Abstract
Lessons Learned
The efficacy of second‐line treatment for advanced non‐small cell lung carcinoma (NSCLC) without a sensitizing driver gene mutation is still unsatisfactory. The combination of apatinib and chemotherapy improved progression‐free survival in the second‐line therapy of advanced NSCLC without a sensitizing mutation.
This study offers a new treatment strategy for second‐line treatment of such patients but requires confirmation in a larger multi‐institutional trial.
Background
This study explored the efficacy and safety of apatinib combined with single‐agent chemotherapy versus single‐agent chemotherapy in the second‐line treatment of advanced non‐small‐cell lung carcinoma (NSCLC) without driver mutations.
Methods
In this double‐arm, open label, exploratory clinical study, we enrolled patients with unresectable locally advanced or advanced NSCLC without driver mutations that had progressed following first‐line chemotherapy. The subjects were allocated into an experimental group and a control group by 2:1. The experimental group received apatinib combined with four cycles of docetaxel or pemetrexed until disease progression, intolerable toxicity, or discontinuation at the patient' request. The control group only received four cycles of docetaxel or pemetrexed. The primary endpoints were progression‐free survival (PFS), and the secondary endpoints were overall survival (OS), disease control rate (DCR), and safety.
Results
Thirty‐seven patients were enrolled. The efficacy of 33 patients was evaluated. The median PFS was 5.47 versus 2.97 months, the DCR was 95% versus 73%, and the objective response rate (ORR) was 27% versus 9% in the experimental versus control group. The OS was still under follow‐up. The most common adverse effects included hypertension, hand‐foot skin reaction (HFSR), and fatigue.
Conclusion
Apatinib combined with single‐agent chemotherapy may be a novel option for second‐line treatment of advanced NSCLC
Discussion
The second‐line treatment of locally advanced or advanced NSCLC without a driver gene predominantly included single‐agent chemotherapy. At present, second‐line chemotherapy is unsatisfactory, and scholars have conducted various investigations, including single‐agent chemotherapy along with antiangiogenesis drugs. According to previous studies, chemotherapy combined with antiangiogenesis agents has the prospect of broad application in the second‐line treatment of cancer.
Apatinib mesylate is a novel small molecular antiangiogenesis, Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) tyrosine kinase inhibitor (TKI) developed in China. The study of apatinib in the treatment of lung cancer has primarily been focused on three lines and after the third‐line treatment with the single agent. The phase II clinical studies of advanced non‐small cell non‐squamous cell carcinoma after the progression of second‐line chemotherapy showed that apatinib alone had a median PFS (mPFS) of 4.7 months and an ORR of 12.2%, both with significant efficacy.
In this study, we observed that the mPFS of the combined treatment group was significantly longer than that of the simple chemotherapy group (5.47 months vs. 2.97 months), reaching our planned primary endpoint (Fig. 2). In addition, our results also demonstrated that the mPFS of the single‐agent chemotherapy group was 2.97 months, which was in agreement with the data reported globally. In the secondary endpoints, the DCR of the combined treatment group was also improved compared with the chemotherapy group. These results indicated that apatinib combined with chemotherapy could improve the PFS and DCR in the second‐line treatment of patients with advanced NSCLC, and hence, further investigation with respect to the prolonged survival period was essential.
Figure 2.
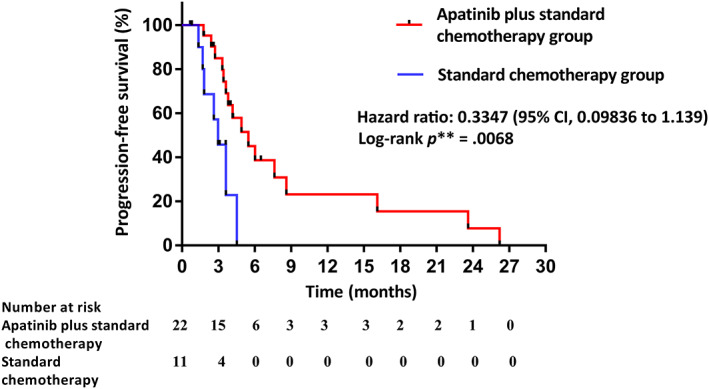
Kaplan‐Meier curves for progression‐free survival between the apatinib plus standard chemotherapy group and standard chemotherapy group. Abbreviation: CI, confidence interval.
