Abstract
The addition of immune checkpoint inhibitors to the armamentarium of cancer therapies has resulted in unprecedented improvement in clinical outcomes for a vast range of malignancies. Because they interfere with the physiologic function of immune checkpoints, such as programmed cell death protein 1 or cytotoxic T‐lymphocyte‐associated protein 4, to promote self‐tolerance, these agents are associated with a unique spectrum of immune‐related adverse events (irAEs). Immune‐mediated endocrinopathies are among the most commonly noted irAEs. Immune‐mediated diabetes is an uncommon irAE but can be associated with significant morbidity if it is not recognized and treated in a time‐sensitive manner. In this manuscript, we present a case based discussion and review of the literature pertaining to immune‐mediated diabetes associated with immune checkpoint blockade.
Key Points
Immune checkpoint inhibitor associated diabetes mellitus often resembles type 1 diabetes mellitus (DM) in its pathophysiology and clinical manifestations. However, some patients may present with type 2 DM or worsening hyperglycemia in the setting of pre‐existent DM.
Early recognition and management is key to preventing life‐threatening events such as diabetic ketoacidosis.
Endocrinology referral and interdisciplinary management should be considered for every patient to optimize glycemic control and to ensure optimal monitoring for long‐term microvascular complications.
Short abstract
This article presents a case‐based discussion and review of the literature pertaining to immune‐mediated diabetes associated with immune checkpoint blockade.
Introduction
The development of immune‐checkpoint inhibitors (ICIs) for cancer treatment has introduced promising new opportunities to the field of oncology. The programmed cell death protein 1 (PD1)/programmed death ligand 1 (PD‐L1) and cytotoxic T‐lymphocyte‐associated protein 4 pathways allow for pathogenic tumor evasion of immunosurveillance, as well as physiologic immune tolerance of normal tissues. Inhibition of these pathways by ICIs, therefore, can result in a diverse array of immune‐related adverse events (irAEs) affecting tissues throughout the body and manifesting with signs and symptoms that resemble autoimmune conditions.
The diversity of organ system involvement in irAEs due to ICI treatment necessitates a multidisciplinary approach to managing these conditions. At the Cleveland Clinic, we conduct a monthly irAE tumor board where patients with challenging irAEs are discussed among oncologists, endocrinologists, rheumatologists, pulmonologists, dermatologists, pathologists, gastroenterologists, hepatologists, neurologists, ophthalmologists, nurses and nurse practitioners in any of these fields, and others. Discussion among experts in various fields relevant to a given irAE case enriches clinicians’ understanding of the pathology involved and allows for collaboration in devising an appropriate treatment approach.
In this article, we describe a case of a patient with metastatic lung adenocarcinoma who developed diabetes mellitus after treatment with pembrolizumab, and the diagnostic workup and management of ICI‐related endocrine toxicities based on collaborative input from our irAE tumor board.
Case Vignette
A 52‐year‐old man was incidentally found to have pulmonary nodules on an abdominal computed tomography scan performed for evaluation of right nephrolithiasis. Following bronchoscopy and a transbronchial biopsy, he was diagnosed with metastatic adenocarcinoma of the lung in mid‐2016. Molecular testing revealed the presence of an EGFR exon 19 insertion mutation, and PD‐L1 tumor proportion score was 5%. He initiated treatment with afatinib and demonstrated a partial response. Unfortunately, he developed progressive disease after 15 months of therapy. Repeat tumor biopsy was performed and was negative for the acquired T790M resistance mutation. His regimen was switched to carboplatin plus pemetrexed plus pembrolizumab, and he received three cycles of chemo‐immunotherapy in early 2018, with a partial response noted on imaging. Routine labs performed after his third cycle revealed an elevated blood glucose of 300 mg/dL compared with previously normal random blood glucose levels. At the time, the patient also complained of blurry vision, polyuria, polydipsia, and fatigue for 2 weeks prior to evaluation.
Pertinent past medical history before initiation of ICI therapy was notable for hypertension, obesity, gastroesophageal reflux, gout, obstructive sleep apnea, and testosterone deficiency. Patient denied any personal or family history of autoimmune disease. Family history was notable for type 2 diabetes mellitus (T2DM) in his mother. Other medications at this time included benzonatate, ondansetron, omeprazole, and lisinopril‐hydrochlorothiazide.
Following this episode of hyperglycemia, the patient was given a glucometer to monitor his blood glucose at home and was referred to endocrinology. The next day, he reported fasting blood glucose levels in the 300s and premeal levels in the 400s. He was subsequently started on 15 units of insulin glargine and 5 units of insulin lispro with meals. Thirty days after initiating insulin, he continued to have poorly controlled blood sugars, with average fasting glucose levels elevated in the 200s and premeal blood glucose in the 150s. His glargine and lispro insulin doses were titrated up significantly in a stepwise manner to 48 units of glargine and 12 units of lispro insulin per day. Because of suboptimal glycemic control despite relatively high doses of insulin, he was sequentially started on pioglitazone, exenetide, and metformin. With this multidrug regimen, he was able to achieve acceptable glycemic control.
