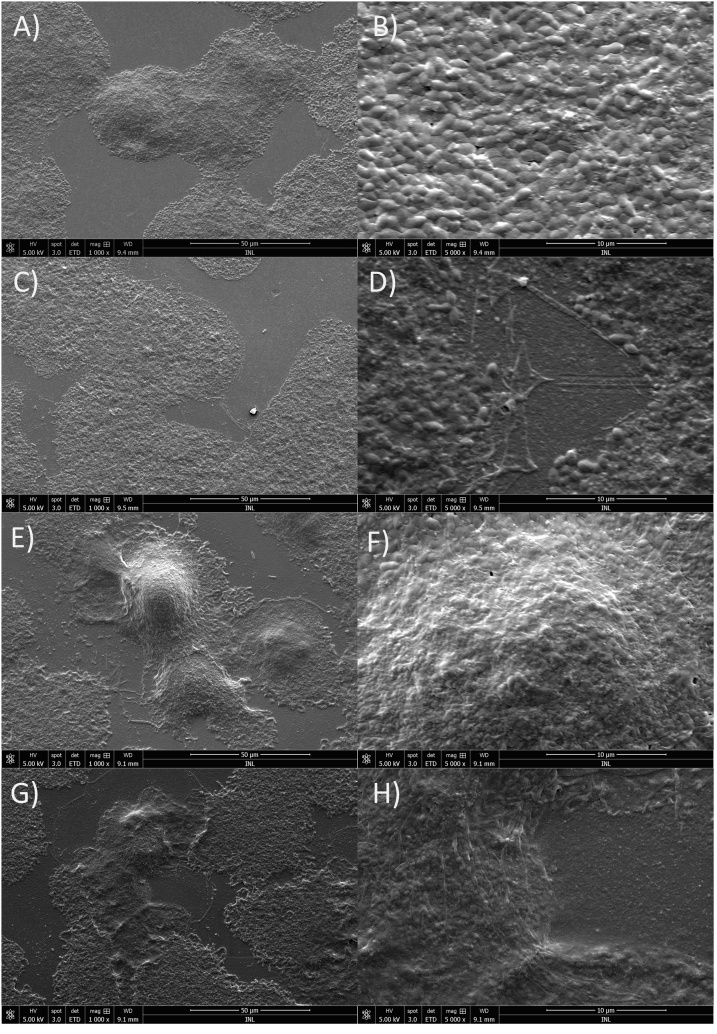
The effect of 2 h of MSlys treatment on 24-h and 48-h-old biofilms evaluated using SEM (Fig. 5) showed cells covering the polycarbonate membrane forming in some areas thick clusters (Fig. 5a and b). MSlys damaged the cells and increased the amount of cell debris in the surface (Fig. 5c and d). The 48-h biofilms were denser with thicker cell clusters (Fig. 5e and f). Once more, MSlys was able to damage these 48-h-old biofilms, increasing the amount of cell debris (Fig. 5g and h).
Fig. 5.
SEM micrographs showing the effect of 2 h treatment with MSlys (4 μM) on 24 h- and 48 h-biofilms of S. pneumoniae R6st: a) and b) 24 h-biofilm control (PBS-treated); c) and d) 24-h biofilm treated with MSlys; e) and f) 48 h-biofilm control (PBS-treated); g) and h) 48 h-biofilm treated with MSlys.
