Abstract
Background
The aim of this study was to investigate the predictive value of early changes in 18F‐fluoroestradiol (FES) positron emission tomography (PET)/computed tomography (CT) during fulvestrant 500 mg therapy in patients with estrogen receptor (ER)‐positive metastatic breast cancer.
Materials and Methods
Patients underwent 18F‐FES PET/CT scans at both baseline (scan 1) and day 28 (scan 2). The maximum standardized uptake value (SUVmax) of all metastatic sites was determined in each scan, and the percentage reduction in SUVmax (ΔSUVmax) was calculated as [(SUVmax on scan 1‐SUVmax on scan 2)/ SUVmax on scan 1] * 100%.
Results
In total, 294 18F‐FES‐positive lesions from 36 patients were identified. The 18F‐FES SUVmax varied widely among lesions (median 5.7; range 1.8–32.4) and patients (median 5.1; range 2.5–13.2). After treatment, the median SUVmax among lesions and patients was 2.1 and 2.1, respectively. The ΔSUVmax ranged from −5.1% to 100%, with a median reduction of 61.3%. Using receiver operating characteristic analysis, the optimal cutoff point to discriminate patients who could derive clinical benefit from fulvestrant was determined to be 38.0%. Patients with a median ΔSUVmax ≥38.0% experienced significantly longer progression‐free survival (PFS) than those with ΔSUVmax <38.0% (28.0 months vs. 3.5 months, p = .003). Multivariate analysis demonstrated that ΔSUVmax ≥38.0% was an independent predictor of PFS benefit in patients receiving fulvestrant therapy.
Conclusion
Changes in SUVmax measured by serial imaging of 18F‐FES PET/CT could be used early to predict PFS benefit in patients receiving fulvestrant therapy.
Implications for Practice
The aim of this study was to evaluate the role of 18F‐fluoroestradiol (FES) positron emission tomography (PET)/computed tomography (CT) in predicting response to fulvestrant 500 mg therapy in patients with hormone receptor‐positive/human epidermal growth receptor 2–negative metastatic breast cancer. This study highlights the utility of FES PET/CT as a predictive factor to discriminate patients who might benefit from fulvestrant. Moreover, these findings showed that this molecular imaging technique might be a potential tool for physicians to make individualized treatment strategies.
Keywords: Metastatic breast cancer, Fulvestrant, Predictive factor, 18F‐FES PET/CT, ΔSUVmax
Short abstract
This study investigated whether changes in 18F‐FES positron emission tomography uptake could be used for the early prediction of treatment response in patients with metastatic breast cancer who received a fulvestrant 500 mg regimen.
Introduction
More than 70% of patients with metastatic breast cancer (MBC) present with hormone receptor (HR)‐positive disease and are candidates for endocrine therapy [1, 2]. Targeting the estrogen receptor (ER) has been established as a treatment option for hormone‐sensitive breast cancer [3].
Fulvestrant, a 17β‐estradiol analog, is an antiestrogen that suppresses estrogen signaling by binding to ER and inducing its degradation [4]. It has estrogen antagonistic activity and no estrogen agonistic effects [5]. Fulvestrant 500 mg was approved as the standard dose for HR‐positive MBC after the CONFIRM study found that fulvestrant 500 mg was associated with improved efficacy compared with the 250‐mg dose in patients who experienced disease recurrence or progression after previous endocrine therapy [6]. The FIRST and FALCON trials further demonstrated the superior efficacy of fulvestrant 500 mg compared with anastrozole in the first‐line setting for HR‐positive MBC [7, 8, 9].
Although fulvestrant 500 mg was associated with improved efficacy in both first‐ and later‐line settings in the abovementioned trials, progression‐free survival (PFS) may range from a few months with a very aggressive disease course to several years without major effects on quality of life [10]. This variation underscores the importance of earlier prediction of treatment response in order to develop individualized treatment strategies.
ER expression levels have been shown to function as an important prognostic factor that can be used to predict the likelihood of response to conventional hormonal therapies [11, 12]. The evaluation of ER status by immunohistochemical staining is the current gold standard. Nevertheless, this method has some limitations, and owing to the heterogeneous nature of breast cancer, patients may present with discordant ER expression between the primary tumor and metastases [13, 14]. Therefore, a single biopsy may not be sufficiently predictive or representative of the ER characteristics of the tumor burden as a whole.
Whole‐body positron emission tomography (PET) is an imaging technology that provides insights for the quantitative assessment of many biological characteristics. For patients with advanced or metastatic ER‐positive disease, the use of ER‐targeting radiopharmaceuticals in PET is a noninvasive method to evaluate ER expression in all metastatic lesions without performing multiple biopsies. Several studies have validated the novel tracer 16a‐18F‐fluoro‐17β‐estradiol (18F‐FES) as a unique approach to quantify and visualize molecular information about ER expression in all metastatic lesions [15, 16, 17]. Studies at our center and others have shown that 18F‐FES uptake correlates with ER expression measured by immunohistochemistry staining, indicating the important role of 18F‐FES PET in the prediction of treatment response to endocrine therapy [15, 18, 19, 20, 21, 22].
The aim of this prospective study was, therefore, to investigate whether changes in 18F‐FES PET uptake could be used for the early prediction of treatment response in patients with MBC who received a fulvestrant 500 mg regimen.
Materials and Methods
Patients
This prospective trial (Clinical trials.gov identifier: NCT03507088) was approved by the Ethics Committee for Clinical Investigation of Fudan University Shanghai Cancer Center. All patients provided written informed consent to participate. Patients with histologically confirmed HR‐positive, human epidermal growth receptor 2 (HER2)‐negative MBC were eligible. Other eligibility criteria were Eastern Cooperative Oncology Group performance status ≤2, life expectancy >3 months, one or more measurable or nonmeasurable lesions, and adequate hematologic, hepatic, renal, and cardiac function. Key exclusion criteria were previous fulvestrant treatment, the presence of life‐threatening visceral metastasis, and central nervous system metastasis. 18F‐FES PET/CT scans have limited value in diagnosing and quantifying liver lesions because of high liver physiological uptake [23]; therefore, patients with liver metastases were also excluded from our study. To avoid false‐negative 18F‐FES results, patients were required to discontinue drugs known to block ER, such as tamoxifen, for a minimum of 5 weeks before participation. However, the use of aromatase inhibitors as the treatment immediately preceding fulvestrant was allowed [24, 25].