In terms of safety, the common adverse effects of the combined treatment group included hypertension, HFSR, and fatigue, which were similar to those observed with other antivascular targeted therapies. Patients with adverse effects were improved symptomatically after supportive treatment or suspension of medication, and no severe adverse event–related deaths occurred. The rate of overall adverse effects of the two groups was not significantly different, indicating that the safety of apatinib combined with single‐agent chemotherapy was satisfactory and that the adverse effects were controllable.
Therefore, the results of our study indicated that the combination of apatinib and chemotherapy significantly improved the PFS time in the second‐line therapy of advanced NSCLC without a sensitizing mutation. It was safe and well tolerated.
Trial Information
| Disease | Lung cancer – NSCLC |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | 1 prior regimen |
| Type of Study | Phase II, randomized |
| PFS | p: .0068, HR: 0.3347 |
| Primary Endpoint | Progression‐free survival |
| Secondary Endpoints | Overall survival, disease control rate, safety |
| Additional Details of Endpoints or Study Design | |
| Study Design and Eligibility Criteria | |
| This study was a double‐arm, open, multicenter, exploratory clinical study that included patients from six centers in Southeast China. The patients who fulfilled the inclusion criteria were stratified by 2:1 and assigned to the experimental and control groups based on factors such as performance status (PS), presence or absence of brain metastasis, and histological type. | |
| The specific inclusion criteria were as follows: locally advanced or advanced primary NSCLC diagnosed by histology or cytology (account to American Joint Committee on Cancer version 7 stage, stage IIIB with unresectable, stage IV) that could not be surgically resected or recurred; age ≥18 years and ≤75 years; no epidermal growth factor receptor–sensitive mutations detected by molecular pathology; negative of anaplastic lymphoma kinase, or unknown; and had experienced progression of disease after chemotherapy or had discontinued therapy owing to intolerable adverse effects. Other inclusion criteria were as follows: at least one measurable lesion; Eastern Cooperative Oncology Group (ECOG) score 0–1; estimated survival time ≥3 months; and adequate hematologic, hepatic, and renal function. Patients with asymptomatic brain metastases were also included. Patients with uncontrolled blood pressure on medication (>140/90 mmHg), those with bleeding tendency, and those receiving thrombolytics or anticoagulants were excluded. The study was approved by the ethics committee. All patients provided written informed consent before participation in the study. | |
| Study Assessments | |
| The baseline assessment of the tumor was performed within 28 days prior to enrollment. Measurable lesions were determined by computed tomography (CT) or magnetic resonance imaging (MRI), followed by radiographic evaluation after every two cycles of treatment until disease progression. Efficacy was evaluated according to the RECIST standards (version 1.1) and divided into complete remission (CR), partial remission (PR), stable disease (SD), and progression of disease (PD). The adverse events were defined and graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (version 4.03) and observed and evaluated at the beginning of the treatment as well as after 28 days. The primary research endpoint was PFS, whereas the secondary endpoints were total OS, DCR, and safety. | |
| Statistical Analyses | |
| Patients were stratified based on ECOG PS, presence or absence of brain metastasis, gender, smoking history, and histological type. In analyzing the data, we performed exploratory analyses comparing the two treatment arms. The primary research endpoint was PFS, whereas the secondary endpoints were OS, DCR, and safety. Between‐group differences in patient characteristics were analyzed using the t test. The differences before and after treatment were determined by analysis of variance or the rank sum test. Median PFS and OS were estimated from Kaplan‐Meier curves. | |
| As to study design and size, this was an investigator‐initiated exploratory study. Referring to the results of the LUME‐Lung 1 trial, the control group of PFS was set to 2.7 months, and the experimental group of PFS was assumed to be 5.2 months. The unilateral threshold value and test efficacy was set to 10% and 80%, respectively, and 49 patients (33 in the experimental group and 16 in the control group) were scheduled to be enrolled. Owing to the geographical and epidemiological factors of the disease, the enrollment was slow and exceeded the expected enrollment time. We terminated the study when 37 patients were enrolled. | |
| Investigator's Analysis | Active and should be pursued further |
Drug Information: Control
| Drug 1 | |
| Generic/Working Name | Pemetrexed sodium for injection |
| Dose | 500 mg/m2 |
| Route | IV |
| Schedule of Administration | |
