Abstract
Background
Companion diagnostic (CDx) testing for patients with advanced non‐small cell lung cancer (aNSCLC) identifies patients more likely to benefit from biomarker‐driven treatments.
Methods
Patients with nonsquamous cell (non‐Sq) aNSCLC from the Flatiron Health database (diagnosed January 1, 2011–May 31, 2018) who had CDx testing were compared with those who had no reported evidence of testing. The association between CDx testing and overall survival was evaluated by unadjusted and adjusted Cox proportional hazards regression models. Logistic regression analysis identified characteristics associated with CDx testing. The revised modified Lung Cancer Prognostic Index and other factors identified a priori were included in the adjusted models.
Results
A total of 17,555 patients with non‐Sq aNSCLC (CDx, n = 14,732; no CDx, n = 2,823) with mean ± SD age of 67.2 ± 10.0 years were included. Most were insured (91.7%) and white (67.1%). Asian patients and those who were never‐smokers were more likely to undergo CDx testing. Those with CDx testing lived longer than those without (median [95% confidence interval (CI)] survival, 13.04 [12.62–13.40] vs. 6.01 [5.72–6.24] months) and had a decreased mortality risk (adjusted hazard ratio [95% CI], 0.72 [0.69–0.76]). A survival advantage was also seen for patients with CDx testing who received biomarker‐driven first‐line therapy.
Conclusion
Patients with non‐Sq aNSCLC who had CDx testing had a greater survival benefit than those without, supporting broader use of CDx testing in routine clinical practice to identify patients more likely to benefit from precision medicine.
Implications for Practice
Companion diagnostic (CDx) testing coupled with biomarker‐driven treatment offers a greater survival benefit for patients with advanced non‐small cell lung cancer (aNSCLC). In this study, patients with nonsquamous aNSCLC from Flatiron Health, a large, real‐world oncology database, with CDx testing had a reduced mortality risk and lived longer than patients without reported evidence of CDx testing; those who received biomarker‐driven therapy as their first line of treatment were likely to survive three times longer than those who did not. These results demonstrate the clinical utility of CDx testing as the first step in treating nonsquamous aNSCLC in real‐world clinical practice.
Keywords: Real‐world, Survival, Mortality, Advanced non‐small cell lung cancer, Companion diagnostic testing
Short abstract
Improved diagnostic and treatment approaches are needed for patients with non‐small cell lung cancer. This article evaluates the testing patterns and outcomes associated with overall companion diagnostic testing in real‐world clinical practice.
Introduction
A tremendous need exists for improved diagnostic and treatment approaches for patients with non‐small cell lung cancer (NSCLC), which has an estimated 5‐year survival rate of 22.7% for all stages and 5.5% among patients with advanced disease [1], including distant metastases to the contralateral lymph nodes (i.e., stage IIIB) or to other parts of the body (stage IV) [2, 3, 4]. Lung cancer is the most commonly diagnosed malignancy and the leading cause of cancer‐related deaths in the world [5]. In the U.S. alone, an estimated 228,150 new cases of lung cancer will be diagnosed in 2019, resulting in an estimated 142,670 deaths, which is more than the number of deaths resulting from breast, prostate, colorectal, and bladder cancers combined [6].
Traditional oncology treatment paradigms typically apply the same therapeutic regimen to patients with the same disease [7]. In contrast, a precision medicine approach involves having a patient undergo companion diagnostic (CDx) testing to identify specific molecular targets, which can aid in selecting biomarker‐driven treatments, such as targeted therapy and cancer immunotherapy, that have a higher likelihood of success [8] while avoiding those that have an increased toxicity risk or lack efficacy [7]. A predominant subtype of NSCLC is adenocarcinoma, with a molecular abnormality, such as ALK receptor tyrosine kinase (ALK), B‐Raf proto‐oncogene, serine/threonine kinase (BRAF), epidermal growth factor receptor (EGFR), KRAS proto‐oncogene, GTPase (KRAS), MET proto‐oncogene, receptor tyrosine kinase (MET), and ROS proto‐oncogene 1, receptor tyrosine kinase (ROS1), being present in approximately 50% of metastatic NSCLC tumors [9, 10]. For some molecular abnormalities, a biomarker‐driven therapy is available and could be used [9, 10], and for programmed cell death 1 ligand 1 (PD‐L1) expression, immunotherapy may be a recommended treatment approach [11]. The National Comprehensive Cancer Network and the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology guidelines recommend molecular testing [12, 13], and previous retrospective analyses have demonstrated that a precision medicine approach may help improve the overall survival rate in patients with advanced cancer [14, 15]. However, more clinical utility evidence assessing the use of various CDx testing is needed to support payer reimbursement, especially in community settings where 85% of patients with cancer are treated [9, 14]. The objective of this study was to evaluate the testing patterns and outcomes associated with overall CDx testing in real‐world clinical practice.
Materials and Methods
Patients and Follow‐Up
We evaluated the clinical value of CDx testing in the real world using the Flatiron Health database [16], a nationwide, longitudinal, demographically and geographically diverse database derived from deidentified electronic health record (EHR) data from approximately 2.2 million patients with cancer undergoing treatment at more than 280 oncology clinics in the U.S. The Flatiron Health database consists of predominantly community‐based clinics (although academic centers are actively being integrated) and is updated monthly, allowing for patients to be followed up over several years from the initial cancer diagnosis. In our study, CDx testing included the first CDx after advanced diagnosis.
Prior to study conduct, institutional review board approval of the study protocol was obtained and included a waiver of informed consent from the Copernicus Group. This retrospective analysis included patients from the Flatiron Health database who were diagnosed with advanced (stage IIIB or IV) NSCLC (aNSCLC) between January 1, 2011, and May 31, 2018 (n = 48,857). The date of advanced diagnosis was deemed the same date of initial diagnosis if the patient was initially identified as having advanced and/or metastatic disease. Otherwise, the date of first recurrence or progressive disease determined the advanced and/or metastatic date using the following hierarchy: pathology report biopsy specimen collection date, physician‐reported biopsy date, radiology scan date with physician confirmation of recurrence or progressive disease, physician‐reported advanced diagnosis date, or definitive surgery date of recurrence or progressive disease site.