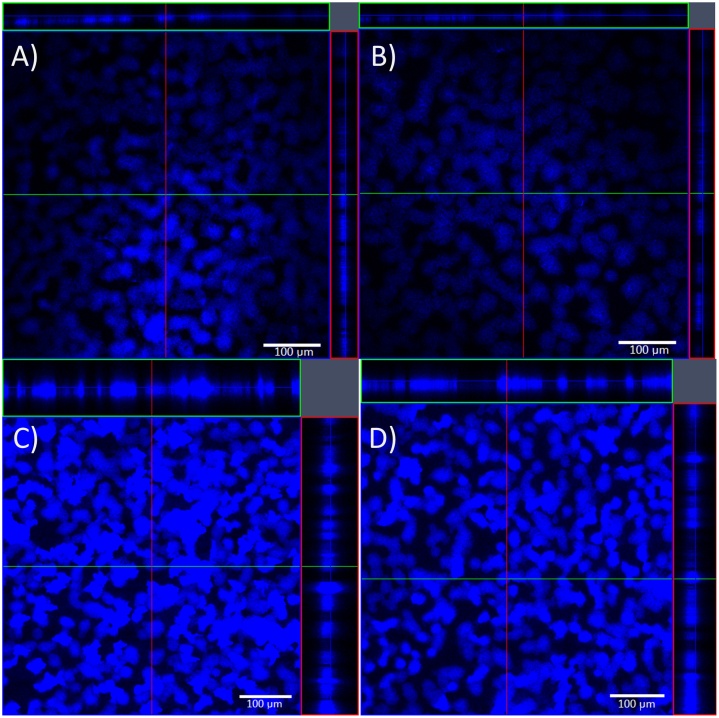
Taking into account the z-axis plot profile (mean>20), the average thickness of the 24-h-old biofilms was 45.33 ± 1.15 μm, and of the 48-h-old was 132.00 ± 28.62 μm. CLSM analysis showed that MSlys caused changes in the biofilm thickness (Fig. 6), indicating that this endolysin lysed pneumococcal cells within the biofilm structures. The average thickness of 24-h-old biofilms after MSlys treatment was 12.67 ± 1.15 μm. The biofilm thickness reduction was not as visible in the 24-h-old biofilms treated with MSlys (see Fig. 6a and b), compared to the 48-h-old treated biofilms that showed an average thickness of 87.00 ± 4.24 μm (see Fig. 6c and d).
Fig. 6.
CLSM micrographs (magnification 100×) showing the effect of 2 h treatment with MSlys (4 μM) on 24 h- and 48 h-biofilms of S. pneumoniae R6st: a) 24 h-biofilm control (PBS-treated); b) 24-h biofilm treated with MSlys; c) 48 h-biofilm control (PBS-treated); d) 48 h-biofilm treated with MSlys.
4. Discussion
The virulent MS1 phage is related to phage Dp-1, presenting an average nucleotide identity of 73.3 % on 62.3 % of the aligned nucleotides [8]. In our study, we recombinantly expressed the endolysin of phage MS1 (MSlys) and Pal endolysin from Dp-1. MSlys and Pal endolysins are similar, being both modular proteins with a catalytic module belonging to the Amidase_5 family and a choline-binding module. The catalytic domain (N-acetylmuramoyl-l-alanine amidase) is responsible for the cell wall degradation through hydrolysis of the amide bond between the muramic acid and the l-alanine. The binding domain is responsible for the attachment to choline residues present in the pneumococcal envelope [30]. In silico analysis showed that the protein sequences differ in the number of cell wall-binding repeats (CW), with MSlys having 5 CW while Pal presents 6. However, the number of CW obtained differs using different software. For instance, Pal shows 6 [15,30], and 7 CW in published literature [31]. Some authors hypothesized that the affinity for choline was related to the number of CW. Still, other studies have refuted this assumption, showing that the increase in the number of CW seems to be associated with a stronger choline-binding, but this still needs to be validated [15].
The antibacterial effect of MSlys was specific for S. pneumoniae, including strains isolated from the middle ear fluid of children with otitis media. It has been demonstrated that most clinical pneumococcal strains express capsule [32]. However, non-encapsulated S. pneumoniae (NESp) have been isolated from patients with otitis media [33,34] and are reported as a potential causative agent of chronic or recurrent otitis media [35]. Although only a few of the middle ear fluid isolates belonging to serotypes 6A/B, 11A and 19 F were identified, MSlys was effective against these capsule expressing strains, as well as against five other encapsulated S. pneumoniae strains (serotypes 1, 4, 15B/C, 19 F) isolated from sputum and blood. Some serotypes identified are covered by the 13-valent pneumococcal conjugate vaccine (1, 4, 6A, 6B, 19 F), which is included in the national immunization program in Portugal since 2015 [36]. MSlys also killed an unencapsulated strain lacking the autolysin gene, showing that its activity is independent of the host autolysin. Besides S. pneumonie, MSlys lysed S. mitis but did not affect other Streptococcus species tested and non-streptococcal species. This specificity is in agreement with previous reports for choline-binding proteins, such as Pal, Cpl-1, and PL3 [31]. Due to the presence of the choline-binding domain, it was already expected that MSlys would be specific for bacteria containing choline in their cell walls. For instance, Pal and Cpl-1 kill at a lower rate Streptococcus oralis and S. mitis, which incorporate choline in their cell walls [9,10], and the chimeric enzyme PL3, derived from Pal, killed S. oralis, Streptococcus pseudopneumoniae, and S. mitis type [37]. The specific binding of MSlys to pneumococcal strains and a few related species is advantageous, since this provides a targeted killing and prevents collateral effects on commensal bacteria and dysbiosis [21]. In contrast, Cpl-7 and Cpl-7S have a different binding domain, composed of 3 identical CW_7 tandem repeats, that confer the ability to degrade pneumococcal cell walls containing either choline or ethanolamine. These endolysins lyse a broader range of bacteria, including S. pneumoniae and other Gram-positive pathogens [38,39].