| The control group received four cycles of docetaxel or pemetrexed single‐agent chemotherapy and were monitored by regular follow‐up. The withdrawal of drugs because of drug‐related toxicity was for <14 days and not more than two times. The patients in the pemetrexed group were treated with 4 mg dexamethasone 1 day before administration, on the same day, and 1 day after administration, twice a day. At least five daily doses of folic acid (350–1000 μg) were administered 7 days prior to the first treatment and continued until 21 days after the final pemetrexed dose. One thousand micrograms of vitamin B12 should also be injected intramuscularly within 7 days prior to the first pemetrexed administration and once every three cycles thereafter. The granulocyte colony‐stimulating factor was permitted for prophylactic use in patients with granulocytopenia events or those that occurred during the previous cycle of treatment. | |
| Drug 2 | |
| Generic/Working Name | Docetaxel injection |
| Dose | 75 mg/m2 |
| Route | IV |
| Schedule of Administration | |
| The control group received four cycles of docetaxel or pemetrexed single‐agent chemotherapy and were monitored by regular follow‐up. The withdrawal of drugs because of drug‐related toxicity was for <14 days and not more than two times. The patients in the docetaxel treatment group received 8 mg dexamethasone 1 day before administration, on the same day, and 1 day after administration, twice a day. The granulocyte colony‐stimulating factor was permitted for prophylactic use in patients with granulocytopenia events or those that occurred during the previous cycle of treatment. | |
Drug Information: Apatinib
| Drug 1 | |
| Generic/Working Name | Apatinib mesylate tablets |
| Drug Type | Small molecule |
| Drug Class | VEGFR |
| Dose | 500 mg per flat dose |
| Route | p.o. |
| Schedule of Administration | |
| The experimental group received 500 mg apatinib once a day orally combined with four cycles of single‐agent docetaxel (75 mg/m2, every 21 days) or pemetrexed chemotherapy (500 mg/m2, every 21 days, non‐squamous cell carcinoma only) until disease progression or intolerable toxicity or in the event of discontinuation upon patient' request. Holding drugs as a result of drug‐related toxicity was allowed for <14 days but not more than two times. The dose of the drugs could be decreased during treatment; apatinib was >375 mg once a day. The granulocyte colony‐stimulating factor was permitted for prophylactic use in patients with granulocytopenia events or those that occurred during the previous cycle of treatment. | |
| Drug 2 | |
| Generic/Working Name | Pemetrexed sodium for injection |
| Dose | 500 mg per flat dose |
| Route | IV |
| Schedule of Administration | |
| The experimental group received 500 mg apatinib once a day orally combined with four cycles of single‐agent docetaxel (75 mg/m2, every 21 days) or pemetrexed chemotherapy (500 mg/m2, every 21 days, non‐squamous cell carcinoma only) until disease progression or intolerable toxicity or in the event of discontinuation upon patient' request. Holding drugs as a result of drug‐related toxicity was for <14 days and not more than two times. The patients in the pemetrexed group were treated with 4 mg dexamethasone 1 day before administration, on the same day, and 1 day after administration, twice a day. At least five daily doses of folic acid (350–1,000 μg) were administered 7 days prior to the first treatment and continued until 21 days after the final pemetrexed dose. One thousand micrograms of vitamin B12 should also be injected intramuscularly within 7 days prior to the first pemetrexed administration and once every three cycles thereafter. The granulocyte colony‐stimulating factor was permitted for prophylactic use in patients with granulocytopenia events or those that occurred during the previous cycle of treatment. | |
| Drug 3 | |
| Generic/Working Name | Docetaxel injection |
| Dose | 75 mg/m2 |
| Route | IV |
| Schedule of Administration | |
| The experimental group received 500 mg apatinib once a day orally combined with four cycles of single‐agent docetaxel (75 mg/m2, every 21 days) or pemetrexed chemotherapy(500 mg/m2, every 21 days, non‐squamous cell carcinoma only) until disease progression or intolerable toxicity or in the event of discontinuation upon patient' request. The patients in the docetaxel treatment group received 8 mg dexamethasone 1 day before administration, on the same day, and 1 day after administration, twice a day. The granulocyte colony‐stimulating factor was permitted for prophylactic use in patients with granulocytopenia events or those that occurred during the previous cycle of treatment. | |
Patient Characteristics: Control
| Number of Patients, Male | 8 |
| Number of Patients, Female | 3 |
| Stage | Stage IIIB with unresectable, stage IV |
| Age | Median (range): 62 (41–74) years |
| Performance Status: ECOG |
0 — 1 1 — 10 2 — 3 — Unknown — |
| Other | |
| During the period from May 30, 2016, to March 27, 2019, 37 patients from six centers were enrolled in the present study. Twenty‐six patients were included in the experimental group, and 11 patients were included in the control group (Fig. 3). The baseline and clinical characteristics of the two groups were well balanced, with no statistically significant differences (Table 1). Regarding the data cutoff (March 27, 2019), 11 patients in the control cohort had tissue or cytology available for testing. | |
| Cancer Types or Histologic Subtypes | Nonsquamous cell, 8; squamous cell, 3 |
Figure 3.
