Abstract
Purpose
We have previously reported an association between high red blood cell distribution width (RDW) and mortality in septic and brain infarction patients. However, no association between RDW and mortality in coronavirus disease 2019 (COVID-19) patients has been reported so far; thus, the objective of this study was to determine if that association exists.
Methods
Prospective and observational study carried out in 8 Intensive Care Units from 6 hospitals of Canary Islands (Spain) including COVID-19 patients. We recorded RDW at ICU admission and 30-day survival.
Results
We found that patients who did not survive (n = 25) compared to surviving patients (n = 118) were older (p = 0.004), showed higher RDW (p = 0.001), urea (p < 0.001), APACHE-II (p < 0.001) and SOFA (p < 0.001), and lower platelet count (p = 0.007) and pH (p = 0.008). Multiple binomial logistic regression analysis showed that RDW was associated with 30-day mortality after controlling for: SOFA and age (OR = 1.659; 95% CI = 1.130–2.434; p = 0.01); APACHE-II and platelet count (OR = 2.062; 95% CI = 1.359–3.129; p = 0.001); and pH and urea (OR = 1.797; 95% CI = 1.250–2.582; p = 0.002). The area under the curve (AUC) of RDW for mortality prediction was of 71% (95% CI = 63–78%; p < 0.001). We did not find significant differences in the predictive capacity between RDW and SOFA (p = 0.66) or between RDW and APACHE-II (p = 0.12).
Conclusions
Our study provides new information regarding the ability to predict mortality in patients with COVID-19. There is an association between high RDW and mortality. RDW has a good performance to predict 30-day mortality, similar to other severity scores (such as APACHE II and SOFA) but easier and faster to obtain.
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation; aPTT, activated Partial Thromboplastin Time; ARDS, Acute Respiratory Distress Syndrome; COPD, Chronic Obstructive Pulmonary Disease; FIO2, Fraction Inspired Oxygen; GCS, Glasgow Coma Scale; INR, International Normalized Ratio; NTproBNP, N-Terminal prohormone of Brain Natriuretic Peptide; PaO2, Pressure of arterial Oxygen; RDW, Red blood cell Distribution Width; SOFA, Sepsis-related Organ Failure Assessment
Keywords: Red blood cell distribution width, COVID-19, Patients, Mortality, Outcome
1. Introduction
The novel coronavirus detected for the first time in December 2019 in Wuhan (China) is named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the disease it causes is called as coronavirus disease 2019 (COVID-19). COVID-19 is an emerging health threat in the world. On the 23rd of May 2020, there were 5,346,876 confirmed cases and 340,869 deaths (6.4%) from COVID-19 [1], [2]. However, the mortality in critically ill patients with COVID-19 was 15–62% [3], [4], [5], [6], [7]. Different factors have been associated with the risk of death, as age, some comorbidities (diabetes mellitus, arterial hypertension, chronic obstructive pulmonary disease, smoking, cardiovascular or cerebrovascular diseases), blood biomarkers (of inflammation, cardiac injury, muscle injury, liver dysfunction, kidney dysfunction and coagulation alterations), and clinical data of severity as the presence of acute respiratory distress syndrome (ARDS) [8], [9], [10], [11], [12], [13], [14], [15], [16].
Red blood cell distribution width (RDW) is a parameter of the haemogram used in the differential diagnosis of anaemia and involves the variability in form and size of red blood cells in the subject [17]. An association between high RDW and mortality has been found in patients with coronary disease [18], liver disease [19], pancreatitis [20] and ischaemic stroke [21], [22]. This association has also been found in septic patients [23], [24], [25]. In one study was found that severe patients showed higher RDW than non-severe COVID-19 patients; however, the criteria of severity were not clearly established and the mortality rate was < 1% in the whole series and < 4% in the severe patients group [26].
We had previously found that RDW at ICU admission was associated with mortality in brain infarction [22] and in septic patients [23], but this association in COVID-19 patients has not been reported so far. Thus, the objective of this study was to determine whether or not there is an association between RDW at ICU admission and mortality.
2. Methods
2.1. Design and subjects
In this prospective and observational study participated 8 Intensive Care Units from 6 hospitals of Canary Islands (Spain). The study was conducted with the approval in all hospitals of the Ethics Committee (Protocol code CHUC-2020-26). The requirement for written informed consent of each patient was waived given that data were prospectively collected, the context of the rapid emergence of this infectious disease and the public health outbreak policy consisting in forbidding patient visits by the Government of Spain.
