Abstract
Background
Matrix metalloproteinase 9 (MMP9) expression in the tumor microenvironment is implicated in multiple protumorigenic processes. Andecaliximab (GS‐5745), a monoclonal antibody targeting MMP9 with high affinity and selectivity, was evaluated in combination with gemcitabine and nab‐paclitaxel in patients with advanced pancreatic adenocarcinoma.
Patients and Methods
This phase I study was completed in two parts: part A was a dose‐finding, monotherapy phase that enrolled patients with advanced solid tumors, and part B examined andecaliximab in combination with chemotherapy in specific patient cohorts. In the cohort of patients with pancreatic adenocarcinoma (n = 36), andecaliximab 800 mg every 2 weeks was administered in combination with gemcitabine and nab‐paclitaxel. Patients were treated until unacceptable toxicity, withdrawal of consent, disease progression, or death. Efficacy, safety, and biomarker assessments were performed.
Results
Andecaliximab combined with gemcitabine and nab‐paclitaxel appeared to be well tolerated and did not demonstrate any unusual toxicities in patients with pancreatic adenocarcinoma. The most common treatment‐emergent adverse events were fatigue (75.0%), alopecia (55.6%), peripheral edema (55.6%), and nausea (50.0%). Median progression‐free survival was 7.8 months (90% confidence interval, 6.9−11.0) with an objective response rate of 44.4% and median duration of response of 7.6 months. Maximal andecaliximab target binding, defined as undetectable, andecaliximab‐free MMP9 in plasma, was observed.
Conclusion
Andecaliximab in combination with gemcitabine and nab‐paclitaxel demonstrates a favorable safety profile and clinical activity in patients with advanced pancreatic adenocarcinoma.
Implications for Practice
The combination of andecaliximab, a novel, first‐in‐class inhibitor of matrix metalloproteinase 9, with gemcitabine and nab‐paclitaxel in patients with advanced pancreatic adenocarcinoma provided a median progression‐free survival of 7.8 months and objective response rate of 44.4%. The majority of systemic biomarkers related to matrix metalloproteinase 9 activity and immune suppression increased at 2 months, whereas biomarkers related to tumor burden decreased. Although this study demonstrates promising results with andecaliximab plus chemotherapy in patients with advanced pancreatic adenocarcinoma, andecaliximab was not associated with a survival benefit in a phase III study in patients with advanced gastric/gastroesophageal junction carcinoma.
Keywords: Andecaliximab, GS‐5745, Matrix metalloproteinase 9, Pancreatic adenocarcinoma
Short abstract
This article presents the results of a phase I study evaluating the safety and efficacy of andecaliximab in advanced pancreatic adenocarcinoma.
Introduction
Matrix metalloproteinases (MMPs) are a family of at least 23 Zn2+‐dependent proteases involved in the degradation and remodeling of the extracellular matrix and basement membrane, as well as activation or inactivation of growth factors, cytokines, and chemokines, in normal and pathologic biological processes [1]. MMP9 is an inducible MMP expressed heterogeneously by tumor epithelia, infiltrating macrophages, neutrophils, other inflammatory cells, fibroblastic stroma, and tumor‐associated endothelial cells. Expression of MMP9 by tumor epithelia has been implicated in many protumorigenic processes and is associated with either loss of tumor suppressor or gain of oncogenic activity as a temporal response either to changes in local tumor environment or during processes such as invasion and proliferation [2, 4]. MMP9 activation can release cytokines, growth factors, and bioactive protein fragments that modulate inflammation, neovascularization, and matrix remodeling [1, 2, 3]. Elevated tumoral MMP9 protein or RNA levels are associated with reduced overall survival in pancreatic and gastric cancer [4, 5, 6, 7].
Early clinical experience with pan‐MMP inhibitors in cancer demonstrated potential efficacy but was associated with dose‐limiting musculoskeletal syndrome [8, 9]. Andecaliximab is a recombinant chimeric IgG4 monoclonal antibody (mAb) that demonstrates high affinity and selectivity for MMP9 [10, 11]. Andecaliximab was engineered without T‐cell epitopes to reduce the risk of immunogenicity [12, 13]. In a colorectal cancer xenograft model, inhibition of tumor‐derived or stroma‐derived MMP9 with a murine mAb targeting the same MMP9 epitope significantly reduced tumor growth and the incidence of metastasis, irrespective of whether stromal or epithelial MMP9 was targeted. This highlights the disease‐associated role of both tumor and stromal cellular sources of MMP9 in tumorigenesis [10].
The overexpression of MMP9 in pancreatic cancer, previous clinical experience with a pan‐MMP inhibitor [8, 9, 14, 15], and data on correlation of MMP9 expression and patient survival provide a rationale for evaluating andecaliximab combined with chemotherapy in pancreatic adenocarcinoma. Even though the incidence of pancreatic adenocarcinoma is low, it remains a devastating disease with a 5‐year survival rate of only 10% in the U.S. [16]. Between 1973 and 2014, the incidence rates of pancreatic cancer increased by 1% per year, leading to projections that it will be the second leading cause of cancer‐related deaths by 2030 [17]. There has been minimal improvement in survival rates over the last few decades [16], highlighting an unmet need for methods of early detection as well as novel therapeutic strategies.