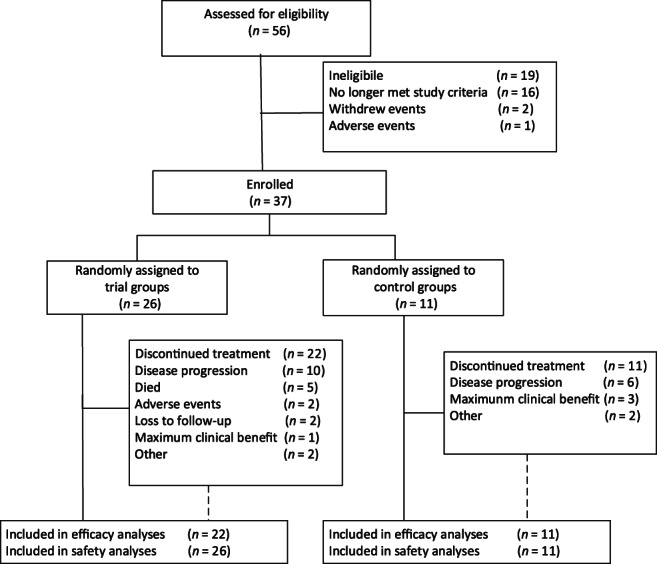
Research flow chart.
Table 1.
Baseline characteristics of patients
| Patient characteristics | Trial group (n = 22) | Control group (n = 11) |
|---|---|---|
| Gender, n (%) | ||
| Male | 18 (82) | 8 (73) |
| Female | 4 (18) | 3 (27) |
| Age, years | ||
| Median | 58.5 | 62 |
| Range | 31–73 | 41–74 |
| Performance status (ECOG), n (%) | ||
| 0 | 2 (9) | 1 (9) |
| 1 | 20 (91) | 10 (91) |
| Operation history, n (%) | ||
| Never had an operation | 3 (14) | 1 (9) |
| Had an operation | 19 (86) | 10 (91) |
| Radiation history, n (%) | ||
| Had radiation | 7 (32) | 5 (45) |
| Never had radiation | 15 (68) | 6 (55) |
| Smoking history, n (%) | ||
| Never smoker | 10 (45) | 6 (55) |
| Smoker | 12 (55) | 5 (45) |
| Pathological type, n (%) | ||
| Non‐squamous cell | 17 (77) | 8 (73) |
| Squamous cell | 5 (23) | 3 (27) |
| Brain metastases, n (%) | ||
| Yes | 2 (9) | 1 (9) |
| No | 20 (91) | 10 (91) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Patient Characteristics: Apatinib
| Number of Patients, Male | 18 |
| Number of Patients, Female | 4 |
| Stage | Stage IIIB with unresectable, stage IV |
| Age | Median (range): 58.5 (31–73) years |
| Performance Status: ECOG |
0 — 2 1 — 20 2 — 3 — Unknown — |
| Other | |
| During the period from May 30, 2016, to March 27, 2019, 37 patients from six centers were enrolled in the present study. Twenty‐six patients were included in the experimental group, and 11 patients were included in the control group (Fig. 3). The baseline and clinical characteristics of the two groups were well balanced, with no statistically significant differences (Table 1). Regarding the data cutoff (March 27, 2019), 26 patients had tissue cytology in the experimental group, 4 of which were not evaluated for efficacy. In patients without evaluation of efficacy, three patients were treated for less than one cycle because of adverse reactions, and one patient had not undergone imaging examination. | |
| Cancer Types or Histologic Subtypes | Nonsquamous cell, 17; squamous cell, 5 |
Primary Assessment Method: Control
| Title | New assessment |
| Title | RECIST response |
| Number of Patients Enrolled | 11 |
| Number of Patients Evaluable for Toxicity | 11 |
| Number of patients Evaluated for Efficacy | 11 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 0 |
| Response Assessment PR | n = 1 |
| Response Assessment SD | n = 7 |
| Response Assessment PD | n = 3 |
| (Median) Duration Assessments PFS | 2.97 months, CI: 2.02–3.92 |
| Outcome Notes | |
| In patients without evaluation of efficacy, three patients were treated for less than one cycle because of adverse reactions, and one patient had not undergone imaging examination. In the apatinib plus standard chemotherapy group, two patients continued to receive the treatment, and seven patients had undergone apatinib therapy for more than 6 months. PFS was significantly longer in the experimental group than in the control group (median PFS 5.47 months [95% confidence interval (CI): 3.21–7.73] vs. 2.97 months [95% CI: 2.02–3.92], hazard ratio [HR]: 0.3347, p = .0068; Fig. 2). ORR and DCR were 27% and 95% for the experimental group compared with 9% and 73% for the control group (Fig. 1; Table 2), respectively. Because the number of patients is too small, therapeutic effect evaluation and statistical analysis of subgroup are not performed temporarily. | |
Figure 1.