Of 48,857 patients with aNSCLC in the Flatiron Health database, a total of 31,302 were excluded from the analysis for the following reasons: <18 years of age (n = 1) or histology other than nonsquamous (non‐Sq) cell (n = 15,114); no visit activity within 120 days of diagnosis or the start of the first line of treatment was >120 days (n = 2,891); invalid CDx test results (n = 560) or CDx test was performed prior to diagnosis (n = 2,124); exclusion was treatment related (n = 10,416); biomarker‐driven treatment was received before diagnosis (n = 116); or, for those who died, the date of death was on or before the start of the first line of treatment (n = 80; Fig. 1). A total of 17,555 eligible patients with non‐Sq aNSCLC were classified into one of two groups: patients who had any actionable CDx test for one of four driver mutations (ALK, BRAF, EGFR, or ROS1 positive or negative) and/or PD‐L1 expression (high, low, or negative) captured by Flatiron (CDx group; n = 14,732) and those who did not have reported evidence of testing, including those with no CDx test or testing status unknown (no‐CDx group; n = 2,823). Comorbid conditions in eligible patients were diagnosed based on the International Classification of Diseases, Ninth and Tenth Revisions criteria [17, 18]. The index date was identified as the start of the first line of treatment, defined as the first eligible administration of a therapy which could potentially begin 14 days prior to the aNSCLC diagnosis date. The end of follow‐up was either defined as the date of death or, for all others, a 90‐day activity window prior to the Flatiron data cutoff was implemented: if there was a visit within the activity window (e.g., laboratory tests, treatment, vitals), the end of follow‐up was the Flatiron data cutoff (May 31, 2018); otherwise, the end of follow‐up was the last visit date. Study design details are shown in Figure 2.
Figure 1.
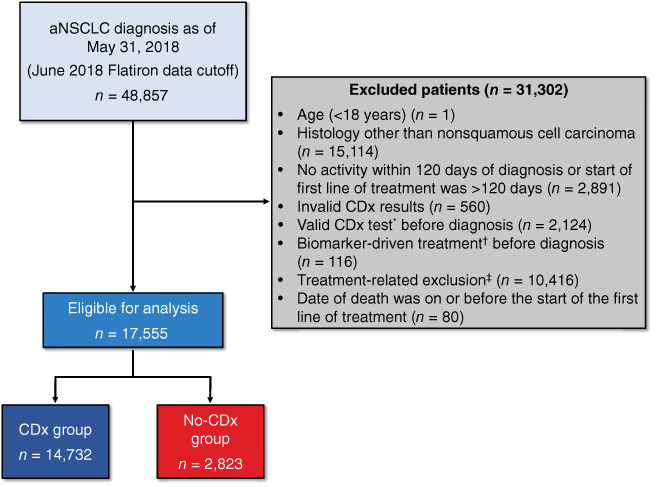
Study attrition. *, Epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), ROS proto‐oncogene 1 (ROS1), B‐Raf proto‐oncogene, serine/threonine kinase (BRAF), programmed cell death 1 ligand 1 (PD‐L1) positive or negative, or PD‐L1 expression high, low, or negative. †, Biomarker‐driven treatment included the following: Gilotrif (afatinib; Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT), Iressa (gefitinib; AstraZeneca Pharmaceuticals LP, Wilmington, DE), Tarceva (erlotinib; distributed by Genentech, Inc., A Member of the Roche Group, South San Francisco, CA), Tagrisso (osimertinib; AstraZeneca Pharmaceuticals LP, Wilmington, DE), Alecensa (alectinib; distributed by Genentech, Inc., A Member of the Roche Group, South San Francisco, CA), Xalkori (crizotinib; Pfizer Labs, Division of Pfizer Inc, New York, NY), Zykadia (ceritinib; Novartis Pharmaceuticals Corporation, East Hanover, NJ), Alunbrig (brigatinib; Takeda Pharmaceutical Company Limited, Cambridge, MA), Keytruda (pembrolizumab; Merck Sharp & Dohme Corp, a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ), Tafinlar (dabrafenib; Novartis Pharmaceuticals Corporation, East Hanover, NJ) in combination with Mekinist (trametinib; Novartis Pharmaceuticals Corporation, East Hanover, NJ), Zelboraf (vemurafenib; distributed by Genentech, Inc., A Member of the Roche Group, South San Francisco, CA), Opdivo (nivolumab; Bristol‐Myers Squibb Company, Princeton, NJ), Tecentriq (atezolizumab; Genentech, Inc., A Member of the Roche Group, South San Francisco, CA), Imfinzi (durvalumab; AstraZeneca Pharmaceuticals LP, Wilmington, DE), or Bavencio (avelumab; EMD Serono, Inc., Rockland, MA). ‡, Treatment‐related exclusions included the following: no evidence of treatment after aNSCLC diagnosis (n = 9,512), received treatment with HER2 inhibitors in any line of therapy at any time (n = 22), received any biomarker‐driven treatment in any line of therapy among patients without CDx testing (n = 882), or received biomarker‐driven treatment before the first CDx test among patients with CDx testing (n = 0). Abbreviations: aNSCLC, advanced non‐small cell lung cancer; CDx, companion diagnostic.
Figure 2.
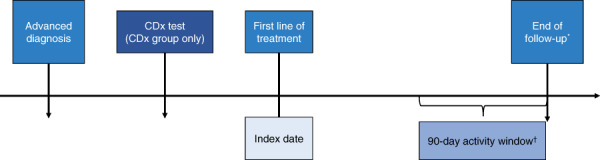
Study design. *, For deaths, the end of follow‐up was the date of death. †, For all others, a 90‐day activity window prior to the Flatiron data cutoff was implemented: if there was a visit (e.g., laboratory tests, treatment, vitals) within the activity window, the end of follow‐up was the Flatiron data cutoff; otherwise, the end of follow‐up was the last visit date. Abbreviation: CDx, companion diagnostic.
Study Objectives
The primary objective of this study was to evaluate overall survival and risk of mortality in patients with non‐Sq aNSCLC who had CDx testing versus those who did not; the secondary objective was to identify factors associated with a greater likelihood of CDx testing use. The overall survival probability for patients in the CDx group who received biomarker‐driven therapy, such as targeted therapy and cancer immunotherapy, as the first line of treatment versus those in the no‐CDx group and for patients who had their first CDx prior to the first line of treatment were also assessed in subgroup analyses.
Statistical Analysis
Demographic and clinical characteristics were summarized overall as well as in both patient groups using descriptive statistics, and between‐group comparisons were conducted using χ2 and independent t tests. Overall survival, determined using mortality as a validated endpoint in the Flatiron database [19], was evaluated using Kaplan‐Meier analysis. Patients in both arms were followed up from the start of the first line of treatment (index date) until death or the last activity date or study end, whichever occurred first. In the CDx arm, the first line of treatment included biomarker‐driven and nontargeted therapy (e.g., chemotherapy, radiation, and immunotherapy); in the no‐CDx arm, the first line of treatment included only nontargeted therapy. The use of immunotherapy was not driven by PD‐L1 status. Right censoring criteria were used such that patients who had activity within 90 days of the May 31, 2018, data cutoff were censored on that date, whereas patients who had their last activity prior to this window were censored at the last activity date. The Kaplan‐Meier analysis also evaluated overall survival in the following subgroups: (a) patients in the CDx group who received biomarker‐driven therapy as the first line of treatment versus those in the no‐CDx group and (b) for patients who had their first CDx prior to the first line of treatment versus those with no CDx test.