To evaluate the safety and efficacy of andecaliximab in advanced solid tumors, we initiated a phase I study (ClinicalTrials.gov identifier: NCT01803282) in two parts: a monotherapy dose‐finding stage (part A) and a combination treatment stage (part B), which combined andecaliximab with chemotherapy regimens in patients with selected tumor types. An andecaliximab dose of 800 mg i.v. every 2 weeks (Q2W) or 1,200 mg i.v. every 3 weeks was selected based on data from the dose‐finding stage of this study and has been previously reported [18]. Gemcitabine with nab‐paclitaxel was selected for combination with andecaliximab in patients with advanced pancreatic ductal adenocarcinoma (PDAC) based on the National Comprehensive Cancer Network guidelines for pancreatic adenocarcinoma (version 3.2017) [19, 20]. In this paper, we present data from the 36 patients with PDAC enrolled in part B of the study.
Subjects, Materials, and Methods
Study Design
This phase I study was divided into two parts: part A was a dose‐finding monotherapy phase enrolling patients with advanced solid tumors, and part B combined andecaliximab with chemotherapy in specific patient expansion cohorts, including patients with advanced PDAC, non‐small cell lung cancer, gastric/esophagogastric junction (GEJ) adenocarcinoma, colorectal adenocarcinoma, and HER2‐negative breast cancer (supplemental online Fig. 1). Planned enrollment was 10 to 40 patients per cohort in part B, sufficient to allow assessment of pharmacokinetics (PK), safety, and tumor response. Local ethics committees/institutional review boards at all participating centers approved the study. All patients gave written informed consent before entering the study. Healthy volunteer controls were enrolled on a separate protocol, and their samples were used for comparison in this study.
Figure 1.
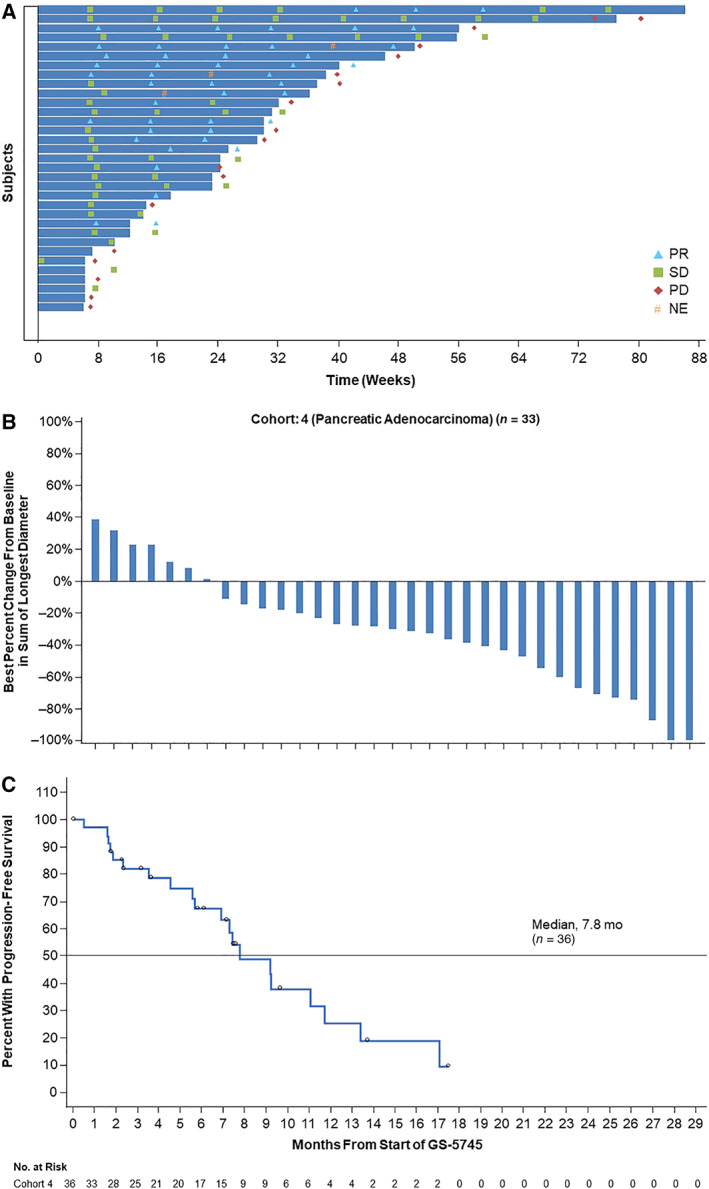
Efficacy summary for patients with pancreatic adenocarcinoma. (A): Exposure and response in individual patients (n = 36). (B): Best percent change from baseline in sum of longest diameter in patients with target lesions (n = 33) at screening. (C): Progression‐free survival.Abbreviations: NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Endpoints
The primary endpoint was safety, evaluated by incidence of adverse events (AEs), assessment of clinical laboratory test findings, physical examination, 12‐lead ECG, and vital sign measurements. Efficacy was an exploratory endpoint, which included investigator‐assessed objective response rate (ORR) and progression‐free survival (PFS). Exploratory biomarker analyses were performed to evaluate the association of each biomarker with clinical outcomes, and the modulation of biomarkers related to mechanism of action and disease progression.
Patient Eligibility
Key inclusion criteria included age ≥ 18 years; histologically confirmed, inoperable, locally advanced or metastatic pancreatic adenocarcinoma that had not been treated in the metastatic setting; Eastern Cooperative Oncology Group performance status of ≤1; life expectancy of >3 months; adequate hematologic, hepatic, and coagulation function; serum creatinine ≤1.5 × upper limit of normal; and willingness to follow adequate precautions to prevent pregnancy. Key exclusion criteria included significant comorbid medical conditions that posed a risk to patient safety or limited study participation, pregnancy or lactation in women, untreated central nervous system metastases, and known human immunodeficiency virus and hepatitis B or C infection.