We included patients with COVID-19 admitted to the ICU. We included only patients with laboratory-confirmed COVID-19 by means of a positive result for COVID-19 nucleic acids by a real-time fluorescence reverse transcription-polymerase chain reaction (RT-PCR) assay of a nasopharyngeal swab sample.
2.2. Variables recorded
We recorded the following variables regarding to demographic and clinical data: sex, age, body max index, and history of diabetes mellitus, chronic renal failure, chronic obstructive pulmonary disease (COPD), ischaemic heart disease, chronic liver disease, smoking cessation, active smoking, arterial hypertension, steroid agents, haematological tumour, solid tumour, and human immunodeficiency virus (HIV). We also recorded temperature, chest radiography findings, Acute Physiology and Chronic Health Evaluation (APACHE)-II score [27], Sepsis-related Organ Failure Assessment [SOFA] score [28] and the development of ARDS [29].
Besides, regarding to laboratory data at ICU admission, we also recorded lactic acid, glucose, sodium, creatinine, urea, protein, albumin, creatine kinase, bilirubin, aspartate transaminase, alanine transaminase, gamma-glutamyl transpeptidase, lactate dehydrogenase, alkaline phosphatase, C-reactive protein, procalcitonin, ferritin, N-terminal prohormone of brain natriuretic peptide (NTproBNP), interleukin-6, haemoglobin, haematocrit, white blood cell, neutrophils, lymphocytes, monocytes, eosinophils, basophils, platelets, international normalized ratio (INR), activated partial thromboplastin time (aPTT), fibrinogen, d-dimer, pressure of arterial oxygen (PaO2), fraction inspired of oxygen (FIO2), arterial pH and RDW at ICU admission.
In regards to ICU treatment, we recorded respiratory support, neuromuscular blockers, prone position, lopinavir/ritonavir, hydroxycloroquine, interferon, tocilizumab, steroid agents, intermittent and continuous renal replacement therapy, and vasopressors. Finally, survival at 30 days was the endpoint study.
2.3. Statistical methods
We used frequencies (percentages) and medians (percentile 25–75) to describe categorical and continuous variables. We used chi-square test and Mann–Whitney U-test to compare categorical and continuous variables between patient groups (surviving and non-surviving). We tested the ability of RDW for mortality prediction by a receiver operating characteristic analysis, and we reported area under curve (AUC), and sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, positive predicted value and negative predicted value for the cut-off of RDW 13.0% (which was Youden J index). We constructed Kaplan–Meier 30-day survival curves using RDW lower and higher to 13.0%. We tested the possible association between RDW and 30-day mortality using multiple logistic regression analysis. As 25 was the number of non-surviving patients at 30 days in our study, we constructed several multiple binomial logistic regression models with only three predictor variables in each model to avoid overfitting effect. We included in the regression analyses those variables with p-value < 0.01 in the comparison between non-surviving and surviving patients. We included RDW, SOFA and age in the first model, RDW, APACHE-II and platelet count in the second model, and RDW, arterial pH and urea in the third model. Odds Ratio and 95% confidence intervals were calculated as measurement of the clinical impact of the predictor variables. Spearman's rank correlation coefficient was used to determine the association between continuous variables. We used the point p < 0.05 for the establishment of significant differences, and the programmes NCSS 2000 (Kaysville, Utah) and SPSS 17.0 (SPSS Inc., Chicago, IL, USA) for the analyses.
3. Results
We found that non-surviving (n = 25) compared to surviving patients (n = 118) were older (p = 0.004) and showed higher APACHE-II (p < 0.001) and SOFA (p < 0.001) scores (Table 1 ). In addition, non-surviving showed at ICU admission lower platelet count (p = 0.007) and arterial pH (p = 0.008), and higher creatinine (p = 0.02), urea (p < 0.001), alkaline phosphatase (p = 0.04), aPTT (p = 0.009) and RDW (p = 0.001) (Table 2 ). Besides, during their ICU stay, non-surviving patients received CRRT (p = 0.002) and vasopressors (p = 0.001) more frequently (Table 3 ).
Table 1.
