Abstract
Background
Peripheral blood parameters are correlated to immune‐checkpoint inhibitor efficacy in solid tumors, such as melanoma and non‐small cell lung cancer. Few data are currently available on the prognostic role of these immune‐inflammatory biomarkers for other solid tumors and immunotherapy combinations.
Material and Methods
From August 2014 to May 2019, 153 patients with metastatic solid tumors were enrolled in phase I clinical trials testing immunotherapy both as single agents and as combinations. Primary endpoint was to evaluate the impact of baseline blood parameters on progression‐free survival (PFS) and overall survival (OS).
Results
The most common tumor types were gastrointestinal, breast, and gynecological cancers (22.9%, 22.2%, and 15.0%, respectively). Higher lactate dehydrogenase (LDH) and derived neutrophil‐to‐lymphocyte ratio (dNLR) were independently associated with reduced PFS (hazard ratio [HR], 1.97; 95% confidence interval [CI], 1.30–2.99; p = .001, and HR, 2.29; 95% CI, 1.39–3.77; p = .001, respectively) and reduced OS (HR, 2.04; 95% CI, 1.26–3.28; p = .004, and HR, 2.06; 95% CI, 1.12–3.79; p = .02, respectively). In the subgroup analysis, (single agent vs. combination), patients at “good” (dNLR <3 and LDH < upper limit of normal [ULN]) and “intermediate and poor” (dNLR >3 and/or LDH > ULN) risk had higher and lower PFS, respectively (p for interaction = .002). Conversely, patients receiving monotherapy presented statistically significant difference in OS according to the risk group, whereas this effect was not observed for those treated with combinations (p for interaction = .004).
Conclusion
Elevated LDH and dNLR are associated with poorer survival outcomes in patients treated with immunotherapy in phase I clinical trials, regardless of tumor type. These parameters represent an easy tool that might be considered as stratification factors in immunotherapy‐based clinical trials.
Implications for Practice
In this retrospective cohort study of 153 patients with metastatic solid tumors treated with immunotherapy in the context of phase I clinical trials, elevated baseline lactate dehydrogenase and derived neutrophil‐to‐lymphocyte ratio were associated with reduced survival regardless of tumor subtype. If prospectively validated, these parameters might represent low‐cost and easy biomarkers that could help patient selection for early phase immunotherapy trials and be applied as a stratification factor in randomized studies testing immunotherapy agents.
Short abstract
This article evaluates the effect of routine baseline blood parameters on outcomes of patients with advanced cancers treated in phase I trials testing immunotherapy agents as monotherapy or in combination.
Background
Phase I clinical trials allow to translate findings from preclinical research into clinical practice [1]. Although they have been historically considered as “toxicity trials” with no therapeutic intent, the deeper understanding of the molecular and immune bases of cancer and the increasing availability of molecular targeted and immunotherapy agents allow us to refine patient selection and to unveil rapidly the potential efficacy of the drugs [2]. This is also demonstrated by the increased number of phase I trials that incorporate phase II extension cohorts to investigate efficacy [3]. The median response rates observed in phase I clinical trials is steadily increasing over time: it is now around 20% (or even higher when a biomarker is used for patient selection) compared with less than 5% in trials conducted in the 1980s [4, 5, 6]. The current patient selection still has several issues and weaknesses. Various prognostic scoring systems based on clinical and blood parameters have been proposed for patients treated in phase I trials [7, 8, 9, 10, 11, 12, 13, 14, 15, 16], whereas most of them have been developed in the context of trials testing cytotoxic and targeted agents. Of note, only a few of these studies focused on patients treated with immunotherapy [14, 15, 16].