Study Treatment
Andecaliximab 800 mg was administered on days 1 and 15 of each 28‐day cycle; gemcitabine and nab‐paclitaxel were administered as follows: gemcitabine 1,000 mg/m2 i.v. on days 1, 8, and 15 of a 28‐day treatment cycle and nab‐paclitaxel 125 mg/m2 i.v. on days 1, 8, and 15 of a 28‐day treatment cycle. Gemcitabine and nab‐paclitaxel were administered immediately after andecaliximab on days 1 and 15 of each cycle. Patients were treated until unacceptable toxicity, withdrawal of consent, disease progression, or death.
Biomarker Samples
Blood samples were collected at screening, at day 1 prior to treatment (baseline [BL]), at the beginning of each treatment cycle, and prior to treatment infusion. Archival formalin‐fixed, paraffin‐embedded tumor specimens were available from 20 of 36 patients.
Pharmacodynamic MMP9‐Binding Assay
Total (bound and free) MMP9 and free MMP9 were measured in serial platelet‐poor plasma samples as previously described [15]. Maximal circulating MMP9 coverage (MMP9 bound to andecaliximab) is achieved when levels of andecaliximab‐free MMP9 are below detection. Healthy volunteer control samples were sourced from a commercial vendor and were not sex and age matched.
Collagen Neoepitope Assays
Collagen 1 (C1M) was measured as previously described [15]; other neoepitopes measured were collagen 3 (C3M), collagen 4 alpha 1 (C4M2), and a basement membrane component laminin alpha 5 in serial serum samples by enzyme‐linked immunosorbent assay (ELISA; Nordic Bioscience A/S, Herlev, Denmark). For the latter two, not all patients’ samples were tested. For association with PFS, the C1M low and high were defined with a cutoff at the median of 79.25 ng/mL.
Serum Biomarker Screen
A total of 131 circulating serum biomarkers were measured by ELISA (R&D Systems, Inc., Minneapolis, MN) and Meso Scale Discovery (Meso Scale Diagnostics, LLC, Rockville, MD). Treatment effect on biomarkers was assessed using percent BL at cycle 3, day 1, by Wilcoxon signed rank test. Multiple testing was controlled for by using the false discovery rate.
Association between biomarker BL value and best overall response was assessed using the machine learning method, random forest, and a receiver operating characteristics curve.
MMP9 Immunohistochemistry
Immunohistochemistry (IHC) for MMP9 was performed using rabbit mAb from Abcam Ab76003 (clone EP1254; Cambridge, UK).
Efficacy Assessments
Disease burden was evaluated at screening by physical examination and radiographic assessment (contrast‐enhanced computed tomography or magnetic resonance imaging) and then at every 8 weeks. Responses were assessed by investigators per RECIST version 1.1 criteria [18]. ORR was defined as the proportion of patients with complete response (CR) or partial response (PR). The Clopper‐Pearson method was used to calculate the exact confidence intervals (CIs) of ORR. PFS was defined as the time interval from the first dose of andecaliximab to the earlier of the first documentation of definitive disease progression or death from any cause, analyzed using Kaplan‐Meier methods.
Safety Assessments
Safety assessments were performed prior to each andecaliximab infusion. AEs were assessed per the Common Terminology Criteria for Adverse Events version 4.03 criteria [19].
Results
Patient Characteristics
Between October 2013 and December 2015, 36 patients with PDAC were enrolled in the study from 10 sites in the U.S. Patient characteristics are summarized in Table 1.
Table 1.
Patient demographics and baseline characteristics
| Characteristic | Value |
|---|---|
| Patients, n | 36 |
| Male, n (%) | 26 (72.2) |
| Age, median (range), yr | 66 (40−83) |
| ECOG PS at screening, n (%) | |
| 0 | 12 (33.3) |
| 1 | 24 (66.7) |
| Disease stage at screening, n (%) | |
| Locally advanced | 8 (22.2) |
| Metastatic | 28 (77.8) |
| Patients with ≥1 prior systemic chemotherapy, n (%) a , b | 5 (13.9) |
| Prior chemotherapy regimens, median (range) | 1 (1−2) |
| Patients with ≥1 prior radiation regimen, n (%) | 3 (8.3) |
5‐Fluorouracil (FU) − containing regimen (n = 3), gemcitabine‐containing regimen (n = 4).
All five patients received chemotherapy in the neoadjuvant or adjuvant setting. One patient was administered a 5‐FU regimen twice, once each in the neoadjuvant and adjuvant settings. A second patient received two separate gemcitabine regimens in the neoadjuvant setting.
Abbreviation: ECOG PS, Eastern Cooperative Oncology Group performance status.
Safety
The median duration of exposure to andecaliximab was 23.6 weeks (range: 0.1−86.1) with a median of 11 doses (range: 1−37) received. The median duration of exposure and median number of doses of gemcitabine with nab‐paclitaxel were as follows: gemcitabine 23.6 weeks (range: 0.1−86.1), 17 doses (range: 1−56), and nab‐paclitaxel 20.6 weeks (range: 0.1−86.1), 15 doses (range: 1−52).
AEs are reported in Table 2. One grade 5 event of duodenal perforation was observed and considered unrelated to andecaliximab, gemcitabine, or nab‐paclitaxel. Treatment‐emergent AEs of any grade observed in ≥50% of all patients included fatigue (75.0%), alopecia (55.6%), peripheral edema (55.6%), and nausea (50.0%). As of August 31, 2016, all patients had discontinued all study treatments.
Table 2.
