Abstract
Alterations in c‐MET, a tyrosine kinase receptor encoded by the MET gene, have been reported in approximately 3% of non‐small cell lung cancer (NSCLC) cases and carry important treatment implications. The best studied genetic alterations are exon 14 skipping and gene amplification; however, gene rearrangement has also been described, and multiple fusion partners have been reported. Recently, in METex14‐mutated NSCLC, multitarget tyrosine kinase inhibitors (TKIs), such as crizotinib and cabozantinib, as well as MET‐selective TKIs, such as tepotinib and capmatinib, have demonstrated durable responses. In this study, we present the case of a 41‐year‐old woman with advanced NSCLC harboring an HLA‐DRB1‐MET gene fusion. The patient was offered successively two different MET multikinase inhibitors, crizotinib and cabozantinib, and the selective inhibitor tepotinib. Each time, including under tepotinib, the patient experienced rapid and complete responses associated with a tremendous improvement in her physical function.
Key Points
To our knowledge, this is the first report of a patient with non‐small cell lung cancer harboring an HLA‐DRB1‐MET gene fusion demonstrating a clinical response to multiple MET inhibitors, including tepotinib.
This finding illustrates the efficacy and rationale to targeting MET regardless of fusion partner and gives insight to pooling of patients with different MET fusion products in trials assessing safety and efficacy of novel molecules.
Short abstract
This article provides a promising report of tepotinib efficacy in a patient with non‐small cell lung cancer harboring an HLA‐DRB1‐MET gene fusion.
Patient Story
The patient is a 43‐year‐old woman (never‐smoker) with no significant medical history except that one of her children has retinoblastoma (negative for germline RB1 mutations). She was diagnosed in September 2016 with locally advanced lung adenocarcinoma (stage IIIA: T2 N2 M0; Fig. 1A). The tumor was negative for programmed death ligand 1 expression (0%), EGFR mutations, and ALK and ROS1 gene rearrangements. The patient was treated with concomitant chemotherapy (cisplatin and vinblastine) and radiotherapy between December 2016 and January 2017 and achieved partial radiologic response. However, during the radiotherapy, she developed severe postradiation dermatitis and grade 3 pneumonitis.
Figure 1.
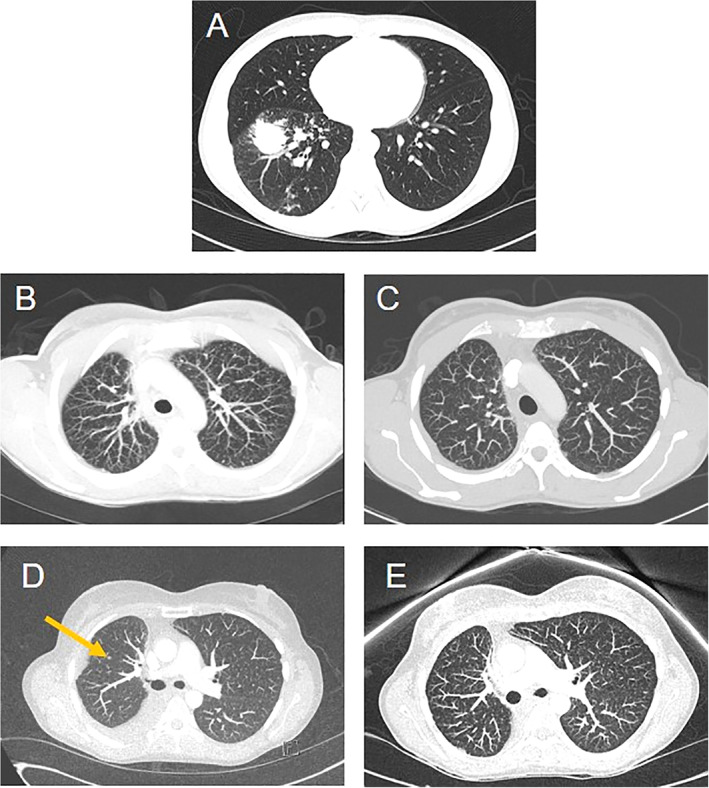
Thoracic computed tomography scans. (A): December 2016: diagnosis of locally advanced (stage IIIA) NSCLC. (B): September 2017: multiple millimeter‐sized lung metastases. (C): November 2017: complete resolution of the lung lesions. (D): May 2019: new secondary lesion in right lung (yellow arrow). (E): August 2019: complete disappearance of the lung metastases.
