Abstract
Lessons Learned
Removal of sonographically abnormal (up to 3) metastatic clipped nodes, without sentinel lymph node biopsy, could accurately predict axillary status in breast cancer patients receiving neoadjuvant chemotherapy.
ypT and the first clipped node status were statistically significant factors for nodal pathologic complete response.
This novel approach requires validation in larger studies.
Background
In patients who have node‐positive breast cancer, neoadjuvant chemotherapy could result in nodal pathologic complete response (pCR) and avoid an axillary lymph node dissection (ALND). Axillary staging, in such cases, can be performed using targeted axillary dissection (TAD) with a low false negative rate. However, identification of sentinel lymph nodes (SLNs) after chemotherapy can be difficult, and currently, it is the standard to remove only one clipped node in TAD. We aimed to determine if removal of all sonographically abnormal metastatic clipped nodes, without SLN biopsy, could accurately predict the axillary status post neoadjuvant chemotherapy.
Methods
Patients with breast cancer with one to three sonographically abnormal metastatic axillary nodes were prospectively recruited. Each abnormal node had histology and clip insertion before neoadjuvant chemotherapy. After chemotherapy, the patients underwent removal of clipped nodes using the Skin Mark clipped Axillary nodes Removal Technique (SMART) and ALND.
Results
Fourteen patients were recruited, having a total of 21 sonographically abnormal metastatic nodes, with nine, three, and two patients having 1, 2, and 3 malignant nodes clipped, respectively. Mean age was 55.5 years; 92.9% and 57.1% of patients had invasive ductal carcinoma and grade III tumors, respectively; and 35.7% patients achieved nodal pCR. The first clipped node predicted the axillary status with a false negative rate of 7.1%. Adding to this another second clipped node, the false negative rate was 0%. Pathologic tumor staging after neoadjuvant chemotherapy (ypT) (p = .0390) and the first clipped node pathological response status (p = .0030) were statistically significant predictors for nodal pCR.
Conclusion
Removal of sonographically abnormal metastatic clipped nodes using SMART, without sentinel lymph node biopsy, could accurately predict axillary status. This finding needs validation in larger studies.
Discussion
The promising axillary nodal response [1] post chemotherapy prompted the consideration of SLN biopsy for axillary staging, to avoid an axillary dissection and its morbidities [2]. However, using sentinel lymph node biopsy alone for axillary staging is associated with a false negative rate (FNR) of 12.6%–14.2% [3, 4]. To decrease the FNR, TAD was introduced [5].
However, TAD still requires the identification of sentinel lymph nodes, which can be challenging after chemotherapy [6]. Furthermore, the sentinel lymph nodes did not corelate with the metastatic clipped node in 23% of cases [5]. Conversely, removal of the clipped node alone, without sentinel lymph node biopsy post chemotherapy, has an FNR of 7% [7]. Currently, only a single clipped node was removed in axillary staging.
This is the first study, to the best of our knowledge, to determine the accuracy of clipping and removal of all sonographically abnormal metastatic nodes seen prior to neoadjuvant chemotherapy, on axillary staging, without SLN biopsy. Only patients with up to 3 sonographically abnormal lymph nodes were included. Each node was clipped with a different clip to aid individual node identification (Fig. 1).
Figure 1.
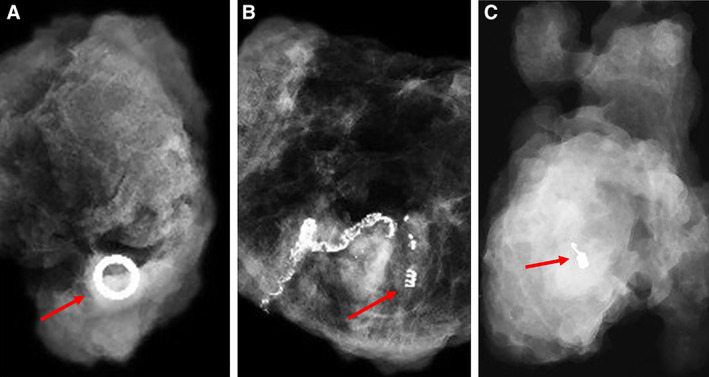
Radiographs of resected clipped node with arrows showing the different types of clips used. (A): UltraCor Twirl. (B): HydroMARK. (C): UltraClip dual trigger.