Treatment‐emergent adverse events of any grade observed in at least 15% of patients and grade 3–4 adverse events observed in at least 5% of patients
| AE | AEs of any grade, n (%) | Grade 3−4 AEs, n (%) |
|---|---|---|
| Any treatment‐emergent AEs | 36 (100.0) | 29 (80.6) |
| Fatigue | 27 (75.0) | 5 (13.9) |
| Alopecia | 20 (55.6) | |
| Edema peripheral | 20 (55.6) | |
| Nausea | 18 (50.0) | |
| Diarrhea | 17 (47.2) | |
| Neutropenia | 12 (33.3) | 9 (25.0) |
| Pyrexia | 12 (33.3) | |
| Anemia | 11 (30.6) | 7 (19.4) |
| Cough | 11 (30.6) | |
| Neuropathy peripheral | 11 (30.6) | |
| Vomiting | 11 (30.6) | |
| Decreased appetite | 10 (27.8) | |
| Dysgeusia | 10 (27.8) | |
| Thrombocytopenia | 10 (27.8) | |
| Hypokalemia | 9 (25.0) | 2 (5.6) |
| Rash | 9 (25.0) | |
| Constipation | 8 (22.2) | 2 (5.6) |
| Dry skin | 8 (22.2) | |
| Dyspnea | 8 (22.2) | |
| Pain in extremity | 8 (22.2) | |
| Paresthesia | 8 (22.2) | |
| Abdominal pain | 7 (19.4) | |
| Anxiety | 7 (19.4) | |
| Dizziness | 7 (19.4) | |
| Myalgia | 7 (19.4) | |
| Weight decreased | 7 (19.4) | |
| Asthenia | 6 (16.7) | |
| Cellulitis | 6 (16.7) | 2 (5.6) |
| Deep vein thrombosis | 6 (16.7) | |
| Neutrophil count decreased | 6 (16.7) | 2 (5.6) |
| Bacteremia | NA | 2 (5.6) |
| Dehydration | NA | 2 (5.6) |
| Hyponatremia | NA | 3 (8.3) |
| Hypoxia | NA | 2 (5.6) |
| Hypertension | NA | 2 (5.6) |
| Pneumonitis | NA | 2 (5.6) |
Abbreviations: AE, adverse event; NA, not applicable.
Table 3.
Investigator‐assessed efficacy
| Response | All patients (n = 36), n (%) |
|---|---|
| CR | 0 |
| PR | 16 (44.4) |
| SD | 13 (36.1) |
| Non‐CR/non‐PD | 0 |
| PD | 4 (11.1) |
| ORR (90% CI), % | 44.4 (30.2−59.4) |
| DOR (90% CI), months | 7.6 (3.9−9.9) a |
| PFS (90% CI), months | 7.8 (6.9−11.0) |
Based on investigator assessment in patients with CR or PR (n = 16).
Abbreviations: CI, confidence interval; CR, complete response; DOR, duration of response; ORR, objective response rate (CR + PR); PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease.
Efficacy
Exposure and response data are shown in Figure 1A (swimmer plot). The ORR was 44.4% (90% CI, 30.2−59.4) with 16 (44.4%) PRs; no CR was observed (Table 3). The percent change in tumor size for patients with measurable disease at BL is described in Figure 1B. Duration of response was 7.6 months, and PFS was 7.8 months (90% CI, 6.9−11; Fig. 1C).
Biomarker Assessments
MMP9, evaluated in archival tumor samples by IHC, was observed in macrophages, neutrophils, and some tumor cells. Examples of MMP9 protein expression are shown in Figure 2A. All PDAC tumor samples were positive for MMP9, but the predominant MMP9‐positive cell population varied by case. Pretreatment plasma MMP9 was elevated in enrolled patients compared with healthy volunteer controls (Fig. 2B). Free circulating MMP9 was detectable in 94% of patients at BL and dropped below the limit of detection in >92% of patients at on‐treatment time points. Total circulating MMP9 was measurable in all patients at all time points for the duration of treatment (Fig. 2C), demonstrating that MMP9 protein was detectable but fully bound to andecaliximab at on‐treatment time points.
Figure 2.