Administration of Fulvestrant
Eligible patients were treated with fulvestrant 500 mg (administered intramuscularly on days 1, 15, and 29 and every 28 days thereafter). For premenopausal women, fulvestrant was given upon administration of a gonadotropin‐releasing hormone agonist. Treatment was continued until progressive disease (PD) by radiologic or clinical assessment, intolerable toxicity, or withdrawal of consent.
18F‐FES PET
All patients underwent two 18F‐FES PET/CT scans to assess ER expression in lesions. The first FES PET/CT scan was performed at baseline (scan 1, within 7 days before treatment), and the second FES PET/CT scan was performed at day 28 ± 2 (scan 2, before administration of the third dose of fulvestrant).
18F‐FES synthesis and quality control were performed as previously described. The specific activity was 2–5 Ci/μmol at the time of injection, and the radiochemical purity was >99%. All patients fasted for at least 4 hours to reduce the uptake of 18F‐FES by the hepatobiliary system and the gastrointestinal tract and to prevent its interference to abdominal and pelvic imaging. An average dose of 222 MBq (6 mCi) of 18F‐FES was injected over 1–2 minutes. All PET/CT scans were performed on a Siemens biograph 16 HR PET/CT in 3‐dimensional, high‐resolution mode 60 minutes after injection. The transaxial intrinsic spatial resolution was 4.1 mm, full‐width at half‐maximum in the center of the field of view. For the PET/CT device, a low‐dose CT scan was first acquired from the proximal thighs to the head to provide data for attenuation correction. A PET emission scan (2–3 minutes per bed) covering the same transverse field of view was obtained immediately after the low‐dose CT scan. The Gaussian filter iteration was used to reconstruct the emission images.
Image Interpretation
Two board‐certified nuclear medicine physicians with more than 5 years of experience independently analyzed images on a multimodality computer platform (Syngo; Siemens, Knoxville, TN). Consensus was reached when the analyses were inconsistent or ambiguous.
Lesions in 18F‐FES PET/CT images were also identified and localized by 18F‐FDG PET/CT (n = 26) or other conventional imaging techniques (six patients underwent chest CT for lung lesions, and four patients underwent bone scan and magnetic resonance imaging [MRI] for bone lesions). Semiquantitative analysis of tumor metabolic activity was obtained using standardized uptake value (SUV) based on body weight. For each lesion, the maximum SUV (SUVmax) was recorded by manually placing an individual region of interest on all consecutive slices that contained the lesion on coregistered and fused transaxial PET/CT images. When there were countless bone lesions, up to five of the most 18F‐FES PET–avid lesions were calculated in each of five regions: skull, chest (including sternum, scapula, clavicle, and rib), long bone, spine, and pelvis. We used the threshold of SUVmax ≥1.8 to define 18F‐FES positivity based on our previous study [18]. For each 18F‐FES‐positive lesion, the SUVmax obtained from baseline scan (scan 1) and scan 2 were analyzed for the percentage reduction in SUVmax (ΔSUVmax), which was calculated as [(SUVmax on scan 1‐SUVmax on scan 2)/ SUVmax on scan 1] * 100%.
Assessment of Treatment Response
Clinical follow‐up, including clinical history, performance status, physical examination, and laboratory tests, was performed every 4 weeks. Tumor response assessment was performed by radiologic imaging every 2 months until PD. For patients with measurable disease, response was defined according to RECIST v1.1 criteria. Patients with only nonmeasurable lesions were considered to have PD when there was unequivocal progression of existing lesions or when new lesions were detected at follow‐up. For patients with bone‐only metastases, the MD Anderson (MDA) criteria were used to define response evaluation [26]. In the absence of radiological PD, patients could develop clinical PD, defined as an overall level of substantial worsening such that the overall tumor burden or complaints increased sufficiently to merit discontinuation of therapy [11]. The primary endpoint of this study was PFS. Secondary endpoints included the objective response rate (ORR, best overall response of patients with either a complete or partial response among those with measurable disease at baseline), clinical benefit rate (CBR, best overall response of patients with a complete response, partial response, or stable disease ≥24 weeks), and overall survival (OS, time interval from fulvestrant treatment to death during follow‐up).
Statistical Analysis
Receiver operating characteristic (ROC) analysis was performed to evaluate the optimal threshold for predicting clinical benefit through 18F‐FES PET. The Kaplan‐Meier method was used to estimate PFS. A subgroup analysis of the PFS data was conducted with image parameters (baseline SUVmax, residual SUVmax (scan 2 SUVmax), ΔSUVmax, and heterogeneity of FES) and the following clinicopathological characteristics: age, menopausal status, disease‐free interval, visceral disease, de novo metastatic disease, prior endocrine therapy for MBC, prior chemotherapy for metastatic disease, and level of responsiveness to endocrine therapy before fulvestrant treatment (primary resistance versus secondary resistance). Primary resistance to endocrine therapy was defined as recurrence occurring during the first 2 years of adjuvant endocrine therapy or disease progression within the first 6 months of first‐line endocrine therapy for advanced disease. Secondary resistance to endocrine therapy was defined as recurrence occurring after the first 2 years of adjuvant endocrine therapy or disease progression after the first 6 months of endocrine therapy for advanced disease. The Cox proportional hazards model was applied for univariate and multivariate analyses. Multivariate analysis with the stepwise model by forward selection was performed with all significant image parameters and clinicopathological characteristics in the univariate analysis. The associations among image parameters and clinical benefit from fulvestrant were analyzed by the Mann‐Whitney U test. Two‐sided p values of less than .05 were considered to indicate statistically significant differences. All statistical analyses were performed using SPSS, version 20.0 (IBM Corporation, Armonk, NY).
