Abstract
Lessons Learned
The results from the liposarcoma cohort of SARC024 confirm previously published data and do not support the routine use of regorafenib in this patient population.
Continued exploration of novel therapies, including combination approaches, is warranted for a patient population in whom limited treatment options exist.
Background
Regorafenib is a multitargeted kinase inhibitor with a kinase profile overlapping, but distinct from, pazopanib, an agent approved for recurrent and metastatic non‐gastrointestinal stromal tumor (GIST), non‐adipocytic soft tissue sarcoma. We conducted a randomized, phase II study of regorafenib versus placebo in refractory liposarcoma patients.
Methods
Patients with advanced or metastatic, treatment‐refractory liposarcoma were randomized 1:1 to receive regorafenib 160 mg or placebo once daily (3 weeks on, 1 week off). Patients with well‐differentiated liposarcoma only were excluded. Crossover for placebo was allowed upon progression. The primary endpoint was progression‐free survival (PFS), according to RECIST version 1.1.
Results
Forty‐eight subjects with liposarcoma (34 dedifferentiated, 12 myxoid/round cell, 2 pleomorphic) were enrolled. Median PFS was 1.87 (95% confidence interval [CI], 0.92–3.67) months for regorafenib versus 2.07 (95% CI, 1.64–3.44) months for placebo; stratified hazard ratio [HR], 0.85 (95% CI, 0.46, 1.58), p = .62. No responses were seen on regorafenib. One PR was observed on placebo. Median overall survival was 6.46 (95% CI, 4.16–23.48) months for regorafenib and 4.89 (95% CI, 3.02–9.77) months for placebo, stratified HR, 0.66 (95% CI, 0.31–1.40), p = .28). Treatment‐related adverse events were similar to the known safety profile of regorafenib.
Conclusion
Regorafenib did not appear to improve PFS in treatment‐refractory liposarcoma. No new significant safety signals were observed.
Discussion
Sarcomas represent a family of mesenchymal neoplasms with varied clinical behavior and outcomes. Liposarcomas represent one of the more common soft tissue sarcomas, with five distinct subtypes being recognized. Despite the recent approval of eribulin and trabectedin for the treatment of advanced disease, effective treatment options remain an area of unmet medical need.
Regorafenib is a multitargeted receptor tyrosine kinase inhibitor (TKI) with activity against a number of important targets, including but not limited to KIT, PDGFR, FGFR‐1, RET, BRAF, and VEGFR1–3 [1]. The Sarcoma Alliance for Research through Collaboration (SARC) performed the SARC024 trial, a multicohort phase II study, in select histologies of soft tissue and bone sarcoma, including liposarcoma (n = 48), osteosarcoma (n = 48), Ewing sarcoma (n = 30), and rhabdomyosarcoma and mesenchymal chondrosarcoma (n = 24 between the two cohorts). The Ewing cohort met its primary endpoint of 8‐week progression‐free survival, and the osteosarcoma cohort showed an improved PFS compared with placebo [2, 3].
This study did not meet its primary endpoint of an improvement in PFS. No responses were seen in subjects who received regorafenib. At the time of the analyses, 46 (96%) patients in each arm experienced a progression event: 23 on regorafenib and 23 on placebo. The PFS between the two arms did not differ (Fig. 1). Subjects randomized to regorafenib had a median PFS of 1.9 (95% CI, 0.9–3.7) months compared with 2.1 (95% CI, 1.6–3.4) months for placebo. The unstratified hazard ratio was 0.93 (95% CI, 0.51–1.69), p = .81. The stratified hazard ratio was 0.85 (95% CI, 0.46–1.58), p = .62. The median OS between the treatment arms did not differ (Fig. 2). Adverse events were consistent with the known side effect profile of regorafenib.
Figure 1.