Demographic and clinical data at ICU admission of non-surviving and surviving patients.
| Survivors (n = 118) | Non-survivors (n = 25) | p Value | |
|---|---|---|---|
| Gender female – n (%) | 65 (55.1) | 18 (72.0) | 0.18 |
| Age (years) – median (p 25–75) | 64 (55–72) | 71 (68–75) | 0.004 |
| Body max index (kg/m2) | 27.7 (25.4–30.1) | 29.4 (24.7–32.7) | 0.91 |
| Diabetes mellitus – n (%) | 35 (29.7) | 7 (28.0) | 0.99 |
| Chronic renal failure – n (%) | 1 (0.8) | 0 | 0.99 |
| COPD – n (%) | 8 (6.8) | 5 (20.0) | 0.052 |
| Ischaemic heart disease – n (%) | 9 (7.6) | 3 (12.0) | 0.44 |
| Chronic liver disease – n (%) | 1 (0.8) | 1 (4.0) | 0.32 |
| Smoking cessation – n (%) | 29 (24.6) | 6 (24.0) | 0.99 |
| Smoking – n (%) | 5 (4.2) | 2 (8.0) | 0.35 |
| Arterial hypertension – n (%) | 51 (43.2) | 12 (48.0) | 0.67 |
| Steroid agents – n (%) | 3 (2.5) | 2 (8.0) | 0.21 |
| Haematological tumour – n (%) | 5 (4.2) | 1 (4.0) | 0.99 |
| Solid tumour – n (%) | 1 (0.8) | 0 | 0.99 |
| Human Immunodeficiency Virus – n (%) | 1 (0.8) | 0 | 0.99 |
| Temperature (°C) – median (p 25–75) | 36.9 (36.0–37.6) | 36.4 (34.6–38.0) | 0.33 |
| Chest radiography findings – n (%) | 0.98 | ||
| - Consolidation only | 20 (16.9) | 4 (16.0) | |
| - Ground glass opacity plus consolidation | 44 (37.3) | 9 (36.0) | |
| - Ground glass opacity only | 54 (45.8) | 12 (48.0) | |
| ARDS – n (%) | 92 (78.0) | 19 (76.0) | 0.80 |
| APACHE-II score – median (p 25–75) | 11 (7–15) | 18 (15–22) | < 0.001 |
| SOFA score – median (p 25–75) | 5 (3–7) | 8 (7–9) | < 0.001 |
COPD = Chronic Obstructive Pulmonary Disease; APACHE = Acute Physiology and Chronic Health Evaluation; SOFA = Sepsis-related Organ Failure Assessment; ARDS = Acute Respiratory Distress Syndrome.
Table 2.
Laboratory data at ICU admission of non-surviving and surviving patients.
| Survivors (n = 118) | Non-survivors (n = 25) | p Value | |
|---|---|---|---|
| Lactic acid (mmol/L) – median (p 25–75) | 1.33 (1.06–1.80) | 1.60 (1.20–2.05) | 0.20 |
| Glucose (g/dL) – median (p 25–75) | 140 (108–189) | 158 (127–249) | 0.14 |
| Sodium (mEq/L)- median (p 25–75) | 138 (135–141) | 139 (135–143) | 0.60 |
| Creatinine (mg/dl) – median (p 25–75) | 0.86 (0.66–1.09) | 1.07 (0.77–1.21) | 0.02 |
| Urea (mg/dl) – median (p 25–75) | 39 (27–54) | 65 (52–85) | < 0.001 |
| Protein (g/L) – median (p 25–75) | 6.4 (5.9–7.0) | 6.2 (5.9–6.7) | 0.49 |
| Albumin (g/L) – median (p 25–75) | 3.2 (2.8–3.5) | 3.4 (3.1–3.9) | 0.25 |
| Creatine kinase (U/L) – median (p 25–75) | 121 (44–258) | 209 (39–316) | 0.63 |
| Total bilirubin (mg/dl) – median (p 25–75) | 0.60 (0.40–1.00) | 0.72 (0.50–1.20) | 0.16 |
| Aspartate transaminase (U/L) – median (p 25–75) | 40 (30–71) | 45 (23–123) | 0.74 |
| Alanine transaminase (U/L) – median (p 25–75) | 38 (27–66) | 31 (19–68) | 0.22 |
| Gamma-glutamyl transpeptidase (U/L) – median (p 25–75) | 55 (35–108) | 102 (39–176) | 0.11 |
| Lactate dehydrogenase (U/L) – median (p 25–75) | 397 (309–475) | 461 (287–561) | 0.19 |
| Alkaline phosphatase (U/L) – median (p 25–75) | 57 (49–79) | 88 (52–117) | 0.04 |
| C-reactive protein (mg/gl) – median (p 25–75) | 26 (13–102) | 24 (14–59) | 0.75 |
| Procalcitonin (ng/ml) – median (p 25–75) | 0.21 (0.09–0.59) | 0.58 (0.16–0.84) | 0.18 |