An adjusted logistic regression analysis was performed to identify characteristics associated with undergoing CDx testing using all patients treated in a community practice setting (n = 16,501; Fig. 3). An adjusted Cox regression survival analysis was conducted using the entire cohort (n = 17,555) to determine factors associated with increased risk of all‐cause mortality. A bivariate regression analysis was conducted prior to the multivariate Cox regression analysis to determine the effects of each factor associated with the outcome variable. Unadjusted and adjusted Cox proportional hazards models were used to determine the effect of confounders on the outcome variable. Statistically significant factors and ratios with a ≥10% change were included in the multivariate model as potential confounders.
Figure 3.
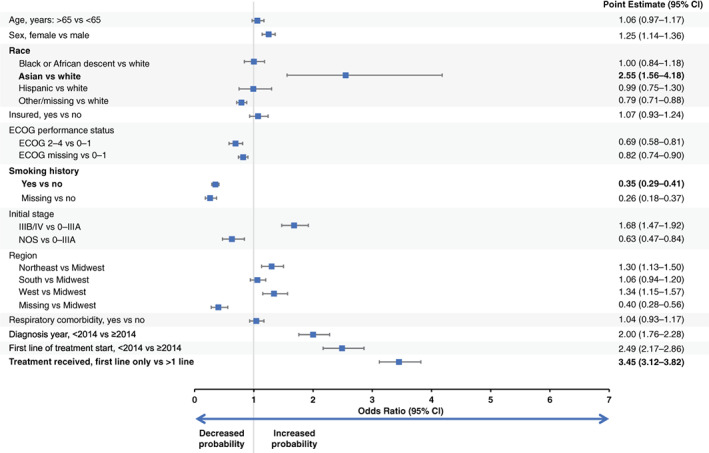
Likelihood of undergoing companion diagnostic testing among patients with nonsquamous advanced non‐small cell lung cancer treated in a community practice setting. All variables were mutually adjusted for each other. Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; NOS, not otherwise specified.
Factors included a priori were based on a revised version of the modified Lung Cancer Prognostic Index (LCPI) [20], a validated tool that uses readily available data in routine practice to standardize baseline risk for patients with NSCLC. The original LCPI included cancer stage, histology, mutation status, performance status, weight loss, smoking history, respiratory comorbidity, sex, and age as prognostic factors [20]. A modified version (m‐LCPI) was developed that excludes performance status (not often collected in routine clinical practice) and respiratory comorbidity (owing to the lack of consistency with which these are reported) [20]. The analysis reported here includes a revised m‐LCPI that excludes weight loss (not consistently recorded in clinical practice) and performance status (not often collected in routine clinical practice and because of the large proportion of patients with missing Eastern Cooperative Oncology Group [ECOG] scores) from the analysis while including the following factors: cancer stage, histology, smoking history, respiratory comorbidity, sex, age, and mutation status [21]. Using the revised m‐LCPI, patients were identified as having “no proven actionable mutation” based on EGFR/ALK/ROS1–negative mutation status, whereas the LCPI was developed prior to the availability of targeted therapies for ROS1 and therefore does not include this mutation. PD‐L1 was not included in either the LCPI or m‐LCPI because it is a predictive biomarker, whereas the rest are prognostic factors, but it was included as a confounder. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Baseline Characteristics of the Patient Population
A total of 17,555 patients with non‐Sq aNSCLC (CDx, n = 14,732 [83.9%]; no CDx, n = 2,823 [16.1%]) and a mean ± SD age at diagnosis of 67.2 ± 10.0 years were included in the analysis (Table 1). Overall, patients were mostly white (67.1%) and had insurance (91.7%). Significant between‐group differences (p < .01) were observed in all baseline demographic, disease, and clinical characteristics, including m‐LCPI components and index scores (Table 1). For example, in the CDx group, a lower proportion of men were tested (48.6% vs. 56.1% in the no‐CDx group), a greater proportion of patients had insurance (92.7% vs. 86.5% in the no‐CDx group), and there was a larger proportion of never‐smokers (18.1% vs. 5.7% in the no‐CDx group).
Table 1.
Patient baseline characteristics
| Characteristic | All Patients With non‐Sq aNSCLC (n = 17,555) | Patients with CDx testing (n = 14,732) | Patients without CDx Testinga (n = 2,823) | p value b | |
|---|---|---|---|---|---|
| Baseline demographics | |||||
| Age, mean (SD), yr | 67.2 (10.0) | 67.1 (10.2) | 67.4 (9.2) | ||
| Sex, n (%) | <.01 | ||||
| Male | 8,740 (49.8) | 7,157 (48.6) | 1,583 (56.1) | ||
| Female | 8,815 (50.2) | 7,575 (51.4) | 1,240 (43.9) | ||
| Race or ethnicity, n (%) | <.01 | ||||
| White | 11,780 (67.1) | 9,937 (67.5) | 1,843 (65.3) | ||
| African descent | 1,403 (8.0) | 1,178 (8.0) | 225 (8.0) | ||
| Asian | 494 (2.8) | 476 (3.2) | 18 (0.6) | ||
| Hispanic or Latino | 640 (3.6) | 549 (3.7) | 91 (3.2) | ||
| Other | 1,213 (6.9) | 1,030 (7.0) | 183 (6.5) | ||
| Unknown | 2,025 (11.5) | 1,562 (10.6) | 463 (16.4) | ||
| Insurance, n (%) | <.01 | ||||
| Yes | 16,094 (91.7) | 13,653 (92.7) | 2,441 (86.5) | ||
| No | 1,461 (8.3) | 1,079 (7.3) | 382 (13.5) | ||
| Baseline disease and clinical characteristics | |||||
| Death, n (%) | <.01 | ||||
| Yes | 11,656 (66.4) | 9,371 (63.6) | 2,285 (80.9) | ||
| No | 5,899 (33.6) | 5,361 (36.4) | 538 (19.1) | ||
| Year of advanced diagnosis, n (%) | <.01 | ||||
| 2011–2013 | 5,375 (30.6) | 3,818 (25.9) | 1,557 (55.2) | ||
| 2014–2018 | 12,180 (69.4) | 10,914 (74.1) | 1,266 (44.8) | ||
| Smoking history, n (%) | <.01 | ||||
| No history of smoking | 2,824 (16.1) | 2,664 (18.1) | 160 (5.7) | ||
| History of smoking | 14,539 (82.8) | 11,944 (81.1) | 2,595 (91.9) | ||
| Missing | 192 (1.1) | 124 (0.8) | 68 (2.4) | ||
| ECOG performance status at diagnosis, n (%) | <.01 | ||||