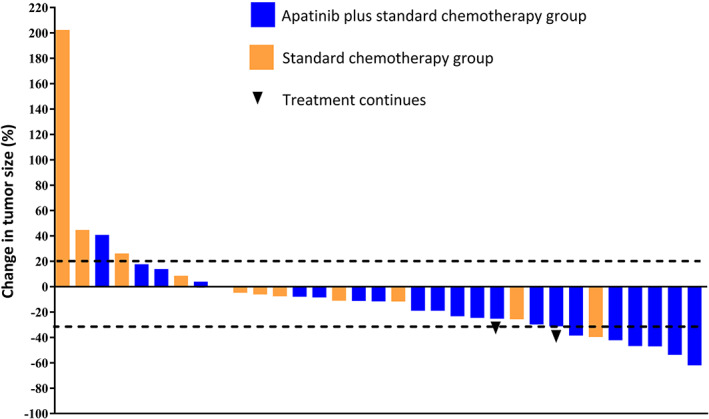
Waterfall plots of the largest percentage changes from the baseline in the sum of the longest tumor diameters for patients in the apatinib plus standard chemotherapy group and standard chemotherapy group.
Table 2.
The best confirmed tumor response of the two groups
| Response | Trial group (n = 22) | Control group (n = 11) |
|---|---|---|
| ORR, % | 27 | 9 |
| DCR, % | 95 | 73 |
| CR, n | 0 | 0 |
| PR, n | 6 | 1 |
| SD, n | 15 | 7 |
| PD, n | 1 | 3 |
Abbreviations: CR, complete remission; DCR, disease control rate; ORR: objective response rate; PD, progression of disease; PR, partial remission; SD, stable disease.
Primary Assessment Method: Apatinib
| Title | RECIST response |
| Number of Patients Enrolled | 26 |
| Number of Patients Evaluable for Toxicity | 26 |
| Number of Patients Evaluated for Efficacy | 22 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 0 |
| Response Assessment PR | n = 6 |
| Response Assessment SD | n = 15 |
| Response Assessment PD | n = 1 |
| (Median) Duration Assessments PFS | 5.47 months, CI: 3.21–7.73 |
| Outcome Notes | |
| In patients without evaluation of efficacy, three patients were treated for less than one cycle because of adverse reactions, and one patient had not undergone imaging examination. In the apatinib plus standard chemotherapy group, two patients continued to receive the treatment, and seven patients underwent apatinib therapy for more than 6 months. PFS was significantly longer in the experimental group than in the control group (median PFS 5.47 months [95% CI: 3.21–7.73] vs. 2.97 months [95% CI: 2.02–3.92], HR: 0.3347, p = .0068; Fig. 2). ORR and DCR were 27% and 95% for the experimental group compared with 9% and 73% for the control group (Fig. 1; Table 2), respectively. Because the number of patients is too small, therapeutic effect evaluation and statistical analysis of subgroup are not performed temporarily. | |
Adverse Events: Control
| All Cycles | |||||||
|---|---|---|---|---|---|---|---|
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
| Cough | 36% | 55% | 9% | 0% | 0% | 0% | 64% |
| Fatigue | 82% | 9% | 9% | 0% | 0% | 0% | 18% |
| Febrile neutropenia | 73% | 9% | 9% | 9% | 0% | 0% | 27% |
| Proteinuria | 91% | 9% | 0% | 0% | 0% | 0% | 9% |
| Nausea | 91% | 9% | 0% | 0% | 0% | 0% | 9% |
| Diarrhea | 91% | 9% | 0% | 0% | 0% | 0% | 9% |
| Lung infection | 91% | 9% | 0% | 0% | 0% | 0% | 9% |
| Hypoalbuminemia | 91% | 9% | 0% | 0% | 0% | 0% | 9% |
| Hepatic infection | 91% | 9% | 0% | 0% | 0% | 0% | 9% |
| Weight loss | 91% | 9% | 0% | 0% | 0% | 0% | 9% |
| Bone marrow hypocellular | 82% | 0% | 9% | 0% | 9% | 0% | 18% |
| Aspiration | 82% | 18% | 0% | 0% | 0% | 0% | 18% |
Adverse Events Legend
The adverse events that were more common in the experimental group than in the control placebo group were fatigue, hand‐foot skin reaction (HFSR), hypertension, chest pain, proteinuria, diarrhea, and nausea. Although most of these adverse events were manageable with supportive treatment or dose reduction, two patients had to cease the treatment because of adverse events. Dose modifications resulting from toxicity were more common in the trial group than in the control group. Dose reduction was mainly attributed to HFSR and hypertension.