In this work, Pal structure analysis using CD agreed with a previous report [37]. The authors theorized that the CD signature of Streptococcus phage endolysins Pal and PL3, with two maximum peaks (at 220−240 nm) and the negative band (peak in PL3 and shoulder in Pal at 200 nm) corresponded to a fingerprint of the Amidase_5 domain. MSlys, which also encodes an Amidase_5 domain, does not present a negative peak or shoulder (at 200 nm). Both MSlys and Pal secondary structures are mostly composed of β-sheets. In a previous study, deconvolution of the CD spectrum showed that the secondary structure content of Pal corresponded to 45 % β-strands, 7% α-helices, 21 % turns, and 24 % unordered [40], being close to the values that were obtained herein. The same authors suggested an influence of choline in the tertiary and quaternary structures. Considering the similarity between Pal and MSlys, the same fact can be hypothesized for MSlys. The rich β-sheet content of Pal and MSlys are different from other pneumococcal endolysins characterized by similar methods, such as Cpl-7 and derived engineered enzymes (Cpl-7S, Cpl-711), which are predicted to have rich α-helix content [38,39,41], but less different from Cpl-1 (19 % α-helices, 32 % β-sheet, 28 % β-turn, 21 % random coil) [42].
MSlys was stable after exposure until 37 °C, with a slight stability decrease starting at 40 °C. MSlys was stable between a pH of 6.0–9.0, just like Pal [31]. These biochemical properties are fundamental, allowing MSlys to kill in conditions found in the middle ear during infection. The mean ear temperature is 36.4 ± 0.61 °C, increasing by 1.0–1.5 °C when feverish [43], while the mean pH of the middle ear fluid of children with otitis media varies between 8.55, 8.33, and 7.92 in the case of mucous, serous-mucous, and serous secretions [44]. Being a choline-binding protein, MSlys might be inhibited by the presence of choline (in the tens of millimolar range) or choline analogs, such as esters of bicyclic amines, that compete with the choline present in the pneumococcal cell wall [45]. Indeed, the activity of MSlys was entirely inhibited by supplementation of the lysis reaction with 20 mM of choline.
Despite the similarity between MSlys and Pal, in this study, the activity of MSlys against S. pneumoniae R6st planktonic cells was significantly better, after 2 h, compared to Pal using 2 and 4 μM endolysin concentrations. MSlys killed faster, decreasing the number of cells considerably after 30 min, and continued to decline until the end of the experiment. Overall, MSlys reduced the number of viable cells by 3.5 logarithmic units, whereas a maximum decrease of 1.93 log10 CFU/mL was obtained using 4 μM of Pal after 2 h. The anti-pneumococcal effect was demonstrated to be concentration-dependent, with a lower concentration of MSlys needed compared to Pal.