At the time of his initial diagnosis of diabetes mellitus (DM), labs revealed a hemoglobin A1C (HbA1C) of 5.7% and a C‐peptide level of 3.0 ng/mL (normal range, 0.8–3.2 ng/mL). Subsequent labs revealed a rapid decline in the C‐peptide levels (1.7 ng/mL at 10 days and 0.2 ng/mL at 4 weeks). Antibody testing was positive for anti‐glutamic acid decarboxylase antibodies (GAD; 32.1 IU/mL) but negative for other anti‐islet cell or insulin antibodies. Four weeks after his diagnosis, the fructosamine level was found to be high at 443 umol/L.
irAE Tumor Board
Clinical Presentation
This patient's clinical presentation of rapid onset of hyperglycemia, diminished C‐peptide level, and presence of anti‐GAD antibodies suggested the diagnosis of ICI‐induced type 1 diabetes mellitus (T1DM). Borderline and normal HbA1C and C‐peptide levels measured immediately after first episode of hyperglycemia further supported the diagnosis of recent onset of diabetes, which was likely pembrolizumab‐mediated T1DM with immune‐mediated β cell destruction, leading to subsequent high fructosamine and low C‐peptide levels measured a month later.
This patient, however, also exhibited extreme insulin resistance, as evidenced by the increasingly high insulin doses necessary to control his diabetes. Insulin resistance is more closely associated with T2DM, although patients with T1DM who are also obese exhibit more insulin resistance and can have higher insulin requirements [1]. As a middle‐aged man with obesity, this patient exhibited a general phenotype more closely aligned with T2DM, which explains his high insulin requirement, but the presence of GAD65 antibodies is consistent with an immune‐mediated mechanism of the development of his diabetes. Because this patient did not have an HbA1C or C‐peptide prior to initiating ICI therapy, we cannot be certain that the diabetogenic processes of diminished insulin production and increased insulin resistance did not begin prior to ICI therapy. Based on the patient's age, body mass index, and family history of T2DM, it is likely that this patient was predisposed to developing insulin resistance and T2DM [2]. A single institution retrospective study has also reported worsening hyperglycemia in the setting of pre‐existing diabetes in patients treated with ICIs [3]. The mechanisms underlying insulin resistance with immune checkpoint blockade are yet to be elucidated.
Endocrine disorders, particularly those affecting the pituitary, thyroid, and adrenal glands, are among the most common irAEs reported with ICIs [4]. Immune‐mediated DM is one of the rarer endocrine irAEs, and among the ICIs, it is most commonly associated with pembrolizumab [4, 5, 6, 7, 8]. The onset of diabetes after initiating ICIs has been reported as early as 3 weeks to a year after the first cycle of ICIs [9, 10]. Symptoms such as polyuria, polydipsia, weight loss, and vomiting should prompt investigation for possible diabetes. Unlike other common irAEs, endocrine toxicities of checkpoint blockade are often irreversible [4]. Although almost all patients require lifelong therapy after developing ICI‐mediated DM, Iyer et al. reported one case in which the patient had complete resolution of diabetes and achieved independence from insulin use [3]. Because of mechanistic similarities with T1DM, a sizeable proportion of patients (over 50% in previous series) present with diabetic ketoacidosis at diagnosis [8].
Pathophysiology
T1DM is characterized by insulin deficiency as a consequence of autoimmune destruction of pancreatic β cells. Whereas patients with type 1A DM often have detectable islet cell antibodies such as anti‐GAD, those with type 1B DM are seronegative upon diagnosis [11]. In a fraction of patients, a state of latent autoimmune diabetes characterized by seropositivity for anti‐islet cell antibodies without insulin dependence may precede overt clinical manifestations of diabetes [12]. Whether such individuals are at a higher risk of immune‐mediated DM with ICIs is yet unknown. Similarly, the association between high risk HLA haplotypes and T1DM induced by ICIs has not been well established. However, some of the cases reported in literature were in fact in individuals with previously described high risk haplotypes [5]. Preclinical evidence suggests that inhibition of the PD1/PD‐L1 pathway may play a role in the pathogenesis of T1DM (Figure 1) [13]. In a study of 37 children with new onset T1DM, Granados et al. reported failure to upregulate PD1 upon T cell activation from the peripheral blood during the acute onset phase of disease [14]. It is therefore plausible that by disrupting one of the key physiologic pathways for preventing T1DM, immune checkpoint blockade can precipitate immune‐mediated destruction of islet cells and, as a consequence, T1DM (Figure 2).
