Short abstract
This commentary provides an overview of four randomized clinical trials on the use of geriatric assessment to guide decisions and interventions for cancer care. These studies show the effect of geriatric interventions on oncological outcomes, including toxicity, quality of life, and survival.
In 2018, the American Society of Clinical Oncology (ASCO) published its geriatric oncology guidelines, calling for the implementation of the geriatric assessment (GA) for all older adults with cancer [1]. However, although high‐quality data supports the use of the GA to identify geriatric syndromes and to predict mortality and chemotherapy toxicity, evidence supporting the implementation of GA‐guided processes for informing cancer treatment is moderate and mostly based on expert consensus [1].
The GA is a multidisciplinary evaluation of an older adult's functional, psychosocial, physical, and cognitive abilities, including comorbidities and medication use, and has been used by geriatricians since the 1950s. Although GA‐guided interventions are effective for improving survival and functional status in several scenarios [2], there is a lack of information regarding the effects of GA‐guided care on traditional “hard” oncological outcomes, such as overall survival (OS), treatment toxicity, or quality of life (QoL) [3]. Fortunately, four randomized clinical trials (RCT) presented at the 2020 ASCO Annual Meeting have provided new evidence showing that the implementation of GA‐guided interventions for older adults with cancer can in fact lead to improvements in QoL and decreased treatment toxicity, without compromising survival (Table 1) [ 4, 5, 6]. These RCTs represent a giant leap forward for geriatric oncology, because they provide a strong foundation that will allow for GA‐guided interventions to become the standard of care for all older adults with cancer.
Table 1.
Summary of randomized controlled trials of geriatric assessment‐guided care presented at the 2020 ASCO Annual Meeting
| Reference | Interventions | Setting | Patients | Outcomes |
|---|---|---|---|---|
| GAIN Li et al. [5] | Intervention group: multidisciplinary GA‐based interventions (physical therapy, nutrition, advanced care planning, occupational therapy, medication reconciliation, referrals for comorbidity care) Usual care group: GA provided to treating oncologist but no interventions offered | Single cancer center in the U.S. with availability of multidisciplinary team with geriatric expertise |
n = 600 Inclusion criteria: age ≥65, any functional status, solid tumors, all stages, starting chemotherapy |
Decreased incidence of severe chemotherapy toxicity (50% vs. 60%, p = .02). Increased advance directive completion (24% vs. 10%, p < .01) |
| GAP‐70 Mohile et al. [4] |
Intervention group: GA‐based recommendations sent to treating oncologists Usual care group: No summary provided to treating oncologists, patients treated according to standard of care |
41 community practice sites in the U.S. Geriatricians unavailable at the practice sites. |
n = 41 centers (718 patients) Inclusion criteria: age >70, ≥1 impaired GA domain, incurable solid tumors or lymphoma, starting new treatment |
Decreased incidence of severe chemotherapy toxicity (50% vs. 71%, p < .01). No differences in 6‐month survival |
| INTEGERATE Soo et al. [6] | Intervention group: patients were comanaged by a geriatrician during oncological treatment. Usual care group: Care directed by oncologist alone | Three Australian cancer centers with availability of geriatricians |
n = 154 Inclusion criteria: age ≥70, solid tumors and lymphoma, candidates for systemic therapy |
Quality of life better in the intervention group at 6 months. Reduced hospitalizations (41% less) and emergency room visits (39% less) |
|
Qian et al. [8] |
Intervention group: perioperative geriatric interventions multidisciplinary GA‐based interventions. Usual care group: GA provided to treating oncologist but no interventions offered | Single cancer center in the U.S. with availability of multidisciplinary team with geriatric expertise |
n = 160 Inclusion criteria: age ≥65, undergoing surgery for GI cancer, any functional status, all stages |
Per‐protocol analysis: decreased hospital stay (8.2 vs. 7.3 days, p = .02); decreased ICU admissions (32% vs. 13%, p = .05). No differences in ITT analysis |
Abbreviations: ASCO, American Society of Clinical Oncology; GA, geriatric assessment; GI, gastrointestinal; ICU, intensive care unit; ITT, intention‐to‐treat
In the GAP‐70 cluster RCT, investigators from the University of Rochester analyzed the effect of providing GA‐guided recommendations to oncologists working in community practices [4]. Forty‐one practices were randomized to the GA intervention or to usual oncologist‐directed care alone. All patients were aged ≥70 years and had advanced cancers (stages III or IV) plus a geriatric deficit or syndrome. Although all patients underwent a GA at baseline, the GA results and a set of GA‐guided recommendations were provided only to oncologists and patients in the practices randomized to the intervention arm. The primary aim of GAP‐70 was treatment‐related toxicity, whereas a key secondary aim was 6‐month OS. Of the 718 included patients, 349 were treated in practices randomized to the intervention arm, and 369 were treated in practices allocated to usual care. Patients in the GA intervention arm were more likely to have gastrointestinal malignancies, and approximately 10% did not receive chemotherapy. Importantly, the patient population included in GAP‐70 was mostly vulnerable or frail, with more than half having limitations in functional status. For the primary outcome of grade 3–5 toxicity, the intervention arm had an absolute reduction of 20% when compared with the usual care arm (50% vs. 71%, risk ratio, 0.74; p < .01). A potential explanation for this is that patients in the intervention arm were significantly more likely to receive a reduced dose of chemotherapy at cycle 1 (49% vs. 35%, p = .016). However, despite dose reductions, no differences in 6‐month OS were observed (71% vs. 74%, p = .33), meaning that the GA intervention led to a reduction in toxicity without affecting survival.