| Ferritin (ng/ml) – median (p 25–75) | 906 (593–1593) | 1391 (977–1843) | 0.18 |
| NTproBNP (pg/ml) – median (p 25–75) | 288 (130–1195) | 3480 (468–6162) | 0.07 |
| Interleukin-6 (pg/ml) – median (p 25–75) | 55 (6–237) | 65 (35–249) | 0.62 |
| Haemoglobin (g/dL) – median (p 25–75) | 13.0 (11.7–14.4) | 12.7 (10.9–14.3) | 0.50 |
| Haematocrit (%) – median (p 25–75) | 39 (35–43) | 38 (34–43) | 0.59 |
| RDW (%) – median (p 25–75) | 13.3 (12.5–14.5) | 14.1 (13.3–16.1) | 0.001 |
| White blood cell – median*103 mm–3 (p 25–75) | 8.3 (6.0–11.8) | 9.4 (5.6–12.8) | 0.64 |
| Neutrophils – median*103 mm–3 (p 25–75) | 7.2 (4.9–10.2) | 7.6 (4.0–10.2) | 0.99 |
| Lymphocytes – median*103 mm–3 (p 25–75) | 0.72 (0.52–1.04) | 0.70 (0.50–1.21) | 0.78 |
| Monocytes – median*103 mm–3 (p 25–75) | 0.42 (0.30–0.63) | 0.40 (0.23–0.52) | 0.24 |
| Eosinophils – median*103 mm–3 (p 25–75) | 0.00 (0.00–0.03) | 0.01 (0.00–0.03) | 0.37 |
| Basophils – median*103 mm–3 (p 25–75) | 0.01 (0.00–0.03) | 0.01 (0.00–0.03) | 0.91 |
| Platelets – median*103 mm–3 (p 25–75) | 243 (173–312) | 198 (121–266) | 0.007 |
| INR – median (p 25–75) | 1.18 (1.08–1.32) | 1.25 (1.16–1.41) | 0.13 |
| aPTT (seconds) – median (p 25–75) | 28 (24–32) | 32 (29–36) | 0.009 |
| Fibrinogen (mg/dl) – median (p 25–75) | 698 (524–810) | 726 (548–894) | 0.47 |
| D-dimer (ng/mL) – median (p 25–75) | 1154 (664–2663) | 1758 (595–11365) | 0.17 |
| PaO2/FI02 ratio – median (p 25–75) | 176 (104–234) | 112 (100–174) | 0.07 |
| Arterial pH – median (p 25–75) | 7.41 (7.34–7.46) | 7.36 (7.29–7.42) | 0.008 |
NTproBNP = N-terminal prohormone of Brain Natriuretic Peptide; RDW = Red blood cell Distribution Width (RDW); INR = International Normalized Ratio; aPTT = Activated Partial Thromboplastin Time; PaO2 = Pressure of arterial Oxygen; FIO2 = Fraction Inspired Oxygen
Table 3.
Treatment data in ICU of non-surviving and surviving patients.
| Survivors (n = 118) | Non-survivors (n = 25) | p Value | |
|---|---|---|---|
| Respiratory support – n (%) | 0.01 | ||
| - Conventional oxygen therapy | 10 (8.5) | 0 | |
| - High-flow nasal cannula | 22 (18.6) | 0 | |
| - Non-invasive mechanical ventilation | 8 (6.8) | 0 | |
| - Invasive mechanical ventilation | 78 (66.1) | 25 (100) | |
| Neuromuscular blockers – n (%) | 79 (66.9) | 20 (80.0) | 0.24 |
| Prone position – n (%) | 46 (39.0) | 14 (56.0) | 0.13 |
| Lopinavir/Ritonavir – n (%) | 111 (94.1) | 23 (92.0) | 0.66 |
| Hydroxychloroquine – n (%) | 113 (95.8) | 25 (100) | 0.59 |
| Interferon Beta 1-B – n (%) | 68 (57.6) | 15 (60.0) | 0.99 |
| Tocilizumab – n (%) | 47 (39.8) | 10 (40.0) | 0.99 |
| Steroid agents – n (%) | 77 (65.3) | 16 (64.0) | 0.99 |
| Intermittent renal replacement therapy – n (%) | 2 (1.7) | 0 | 0.99 |
| Continuous renal replacement therapy – n (%) | 11 (9.3) | 9 (36.0) | 0.002 |
| Vasopressors – n (%) | 77 (65.3) | 24 (96.0) | 0.001 |
Multiple binomial logistic regression analysis showed that RDW was associated with 30-day mortality after controlling for: SOFA and age (OR = 1.659; 95% CI = 1.130–2.434; p = 0.01), APACHE-II and platelet count (OR = 2.062; 95% CI = 1.359–3.129; p = 0.001), and arterial pH and urea (OR = 1.797; 95% CI = 1.250–2.582; p = 0.002) (Table 4 ).
Table 4.