Monoclonal antibodies targeting cytotoxic T‐lymphocyte–associated antigen‐4, programmed cell death protein‐1 (PD‐1), and PD‐ligand 1 (PD‐L1), also defined as immune checkpoint inhibitors (ICIs), revolutionized the treatment of several solid tumors [17, 18, 19, 20, 21, 22, 23]. However, the efficacy of ICIs is limited to a small proportion of patients, and, at present, no validated and reliable predictive biomarkers of response or resistance to immunotherapy have been identified. Routine blood parameters, including neutrophils, platelets, lactate dehydrogenase (LDH), and albumin levels, as well as other biomarkers, such as neutrophil‐to‐lymphocyte ratio (NLR) and derived neutrophil‐to‐lymphocyte ratio (dNLR), have been associated with worse outcomes in patients with cancer [24, 25, 26, 27, 28, 29]. Interestingly, some of these parameters, namely LDH, NLR, and dNLR, have demonstrated to predict efficacy to ICIs in patients with melanoma and non‐small cell lung cancer (NSCLC) [30, 31, 32, 33, 34, 35, 36]. However, limited information is currently available on the predictive and prognostic role of these blood parameters in other tumor types treated with immunotherapy. Recently, novel agents targeting immune inhibitory and costimulatory molecules have been developed and are currently under evaluation in phase I studies, either as monotherapy or in combination [37]. No data are currently available on the impact of these parameters in patients treated with next‐generation immunotherapy agents. Our study aims at evaluating the impact of routine baseline blood parameters on outcomes of patients with advanced cancers treated in phase I trials testing immunotherapy agents either as monotherapy or in combination.
Material and methods
Study Population
We retrospectively reviewed clinical outcomes of all consecutive patients with advanced and metastatic solid tumors treated with immunotherapy at the Early Drug Development Unit of the European Institute of Oncology (Milan, Italy) from August 2014 until May 2019. All patients included were enrolled in phase I trials investigating immunotherapy‐based treatments, such as single‐agent immunotherapy, combinations of two immunotherapeutics, or combinations of immunotherapy with targeted therapies. A detailed list of all experimental treatments is reported in the supplementary material (supplemental online Table 1). Demographic, clinical, and pathological patient characteristics were retrieved from medical records. The study protocol was approved by the Internal Review Board and the Local Ethics Committee and was conducted in accordance with the Declaration of Helsinki. All patients signed an informed consent to allow the use of their data for research purposes.
Blood Parameters and Ratios
Patients’ laboratory values at baseline (within 14 days before starting treatment) included complete blood count, LDH (U/L), and albumin (g/dL) levels. The following parameters were calculated: (a) NLR by dividing neutrophil by lymphocyte counts, (b) platelet‐to‐lymphocyte ratio (PLR) by dividing platelet by lymphocyte counts, and (c) lymphocyte‐to‐monocyte ratio (LMR) by dividing lymphocyte by monocyte counts. dNLR was defined as neutrophils/(leucocytes‐neutrophils). The thresholds for NLR (greater than 6), dNLR (greater than 3), PLR (greater than 300), and LMR (greater than 3), were set according to data available in literature [14, 15, 16, 30, 31, 32, 33, 34, 35, 36]. LDH and albumin values were categorized in high or low when detected as greater or lower than institutional laboratory range of normal limits (upper limit of normal [ULN] and lower limit of normal, respectively).
To better identify patients at high‐risk of progression or death, we combined LDH greater than ULN and dNLR greater than 3 to separate patients in two different risk groups, “good” (0 factors) and “intermediate and poor” (1 or 2 factors), as previously described [31].
Study objectives
The main objectives of the current study were to evaluate the impact of NLR, dNLR, PLR, and LMR as well as of LDH and albumin values on survival outcomes, namely progression‐free survival (PFS) and overall survival (OS), of patients with cancer treated with immunotherapy in the context of phase I trials. Secondary objective was to investigate the impact of these parameters on response rates, namely overall response rate (ORR) and clinical benefit rate (CBR).
Statistical Analysis
Descriptive statistics were used to analyze and report patients’ characteristics. Clinical and biological variables were stratified into categories whenever reasonable to preserve statistical power and feasibility of data collection. Differences between groups were compared by the χ2 test, Fisher's exact test, or Wilcoxon‐Mann‐Whitney test, as appropriate. Correlations of blood parameters and derived ratios were analyzed by nonparametric Spearman's rank correlation test. ORR and CBR were defined as the proportion of patients who achieved a complete (CR) or partial response (PR) and CR, PR, or stable disease (SD) as best response, respectively. Factors associated with ORR and CBR were tested with logistic regression in univariate and multivariate analyses.
