Abstract
Background
Precision oncology uses molecular profiling of tumors to identify biomarker‐tailored therapies for patients in the hope of improving outcomes. Typically, only a minority of patients receives evaluable matched treatment. This study explored the reasons for attrition on a precision medicine trial.
Materials and Methods
Study participants were 190 adult patients who consented to the I‐PREDICT (Investigation of molecular Profile‐Related Evidence Determining Individualized Cancer Therapy) trial. Patients had metastatic and/or unresectable incurable malignancies. Patients who were not evaluable were analyzed.
Results
Of consented patients, 44% were not evaluable. Men were twice as likely to be not evaluable as women. Prominently, 45% of patients who were not evaluable dropped off because of death, hospice referral, or decline in organ function.
Conclusion
Health deterioration of consented patients is a significant barrier to being evaluable on the I‐PREDICT trial. These data suggest that patients are enrolled on precision oncology trials too late in their disease course or with excessive disease burden.
Short abstract
Few studies have focused on patient attrition in the context of personalized oncology studies. This article examines factors contributing to the loss of patients enrolled in a precision medicine trials.
Introduction
Genome‐driven cancer care is predicated on the presence of actionable alterations for which targeted therapies exist. Molecular profiling of tumors has become more common. Studies have demonstrated that profiling identifies actionable alterations in 40%–95% of patients [1, 2, 3, 4, 5, 6, 7, 8, 9, 10]. However, only 5% to ∼50% of eligible patients were treated with matched therapies [1, 2, 3, 4, 5, 6, 7, 8, 9, 10].
Limited studies have explored this low rate of matching and treatment in precision oncology trials. Common barriers include the discretion of treating oncologists, access to drugs, and the timing of profiling in advanced disease [1, 2, 3, 4, 5, 6]. The current study investigated patient attrition in the Investigation of molecular Profile‐Related Evidence Determining Individualized Cancer Therapy (I‐PREDICT) [10] trial.
Materials and Methods
I‐PREDICT Trial
The I‐PREDICT trial (ClinicalTrials.gov Identifier: NCT02534675) uses genomic profiling to match patients to treatment [10]. Next‐generation sequencing from Foundation Medicine profiled tumors (Cambridge, MA, http://www.foundationmedicine.com). These assays have been previously described [10]. Based on profiling results, a molecular tumor board recommended therapies to treating oncologists. All patients consented to an institutional review board–approved protocol.
Participants
The first 190 enrolled patients, beginning February 13, 2015, at the University of California, San Diego Moores Cancer Center site were included. Eligibility criteria for the I‐PREDICT trial have been previously outlined [10]. Participants were adults (age ≥ 18 years) with an incurable metastatic or unresectable malignancy that was treatment naïve and with ≥50% 2‐year mortality, or previously treated that had failed standard therapies or had no standard therapy.
Data Analysis
A secondary analysis of the I‐PREDICT trial data was performed. Demographic and clinicopathologic characteristics were described for patients who were not evaluable and those who were evaluable. Patients who were not evaluable were subdivided: untreated (since consent) and treated (with ≥1 dose of anticancer drug after consent; see supplemental online Materials and Methods).
Results
Patient Characteristics
Of the 190 total patients, the median age was 62 years (range: 21–93 years); 59% were women (n = 112); and 66% were White (n = 125). More than half had gastrointestinal cancers (n = 103, 54%). Most patients had received prior treatment (n = 123, 65%). Of these, the median number of prior lines of therapy was 2 (range: 1–11 therapies). At enrollment, 57 patients (30%) had excellent performance status. Overall, 56 patients (29%) died within 6 months, and 33 (17%) within 3 months of consent. In this cohort, 4% were awaiting treatment (n = 8), 52% were evaluable (n = 99), and 44% were not evaluable (n = 83). Of the 83 patients who were not evaluable, 28% were treated (n = 23) and 72% were untreated (n = 60; Table 1; Fig. 1).
Table 1.