Multiple logistic regression analyses to predict mortality at 30 days.
| Odds Ratio | 95% Confidence interval | p-Value | |
|---|---|---|---|
| Model 1 | |||
| RDW (%) | 1.659 | 1.130–2.434 | 0.01 |
| SOFA score (point) | 1.251 | 1.056–1.483 | 0.01 |
| Age (year) | 1.072 | 1.010–1.139 | 0.02 |
| Model 2 | |||
| RDW (%) | 2.062 | 1.359–3.129 | 0.001 |
| APACHE-II (point) | 1.184 | 1.092–1.282 | <0.001 |
| Platelet count (each 10.000 mm–3) | 0.945 | 0.895–0.998 | 0.04 |
| Model 3 | |||
| RDW (%) | 1.797 | 1.250–2.582 | 0.002 |
| Arterial pH (point) | 1.188 | 0.446–3.166 | 0.73 |
| Urea (mg/dl) | 1.007 | 0.996–1.017 | 0.20 |
RDW = Red blood cell Distribution Width; SOFA = Sepsis-related Organ Failure Assessment; APACHE = Acute Physiology and Chronic Health Evaluation.
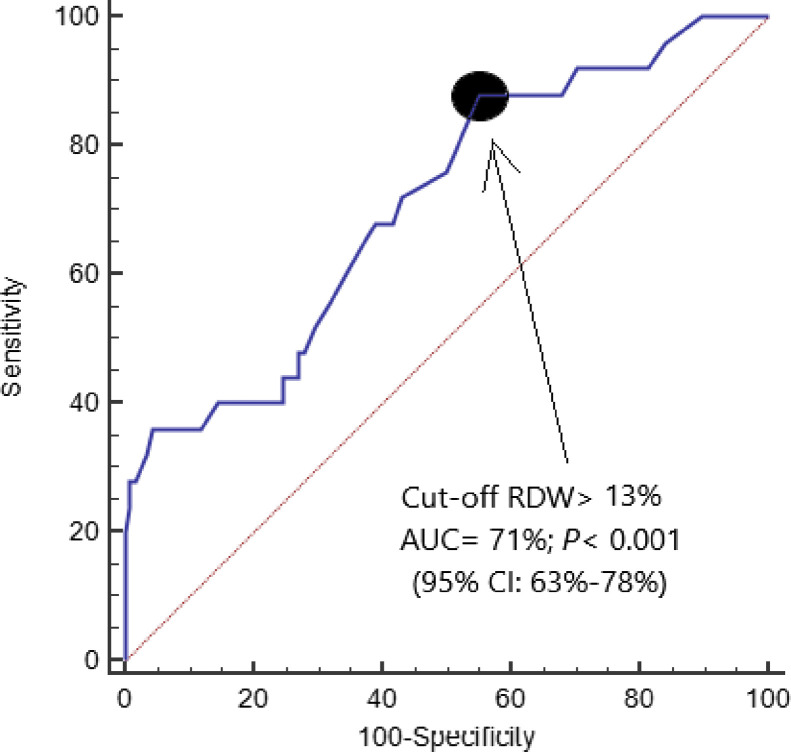
The AUC of RDW for mortality prediction was 71% (95% CI = 63–78%; p < 0.001) (Fig. 1 ). The RDW cut-off of 13.0% showed sensitivity 88% (69–98%), specificity 45% (36–54%), positive likelihood ratio 1.6 (1.3–2.0), negative likelihood ratio 0.3 (0.1–0.8), positive predicted value 25% (21–30%) and negative predicted value 95% (86–98%). On the other hand, the AUC for mortality prediction by SOFA score and APACHE II score were of 74% (95% CI = 65–81%; p < 0.001) and 83% (95% CI = 75–89%; p < 0.001) respectively. We did not find significant differences in the AUC between RDW and SOFA (p = 0.66), RDW and APACHE-II (p = 0.12) or APACHE-II and SOFA (p = 0.13).
Fig. 1.
Receiver operating characteristic analysis using RDW for prediction of mortality at 30 days.