All patients were followed‐up until death, loss of contact, or time of data lock, which was set on January 1, 2020. Each patient's living status (dead or alive), disease progression (occurred or not), date of disease progression, and date of death or last follow‐up were recorded for survival analyses. PFS was calculated from experimental treatment start to the date of radiological or clinical documentation of progressive disease (PD), last follow‐up or death, whichever occurred first (censored at last follow‐up for patients alive and without PD). OS was calculated from experimental treatment start to the date of death or last follow‐up (censored at last follow‐up for patients alive). Kaplan‐Meier method and Cox proportional‐hazards model were used for survival analyses. The reverse Kaplan‐Meier method was used for median follow‐up quantification [38]. Hazard ratios (HRs) together with 95% confidence intervals (CI) were provided. At multivariate analyses, the choice of the covariates to adjust for was based on their clinical relevance and statistical significance in a univariate analysis (p ≤ .1). An interaction term was included in the statistical models when subgroup analyses were performed. Statistical significance threshold was set to a two‐tailed 0.05 value. Statistical analyses were carried out using R (version 3.5.3) and R Studio (version 1.1.456).
Results
Patients’ Characteristics
A total of 153 patients were included in the study. All included patients received at least one dose of experimental therapy. Baseline patients’ characteristics are summarized in Table 1. Median age was 58 years (range, 32–80). Ninety‐one (59.5%) were women and 62 (40.5%) were men. At baseline, performance status (PS) of the Eastern Cooperative Oncology Group (ECOG) was 0 or ≥ 1 in 53.6% and 46.4% of cases, respectively. The most common tumor types were gastrointestinal, breast, and gynecological cancers (22.9%, 22.2%, and 15.0%, respectively). Sixty‐four (41.8%) patients had more than two metastatic sites, with visceral disease dissemination in more than 70% of cases. Sixty patients (39.2%) received more than two previous lines of systemic therapy in the metastatic setting. Of note, 18 patients (11.8%) were pretreated with immune checkpoint inhibitors. Experimental therapies administered were represented by single‐agent immunotherapy, combination of two or more immunotherapeutics, and combination of immunotherapy with targeted agents (38.6%, 54.9%, and 6.5%, respectively). PD‐L1 status was reported in 11 cases only (data not shown), and therefore, we decided to remove this variable from the statistical analyses.
Table 1.
Patients’ characteristics
| Variable | Overall (n = 153), n (%) |
|---|---|
| Age, median (min–max), yr | 58 (32–80) |
| Age, yr | |
| ≥65 | 46 (30.1) |
| <65 | 107 (69.9) |
| Sex | |
| Female | 91 (59.5) |
| Male | 62 (40.5) |
| PS ECOG | |
| 0 | 82 (53.6) |
| ≥1 | 71 (46.4) |
| Primary tumor | |
| Gastrointestinal | 35 (22.9) |
| Breast | 34 (22.2) |
| Gynecologic | 23 (15.0) |
| Head and neck | 16 (10.5) |
| Lung | 12 (7.8) |
| Melanoma and other skin cancers | 10 (6.5) |
| Mesothelioma | 12 (7.8) |
| Neuroendocrine | 1 (0.7) |
| Hematologic | 1 (0.7) |
| Genitourinary | 9 (5.9) |
| Metastatic site(s) | |
| Lymph nodes | 17 (11.1) |
| Bone | 2 (1.3) |
| Lymph nodes + bone | 3 (2.0) |
| Visceral | 112 (73.2) |
| Other | 19 (12.4) |
| Visceral metastases | |
| No | 41 (26.8) |
| Yes | 112 (73.2) |
| Brain metastases | |
| No | 147 (96.1) |
| Yes | 6 (3.9) |
| Liver metastases | |
| No | 103 (67.3) |
| Yes | 50 (32.7) |
| Number of metastatic sites | |
| ≤2 | 89 (58.2) |
| >2 | 64 (41.8) |
| Previous lines of systemic therapy | |
| ≤2 | 93 (60.8) |
| >2 | 60 (39.2) |
| Previous immunotherapy | |
| No | 135 (88.2) |
| Yes | 18 (11.8) |
| Type of experimental therapy | |
| IO single agent | 59 (38.6) |
| IO + IO combination | 84 (54.9) |
| IO + target agent combination | 10 (6.5) |
Abbreviations: IO, immunotherapy; PS ECOG, performance status according to Eastern Cooperative Oncology Group.