| 0–1 | 6,226 (35.5) | 5,447 (37.0) | 779 (27.6) | ||
| 2–4 | 1,338 (7.6) | 1,089 (7.4) | 249 (8.8) | ||
| Missing | 9,991 (56.9) | 8,196 (55.6) | 1,795 (63.6) | ||
| Stage at initial NSCLC diagnosis, n (%) c | |||||
| Early stage | 1,756 (10.0) | 1,345 (9.1) | 411 (14.6) | <.01 | |
| Advanced stage | 15,476 (88.2) | 13,181 (89.5) | 2,295 (81.3) | ||
| Missing | 323 (1.8) | 206 (1.4) | 117 (4.1) | ||
| Year treatment initiated and line of therapy | |||||
| Year first line of treatment began, n (%) d | <.01 | ||||
| 2011–2014 | 7,807 (44.5) | 5,803 (39.4) | 2,004 (71.0) | ||
| 2015–2018 | 9,748 (55.5) | 8,929 (60.6) | 819 (29.0) | ||
| Patients receiving any therapy, n (%) | <.01 | ||||
| 1 line (first line) only | 9,571 (54.5) | 7,451 (50.6) | 2,120 (75.1) | ||
| ≥1 line | 7,984 (45.5) | 7,281 (49.4) | 703 (24.9) | ||
| Patients receiving biomarker‐driven therapy, n (%) e | – | ||||
| Total | 7,784 (44.3) | 7,784 (52.8) | 0 | ||
| 1 line only | 3,016 (17.2) | 3,016 (20.5) | 0 | ||
| ≥1 line | 4,768 (27.2) | 4,768 (32.4) | 0 | ||
| None | 9,771 (55.7) | 6,948 (47.2) | 2,823 (100) | ||
| First CDx testing in relation to start of first line of treatment, n (%) | – | ||||
| Before or at start of first line of treatment | 11,183 (63.7) | 11,183 (75.9) | 0 | ||
| After start of first line of treatment | 3,549 (20.2) | 3,549 (24.1) | 0 | ||
| First CDx test result date missing | 2,823 (16.1) | 0 | 2,823 (100) | ||
| m‐LCPI patient components and groups | |||||
| Stage at initial diagnosis, n (%) f | LCPI points | <.01 | |||
| I | 0 | 858 (4.9) | 672 (4.6) | 186 (6.6) | |
| II | 2 | 408 (2.3) | 325 (2.2) | 83 (2.9) | |
| IIIA | 4 | 490 (2.8) | 348 (2.4) | 142 (5.0) | |
| IIIB | 6 | 1,784 (10.2) | 1,349 (9.2) | 435 (15.4) | |
| IV | 8 | 13,692 (78.0) | 11,832 (80.3) | 1,860 (65.9) | |
| NSCLC NOS | 3 | 323 (1.8) | 206 (1.4) | 117 (4.1) | |
| No proven actionable mutation, n (%) g | 3 | 13,378 (76.2) | 10,555 (71.6) | 2,823 (100) | <.01 |
| Ever smoker, n (%) | 2 | 14,539 (82.8) | 11,944 (81.1) | 2,595 (91.9) | <.01 |
| Respiratory comorbidity, n (%) | 2 | 4,413 (25.1) | 3,836 (26.0) | 577 (20.4) | <.01 |
| Male, n (%) | 1 | 8,740 (49.8) | 7,157 (48.6) | 1,583 (56.1) | <.01 |
| Age group, years, n (%) | <.01 | ||||
| ≤ 50 | 0 | 962 (5.5) | 846 (5.7) | 116 (4.1) | |
| > 50–≤70 | 1 | 9,442 (53.8) | 7,910 (53.7) | 1,532 (54.3) | |
| > 70–≤90 | 2 | 7,151 (40.7) | 5,976 (40.6) | 1,175 (41.6) | |
| > 90 | 3 | 0 | 0 | 0 | |
| m‐LCPI group | <.01 | ||||
| 1 | ≤8 | 1,162 (6.6) | 979 (6.6) | 183 (6.5) | |
| 2 | 9–11 | 2,852 (16.2) | 2,562 (17.4) | 290 (10.3) | |
| 3 | 12–14 | 6,068 (34.6) | 5,109 (34.7) | 959 (34.0) | |
| 4 | ≥15 | 7,473 (42.6) | 6,082 (41.3) | 1,391 (49.3) | |
No reported evidence of testing.
The p value applies to the comparison between patients with CDx testing, defined as the first actionable CDx test for one of four driver mutations (ALK, BRAF, EGFR, or ROS1 positive or negative) and/or PD‐L1 expression (high, low, or negative) and included subsequent CDx tests within 30 days of the first test, versus those with no CDx testing.
Early stage includes occult, 0, I, IIA, IIB, and IIIA; advanced stage includes IIIB and IV; missing includes missing and III (A/B not indicated).
As per Flatiron line of therapy business rules, the first line of treatment could potentially begin 14 days prior to aNSCLC diagnosis date; therefore, a patient diagnosed within the first 2 weeks of January 2011 could have started their first line of treatment in December 2010.
Biomarker‐driven therapy included the following: Gilotrif (afatinib; Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT), Iressa (gefitinib; AstraZeneca Pharmaceuticals LP, Wilmington, DE), Tarceva (erlotinib; distributed by Genentech, Inc., A Member of the Roche Group, South San Francisco, CA), Tagrisso (osimertinib; AstraZeneca Pharmaceuticals LP, Wilmington, DE), Alecensa (alectinib; distributed by Genentech, Inc., A Member of the Roche Group, South San Francisco, CA), Xalkori (crizotinib; Pfizer Labs, Division of Pfizer Inc, New York, NY), Zykadia (ceritinib; Novartis Pharmaceuticals Corporation, East Hanover, NJ), Alunbrig (brigatinib; Takeda Pharmaceutical Company Limited, Cambridge, MA), Keytruda (pembrolizumab; Merck Sharp & Dohme Corp, a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ), Tafinlar (dabrafenib; Novartis Pharmaceuticals Corporation, East Hanover, NJ) in combination with Mekinist (trametinib; Novartis Pharmaceuticals Corporation, East Hanover, NJ), Zelboraf (vemurafenib; distributed by Genentech, Inc., A Member of the Roche Group, South San Francisco, CA), Opdivo (nivolumab; Bristol‐Myers Squibb Company, Princeton, NJ), Tecentriq (atezolizumab; Genentech, Inc., A Member of the Roche Group, South San Francisco, CA), Imfinzi (durvalumab; AstraZeneca Pharmaceuticals LP, Wilmington, DE), or Bavencio (avelumab; EMD Serono, Inc., Rockland, MA).
Stage I includes occult, 0, I, IA, and IB; stage II includes II, IIA, and IIB.
Includes EGFR/ALK/ROS1 negative; includes PD‐L1 expression–based results low (1%–49% staining) or negative (<1%) as per National Comprehensive Cancer Network guidelines for treatment or biomarker status negative; includes no mutation tested (e.g., mutation not tested, test information missing).
