Abstract
Purpose
The purpose of this study was to explore the clinical features, risk factors, and outcomes of mixed Candida albicans/bacterial bloodstream infections (mixed-CA/B-BSIs) compared with monomicrobial Candida albicans bloodstream infection (mono-CA-BSI) in adult patients in China.
Methods
All hospitalized adults with Candida albicans bloodstream infection (CA-BSI) were recruited for this retrospective observational study from January 1, 2013, to December 31, 2018.
Results
Of the 117 patients with CA-BSI, 24 patients (20.5%) had mixed-CA/B-BSIs. The most common copathogens were coagulase-negative Staphylococcus (CNS) (24.0%), followed by Klebsiella pneumoniae (20.0%) and Staphylococcus aureus (16.0%). In the multivariable analysis, a prior ICU stay > 2 days (adjusted odds ratio [OR], 7.445; 95% confidence interval [CI], 1.152–48.132) was an independent risk factor for mixed-CA/B-BSIs. Compared with patients with mono-CA-BSI, patients with mixed-CA/B-BSIs had a prolonged length of mechanical ventilation [17.5 (4.5, 34.8) vs. 3.0 (0.0, 24.5), p = 0.019] and prolonged length of ICU stay [22.0 (14.3, 42.2) vs. 8.0 (0.0, 31.5), p = 0.010]; however, mortality was not significantly different.
Conclusions
There was a high rate of mixed-CA/B-BSIs cases among CA-BSI cases, and CNS was the predominant coexisting species. A prior ICU stay > 2 days was an independent risk factor for mixed -CA/B-BSIs. Although there was no difference in mortality, the outcomes of patients with mixed -CA/B-BSIs, including prolonged length of mechanical ventilation and prolonged length of ICU stay, were worse than those with mono-CA-BSI; this deserves further attention from clinicians.
Keywords: Candidemia, Bloodstream infections, Mixed Candida/bacterial bloodstream infections, Candida albicans, Mortality, Risk factor
Introduction
In critically ill patients, bloodstream infection (BSI) is an important cause of morbidity and mortality. Candidemia is a leading cause of healthcare-associated BSI, with all-cause in-hospital mortality reaching 30% in the United States [1–3]. With the extensive use of antibiotics and immunosuppressants and rapid increases in the applications of invasive medical examinations and treatments, Candida has gradually become a significant pathogen responsible for BSI, with a crude average incidence of 8.7 per 100,000 population [2]. Patients with candidemia have many typical risk factors, including recent surgery, use of broad-spectrum antibiotics, presence of a central venous catheter (CVC), and injection drug use [2].
Although many candidemia cases are monomicrobial, mixed Candida/bacterial BSIs account for 18–56%, as previously reported [4–8]. In these studies [4–6, 8], the following limitations existed: (1) Although the clinical significance and prognosis of mixed Candida/bacterial BSIs versus monomicrobial candidemia were investigated, few reports focused on specific Candida species, such as C. albicans. (2) A recent study reported that patients with mixed Candida/bacterial BSIs had a worse prognosis than patients with monomicrobial candidemia (45.0% crude mortality vs. 17.8% crude mortality, p < 0.05) [8], while other studies did not observe a similar mortality rate [4–6]. The discrepancies in this clinical outcome among different studies are not understood. (3) Some risk factors associated with mixed Candida/bacterial BSIs, such as prolonged length of prior hospital stay (≥7 weeks), septic shock at the time of candidemia, a high acute physiology chronic health evaluation (APACHE) II score, and use of antibiotics, are supported by data mainly from Korea and Spain. Whether these risk factors also apply in other countries, such as China, is unknown. Therefore, it is necessary to investigate the clinical features of mixed Candida/bacterial BSIs involving specific Candida species in China.
Although an increase in the proportion of non-albicans Candida species was observed in some epidemiologic studies [9, 10], C. albicans is still the most common species isolated from patients with candidemia, followed by C. glabrata and C. parapsilosis [2–4, 10, 11]. The following differences were found among different Candida spp. (1) Different distributions were observed among different Candida spp. C. albicans is the predominant species associated with ICU-related infections, while C. glabrata is most commonly associated with gastrointestinal tract diseases [11]. (2) Different resistance rates to common antifungal agents have been observed between C. albicans and non-albicans Candida species. Non-albicans Candida species are more likely to be resistant to fluconazole than C. albicans [11]. (3) Different outcomes, such as mortality, also exist between non-albicans candidemia and C. albicans infection [9, 10]. Whether there are differences in the sensitivity to antifungal agents, the severity of illness or mortality between those with mixed- CA/B-BSIs and mono-CA-BSI, and which factors are associated with mixed-CA/B-BSIs are not well known. Given that C. albicans is the most common species responsible for candidemia and few reports about mixed-candidemia involving a specific Candida species, such as C. albicans, exist, we performed the study to investigate the clinical characteristics of, risk factors for and outcomes of mixed-CA/B-BSIs compared with mono-CA-BSI.
Material and methods
Patients and study design
From January 2013 to December 2018, we conducted a single-center retrospective cohort study at the Second Affiliated Hospital of Zhejiang University School of Medicine, a teaching hospital with 3200 beds in Hangzhou, China. The study was approved by the Human Ethics Board of the Ethics Committee of the Second Affiliated Hospital of Zhejiang University Medical College. Due to its retrospective nature, the Ethics Committee determined that patient consent was not required.
Patients positive for C. albicans according to blood culture were recruited. Candidemia that occurred 30 days after the initial episode was considered a new case [10]. The exclusion criteria were as follows: a) age < 18 years old; b) incomplete or missing case data; and c) the presence of nonpathogenic C. albicans. Common skin flora (e.g., Bacillus spp., Corynebacterium spp., Micrococcus spp., Streptococci, Lactobacillus spp. and CNS) were considered pathogenic only when they were present in two or more consecutive blood cultures from at least two separate blood draws or from two separate sites on the same or two consecutive calendar days. Moreover, at the time of specimen collection, the patients must have at least one of the following signs or symptoms: fever (> 38.0 °C), chills, or hypotension [12, 13]. Thus, a total of 147 blood culture specimens containing C. albicans were initially screened, and a final total of 117 cases were recruited, with 24 cases of mixed-CA/B-BSIs and 93 cases of mono-CA-BSI (Fig. 1).
Fig. 1.