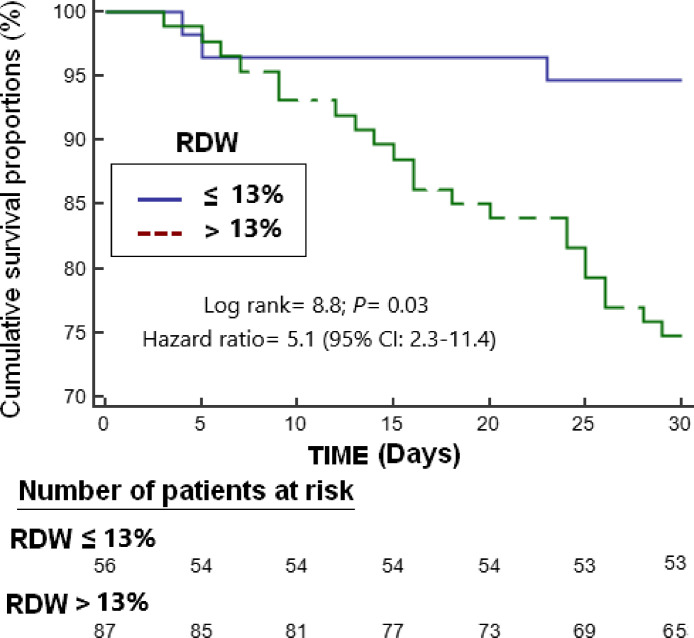
Survival analysis showed that patients with RDW > 13.0% had higher 30-day mortality than patients with lower RDW (Hazard ratio = 5.1; 95% CI = 2.3–11.4; p = 0.03) (Fig. 2 ). We found a trend towards a positive association between RDW and IL-6 (n = 31; rho = 0.28; p = 0.12). We found a positive association between age and RDW (n = 143; rho = 0.22; p = 0.01).
Fig. 2.
Survival curves at 30 days using red blood cell distribution width (RDW) higher or lower than 13.0%.
4. Discussion
The main findings of our study were as follows: First, non-surviving COVID-19 patients show higher RDW at ICU admission. Second, there is an association between high RDW and mortality. Third, RDW values greater than 13% have good performance to predict 30-day mortality. Fourth, RDW has an ability to predict mortality similar to other severity scores (such as APACHE II and SOFA) but easier and faster to obtain.
In a previous study it was found that severe COVID-19 patients showed higher RDW; however, the criteria of severity were not clearly established, no patient died in the non-severe patients group and only one died in the severe patients group (mortality group < 4%) [26]. Thus, our study report interesting novelties about the potential role of RDW at ICU admission as prognostic biomarker in COVID-19 patients.
The mortality in our series of critically ill patients (17.5%) was in the lower limit of those reported in other series (15–62%) [3], [4], [5], [6], [7]. In a study carried out in Spain, critically ill patients admitted to ICU (APACHE-II 15 ± 5; SOFA 7 ± 3) had a 28-day mortality rate of 15% [3], similar to that in our series.
In addition to RDW, we also found differences between surviving and non-surviving patients in age, urea, creatinine, SOFA, APACHE-II, platelet count, aPTT; and these findings are in line with the results of previous studies [8], [9], [10], [11], [12], [13], [14], [15], [16]. A new and interesting finding of our study was that we did not find significant differences in the predictive capacity of mortality provided by RDW, SOFA and APACHE-II; however, RDW is an easier and faster predictor to obtain. Previously, there was found that RDW increased with age [30]; and we found a positive association between age and RDW in our series of COVID-19 patients.
Some studies have showed an association between RDW and inflammatory cytokines as tumour necrosis factor (TNF)-alpha, interleukin (IL)-1 and IL-6 [31], [32], and between RDW and oxidative stress [32], [33]. Previously, we have reported in patients with sepsis [23] or brain infarction [22] an association between RDW and blood levels of TNF-alpha and malondialdehyde (which appears by lipid peroxidation of cellular membrane phospholipids). We speculated that the association between RDW and mortality of COVID-19 patients found in our study could be due to a higher degree of inflammation and oxidation in those patients who end up dying. In our study of COVID-19 patients a trend towards a positive association between RDW and IL-6 was found, although possibly the limited number of patients in whom IL-6 was determined (n = 31) could have contributed to the absence of a significant association. Another limitation of our study is that we do not have data on other inflammatory cytokines or markers of oxidative damage. The limited number of deaths prevented the inclusion of more variables in the same regression model and was another limitation of our study. Data about the symptoms onset is lacking and this was another limitation in our study.
There are two methods of RDW assessment, RDW-coefficient variation (RDW-CV) and RDW-standard deviation (RDW-SD). RDW-CV is calculated by dividing the standard deviation of the mean erythrocyte volume by the mean erythrocyte volume and multiplying it by 100 to obtain a percentage. RDW-SD is an actual measurement of RDW. However, RDW-SD was not reported in our study and this is another limitation. In addition, RDW may vary depending on the analyser used [34]. Different analysers (Sysmex XN-1000, Beckman Coulter DxH-800, CELL-DYN Sapphire) were used in each hospital and in the same hospital in our study; thus, we cannot determine whether there were differences on RDW according to the analyser used.