Abbreviations: CDx, companion diagnostic; ECOG, Eastern Cooperative Oncology Group; m‐LCPI, modified Lung Cancer Prognostic Index; non‐Sq aNSCLC, nonsquamous advanced non‐small cell lung cancer; NOS, not otherwise specified.
In this study, a higher proportion (62.2%) of patients with non‐Sq aNSCLC received their first CDx testing in 2014–2018, compared with 21.7% of patients in 2011–2013; a similar trend was observed for PD‐L1–only testing (29.4% in 2014–2018 vs. 1.0% in 2011–2013). Most (53.6%) received a first CDx test for ALK, BRAF, EGFR, or ROS1 mutations (without PD‐L1), whereas only 0.9% received PD‐L1 only and 29.4% had a PD‐L1 test in addition to the other biomarkers.
Overall, 30% of patients had positive test results for any of the biomarkers, including PD‐L1, and 9.1% were positive for PD‐L1 only. Patient‐level testing patterns demonstrated that 93.4% were tested for EGFR (n = 13,767), 86.0% for ALK (n = 12,671), 43.5% for ROS1 (n = 6,410), 28.4% for PD‐L1 (n = 4,182), and 25.5% for BRAF (3,757). Testing for any biomarker occurred within 30 days from the aNSCLC diagnosis date for 72.2% of patients; 35.6% were tested more than 30 days from diagnosis. The median time from diagnosis to the first biomarker test was 19.0 (interquartile range, 10.5–33.0) days.
The median (interquartile range) time to start of first line of treatment following the advanced diagnosis was 33 (21–49) days. A greater proportion of patients in the CDx group (49.4%) received more than one line of therapy than in the no‐CDx group (24.9%); approximately 75.9% of patients in the CDx group had received their CDx test result before or at the start of the first line of treatment (Table 1).
Factors Associated with Increased Likelihood of Having CDx Testing
Factors associated with an increased probability of CDx testing are presented in Figure 3 for patients with non‐Sq aNSCLC treated in a community practice setting (n = 16,501). Of interest, female patients, those with first line of treatment in 2014 or later, and those who received more than one line of therapy were more likely to have CDx testing (point estimate [95% confidence interval (CI)], 1.25 [1.14–1.36], 2.49 [2.17–2.86], and 3.45 [3.12–3.82], respectively). In contrast, smokers were less likely to undergo CDx testing, with a point estimate (95% CI) of 0.35 (0.29–0.41). Although the number of patients was small (n = 473 [2.9%]), Asian patients were more likely to undergo CDx testing than white patients (point estimate [95% CI], 2.55 [1.56–4.18]). Regional differences were also observed: patients treated in the Northeast or West were more likely to undergo CDx testing than those treated in the Midwest (point estimate [95% CI], 1.30 [1.13–1.50] and 1.34 [1.15–1.57], respectively).
Patients with CDx Testing Lived Longer and Had a Decreased Mortality Risk
Among all patients with non‐Sq aNSCLC in our analysis (n = 17,555), patients with CDx testing had a longer median survival than those without testing (median overall survival [95% CI], 13.04 [12.62–13.40] months vs. 6.01 [5.72–6.24] months; Fig. 4).
Figure 4.
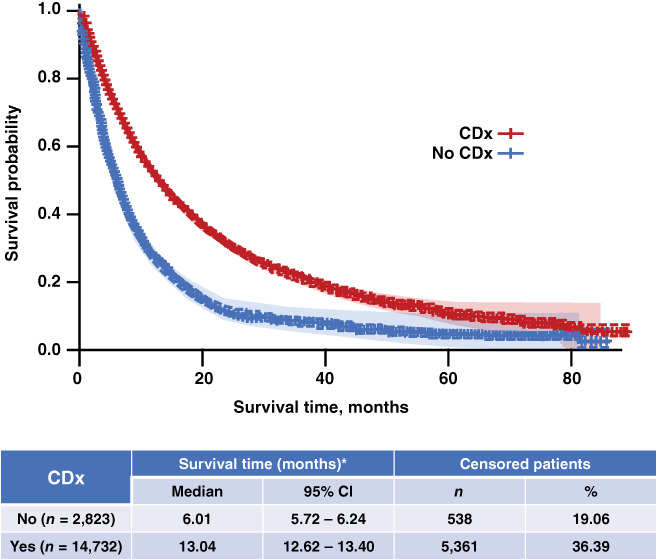
Overall survival in patients with nonsquamous advanced non‐small cell lung cancer with and without CDx testing. *, Survival time in months was calculated from the index date to the end of follow‐up. For deaths, the end of follow‐up was the date of death. For all others, a 90‐day activity window prior to the Flatiron data cutoff was implemented: if there was a visit (e.g., laboratory tests, treatment, vitals) within the activity window, the end of follow‐up was the Flatiron data cutoff; otherwise, the end of follow‐up was the last visit date. Abbreviations: CDx, companion diagnostic; CI, confidence interval.
Patients with CDx testing had a decreased mortality risk, with an unadjusted hazard ratio (HR) of 0.54 (95% CI, 0.52–0.57; Fig. 5). The significant reduction in mortality associated with CDx testing remained after adjusting for different combinations of factors in three adjusted models (Fig. 5): age at diagnosis, sex, stage at initial non‐Sq NSCLC diagnosis, and smoking history (adjusted HR [95% CI], 0.57 [0.54–0.59]); age at diagnosis, sex, stage at initial diagnosis, smoking history, respiratory comorbidities, and line of therapy (adjusted HR [95% CI], 0.71 [0.68–0.75]); and m‐LCPI by index groups (including mutation status), PD‐L1 expression levels, and line of therapy (adjusted HR [95% CI], 0.72 [0.69–0.76]).
Figure 5.
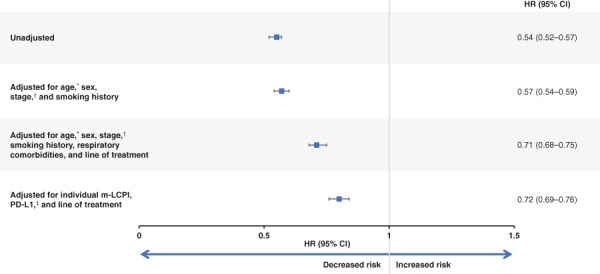
Multivariable hazard ratios for all‐cause mortality in patients with nonsquamous advanced non‐small cell lung cancer (aNSCLC) with and without companion diagnostic testing. *, Age at aNSCLC diagnosis (<65/≥65 years). †, Early stage includes occult, 0, I, IIA, IIB, and IIIA; advanced stage includes IIIB and IV; missing includes missing and III (A/B not indicated). ‡, PD‐L1 expression–based results incorporate only percent staining and expression‐level variables, with percent staining results given priority over expression‐level results. They are categorized as follows: high = percent staining >50% or expression‐level high; low = percent staining of 1%–49%, expression‐level moderate, and/or expression‐level low; negative = percent staining <1%; missing = percent staining and expression‐level missing. Abbreviations: CI, confidence interval; HR, hazard ratio; m‐LCPI, modified Lung Cancer Prognostic Index; PD‐L1, programmed cell death 1 ligand 1.