Abbreviation: NC/NA, no change from baseline/no adverse event.
Adverse Events: Apatinib
| All Cycles | |||||||
|---|---|---|---|---|---|---|---|
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
| Fatigue | 42% | 35% | 19% | 4% | 0% | 0% | 58% |
| Cough | 61% | 35% | 4% | 0% | 0% | 0% | 39% |
| Weight loss | 92% | 0% | 8% | 0% | 0% | 0% | 8% |
| Nausea | 88% | 4% | 8% | 0% | 0% | 0% | 12% |
| Diarrhea | 88% | 8% | 0% | 4% | 0% | 0% | 12% |
| Proteinuria | 88% | 12% | 0% | 0% | 0% | 0% | 12% |
| Chest pain—cardiac | 88% | 12% | 0% | 0% | 0% | 0% | 12% |
| Hypertension | 80% | 8% | 8% | 4% | 0% | 0% | 20% |
| Febrile neutropenia | 77% | 15% | 4% | 0% | 4% | 0% | 23% |
| Mucositis oral | 92% | 4% | 4% | 0% | 0% | 0% | 8% |
| Lung infection | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Bone marrow hypocellular | 92% | 4% | 0% | 4% | 0% | 0% | 8% |
| Hypoalbuminemia | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Hepatic infection | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Aspiration | 84% | 12% | 4% | 0% | 0% | 0% | 16% |
| Skin and subcutaneous tissue disorders—hand‐foot skin reaction | 62% | 23% | 0% | 15% | 0% | 0% | 38% |
| Rash maculo‐papular | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
Abbreviation: NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Active and should be pursued further |
The second‐line treatment of locally advanced or advanced non‐small cell lung carcinoma (NSCLC) without a driver gene predominantly includes single‐agent chemotherapy [1, 2]. According to one meta‐analysis, the median objective response rate is approximately 6.8% (range, 0.8–12.2), whereas the median disease control rate (DCR) was 42.4% (range, 30.9–58.5), and the median survival time was 6.6 (range, 5.4–10.2) months in the second‐line chemotherapy for advanced NSCLC [3]. Thus, second‐line chemotherapy is overall unsatisfactory, and various investigations have been conducted in an effort to improve on these data, including single‐agent chemotherapy along with antiangiogenesis drugs [4, 5]. According to previous studies, chemotherapy combined with antiangiogenesis therapy has the possibility of broad application in second‐line treatment but needs further randomized data for confirmation [6, 7, 8, 9, 10, 11].
The study included patients with non‐small cell lung cancer, including squamous cell and adenocarcinoma. According to previous research data and clinical guidelines (National Comprehensive Cancer Network and Chinese Society of Clinical Oncology, etc.), pemetrexed and docetaxel have been the standard second‐line treatment for patients with non‐small cell lung cancer. The data from this study were consistent with the previous data of chemotherapy for second‐line treatment of NSCLC.