Results
Patient Characteristics
From March 24, 2016, to June 28, 2018, our study enrolled 46 patients. After excluding 10 patients who were found to be ineligible, 36 patients were included in the full analysis set (Fig. 1). The patients’ baseline demographic and disease characteristics are summarized in Table 1. The median patient age at the start of fulvestrant treatment was 55.5 years (range 31–78), and bone was the most common metastatic site (63.9%). Baseline visceral metastases were present in 15 patients (41.7%). In total, 21 patients met the criteria for measurable disease according to RECIST v1.1 at baseline, and the remaining 15 patients had nonmeasurable nodal or visceral involvement or bone‐only disease. Most (n = 27) patients had not received previous endocrine treatment for metastatic disease, and 5 patients had received prior palliative chemotherapy. In addition, the last endocrine therapy prior to fulvestrant was an aromatase inhibitor for 13 patients and an antiestrogen for 7 patients.
Figure 1.
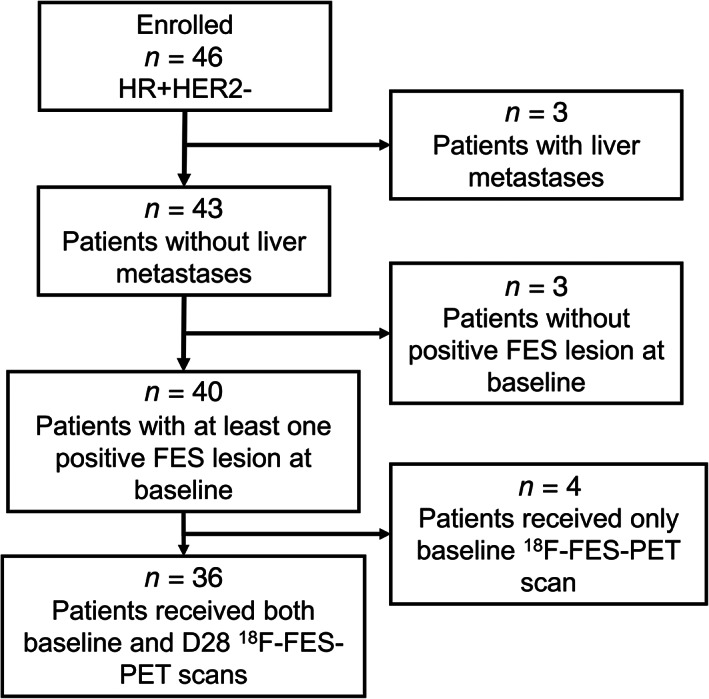
Patient flowchart for inclusion and exclusion.Abbreviations: FES, fluoroestradiol; HER2, human epidermal growth receptor 2; HR, hormone receptor; PET, positron emission tomography.
Table 1.
Patient demographic and disease characteristics at baseline
| Characteristics | Patients (n = 36) | % |
|---|---|---|
| Median age (range), years | 55.5 (31–78) | |
| Menopausal status | ||
| Premenopausal a | 8 | 22.2 |
| Postmenopausal | 28 | 77.8 |
| Disease‐free interval, years b | ||
| >5 | 18 | 50.0 |
| ≤5 | 7 | 19.4 |
| Pathologic type | ||
| Invasive ductal carcinoma | 33 | 91.7 |
| Invasive lobular carcinoma | 2 | 5.6 |
| Tubular carcinoma | 1 | 2.7 |
| PgR status | ||
| Positive | 32 | 88.9 |
| Negative | 4 | 11.1 |
| Metastatic sites | ||
| Nonvisceral | 21 | 58.3 |
| Bone | 23 | 63.9 |
| Bone‐only | 2 | 5.6 |
| Visceral disease | 15 | 41.7 |
| Any lung | 11 | 30.6 |
| Pleural | 5 | 13.9 |
| Peritoneum | 1 | 2.8 |
| No. of disease sites | ||
| 1–3 | 9 | 25.0 |
| 4–6 | 9 | 25.0 |
| 7–9 | 7 | 19.4 |
| ≥10 | 11 | 30.6 |
| De novo metastatic disease | 11 | 30.6 |
| Adjuvant ET | ||
| Antiestrogen | 14 | 38.9 |
| Aromatase inhibitor | 4 | 11.1 |
| Antiestrogen followed by aromatase inhibitor | 2 | 5.6 |
| None | 5 | 13.9 |
| Prior ET for metastatic disease | ||
| None | 27 | 75.0 |
| Yes | 9 | 25.0 |
| Prior ET type for metastatic disease | ||
| Antiestrogen ± LH‐RH analog | 1 | 2.8 |
| Aromatase inhibitor ± LH‐RH analog | 8 | 22.2 |
| Prior sensitivity to ET | ||
| Primary resistance | 1 | 2.7 |
| Secondary resistance | 23 | 63.8 |
| Prior chemotherapy for metastatic disease | ||
| None | 31 | 86.1 |
| Yes | 5 | 13.9 |
| Treatment immediately preceding fulvestrant | ||
| None | 12 | 33.3 |
| Chemotherapy | 4 | 11.1 |
| Antiestrogen | 7 | 19.4 |
| Aromatase inhibitor | 13 | 36.1 |
| Progression‐free survival | ||
| Events | 19 (range 1.8–28.0 months) | 52.8 |
| Censored | 17 (range 5.6–19.4 months) | 47.2 |
| With negative 18F‐FES lesions | ||
| None | 26 | 72.2 |
| Yes | 10 | 27.8 |
For premenopausal women, fulvestrant was given upon the administration of gonadotropin‐releasing hormone agonist.
Patients with stage IV breast cancer at initial diagnosis were excluded (n = 11).
Abbreviations: ER, estrogen receptor; ET, endocrine therapy; FES, fluoroestradiol; LH‐RH, luteinizing hormone releasing hormone; PgR, progesterone receptor.