S. pneumoniae biofilms are a significant concern, having been identified in the middle ear mucosa specimens of children diagnosed with chronic otitis media with effusion [46] and in adenoid samples from children with recurrent acute otitis media [47]. MSlys was able to kill cells from 24 and 48-h-old biofilms, reducing the viable cell counts after 2 h by approximately 1.5 (96.84 %) and 1.8 log10 CFU/mL (98.42 %), respectively. Pal was previously reported to reduce nearly 90 % of biofilm-cells after 4 h of treatment [31]. However, the authors decided to use strain P046 (a double lytA lytC mutant of the R6 strain), which is unable to autolyze, for their biofilm assays (96-well plates, 14–16 h, 34 °C, 5 % CO2). Although the method of biofilm formation used was different, increased activity of MSlys against 24-h biofilms and already after 2 h was observed in this study compared to reported for Pal. In a subsequent investigation, treatment of S. pneumoniae P046 24-h-old biofilms with the engineered endolysin Cpl-711 at 1 μg/mL for 2 h killed about 4 logs of the bacterial population. At the same time, Cpl-1 and Cpl-7 reduced biofilms cells by approximately 1.5 logarithmic units [41]. The activity of the engineered endolysin is undoubtedly much higher than MSlys. However, the values obtained with the natural phage-encoded enzymes Cpl-1 and Cpl-7 are comparable to the ones observed for MSlys. The synergy between the enzymes Cpl-711 and PL3 against S. pneumoniae biofilms formed at 34 °C for 14−16 h was also reported [48]. Treatment with the combination of 0.5 × MIC of the endolysins for 1 h reduced cells in biofilms by more than 4.0 log10 CFU/mL, representing an increase of 3.6 logs compared to the sum of activities of the individual Cpl-711 or PL3 treatments. So, combining MSlys with another endolysin can be another strategy to enhance their antibacterial activity against pneumococcal biofilms. The potential of MSlys against pneumococcal biofilms was corroborated by SEM, where damaged cells and an increase of cell debris due to the endolysin were observed, and also by CLSM, where a significant difference in biofilm thickness was observed between treated and control samples. This result seems to be similar to the one obtained by previous authors [31]. These authors showed that two S. pneumoniae endolysins disintegrated biofilm, analysed by crystal violet (CV) staining of the total biofilm biomass, resulting in a reduction of around 70 % (Cpl-7), and 55 % (Cpl-1). Due to the similarity between MSlys and Pal, one would expect that their action towards biofilms would be comparable. However, this was not observed since Pal did not damage the biofilm structures as analyzed both by CLSM and CV staining [31].
During the last few years, protein engineering has improved properties of S. pneumoniae endolysins, i.e., has amplified their antimicrobial activity, increased their plasma half-time, and their capacity to pass through the negatively charged bacterial envelope. These improvements have been accomplished by the inclusion of new residues (Cpl-1 dimer) [49], inversion of the charge of the cell wall binding domain (Cpl-7S) [39], and domain swapping/fusion (Cpl-711, PL3) [37,41]. The natural MSlys is not as efficient as engineered endolysins; nevertheless, it presents excellent features against S. pneumoniae.
In summary, we characterized a novel natural pneumococcal endolysin, which is structurally very similar to Pal. MSlys was shown to be very effective against S. pneumoniae planktonic and biofilm cells. Furthermore, we showed that its activity is specific for S. pneumoniae and S. mitis, lysing pneumococcal strains isolated from otitis media infections. Also, MSlys was shown to be active in conditions commonly found in the middle ear during disease.
Funding
MDS acknowledges the Portuguese Foundation for Science and Technology (FCT) grant (SFRH/BD/128825/2017). This study was supported by the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UID/BIO/04469/2019unit and BioTecNorte operation (NORTE-01-0145-FEDER-000004) funded by the European Regional Development Fund under the scope of Norte2020 - Programa Operacional Regional do Norte. This project also received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 713640. This study was also supported by the grant PTDC/CVT-CVT/29628/2017 [POCI-01-0145-FEDER-029628].