Baseline Blood Parameters
Baseline blood parameters values are summarized in supplemental online Table 2. Baseline albumin was available for all patients, whereas LDH was available for 76.5% (n = 117). LDH levels were higher in patients with visceral metastases (343.5 vs. 279.7 U/L; p = .036) and in those previously treated with more than two lines of systemic therapy (412.4 vs. 282.9 U/L; p = .025; supplemental online Fig. 1). No other significant associations between baseline blood parameters and patient characteristics were found (data not shown). The association between NLR and dNLR assessed by Spearman's rank correlation analysis was 0.913 (p < .001). Correlations between NLR, dNLR, PLR, LMR, and LDH values and albumin values are described in supplemental online Table 3.
Patients’ outcomes
Median follow‐up was 25.9 months (95% CI, 22.3–29.6). In the overall population (n = 153), we observed 2 CRs and 17 PRs, with an ORR of 11.8%. Thirty‐four patients had SD as best response, leading to a CBR of 34.9% (supplemental online Table 4). At the time of data analysis, 138 patients (90.2%) experienced PD, and 117 (77.0%) had died. Median PFS and OS were 2.43 (95% CI, 1.92–2.94) and 9.47 (95% CI, 6.57–12.38) months, respectively.
Impact of Baseline Blood Parameters and Clinical Variables on ORR and CBR
Baseline blood parameters were not significantly associated with ORR (Table 2). Of note, PS ECOG ≥1, more than two prior lines of treatment, and more than two metastatic sites were associated with reduced ORR (supplemental online Fig. 2). PS ECOG and previous treatments retained significance in multivariate analysis (OR, 0.28; 95% CI, 0.09–0.94; p = .039, and OR, 0.18; 95% CI, 0.04–0.82; p = .027, respectively; Table 2). Conversely, LDH greater than ULN was associated with reduced CBR at univariate analysis (LDH < ULN vs. > ULN: 51.6% vs. 20.8%, OR, 0.25; 95% CI, 0.1–0.55; p < .001). In multivariate logistic regression analysis, LDH greater than UNL confirmed to be independently associated with reduced CBR (OR, 0.25; 95% CI, 0.1–0.62; p = .003; supplemental online Fig. 2; Table 3).
Table 2.