Treatment Outcome
The data cutoff date for the primary analysis was February 20, 2019, when 19 PFS events were recorded. The median PFS for the entire group was 13.1 months. The minimal PFS was 1.8 months, and no patient experienced PD prior to receiving their second scan of FES‐PET. Fulvestrant induced 1 complete response and 10 partial responses. Nineteen patients had stable disease ≥24 weeks. In patients with measurable disease, the ORR was 30.6% (11/36) with fulvestrant, and the CBR was 83.3% (30/36) for the entire group. Among patients with nonmeasurable disease, 9 out of the 15 had PD events at the data cutoff for PFS analysis, including 7 patients with newly emerged lesions, 1 patient with obvious increase in the range of osteolytic destruction, and 1 patient was defined as clinical PD as a result of evident expansion of chest wall skin erythema and a threefold increase of tumor marker CA153. Median OS could not be calculated because of insufficient follow‐up time. At the data cutoff, no patient in the cohort had died. Generally, fulvestrant was well tolerated in all patients, and no patients withdrew during follow‐up.
18F‐FES PET/CT Analysis
A total of 294 positive lesions were identified at baseline, including 164 bone lesions, 92 lymph node metastases, 17 lung metastases, 9 breast lesions, 6 pleural metastases, 1 peritoneal lesion, and 5 soft tissue lesions (supplemental online Table 1). In addition, 18F‐FDG and other conventional imaging technologies revealed 15 18F‐FES‐negative lesions (8 bone lesions, 2 lymph node metastases, 3 lung metastases, 1 pleural metastasis, and 1 breast lesion).
Heterogeneity of FES avidity was observed among lesions within individual patients. Twenty‐six patients (72.2%) had homogeneously 18F‐FES‐positive lesions, and the remaining 10 patients (27.8%) had both 18F‐FES‐positive and 18F‐FES‐negative lesions. Nine out of the 10 patients underwent FDG‐PET scan at baseline. All the FES‐negative lesions were FDG‐avid in these 9 patients. Meanwhile, baseline bone scan and MRI were performed on the remaining patient, who presented with bone‐only metastases. Her FES‐negative lesions manifested as nuclide accumulation on bone scan and osteolytic destruction on MRI. Baseline 18F‐FES uptake (SUVmax) varied widely among lesions (median 5.7; range 1.8–32.4) and patients (median 5.1; range 2.0–13.2). At scan 2, the residual median SUVmax among lesions and patients was 2.1 (range 0–7.2) and 2.1 (range 0–4.8), respectively. For the 36 patients that formed the entire cohort, the SUV reduction ranged from −5.1% to 100%, with a median of 61.3%.
Predictive Value of 18F‐FES PET for Response to Fulvestrant Treatment
Both baseline and residual 18F‐FES uptake in lesions was similar between patients with clinical benefit from fulvestrant and patients with PD (baseline median SUVmax, 5.1 vs. 4.4, p = .270; median SUVmax on scan 2, 2.1 vs. 2.2, p = .308). Neither of the two 18F‐FES PET image parameters predicted PFS (Fig. 2A, 2B; all log‐rank p > .05). Interestingly, we found that even when we used a lower threshold of SUVmax ≥1.5 to define FES positivity, the pretherapy FES uptake still yielded nonsignificant results for predicting PFS (p = .628; supplemental online Fig. 1). Furthermore, median PFS was nearly doubled in patients with purely 18F‐FES‐positive disease compared with those with heterogeneous ER expression (14.6 months vs. 7.2 months; Fig. 2C). However, this was of borderline significance (p = .081).
Figure 2.
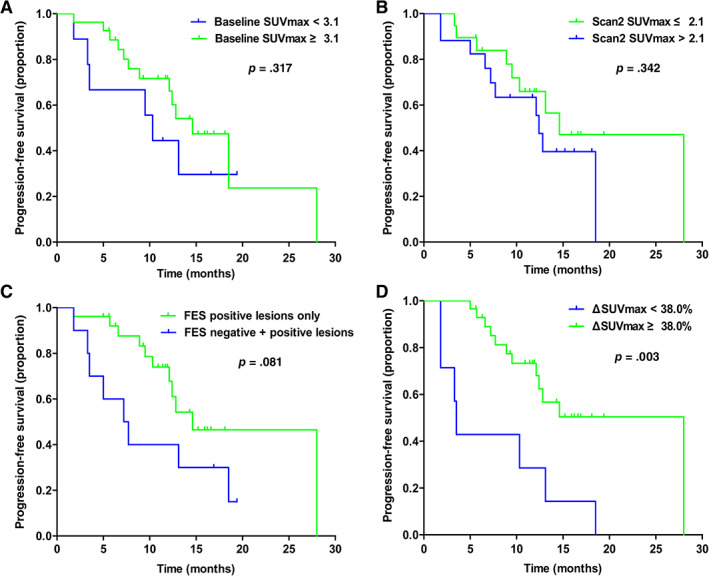
Kaplan‐Meier curves of progression‐free survival for patients with different imaging parameters. (A): For patients stratified by baseline SUVmax. (B): For patients stratified by scan 2 SUVmax. (C): For patients stratified by the presence or absence of 18F‐FES‐negative lesions. (D): For patients stratified by ΔSUVmax.Abbreviations: ΔSUVmax, change in SUVmax; FES, fluoroestradiol; SUVmax, maximum standard uptake value.
The relative decrease in 18F‐FES uptake and its relationship with response are shown in Fig. 3. Patients with a clinical benefit from fulvestrant had a significantly greater ΔSUVmax than those with PD (61.7% vs. 31.3%; p = .042). Representative patients with complete and incomplete reductions in 18F‐FES uptake at scan 2 are shown in Fig. 4. Using ROC curve analysis, the optimal threshold of ΔSUVmax for discriminating clinical efficacy was determined to be 38.0% with sensitivity, specificity, and area under the curve values of 90.0%, 66.7%, and 0.767, respectively. The Kaplan‐Meier plots indicated that patients with a ≥ 38% decrease in 18F‐FES uptake had a significantly longer PFS than patients with a < 38.0% decrease (28.0 months vs. 3.5 months; p = .003; Fig. 2D). Of note, the proportion of patients receiving fulvestrant as first‐line endocrine therapy with a < 38.0% decrease in median FES uptake (5 of 7, 71.4%) was comparable to that of patients with a ≥ 38% decrease (22 of 29, 75.8%).
Figure 3.