The GAIN RCT studied the effect of a multidisciplinary team (MDT) GA‐guided intervention on the outcomes of patients with solid tumors treated at a single cancer center in southern California [5]. In GAIN, patients aged ≥65 years with solid tumors (any stage) starting a new line of chemotherapy were randomized 2:1 to the MDT GA intervention or to usual oncologist‐guided care. Patients in the intervention arm underwent a GA that was then reviewed by the MDT, which implemented tailored interventions. In the usual care arm, the treating oncologist received the GA results, but no interventions were implemented. As in GAP‐70, GAIN's primary aim was treatment‐related toxicity, whereas secondary aims included advance directive (AD) completion and resource use (emergency department [ED] visits and hospitalizations). Six‐hundred and twenty patients were included, of which 413 were randomized to the intervention arm and 207 to the control arm. Most patients had advanced cancer, and approximately half had impairments in activities of daily living. GAIN was a positive trial, because grade 3–5 chemotherapy toxicity was reduced by 10% in the intervention arm compared with the usual care arm (50% vs. 60%, p = .02). GAIN also showed an increase in AD completion for patients randomized to the intervention (70% vs. 59%, p < .01). However, no differences between arms were found for hospitalizations or ED visits. Survival outcomes are awaited.
The third trial was INTEGERATE, which investigated the effect of geriatrician‐oncologist comanagement on the QoL of older adults with solid tumors or lymphoma starting a new treatment line (including targeted therapy) [6]. Patients aged ≥70 years treated at three Australian hospitals were randomized in a 1:1 fashion to an integrated onco‐geriatric care intervention or to usual care. Patients in the intervention arm got a GA followed by standardized personalized interventions and referrals, including supportive care information, encouragement of physical activity, management of comorbidities, medication reconciliation, and advance care planning. Meanwhile, patients in the control arm were managed by their treating oncologist without input from geriatrics. In contrast with both GAIN and GAP‐70, INTEGERATE's main outcome was QoL measured using the Elderly Functional Index (ELFI), which was developed and validated by the authors [7]. Additional outcomes included health care use, treatment delivery, and survival. One‐hundred and fifty‐four patients were included, of whom 76 were randomized to the intervention arm and 78 were randomized to the control arm. Almost a third of patients in both arms had advanced lung cancer, and the intent of treatment was palliative in 67%. At 24 weeks follow‐up, patients in the intervention arm had better ELFI QoL scores than those in the usual care arm (73.1 vs. 64.6, p = .04). Interestingly, patients in the control arm had a large decline in QoL during the first 3 months after the start of treatment. In contrast with GAIN, INTEGERATE showed a deep decline in health care use in the intervention arm (RR, 0.61 for ED visits and RR, 0.59 for unplanned hospitalizations).