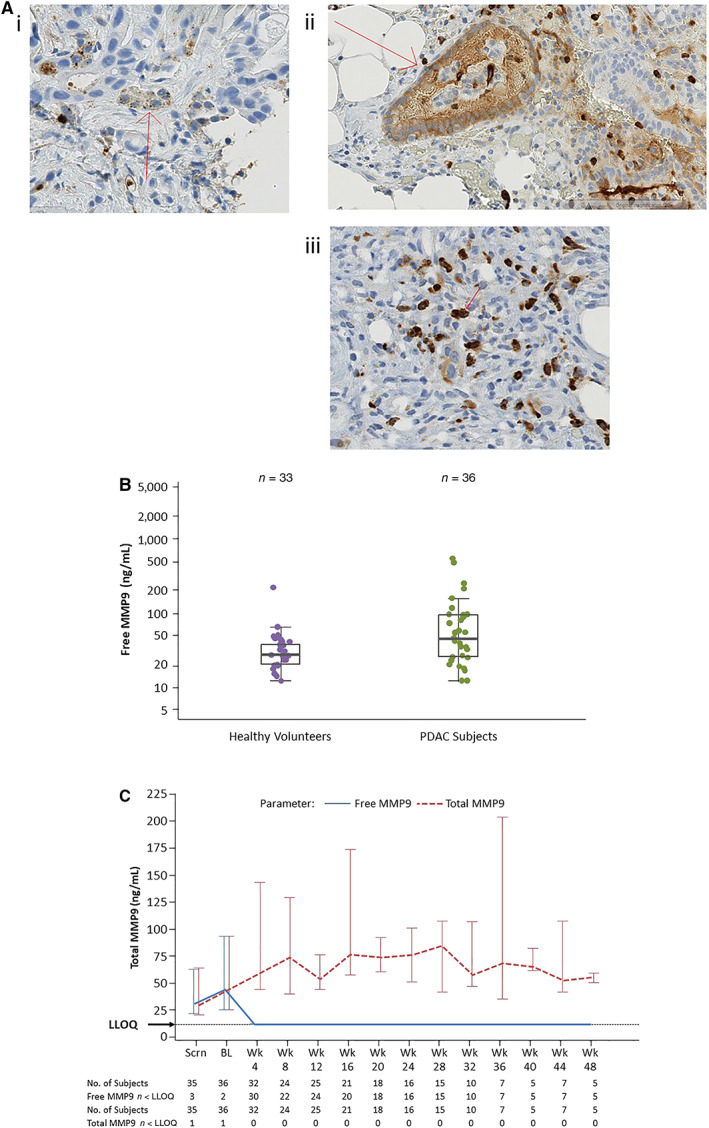
MMP9 expression in baseline tumor tissue and plasma and pharmacodynamic effect of andecaliximab combined with gemcitabine and nab‐paclitaxel. (A): Examples of MMP9 protein expression. (B): MMP9 is elevated in plasma of patients with PDAC compared with healthy volunteers. (C): Upon treatment with andecaliximab plus chemotherapy, free MMP9 was bound by andecaliximab, whereas total MMP9 remained unchanged.Abbreviations: BL, baseline; LLOQ, lower limit of quantitation; MMP9, matrix metalloproteinase 9; PDAC, pancreatic ductal adenocarcinoma; Scrn, screening.
MMP9 cleaves extracellular matrix proteins, including collagens and basement membrane components such as laminin. Fragments of these proteins (neoepitopes) are detectable in blood and may serve as markers of proteolytic activity. BL serum concentrations of C1M, C3M, and C4M2 neoepitopes were significantly higher in enrolled patients with PDAC than in healthy volunteer controls (Fig. 3A). The pharmacodynamic effect of andecaliximab combined with gemcitabine and nab‐paclitaxel was evaluated. Although the mean C1M percent BL decreased over time, the decrease was not significant at any time point (Fig. 3B). No significant association of BL C1M with clinical response to andecaliximab in combination with gemcitabine and nab‐paclitaxel was observed (Fig. 3C). When patients were divided into C1M‐high and C1M‐low groups by median BL C1M, the high group had shorter PFS (median 6.93 months vs. 9.17 months; hazard ratio, 0.5), although this was not significant (p = .14) (Fig. 3D).
Figure 3.
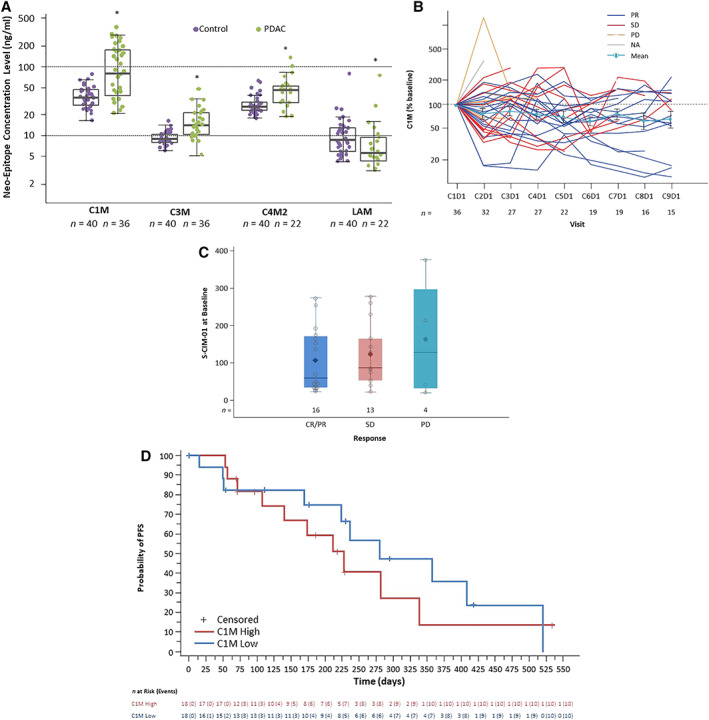
Evaluation of collagen cleavage fragments as pharmacodynamic biomarkers of andecaliximab combined with gemcitabine and nab‐paclitaxel. (A): The median baseline levels of C1M, C3M, and C4M2 neoepitopes were higher in the serum of patients with PDAC than in healthy volunteers. *p < .001. (B): C1M on‐treatment downward trend persisted over time. (C): No significant association of baseline C1M with clinical response. (D): Kaplan‐Meier plot of PFS by C1M. No statistically significant difference between the C1M‐low and C1M‐high groups. C1M low and high are defined with cutoff at C1M median of 79.25 ng/mL.Abbreviations: C1M, collagen 1; C3M, collagen 3; C4M2, collagen 4 alpha 1; CxD1, cycle x day 1; LAM, laminin alpha 5; NA, not applicable; PD, progressive disease; PDAC, pancreatic ductal adenocarcinoma; PFS, progression‐free survival; PR, partial response; S‐C1M‐01, C1M is a collagen neoepitope and a putative product of Collagen 1 (C1) cleavage by MMP9 measured in serum; SD, stable disease.