Finally, in the subpopulation analyses, patients who had CDx testing and biomarker‐driven therapy as the first line of treatment had a much greater survival benefit than those who did not undergo CDx testing, with a median (95% CI) survival of 18.00 (16.66–19.12) versus 6.01 (5.72–6.24) months, respectively (Fig. 6). Patients who had their first CDx on or prior to the first line of treatment also had a longer median overall survival than those not tested (median [95% CI], 12.78 [12.32–13.14] vs. 6.01 [5.72–6.24] months).
Figure 6.
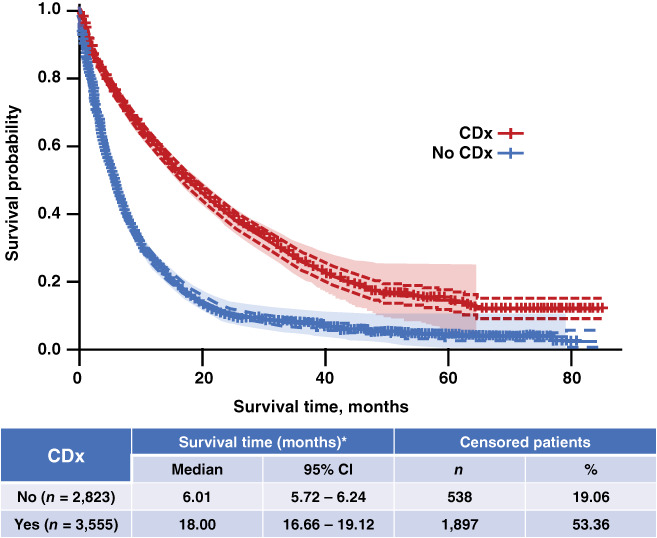
Overall survival in patients with nonsquamous advanced non‐small cell lung cancer in the CDx group who received biomarker‐driven therapy as the first line of treatment versus those without CDx testing. *, Survival time in months was calculated from the index date to the end of follow‐up. For deaths, the end of follow‐up was the date of death. For all others, a 90‐day activity window prior to the Flatiron data cutoff was implemented: if there was a visit (e.g., laboratory tests, treatment, vitals) within the activity window, the end of follow‐up was the Flatiron data cutoff; otherwise, the end of follow‐up was the last visit date. Abbreviations: CDx, companion diagnostic; CI, confidence interval.
Discussion
Despite level 1 evidence supporting the benefits of biomarker‐driven therapeutic approaches and consensus among national and international guidelines for the routine use of CDx testing in aNSCLC, it is not clear whether this evidence has translated to practice in real‐world settings; in our study, only 44% of adult patients in the Flatiron Health database with a diagnosis of non‐Sq aNSCLC as of May 31, 2018, were tested (n = 14,732 patients in the CDx group and 33,742 adult patients with non‐Sq aNSCLC diagnosis). To our knowledge, this is the largest real‐world study that used Flatiron Health EHR data collected as part of routine clinical practice to evaluate patterns of and outcomes with CDx testing. Our findings showed that patients with CDx testing had a reduced mortality risk compared with those not tested. Furthermore, those who had CDx testing and biomarker‐driven therapy as the first line of treatment experienced a 3‐fold greater survival benefit than those not tested. Our results reaffirm various studies reporting the benefits of identifying individual biomarkers and associated treatment [14, 22, 23], demonstrating that CDx testing should be an integral component of the diagnostic and treatment paradigm for all patients with non‐Sq aNSCLC.
In this analysis, patients with non‐Sq aNSCLC from the Flatiron Health database who underwent CDx testing had significant improvements in overall survival compared with those who did not undergo testing: findings that are consistent with a number of other trials in patients with various forms of cancer [14, 22, 23]. In our study, the significant benefits associated with undergoing CDx testing remained after adjusting for multiple factors, including age at diagnosis, sex, stage at diagnosis, smoking history, respiratory comorbidities, line of therapy, m‐LCPI group (which considers actionable mutations), and PD‐L1 expression (Fig. 5). Furthermore, validated prognostic tools play an important role in identifying baseline risk factors in a standardized manner that could affect treatment response and patient outcomes, further guiding treatment decisions. We used a revised version of the m‐LCPI, which is a simple, standardized tool with real‐world relevance to support clinical decision making.
Our study also identified several factors associated with increased CDx testing, including female sex, nonsmoker status, stage at diagnosis, diagnosis received after 2014, and Asian race. These findings are consistent with previous research demonstrating that testing may be driven by patient phenotypes [24]. For example, in NSCLC, EGFR mutations are more common in female patients and never‐smokers, and approximately 20%–40% of Asian patients with NSCLC have EGFR mutations [25], which may drive increased use of CDx testing in these patients. Given the benefit of CDx testing observed in this study, there may be additional opportunities to improve testing rates in specific subpopulations who may be less likely to be tested.
The use of CDx testing can help identify patients most likely to have a positive response to a particular therapy based on the assessment of specific molecular targets. The success of this strategy has been proven, with a number of approved biomarker‐driven treatments and CDx assays currently available [26, 27]. In our study, the subgroup of patients who had CDx testing and biomarker‐driven therapy as the first line of treatment lived longer than those who did not have any CDx testing (median survival of 18.0 versus 6.01 months, respectively). Furthermore, the data used in this study were collected beginning in 2011; the availability of CDx tests and biomarker‐driven therapies has substantially increased over time. Similarly, in this study, the percentage of patients with testing also increased over time and was quite high (62.2% in 2014–2018 vs. 21.7% in 2011–2013). Despite a larger proportion of patients in the no‐CDx arm (71.0%) than in the CDx arm (39.4%) receiving first line of treatment prior to 2014 and the first line of treatment year being significantly predictive of being CDx tested (odds ratio, 2.49), these were not associated with survival and therefore not carried forward in multivariate models.
The improved outcomes in patients with CDx testing and biomarker‐driven therapy demonstrate the benefit of a precision medicine treatment approach in those with non‐Sq aNSCLC and highlight the need for additional therapies targeting new mutations. Presently, therapies targeting molecular alterations (e.g., NTRK, RET, and KRAS) [28, 29, 30, 31] were not available at the time of treatment for our analysis; as new therapies become available, additional research may be needed to reassess the clinical benefit of CDx testing. Moreover, our analysis did not investigate sequential testing and the influence of treatment changes due to mutations following the first line of treatment. Based on our current findings, one might hypothesize that evidence supporting the benefits of CDx testing and biomarker‐driven therapy on survival may only increase over time (and hence our results may be conservative). Furthermore, additional research is needed to understand if patients who may have benefited from biomarker‐driven therapy did not receive such therapy because of a delay in or lack of access to CDx testing or if patient adherence to medication use not otherwise captured by Flatiron, even in patients with CDx testing, may also influence results. Last, as availability of biomarkers and targeted treatment evolve over time, future studies are needed to evaluate individual treatment response in the real‐world setting, including the effectiveness of biomarker‐driven therapy on survival, in patients with CDx testing.