Intuitively, targeted assessment of nodes, which were proven metastatic before neoadjuvant chemotherapy, should predict reliably nodal response [7]. Based on the first clipped node, our FNR was similar to reported literature [7]. However, as chemotherapy can cause a non‐uniform nodal response, evaluation of more clipped metastatic nodes could logically give a better representation of the true axillary status. Our FNR was further decreased with assessment of a second clipped node. Assessment of the clipped nodes also avoided the problems associated with sentinel lymph node identification, secondary to aberrant lymphatics post chemotherapy.
In our study, ypT and the first clipped node status correlated with nodal pCR, which were consistent with literature [7, 8], but a significant relationship between estrogen receptor (ER) status and nodal pCR was not seen. This could be explained by 20% of our patients with ER‐positive disease having weak positivity.
Strengths of this study include a detailed documentation of the individual clipped nodes with radiological and pathological correlation. Limitations include a small sample size and additional cost that may be incurred secondary to multiple nodal localization devices; however, this may be negated by the cost of sentinel lymph node biopsy, which was omitted. SMART is a novel inexpensive technique [9] that can be used for removal of multiple clipped nodes, but it has limitations. A cost analysis will require future studies.
Trial Information
| Disease | Breast cancer |
| Stage of Disease/Treatment | Neoadjuvant |
| Prior Therapy | None |
| Type of Study – 1 | Phase II |
| Type of Study – 2 | Single arm |
| Primary Endpoint | False negative rate of clipped nodes on axillary staging |
| Secondary Endpoint | Factors affecting nodal pathologic complete response |
| Additional Details of Endpoints or Study Design | |
|
Newly diagnosed patients with invasive breast cancer going for neoadjuvant chemotherapy with histologically proven metastatic axillary nodal burden and one to three abnormal lymph nodes on ultrasound were recruited. Patients with stage IV disease or more than three abnormal lymph nodes on ultrasound were excluded. All recruited patients underwent a dedicated axillary ultrasound in addition to their mammogram and breast ultrasound evaluation. An abnormal axillary lymph node was defined as having any of the following sonographic features: cortical thickness more than 3 mm or marked fatty hilar effacement. In these patients, the sonographically most suspicious lymph node was first biopsied. If proven metastatic, this particular lymph node would be clipped at a second setting and designated as the first clipped node. During the same second setting, the rest of the sonographically abnormal lymph nodes would undergo ultrasound guided fine needle cytology and clipping was performed for all malignant nodes. Patients with multiple nodes would have each node clipped with a different type of metallic marker to aid individual node identification. The clips used included UltraCor Twirl, HydroMARK, and UltraClip dual trigger markers. The choice of clip was determined by the performing radiologist's preference. After neoadjuvant chemotherapy, all patients underwent SMART and ALND. The clip in the clipped node specimen was confirmed by specimen x‐ray and pathological examination. All patients then underwent neoadjuvant chemotherapy. | |
| Investigator's Analysis | Active and should be pursued further |
Patient Characteristics
| Number of Patients, Male | 0 |
| Number of Patients, Female | 14 |
| Age | Median (range): 57.5 (27–74) years |
| Number of Prior Systemic Therapies | Median (range): nil |
| Performance Status: ECOG |
0 — 14 1 — 2 — 3 — Unknown — |
| Cancer Types or Histologic Subtypes | Invasive ductal cancer, 13; invasive lobular cancer, 1 |
Primary Assessment Method
| Title | New assessment |
| Number of Patients Enrolled | 14 |
| Evaluation Method and Outcome Notes | |
|
The primary endpoint of FNR was calculated by correlating the pathological result of each clipped lymph node specimen (removed via SMART) with the patients’ ypN status. For clipped nodes that were not retrieved via SMART, the clip was specifically identified in the ALND specimen and analyzed for nodal pathological response. For the secondary endpoint of identifying factors affecting nodal pCR, Fisher's exact test (p < .05) was used to compare categorical demographic, radiological and pathological variables between patients and clips with and without nodal pCR. The sample size was small as this was a pilot study of a novel surgical approach. Fisher's exact test was used to compare categorical demographic, radiological and pathological variables between patient groups with and without nodal pCR. It was also used for comparison of various features between the individually clipped nodes with and without pCR. Statistical significance was set at p < .05. SAS V9.4 statistical software (SAS Inc, Cary, NC) was used to perform the analysis. All 21 metastatic clipped nodes in 14 patients were removed but SMART failed to localize 3 clipped nodes and they were only retrieved from the ALND specimens. There was no surgical complications associated with SMART or ALND. The first clipped node predicted the axillary status with a FNR of 7.1%. Based on this and another second clipped node, the FNR was 0%. ypT (p = .0390) and the first clipped node status (p = .0030) were statistically significant predictors for nodal pCR (Table 1). Table 2 shows detailed characteristics of the 21 clipped nodes. | |
Table 1.