Univariate and multivariate logistic regression analysis for overall response rate (n = 152)
| Variable | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| ORR, % | OR | 95% CI | P value | OR | 95% CI | P value | |
| Experimental therapy | |||||||
| IO monotherapy | 11.9 | Ref | |||||
| IO combination | 11.8 | 0.99 | 0.37–2.86 | .995 | |||
| Age | |||||||
| <65 | 10.4 | Ref | |||||
| ≥65 | 15.2 | 1.55 | 0.54–4.24 | .399 | |||
| Sex | |||||||
| Female | 8.9 | Ref | |||||
| Male | 16.1 | 1.97 | 0.73–5.48 | .180 | |||
| PS ECOG | |||||||
| 0 | 17.1 | Ref | |||||
| ≥1 | 5.6 | 0.29 | 0.08–0.84 | .035 | 0.28 | 0.09–0.94 | .039 |
| Visceral metastases | |||||||
| No | 14.6 | Ref | |||||
| Yes | 10.8 | 0.71 | 0.25–2.16 | .519 | |||
| Metastatic sites | |||||||
| ≤2 | 17.0 | Ref | |||||
| >2 | 4.7 | 0.24 | 0.05–0.77 | .029 | 0.28 | 0.08–1.07 | .062 |
| Prior lines of therapy | |||||||
| ≤2 | 17.4 | Ref | |||||
| >2 | 3.3 | 0.16 | 0.03–0.61 | .019 | 0.18 | 0.04–0.82 | .027 |
| LDH | |||||||
| < ULN | 19.4 | Ref | |||||
| > ULN | 11.3 | 0.53 | 0.17–1.49 | .242 | |||
| NLR | |||||||
| <6 | 13.6 | Ref | |||||
| ≥6 | 3.7 | 0.24 | 0.01–1.28 | .180 | |||
| dNLR | |||||||
| <3 | 13.2 | Ref | |||||
| ≥3 | 6.7 | 0.47 | 0.07–1.78 | .331 | |||
| PLR | |||||||
| <300 | 12.2 | Ref | |||||
| ≥300 | 10.8 | 0.87 | 0.24–2.64 | .823 | |||
| LMR | |||||||
| <3 | 11.8 | Ref | |||||
| ≥3 | 12.1 | 1.03 | 0.28–3.14 | .955 | |||
| Albumin | |||||||
| ≤3.5 | 5.3 | Ref | |||||
| >3.5 | 12.8 | 2.64 | 0.492–48.96 | 0.360 | |||
Abbreviations: CI, confidence interval; dNLR, derived neutrophil‐to‐lymphocyte ratio; IO, immunotherapy; LMR, lymphocyte‐to‐monocyte ratio; OR, odds ratio; ORR, overall response rate; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; PS ECOG, performance status according to Eastern Cooperative Oncology Group; ULN, upper limit of normal.
Table 3.
Univariate and multivariate logistic regression analysis for clinical benefit rate (n = 152)
| Variable | CBR, % | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | ||
| Experimental therapy | |||||||
| IO monotherapy | 28.8 | Ref | |||||
| IO combination | 38.7 | 1.56 | 0.781–3.194 | .214 | |||
| Age | |||||||
| <65 | 29.2 | Ref | |||||
| ≥65 | 47.8 | 2.22 | 1.086–4.552 | .029 | 2.91 | 1.16–7.31 | .023 |
| Sex | |||||||
| Female | 26.7 | Ref | |||||
| Male | 46.8 | 2.42 | 1.226–4.828 | .011 | 2.19 | 0.91–5.24 | .079 |
| PS ECOG | |||||||
| 0 | 37.0 | Ref | |||||
| ≥1 | 32.4 | 0.81 | 0.414–1.591 | .549 | |||
| Visceral metastases | |||||||
| No | 43.9 | Ref | |||||
| Yes | 31.5 | 0.59 | 0.282–1.235 | .158 | |||
| Metastatic site | |||||||
| ≤2 | 45.5 | Ref | |||||
| >2 | 20.3 | 0.31 | 0.142–0.628 | .002 | 0.5 | 0.2–1.25 | .139 |
| Prior lines of therapy | |||||||
| ≤2 | 43.5 | Ref | |||||
| >2 | 21.7 | 0.36 | 0.167–0.739 | .007 | 0.43 | 0.15–1.19 | .105 |
| LDH | |||||||
| <ULN | 51.6 | Ref | |||||
| >ULN | 20.8 | 0.25 | 0.104–0.550 | <.001 | 0.25 | 0.1–0.62 | .003 |
| NLR | |||||||
| <6 | 33.6 | Ref | |||||
| ≥6 | 40.7 | 1.36 | 0.567–3.167 | .481 | |||
| dNLR | |||||||
| <3 | 35.5 | Ref | |||||
| ≥3 | 30.0 | 0.78 | 0.314–1.803 | .568 | |||
| PLR | |||||||
| <300 | 32.2 | Ref | |||||
| ≥300 | 43.2 | 1.61 | 0.745–3.429 | .221 | |||
| LMR | |||||||
| <3 | 33.6 | Ref | |||||
| ≥3 | 39.4 | 1.28 | 0.570–2.825 | .538 | |||
| Albumin | |||||||
| ≤3.5 | 26.3 | Ref | |||||
| >3.5 | 36.1 | 1.58 | 0.566–5.135 | .406 | |||
Abbreviations: CBR, clinical benefit rate; CI, confidence interval; dNLR, derived neutrophil‐to‐lymphocyte ratio; IO, immunotherapy; LMR, lymphocyte‐to‐monocyte ratio; OR, odds ratio; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; PS ECOG, performance status according to Eastern Cooperative Oncology Group; ULN, upper limit of normal.