As the patient was young and a nonsmoker, a large‐scale genetic sequencing panel was used to explore other rare genetic drivers, which included an anchored multiplex polymerase chain reaction–based targeted fusion assay using next‐generation sequencing [1].
Molecular Tumor Board
The fusion assay revealed an in‐frame transcript involving HLA‐DRB1 exon 4 (ENST00000360004) and MET exon 15 (ENST00000318493; Fig. 2A). MET rearrangement was confirmed by a MET break‐apart fluorescence in situ hybridization assay ([2]B). The HLA‐DRB1‐MET gene fusion, which retains the immunoglobulin‐like domain of HLA‐DRB1 and the kinase domain of MET (Fig. 2C), is presumed to lead to oncogenic activation of MET. The HLA‐DRB1‐MET fusion gene that we identified in our patient has been reported only once before in a case of a 74‐year‐old woman who developed recurrent metastatic non‐small cell lung cancer (NSCLC) [2]. This patient was treated with crizotinib and experienced a rapid and complete response, and her disease had remained stable for 8 months at the time of publication. In contrast, although our patient initially responded well to crizotinib, her disease progressed to symptomatic cerebral metastases within 6 months of initiating treatment. The presence of this fusion in our patient's tumor suggested that it may be sensitive to treatment with MET inhibitors.
Figure 2.
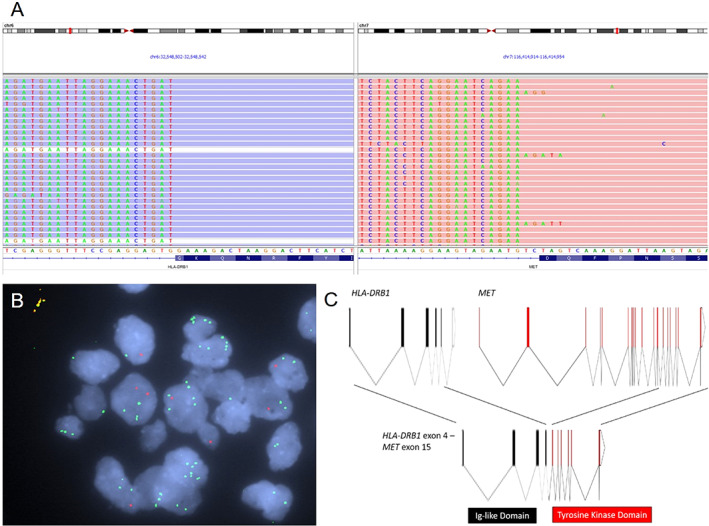
Molecular analysis of the tumor. (A): Screenshot of the Integrative Genomics Viewer from the next‐generation sequencing fusion assay showing abundant supporting RNA reads with sequences starting from HLA‐DRB1 exon 4 and continuing to MET exon 15, indicating the presence of the HLA‐DRB1‐MET gene fusion. (B): Break‐apart fluorescence in situ hybridization assay showing the separation of MET 5' green probes from 3' red probes, confirming MET translocation. (C): Schematic of intron‐exon structures of the HLA‐DRB1 and MET genes and the HLA‐DRB1‐MET gene fusion.
Abbreviation: Ig, immunoglobulin.
Although still considered an emerging biomarker in NSCLC, alterations in MET are becoming established not only as important oncogenic drivers but as acquired resistance mechanisms to EGFR‐targeted tyrosine kinase inhibitors (TKIs) in patients with EGFR mutations [3]. The best studied genetic alterations in MET in NSCLC are exon 14 skipping (METex14; 3%–4% of cases) and gene amplification (1%–5% of cases) [4]. Compared with gene amplification and METex14 mutations, reports of MET fusions are relatively rare [5]. Lack of expression of exon 14 leads to absence of the juxtamembrane region, which contains multiple sites for negative regulation [3]. Multiple MET fusion partners have been identified in NSCLC, including KIF5B [6, 7], STARD3NL [7], HLA‐DRB1 [2], UBE2H [8], and ATXN7L1 [9]. It is thought that MET fusions that include the 3' MET kinase domain by fusing at exon 15 (e.g., fusions with 5' partners KIF5B, STARD3NL, HLA‐DRB1) may become activated because of constitutive dimerization of MET [10]. It has also been suggested that MET kinase domain fusions may recapitulate the mechanism of METex14 mutants owing to loss of negative regulation in the juxtamembrane region [8].