Finally, Qian et al. studied the effect of a perioperative geriatric intervention on the surgical outcomes of older patients with gastrointestinal cancer [8]. Patients aged ≥65 years who were scheduled for surgery at Massachusetts General Hospital were randomized in a 1:1 fashion to a geriatrician‐led intervention (including management of nutrition, comorbidity, psychological issues, functional status, polypharmacy, postoperative complications, delirium prevention, and discharge planning) or usual care alone. The study's main outcome was hospital length of stay (LOS), whereas secondary outcomes were intensive care unit (ICU) use, readmissions, and various patient‐reported outcomes (symptoms and QoL). One‐hundred and sixty patients were enrolled, of whom 82 were randomized to the intervention and 78 to the control arm. Importantly, only 30 patients allocated to the geriatric intervention went on to receive it as stated in the protocol. The median age was 72 years, and half of the patients in both arms had pancreatic cancer. In the intention‐to‐treat population, no differences were found in LOS, ICU use, or readmissions between arms. However, patients who received the full geriatric intervention had reduced LOS (8.2 vs. 5.9 days, p = .02), were less likely to be admitted to the ICU (32% vs. 13%, p = .049), and had an improvement in symptoms at 60 days. This trial highlights the challenges of implementing complex multicomponent interventions in the busy and highly dynamic perioperative scenario, even in a center with a large availability of specialized personnel and resources.
Although the four studies used different geriatric oncology models of care, they all showed that GA‐guided interventions improve key outcomes for older patients with cancer. This level 1 evidence shows that GA‐directed interventions can provide benefits to patients across various settings with differing resources and infrastructure (Fig. 1) [9]. In addition, although all studies used different measurements and tools to assess patients, the types of findings, interventions, and outcomes were similar. This might be related to the fact that all of the used assessments measure cumulative deficits on broad geriatric domains, which in turn represent a measurement of frailty [10]. An important finding, which was particularly notable in the GAP‐70 trial, was that although patients who received GA‐guided care were less likely to get full‐dose chemotherapy, this did not have a detrimental effect on OS [4]. This mimics the results of disease‐specific chemotherapy RCT in geriatric oncology, such as ESOGIA in non‐small cell lung cancer or GO2 in gastric cancer, which have shown that reduced dosing, or even omission of systemic treatment, may lead to reduced toxicity without compromising survival [11, 12]. As with all RCT of complex multicomponent interventions, it will be difficult to understand which specific component influenced outcomes the most, and we will have to wait for publication of the full results to have a better understanding of this issue.
Figure 1.
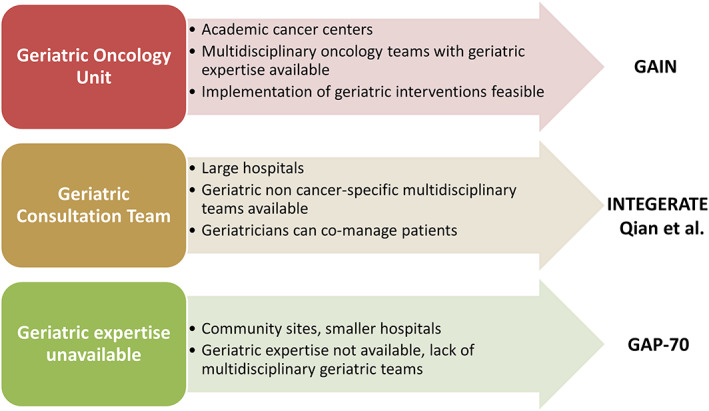
Potential for the implementation of the findings from the four randomized clinical trials into diverse geriatric oncology models of care.
It is probable that these studies will have an effect on geriatric oncology, similar to the effect that the publication of the seminal study by Temel et al. had on supportive and palliative care [13], and lead to an earlier integration of specialists with geriatric expertise into cancer care teams or training of oncologists to perform the GA. However, as we discuss these very relevant results, we should also keep in mind that there are still many barriers to the large‐scale implementation of geriatric oncology care, including health care system‐level organizational issues, lack of time, limited staffing, lack of training and familiarity with available tools, and unclear reimbursement rules [14, 15]. So, just as with supportive and palliative care, there is significant risk that despite growing evidence of its effectiveness, GA‐guided care could fail to translate into improved outcomes for most older adults with cancer because of a lack of widespread integration into daily practice [16]. This represents both a challenge and an opportunity for researchers in geriatric oncology, who should strive to design robust studies aimed at establishing the best strategies for the implementation of GA‐guided care and geriatric interventions in everyday clinical practice across multiple settings with differing availability of resources.