In the tumor microenvironment, MMP9 cleaves and activates substrates such as vascular endothelial growth factor and transforming growth factor beta that promote tumor growth through angiogenesis and immune suppression [5, 20]. The effect of andecaliximab in combination with gemcitabine and nab‐paclitaxel on systemic biomarkers related to MMP9 activity and immune suppression and activation was explored in a serum biomarker screen. After 2 months of treatment, the predominant trend was an increase in circulating biomarkers over time; however, a small number of factors, including cancer antigen (CA)‐125, CA‐50, and thyroid peroxidase (TPO) decreased (Fig. 4). Circulating biomarkers at BL were evaluated for correlation with response, which identified thymus and activation regulated chemokine, cutaneous T‐cell–attracting chemokine, monocyte chemoattractant protein 4, cystatin‐C, monocyte‐specific chemokine 3, C‐X‐C motif chemokine 5 (or epithelial‐derived neutrophil‐activating peptide‐78), and T‐lymphocyte–secreted protein (chemokine C‐C motif ligand 1 or I‐309) as higher in responders (CR and PR) than nonresponders (stable disease and progressive disease) (p < .05, supplemental online Table 1). A multivariate analysis that included all screened biomarkers failed to identify any that could distinguish responders from nonresponders (data not shown).
Figure 4.
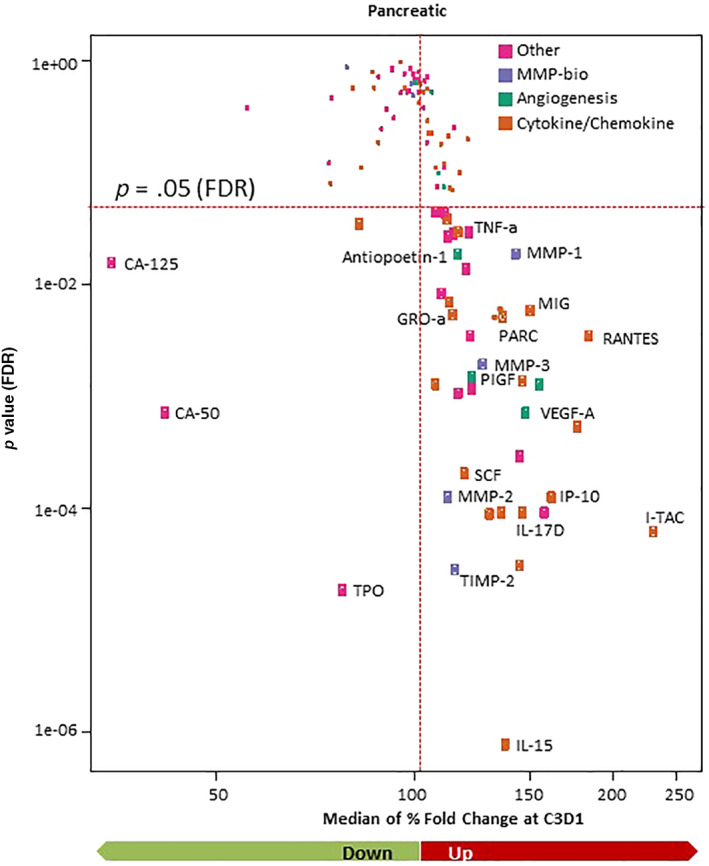
Assessment of circulating factors. A small number of circulating factors decreased from baseline to C3D1, whereas the majority of circulating factors increased. Cytokines that were significantly changed (FDR < 0.05) are named and marked with larger points.Abbreviations: C3D1, cycle 3 day 1; CA, cancer antigen; FDR, false discovery rate; GRO‐a, growth‐regulated oncogene alpha; IL, interleukin; IP‐10, interferon gamma–induced protein 10; MIG, monokine induced by gamma; MMP, matrix metalloproteinase; PARC, pulmonary and activation‐regulated chemokine; PIGF, placental growth factor; RANTES, regulated on activation, normal T‐cell expressed and secreted; SCF, stem cell factor; TIMP‐2, tissue inhibitor of metalloproteinases 2; TNF‐a, tumor necrosis factor alpha; TPO, thyroid peroxidase; VEGF‐A, vascular endothelial growth factor A.
Discussion
Andecaliximab is a novel, highly selective antibody inhibitor of MMP9. The purpose of this study was to assess the safety and efficacy of andecaliximab in combination with chemotherapy in patients with advanced solid tumors. In the dose‐finding stage of this study [15], andecaliximab monotherapy was well tolerated when administered at doses of 200, 600, and 1,800 mg i.v. Q2W, and no dose‐limiting toxicity was observed at any dose. Based on this, a dose of 800 mg Q2W was selected because it was expected to achieve plasma concentrations in the linear range of the PK profile and to achieve adequate steady trough concentrations to saturate target‐mediated drug disposition. In the current cohort of patients with advanced PDAC, treatment of 800 mg Q2W was demonstrated to achieve complete peripheral target coverage. The combination of andecaliximab with gemcitabine and nab‐paclitaxel appeared to be well tolerated without new and unexpected safety signals. The most frequently reported AEs were fatigue, alopecia, peripheral edema, and nausea. In contrast to the pan‐MMP inhibitor marimastat [9, 10], andecaliximab was not associated with treatment‐emergent musculoskeletal syndrome. The safety profile of andecaliximab in combination with gemcitabine and nab‐paclitaxel appeared similar to the previously characterized toxicity profile for gemcitabine plus nab‐paclitaxel in patients with pancreatic cancer, with fatigue, alopecia, and nausea being the most frequently observed AEs [16].