Demographic characteristics of the patients in the study
| Clinical features | Patients with nodal pCR (n = 5), n (%) | Patients without nodal pCR (n = 9), n (%) | p value a |
|---|---|---|---|
| Age, yr | .5804 | ||
| ≤50 | 1 (20) | 4 (44.4) | |
| >50 | 4 (80) | 5 (55.6) | |
| BMI, kg/m2 | .5804 | ||
| <18.5 | 0 (0) | 2 (22.2) | |
| 18.5–24.9 | 3 (60) | 6 (66.7) | |
| 25–29.9 | 1(20) | 1 (11.1) | |
| ≥30 | 1 (20) | 0 (0) | |
| Sonographic features | |||
| Breast tumor size, mm b | .2358 | ||
| ≤20 | 2 (40) | 0 (0) | |
| >20 to ≤50 | 1 (20) | 5 (55.6) | |
| >50 | 2 (40) | 4 (44.4) | |
| No. of abnormal lymph nodes on ultrasound | 1.0000 | ||
| 1 | 3 (60) | 4 (44.5) | |
| 2 | 1 (20) | 2 (22.2) | |
| 3 | 1 (20) | 3 (33.3) | |
| Pathological features | |||
| Breast tumour histology | 1.0000 | ||
| Ductal | 5 (100) | 8 (88.9) | |
| Lobular | 0 (0) | 1 (11.1) | |
| Grade | .3007 | ||
| I | 0 (0) | 0 (0) | |
| II | 1 (20) | 5 (55.6) | |
| III | 4 (80) | 4 (44.4) | |
| Estrogen receptor | .0949 | ||
| Positive | 2 c (40) | 8 c (88.9) | |
| Negative | 3 (60) | 1 (11.1) | |
| Progesterone receptor | .2657 | ||
| Positive | 1 (20) | 6 (66.7) | |
| Negative | 4 (80) | 3 (33.3) | |
| Human epidermal growth factor receptor 2 | .2657 | ||
| Positive | 4 (80) | 3 (33.3) | |
| Negative | 1(20) | 6 (66.7) | |
| ypT status | .0390 | ||
| ypTpcr | 1 (20) | 0 (0) | |
| ypTis | 3 (60) | 0 (0) | |
| ypT1 | 1 (20) | 4 (44.5) | |
| ypT2 | 0 (0) | 2 (22.2) | |
| ypT3 | 0 (0) | 2 (22.2) | |
| ypT4 | 0 (0) | 1 (11.1) | |
| ypN status | NA | ||
| ypN0 | 5 (100) | 0 (0) | |
| ypN1 | 0 (0) | 4 (44.4) | |
| ypN2 | 0 (0) | 5 (55.6) | |
| ypN3 | 0 (0) | 0 (0) | |
| Status of first clipped node | .0030 | ||
| Metastatic | 0 (0) | 8 (88.9) | |
| Non metastatic | 5 (100) | 1 (11.1) |
Fisher's exact test.
If multifocal and centric disease was present sonographically, the breast size measurement will be based on the largest size of all lesions.
One of patients from nodal pCR and non pCR group had weakly positive estrogen receptor status respectively.
Abbreviations: BMI, body mass index; NA, not applicable; pCR, pathological complete response.
Table 2.