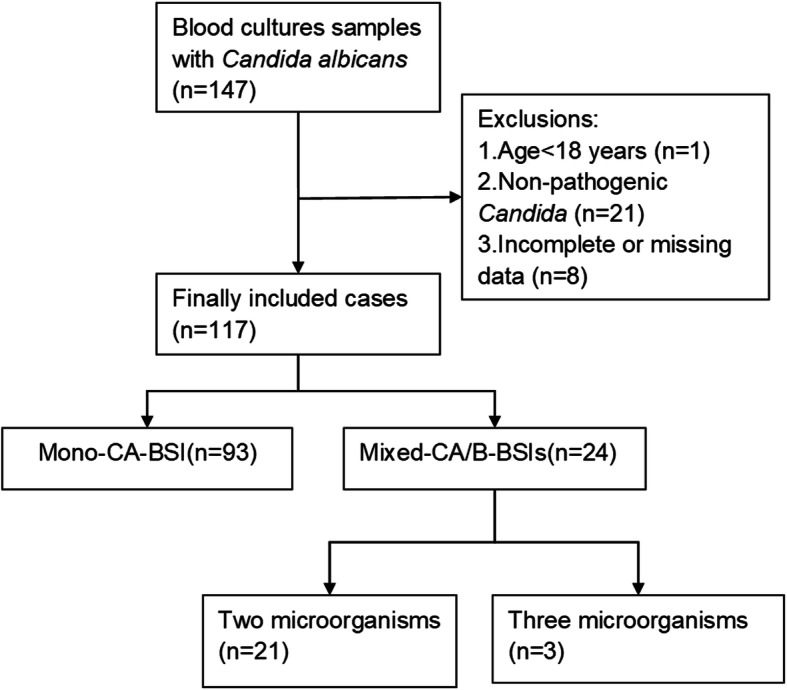
Flowchart of study participant enrollment
Data collection
Patient data were collected from electronic medical records. Patient characteristics included age, sex, severity of illness, sequential organ failure assessment (SOFA) score, and APACHE II score in the first 24 h following candidemia onset. Data regarding prior ICU stay, prior hospital stay, underlying diseases, immune status, hospitalization ward, life-sustaining treatments ≥24 h, prior use of antibiotics or antifungal agents, previous treatments such as surgical procedures, source control methods and outcomes (length of hospital stay, length of ICU stay, septic shock after the onset of BSI and 28-day mortality after the onset of BSI) were collected. Microbiological data, such as copathogens in mixed-CA/B-BSIs, likely sources of BSI and sensitivity to antimycotics, were also recorded.
Species identification and antimycotic sensitivity test
Blood samples were collected following rigorous skin disinfection to obtain at least two sets of aerobic and anaerobic blood cultures (10–20 mL per bottle) when the patients were suspected of BSI with clinical manifestations, i.e., fever > 38.0 °C, chills, hypotension, low-grade fever at 38 °C or even hypothermia if sepsis was suspected [12]. Species identification of both bacteria and yeasts was performed by matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonik GmbH, Bremen, Germany). Antimicrobial susceptibility testing for bacteria and yeasts was carried out with a Vitek 2 Compact system and ATB FUNGUS 3 panel (bioMerieux, France), respectively. The results for bacteria and C. albicans were interpreted according to breakpoints defined by the Clinical Laboratory Standards Institute [14, 15]. Because echinocandins were not included in the ATB FUNGUS 3 panel, the results of caspofungin susceptibility were unknown.
Definitions
Candidemia was defined as the isolation of Candida in blood culture accompanied by fever, chills or hypotension and other corresponding clinical symptoms and signs and the exclusion of specimen contamination [16]. If the identified Candida species was C. albicans, CA-BSI was considered. Mixed-CA/B-BSIs were defined as the isolation of a bacterial organism from blood cultures obtained within 48 h before or after the onset of CA-BSI [9]. An infection was considered a healthcare-associated infection (HAI) if the date of the event (specific infection criteria) occurred on or after the 3rd calendar day of admission in an inpatient department; the day of admission to the inpatient department was regarded as calendar day 1 [13]. A definitive diagnosis of catheter-related bloodstream infection (CRBSI) required that the same organism was cultivated from at least one percutaneous blood culture and catheter tip culture or that two cultured blood samples (one from a catheter hub and the other from a peripheral vein) met the CRBSI criteria for quantitative blood culture or differential time to positivity [17]. If no other primary source of infection for candidemia can be assigned as secondary is found, a primary BSI with Candida is identified [12]. The timing of administration of antifungal therapy was defined as the interval between the time at which the first C. albicans-positive blood sample for culture was drawn and the time at which antifungal treatment was first administered [18]. Antifungal therapy was considered appropriate if the isolated Candida spp. was sensitive to the chosen antifungal agent and the antifungal agent was administered with an adequate dosage (for example, fluconazole was administered with a loading dose of 800 mg [12 mg/kg] followed by 400 mg [6 mg/kg] daily, and caspofungin was administered with a loading dose of 70 mg followed by 50 mg daily) [3, 19]. A delay in empiric antifungal treatment was considered when initial administration occurred more than 12 h after the first positive blood sample [18]. Appropriate antibiotic therapy was defined as antibiotic therapy for bacteremia, where applicable, and sensitivity of the pathogen to the agent [20]. Septic shock was consistent with the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) [21].
Statistical analyses
Statistical analysis was performed with SPSS 20.0 software (IBM Corp, Armonk, NY, USA). Continuous variables are presented as the means ± standard deviations if the data were normally distributed and as medians and interquartile ranges (IQRs) if the data were nonnormally distributed. Continuous variables were compared by Student’s t-test or the Mann-Whitney U test, and enumerated variables were compared by the Pearson χ2 or Fisher’s exact test, where appropriate. Variables with p-values < 0.05 in the univariate analysis were entered into the multivariable model. Continuous variables were treated as dichotomous variables based on the Youden index. The multivariate analysis was performed with logistic regression to identify independent risk factors for mixed-CA/B-BSIs. Kaplan-Meier survival estimates were used to generate the survival curves. Differences between survival curves were assessed with log-rank tests. A two-tailed p < 0.05 was considered statistically significant.
Results
Demographics and clinical characteristics
The median age was 68 years (IQR, 59–75 years), and 58.1% (68/117) of the patients were male. Mono-CA-BSI and mixed-CA/B-BSIs were responsible for 93/117 (79.5%) and 24/117 (20.5%) cases, respectively. The most common ward associated with CA-BSI occurrence was the ICU (66.7%), followed by the surgical ward (23.9%) and medical ward (9.4%). Solid tumors were the most common comorbidity (28.2%), followed by diabetes mellitus (23.9%). There were no significant differences in age, sex, immune status, or illness severity between the two groups. In the surgical patients and ICU patients, 65.8% (77/117) and 66.7% (78/117) episodes were documented, respectively. Other common predisposing factors for candidemia included CVC insertion (106/117, 90.6%), urethral catheter insertion (106/117, 90.6%), prior antibiotic exposure (93/117, 79.5%) and total parenteral nutrition (TPN) (85/117, 72.6%). Compared with the mono-CA-BSI group, the mixed-CA/B-BSIs group had a longer ICU stay before candidemia onset [12.0 (8.0,17.8) vs. 1.0 (0.0,11.0) days, p = 0.001], longer hospital stay before candidemia onset [19.0 (12.0,30.8) vs. 12.0 (2.0,26.5) days, p = 0.031], longer duration of mechanical ventilation before candidemia onset [11.0 (0.3,24.5) vs. 1.0 (0.0,10.0) days, p = 0.013], and longer prior antibiotic exposure before candidemia onset [17.0 (10.3,28.8) vs. 8.0 (1.0,20.5) days, p = 0.007]; additionally, they were more likely to have an indwelling hemodialysis catheter [41.7% vs. 18.3%, p = 0.015] and presence of two or more central venous catheters [50.0% vs. 25.8%, p = 0.022], and they had higher rates of life-sustaining treatments such as invasive mechanical ventilation (81.8% vs. 54.7%, p = 0.020) and continuous renal replacement therapy (CRRT) (41.7% vs. 21.5%, p = 0.044). Nonetheless, there were no significant differences in the proportions of surgical patients, blood transfusion, TPN, or hypoproteinemia (see Table 1). The main source of CA-BSI was CVCs (29.1%, 34/117), followed by intra-abdominal catheters (20.5%, 24/117). The main sources of mixed-CA/B-BSIs were CVCs (29.2%, 7/24) and primary sources (29.2%, 7/24). The main source of mono-CA-BSI was CVCs (29.0%, 27/93), followed by intra-abdominal catheters (19.4%, 18/93). Compared with the sources of Candida in mono-CA-BSI, the sources of Candida in mixed-CA/B-BSIs were not significantly different, as shown in Table 2. Regarding infection source control, the rate of CVC removal within 48 h after the first positive sample in the mixed CA/B-BSI group was higher than that in the mono-CA-BSI group (54.2% vs. 29.0%, p = 0.021), but there was no significant difference in the rate of fungal collection from drainage fluid between the two groups (20.8% vs. 15.1%, p = 0.708) (see Table 2).