Figure 1.
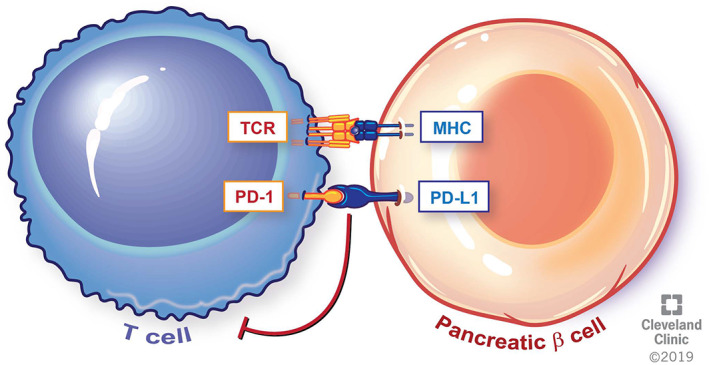
Physiologic interaction between pancreatic β cell and T cells. Engagement of the PD1/PD‐L1 axis leads to self‐tolerance and immune homeostasis. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2019. All Rights Reserved.
Figure 2.
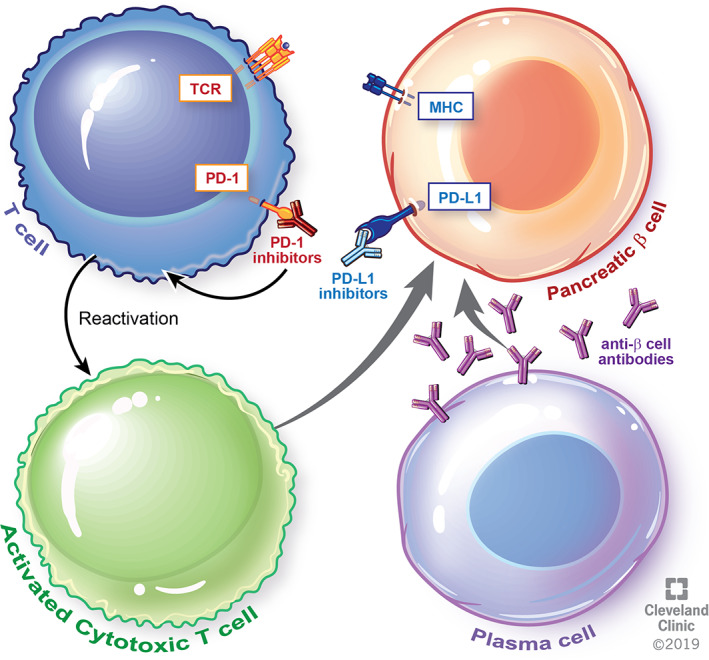
Disruption of the physiologic immune tolerance mechanisms due to immune checkpoint blockade leads to reactivation of self‐reactive T cells and immune mediated destruction of the pancreatic β cells resulting in diabetes. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2019. All Rights Reserved.
Diagnostic Evaluation
In clinical practice, hemoglobin A1C testing prior to initiating ICIs is not routinely recommended. However, American Society of Clinical Oncology (ASCO) guidelines do recommend measuring blood glucose levels at baseline and with every treatment cycle for 12 weeks, following which it can be monitored every 3–6 weeks [15]. Until the association between pre‐existing high risk HLA haplotypes or latent autoimmune diabetes and postcheckpoint diabetes is clearly defined with further investigations, testing for either should not be routinely performed before therapy.
In patients who have clinical symptoms or laboratory abnormalities suggestive of DM (hyperglycemia, anion gap metabolic acidosis, ketoacidosis), antibody testing as well as insulin and C‐peptide levels may aide the distinction between types 1 and 2 DM. Antibodies against GAD65, anti‐insulin, anti‐islet cell A, and zinc transporter 8 are specific for autoimmune diabetes [15, 16]. The lack of endogenous insulin secretion due to islet cell destruction in ICI‐mediated DM often manifests as a rapid decline in C peptide levels with time. In contrast to T2DM, hemoglobin A1C may not be elevated upon initial evaluation in patients with ICI‐induced DM due to the rapid onset of hyperglycemia. For estimating short‐term glycemic control, serum fructosamine may be used. Because the turnover of serum albumin is more rapid in comparison with hemoglobin, serum frustosamine usually estimates glycemic control over a shorter period of time (1–2 weeks) [17]. Long‐term blood glucose control should be monitored with periodic hemoglobin A1C testing. For patients with T2DM or those with T1DM for over 5 years, annual screening for albuminuria should be performed.