In addition, haemoglobin disorders [35], myelodysplastic syndromes [36] and red blood cell (RBC) transfusion increased RDW values [37]; however, we are aware no patients in our series suffered of those disorders or received RBC transfusion before RDW determination.
One of the strengths of the study is that the association between RDW at ICU admission and mortality was found in all regression models, and that this association is in line with the results we found in previous studies in septic or cerebral ischaemic patients [22], [23], as well as the results found by other researchers in patients with coronary heart disease [18], liver disease [19] or pancreatitis [20].
The results of our study suggest that RDW, a laboratory parameter that is automatically provided in a conventional haemogram could be very useful in estimating the probability of death in the population of patients with COVID-19. Further studies that include a large number of patients and explore the association of RDW with oxidative stress and inflammatory markers are needed to confirm these results.
5. Conclusions
Our study provides new information regarding the ability to predict mortality in patients with COVID-19. Compared with surviving, non-surviving COVID-19 patients show higher RDW at ICU admission. There is an association between high RDW and mortality. RDW has a good performance to predict 30-day mortality, similar to other severity scores (such as APACHE II and SOFA) but easier and faster to obtain.
Consent for publication
Not applicable.
Compliance with ethical standards
The study was conducted with the approval in all hospitals of the Ethics Committee (Protocol code CHUC-2020-26). The requirement for written informed consent of each patient was waived given that data were prospectively collected, the context of the rapid emergence of this infectious disease and the public health outbreak policy of forbidding patient visits by the Government of Spain.
Availability of data and materials
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Disclosure of interest
The authors declare that they have no competing interests.
Funding
This study was supported by a grant from the Instituto de Salud Carlos III (PI-18-00500) (Madrid, Spain) and co-financed by the European Regional Development Fund (ERDF).
Author contributions
LL conceived, designed and coordinated the study, participated in data acquisition and interpretation, and drafted the manuscript.
MMM, MA, JSV, AP, JAMR, LRG, SL, AF, AFGR, MM, VG, JAF, MAR, MRR, JGOC, LG, TC, ROL, NO, ARP and CD participated in acquisition of data.
AJ participated in the interpretation of data.
All authors revised the manuscript critically for important intellectual content and made the final approval of the version to be published.
Acknowledgments
This study was supported by a grant from the Instituto de Salud Carlos III (PI-18-00500) (Madrid, Spain) and co-financed by the European Regional Development Fund (ERDF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Meters. Coronavirus disease (COVID-19). https://www.worldometers.info/coronavirus/coronavirus-cases [assessed 23.05.20].
- 2.World Health Organization. Coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [assessed 23.05.20].
- 3.Barrasa H., Rello J., Tejada S., Martín A., Balziskueta G., Vinuesa C., et al. Alava COVID19 Study Investigators Anaesth Crit Care Pain Med. 2020;(April) doi: 10.1016/j.accpm.2020.04.001. S2352-5568(20)30064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;(April) doi: 10.1001/jama.2020.5394. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., et al. Covid-19 in critically ill patients in the Seattle region – case series. N Engl J Med. 2020 Mar 30 doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;(March) doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;(February) doi: 10.1016/S2213-2600(20)30079-5. pii: S2213-2600(20)3007.9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 Mar 3 doi: 10.1007/s00134-020-05991-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W., Tao Z.W., Lei W., Ming-Li Y., Kui L., Ling Z., et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020 Feb 28 doi: 10.1097/CM9.0000000000000775. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan China: a retrospective cohort study. Lancet. 2020;395(March (10229)):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368(March):m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC COVID-19 Response Team Severe outcomes among patients with Coronavirus Disease 2019 (COVID-19) – United States. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020 Mar 13 doi: 10.1001/jamainternmed.2020.0994. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Q., Meng M., Kumar R., Wu Y., Huang J., Lian N., et al. The impact of COPD and smoking history on the severity of Covid-19: a systemic review and meta-analysis. J Med Virol. 2020 Apr 15 doi: 10.1002/jmv.25889. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;(April) doi: 10.1515/cclm-2020-0369. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Du R.H., Liang L.R., Yang C.Q., Wang W., Cao T.Z., Li M., et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020:55. doi: 10.1183/13993003.00524-2020. pii: 2000524 [Print 2020 May] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aslan D., Gümrük F., Gürgey A., Altay C. Importance of RDW value in differential diagnosis of hypochrome anemias. Am J Hematol. 2002;69:31–33. doi: 10.1002/ajh.10011. [DOI] [PubMed] [Google Scholar]