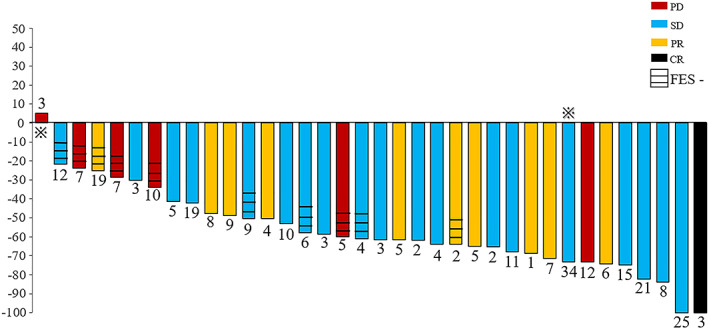
Waterfall plot showing the relative changes in tumor 18F‐FES uptake in individual patients treated with 500 mg of fulvestrant on follow‐up scans compared with baseline. FES ‐, presence of 18F‐FES negative lesion(s). The number on each bar represents the number of lesions per patient.  , the patient is also presented in Figure 4.Abbreviations: CR, complete response; FES, fluoroestradiol; PD, progressive disease; PR, partial response; SD, stable disease.
, the patient is also presented in Figure 4.Abbreviations: CR, complete response; FES, fluoroestradiol; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 4.
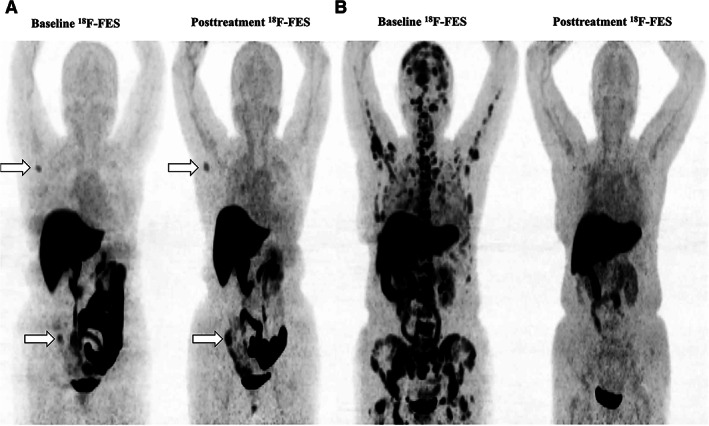
Representatives images of changes in 18F‐FES uptake in the tumor during treatment. (A): Incomplete reduction of 18F‐FES uptake. A 50‐year‐old premenopausal woman with invasive ductal breast cancer, who presented with bone and lymph node metastases after 42 months of adjuvant endocrine therapy (goserelin plus aromatase inhibitor). The patient received fulvestrant therapy as first‐line endocrine therapy and had a median change in maximum standard uptake value (ΔSUVmax) of −5.1% and a progression‐free survival (PFS) of 1.8 months. This patient is shown by the first bar in Figure 3. (B): Extensive reduction of 18F‐FES uptake. A 51‐year‐old postmenopausal woman with invasive ductal breast cancer, who presented with lung and bone metastases after a 7‐year disease‐free interval (completed 5 years of adjuvant tamoxifen therapy). The patient received fulvestrant therapy as first‐line endocrine therapy and had a median ΔSUVmax of 73.2% and a PFS of 11.7+ months. The patient's best response was stable disease. This patient is shown by the 29th bar in Figure 3.Abbreviation: FES, fluoroestradiol.
Univariate and Multivariate Analyses
To further validate the correlation between early changes in 18F‐FES uptake and fulvestrant treatment response, all image parameters and clinicopathologic factors were included in the Cox proportional hazards regression model (Table 2). ΔSUVmax were significantly related to PFS (p = .006). When we further corrected for these factors in the multivariate analysis, ΔSUVmax remained statistically significant in predicting PFS (hazard ratio 0.26, 95% confidence interval 0.10–0.68, p = .006).
Table 2.
Univariate and multivariate analysis of PFS among included patients
| Parameters | No. | Events | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|---|
| Median PFS (95% CI) | Log‐rank p value | HR (95% CI) | p value | HR (95% CI) | p value | |||
| Age, years | — | — | ||||||
| <65 | 26 | 16 | 12.1 (8.0–16.2) | .048 | 1 | .061 | ||
| ≥65 | 10 | 3 | 18.5 (12.7–24.3) | 0.30 (0.85–1.06) | ||||
| Menopausal status | — | — | ||||||
| Premenopausal | 8 | 5 | 10.3 (3.9–16.7) | .446 | 1 | .447 | ||
| Postmenopausal | 28 | 14 | 14.6 (9.7–19.5) | 0.67 (0.24–1.90) | ||||
| Disease‐free interval, years | — | — | ||||||
| ≤5 | 7 | 6 | 7.2 (3.4–11.0) | .096 | 1 | .109 | ||
| >5 | 18 | 7 | 18.5 (12.5–24.5) | 0.38 (0.12–1.24) | ||||
| Visceral disease | — | — | ||||||
| No | 21 | 10 | 13.1 (11.5–14.7) | .716 | 1 | .717 | ||
| Yes | 15 | 9 | 14.6 (3.3–25.9) | 0.84 (0.32–2.21) | ||||
| De novo metastatic disease | — | — | ||||||
| No | 25 | 13 | 14.6 (9.5–19.7) | .842 | 1 | .843 | ||
| Yes | 11 | 6 | 12.4 (7.6–17.2) | 1.11 (0.41–3.00) | ||||
| Prior palliative chemotherapy | — | — | ||||||
| No | 31 | 14 | 14.6 (10.9–18.3) | .467 |
1 1.51 (0.49–4.66) |
.470 | ||
| Yes | 5 | 5 | 12.4 (2.3–22.5) | |||||
| Number of prior lines of palliative ET | — | — | ||||||
| 0 | 27 | 11 | 18.5 (12.3–24.7) | .118 | 1 | .127 | ||
| ≥1 | 9 | 8 | 12.1 (7.7–16.5) | 2.12 (0.81–5.55) | ||||
| ΔSUVmax | ||||||||
| <38.0% | 7 | 7 | 3.5 (3.0–4.0) | .003 | 1 | .006 | 1 | .006 |
| ≥38.0% | 29 | 12 | 28.0 (not reached) | 0.26 (0.10–0.68) | 0.26 (0.10–0.68) | |||
| Baseline SUVmax | ||||||||
| <3.1 | 9 | 6 | 10.3 (8.0–12.6) | .317 | 1 | .323 | — | — |
| ≥3.1 | 27 | 13 | 14.6 (10.8–18.4) | 0.61 (0.23–1.63) | ||||
| Scan 2 SUVmax | ||||||||
| ≤2.1 | 19 | 9 | 14.6 (7.4–21.8) | .342 | 1 | .347 | — | — |
| >2.1 | 17 | 10 | 12.4 (11.3–13.5) | 1.57 (0.62–3.98) | ||||
| With negative 18F‐FES lesions | ||||||||
| No | 26 | 11 | 14.6 (8.4–20.8) | .081 | 1 | .089 | — | — |
| Yes | 10 | 8 | 7.2 (3.0–11.4) | 2.29 (0.88–5.93) | ||||
Bold type indicates significance.