Table 1.
Baseline characteristics of the patients with mono-CA-BSI or mixed-CA/B-BSIs
| Characteristics | Total(n = 117) | Mono-CA-BSI(n = 93) | Mixed-CA/B-BSIs(n = 24) | Pvalue |
|---|---|---|---|---|
| Age, median years (IQR) | 68 (59,75) | 69 (59,76) | 64 (47,74) | 0.399 |
| Male sex [n (%)] | 68 (58.1%) | 53 (56.9%) | 15 (62.5%) | 0.626 |
| APACHE II score at the onset of candidemia (IQR) | 17.0 (11.5,24.5) | 17.0 (12.0,24.0) | 17.5 (10.0,26.5) | 0.863 |
| SOFA score at the onset of candidemia (IQR) | 6.0 (2.0,9.0) | 5.0 (2.0,9.0) | 6.5 (2.0,9.8) | 0.494 |
| Prior ICU stay (days) (IQR) | 3.0 (0.0,14.0) | 1.0 (0.0,11.0) | 12.0 (8.0,17.8) | 0.001 |
| Prior hospital stay (days) (IQR) | 14.0 (4.5,27.5) | 12.0 (2.0,26.5) | 19.0 (12.0,30.8) | 0.031 |
| Prior ventilation mechanical ventilation (days) (IQR) | 1.0 (0.0,13.0) | 1.0 (0.0,10.0) | 11.0 (0.3,24.5) | 0.013 |
| Underlying disease [n (%)] | ||||
| Diabetes mellitus | 28 (23.9%) | 23 (24.7%) | 5 (20.8%) | 0.690 |
| Chronic cardiac dysfunction | 24 (20.5%) | 16 (17.2%) | 8 (33.3%) | 0.144 |
| Chronic obstructive pulmonary disease | 5 (4.3%) | 5 (5.4%) | 0 (0%) | 0.552 |
| Chronic renal insufficiency | 9 (7.7%) | 8 (8.6%) | 1 (4.2%) | 0.766 |
| Chronic hepatic insufficiency | 14 (12.0%) | 13 (14.0%) | 1 (4.2%) | 0.333 |
| Solid tumour | 33 (28.2%) | 28 (30.1%) | 5 (20.8%) | 0.368 |
| Haematological malignancy | 1 (0.9%) | 1 (1.1%) | 0 (0%) | > 0.999 |
| Trauma | 19 (16.2%) | 14 (15.1%) | 5 (20.8%) | 0.708 |
| Burn injury | 4 (3.4%) | 2 (2.2%) | 2 (8.3%) | 0.186 |
| Transplant | 14 (12.0%) | 11 (11.8%) | 3 (12.5%) | > 0.999 |
| Immunocompromised [n (%)] | ||||
| Immunosuppressant therapy | 6 (5.1%) | 6 (6.5%) | 0 (0.0%) | 0.448 |
| Steroid therapy | 6 (5.1%) | 6 (6.5%) | 0 (0.0%) | 0.448 |
| Chemotherapy/radiation | 7 (6.0%) | 7 (7.5%) | 0 (0.0%) | 0.366 |
| Neutropenia | 4 (3.4%) | 3 (3.2%) | 1 (4.2%) | > 0.999 |
| Blood transfusion [n (%)] | 40 (34.2%) | 30 (32.2%) | 10 (41.7%) | 0.386 |
| Hospitalization ward [n (%)] | ||||
| Medical | 11 (9.4%) | 11 (11.8%) | 0 (0.0%) | 0.168 |
| Surgical | 28 (23.9%) | 24 (25.8%) | 4 (16.7%) | 0.349 |
| ICU | 78 (66.7%) | 58 (62.4%) | 20 (83.3%) | 0.052 |
| Nosocomial infection [n (%)] | 112 (95.7%) | 88 (94.6%) | 24 (100%) | 0.552 |
| Life-sustaining treatments ≥24 h [n (%)] | ||||
| Invasive mechanical ventilation | 65 (60.2%) | 47 (54.7%) | 18 (81.8%) | 0.020 |
| Vasopressor | 45 (38.5%) | 34 (36.6%) | 11 (45.8%) | 0.405 |
| CRRT | 30 (25.6%) | 20 (21.5%) | 10 (41.7%) | 0.044 |
| Catheterisation a [n (%)] | ||||
| Central venous catheterb | 106 (90.6%) | 83 (89.2%) | 23 (95.8%) | 0.553 |
| Hemodialysis catheterc | 27 (23.1%) | 17 (18.3%) | 10 (41.7%) | 0.015 |
| PICC | 13 (11.1%) | 10 (10.8%) | 3 (12.5%) | > 0.999 |
| Peripheral arterial catheters | 37 (31.6%) | 29 (31.2%) | 8 (33.3%) | 0.840 |
| Drainage tube | 77 (65.8%) | 59 (63.4%) | 18 (75.0%) | 0.287 |
| Urethral catheter | 106 (90.6%) | 85 (91.4%) | 21 (87.5%) | 0.848 |
| Presence of two or more central venous catheters | 36 (30.8%) | 24 (25.8%) | 12 (50.0%) | 0.022 |
| Total parenteral nutrition [n (%)] | 85 (72.6%) | 65 (69.9%) | 20 (83.3%) | 0.188 |
| Hypoproteinemia [n (%)] | 49 (41.9%) | 37 (39.8%) | 12 (50.0%) | 0.366 |
| Surgery [n (%)] | 77 (65.8%) | 59 (63.4%) | 18 (75.0%) | 0.287 |
| Abdominal | 39 (33.3%) | 32 (34.4%) | 7 (29.2%) | 0.627 |
Notes: Bold, indicates P < 0.05
Abbreviations: IQR interquartile range, COPD chronic obstructive pulmonary disorder, SOFA sequential organ failure assessment, APACHE acute physiology and chronic health evaluation, ICU intensive care unit, CRRT continuous renal replacement therapy, PICC Peripherally inserted central catheters
aIncluded patients who were required to be catheterised within 2 weeks of the first positive sample, regardless of whether or not the catheter was removed before diagnosis
bNon-tunneled central venous catheters such as subclavian, internal jugular and femoral venous catheters excluding hemodialysis catheter and PICC
cNon-tunneled temporary dialysis catheter
Table 2.