Although guidelines for establishing successful precision medicine programs have been published and may be used to implement this type of approach in community practices, only 16% of oncology practitioners reported “always” performing CDx testing in a survey conducted by the National Comprehensive Cancer Network; 29% reported “rarely” to “never,” and up to 55% reported “often” to “sometimes” [32]. Several potential barriers may limit access to CDx testing in the community setting, in which the majority of patients are treated. First, selecting the most appropriate test for use in a nonresearch setting is challenging for many physicians, and turnaround time may be as long as 2 weeks [9]. Second, once a molecular mutation is identified, criteria for determining whether that mutation is actionable differ in the research and clinical settings and may be interpreted differently by different practitioners [9]. Moreover, given the complexity associated with incorporating precision medicine into the health care system, there is a need for molecular tumor boards to be part of current oncology practice [33, 34]. Molecular tumor boards provide clinicians with the opportunity to discuss tumor profiling and targeted therapeutic options with a multidisciplinary team; this improves understanding of CDx testing options, molecular profiling results, and therapeutic options for druggable alterations, thus increasing the efficiency of using precision medicine as part of patient care [33, 34, 35]. In addition, evidence demonstrating improved survival rates along with equivalent cost‐per‐week metrics are needed and may help to increase benefit availability through insured programs.
This study has several advantages over other precision medicine trials. Whereas previous trials were usually small [36], making it difficult to generalize findings to other patients, this study comprised a large cohort of patients with non‐Sq aNSCLC (n = 17,555) who were treated primarily in a community‐based setting for an evaluation period of up to 7 years (January 1, 2011–May 31, 2018). Using EHRs from the Flatiron Health database enabled longer‐term follow‐up of well‐defined cohorts in a real‐world setting. Moreover, we were able to adjust for some factors that are not typically included or available within patient registry data. Another advantage is that our study incorporated any type of CDx testing, increasing the likelihood of including biomarkers that are likely screened for by clinicians in real‐world clinical practice settings. By contrast, patients in other precision medicine trials were usually selected based on a single biomarker or a biomarker that is not routinely evaluated in community‐based practice settings [36].
Despite the advantages outlined here, this study had several limitations. First, overall survival in our study was assessed using real‐world data, which may have been susceptible to unobserved biases that influenced the selection of treatment or type of CDx test. Second, patients in the no‐CDx group may have undergone CDx testing outside of the Flatiron network or testing may not have been documented in the Flatiron EHR. Third, granular details regarding non‐Sq histology subtypes, including adenocarcinomas, large cell carcinomas, and other rare histologies, were not available and, thus, were not included in this analysis. Although performance status is clinically driven and a useful tool for assessing disease progression, ECOG scores were missing in approximately 57% of the patients with non‐Sq aNSCLC included in our analysis. Even though the Flatiron Health database is derived largely from community clinics, making the results more generalizable to patients treated in nonacademic practice settings, the population included in our analysis was predominantly white and had insurance. Therefore, the results may not be representative of populations outside the U.S., although they are representative of real‐world challenges faced by minorities in having access to care. There was also a potential for unmeasured bias because of the selection of patients based on the availability of their testing data. There may have been physician channeling bias for selection of CDx testing and/or therapy, for which we were unable to adjust. Because of the nature of routinely collected data in clinical practice, there is a potential for underreporting of comorbidities by patients or in the EHR, incomplete or unstructured oral prescription documentation in the EHR, and/or associated missing data, such as Eastern Cooperative Oncology Group performance status; however, routinely collected data provide valuable insights to inform clinical decisions.
Conclusion
Because the prognosis for patients with aNSCLC is generally very poor [1], a dire need exists for earlier initiation of care through diagnostic testing and biomarker‐driven treatment strategies. Based on this, it is evident that a standard one‐size‐fits‐all approach in the treatment of cancer and many other disease areas does not always result in optimal patient outcomes. Precision medicine, in contrast, is an expanding treatment approach that can help match patients to the most effective treatments sooner based on an individual's characteristics, including specific molecular targets likely to respond to biomarker‐driven therapies. Results from this analysis demonstrate that many patients are not routinely tested, and the use of CDx testing among patients with non‐Sq aNSCLC was associated with a reduced risk of mortality compared with those not undergoing CDx testing. Thus, it is imperative that health care providers and payers prioritize timely patient access to CDx testing, incorporate it as a critical first step to inform individualized treatment strategies, and use it as an opportunity to leverage multidisciplinary molecular tumor boards to improve management and timely delivery of precision medicine for patients with non‐Sq aNSCLC.
Author Contributions
Conception and design: Ani John, Roma A. Shah, William B. Wong, Marliese Alexander, Charles E. Schneider
Collection and assembly of data: Ani John, Roma A. Shah, William B. Wong, Marliese Alexander
Data analysis and interpretation: Ani John, Roma A. Shah, William B. Wong, Marliese Alexander
Manuscript writing: Ani John, Roma A. Shah, William B. Wong, Marliese Alexander, Charles E. Schneider
Final approval of manuscript: Ani John, Roma A. Shah, William B. Wong, Marliese Alexander, Charles E. Schneider
Disclosures
Ani John: Roche Diagnostics Information Solutions, a division of Roche Diagnostics (E); Roma A. Shah: Roche Diagnostics Information Solutions, a division of Roche Diagnostics (E); William B. Wong: Genentech, Inc., a member of the Roche Group; Charles E. Schneider: Roche Sequencing Solutions, Inc., a division of Roche Diagnostics; Marliese Alexander indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
This study was sponsored by Roche Diagnostics, Pleasanton, CA. Writing and editorial assistance was provided by Angela Morris, Ph.D., and Liz LaFlamme, Ph.D., of Health Interactions, Inc, and funded by Roche Diagnostics. We would like to thank Hamid Gari for his scientific contribution to this study.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Noone AM, Howlader N, Krapcho M, et al. SEER cancer statistics review, 1975‐2015: Lung and bronchus cancer. Available at https://seer.cancer.gov/archive/csr/1975_2015/results_merged/sect_15_lung_bronchus.pdf. Accessed October 27, 2019.