Kaplan‐Meier curves for progression‐free survival with p value from stratified log rank test. The stratification factors are prior lines of therapy (1 vs. 2 or more) and WHO performance status (0–1 vs. 2). Abbreviations: mPFS, median progression‐free survival; WHO, World Health Organization.
Figure 2.
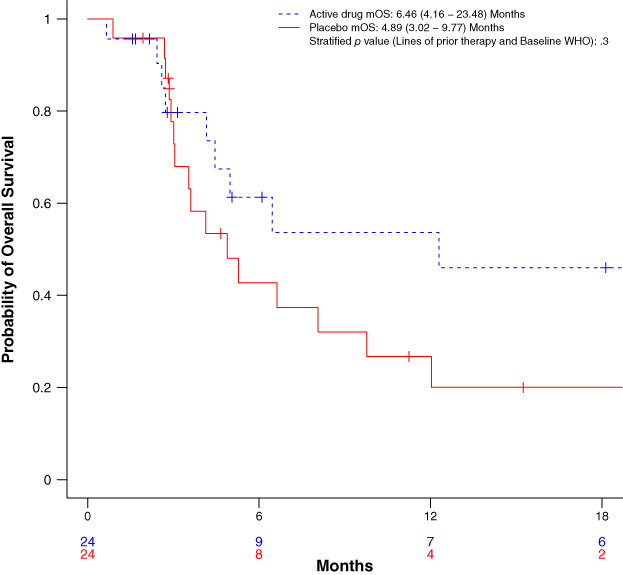
Kaplan‐Meier curves for overall survival with p value from stratified log rank test. The stratification factors are prior lines of therapy (1 vs. 2 or more) and WHO performance status (0–1 vs. 2). Abbreviations: mOS, median overall survival; WHO, World Health Organization.
These results are consistent with two prior studies exploring a multitargeted TKI in advanced soft tissue sarcomas, both of which included liposarcoma cohorts: REGOSARC, a randomized, double‐blind, placebo‐controlled, phase II trial exploring safety and efficacy of regorafenib in four advanced soft tissue sarcoma cohorts [4], and EORTC 62043, a phase II trial of the multitargeted TKI pazopanib in advanced soft tissue sarcomas [5]. Exploration of novel therapies, including combination approaches, is warranted for this patient population with limited treatment options.
Trial Information
| Disease | Sarcomas – adult |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | 1 prior regimen |
| Type of study | Phase II, randomized |
| PFS | p = .616, HR: 0.85 |
| Primary Endpoint | Progression‐free survival |
| Secondary Endpoints | Overall survival, overall response rate, toxicity |
| Additional Details of Endpoints or Study Design | |
|
The primary endpoint of the study was PFS, which was defined as the time from randomization to radiographic tumor progression (assessed by RECIST 1.1) or death from any cause, whichever occurs first. Progression is defined, according to RECIST 1.1 criteria, as a 20% increase from nadir and a minimum of 5 mm increase over the lowest sum, the appearance of one or more new target or nontarget lesions, or unequivocal progression of existing nontarget lesions determined by radiographic assessment. For patients alive without progression, patients were censored at the time of their last tumor assessment. The study was powered to detect a difference of at least a 3‐month improvement in median PFS. The anticipated median PFS for the placebo arm was 2 months, with a minimum detectable median PFS in the treatment arm of 5 months, for a minimum detectable HR of 0.40 (treatment/control group). A total of 42 PFS events were needed to detect a targeted difference with 90% power and 5% one‐sided significance level. A sample size of 48 eligible patients was expected to be randomized to achieve the 42 required PFS events. Secondary endpoints included overall survival (OS), overall response rate (ORR), and adverse events (AEs). OS was defined as the time from randomization until death from any cause, and patients who have not died were censored at the date of last contact. Overall response rate (ORR as assessed by RECIST 1.1) was defined as the portion of patients evaluable for response who achieved partial response or better. AEs were assessed according to National Cancer Institute's Common Terminology Criteria for Adverse Events version 4.03. Analyses