The source of candidemia, prior antibiotic and antifungal therapy of the mono-CA-BSI compared with the Mixed-CA/B-BSIs
| Variable | Total(n = 117) | Mono-CA-BSI(n = 93) | Mixed-CA/B-BSIs(n = 24) | P value |
|---|---|---|---|---|
| Source of candidaemia [n (%)] | ||||
| Definitive CVC-related | 34 (29.1%) | 27 (29.0%) | 7 (29.2%) | 0.990 |
| Intra-abdominal | 24 (20.5%) | 18 (19.4%) | 6 (25.0%) | 0.744 |
| Primary | 22 (18.8%) | 15 (16.1%) | 7 (29.2%) | 0.244 |
| Lower respiratory tract | 12 (10.3%) | 11 (11.8%) | 1 (4.2%) | 0.468 |
| Urinary tract infection | 7 (6.0%) | 6 (6.5%) | 1 (4.2%) | > 0.999 |
| Gastrointestinal tract | 6 (5.1%) | 6 (6.5%) | 0 (0.0%) | 0.344 |
| Skin and Soft tissue | 5 (4.3%) | 4 (4.3%) | 1 (4.2%) | > 0.999 |
| Meningitis | 3 (2.6%) | 2 (2.2%) | 1 (4.2%) | 0.501 |
| Endocardium | 2 (1.7%) | 2 (2.2%) | 0 (0.0%) | > 0.999 |
| Osteoarthritis | 1 (0.9%) | 1 (1.1%) | 0 (0.0%) | > 0.999 |
| Source control [n (%)] | ||||
| Removal of contaminated lines a | 40 (34.2%) | 27 (29.0%) | 13 (54.2%) | 0.021 |
| Draining of fungal collection | 19 (16.2%) | 14 (15.1%) | 5 (20.8%) | 0.708 |
| Days of prior antibiotic exposure (IQR) | 11.0 (3.0,22.0) | 8.0 (1.0,20.5) | 17.0 (10.3,28.8) | 0.007 |
| Prior antibiotic exposure b [n (%)] | 93 (79.5%) | 69 (74.2%) | 24 (100.0%) | 0.012 |
| Cephalosporins | 33 (28.2%) | 25 (26.9%) | 8 (33.3%) | 0.531 |
| Carbapenems | 49 (41.9%) | 41 (44.1%) | 8 (33.3%) | 0.341 |
| Penicillins | 25 (21.4%) | 19 (20.4%) | 6 (25.0%) | 0.626 |
| Quinolones | 4 (3.4%) | 4 (4.3%) | 0 (0.0%) | 0.580 |
| Initial antifungal agent [n (%)] | ||||
| Fluconazole | 40 (34.2%) | 32 (34.4%) | 8 (33.3%) | 0.921 |
| Echinocandin | 46 (39.3%) | 36 (38.7%) | 10 (41.7%) | 0.791 |
| Voriconazole | 11 (9.4%) | 9 (9.7%) | 2 (8.3%) | > 0.999 |
| Prior antifungal exposure [n (%)] | 10 (8.5%) | 6 (6.4%) | 4 (16.7%) | 0.235 |
| Appropriate Antifungal therapy c [n (%)] | 43 (36.8%) | 35 (37.6%) | 8 (33.3%) | 0.697 |
| Delay in initiation of empiric antifungal treatment d [n (%)] | 100 (85.5%) | 82 (88.2%) | 18 (75.0%) | 0.103 |
Abbreviations: CVC central venous catheter, PICC Peripherally inserted central catheters, CRBSI catheter-related bloodstream infection;
aCentral venous catheter removed within 48 h after the first positive sample
bAll patients receiving systemic drug therapy for ≥3 days within 2 weeks prior to candidaemia onset
cAntifungal therapy was defined as appropriate if the isolated Candida spp. was sensitive to the chosen antifungal agent, and the antifungal agent was used with adequate dosages (like Fluconazole: loading dose of 800 mg [12 mg/kg], then 400 mg [6 mg/kg] daily; Caspofungin: loading dose of 70 mg, then 50 mg daily)
dThe delay of empiric antifungal treatment was considered as initial use more than 12 h after the report of first positive blood sample
A high rate of delay of initiation of empiric antifungal treatment (85.5%) was observed among all patients, and no difference was found between the mixed-CA/B-BSIs (75.0%) and mono-CA-BSI (88.2%) groups. In addition, the total rate of appropriate antifungal therapy was less than 50% (36.8%), and it was similar between patients with mixed-CA/B-BSIs (33.3%) and those with mono-CA-BSI (37.6%), as shown in Table 2. The proportions of empiric treatment and appropriate antibiotic therapy for bacteremia in mixed-CA/B-BSIs patients were 33% (8/24) and 70% (17/24), respectively.
Antifungal susceptibility
The isolated C. albicans in both groups exhibited 100% susceptibility to amphotericin B, voriconazole, and no resistance to fluconazole was observed. Notably, in the mono-CA-BSI and mixed CA/B-BSIs groups, 11 (24.4%) and 2 (13.3%) cases were completely resistant to ketoconazole, respectively. There was no significant difference between the two groups in the in-vitro antifungal susceptibility test results, as shown in Table 3. Because the drug sensitivity kit used in our current microbiology laboratory does not include echinocandins, the specific drug sensitivity of C. albicans to echinocandins was unclear.
Table 3.