In a phase III trial, gemcitabine plus nab‐paclitaxel was associated with a median PFS of 5.5 months, response rate of 23%, and median overall survival (OS) of 8.5 months [19]. Oxaliplatin, irinotecan, fluorouracil, and leucovorin (FOLFIRINOX) demonstrated a median PFS of 6.4 months and median OS of 11.1 months in patients with metastatic pancreatic cancer [21]. Given this previously reported literature, the clinical activity of andecaliximab in combination with gemcitabine and nab‐paclitaxel (ORR of 44% and median PFS of 7.8 months) was promising.
As expected, MMP9 protein was observed in macrophages and neutrophils, both of which are infiltrating sources of MMP9 in the tumor microenvironment. MMP9 cleaves extracellular matrix proteins, and the hypothesis that peripherally detected cleaved collagens could demonstrate MMP9 activity in the tumor was tested. Peripheral cleaved collagens were higher in patients with PDAC prior to treatment but were not associated with response or PFS, nor were they consistently modulated by treatment. The impact of andecaliximab combined with gemcitabine and nab‐paclitaxel on systemic biomarkers related to MMP9 activity and immune suppression and activation was explored. The majority of the factors increased at 2 months, which may relate to response to chemotherapy. Consistent with clinical response, biomarkers related to tumor burden (CA‐125, CA‐50, and TPO) decreased. A few BL biomarkers that included cytokines involved in trafficking neutrophils and macrophages, sources of MMP9 that are generally associated with an unfavorable prognosis in cancer [22, 25], were significantly higher in the response (CR + PR) group.
Conclusion
Andecaliximab in combination with gemcitabine and nab‐paclitaxel demonstrated a favorable safety profile and clinical activity in patients with advanced PDAC. However, the combination of andecaliximab with chemotherapy was evaluated in a global phase III study in patients with advanced gastric/GEJ cancer, and there was no survival benefit associated with andecaliximab (ClinicalTrials.gov identifier: NCT02545504). Clinical development of andecaliximab has been discontinued.
Author Contributions
Conception/design: Johanna Bendell, Carrie Baker Brachmann, Marianna Zavodovskaya, Dung Thai, Pankaj Bhargava, Alexander Starodub
Provision of study material or patients: Johanna Bendell, Sunil Sharma, Manish R. Patel, Kevin Windsor, Zev A. Wainberg, Michael Gordon, Jorge Chaves, Jordan Berlin, Manish A. Shah, Saad A. Khan, Alexander Starodub
Collection and/or assembly of data: Johanna Bendell, Sunil Sharma, Manish R. Patel, Kevin Windsor, Zev A. Wainberg, Michael Gordon, Jorge Chaves, Jordan Berlin, Manish A. Shah, Saad A. Khan, Alexander Starodub
Data analysis and interpretation: Johanna Bendell, Carrie Baker Brachmann, Marianna Zavodovskaya, JieJane Liu, Dung Thai, Alexander Starodub
Manuscript writing: Johanna Bendell, Carrie Baker Brachmann, Marianna Zavodovskaya, JieJane Liu, Dung Thai, Alexander Starodub
Final approval of manuscript: Johanna Bendell, Sunil Sharma, Manish R. Patel, Kevin Windsor, Zev A. Wainberg, Michael Gordon, Jorge Chaves, Jordan Berlin, Carrie Baker Brachmann, Marianna Zavodovskaya, JieJane Liu, Dung Thai, Pankaj Bhargava, Manish A. Shah, Saad A. Khan, Alexander Starodub
Disclosures
Johanna Bendell: Gilead, Genentech/Roche, Bristol‐Myers Squibb, Eli Lilly & Co., Merck, MedImmune, Celgene, Taiho, Novartis, OncoMed, Boehringer Ingelheim, ARMO, Ipsen Oncogenex, FORMA (other—food, beverage, and travel reimbursement); Sunil Sharma: Elevar (LSK BioPharma), Salarius Pharmaceuticals, Iterion Therapeutics, Proterus Therapeutics, ConverGene, HLB‐Korea, Stingray Therapeutics (OI); Zev A. Wainberg: Merck, Eli Lilly & Co., Bayer, Five Prime (C/A), Ipsen, Five Prime, Gilead, Merck (RF); Michael Gordon: Imaging Endpoints, Tracon, Deciphera, Salarius (H); Jordan Berlin: AstraZeneca, LSK Pharmaceuticals, QED, Clovis, Ipsen (SAB), Novartis (Array), Abbvie, Immunomedics, Taiho, Genentech/Roche, Bayer, Eli Lilly & Co., Incyte, Pharmacyclics, Five Prime, Loxo, EMD Serono, Boston Biomedical, PsiOxus, Macrogenics, Boston Biomedical, Symphogen, LSK, Pfizer (RF); Carrie Baker Brachmann: Gilead Sciences (E, OI); Marianna Zavodovskaya: Gilead Sciences (E, OI); JieJane Liu: Gilead Sciences (E, OI); Dung Thai: Gilead Sciences (E, OI); Pankaj Bhargava: Gilead Sciences (E, OI); Manish A. Shah: Sanofi Aventis, Roche, Boston Biomedical, Merck (RF), Eli Lilly & Co. (SAB); Saad A. Khan: Genentech, Foundation Medicine (H); Alexander Starodub: Sandoz, Bayer (C/A), Bristol‐Myers Squibb (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Figure S1 Full study schema (ClinicalTrials.gov Identifier: NCT01803282)
Table S1 Cytokine median baseline level (ng/mL) vs best overall response
Acknowledgments
This research was supported by Gilead Sciences, Inc., Foster City, CA. Professional medical writing assistance was provided by Impact Communications (New York, NY) and was supported by Gilead Sciences, Inc. The authors also thank the study site staff and all patients and their families for their participation in the study.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Hijova E. Matrix metalloproteinases: Their biological functions and clinical implications. Bratisl Lek Listy 2005;106:127–132. [PubMed] [Google Scholar]