were performed on an intent to treat basis. Time‐to‐event outcomes were compared between treatment groups using a one‐sided stratified log rank test with stratification for World Health Organization (WHO) performance status (0–1 vs. 2) and number of prior therapies (1 vs. >2). Proportions were compared using Fisher's exact test. Logistic regression and Cox proportional hazard models, with stratification variables included, were used to generate estimates of odds ratios and hazard ratios, respectively. Kaplan‐Meier curves were generated for time‐to‐event data. Point estimates and corresponding 95% CIs are provided as estimates of effects. The data were last updated on March 15, 2019, and were analyzed using R (version 3.5.2) and SAS 9.4 (SAS Institute, Cary, NC). | |
| Investigator's Analysis | Inactive because results did not meet primary endpoint |
Drug Information: Placebo
| Generic/Working Name | Placebo |
| Schedule of Administration | Four tablets ("matching placebo") p.o. daily for adult subjects on days 1–21, followed by a 7‐day rest period. Cycles were repeated every 28 days |
Drug Information: Regorafenib
| Generic/Working Name | Regorafenib |
| Trade Name | Stivarga |
| Company Name | Bayer |
| Drug Type | Small molecule |
| Drug Class | VEGFR |
| Dose | 160 mg per flat dose |
| Route | p.o. |
| Schedule of Administration | 160 mg (4 x 40 mg tablets) p.o. daily for adult subjects on days 1–21, followed by a 7‐day rest period. Cycles were repeated every 28 days |
Patient Characteristics: Placebo
| Number of Patients, Male | 18 (75%) |
| Number of Patients, Female | 6 (25%) |
| Stage | 92% (n = 22) with metastatic disease |
| Age | Median (range): 64.17 years (37.94–78.73) |
| Number of prior systemic therapies | Median (range): 2 (1–4) |
| Performance Status: WHO |
0 — 10 1 — 13 2 — 1 3 — 0 |
| Ethnicity |
Hispanic or Latino: 1 (4%) Not Hispanic or Latino: 22 (92%) Unknown: 1 (4%) |
| Race |
American Indian or Alaskan Native: 0 (0%) Asian: 3 (13%) Black or African Heritage: 1 (4%) White: 20 (83%) Unknown: 0 (0%) |
| Tumor Location |
Abdominopelvic/retroperitoneal: 17 (71%) Extremity/limb girdle: 6 (25%) Upper torso/thorax: 1 (4%) |
| Cancer Types or Histologic Subtypes |
Dedifferentiated liposarcoma 16 (67%) Myxoid and/or round cell liposarcoma 7 (29%) Pleomorphic liposarcoma 1 (4%) |
Patient Characteristics: Regorafenib
| Number of Patients, Male | 10 (42%) |
| Number of Patients, Female | 14 (58%) |
| Stage | 83% (n = 20) with metastatic disease |
| Age | Median (range): 61.22 years (27.03–79.65) |
| Number of Prior Systemic Therapies | Median (range): 1 (1–4) |
| Performance Status: WHO |
0 — 8 1 — 14 2 — 2 3 — 0 |
| Ethnicity |
Hispanic or Latino: 0 (0%) Not Hispanic or Latino: 23 (96%) Unknown: 1 (4%) |
| Race |
American Indian or Alaskan Native: 1 (4%) Asian: 0 (0%) Black or African Heritage: 5 (21%) White: 16 (67%) Unknown: 2 (8%) |
| Tumor Location |
Abdominopelvic/retroperitoneal: 18 (75%) Extremity/limb girdle: 4 (17%) Upper torso/thorax: 2 (8%) |
| Cancer Types or Histologic Subtypes |
Dedifferentiated liposarcoma, 18 (75%) Myxoid and/or round cell liposarcoma, 5 (21%) Pleomorphic liposarcoma, 1 (4%) |
Primary Assessment Method (Placebo)