Characteristics of the 21 metastatic clipped nodes
| Clinical features | Clipped nodes with pCR (n = 9), n (%) | Clipped nodes without pCR (n = 12), n (%) | p value a |
|---|---|---|---|
| Type of clip | .3023 | ||
| UltraCor Twirl | 5 (55.6) | 8 (66.7) | |
| hydroMARK | 2 (22.2) | 4 (33.3) | |
| UltraClip dual trigger | 2 (22.2) | 0 (0) | |
| Sonographic features before NACT | |||
| Clipped node size, b mm | .7299 | ||
| <10 | 2 (22.2) | 2 (16.6) | |
| 10–20 | 5 (55.6) | 5 (41.7) | |
| >20 | 2 (22.2) | 5 (41.7) | |
| Clipped node cortex size, b mm | 1.0000 | ||
| <5 | 3 (33.3) | 5 (41.7) | |
| 5–10 | 4 (44.5) | 4 (33.3) | |
| >10 | 2 (22.2) | 3 (25.0) | |
| Sonographic features after NACT | |||
| Clipped node size, b mm | .8380 | ||
| <5 | 3 (33.3) | 6 (50.0) | |
| 5–9 | 5 (55.6) | 4 (33.3) | |
| >9 | 1 (11.1) | 2 (16.7) | |
| Clipped node cortex size, b mm | 1.0000 | ||
| <0.5 | 3 (33.3) | 5 (41.7) | |
| 0.5–1 | 1 (11.1) | 1 (8.3) | |
| >1 | 5 (55.6) | 6 (50.0) | |
| Pathological features | |||
| No. of nodes retrieved per clipped node specimen c | .1593 | ||
| 1 | 1 (14.2) | 6 (54.5) | |
| 2 | 3 (42.9) | 4 (36.4) | |
| >2 | 3 (42.9) | 1 (9.1) |
Fisher's exact test.
Based on the largest size dimension.
Based on 18 clipped nodes retrieved by SMART.
Abbreviations: NACT, neoadjuvant chemotherapy; pCR, pathological complete response.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Active and should be pursued further |
Conventionally, axillary lymph node dissection (ALND) is the standard of care in patients with nodal disease from breast cancer. However, as neoadjuvant chemotherapy can result in axillary nodal resolution in about 40% of these patients [1], it has prompted the use of sentinel lymph node (SLN) biopsy for axillary staging, reserving ALND only for those patients with positive staging. As a result, it is crucial to have accurate staging of the axilla with a low false negative rate so as not to miss residual nodal disease that could lead to a recurrence.
However, the use of sentinel lymph node biopsy alone after neoadjuvant chemotherapy has a false negative rate (FNR) of 12.6%–14.2% [3, 4]. Although the FNR is lowered with dual mapping modalities, the reported pooled FNR of 11% still remained higher than the prespecified threshold of 10% [6]. In addition, although the FNR could be further reduced to 4% with removal of three or more SLNs [6], this may not be achievable in all patients, with only 13%–57.1% of patients in some prospective studies having three or more SLNs [6].
To reduce the FNR associated with SLN biopsy alone in axillary staging, targeted axillary dissection, which involves the removal of clipped metastatic nodes and SLN, has been described.
However, with targeted axillary dissection, the identification of sentinel lymph nodes remained challenging. Chemotherapy can result in fibrosis, fat necrosis, and granulation tissue formation, which leads to aberrant lymphatic pathways [10, 11]. As a result, a sentinel lymph node identification rate of 77.9% to 98% [6] post neoadjuvant chemotherapy has been reported. Conversely, this limitation is not applicable to the clipped node as identification of the clipped node is independent of the lymphatic alterations post chemotherapy. In addition, the clipped node is not one of the sentinel lymph nodes in 23% of cases [5].
In contrast, targeted removal of the clipped node alone without sentinel lymph node biopsy was able to accurately predict the axillary status with a FNR of 7% [7], which was similar to our FNR of first clipped node removal.
Previous axillary staging studies however, only examined the clipping of a single metastatic node. In this study, we evaluated if the novel approach of clipping and removal of all the sonographically abnormal and metastatic proven nodes can further reduce the FNR. Intuitively, targeted assessment of the nodes, which have been proven metastatic prior to neoadjuvant chemotherapy, should be able to predict the axillary response, as demonstrated by the marking axillary lymph nodes with radioactive iodine seeds (MARI) procedure with a single clipped node [7]. However, as chemotherapy can cause a nonuniform differential axillary nodal response, evaluation of more clipped metastatic nodes could logically give a better representation of the true axillary status. This was demonstrated in our study, with 100% accuracy achieved with the removal of a second clipped node.