- 18.Abrahan L.L., 4th, Ramos J.D.A., Cunanan E.L., Tiongson M.D.A., Punzalan F.E.R. Red cell distribution width and mortality in patients with acute coronary syndrome: a meta-analysis on prognosis. Cardiol Res. 2018;9:144–152. doi: 10.14740/cr732w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milas G.P., Karageorgiou V., Cholongitas E. Red cell distribution width to platelet ratio for liver fibrosis: a systematic review and meta-analysis of diagnostic accuracy. Expert Rev Gastroenterol Hepatol. 2019;13:877–891. doi: 10.1080/17474124.2019.1653757. [DOI] [PubMed] [Google Scholar]
- 20.Goyal H., Awad H., Hu Z.D. Prognostic value of admission red blood cell distribution width in acute pancreatitis: a systematic review. Ann Transl Med. 2017;5:342. doi: 10.21037/atm.2017.06.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song S.Y., Hua C., Dornbors D., III, Kang R.J., Zhao X.X., Du X., et al. Baseline red blood cell distribution width as a predictor of stroke occurrence and outcome: a comprehensive meta-analysis of 31 studies. Front Neurol. 2019;10:1237. doi: 10.3389/fneur.2019.01237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorente L., Martín M.M., Abreu-González P., Pérez-Cejas A., González-Rivero A.F., Ramos-Gómez L., et al. Early mortality of brain infarction patients and red blood cell distribution width. Brain Sci. 2020:10. doi: 10.3390/brainsci10040196. pii: E196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorente L., Martín M.M., Abreu-González P., Solé-Violán J., Ferreres J., Labarta L., et al. Red blood cell distribution width during the first week is associated with severity and mortality in septic patients. PLOS ONE. 2014;9:e105436. doi: 10.1371/journal.pone.0105436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu Z.D., Lippi G. Montagnana M3 diagnostic and prognostic value of red blood cell distribution width in sepsis: a narrative review. Clin Biochem. 2020;77:1–6. doi: 10.1016/j.clinbiochem.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Orfanu A.E., Popescu C., Leuștean A., Negru A.R., Tilişcan C., Aramă V., et al. The importance of haemogram parameters in the diagnosis and prognosis of septic patients. J Crit Care Med (Targu Mures) 2017;3:105–110. doi: 10.1515/jccm-2017-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong J., Ou J., Qiu X., Jie Y., Chen Y., Yuan L., et al. A tool to early predict severe corona virus disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020;(April) doi: 10.1093/cid/ciaa443. pii: ciaa443 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 28.Vincent J.L., Moreno R., Takala J., Willatts S., De Mendonça A., Bruining H., et al. The Sepsis-related Organ Failure Assessment (SOFA) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 29.ARDS Definition Task Force, Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann J.J., Nabbe K.C., van den Broek N.M. Effect of age and gender on reference intervals of red blood cell distribution width (RDW) and mean red cell volume (MCV) Clin Chem Lab Med. 2015;53:2015–2019. doi: 10.1515/cclm-2015-0155. [DOI] [PubMed] [Google Scholar]
- 31.Pierce C.N., Larson D.F. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion. 2005;20:83–90. doi: 10.1191/0267659105pf793oa. [DOI] [PubMed] [Google Scholar]
- 32.Scharte M., Fink M.P. Red blood cell physiology in critical illness. Crit Care Med. 2003;31:S651–S657. doi: 10.1097/01.CCM.0000098036.90796.ED. [DOI] [PubMed] [Google Scholar]
- 33.Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal. 2008;10:1923–1940. doi: 10.1089/ars.2008.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lippi G., Pavesi F., Bardi M., Pipitone S. Lack of harmonization of red blood cell distribution width (RDW). Evaluation of four hematological analyzers. Clin Biochem. 2014;47:1100–1103. doi: 10.1016/j.clinbiochem.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann J.J., Urrechaga E., Aguirre U. Discriminant indices for distinguishing thalassemia and iron deficiency in patients with microcytic anemia: a meta-analysis. Clin Chem Lab Med. 2015;53:1883–1894. doi: 10.1515/cclm-2015-0179. [DOI] [PubMed] [Google Scholar]
- 36.Baba Y., Saito B., Shimada S., Yohei S., So M., Maasa A., et al. Association of red cell distribution width with clinical outcomes in myelodysplastic syndrome. Leuk Res. 2018;67:56–59. doi: 10.1016/j.leukres.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Spadaro S., Taccone F.S., Fogagnolo A., Federico F., Sabino S., Riccardo R., et al. The effects of blood transfusion on red blood cell distribution width in critically ill patients: a pilot study. Transfusion. 2018;58:1863–1869. doi: 10.1111/trf.14759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.