| Title | Progression‐free survival (PFS) |
| Number of Patients Evaluated for Efficacy | 24 |
| Evaluation Method | Kaplan‐Meier method |
| (Median) Duration Assessments PFS | 2.07 months, CI: 1.64–3.44 |
Secondary Assessment Method (Placebo)
| Title | Objective response (ORR) |
| Number of Patients Evaluated for Efficacy | 22 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment PR | n = 1 (4.5%) |
| Title | Overall survival (OS) |
| Number of Patients Evaluated for Efficacy | 24 |
| Evaluation Method | Kaplan‐Meier method |
| (Median) Duration Assessments OS | 4.89 months, CI: 3.02–9.77 |
Primary Assessment Method (Regorafenib)
| Title | Progression‐free survival (PFS) |
| Number of Patients Evaluated for Efficacy | 24 |
| Evaluation Method | Kaplan‐Meier method |
| (Median) Duration Assessments PFS | 1.87 months, CI: 0.92–3.67 |
| Outcome Notes |
PFS (regorafenib vs. placebo; unstratified) HR, 0.93 (CI 0.51, 1.69); p = .81 PFS (regorafenib vs. placebo; stratified) HR, 0.85 (95% CI 0.46, 1.58); p = .616 |
Secondary Assessment Method (Regorafenib)
| Title | Overall response rate (ORR) |
| Number of Patients Evaluated for Efficacy | 20 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 0 (0%) |
| Title | Overall survival (OS) |
| Number of Patients Evaluated for Efficacy | 24 |
| Evaluation Method | Kaplan‐Meier method |
| (Median) Duration Assessments OS | 6.46 months, CI: 4.16–23.77 |
| Outcome Notes |
OS (regorafenib vs. placebo; unstratified) HR, 0.60 (95% CI 0.29, 1.25); p = .171 OS (regorafenib vs. placebo; stratified) HR, 0.66 (95% CI 0.31, 1.40); p = .275 |
Adverse Events (Placebo)
| All Cycles | |||||||
|---|---|---|---|---|---|---|---|
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
| Abdominal pain | 92% | 0% | 0% | 8% | 0% | 0% | 8% |
| Anemia | 83% | 0% | 0% | 17% | 0% | 0% | 17% |
| Anorexia | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Blood bilirubin increased | 92% | 0% | 0% | 8% | 0% | 0% | 8% |
| Diarrhea | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Fatigue | 83% | 0% | 0% | 17% | 0% | 0% | 17% |
| Generalized muscle weakness | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Hypertension | 84% | 0% | 8% | 8% | 0% | 0% | 16% |
| Hypoalbuminemia | 84% | 0% | 8% | 8% | 0% | 0% | 16% |
| Hypocalcemia | 92% | 0% | 8% | 0% | 0% | 0% | 8% |
| Hypomagnesemia | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Hyponatremia | 96% | 0% | 0% | 0% | 4% | 0% | 4% |
| Hypophosphatemia | 92% | 0% | 0% | 8% | 0% | 0% | 8% |
| Lipase increased | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Nausea | 79% | 13% | 0% | 8% | 0% | 0% | 21% |
| Pain | 92% | 0% | 0% | 8% | 0% | 0% | 8% |
| Palmar‐plantar erythrodysesthesia syndrome | 100% | 0% | 8% | 0% | 0% | 0% | 0% |
| Rash maculo‐papular | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Skin and subcutaneous tissue disorders | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
Adverse events occurring in >5% of subjects at any time during study treatment according to initial randomization. There were three nondrug‐related grade 5 events in the cohort initially randomized to placebo.
Abbreviation: NC/NA, no change from baseline/no adverse event; CI: confidence intervals.