- 2. Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase b or matrix metalloproteinase‐9 (MMP‐9): The next decade. Crit Rev Biochem Mol Biol 2013;48:222–272. [DOI] [PubMed] [Google Scholar]
- 3. Farina AR, Mackay AR. Gelatinase B/MMP‐9 in tumour pathogenesis and progression. Cancers (Basel) 2014;6:240–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen J, Chen LJ, Zhou HC et al. Prognostic value of matrix metalloproteinase‐9 in gastric cancer: A meta‐analysis. Hepatogastroenterology 2014;61:518–524. [PubMed] [Google Scholar]
- 5. Liu YF, Guo S, Zhao R et al. Correlation of vascular endothelial growth factor expression with tumor recurrence and poor prognosis in patients with pn0 gastric cancer. World J Surg 2012;36:109–117. [DOI] [PubMed] [Google Scholar]
- 6. Yang Q, Ye ZY, Zhang JX et al. Expression of matrix metalloproteinase‐9 mRNA and vascular endothelial growth factor protein in gastric carcinoma and its relationship to its pathological features and prognosis. Anat Rec (Hoboken) 2010;293:2012–2019. [DOI] [PubMed] [Google Scholar]
- 7. Xu Y, Li Z, Jiang P et al. The co‐expression of MMP‐9 and Tenascin‐c is significantly associated with the progression and prognosis of pancreatic cancer. Diagn Pathol 2015;10:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sparano JA, Bernardo P, Stephenson P et al. Randomized phase III trial of marimastat versus placebo in patients with metastatic breast cancer who have responding or stable disease after first‐line chemotherapy: Eastern Cooperative Oncology Group trial E2196. J Clin Oncol 2004;22:4683–4690. [DOI] [PubMed] [Google Scholar]
- 9. Bramhall SR, Hallissey MT, Whiting J et al. Marimastat as maintenance therapy for patients with advanced gastric cancer: A randomised trial. Br J Cancer 2002;86:1864–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marshall DC, Lyman SK, McCauley S et al. Selective allosteric inhibition of MMP9 is efficacious in preclinical models of ulcerative colitis and colorectal cancer. PLoS One 2015;10:e0127063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Appleby TC, Greenstein AE, Hung M et al. Biochemical characterization and structure determination of a potent, selective antibody inhibitor of human MMP9. J Biol Chem 2017;292:6810–6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baker MP, Reynolds HM, Lumicisi B et al. Immunogenicity of protein therapeutics: The key causes, consequences and challenges. Self Nonself 2010;1:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perry L, Jones T, Baker M. New approaches to prediction of immune responses to therapeutic proteins during preclinical development. Drugs R D 2008;9:385–396. [DOI] [PubMed] [Google Scholar]
- 14. Lekstan A, Lampe P, Lewin‐Kowalik J et al. Concentrations and activities of metalloproteinases 2 and 9 and their inhibitors (TIMPs) in chronic pancreatitis and pancreatic adenocarcinoma. J Physiol Pharmacol 2012;63:589–599. [PubMed] [Google Scholar]
- 15. Jones LE, Humphreys MJ, Campbell F et al. Comprehensive analysis of matrix metalloproteinase and tissue inhibitor expression in pancreatic cancer: Increased expression of matrix metalloproteinase‐7 predicts poor survival. Clin Cancer Res 2004;10:2832–2845. [DOI] [PubMed] [Google Scholar]
- 16. National Cancer Institute, Surveillance, Epidemiology, and End Results Program . Cancer stat facts: Pancreatic cancer. National Cancer Institute Web site. Available at https://seer.cancer.gov/statfacts/html/pancreas.html. Accessed July 16, 2020. [Google Scholar]
- 17. Saad AM, Turk T, Al‐Husseini MJ et al. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER‐based study. BMC Cancer 2018;18:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shah MA, Starodub A, Sharma S et al. Andecaliximab/GS‐5745 alone and combined with mFOLFOX6 in advanced gastric and gastroesophageal junction adenocarcinoma: Results from a phase I study. Clin Cancer Res 2018;24:3829–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Von Hoff DD, Ervin T, Arena FP et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Comprehensive Cancer Network . Pancreatic Adenocarcinoma. Version 3.2017. Plymouth Meeting, PA: National Comprehensive Cancer Network, 2017.
- 21. Conroy T, Desseigne F, Ychou M et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 22. Li A, King J, Moro A, et al. Overexpression of CXCL5 is associated with poor survival in patients with pancreatic cancer. Am J Pathol 2011;178(3):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu B, Fan H, Lv X, Chen S, Shao Z. Prognostic significance of CXCL5 expression in cancer patients: a meta‐analysis. Can Cell Int 2018;18:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Y, Cai Y, Liu L, Wu Y, Xiong X. Crucial biological functions of CCL7 in cancer. PeerJ 2018;6:e4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Okugawa Y, Toiyama Y, Mohri Y, et al. Elevated serum concentration of monocyte chemotactic protein 4 (MCP‐4) as a novel non‐invasive prognostic and predictive biomarker for detection of metastasis in colorectal cancer. J Surg Oncol 2016;114(4):483–489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Figure S1 Full study schema (ClinicalTrials.gov Identifier: NCT01803282)
Table S1 Cytokine median baseline level (ng/mL) vs best overall response