In vitro antifungal susceptibility of C. albicans between mono-CA-BSI and mixed-CA/B-BSIs
| Candida species | Antifungalagent | mono-CA-BSI(n = 93) | mixed-CA/B-BSIs(n = 24) | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of strains | Drug sensitivity | Number of strains | Drug sensitivity | |||||||
| S | I | R | S | I | R | |||||
| C.albicans | Fluconazole(n = 104) a | 85(91.3%) | 81(95.3%) | 4(4.7%) | 0 | 19(79.1%) | 19(100.0%) | 0 | 0 | > 0.999 |
| Clotrimazole(n = 69) a | 55(59.1%) | 54(98.2%) | 0 | 1(1.8%) | 15(62.5%) | 14(93.3%) | 0 | 0 | 0.901 | |
| Ketoconazole(n = 60) a | 45(48.3%) | 19(42.2%) | 15(33.3%) | 11(24.4%) | 15(62.5%) | 7(46.7%) | 6(40.0%) | 2(13.3%) | 0.764 | |
| Itraconazole(n = 111) a | 89(95.7%) | 86(96.6%) | 1(1.1%) | 2(2.2%) | 22(91.7%) | 21(95.5%) | 1(4.5%) | 0 | > 0.999 | |
| Amphotericin B (n = 111) a | 90(96.8%) | 90(100.0%) | 0 | 0 | 21(87.5%) | 21(100.0%) | 0 | 0 | > 0.999 | |
| Nystatin(n = 68) a | 56(60.2%) | 55(98.2%) | 1(1.8%) | 0 | 12(50.0%) | 12(100.0%) | 0 | 0 | > 0.999 | |
| 5-fluorocytosine(n = 38) a | 31(33.3%) | 30(96.8%) | 0 | 1(3.2%) | 7(29.1%) | 7(100.0%) | 0 | 0 | > 0.999 | |
| Voriconazole(n = 103) a | 82(88.2%) | 82(100.0%) | 0 | 0 | 21(87.5%) | 21(100.0%) | 0 | 0 | > 0.999 | |
S sensitive, I intermediary, R resistant
aNot all agents listed tested in all isolates
Independent risk factors for mixed-CA/B-BSIs
Variables with p-value of < 0.05, including a prior hospital stay> 7 days, a prior ICU stay> 2 days, prior antibiotic exposure> 7 days, prior mechanical ventilation> 2 days, an indwelled hemodialysis catheter and the presence of two or more CVCs at the time of onset of candidemia, were entered into the multivariable logistic regression model to identify factors associated with mixed-CA/B-BSIs. As shown in Table 4, the only independent risk factor for mixed-CA/B-BSIs was a prior ICU stay > 2 days (adjusted odds ratio [OR], 7.445; 95% confidence interval [CI], 1.152–48.132).
Table 4.
Multivariable logistic regression of factors associated with mixed-CA/B-BSIs
| Risk factors | B | S.E. | Wald | P value | OR(95% CI) |
|---|---|---|---|---|---|
| Prior hospital stay> 7 days | 0.787 | 1.581 | 0.248 | 0.618 | 2.198 (0.099,48.740) |
| Prior ICU stay> 2 days | 2.008 | 0.952 | 4.444 | 0.035 | 7.445 (1.152,48.132) |
| Prior antibiotic exposure> 7 days | 1.289 | 1.176 | 1.203 | 0.273 | 3.631 (0.362,36.383) |
| Prior mechanical ventilation> 2 days | −0.469 | 0.809 | 0.336 | 0.562 | 0.626 (0.128,3.057) |
| Hemodialysis catheter | 0.707 | 0.913 | 0.600 | 0.439 | 2.028 (0.339,12.133) |
| Two or more central venous catheters | 0.525 | 0.889 | 0.348 | 0.555 | 1.690 (0.296,9.652) |
| Constant | −4.519 | 1.147 | 15.517 | 0.000 | 0.011(−) |
Notes: Bold, indicates P < 0.05
Abbreviations: B coefficient, S.E. standard error, Wald Wald test statistic, OR odds ratio, CI confidence interval, ICU intensive care unit, CRRT continuous renal replacement therapy
Species distributions of concomitant bacteria isolated from the mixed-CA/B-BSIs
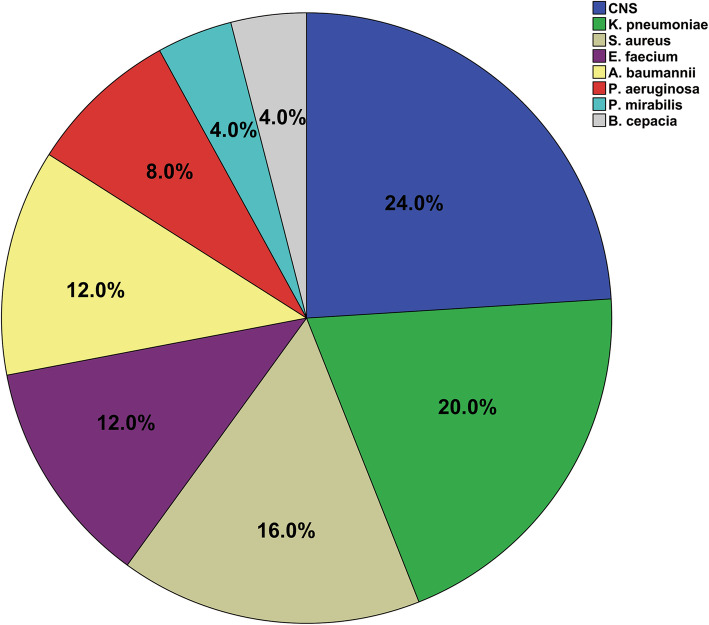
The most common copathogens were gram-positive bacteria (52.0%), followed by gram-negative bacteria (48.0%). In terms of the exact microorganisms, the most frequent pathogen was CNS (24.0%), followed by Klebsiella pneumoniae (K. pneumoniae) (20.0%) and Staphylococcus aureus (S. aureus) (16.0%). The detailed distribution of concomitant bacterial species in mixed-CA/B-BSIs is shown in Fig. 2.
Fig. 2.
Species distributions of concomitant bacteria isolated from the mixed-CA/B-BSIs
Outcomes
The median length of ICU stay was 14 days (IQR, 1.0–33.0), and the median length of hospital stay was 33 days (IQR, 18.0–56.0). Compared with patients with mono-CA-BSI, patients with mixed -CA/B-BSIs had a prolonged length of ICU stay [8.0 (0.0, 31.5) vs. 22.0 (14.3, 42.2) days, p = 0.010] and longer mechanical ventilation time [3.0 (0.0, 24.5) vs. 17.5 (4.5, 34.8) days, p = 0.019]. The incidence of septic shock, 28-day and 60-day mortality, and in-hospital mortality in patients with mixed-CA/B-BSIs were not different from those in patients with mono-CA-BSI (Table 5, Fig. 3).
Table 5.
Comparison of outcomes between mono-CA-BSI and mixed-CA/B-BSIs
| Outcomes | Total(n = 117) | mono-CA-BSI(n = 93) | mixed-CA/B-BSIs(n = 24) | P value |
|---|---|---|---|---|
| Total ICU stay days (IQR) | 14.0 (1.0, 33.0) | 8.0 (0.0, 31.5) | 22.0 (14.3, 42.2) | 0.010 |
| Total Hospitalization days (IQR) | 33.0 (18.0, 56.0) | 33.0 (15.0,51.0) | 31.5 (23.0,66.0) | 0.217 |
| Total mechanical ventilation days (IQR) | 6.0 (0.0,30.5) | 3.0 (0.0,24.5) | 17.5 (4.5,34.8) | 0.019 |
| Septic shock (n,%) | 40 (34.2%) | 31 (33.3%) | 9 (37.5%) | 0.701 |
| 28-day mortality (n,%) | 41 (35.0%) | 31 (33.3%) | 10 (41.7%) | 0.446 |
| 60-day mortality (n,%) | 46 (39.3%) | 34 (36.6%) | 12 (50.0%) | 0.229 |
| In-hospital mortality (n,%) | 50 (42.7%) | 37 (39.8%) | 13 (54.2%) | 0.204 |
Notes: Bold, indicates P < 0.05
Abbreviations: ICU intensive care unit, IQR interquartile range
Fig. 3.