Recently, case studies and early‐phase trials in METex14‐mutated NSCLC have demonstrated durable responses to multitarget TKIs, such as crizotinib and cabozantinib [11, 12, 13], as well as MET‐selective TKIs, such as tepotinib and capmatinib [14, 15, 16]. MET TKIs may also improve overall survival in patients with METex14 mutations [17]. Although several therapies targeting MET are under development for selected cancers, only cabozantinib and crizotinib are approved in the U.S., Europe, and Canada.
The choice of MET kinase inhibitor may be important to consider when treating NSCLC with central nervous system (CNS) metastasis, given that these inhibitors vary in their ability to cross the blood‐brain barrier. Despite producing objective responses in the primary tumor, crizotinib has limited activity in treating brain metastases as a result of poor CNS penetration [18]. In contrast, cabozantinib led to rapid intracranial responses in a case report of METex14‐mutated NSCLC resistant to crizotinib [13]. Capmatinib also had activity in patients with brain metastases in a phase II trial in previously treated NSCLC with METex14 mutations [16, 19].
Patient Update
Seven months after completing first‐line treatment with combination radiochemotherapy (August 2017), follow‐up scans revealed that the patient had progressive disease. This was evidenced by an increase in the size of the primary tumor and the appearance of multiple subcentimetric bilateral lung and brain metastases (Figs. 1B, 3A), as well as centimetric lesions in the bone (2 lesions) and liver (1 lesion). The patient was started on crizotinib 250 mg twice daily in October 2017 and had an excellent clinical response after only a few weeks of treatment. Radiologic scans from November 2017 revealed complete resolution of the lung and brain lesions (Figs. 1C, 3B). Tolerance to crizotinib was excellent without any major adverse events, allowing the patient to return to work part time as a teacher.
Figure 3.
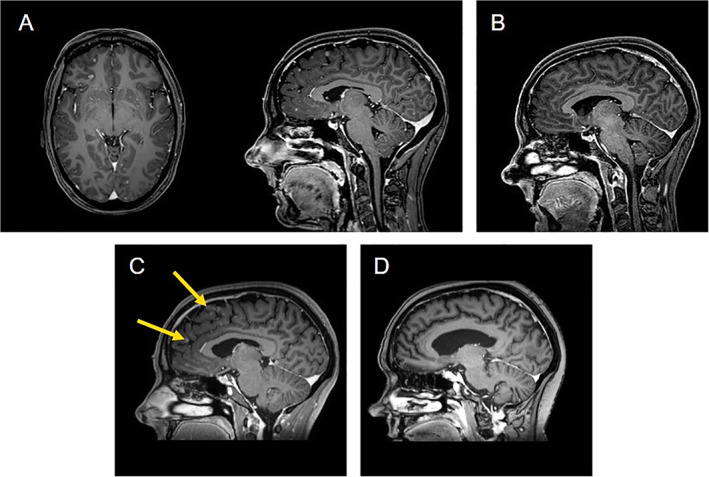
Brain magnetic resonance imaging scans. (A): August 2017: multiple disseminated brain metastases. (B): November 2017: complete resolution of the brain metastases. (C): May 2018: new secondary brain lesions (yellow arrow). (D): September 2018: complete resolution of the brain metastases.
The patient's disease recurred in May 2018, when she presented with symptomatic cerebral metastases (ataxia, dysphagia, fatigue, global psychomotor retardation, Eastern Cooperative Oncology Group [ECOG] performance status [PS] of 3; Fig. 3C), new infracentimetric lung lesions in the right parenchyma (8 mm and 6 mm), with right pleural effusion. At that time, the liver metastasis remained stable. The patient stopped work. Attempts to biopsy the lung lesions or obtain pleural fluid from the effusion failed to produce sufficient material to perform analyses to identify mechanisms of resistance and were further complicated by the presence of a grade 2 pneumothorax. There was no liquid biopsy performed either. The patient received whole brain radiation therapy (WBRT; 20 Gy in 5 fractions) with oral dexamethasone (4 mg four times daily) in June 2018. She experienced partial clinical improvement with respect to ataxia and dysphagia but still had neurological symptoms and required daily assistance for activities of daily living. She was then initiated on a second MET inhibitor, tepotinib, 500 mg once daily in July 2018 for 1 month (Special Access Program approval; supply from EMD Serono, a company of Merck KGaA). The dosage was then increased to 1,000 mg daily in August 2018. After 2 months on tepotinib, she showed significant improvement in her general and neurological status, and imaging showed a complete response in the brain (Fig. 3D), lung, and liver, without any adverse events. The patient was able to return to work part time.