Adverse Events (Regorafenib)
| All Cycles | |||||||
|---|---|---|---|---|---|---|---|
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All Grades |
| Abdominal pain | 87% | 0% | 0% | 13% | 0% | 0% | 13% |
| Anemia | 92% | 0% | 0% | 8% | 0% | 0% | 8% |
| Anorexia | 92% | 0% | 0% | 8% | 0% | 0% | 8% |
| Blood bilirubin increased | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Diarrhea | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Fatigue | 92% | 0% | 0% | 8% | 0% | 0% | 8% |
| Generalized muscle weakness | 92% | 0% | 0% | 8% | 0% | 0% | 8% |
| Hypertension | 70% | 0% | 17% | 13% | 0% | 0% | 30% |
| Hypoalbuminemia | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Hypocalcemia | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Hypomagnesemia | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Hyponatremia | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Hypophosphatemia | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Lipase increased | 92% | 0% | 0% | 8% | 0% | 0% | 8% |
| Nausea | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Pain | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Palmar‐plantar erythrodysesthesia syndrome | 71% | 0% | 21% | 8% | 0% | 0% | 29% |
| Rash maculo‐papular | 87% | 0% | 0% | 13% | 0% | 0% | 13% |
| Skin and subcutaneous tissue disorders | 92% | 0% | 8% | 0% | 0% | 0% | 8% |
Adverse events occurring in >5% of subjects at any time during study treatment according to initial randomization. There was one nondrug‐related grade 5 event in the cohort initially randomized to regorafenib.
Abbreviation: NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Inactive because results did not meet primary endpoint |
Liposarcomas represent one of the more common soft tissue sarcoma subtypes, with the World Health Organization recognizing atypical lipomatous tumor/well‐differentiated liposarcoma, dedifferentiated liposarcoma, pleomorphic liposarcoma, myxoid liposarcoma, and liposarcoma as distinct entities [6]. Despite the recent approval of eribulin and trabectedin for the treatment of advanced disease, liposarcoma treatment remains an area of unmet medical need. [7, 8, 9, 10]. Tyrosine kinase inhibitors have been explored and approved in a variety of metastatic soft tissue sarcomas, including imatinib, sunitinib, and regorafenib in gastrointestinal tumors (GISTs) and pazopanib in non‐GIST and nonadipocytic sarcomas [11, 12, 13, 14]. In a multicohort phase II study, pazopanib's efficacy in liposarcomas was limited [5]. As a result, liposarcomas were excluded from the phase III PALETTE study that led to regulatory approval [14]. However, a recent, single‐arm, prospective phase II study revealed potential activity of pazopanib in liposarcoma based on an encouraging progression‐free survival rate at 12 weeks [15]. As a result, there has been continued interest in exploring tyrosine kinase inhibitor (TKI)‐based therapy in various histologic subtypes of sarcoma.
The Sarcoma Alliance for Research through Collaboration (SARC) performed the SARC024 trial (NCT02048371), a multicohort phase II study, in select histologies of soft tissue and bone sarcoma, including liposarcoma, osteosarcoma, and Ewing sarcoma. This phase II study of regorafenib versus placebo in liposarcoma did not meet its primary endpoint of an improvement in PFS. In addition, no responses were seen in subjects who received regorafenib, and no statistically significant differences were noted in PFS or OS. These results confirm the results of the previously published randomized phase II REGOSARC study of multitargeted TKI regorafenib and the phase II EORTC 62043 study of the multitargeted TKI pazopanib in advanced soft tissue sarcomas [4, 5]. Reasons for the limited activity of multitargeted TKIs in liposarcoma from randomized prospective data published to date remain unknown. Despite the available data, there has been continued interest in exploring multitargeted TKI therapies in liposarcoma. Anlotinib, a multitargeted antiangiogenic inhibitor, has recently been explored in a phase II study in patients with metastatic soft tissue sarcoma [16]. Multiple cohorts of soft tissue sarcoma were enrolled, including liposarcomas. Among the liposarcoma cohort, a progression free rate at 12 weeks of 63% was observed, with a median PFS of 5.6 months and OS of 12 months. These results are encouraging but limited by the small number of patients with liposarcoma enrolled and the generalizability of the patient population (Chinese only). Although these appear to be the most promising results for a TKI to date in liposarcoma, the ongoing, phase III APROMISS study is exploring anlotinib in limited histologic subtypes, specifically leiomyosarcoma, synovial sarcoma, and alveolar soft part sarcoma (NCT03016819).