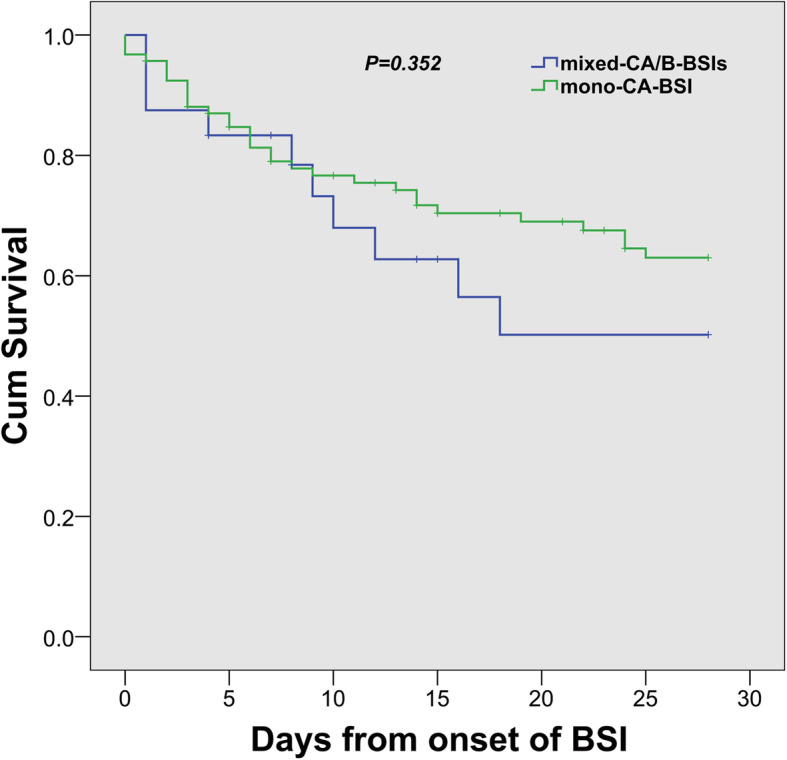
Kaplan-Meier estimates of survival in patients with mixed Candida albicans/bacterial bloodstream infections and monomicrobial Candida albicans bloodstream infection
Discussion
Polymicrobial bacteremia has been reported in previous studies, which was observed in 23.5 and 48.0% of patients with Acinetobacter baumannii bacteremia and K. pneumoniae bacteremia, respectively [22, 23]. In terms of enterococcal BSIs, 34.8% of cases (157/451) had coinfection with other pathogens, such as CNS, A. baumannii, or K. pneumoniae [24]. The current report found that the incidence of mixed- CA/B-BSIs was 20.5%. A similar proportion of mixed-CA/B-BSIs among CA-BSIs was reported in developed regions of Europe, such as Spain (18%) [4], Asia, such as South Korea (23%) [5], and China (20%) [8]. These results suggest that relatively high proportions of specific polymicrobial BSIs are observed not only in bacterial BSIs but also in candidemia and CA-BSIs.
Similar risk factors were found to be associated with mixed-CA/B-BSIs in previous studies [4, 5], including a prolonged ICU stay, a prolonged hospital stay before candidemia onset, antimicrobial administration, the presence of an indwelling hemodialysis catheter, the presence of two or more central venous catheters, and the existence of organ dysfunction/failure (e.g., the need for invasive mechanical ventilation or CRRT) (Table 1). However, there were no differences in the APACHE II score and SOFA score between groups (Table 1), which might reflect similar severities of comorbid diseases. Although septic shock at the time of candidemia was positively associated with mixed Candida/bacterial BSIs in a previous study [5], it was not independently associated with mixed Candida/bacterial BSIs in the current study. This might be partly explained by the similar rate of appropriate antifungal therapy in both groups and a high rate of antibiotic administration (70%) and high rate of CVC removal within 48 h after the first positive sample (54.2%) in the mixed-CA/B-BSIs group (Table 2). Previous work demonstrated that more than 20.2% of nosocomial BSIs in the ICU were polymicrobial BSIs [25, 26], which is consistent with our finding that a prior ICU stay > 2 days was an independent risk factor for mixed-CA/B-BSIs. The high incidence of polymicrobial BSI in the ICU might be explained by a suboptimal host defense altered by underlying diseases, an increased number of artificial/invasive procedures, or the application of immunosuppressive therapy in critically ill patients [26]. These results indicate that patients in the ICU are not only susceptible to BSI but also vulnerable to polymicrobial BSI, including mixed-CA/B-BSIs.
In the current study, gram-positive bacteria (52%) were the main copathogens in mixed-CA/B-BSIs, followed by gram-negative bacteria (48%). Among all the specific coexisting species, CNS was the predominant (24%) species, consistent with previous reports [5]. Following CNS, the most prevalent copathogen species were K. pneumoniae (20%) and S. aureus (16%) (Fig. 2). This might be partly explained by the fact that the primary source of mixed-CA/B-BSIs is a CVC (29.2%). It has been demonstrated that the polymicrobial biofilms formed by C. albicans and Staphylococcus epidermidis in vitro are commonly found in catheter-associated infection cases [27]. Although the main copathogen in Kim’s study was also CNS, the second most common pathogens were Enterococcus spp. and S. aureus [5]. The gastrointestinal tract (35%) was the most common source of mixed-CA/B-BSIs in Kim’s research, while it accounted for only 5.1% of infections in the current study (Table 2). It is well known that a common source of enterococcal bacteremia is the gastrointestinal tract [28]. Consistent with a previous study that found a high proportion of K. pneumoniae (15.2%) among BSIs [29], K. pneumoniae was the second most common copathogen in mixed-CA/B-BSIs in our research; this might be partly due to the fact that K. pneumoniae has been a leading cause of HAIs over the past few decades [30]. Consistent with a previous report [6], S. aureus was the third most common isolated organism in conjunction with C. albicans in mixed-CA/B-BSIs cases. S. aureus formed a substantial polymicrobial biofilm in the presence of C. albicans [31].
Although patients with mixed-CA/B-BSIs had worse outcomes (e.g., prolonged lengths of ICU stay and prolonged mechanical ventilation time) than those with mono-CA-BSI, 28-day mortality (41.7% vs. 33.3%, P = 0.446), 60-day mortality (50.0% vs. 36.6%, P = 0.229) and in-hospital mortality (54.2% vs. 39.8%, P = 0.204) were similar between the two groups (Table 5, Fig. 3), similar to previous studies [4, 5, 7]. In contrast, previous studies showed polymicrobial BSI was associated with a 2.2 fold risk for increased 90-day mortality in patients with community-onset BSI [32], and also promoted an increase in 28-day mortality [33]. In our study, we found no correlation between mixed-CA/B-BSIs and mortality, which might be due to similar chronic comorbidities, a similar severity of illness at the onset of candidemia, a similar rate of fungal collection from drainage fluid, a similar delay in the initiation of empiric antifungal treatment (75% vs. 88.2% P = 0.697) and a similar rate of appropriate antifungal therapy administration (33.3% vs. 37.6%, P = 0.697) (Table 2).