The caveat of this novel approach is that only nodes that were sonographically visible could be targeted. Axillary ultrasound has a pooled diagnostic sensitivity of 79.6% and specificity of 98.3% [12], so ultrasound may not be able to detect all nodal disease. Despite so, after neoadjuvant chemotherapy, a single clipped metastatic node was representative of the axillary nodal status with a 7% FNR [7]. Removal of more clipped nodes could further reduce the FNR, as shown in our study.
Specific tumor subtypes have been associated with increased nodal resolution rates [13]. In our study however, only ypT and the first clipped node status were found to be statistically correlated with nodal resolution. These findings were consistent with the literature, which also showed a higher correlation of nodal resolution with breast tumor pathologic resolution [8] and that the clipped node was highly predictive of the nodal status [7]. Although our study could not demonstrate a significant relationship between estrogen receptor (ER) status and nodal resolution, this could be due to our small numbers and a significant number (20%) of our ER‐positive patients having weak ER positivity.
The relationship between the number of sonographically abnormal nodes prior to neoadjuvant chemotherapy and nodal resolution is unclear. It has been shown that in patients with early breast cancer with a greater number of sonographically abnormal nodes, the axillary nodal burden is also higher [14, 15], hence resulting in a higher likelihood of ALND in the era post‐Z11 trial. In the neoadjuvant setting, however, it is not fully evaluated if this higher nodal burden in patients with a greater number of sonographically abnormal nodes would also translate into a lower rate of nodal resolution. There is however some implication that the number of sonographically abnormal nodes seen after receiving neoadjuvant chemotherapy is a predictive factor for residual nodal disease [16]. If proven true, the number of sonographically abnormal nodes could be used, in conjunction with the tumor subtypes, to better select the good responders suitable for axillary preservation. The cutoff number of sonographically abnormal nodes however remains unknown.
In our study, the maximum of three sonographically abnormal nodes was used as an inclusion criteria cutoff, as it was deemed logistically and clinically cumbersome to clip and remove more than three axillary nodes.
Strengths of this paper included a prospective study with detailed documentation of the individual nodes clipped and removed with radiological and pathological correlation. This is also the first study, to the best of our knowledge, that clipped multiple nodes based on ultrasound interpretation, without sentinel lymph node biopsy.
Limitations included a small sample size and that no frozen section was performed intraoperatively for the clipped node specimens to determine the concordance of frozen section findings to that of histopathology. In addition, our study findings may not be applicable to patients with more than three abnormal nodes seen on axillary ultrasound.
An implication of our study is that localization of more than one node may result in additional cost as a result of the multiple nodal localization devices. However, because sentinel lymph node biopsy was omitted, this additional cost may be negated by the cost of the sentinel lymph node biopsy mapping agents [17]. Skin mark clipped axillary nodes removal technique (SMART) is a recently described technique [9] that could be used for the removal of multiple clipped nodes with minimal cost. However, it is not ideal for the removal of more than two deeply seated clipped nodes. This cost comparison analysis of removal of multiple clipped nodes versus targeted axillary dissection will need evaluation in future studies.
In conclusion, our pilot study showed that removal of all sonographically abnormal metastatic axillary nodes, without sentinel node biopsy, could accurately stage the axilla post neoadjuvant chemotherapy.
Disclosures
The authors indicated no financial relationships.