Abbreviations: —, no data; ΔSUVmax, median change in maximum standard uptake value CI, confidence interval; ET, endocrine therapy; FES, fluoroestradiol; HR, hazard ratio; MBC, metastatic breast cancer; PFS, progression‐free survival; SUVmax, maximum standard uptake value.
Discussion
To our knowledge, this is the largest prospective study to evaluate the role of 18F‐FES PET/CT in predicting the response to fulvestrant 500 mg therapy in patients with HR+/HER2− MBC. Our results showed the advantage of serial FES PET/CT scans during treatment for the early prediction of response: patients with ΔSUVmax ≥38.0% experienced significantly longer PFS than those with reductions of less than 38.0%.
Despite the significant efficacy of fulvestrant therapy for the treatment of ER‐positive breast cancer, a considerable number of patients develop fulvestrant resistance. However, it takes several months for traditional imaging techniques to reliably measure treatment effects. Therefore, the early prediction of treatment response would be very valuable in clinical practice.
18F‐FES PET/CT has been shown to hold potential for assessing in vivo ER expression, predicting response to hormone therapy and adjuvant chemotherapy, evaluating effective ER blockade, pharmacodynamic monitoring of novel ER blockade, and assisting in individualized treatment strategy decision‐making [16, 17, 27, 28, 29]. In a retrospective study of 11 patients who underwent fulvestrant therapy, the mean decrease in 18F‐FES uptake in tumors was 49% [24]. However, the inadequate dose of fulvestrant and unconfirmed follow‐up 18F‐FES PET scans made it difficult to draw a conclusion regarding the relationship between the reduction in FES uptake and treatment response.
In accordance with another FES PET study [30], our results showed that ΔSUVmax, not baseline or residual 18F‐FES uptake, in tumors was correlated with treatment outcome. However, our study differed from this previous study in several respects. First, in the previous study, the follow‐up scan on day 28 (scan 2) was available for 12 of 16 patients, and that on day 84 (scan 3) was available for 9 of 16 patients. Their results showed that residual tumor 18F‐FES uptake was similar between scan 2 and scan 3. Therefore, we predefined the follow‐up scan at day 28, which was performed uniformly for all patients. Second, the cutoff point of 75% in the previous study was predefined on the basis of a previous retrospective study and was shown to be close to the optimal cutoff point by ROC analysis (76%). In our study, the cutoff point was determined by ROC analysis and further validated by the Cox proportional hazards regression model. Moreover, 4 of 16 patients in the previous study withdrew from tamoxifen treatment shortly before the baseline 18F‐FES PET scan. Because tamoxifen can compete for binding to ER, it potentially lowers 18F‐FES uptake [24] and interferes with the detection results. Although seven patients in our study received antiestrogens prior to fulvestrant, the minimum interval since the last use of antiestrogens was more than 6 months, thus minimizing the impact of recent therapies on baseline 18F‐FES uptake. Lastly, owing to the high physiologic background of 18F‐FES uptake in healthy liver [23], patients with liver metastases were excluded from our study as the quantification of 18F‐FES uptake in liver metastases was not feasible.
The main goal of our study was to examine whether 18F‐FES PET/CT could serve as an early predictor of treatment response. We found that ΔSUVmax greater than 38% was significantly associated with improved PFS. In contrast to traditional imaging techniques such as CT, which may take several months to reliably measure treatment effects [31], serial 18F‐FES PET scans could discriminate patients with disease that is sensitive or resistant to fulvestrant as early as 28 days after treatment. Moreover, patients who received fulvestrant as first‐line therapy generally benefited more than those who received fulvestrant as later‐line therapy, but our present study showed that classification using ΔSUVmax had more reliable predictive value than classification using lines of therapy. The improvement was observed in both the multivariate and univariate analyses. For patients with ΔSUVmax less than 38%, an individualized treatment strategy could be recommended, such as increasing the dose of fulvestrant [32] or adding targeted drugs such as everolimus [33] or cyclin‐dependent kinase 4 and 6 inhibitors [34, 35, 36]. Currently, an imaging biomarker trial prospectively applying 18F‐FES PET to guide therapy selection for patients with ER‐positive MBC is being performed at our center.
Another interesting aspect was the heterogeneity of ER expression in patients. There were differences in ER expression among the primary and metastatic sites, as well as intratumoral heterogeneity of receptor content within the same lesion [37, 38, 39]. In addition, ER status may change in a patient during follow‐up as a result of either treatment or genetic/epigenetic loss of the receptor [40]. In our study, intratumoral heterogeneity of ER expression was identified in 10 patients. The data demonstrated that patients with purely 18F‐FES‐positive lesions experienced a longer PFS than those with both 18F‐FES‐positive and 18F‐FES‐negative lesions. However, because the number of included patients was limited, the difference was of borderline significance. Our findings are consistent with those of a previous study [41], which showed that patients with heterogeneous FES uptake suffered from poorer clinical outcomes (supplemental online Fig. 2). Therapeutic failure may be attributable to the loss of ER expression, as higher ER expression levels have been shown to be associated with greater clinical benefit from endocrine therapy [42]. Moreover, another study has suggested the feasibility of analyzing an average SUVmax of the hottest 3–5 lesions instead of all FES‐positive lesions [21]. However, considering the heterogeneity of ER expression, we hope to see future studies with larger sample sizes to further compare these two analysis methods and to illustrate the predictive value of heterogeneous ER expression. To appreciate our findings, some strengths and limitations should be mentioned. Our preliminary results showed the potential of molecular imaging to predict treatment response in patients undergoing fulvestrant therapy. The relatively homogeneous study population and adequate follow‐up time support the validity and objectivity of our conclusions. In addition, our results provide a theoretical basis for individualized treatment strategies in clinical practice.