Without a doubt, the 2020 ASCO Annual Meeting represented a leap forward for the science of geriatric oncology. We now have strong, high‐quality, level 1 evidence supporting the implementation of GA‐guided processes for informing cancer treatment across diverse settings and different models of care. The next step must now be to translate this scientific evidence into global practice, policy, and population health in order to achieve the dream of the late Dr. Arti Hurria: “that one day, all older adults with cancer will receive personalized tailored care, utilizing evidence‐based medicine with a multidisciplinary approach.”
Disclosures
Matti Aapro: Accord Healthcare, Amgen, Bayer, Bristol‐Myers Squibb, Celgene, Cephalon, Chugai, Clinigen, Eisai, Genomic Health, GlaxoSmithKline, Helsinn, Hospira, Ipsen, J&J, Kyowa, Merck, Novartis, OrthoBiotech, Pfizer, PierreFabre, Roche, Sandoz, Sanofi, Tesaro, Taiho, Teva, Vifor (C/A, H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Mohile SG, Dale W, Somerfield MR et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol 2018;36:2326–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stuck AE, Siu AL, Wieland GD, et al. Comprehensive geriatric assessment: A meta‐analysis of controlled trials. Lancet 1993;342:1032–1036. [DOI] [PubMed] [Google Scholar]
- 3. Li D, Soto‐Perez‐de‐Celis E, Hurria A. Geriatric assessment and tools for predicting treatment toxicity in older adults with cancer. Cancer J 2017;23:206–210. [DOI] [PubMed] [Google Scholar]
- 4. Mohile SG, Mohamed MR, Culakova E et al. A geriatric assessment (GA) intervention to reduce treatment toxicity in older patients with advanced cancer: A University of Rochester Cancer Center NCI community oncology research program cluster randomized clinical trial (CRCT). J Clin Oncol 2020;38:12009a. [Google Scholar]
- 5. Li D, Sun CL, Kim H et al. Geriatric assessment‐driven intervention (GAIN) on chemotherapy toxicity in older adults with cancer: A randomized controlled trial. J Clin Oncol 2020;38:12010a. [Google Scholar]
- 6. Soo WK, King M, Pope A et al. Integrated geriatric assessment and treatment (INTEGERATE) in older people with cancer planned for systemic anticancer therapy. J Clin Oncol 2020;38:12011a. [Google Scholar]
- 7. Soo WK, King M, Pope A et al. ELderly Functional Index (ELFI): Validation of a self‐reported measure of functional status in cancer patients. J Clin Oncol 2020;38:e19126a. [Google Scholar]
- 8. Qian CL, Knight HP, Ferrone CR et al. Randomized trial of a perioperative geriatric intervention for older adults with cancer. J Clin Oncol 2020;38(15 suppl):12012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wildiers H, Heeren P, Puts M et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guerard EJ, Deal AM, Chang Y et al. Frailty index developed from a cancer‐specific geriatric assessment and the association with mortality among older adults with cancer. J Natl Compr Canc Netw 2017;15:894–902. [DOI] [PubMed] [Google Scholar]
- 11. Corre R, Greillier L, Le Caër H et al. Use of a comprehensive geriatric assessment for the management of elderly patients with advanced non‐small‐cell lung cancer: The phase III randomized ESOGIA‐GFPC‐GECP 08‐02 study. J Clin Oncol 2016;34:1476–1483. [DOI] [PubMed] [Google Scholar]
- 12. Hall PS, Swinson D, Waters JS et al. Optimizing chemotherapy for frail and elderly patients (pts) with advanced gastroesophageal cancer (aGOAC): The GO2 phase III trial. J Clin Oncol 2019;37:4006a. [Google Scholar]
- 13. Temel JS, Greer JA, Muzikansky A et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med 2010;363:733–742. [DOI] [PubMed] [Google Scholar]
- 14. Plotkin E, Lucas L, Burhenn PS et al. Geriatric assessment adoption in community cancer centers: Trends, barriers, and recommendations. Innov Aging 2019;3(suppl):S122. [Google Scholar]
- 15. Kenis C, Heeren P, Decoster L et al. A Belgian survey on geriatric assessment in oncology focusing on large‐scale implementation and related barriers and facilitators. J Nutr Health Aging 2016;20:60–70. [DOI] [PubMed] [Google Scholar]
- 16. Knaul FM, Farmer PE, Krakauer EL et al. Alleviating the access abyss in palliative care and pain relief‐an imperative of universal health coverage: the Lancet Commission report. Lancet 2018;391:1391–1454. [DOI] [PubMed] [Google Scholar]