The present study has some limitations. First, this was a single-center study, and therefore, the results and conclusions might be influenced by local ecology, management practices, infection control policies, and susceptibility patterns. Second, some critical factors of mixed-CA/B-BSIs might have been missed because of the retrospective design. For example, the corticosteroid dosage and treatment course were not precisely obtained; thus, the immune status due to corticosteroids was unclear. We did not get information about antifungal prophylaxis and follow-up blood cultures to confirm pathogen clearance; thus, the duration of candidemia could not be obtained accurately. Third, although culture-based methods remain the gold standard to identify the causative microorganism in sepsis cases, they are notoriously insensitive, leading to challenges in implementing early interventions [34]. Nonculture diagnostic tests, such as antigen, antibody, or β-D-glucan detection assays; polymerase chain reaction (PCR) assays; and next-generation sequencing (NGS) methods, are now being performed in clinical practice as supplements to blood culture and might provide an early and/or highly sensitive diagnosis of BSI [35–37]. Finally, although this is the first report on the risk factors for and clinical outcomes of mixed-CA/B-BSIs compared with mono-CA-BSI, the relatively small sample size may impact the confidence intervals (CIs) and analysis of risk factors. Thus, further large-scale, multicenter, prospective studies are needed.
Conclusions
Among the total CA-BSIs, mixed-CA/B-BSIs were not rare, and CNS was the predominant coexisting species in mixed-CA/B-BSIs. A prior ICU stay > 2 days was an independent risk factor for mixed-CA/B-BSIs. Although there was no difference in mortality, the prognosis of adult patients with mixed-CA/B-BSIs, including prolonged length of mechanical ventilation and prolonged length of ICU stay, was worse than that in patients with mono-CA-BSI.
Acknowledgements
Not applicable.
Abbreviations
- CA-BSI
Candida albicans bloodstream infection
- mono-CA-BSI
Monomicrobial Candida albicans bloodstream infection
- mixed-CA/B-BSIs
Mixed Candida albicans/bacterial bloodstream infections
- BSI
Bloodstream infection
- IQR
Interquartile range
- CRBSI
Catheter-related bloodstream infection
- CRRT
Continuous renal replacement therapy
- CVC
Central venous catheter
- COPD
Chronic obstructive pulmonary disorder
- SOFA
Sequential organ failure assessment
- APACHE
Acute physiology and chronic health evaluation
- ICU
Intensive care unit
- OR
Odds ratio
- CI
Confidence interval
- CNS
Coagulase-negative Staphylococcus
- K. pneumoniae
Klebsiella pneumoniae
- S. aureus
Staphylococcus aureus
- A. baumannii
Acinetobacter baumannii
- E. faecium
Enterococcus faecium
- P. aeruginosa
Pseudomonas aeruginosa
- B. cepacia
Burkholderia cepacia
- P. mirabilis
Proteus mirabilis
- S. epidermidis
Staphylococcus epidermidis
- CLSI
Clinical and Laboratory Standards Institute
Authors’ contributions
GZ, W C and ZD designed the study, revised the manuscript and gave final approval of the version to be published; LZ, SZ and KT coordinated the writing and preparation of the manuscript and collected/analyzed the data. FZ, CZ, KZ, JC, HZ, YW, BT and ZZ collected and analyzed the data. The author(s) read and approved the final manuscript.
Funding
This manuscript was funded by the National Natural Science Foundation of China (No. 81971871, GS Zhang; No. 81772110, ZC Zhang; No. 81901941, SF Zhang) and the Natural Science Foundation of Zhejiang Province (No. LY19H150007, GS Zhang).
Availability of data and materials
We declare that the data supporting the conclusions of this article are fully described within the article, and the database is available from the first author (lizhong975717720@foxmail.com) upon reasonable request.
Ethics approval and consent to participate
This study was approved by the Human Ethics Board of the Ethics Committee of the Second Affiliated Hospital of Zhejiang University Medical College (reference number 2019–191). We ensured that the patient data remained confidential and complied with the Declaration of Helsinki. Due to its retrospective nature, the Ethics Committee determined that patient consent was not required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Li Zhong, Shufang Zhang and Kankai Tang contributed equally to this work.
Contributor Information
Wei Cui, Email: zricu@zju.edu.cn.
Zhaohui Dong, Email: 1409640178@qq.com.
Gensheng Zhang, Email: genshengzhang@zju.edu.cn.
References
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Toda M, Williams SR, Berkow EL, Farley MM, Harrison LH, Bonner L, Marceaux KM, Hollick R, Zhang AY, Schaffner W, et al. Population-based active surveillance for culture-confirmed Candidemia - four sites, United States, 2012-2016. MMWR Surveill Summ (Washington, DC : 2002) 2019;68(8):1–15. doi: 10.15585/mmwr.ss6808a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das I, Nightingale P, Patel M, Jumaa P. Epidemiology, clinical characteristics, and outcome of candidemia: experience in a tertiary referral center in the UK. Int J Infect Dis. 2011;15(11):e759–e763. doi: 10.1016/j.ijid.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Bouza E, Burillo A, Munoz P, Guinea J, Marin M, Rodriguez-Creixems M. Mixed bloodstream infections involving bacteria and Candida spp. J Antimicrob Chemother. 2013;68(8):1881–1888. doi: 10.1093/jac/dkt099. [DOI] [PubMed] [Google Scholar]
- 5.Kim SH, Yoon YK, Kim MJ, Sohn JW. Risk factors for and clinical implications of mixed Candida/bacterial bloodstream infections. Clin Microbiol Infect. 2013;19(1):62–68. doi: 10.1111/j.1469-0691.2012.03906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klotz SA, Chasin BS, Powell B, Gaur NK, Lipke PN. Polymicrobial bloodstream infections involving Candida species: analysis of patients and review of the literature. Diagn Microbiol Infect Dis. 2007;59(4):401–406. doi: 10.1016/j.diagmicrobio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Pulimood S, Ganesan L, Alangaden G, Chandrasekar P. Polymicrobial candidemia. Diagn Microbiol Infect Dis. 2002;44(4):353–357. doi: 10.1016/S0732-8893(02)00460-1. [DOI] [PubMed] [Google Scholar]
- 8.Chen XC, Xu J, Wu DP. Clinical characteristics and implications of mixed candida/bacterial bloodstream infections in patients with hematological diseases. Eur J Clin Microbiol Infect Dis. 2020;39(8):1445–1452. doi: 10.1007/s10096-020-03863-2. [DOI] [PubMed] [Google Scholar]