Impact of Blood Parameters and Clinical Variables on PFS and OS
At univariate survival analysis, both normal LDH (p = .019) and lower dNLR (p = .006) were associated with longer PFS (supplemental online Table 5). Among clinical variables, younger age (p = .011), female gender (p = .008), more than two metastatic sites (p = .001), and more than two prior lines of therapy (p = .006) were associated with shorter PFS (supplemental online Table 5). Furthermore, PS ECOG ≥1 (p = .009), more than two prior lines of therapy (p = .019), higher LDH (p = .005), and dNLR >3 (p = .03) were correlated with worse OS. At multivariate Cox regression analysis, both higher LDH and dNLR were independently associated with reduced PFS (HR, 1.97; 95% CI, 1.30–2.99; p = .001 and HR, 2.29; 95% CI, 1.39–3.77; p = .001, respectively) and OS (HR, 2.06; 95% CI, 1.26–3.28; p = .004 and HR, 2.06; 95% CI, 1.12–3.79; p = .02, respectively; Figs. 1, 2; supplemental online Tables 5, 6). In multivariate analysis, more than two prior lines of therapy were associated with shorter OS, and with a trend toward shorter PFS. Of note, we tried to evaluate the impact of liver metastases as independent variable in our analyses (data not shown), without any statistically significant result.
Figure 1.
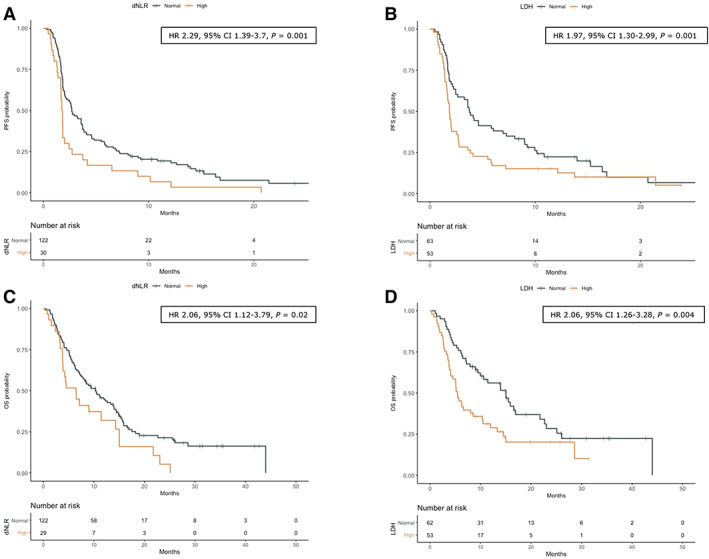
OS and PFS according to dNLR (A, C) and LDH level (B, D). +, indicates patients censored at the time of data cut off and analysis. HRs with relative 95% CIs are referred to Cox multivariable analysis.
Abbreviations: CI, confidence interval; dNLR, derived neutrophil‐to‐lymphocyte ratio; HR, hazard ratio; LDH, lactate dehydrogenase; OS, overall survival; PFS, progression‐free survival.
Figure 2.