Inevitably, our study has several limitations. First, although the sample size was relatively large for an observational study, the study was conducted in a single center, and external validation is required. Second, it is difficult to use 18F‐FES to diagnose and assess treatment response in ER‐negative metastases. 18F‐FES‐negative sites may have been missed, resulting in a low estimate of 18F‐FES‐negative sites. Furthermore, it is difficult to diagnose liver lesions using 18F‐FES PET/CT because of high liver physiological uptake. Finally, we could not analyze the change in ER expression by pathology to compare the results with 18F‐FES PET/CT imaging. However, it is not feasible to observe changes in ER expression in the entire tumor by pathology, and ER expression may be heterogeneous in tumor lesions.
Conclusion
Our data demonstrated that 18F‐FES PET/CT could be used to discriminate patients who might benefit from fulvestrant and that it has the ability to predict treatment response early in patients undergoing endocrine therapy. Given these findings, an individualized treatment strategy should be recommended. Future prospective clinical trials are needed to further validate the results.
Author Contributions
Conception/design: Min He, Yingjian Zhang, Zhongyi Yang, Zhiming Shao
Provision of study material or patients: Min He, Yin Liu, Guangyu Liu, Zhonghua Wang
Collection and/or assembly of data: Min He, Cheng Liu, Zhongyi Yang, Zhonghua Wang
Data analysis and interpretation: Min He, Cheng Liu, Qin Shi, Yuyun Sun, Yongping Zhang, Xiaoping Xu, Huiyu Yuan, Zhonghua Wang
Manuscript writing: Min He, Cheng Liu, Zhongyi Yang, Zhiming Shao
Final approval of manuscript: Min He, Cheng Liu, Qin Shi, Yuyun Sun, Yongping Zhang, Xiaoping Xu, Huiyu Yuan, Yingjian Zhang, Yin Liu, Guangyu Liu, Genhong Di, Zhongyi Yang, Zhonghua Wang, Zhiming Shao
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information.
Appendix S2. Supporting Information.
Acknowledgments
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. This study was supported by the National Natural Science Foundation of China (81802638), the Shanghai Committee of Science and Technology Funds (18ZR1407500), and the Shanghai Engineering Research Center of Molecular Imaging Probes (19DZ2282200).
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Contributor Information
Zhongyi Yang, Email: yangzhongyi21@163.com.
Zhonghua Wang, Email: zhonghuawang_95@yeah.net.
Zhiming Shao, Email: zhiimin_shao@yeah.net.
References
- 1. Lobbezoo DJ, van Kampen RJ, Voogd AC et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2‐positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat 2013;141:507–514. [DOI] [PubMed] [Google Scholar]
- 2. Cardoso F, Costa A, Senkus E et al. 3rd ESO‐ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann Oncol 2017;28:3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maximov PY, Lee TM, Jordan VC. The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr Clin Pharmacol 2013;8:135–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res 1991;51:3867‐3873. [PubMed] [Google Scholar]
- 5. Robertson JF, Lindemann J, Garnett S et al. A good drug made better: The fulvestrant dose‐response story. Clin Breast Cancer 2014;14:381–389. [DOI] [PubMed] [Google Scholar]
- 6. Di Leo A, Jerusalem G, Petruzelka L et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor‐positive advanced breast cancer. J Clin Oncol 2010;28:4594–4600. [DOI] [PubMed] [Google Scholar]
- 7. Robertson JFR, Bondarenko IM, Trishkina E et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor‐positive advanced breast cancer (FALCON): An international, randomised, double‐blind, phase 3 trial. Lancet 2016;388:2997–3005. [DOI] [PubMed] [Google Scholar]
- 8. Robertson JF, Lindemann JP, Llombart‐Cussac A et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first‐line treatment of advanced breast cancer: Follow‐up analysis from the randomized 'FIRST' study. Breast Cancer Res Treat 2012;136:503–511. [DOI] [PubMed] [Google Scholar]
- 9. Ellis MJ, Llombart‐Cussac A, Feltl D et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first‐line treatment of advanced breast cancer: Overall survival analysis from the phase II FIRST Study. J Clin Oncol 2015;33:3781–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang J, Xu B. Efficacy and safety of fulvestrant in postmenopausal patients with hormone receptor‐positive advanced breast cancer: A systematic literature review and meta‐analysis. Breast Cancer Res Treat 2018;171:535–544. [DOI] [PubMed] [Google Scholar]
- 11. Linden HM, Stekhova SA, Link JM et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol 2006;24:2793–2799. [DOI] [PubMed] [Google Scholar]
- 12. Fisher ER, Anderson S, Dean S et al. Solving the dilemma of the immunohistochemical and other methods used for scoring estrogen receptor and progesterone receptor in patients with invasive breast carcinoma. Cancer 2005;103:164–173. [DOI] [PubMed] [Google Scholar]
- 13. Hammond ME, Hayes DF, Dowsett M et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010;28:2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amir E, Clemons M, Purdie CA et al. Tissue confirmation of disease recurrence in breast cancer patients: Pooled analysis of multi‐centre, multi‐disciplinary prospective studies. Cancer Treat Rev 2012;38:708–714. [DOI] [PubMed] [Google Scholar]
- 15. Peterson LM, Mankoff DA, Lawton T et al. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F‐fluoroestradiol. J Nucl Med 2008;49:367–374. [DOI] [PubMed] [Google Scholar]
- 16. Liao GJ, Clark AS, Schubert EK et al. 18F‐fluoroestradiol PET: Current status and potential future clinical applications. J Nucl Med 2016;57:1269–1275. [DOI] [PubMed] [Google Scholar]
- 17. van Kruchten M, de Vries EGE, Brown M et al. PET imaging of oestrogen receptors in patients with breast cancer. Lancet Oncol 2013;14:e465–e475. [DOI] [PubMed] [Google Scholar]