The I‐PREDICT trial: characteristics of consented patients (University of California, San Diego site)
| Parameter a | Evaluable | Not evaluable | Group difference, p value g | Univariable (not evaluable vs. evaluable), OR, 95% CI, p value g , h | Multivariable (not evaluable vs. evaluable), OR, 95% CI, p value g , h | Awaiting treatment i |
|---|---|---|---|---|---|---|
| Consented, n = 190 b | 99 (52%) | 83 (44%) | 8 (4%) | |||
| Age, years | .51 | |||||
| Median = 62 (range: 21–93) | 62 (21–93) | 63 (27–93) | 59 (41–82) | |||
| <62, n = 94 (49%) | 50 (53%) | 39 (42%) | reference | 5 (5%) | ||
| ≥62, n = 96 (51%) | 49 (51%) | 44 (46%) | 1.2, 0.6–2.1, .64 | 3 (3%) | ||
| Gender | .04 | |||||
| Female, n = 112 (59%) | 64 (57%) | 41 (37%) | reference | reference | 7 (6%) | |
| Male, n = 78 (41%) | 35 (45%) | 42 (54%) | 1.9, 1.0–3.4, .04 | 2.0, 1.1–3.9, .03 | 1 (1%) | |
| Ethnicity/Race | .34 | |||||
| White, n = 125 (66%) | 68 (54%) | 50 (40%) | 0.8, 0.4–1.6, .56 | 7 (6%) | ||
| Hispanic, n = 22 (11%) | 9 (41%) | 13 (59%) | 1.6, 0.6–4.6, .38 | 0 | ||
| Other, n = 43 (23%) c | 22 (51%) | 20 (47%) | reference | 1 (2%) | ||
| Tumor type d | .36 | |||||
| Gastrointestinal, n = 103 (54%) | 50 (49%) | 49 (47%) | 1.6, 0.8–3.1, .16 | 1.5, 0.8–2.9, .24 | 4 (4%) | |
| Gynecological, n = 27 (14%) | 13 (48%) | 12 (45%) | 1.5, 0.6–3.9, .39 | 2 (7%) | ||
| Other, n = 60 (32%) | 36 (60%) | 22 (37%) | reference | reference | 2 (3%) | |
| Treatment status before trial | .31 | |||||
| Prior treatment, n = 123 (65%) | 68 (55%) | 51 (42%) | reference | 4 (3%) | ||
| Treatment naïve, n = 67 (35%) | 31 (46%) | 32 (48%) | 1.4, 0.7–2.5, .31 | 4 (6%) | ||
| Prior therapies e | .94 | |||||
| Median = 2 (range: 1–11) | 2 (1–11) | 2 (1–7) | 1 (1–4) | |||
| <2, n = 94 (49%) | 28 (58%) | 18 (38%) | reference | 2 (4%) | ||
| ≥2, n = 96 (51%) | 40 (53%) | 33 (44%) | 1.3, 0.6–2.7, .52 | 2 (3%) | ||
| ECOG status f | .24 | |||||
| 0, n = 57 (30%) | 33 (58%) | 21 (37%) | reference | 3 (5%) | ||
| ≥1, n = 133 (70%) | 66 (50%) | 62 (46%) | 1.5, 0.7–2.9, .24 | 5 (4%) | ||
| Death after consent | ||||||
| <3 months, n = 33 (17%) | 16 (48%) | 17 (52%) | .45 | 0 | ||
| <6 months, n = 56 (29%) | 28 (50%) | 28 (50%) | .43 | 0 |
Data are presented as n (%), unless otherwise stated.
All parameters were from the time of consent.
Only patients consented at the University of California, San Diego site. There was a total of 190 patients. These included 182 evaluable and not evaluable patients and 8 awaiting treatment.
Includes non‐Hispanic ethnicity of Asian, Black or African American, other, and declined to state races.
Gastrointestinal tumor type includes 28 hepatobiliary and pancreatic cancers. Other tumor types are all tumor types other than gastrointestinal and gynecological. A detailed profile of tumor types is in supplemental online Table 1.
Number of prior systemic therapies, including adjuvant or neoadjuvant, only among patients receiving prior treatment before enrollment in the I‐PREDICT trial (n = 123, 65%).
ECOG performance status.
Comparison between evaluable and not evaluable patients; excludes eight patients awaiting treatment for less than 6 months.
Association between not evaluable status and parameter. Parameters in the univariable analysis with p ≤ .2 were included in the multivariable analysis; evaluable status = outcome reference.
Not yet determined whether evaluable or not evaluable as of September 26, 2017.
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; OR, odds ratio.
Figure 1.