There are several ongoing studies actively enrolling and exploring a number of novel therapies in liposarcoma, including, but not limited to, cabozantinib (NCT01913652) and sitravatinib (NCT02978859). The phase II/III SEAL trial, exploring a selective inhibitor of nuclear export (selinexor) versus placebo, in dedifferentiated liposarcomas, is continuing actively enrolling (NCT02606461). Given the known amplification of CDK4 in the majority of dedifferentiated liposarcomas, there has been continued interest in exploring CDK4 inhibition, with trials completed using palbociclib and abemaciclib [17, 18]. Pembrolizumab has been explored in a cohort of patients with liposarcoma, and although initial data suggested modest activity, a recent update failed to confirm the results [19, 20].
Continued exploration is warranted in this patient population with limited treatment options. Inclusion of embedded correlatives to identify potential biomarkers of response with a goal of providing individualized therapies most likely to provide benefit and improve patient outcomes should be emphasized.
Disclosures
Richard F. Riedel: Limbguard, LLC (E, IP [Spouse]), Bayer, Blueprint, EISAI, EMD Serono, Janssen, Lilly, Loxo, Nanocarrier (C/A), AADi, AROG, Glaxo Smith Klein, Karyopharm, Ignyta, Immune Design, Lilly, NanoCarrier, Novartis, Oncternal, Philogen, Plexxikon, Roche, Springworks, Threshold, Tracon (RF); Karla V. Ballman: Patent for a prostate cancer classifier (IP), Takeda (C/A, DMSB member for lung cancer trial), Janssen Pharmaceuticals (ET); Yao Lu:; Steven Attia: Bayer (RF); Elizabeth T. Loggers:; Kristen N. Ganjoo:; Michael B. Livingston:; Warren Chow: GSK (H), AdvenChen (RF); Jennifer Wright: Eli Lilly & Co. (E, OI); John H. Ward:; Daniel Rushing:; Scott H. Okuno:; Damon R. Reed: Pfizer/Merck (C/A), Salarius (RF); David A. Liebner:; Vicki L. Keedy: Karyopharm, Daiichi Sankyo (C/A), Medpacto, Plexxikon, Daiichi Sankyo, Lilly, BioMed Valley, Immune Design, GSK, Tracon, Advenchen, Bayer, Adaptimmune (RF); Leo Mascarenhas: Bayer (C/A), AstraZeneca, Lilly, Bayer; Salarius (RF), Thermo Fisher Scientific (Other); Lara E. Davis: Eisai, Novartis, BTG (RF); Christopher Ryan:; Denise K. Reinke:; Robert G. Maki: Bayer, Deciphera, Foundation Medicine, Karyopharm, Physicans' Education Resource, Presage, Springworks, American Society for Clinical Oncology (C/A), UptoDate, Springer (royalties). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
We acknowledge and thank the patients, families, participating institutions and associated study staff, and SARC leadership and staff who participated in and supported this study.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: NCT02048371
- Sponsors: SARC, with support from Bayer HealthCare Pharmaceuticals (Berlin, Germany)
- Principal Investigator: Richard F. Riedel
- IRB Approved: Yes
References
- 1. Wilhelm SM, Dumas J, Adnane L et al. Regorafenib (BAY 73‐4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245–255. [DOI] [PubMed] [Google Scholar]
- 2. Attia S, Bolejack V, Ganjoo KN et al. A phase II trial of regorafenib (REGO) in patients (pts) with advanced Ewing sarcoma and related tumors (EWS) of soft tissue and bone: SARC024 trial results. J Clin Oncol 2017; 35(suppl):11005a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davis LE, Bolejack V, Ryan CW et al. Randomized double‐blind phase ii study of regorafenib in patients with metastatic osteosarcoma. J Clin Oncol 2019;37:1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mir O, Brodowicz T, Italiano A et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): A randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Oncol 2016;17:1732–1742. [DOI] [PubMed] [Google Scholar]