CRediT authorship contribution statement
Maria Daniela Silva: Conceptualization, Methodology, Investigation, Writing - original draft. Hugo Oliveira: Investigation. Alberta Faustino: Investigation. Sanna Sillankorva: Conceptualization, Methodology, Writing - review & editing, Funding acquisition, Resources, Supervision.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
The authors acknowledge the Department of Otolaryngology of Hospital de Braga and Trofa Saúde Hospital Braga Centro and Braga Sul for collecting the middle ear fluid samples of children with otitis media. The authors acknowledge Dr. Pedro García from both Centro de Investigaciones Biológicas (CSIC) and CIBER de Enfermedades Respiratorias, Madrid, Spain, for providing the double lytA lytC R6 mutant S. pneumoniae P046 strain.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00547.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Weiser J.N., Ferreira D.M., Paton J.C. Streptococcus pneumoniae: transmission, colonization and invasion. Nat. Rev. Microbiol. 2018;16:355–367. doi: 10.1038/s41579-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade A.L., Toscano C.M., Minamisava R., Costa P.S., Andrade J.G. Pneumococcal disease manifestation in children before and after vaccination: what’s new? Vaccine. 2011;29:C2–C14. doi: 10.1016/j.vaccine.2011.06.096. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization WHO Pneumococcal conjugate vaccine for childhood immunization - WHO position paper. Bull. Epidemiol. Inf. Receiv. 2007;82:93–104. http://www.ncbi.nlm.nih.gov/pubmed/17380597 [PubMed] [Google Scholar]
- 4.Donlan R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costerton J.W. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 6.Tacconelli E., Magrini N., Kahlmeter G., Singh N. World Heal. Organ; 2017. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; pp. 1–7. [Google Scholar]
- 7.López R., García E. Recent trends on the molecular biology of pneumococcal capsules, lytic enzymes, and bacteriophage. FEMS Microbiol. Rev. 2004;28:553–580. doi: 10.1016/j.femsre.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Kot W., Sabri M., Gingras H., Ouellette M., Tremblay D.M., Moineau S. Complete genome sequence of Streptococcus pneumoniae virulent Phage MS1. Genome Announc. 2017;5:4–5. doi: 10.1128/genomeA.00333-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loeffler J.M. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science. 2001;294:2170–2172. doi: 10.1126/science.1066869. [DOI] [PubMed] [Google Scholar]
- 10.Loeffler J.M., Fischetti V.A. Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and -resistant Streptococcus pneumoniae strains. Antimicrob. Agents Chemother. 2003;47:375–377. doi: 10.1128/AAC.47.1.375-377.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Djurkovic S., Loeffler J.M., Fischetti V.A. Synergistic killing of Streptococcus pneumoniae with the bacteriophage lytic enzyme Cpl-1 and penicillin or gentamicin depends on the level of penicillin resistance. Antimicrob. Agents Chemother. 2005;49:1225–1228. doi: 10.1128/AAC.49.3.1225-1228.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loeffler J.M., Djurkovic S., Fischetti V.A. Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect. Immun. 2003;71:6199–6204. doi: 10.1128/IAI.71.11.6199-6204.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Entenza J.M., Loeffler J.M., Grandgirard D., Fischetti V.A., Moreillon P. Therapeutic effects of bacteriophage Cpl-1 lysin against Streptococcus pneumoniae endocarditis in rats. Antimicrob. Agents Chemother. 2005;49:4789–4792. doi: 10.1128/AAC.49.11.4789-4792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCullers J.A., Karlström Å., Iverson A.R., Loeffler J.M., Fischetti V.A. Novel strategy to prevent otitis media caused by colonizing Streptococcus pneumoniae. PLoS Pathog. 2007;3:e28. doi: 10.1371/journal.ppat.0030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maestro B., Sanz J. Choline binding proteins from Streptococcus pneumoniae: a dual role as enzybiotics and targets for the design of new antimicrobials. Antibiotics. 2016;5:21. doi: 10.3390/antibiotics5020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finn R.D., Coggill P., Eberhardt R.Y., Eddy S.R., Mistry J., Mitchell A.L., Potter S.C., Punta M., Qureshi M., Sangrador-Vegas A., Salazar G.A., Tate J., Bateman A. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 2016;44:D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sigrist C.J.A., De Castro E., Cerutti L., Cuche B.A., Hulo N., Bridge A., Bougueleret L., Xenarios I. New and continuing developments at PROSITE. Nucleic Acids Res. 2013 doi: 10.1093/nar/gks1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gasteiger E., Hoogland C., Gattiker A., Duvaud S., Wilkins M.R., Appel R.D., Bairoch A. Proteomics Protoc. Handb. Humana Press; Totowa, NJ: 2005. Protein identification and analysis tools on the ExPASy server; pp. 571–607. [DOI] [Google Scholar]