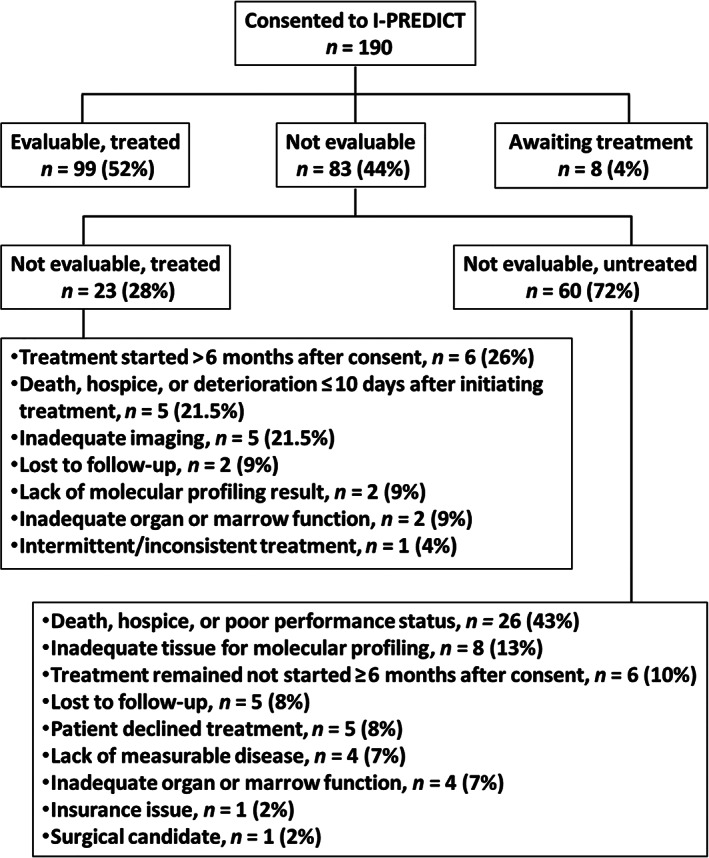
Reasons for being not evaluable in the I‐PREDICT trial (University of California, San Diego site).
Characteristics Associated with Being Not Evaluable
Of the 83 patients who were not evaluable, there were more men (54%) than women (37%; p = .04). Patients with gastrointestinal cancer trended to be not evaluable (p = .16). However, only gender was independently associated with not evaluable status; men were twice as likely to be not evaluable as women (odds ratio = 2.0, 95% confidence interval: 1.1–3.9, p = .03, multivariable analysis; Table 1).
Reasons for Being Not Evaluable
The most common reason for being not evaluable was the deteriorating health of patients, which led to early discontinuation of treatment, hospice care, or death (n = 31, 37% of 83 patients who were not evaluable), plus another 7% who had inadequate organ function (n = 6 of 83 patients). Hence, health decline explained 45% of patients who were not evaluable (n = 37 of 83 patients). Treatment delays, usually for personal reasons, accounted for 14% of patients (n = 12 of 83 patients). Only 12% experienced molecular profiling issues (n = 10 of 83 patients), and 8% were lost to follow‐up (n = 7 of 83 patents). Notably, only 1 patient had insufficient insurance coverage (1.2% of 83 patients; Fig. 1).
Discussion
Growing evidence indicates that matched molecularly targeted therapies may yield improved cancer outcomes [2, 4, 5, 6, 7]. Nevertheless, most patients in precision medicine trials remain untreated/unmatched [1, 2, 3, 4, 5, 6, 7, 8, 9, 10]. We explored patient attrition in the I‐PREDICT trial, which uses genomic sequencing to navigate patients to therapy [10]. Of 190 consecutively enrolled patients, 44% were not evaluable (n = 83). Only male gender was independently associated with not evaluable status (p = .03, multivariable analysis). Prominently, 45% of attrition (n = 37 of the 83 patients who were not evaluable; 19% of 190 consented patients) was attributable to declining health. Other studies also reported that patients were frequently not evaluable on precision medicine trials because of death or hospice transfer [2, 4, 8, 9].
Studies have also reported that patient access to matched clinical trials/therapies was hindered by extensive inclusion criteria, insurance denial, travel restrictions, and lack of available protocols [2, 5–7]. In contrast, only one I‐PREDICT patient dropped off owing to lack of insurance coverage, and drug access was not a significant barrier in the I‐PREDICT trial. Clinical trial navigators and medication acquisition specialists, who are devoted to ensuring that patients receive treatment, and a just‐in‐time molecular tumor board are incorporated into the workflow of the trial to circumvent these barriers.
The treatment rate in the I‐PREDICT cohort was high for a precision medicine trial (52%). This may be partly explained by the few molecular profiling issues experienced in the I‐PREDICT trial (5%, n = 10 of 190 consented patients). In addition to the design features of the trial discussed above, identifying actionable alterations in I‐PREDICT patients may have been facilitated by using a large gene panel as well as blood‐based sequencing. Studies have shown that such assays can identify actionable alterations in up to 90% of patients [2, 4], suggesting that the treatment rate can still be improved.
Conclusion
Health deterioration of patients after consent is a significant barrier to being evaluable on the current genome‐driven precision oncology trial (I‐PREDICT) [10]. Studies should investigate tumor burden, pace of progression, and other features that might correlate with imminent worsening. Consideration should be given to ensuring that patients are enrolled on precision medicine studies before their condition is in rapid decline.