- 2. Young JL, Jr., Roffers SD, Gloeckler Ries AG. et al, eds. SEER Summary Staging Manual ‐ 2000: Codes and Coding Instructions. Bethesda, MD: National Institutes of Health; 2001. Available at https://seer.cancer.gov/tools/ssm/ssm2000/SSSM2000-122012.pdf. Accessed October 27, 2019. [Google Scholar]
- 3. Lung cancer survival rates. American Cancer Society; Available at https://www.cancer.org/content/cancer/en/cancer/lung‐cancer/detection‐diagnosis‐staging/survival‐rates.html. Accessed October 27, 2019. [Google Scholar]
- 4.Lung cancer 101: Types and staging of lung cancer. Lungcancer.org. Available at https://www.lungcancer.org/find_information/publications/163‐lung_cancer_101/268‐types_and_staging. Accessed October 27, 2019.
- 5. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 6. Common cancer types. National Cancer Institute. Available at https://www.cancer.gov/types/common-cancers. Accessed September 27, 2019. [Google Scholar]
- 7. Levit LA, Kim ES, McAneny BL et al. Implementing precision medicine in community‐based oncology programs: Three models. J Oncol Pract 2019;15:325–329. [DOI] [PubMed] [Google Scholar]
- 8. Mok TS. Personalized medicine in lung cancer: What we need to know. Nat Rev Clin Oncol 2011;8:661–668. [DOI] [PubMed] [Google Scholar]
- 9. Ersek JL, Black LJ, Thompson MA et al. Implementing precision medicine programs and clinical trials in the community‐based oncology practice: Barriers and best practices. Am Soc Clin Oncol Educ Book 2018;38:188–196. [DOI] [PubMed] [Google Scholar]
- 10. Pao W, Girard N. New driver mutations in non‐small‐cell lung cancer. Lancet Oncol 2011;12:175–180. [DOI] [PubMed] [Google Scholar]
- 11. Hunter KA, Socinski MA, Villaruz LC. PD‐L1 testing in guiding patient selection for PD‐1/PD‐L1 inhibitor therapy in lung cancer. Mol Diagn Ther 2018;22:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Engstrom PF, Bloom MG, Demetri GD et al. NCCN molecular testing white paper: Effectiveness, efficiency, and reimbursement. J Natl Compr Canc Netw 2011;9(suppl 6):S1–S16. [DOI] [PubMed] [Google Scholar]
- 13. Lindeman NI, Cagle PT, Aisner DL et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol 2018;13:323–358. [DOI] [PubMed] [Google Scholar]
- 14. Haslem DS, Van Norman SB, Fulde G et al. A retrospective analysis of precision medicine outcomes in patients with advanced cancer reveals improved progression‐free survival without increased health care costs. J Oncol Pract 2017;13:e108–e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haslem DS, Chakravarty I, Fulde G et al. Precision oncology in advanced cancer patients improves overall survival with lower weekly healthcare costs. Oncotarget 2018;9:12316–12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Health Flatiron. Available at https://flatiron.com/real-world-evidence/. Accessed September 27, 2019.
- 17. International Classification of Diseases , ninth revision. Centers for Disease Control and Prevention. National Center for Health Statistics (ICD‐9). Available at https://www.cdc.gov/nchs/icd/icd9.htm. Accessed September 27, 2019.
- 18. National Center for Health Statistics . International Classification of Diseases, tenth revision (ICD‐10). Centers for Disease Control and Prevention. Available at https://www.cdc.gov/nchs/icd/icd10.htm. Accessed October 27, 2019. [Google Scholar]
- 19. Curtis MD, Griffith SD, Tucker M et al. Development and validation of a high‐quality composite real‐world mortality endpoint. Health Serv Res 2018;53:4460–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alexander M, Wolfe R, Ball D et al. Lung cancer prognostic index: A risk score to predict overall survival after the diagnosis of non‐small‐cell lung cancer. Br J Cancer 2017;177:744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. John A, Shah R, Wong WB et al. Value of precision medicine in advanced NSCLC: Real‐world outcomes associated with the use of companion diagnostics. J Clin Oncol 2019;37(suppl 15):e20015a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsimberidou AM, Iskander NG, Hong DS et al. Personalized medicine in a phase I clinical trials program: The MD Anderson Cancer Center Initiative. Clin Cancer Res 2012;18:6373–6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kris MG, Johnson BE, Berry LD et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Illei PB, Wong W, Wu N et al. ALK testing trends and patterns among community practices in the United States. JCO Precis Oncol 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 25. Lim E, Lee H, Bae E et al. Economic evaluation of companion diagnostic testing for EGFR mutations and first‐line targeted therapy in advanced non‐small cell lung cancer patients in South Korea. PLoS One 2016;11:e0160155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scheerens H, Malong A, Bassett K et al. Current status of companion and complementary diagnostics. Clin Transl Sci 2017;10:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olsen D, Jørgensen JT. Companion diagnostics for targeted cancer drugs – Clinical and regulatory aspects. Front Oncol 2014;4:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ClinicalTrials.gov. Merestinib in non‐small cell lung cancer and solid tumors. Available at https://clinicaltrials.gov/ct2/show/NCT02920996. Accessed September 27, 2019. [Google Scholar]
- 29. ClinicalTrials.gov . Cabozantinib in patients with RET fusion‐positive advanced non‐small cell lung cancer and those with other genotypes: ROS1 or NTRK fusions or increased MET or AXL activity. Available at https://clinicaltrials.gov/ct2/show/NCT01639508. Accessed September 27, 2019.
- 30. ClinicalTrials.gov . Trial of trametinib and ponatinib in patients with KRAS mutant advanced non‐small cell lung cancer. Available at https://clinicaltrials.gov/ct2/show/NCT03704688. Accessed September 27, 2019.
- 31. Roman M, Baraibar I, López I et al. KRAS oncogene in non‐small cell lung cancer: Clinical perspectives on the treatment of an old target. Mol Cancer 2018;17:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guinto JB. NCCN Trends™: An evaluation of the use of companion diagnostic testing and targeted therapies. Available at https://www.nccn.org/about/news/ebulletin/ebulletindetail.aspx?ebulletinid=61. Accessed September 27, 2019.
- 33. Rolfo C, Manca P, Salgado R et al. Multidisciplinary molecular tumour board: A tool to improve clinical practice and selection accrual for clinical trials in patients with cancer. ESMO Open 2018;3:e000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. El Saghir NS, Charara RN, Kreidieh FY et al. Global practice and efficiency of multidisciplinary tumor boards: Results of an American Society of Clinical Oncology international survey. J Glob Oncol 2015;1:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van der Velden DL, van Herpen CML, van Laarhoven HWM et al. Molecular tumor boards: Current practice and future needs. Ann Oncol 2017;28:3070–3075. [DOI] [PubMed] [Google Scholar]
- 36. Lewis JRR, Kerridge I, Lipworth W. Use of real‐world data for the research, development, and evaluation of oncology precision medicines. JCO Prec Oncol 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