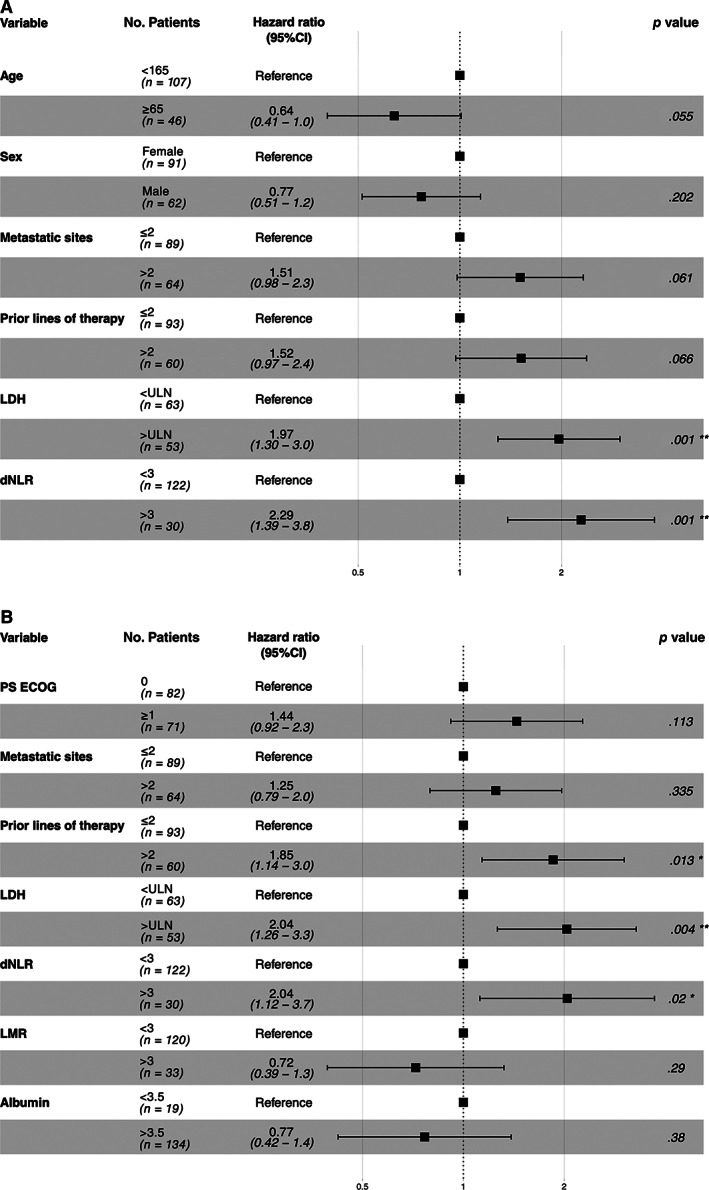
Forest plots summarizing the results of multivariable analysis of patients’ progression‐free survival (A) and overall survival (B). *, <0.05; **, <0.01.
Abbreviations: CI, confidence interval; dNLR, derived neutrophil‐to‐lymphocyte ratio; LDH, lactate dehydrogenase; LMR, lymphocyte‐to‐monocyte ratio; PS ECOG, performance status for Eastern Cooperative Oncology Group.
Immune index
Combining LDH greater than ULN and dNLR greater than 3 identifies two groups of patients with different outcomes. Baseline LDH greater than ULN and dNLR greater than 3, which were independently associated with reduced PFS and OS in Cox proportional hazard regression models, were combined to include patients in two different risk groups: good (0 factors) and intermediate and poor (1 or 2 factors). Thirty‐six patients without baseline LDH were excluded. Among the 115 evaluable patients, 46 (40.0%) had good risk score, whereas 69 (60.0%) had an intermediate and poor risk. The two groups were well balanced according to clinical and pathological characteristics, without statistically significant differences (supplemental online Table 7).