The patient remained progression free for 9 months while on tepotinib until May 2019, when she developed new lung micronodules with pleural effusion (Fig. 1D) and a new liver lesion. There were no clinical or radiological signs of worsening cerebral involvement. She started treatment with cabozantinib 60 mg daily as third‐line treatment in June 2019. The first radiological evaluation in August 2019 demonstrated complete resolution of the pleural effusion and the micronodules (Fig. 1E), whereas the liver lesion remained stable. Brain imaging was also free of recurrence. Cabozantinib was well tolerated, with the main adverse event being grade 1 xerostomia. At last follow‐up in November 2019, the patient was asymptomatic, in good physical condition (ECOG PS of 1), and still on cabozantinib. The timeline of the patient's disease course and interventions is illustrated in Figure 4.
Figure 4.
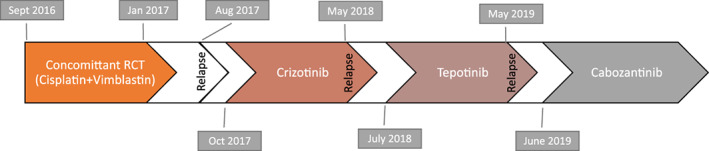
Timeline of the successive treatments. Note that the patient received whole brain radiation therapy with dexamethasone following relapse in May 2018 and prior to initiation of tepotinib in July 2018. The patient had not progressed on cabozantinib as of November 2019.
Abbreviation: RCT, radiochemotherapy.
To our knowledge, this is the first report of tepotinib efficacy in a patient with NSCLC harboring an HLA‐DRB1‐MET gene fusion. This patient had an almost complete remission while on tepotinib, including in the brain, with a sustainable response of almost 9 months, although response in the brain is difficult to assess considering the sequential administration following WBRT. Tepotinib was very well tolerated by the patient even at 1,000 mg daily, and she did not have any related adverse events. Doses of up to 1,400 mg daily were previously shown to be well tolerated in phase I studies [20]. This efficacy is in line with interim data from the ongoing phase II VISION trial in patients with NSCLC with METex14 mutations, in which tepotinib 500 mg daily was shown to have durable clinical activity and a favorable safety profile, and patients with brain metastases at baseline benefitted equally from treatment [15]. The patient experienced a tremendous improvement in her physical function and quality of life and even expressed the desire to return to work.
Glossary of Genomic Terms and Nomenclature
- ALK
anaplastic lymphoma kinase
- ATXN7L1
Ataxin 7 Like 1
- EGFR
epidermal growth factor receptor
- HLA‐DRB1
major histocompatibility complex, class II, DR beta 1
- KIF5B
Kinesin Family Member 5B
- MET
mesenchymal‐epithelial transition factor
- ROS1
ROS proto‐oncogene
- STARD3NL
STARD3 N‐Terminal Like
- UBE2H
Ubiquitin Conjugating Enzyme E2H
Author Contributions
Conception/design: Marie Florescu
Provision of study material or patients: Anthony J. Iafrate, Danh Tran‐Thanh, Charles Leduc
Collection and/or assembly of data: Félix Blanc‐Durand, Raafat Alameddine
Data analysis and interpretation: Félix Blanc‐Durand, Anthony J. Iafrate, Ying‐Chun Lo, Marie Florescu
Manuscript writing: Félix Blanc‐Durand, Raafat Alameddine, Marie Florescu
Final approval of manuscript: Félix Blanc‐Durand, Raafat Alameddine, Anthony J. Iafrate, Danh Tran‐Thanh, Ying‐Chun Lo, Normand Blais, Bertrand Routy, Mustapha Tehfé, Charles Leduc, Phillipe Romeo, Phillipe Stephenson, Marie Florescu
Disclosures
Anthony Iafrate: ArcherDx (OI), Repare Therapeutics (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
The patient gave consent for images and clinical information relating to her case to be reported in this medical publication.