In this study, we observed that the median progression‐free survival (mPFS) of the combined treatment group was significantly longer than that of the simple chemotherapy group (5.47 months vs. 2.97 months), reaching the primary endpoints. In the CheckMate 057 trial, mPFS was not favorable to nivolumab compared with docetaxel (median, 2.3 months and 4.2 months, respectively), although the rate of progression‐free survival at 1 year was higher with nivolumab than with docetaxel (19% and 8%, respectively) [12]. In the REVEL trial, the mPFS was 4.5 months for the ramucirumab plus docetaxel group compared with 3.0 months for the docetaxel group [13]. In the LUME‐Lung 1 trial, the mPFS was 3.4 months versus 2.7 months in the docetaxel plus nintedanib group versus the docetaxel plus placebo group [14]. In addition, our results also demonstrated that the mPFS of the single‐agent chemotherapy group was 2.97 months, which was in agreement with the data reported globally [12, 15, 16]. In the secondary endpoints, the DCR of the combined treatment group was also improved compared with the chemotherapy group. These results indicated that apatinib‐combined chemotherapy could improve the PFS and DCR in the second‐line treatment of patients with advanced NSCLC, and hence, further investigation with respect to the prolonged survival period was essential.
Previous studies on antiangiogenesis therapeutic drugs mostly excluded patients with squamous cell carcinoma because of the potential risk of life‐threatening pulmonary hemorrhage [17, 18, 19, 20]. However, the present study included patients with lung squamous cell carcinoma, but no fatal bleeding events were observed. Owing to the small number of cases in this study, a larger sample size study was imperative to further elucidate the efficacy and safety of apatinib in patients with lung squamous cell carcinoma.
In terms of safety, the main adverse effects of the combined treatment group included hypertension, hand‐foot skin reaction, fatigue, rash, diarrhea, proteinuria, and bone marrow suppression, which were similar to those observed in other antivascular targeted therapies [14, 21, 22, 23]. The symptoms of patients with adverse effects were improved after symptomatic treatment or suspension of medication, and no severe adverse event–related deaths occurred. The rate of overall adverse effects of the two groups was not significantly different, indicating that the safety of apatinib combined with single‐agent chemotherapy was satisfactory and that the adverse effects were controllable.
Therefore, the results of our study suggested that the combination of apatinib and chemotherapy significantly improved the PFS in the second‐line therapy of advanced NSCLC without a sensitizing mutation. It was safe and well tolerated.
This study was an investigator‐initiated exploratory study. Based on the LUME‐Lung 1 trial, PFS for the control group was expected to be 2.7 months, and if successful, the experimental group PFS would be or exceed 5.2 months. The study planned to enroll 49 patients (33 in the experimental group and 16 in the control group), but enrollment was slow and exceeded the expected enrollment time. We terminated the study when 37 patients were enrolled. We consider the results of this study to be valid, as the control group data were consistent with those of previous clinical studies, and the experimental group had significant survival benefits compared with the control group. Although the sample size is small, it still has certain guiding significance for clinical treatment.
Disclosures
The authors indicated no financial relationships.
Figure and Tables
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: NCT03256721
- Sponsor: Zongyang Yu
- Principal Investigator: Zongyang Yu
- IRB Approved: Yes
References
- 1. Hanna N, Johnson D, Temin S et al. Systemic therapy for stage IV non‐small‐cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2017;35:3484–3515. [DOI] [PubMed] [Google Scholar]
- 2. Reck M, Popat S, Reinmuth N et al. Metastatic non‐small‐cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2014;25(suppl 3):iii27–iii39. [DOI] [PubMed] [Google Scholar]
- 3. Hotta K, Fujiwara Y, Kiura K et al. Relationship between response and survival in more than 50,000 patients with advanced non‐small cell lung cancer treated with systemic chemotherapy in 143 phase III trials. J Thorac Oncol 2007;2:402–407. [DOI] [PubMed] [Google Scholar]
- 4. Herbst RS, O'Neill VJ, Fehrenbacher L et al. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small‐cell lung cancer. J Clin Oncol 2007;25:4743–4750. [DOI] [PubMed] [Google Scholar]