- 9.Antoniadou A, Torres HA, Lewis RE, Thornby J, Bodey GP, Tarrand JP, Han XY, Rolston KV, Safdar A, Raad II, et al. Candidemia in a tertiary care cancer center: in vitro susceptibility and its association with outcome of initial antifungal therapy. Medicine. 2003;82(5):309–321. doi: 10.1097/01.md.0000091182.93122.8e. [DOI] [PubMed] [Google Scholar]
- 10.Almirante B, Rodriguez D, Park BJ, Cuenca-Estrella M, Planes AM, Almela M, Mensa J, Sanchez F, Ayats J, Gimenez M, et al. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J Clin Microbiol. 2005;43(4):1829–1835. doi: 10.1128/JCM.43.4.1829-1835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajendran R, Sherry L, Nile CJ, Sherriff A, Johnson EM, Hanson MF, Williams C, Munro CA, Jones BJ, Ramage G. Biofilm formation is a risk factor for mortality in patients with Candida albicans bloodstream infection-Scotland, 2012-2013. Clin Microbiol Infect. 2016;22(1):87–93. doi: 10.1016/j.cmi.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC . Bloodstream infection event (central line-associated bloodstream infection and non-central line-associated bloodstream infection) Atlanta: CDC; 2015. [Google Scholar]
- 13.CDC . Identifying Healthcare-associated Infections (HAI) for NHSN Surveillance. Atlanta: CDC; 2015. [Google Scholar]
- 14.Institute CaLS . Reference method for broth dilution antifungal susceptibility testing of yeasts, third informational supplement. Wayne: M27-A3; 2008. [Google Scholar]
- 15.Institute CaLS . Performance standards for antimicrobial susceptibility testing. 28. Wayne: supplement M100; 2018. [Google Scholar]
- 16.Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, Denning DW, Donnelly JP, Edwards JE, Erjavec Z, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34(1):7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 17.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49(9):3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(5):503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zasowski EJ, Claeys KC, Lagnf AM, Davis SL, Rybak MJ. Time is of the essence: the impact of delayed antibiotic therapy on patient outcomes in hospital-onset Enterococcal bloodstream infections. Clin Infect Dis. 2016;62(10):1242–1250. doi: 10.1093/cid/ciw110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for Sepsis and septic shock (Sepsis-3) Jama. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang YC, Ku WW, Yang YS, Kao CC, Kang FY, Kuo SC, Chiu CH, Chen TL, Wang FD, Lee AY. Is Polymicrobial Bacteremia an Independent Risk Factor for Mortality in Acinetobacter baumannii Bacteremia? J Clin Med. 2020;9(1). [DOI] [PMC free article] [PubMed]
- 23.Liu Q, Wu J, Wang Z, Wu X, Wang G, Ren J. Polymicrobial bacteremia involving Klebsiella pneumoniae in patients with complicated intra-abdominal infections: frequency, co-pathogens, risk factors, and clinical outcomes. Surg Infect. 2019;20(4):317–325. doi: 10.1089/sur.2018.207. [DOI] [PubMed] [Google Scholar]
- 24.Zheng C, Cai J, Liu H, Zhang S, Zhong L, Xuan N, Zhou H, Zhang K, Wang Y, Zhang X, et al. Clinical characteristics and risk factors in mixed-Enterococcal bloodstream infections. Infect Drug Resist. 2019;12:3397–3407. doi: 10.2147/IDR.S217905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sancho S, Artero A, Zaragoza R, Camarena JJ, Gonzalez R, Nogueira JM. Impact of nosocomial polymicrobial bloodstream infections on the outcome in critically ill patients. Eur J Clin Microbiol Infect Dis. 2012;31(8):1791–1796. doi: 10.1007/s10096-011-1503-8. [DOI] [PubMed] [Google Scholar]
- 26.Rello J, Quintana E, Mirelis B, Gurgui M, Net A, Prats G. Polymicrobial bacteremia in critically ill patients. Intensive Care Med. 1993;19(1):22–25. doi: 10.1007/BF01709273. [DOI] [PubMed] [Google Scholar]
- 27.Adam B, Baillie GS, Douglas LJ. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J Med Microbiol. 2002;51(4):344–349. doi: 10.1099/0022-1317-51-4-344. [DOI] [PubMed] [Google Scholar]
- 28.Billington EO, Phang SH, Gregson DB, Pitout JD, Ross T, Church DL, Laupland KB, Parkins MD. Incidence, risk factors, and outcomes for Enterococcus spp. blood stream infections: a population-based study. Int J Infect Dis. 2014;26:76–82. doi: 10.1016/j.ijid.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Chen S, Liu S, Yuan X, Mai H, Lin J, Wen F. Etiology, drug sensitivity profiles and clinical outcome of bloodstream infections: a retrospective study of 784 pediatric patients with hematological and neoplastic diseases. Pediatr Hematol Oncol. 2019;36(8):482–493. doi: 10.1080/08880018.2019.1667462. [DOI] [PubMed] [Google Scholar]
- 30.Moradigaravand D, Martin V, Peacock SJ, Parkhill J. Evolution and Epidemiology of Multidrug-Resistant Klebsiella pneumoniae in the United Kingdom and Ireland. mBio. 2017;8(1). [DOI] [PMC free article] [PubMed]
- 31.Harriott MM, Noverr MC. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: effects on antimicrobial resistance. Antimicrob Agents Chemother. 2009;53(9):3914–3922. doi: 10.1128/AAC.00657-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yo CH, Hsein YC, Wu YL, Hsu WT, Ma MH, Tsai CH, Chen SC, Lee CC. Clinical predictors and outcome impact of community-onset polymicrobial bloodstream infection. Int J Antimicrob Agents. 2019. [DOI] [PubMed]
- 33.Pavlaki M, Poulakou G, Drimousis P, Adamis G, Apostolidou E, Gatselis NK, Kritselis I, Mega A, Mylona V, Papatsoris A, et al. Polymicrobial bloodstream infections: epidemiology and impact on mortality. J Glob Antimicrob Resist. 2013;1(4):207–212. doi: 10.1016/j.jgar.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, et al. Clinical practice guideline for the Management of Candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1–50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chibabhai V, Fadana V, Bosman N, Nana T. Comparative sensitivity of 1,3 beta-D-glucan for common causes of candidaemia in South Africa. Mycoses. 2019;62(11):1023–1028. doi: 10.1111/myc.12982. [DOI] [PubMed] [Google Scholar]
- 36.McKeating C, White PL, Posso R, Palmer M, Johnson E, McMullan R. Diagnostic accuracy of fungal PCR and β-d-glucan for detection of candidaemia: a preliminary evaluation. J Clin Pathol. 2018;71(5):420–424. doi: 10.1136/jclinpath-2017-204692. [DOI] [PubMed] [Google Scholar]
- 37.Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. 2019;20(6):341–355. doi: 10.1038/s41576-019-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We declare that the data supporting the conclusions of this article are fully described within the article, and the database is available from the first author (lizhong975717720@foxmail.com) upon reasonable request.