- 18. Yang Z, Sun Y, Xu X et al. The assessment of estrogen receptor status and its intratumoral heterogeneity in patients with breast cancer by using 18F‐fluoroestradiol PET/CT. Clin Nucl Med 2017;42:421–427. [DOI] [PubMed] [Google Scholar]
- 19. Gong C, Yang Z, Sun Y et al. A preliminary study of (18)F‐FES PET/CT in predicting metastatic breast cancer in patients receiving docetaxel or fulvestrant with docetaxel. Sci Rep 2017;7:6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin FI, Gonzalez EM, Kummar S et al. Utility of (18)F‐fluoroestradiol ((18)F‐FES) PET/CT imaging as a pharmacodynamic marker in patients with refractory estrogen receptor‐positive solid tumors receiving Z‐endoxifen therapy. Eur J Nucl Med Mol Imaging 2017;44:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kurland BF, Peterson LM, Lee JH et al. Estrogen receptor binding (18F‐FES PET) and glycolytic activity (18F‐FDG PET) predict progression‐free survival on endocrine therapy in patients with ER+ breast cancer. Clin Cancer Res 2017;23:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heidari P, Deng F, Esfahani SA et al. Pharmacodynamic imaging guides dosing of a selective estrogen receptor degrader. Clin Cancer Res 2015;21:1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mankoff DA, Peterson LM, Tewson TJ et al. [18F]fluoroestradiol radiation dosimetry in human PET studies. J Nucl Med 2001;42:679–684. [PubMed] [Google Scholar]
- 24. Linden HM, Kurland BF, Peterson LM et al. Fluoroestradiol positron emission tomography reveals differences in pharmacodynamics of aromatase inhibitors, tamoxifen, and fulvestrant in patients with metastatic breast cancer. Clin Cancer Res 2011;17:4799–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Kruchten M, Glaudemans AW, de Vries EF et al. PET imaging of estrogen receptors as a diagnostic tool for breast cancer patients presenting with a clinical dilemma. J Nucl Med 2012;53:182–190. [DOI] [PubMed] [Google Scholar]
- 26. Hamaoka T, Madewell JE, Podoloff DA et al. Bone imaging in metastatic breast cancer. J Clin Oncol 2004;22:2942–2953. [DOI] [PubMed] [Google Scholar]
- 27. Sun Y, Yang Z, Zhang Y et al. The preliminary study of 16alpha‐[18F]fluoroestradiol PET/CT in assisting the individualized treatment decisions of breast cancer patients. PLoS One 2015;10:e0116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang Z, Sun Y, Xue J et al. Can positron emission tomography/computed tomography with the dual tracers fluorine‐18 fluoroestradiol and fluorodeoxyglucose predict neoadjuvant chemotherapy response of breast cancer?–A pilot study. PLoS One 2013;8:e78192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Y, Ayres KL, Goldman DA et al. (18)F‐fluoroestradiol PET/CT measurement of estrogen receptor suppression during a phase I trial of the novel estrogen receptor‐targeted therapeutic GDC‐0810: Using an imaging biomarker to guide drug dosage in subsequent trials. Clin Cancer Res 2017;23:3053–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Kruchten M, de Vries EG, Glaudemans AW et al. Measuring residual estrogen receptor availability during fulvestrant therapy in patients with metastatic breast cancer. Cancer Discov 2015;5:72–81. [DOI] [PubMed] [Google Scholar]
- 31. Cardoso F, Harbeck N, Fallowfield L et al. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2012;23(suppl 7):vii11–19. [DOI] [PubMed] [Google Scholar]
- 32. Young OE, Renshaw L, Macaskill EJ et al. Effects of fulvestrant 750 mg in premenopausal women with oestrogen‐receptor‐positive primary breast cancer. Eur J Cancer 2008;44:391–399. [DOI] [PubMed] [Google Scholar]
- 33. O'Shaughnessy J, Thaddeus Beck J, Royce M. Everolimus‐based combination therapies for HR+, HER2‐ metastatic breast cancer. Cancer Treat Rev 2018;69:204–214. [DOI] [PubMed] [Google Scholar]
- 34. O'Leary B, Cutts RJ, Liu Y et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA‐3 trial. Cancer Discov 2018;8:1390–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sledge GW Jr, Toi M, Neven P et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2‐ advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017;35:2875–2884. [DOI] [PubMed] [Google Scholar]
- 36. Slamon DJ, Neven P, Chia S et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor‐positive, human epidermal growth factor receptor 2‐negative advanced breast cancer: MONALEESA‐3. J Clin Oncol 2018;36:2465–2472. [DOI] [PubMed] [Google Scholar]
- 37. Amir E, Miller N, Geddie W et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol 2012;30:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aurilio G, Disalvatore D, Pruneri G et al. A meta‐analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur J Cancer 2014;50:277–289. [DOI] [PubMed] [Google Scholar]
- 39. van Netten JP, Armstrong JB, Carlyle SJ et al. Cellular distribution patterns of estrogen receptor in human breast cancer. Eur J Cancer Clin Oncol 1988;24:1899–1901. [DOI] [PubMed] [Google Scholar]
- 40. Yoshida T, Eguchi H, Nakachi K et al. Distinct mechanisms of loss of estrogen receptor alpha gene expression in human breast cancer: Methylation of the gene and alteration of trans‐acting factors. Carcinogenesis 2000;21:2193–2201. [DOI] [PubMed] [Google Scholar]
- 41. Peterson LM, Kurland BF, Schubert EK et al. A phase 2 study of 16alpha‐[18F]‐fluoro‐17beta‐estradiol positron emission tomography (FES‐PET) as a marker of hormone sensitivity in metastatic breast cancer (MBC). Mol Imaging Biol 2014;16:431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Davies C, Godwin J, Gray R et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient‐level meta‐analysis of randomised trials. Lancet 2011;378:771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information.
Appendix S2. Supporting Information.