- 5. Sleijfer S, Ray‐Coquard I, Papai Z et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: A phase II study from the European organisation for research and treatment of cancer‐soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol 2009;27:3126–3132. [DOI] [PubMed] [Google Scholar]
- 6. Fletcher CD, Hogendoorn P, Mertens F et al. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon, France: IARC Press; 2013. [Google Scholar]
- 7. Schöffski P, Chawla S, Maki RG et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open‐label, multicentre, phase 3 trial. Lancet 2016;387:1629–1637. [DOI] [PubMed] [Google Scholar]
- 8. Osgood CL, Chuk MK, Theoret MR,et al. FDA approval summary: Eribulin for patients with unresectable or metastatic liposarcoma who have received a prior anthracycline‐containing regimen. Clin Cancer Res 2017;23:6384–6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Demetri GD, von Mehren M, Jones RL et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: Results of a phase III randomized multicenter clinical trial. J Clin Oncol 2016;34:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barone A, Chi DC, Theoret MR et al. FDA approval summary: Trabectedin for unresectable or metastatic liposarcoma or leiomyosarcoma following an anthracycline‐containing regimen. Clin Cancer Res 2017;23:7448–7453. [DOI] [PubMed] [Google Scholar]
- 11. Blanke CD, Rankin C, Demetri GD et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626–632. [DOI] [PubMed] [Google Scholar]
- 12. Demetri GD, van Oosterom AT, Garrett CR et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet. 2006;368:1329–1338. [DOI] [PubMed] [Google Scholar]
- 13. Demetri GD, Reichardt P, Kang YK et al; GRID study investigators. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet. 2013. Jan 26;381:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Graaf WT, Blay JY, Chawla SP et al; Soft Tissue EORTC and Bone Sarcoma Group ; PALETTE study group . Pazopanib for metastatic soft‐tissue sarcoma (PALETTE): A randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet 2012;379:1879–1886. [DOI] [PubMed] [Google Scholar]
- 15. Samuels BL, Chawla SP, Somaiah N et al. Results of a prospective phase 2 study of pazopanib in patients with advanced intermediate‐grade or high‐grade liposarcoma. Cancer 2017;123:4640–4647. [DOI] [PubMed] [Google Scholar]
- 16. Chi Y, Fang Z, Hong X et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft‐tissue sarcoma. Clin Cancer Res 2018;24:5233–5238. [DOI] [PubMed] [Google Scholar]
- 17. Dickson MA, Schwartz GK, Keohan ML et al. Progression‐free survival among patients with well‐differentiated or dedifferentiated liposarcoma treated with CDK4 inhibitor palbociclib: A phase 2 clinical trial. JAMA Oncol 2016;2:937–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dickson MA, Koff A, D'Angelo SP et al. Phase 2 study of the CDK4 inhibitor abemaciclib in dedifferentiated liposarcoma. J Clin Oncol 2019;37(suppl):11004a. [Google Scholar]
- 19. Tawbi HA, Burgess M, Bolejack V et al. Pembrolizumab in advanced soft‐tissue sarcoma and bone sarcoma (SARC028): A multicentre, two‐cohort, single‐arm, open‐label, phase 2 trial. Lancet Oncol 2017;18:1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burgess MA, Bolejack V, Schuetze S et al. Clinical activity of pembrolizumab (P) in undifferentiated pleomorphic sarcoma (UPS) and dedifferentiated/pleomorphic liposarcoma (LPS): Final results of SARC028 expansion cohorts. J Clin Oncol 2019;37(suppl);11015a. [Google Scholar]