Management
In addition to endocrinology referral and the aforementioned medication regimen, pembrolizumab was held for our patient at the time of diagnosis of immune‐mediated DM. The ASCO Clinical Practice Guidelines recommend holding ICIs until glucose control is achieved for patients with grade 3 or higher ICI‐mediated hyperglycemia (defined as fasting glucose >250 mg/dL) and severe symptoms with medically significant or life‐threatening consequences. For patients without any evidence of ketosis or serologic evidence of T1DM, ICIs may be continued along with initiating appropriate therapy for diabetes [15].
Endocrinology referral and close follow up should be considered for all patients with endocrine irAEs. For patients with ICI‐induced diabetes with positive antibodies and low or decreasing C‐peptide levels, insulin is the mainstay of treatment. Patients with T1DM usually require lower doses of insulin because the primary defect is insulin production, and insulin sensitivity is usually preserved. In patients who have features of insulin resistance such as obesity or acanthosis nigricans, other antidiabetic agents that improve insulin sensitivity or may cause weight loss will be of benefit. These agents include metformin, GLP‐1 agonists, SGLT‐2 inhibitors, and the glitazones. Annual eye and foot examinations should also be performed to monitor for diabetic retinopathy and peripheral neurovascular complications of DM.
Patient Update
This patient continues treatment with maintenance pemetrexed plus pembrolizumab without any additional toxicity. His last hemoglobin A1C was 8 on the regimen mentioned before.
Disclosures
Nathan Pennell: Merck, Astrazeneca, Bristol‐Myers Squibb, Cota, and Eli Lilly & Co. (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Polsky S, Ellis SL. Obesity, insulin resistance, and type 1 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes 2015;22:277–282. [DOI] [PubMed] [Google Scholar]
- 2. Scott RA, Langenberg C, Sharp SJ et al; InterAct Consortium. The link between family history and risk of type 2 diabetes is not explained by anthropometric, lifestyle or genetic risk factors: The EPIC‐InterAct study. Diabetologia 2013;56:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iyer P, Best C, Lavis V. Checkpoint inhibitor mediated insulin dependent diabetes: A cancer center experience. Endocrine Rev 2018;39a. [Google Scholar]
- 4. Sznol M, Postow MA, Davies MJ et al. Endocrine‐related adverse events associated with immune checkpoint blockade and expert insights on their management. Cancer Treat Rev 2017;58:70–76. [DOI] [PubMed] [Google Scholar]
- 5. Chae YK, Chiec L, Mohindra N et al. A case of pembrolizumab‐induced type‐1 diabetes mellitus and discussion of immune checkpoint inhibitor‐induced type 1 diabetes. Cancer Immunol Immunother 2017;66:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaudy C, Clévy C, Monestier S et al. Anti‐PD1 pembrolizumab can induce exceptional fulminant type 1 diabetes. Diabetes Care 2015;38:e182–e183. [DOI] [PubMed] [Google Scholar]
- 7. Hughes J, Vudattu N, Sznol M et al. Precipitation of autoimmune diabetes with anti‐PD‐1 immunotherapy. Diabetes Care 2015;38:e55–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kotwal A, Haddox C, Block M et al. Immune checkpoint inhibitors: An emerging cause of insulin‐dependent diabetes. BMJ Open Diabetes Res Care 2019;7:e000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hofmann L, Forschner A, Loquai C et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side‐effects of anti‐PD‐1 therapy. Eur J Cancer 2016;60:190–209. [DOI] [PubMed] [Google Scholar]
- 10. Okamoto M, Okamoto M, Gotoh K et al. Fulminant type 1 diabetes mellitus with anti‐programmed cell death‐1 therapy. J Diabetes Investig 2016;7:915–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chiang JL, Kirkman MS, Laffel LMB et al. Type 1 diabetes through the life span: A position statement of the American Diabetes Association. Diabetes Care 2014;37:2034–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naik RG, Brooks‐Worrell BM, Palmer JP. Latent autoimmune diabetes in adults. J Clin Endocrinol Metab 2009;94:4635–4644. [DOI] [PubMed] [Google Scholar]
- 13. Kochupurakkal NM, Kruger AJ, Tripathi S et al. Blockade of the programmed death‐1 (PD1) pathway undermines potent genetic protection from type 1 diabetes. PLoS One 2014;9:e89561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Granados HM, Draghi A 2nd, Tsurutani N et al. Programmed cell death‐1, PD‐1, is dysregulated in T cells from children with new onset type 1 diabetes. PLoS One 2017;12:e0183887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brahmer JR, Lacchetti C, Schneider BJ et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Puzanov I, Diab A, Abdallah K et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wright LAC, Hirsch IB. The challenge of the use of glycemic biomarkers in diabetes: Reflecting on hemoglobin A1C, 1,5‐anhydroglucitol, and the glycated proteins fructosamine and glycated albumin. Diabetes Spectrum 2012;25:141–148. [Google Scholar]