Tables
Acknowledgments
This study was funded by KKH Health Fund (KKHHF/2017/001). We thank Bard and Medquest for providing their products, UltraCor Twirl, Ultraclip dual trigger, and HydroMARK markers, respectively, for this study. Bard and Medquest, however, had no part in the design of the study. We also thank Miss Ang Mui Ee and Miss Me Me Win Htein for their administrative assistance and Dr. Eileen Lew as the mentor of this grant.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: NCT03878017
- Sponsor: KKH Health Fund (KKHHF/2017/001)
- Principal Investigator: Geok Hoon Lim
- IRB Approved: Yes
References
- 1. Boughey JC, Ballman KV, Le‐Petross HT et al. Identification and resection of clipped node decreases the false‐negative rate of sentinel lymph node surgery in patients presenting with node‐positive breast cancer (T0–T4, N1–N2) who receive neoadjuvant chemotherapy: Results from ACOSOG Z1071 (Alliance). Ann Surg 2016;263:802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wernicke AG, Shamis M, Sidhu KK et al. Complication rates in patients with negative axillary nodes 10 years after local breast radiotherapy after either sentinel lymph node dissection or axillary clearance. Am J Clin Oncol 2013;36:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boughey JC, Suman VJ, Mittendorf EA et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node‐positive breast cancer. The ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013;310:1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuehn T, Bauerfeind I, Fehm T et al. Sentinel‐lymph‐node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicenter cohort study. Lancet Oncol 2013;14:609–618. [DOI] [PubMed] [Google Scholar]
- 5. Caudle AS, Yang WT, Krishnamurthy S et al. Improved axillary evaluation following neoadjuvant therapy for patients with node‐positive breast cancer using selective evaluation of clipped nodes: Implementation of targeted axillary dissection. J Clin Oncol 2016; 34:1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tee SR, Devane LA, Evoy D et al. Meta‐analysis of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with initial biopsy‐proven node‐positive breast cancer. Br J Surg 2018;105:1541–1552. [DOI] [PubMed] [Google Scholar]
- 7. Donker M, Straver ME, Wesseling J et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: The MARI procedure. Ann Surg. 2015; 261:378–382. [DOI] [PubMed] [Google Scholar]
- 8. Tadros AB, Yang WT, Krishnamurthy S et al. Identification of patients with documented pathologic complete response in the breast after neoadjuvant chemotherapy for omission of axillary surgery. JAMA Surg 2017;152:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lim GH, Teo SY, Gudi M et al. Initial results of a novel technique of clipped node localization in breast cancer patients postneoadjuvant chemotherapy: Skin Mark clipped Axillary nodes Removal Technique (SMART trial). Cancer Med 2020;9:1978–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fisher B, Brown A, Mamounas E et al. Effect of preoperative chemotherapy on local–regional disease in women with operable breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B‐18. J Clin Oncol 1997;15:2483–2493. [DOI] [PubMed] [Google Scholar]
- 11. Sharkey FE, Addington SL, Fowler LJ et al. Effects of preoperative chemotherapy on the morphology of resectable breast carcinoma. Mod Pathol 1996;9:893–900. [PubMed] [Google Scholar]
- 12. Upadhyaya VS, Lim GH, Chan EYK et al. Evaluating the preoperative breast cancer characteristics affecting the accuracy of axillary ultrasound staging. Breast J 2020;26:162–167. [DOI] [PubMed] [Google Scholar]
- 13. Liedtke C, Kolberg HC, Kerschke L et al. Systematic analysis of parameters predicting pathological axillary status (ypN0 vs. ypN+) in patients with breast cancer converting from cN+ to ycN0 through primary systemic therapy (PST). Clin Exp Metastasis 2018;35:777–783. [DOI] [PubMed] [Google Scholar]
- 14. Lim GH, Upadhyaya VS, Acosta HA et al. Preoperative predictors of high and low axillary nodal burden in Z0011 eligible breast cancer patients with a positive lymph node needle biopsy result. Eur J Surg Oncol 2018;44:945–950. [DOI] [PubMed] [Google Scholar]
- 15. Lim GH, Teo SY, Allen JC Jr et al. Determining whether high nodal burden in early breast cancer patients can be predicted preoperatively to avoid sentinel lymph node biopsy. J Breast Cancer 2019;22:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Candelaria RP, Bassett RL, Symmans WF et al. Performance of mid‐treatment breast ultrasound and axillary ultrasound in predicting response to neoadjuvant chemotherapy by breast cancer subtype. The Oncologist 2017;22:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang L, Yu JM, Wang YS et al. Preoperative lymphoscintigraphy predicts the successful identification but is not necessary in sentinel lymph nodes biopsy in breast cancer. Ann Surg Oncol 2007;14:2215–2220. [DOI] [PubMed] [Google Scholar]