The development of this publication was financially supported by EMD Inc Canada, a business of Merck KGaA, Darmstadt, Germany, through an independent medical writing grant. The views and opinions described in this publication do not necessarily reflect those of the grantor.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Zheng Z, Liebers M, Zhelyazkova B et al. Anchored multiplex PCR for targeted next‐generation sequencing. Nat Med 2014;20:1479–1484. [DOI] [PubMed] [Google Scholar]
- 2. Davies KD, Ng TL, Estrada‐Bernal A, Le AT, Ennever PR, Camidge DR et al. Dramatic response to crizotinib in a patient with lung cancer positive for an HLA‐DRB1‐MET gene fusion. JCO Precis Oncol 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duplaquet L, Kherrouche Z, Baldacci S et al. The multiple paths towards MET receptor addiction in cancer. Oncogene 2018;37:3200–3215. [DOI] [PubMed] [Google Scholar]
- 4. Tong JH, Yeung SF, Chan AWH et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non‐small cell lung carcinoma with poor prognosis. Clin Cancer Res 2016;22:3048–3056. [DOI] [PubMed] [Google Scholar]
- 5. Drilon A, Cappuzzo F, Ou SHI et al. Targeting MET in lung cancer: Will expectations finally be MET? J Thorac Oncol 2017;12:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cho JH, Ku BM, Sun JM et al. KIF5B‐MET gene rearrangement with robust antitumor activity in response to crizotinib in lung adenocarcinoma. J Thorac Oncol 2018;13:e29–e31. [DOI] [PubMed] [Google Scholar]
- 7. Plenker D, Bertrand M, de Langen AJ et al. Structural alterations of MET trigger response to MET kinase inhibition in lung adenocarcinoma patients. Clin Cancer Res 2018;24:1337–1343. [DOI] [PubMed] [Google Scholar]
- 8. Zhu YC, Wang WX, Song ZB et al. MET‐UBE2H fusion as a novel mechanism of acquired EGFR resistance in lung adenocarcinoma. J Thorac Oncol 2018;13:e202–e204. [DOI] [PubMed] [Google Scholar]
- 9. Zhu YC, Wang WX, Xu CW et al. Identification of a novel crizotinib‐sensitive MET–ATXN7L1 gene fusion variant in lung adenocarcinoma by next generation sequencing. Ann Oncol 2018;29:2392–2393. [DOI] [PubMed] [Google Scholar]
- 10. Stransky N, Cerami E, Schalm S et al. The landscape of kinase fusions in cancer. Nat Commun 2014;5:4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paik PK, Drilon A, Fan PD et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov 2015;5:842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drilon A, Clark JW, Weiss J et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nat Med 2020;26:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klempner SJ, Borghei A, Hakimian B et al. Intracranial activity of cabozantinib in MET exon 14‐positive NSCLC with brain metastases. J Thorac Oncol 2017;12:152–156. [DOI] [PubMed] [Google Scholar]
- 14. Frampton GM, Ali SM, Rosenzweig M et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015;5:850–859. [DOI] [PubMed] [Google Scholar]
- 15. Paik PK, Veillon R, Cortot AB et al. Phase II study of tepotinib in NSCLC patients with METex14 mutations. J Clin Oncol 2019;37(suppl)9005a. [Google Scholar]
- 16. Wolf J, Seto T, Han JY et al. Capmatinib (INC280) in METΔex14‐mutated advanced non‐small cell lung cancer (NSCLC): Efficacy data from the phase II GEOMETRY mono‐1 study. J Clin Oncol 2019;37(suppl):9004a. [Google Scholar]
- 17. Awad MM. Impaired c‐met receptor degradation mediated by MET exon 14 mutations in non‐small‐cell lung cancer. J Clin Oncol 2016;34:879–881. [DOI] [PubMed] [Google Scholar]
- 18. Costa DB, Shaw AT, Ou SHI et al. Clinical experience with crizotinib in patients with advanced ALK‐rearranged non–small‐cell lung cancer and brain metastases. J Clin Oncol 2015;33:1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vansteenkiste JF, Van De Kerkhove C, Wauters E et al. Capmatinib for the treatment of non‐small cell lung cancer. Expert Rev Anticancer Ther 2019;19:659–671. [DOI] [PubMed] [Google Scholar]
- 20. Falchook GS, Kurzrock R, Amin HM et al. First‐in‐man phase I trial of the selective MET inhibitor tepotinib in patients with advanced solid tumors. Clin Cancer Res 2020;26:1237–1246. [DOI] [PubMed] [Google Scholar]