- 19.Larkin M.A., Blackshields G., Brown N.P., Chenna R., Mcgettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., Thompson J.D., Gibson T.J., Higgins D.G. Clustal W and Clustal X version 2.0. Bioinformatics. 2007 doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J.F., Russell D.W. third edition. Cold Spring Harbor Laboratory Press; 2001. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- 21.Harhala M., Nelson D., Miernikiewicz P., Heselpoth R., Brzezicka B., Majewska J., Linden S., Shang X., Szymczak A., Lecion D., Marek-Bukowiec K., Kłak M., Wojciechowicz B., Lahutta K., Konieczny A., Dąbrowska K. Safety studies of pneumococcal endolysins Cpl-1 and Pal. Viruses. 2018;10:638. doi: 10.3390/v10110638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliveira H., Vilas Boas D., Mesnage S., Kluskens L.D., Lavigne R., Sillankorva S., Secundo F., Azeredo J. Structural and enzymatic characterization of ABgp46, a novel phage endolysin with broad anti-Gram-negative bacterial activity. Front. Microbiol. 2016;7:1–9. doi: 10.3389/fmicb.2016.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Compton L.A., Johnson W.C. Analysis of protein circular dichroism spectra for secondary structure using a simple matrix multiplication. Anal. Biochem. 1986;155:155–167. doi: 10.1016/0003-2697(86)90241-1. [DOI] [PubMed] [Google Scholar]
- 24.Van Stokkum I.H.M., Spoelder H.J.W., Bloemendal M., Van Grondelle R., Groen F.C.A. Estimation of protein secondary structure and error analysis from circular dichroism spectra. Anal. Biochem. 1990 doi: 10.1016/0003-2697(90)90396-Q. [DOI] [PubMed] [Google Scholar]
- 25.Whitmore L., Wallace B.A. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004 doi: 10.1093/nar/gkh371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGuffin L.J., Bryson K., Jones D.T. The PSIPRED protein structure prediction server. Bioinformatics. 2000 doi: 10.1093/bioinformatics/16.4.404. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention, Conventional PCR Serotype Deduction Protocols, (n.d.). https://www.cdc.gov/streplab/pneumococcus/resources.html.
- 28.Pai R., Gertz R.E., Beall B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniaeisolates. J. Clin. Microbiol. 2006;44:124–131. doi: 10.1128/JCM.44.1.124-131.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merritt J.H., Kadouri D.E., O’Toole G.A. Curr. Protoc. Microbiol. 2005. Growing and analyzing static biofilms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheehan M.M., García J.L., López R., García P. The lytic enzyme of the pneumococcal phage Dp-1: a chimeric lysin of intergeneric origin. Mol. Microbiol. 1997;25:717–725. doi: 10.1046/j.1365-2958.1997.5101880.x. [DOI] [PubMed] [Google Scholar]
- 31.Domenech M., Garciá E., Moscoso M. In vitro destruction of Streptococcus pneumoniae biofilms with bacterial and phage peptidoglycan hydrolases. Antimicrob. Agents Chemother. 2011;55:4144–4148. doi: 10.1128/AAC.00492-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurola P., Erkkilä L., Kaijalainen T., Palmu A.A., Hausdorff W.P., Poolman J., Jokinen J., Kilpi T.M., Leinonen M., Saukkoriipi A. Presence of capsular locus genes in immunochemically identified encapsulated and unencapsulated Streptococcus pneumoniae sputum isolates obtained from elderly patients with acute lower respiratory tract infection. J. Med. Microbiol. 2010;59:1140–1145. doi: 10.1099/jmm.0.016956-0. [DOI] [PubMed] [Google Scholar]