- 5. Sandler A, Gray R, Perry MC et al. Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer. N Engl J Med 2006;355:2542–2550. [DOI] [PubMed] [Google Scholar]
- 6. de Boer RH, Arrieta O, Yang CH et al. Vandetanib plus pemetrexed for the second‐line treatment of advanced non‐small‐cell lung cancer: A randomized, double‐blind phase III trial. J Clin Oncol 2011;29:1067–1074. [DOI] [PubMed] [Google Scholar]
- 7. Heist RS, Wang X, Hodgson L et al. CALGB 30704 (Alliance): A randomized phase II study to assess the efficacy of pemetrexed or sunitinib or pemetrexed plus sunitinib in the second‐line treatment of advanced non‐small‐cell lung cancer. J Thorac Oncol 2014;9:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Herbst RS, Sun Y, Eberhardt WE et al. Vandetanib plus docetaxel versus docetaxel as second‐line treatment for patients with advanced non‐small‐cell lung cancer (ZODIAC): A double‐blind, randomised, phase 3 trial. Lancet Oncol 2010;11:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramlau R, Gorbunova V, Ciuleanu TE et al. Aflibercept and docetaxel versus docetaxel alone after platinum failure in patients with advanced or metastatic non‐small‐cell lung cancer: A randomized, controlled phase III trial. J Clin Oncol 2012;30:3640–3647. [DOI] [PubMed] [Google Scholar]
- 10. Sheng J, Yang Y, Ma Y et al. The efficacy of combining antiangiogenic agents with chemotherapy for patients with advanced non‐small cell lung cancer who failed first‐line chemotherapy: A systematic review and meta‐analysis. PLoS One 2015;10:e0127306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takeda M, Yamanaka T, Seto T, et al. Bevacizumab beyond disease progression after first‐line treatment with bevacizumab plus chemotherapy in advanced nonsquamous non‐small cell lung cancer (West Japan Oncology Group 5910L): An open‐label, randomized, phase 2 trial. Cancer 2016;122:1050–1059. [DOI] [PubMed] [Google Scholar]
- 12. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garon EB, Ciuleanu TE, Arrieta O et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second‐line treatment of stage IV non‐small‐cell lung cancer after disease progression on platinum‐based therapy (REVEL): A multicentre, double‐blind, randomised phase 3 trial. Lancet 2014;384:665–673. [DOI] [PubMed] [Google Scholar]
- 14. Reck M, Kaiser R, Mellemgaard A et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non‐small‐cell lung cancer (LUME‐Lung 1): A phase 3, double‐blind, randomised controlled trial. Lancet Oncol 2014;15:143–155. [DOI] [PubMed] [Google Scholar]
- 15. Hanna N, Shepherd FA, Fossella FV et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non‐small‐cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589–1597. [DOI] [PubMed] [Google Scholar]
- 16. Shepherd FA, Dancey J, Ramlau R et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non‐small‐cell lung cancer previously treated with platinum‐based chemotherapy. J Clin Oncol 2000;18:2095–2103. [DOI] [PubMed] [Google Scholar]
- 17. Bagley SJ, Talento S, Mitra N et al. Comparative effectiveness of carboplatin/pemetrexed with versus without bevacizumab for advanced nonsquamous non‐small cell lung cancer. J Natl Compr Canc Netw 2019;17:469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson DH, Fehrenbacher L, Novotny WF et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non‐small‐cell lung cancer. J Clin Oncol 2004;22:2184–2191. [DOI] [PubMed] [Google Scholar]
- 19. Ramalingam SS, Dahlberg SE, Belani CP et al. Pemetrexed, bevacizumab, or the combination as maintenance therapy for advanced nonsquamous non‐small‐cell lung cancer: ECOG‐ACRIN 5508. J Clin Oncol 2019:37:2360–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wan X, Luo X, Tan C et al. First‐line atezolizumab in addition to bevacizumab plus chemotherapy for metastatic, nonsquamous non‐small cell lung cancer: A United States‐based cost‐effectiveness analysis. Cancer 2019;125:3526–3534. [DOI] [PubMed] [Google Scholar]
- 21. Chen XZ. Anlotinib for refractory advanced non‐small cell lung cancer in china. JAMA Oncol 2019;5:116–117. [DOI] [PubMed] [Google Scholar]
- 22. Groen HJ, Socinski MA, Grossi F et al. A randomized, double‐blind, phase II study of erlotinib with or without sunitinib for the second‐line treatment of metastatic non‐small‐cell lung cancer (NSCLC). Ann Oncol 2013;24:2382–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reck M, Mellemgaard A, Novello S et al. Change in non‐small‐cell lung cancer tumor size in patients treated with nintedanib plus docetaxel: Analyses from the phase III LUME‐Lung 1 study. Onco Targets Ther 2018;11:4573–4582. [DOI] [PMC free article] [PubMed] [Google Scholar]