Disclosures
Jason K. Sicklick: Foundation Medicine, Inc., Novartis Pharmaceuticals, Blueprint Medicines, Amgen, Inc. (RF), Loxo Oncology, Inc., Biotheranostics, Deciphera Pharmaceuticals, Grand Rounds (C/A), La Hoffman‐Roche (H); Shumei Kato: Foundation Medicine, Inc. (C/A); Vincent A. Miller: Memorial Sloan Kettering Cancer Center (IP), Roche Foundation Medicine, Inc., Revolution Medicines, Mirati Therapeutics, Inc. (OI); Razelle Kurzrock: Incyte Corporation, Genentech, Inc., Konica Minolta, Inc., Merck Serono, Pfizer, Inc., Sequenom, Foundation Medicine, Inc., Grifols S.A., Guardant Health (RF), Loxo Oncology, Inc., NeoMed, Inc., XBiotech, Actuate Therapeutics, Inc. (C/A), Roche (ET), IDbyDNA, Inc., CureMatch, Inc., CureMatrix, Inc. (OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information.
Supplemental Table 1 Tumor Types of Consented Patients in the I‐PREDICT Trial (University of California San Diego site)
Acknowledgments
This work was supported in part by Foundation Medicine, Inc. (J.K.S. and R.K.), as well as the Joan and Irwin Jacobs Philanthropic Fund (R.K.), the Jon Schneider Memorial Cancer Research Fund (J.K.S.), and the National Cancer Institute at the National Institutes of Health (grant number P30CA023100; J.K.S. and R.K.). We also acknowledge the support of the National Institutes of Health (grant numbers K08CA168999, R21CA192072, and R01CA226803), as well as Pedal the Cause, David Foundation, and Kristen Ann Carr Fund (J.K.S.).
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Disclosures of potential conflicts of interest may be found at the end of this article.
Contributor Information
Jason K. Sicklick, Email: jsicklick@health.ucsd.edu.
Razelle Kurzrock, Email: rkurzrock@health.ucsd.edu.
References
- 1. Tsimberidou AM, Iskander NG, Hong DS et al. Personalized medicine in a phase I clinical trials program: The MD Anderson Cancer Center initiative. Clin Cancer Res 2012;18:6373–6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schwaederle M, Parker BA, Schwab RB et al. Precision oncology: The UC San Diego Moores Cancer Center PREDICT experience. Mol Cancer Ther 2016;15:743–752. [DOI] [PubMed] [Google Scholar]
- 3. Meric‐Bernstam F, Brusco L, Shaw K et al. Feasibility of large‐scale genomic testing to facilitate enrollment onto genomically matched clinical trials. J Clin Oncol 2015;33:2753–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wheler JJ, Janku F, Naing A et al. Cancer therapy directed by comprehensive genomic profiling: A single center study. Cancer Res 2016;76:3690–3701. [DOI] [PubMed] [Google Scholar]
- 5. Massard C, Michiels S, Ferte C et al. High‐throughput genomics and clinical outcome in hard‐to‐treat advanced cancers: Results of the MOSCATO 01 trial. Cancer Discov 2017;7: 586–595. [DOI] [PubMed] [Google Scholar]
- 6. Tsimberidou A‐M, Hong DS, Ye Y et al. Initiative for molecular profiling and advanced cancer therapy (IMPACT): An MD Anderson precision medicine study. JCO Precis Oncol 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stockley TL, Oza AM, Berman HK et al. Molecular profiling of advanced solid tumors and patient outcomes with genotype‐matched clinical trials: The Princess Margaret IMPACT/COMPACT trial. Genome Med 2016;8:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Le Tourneau C, Delord JP, Goncalves A et al. Molecularly targeted therapy based on a tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicenter, open‐label, proof‐of‐concept, randomised, controlled phase 2 trial. Lancet Oncol 2015;16:1324–1334. [DOI] [PubMed] [Google Scholar]
- 9. Tredan O, Wang Q, Pissaloux D et al. Molecular screening program to select molecular‐based recommended therapies for metastatic cancer patients: Analysis from the ProfiLER trial. Ann Oncol 2019;30:757–765. [DOI] [PubMed] [Google Scholar]
- 10. Sicklick JK, Kato, S , Okamura R et al. Molecular profiling of cancer patients enables personalized combination therapy: The I‐PREDICT study. Nat Med 2019;25:744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information.
Supplemental Table 1 Tumor Types of Consented Patients in the I‐PREDICT Trial (University of California San Diego site)