- 33.Xu Q., Kaur R., Casey J.R., Sabharwal V., Pelton S., Pichichero M.E. Nontypeable Streptococcus pneumoniae as an otopathogen. Diagn. Microbiol. Infect. Dis. 2011;69:200–204. doi: 10.1016/j.diagmicrobio.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keller L.E., Robinson D.A., McDaniel L.S. Nonencapsulated Streptococcus pneumoniae: emergence and pathogenesis. MBio. 2016;7:1–12. doi: 10.1128/mBio.01792-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murrah K.A., Pang B., Richardson S., Perez A., Reimche J., King L., Wren J., Swords W.E. Nonencapsulated Streptococcus pneumoniae causes otitis media during single-species infection and during polymicrobial infection with nontypeable Haemophilus influenzae. Pathog. Dis. 2015;73:1–8. doi: 10.1093/femspd/ftu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Portuguese Directorate-General of Health . Norma Da Direção Geral Da Saúde; 2017. Programa Nacional de Vacinação; p. 1.http://www.dgs.pt/upload/membro.id/ficheiros/i018596.pdf [Google Scholar]
- 37.Blázquez B., Fresco-Taboada A., Iglesias-Bexiga M., Menéndez M., García P. PL3 amidase, a tailor-made lysin constructed by domain shuffling with potent killing activity against pneumococci and related species. Front. Microbiol. 2016;7:1–13. doi: 10.3389/fmicb.2016.01156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bustamante N., Campillo N.E., García E., Gallego C., Pera B., Diakun G.P., Sáiz J.L., García P., Díaz J.F., Menéndez M. Cpl-7, a lysozyme encoded by a pneumococcal bacteriophage with a novel cell wall-binding motif. J. Biol. Chem. 2010;285:33184–33196. doi: 10.1074/jbc.M110.154559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Díez-Martínez R., De Paz H., Bustamante N., García E., Menéndez M., García P. Improving the lethal effect of Cpl-7, a pneumococcal phage lysozyme with broad bactericidal activity, by inverting the net charge of its cell wall-binding module. Antimicrob. Agents Chemother. 2013;57:5355–5365. doi: 10.1128/AAC.01372-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varea J., Monterroso B., Sáiz J.L., López-Zumel C., García J.L., Laynez J., García P., Menéndez M. Structural and thermodynamic characterization of Pal, a phage natural chimeric lysin active against Pneumococci. J. Biol. Chem. 2004;279:43697–43707. doi: 10.1074/jbc.M407067200. [DOI] [PubMed] [Google Scholar]
- 41.Diez-Martinez R., De Paz H.D., Garcia-Fernandez E., Bustamante N., Euler C.W., Fischetti V.A., Menendez M., Garcia P. A novel chimeric phage lysin with high in vitro and in vivo bactericidal activity against Streptococcus pneumoniae. J. Antimicrob. Chemother. 2015;70:1763–1773. doi: 10.1093/jac/dkv038. [DOI] [PubMed] [Google Scholar]
- 42.Sanz J.M., García J.L. Structural studies of the lysozyme coded by the pneumococcal phage Cp-1. Conformational changes induced by choline. Eur. J. Biochem. 1990;187:409–416. doi: 10.1111/j.1432-1033.1990.tb15319.x. [DOI] [PubMed] [Google Scholar]
- 43.Levander M.S., Grodzinsky E. Variation in normal ear temperature. Am. J. Med. Sci. 2017;354:370–378. doi: 10.1016/j.amjms.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 44.Wezyk M.T., Makowski A. pH of fluid collected from middle ear in the course of otitis media in children. Otolaryngol. Pol. 2000;54:131–133. http://www.ncbi.nlm.nih.gov/pubmed/10961068 [PubMed] [Google Scholar]
- 45.Maestro B., González A., García P., Sanz J.M. Inhibition of pneumococcal choline-binding proteins and cell growth by esters of bicyclic amines. FEBS J. 2007;274:364–376. doi: 10.1111/j.1742-4658.2006.05584.x. [DOI] [PubMed] [Google Scholar]
- 46.Hall-Stoodley L., Hu F.Z., Gieseke A., Nistico L., Nguyen D., Hayes J., Forbes M., Greenberg D.P., Dice B., Burrows A., Wackym P.A., Stoodley P., Post J.C., Ehrlich G.D., Kerschner J.E. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoa M., Tomovic S., Nistico L., Hall-Stoodley L., Stoodley P., Sachdeva L., Berk R., Coticchia J.M. Identification of adenoid biofilms with middle ear pathogens in otitis-prone children utilizing SEM and FISH. Int. J. Pediatr. Otorhinolaryngol. 2009;73:1242–1248. doi: 10.1016/j.ijporl.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 48.Vázquez R., García P. Synergy between two chimeric lysins to kill Streptococcus pneumoniae. Front. Microbiol. 2019;10:1–10. doi: 10.3389/fmicb.2019.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Resch G., Moreillon P., Fischetti V.A. A stable phage lysin (Cpl-1) dimer with increased antipneumococcal activity and decreased plasma clearance. Int. J. Antimicrob. Agents. 2011;38:516–521. doi: 10.1016/j.ijantimicag.2011.08.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.