ORR was numerically higher in the good risk group than in the intermediate and poor (21.7% vs. 11.6%), without reaching the statistical significance (unadjusted OR, 0.46; 95% CI, 0.17–1.27; p = .134; adjusted OR, 0.38; 95% CI 0.11–1.26; p = .113; supplemental online Table 8; supplemental online Fig. 3). In contrast, CBR was significantly higher in the “good risk” group as compared with the intermediate and poor (54.3% vs. 24.6%; OR, 0.21; 95% CI, 0.08–0.54; p = .001; supplemental online Table 8; supplemental online Fig. 3). Median PFS was 4.60 months (95% CI, 3.58–9.23) and 1.84 months (95% CI, 1.68–2.43) for the good and intermediate and poor groups, respectively (unadjusted HR, 1.98; 95% CI, 1.31–2.99; p = .001; adjusted HR, 2.46; 95% CI, 1.58–3.84; p < .001; supplemental online Table 9; Fig. 3). Similarly, median OS was significantly higher (15.89 months; 95% CI, 9.47–not reached vs. 5.9; 95% CI, 4.51–11.4) in the good risk group than in the intermediate and poor group (unadjusted HR, 2.08; 95% CI, 1.30–3.35; p = .002; adjusted HR, 2.11; 95% CI, 1.30–3.44, p = .003; supplemental online Table 9; Fig. 3).
Figure 3.
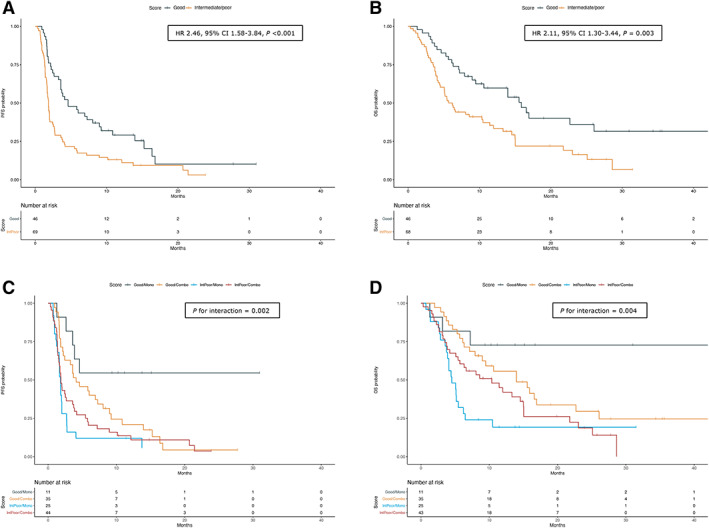
Kaplan‐Meier survival curves for PFS and OS according to IPI score (A, B) and stratified for type of experimental therapy (C, D). In (A) and (B), HRs with relative 95% CIs are referred to Cox multivariable analysis. In (C) and (D), p is referred to interaction term. +, indicates patients censored at the time of data cut off and analysis.
Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression‐free survival.
To evaluate whether these risk group presented different outcomes according to the treatments (monotherapy vs. combination), a subgroup analysis was performed. The good risk and intermediate and poor groups had higher and lower PFS, respectively (p for interaction = .002), regardless of the experimental therapy received. Differently, patients who received immunotherapy single agents presented statistically significant difference in OS according to the risk groups, whereas this effect was not observed for those treated with immunotherapy combinations (p for interaction = .004; supplemental online Table 10; Fig. 3).
Discussion
Our study showed that elevated baseline LDH and dNLR greater than 3 are associated with shorter PFS and OS in patients enrolled in phase I clinical trials testing immunotherapy, regardless of tumor type. When combined, these parameters were able to stratify patients at good and “poor” prognosis. Interestingly, in the subgroup analysis, the survival benefit for patients at good risk (0 factors) was confirmed only for patients treated with immunotherapy single agents but not for those who had received immunotherapy combinations.
The introduction of ICIs‐based immunotherapy represented a real breakthrough for the treatment of cancer, changing the therapeutic algorithms of several tumors [17, 18, 19, 20, 21, 22, 23]. However, this transformative effect was limited to small number of patients and was much less evident in some other malignancies, including breast cancer and microsatellite stable colorectal cancer [39, 40, 41]. Despite the traditional view of phase I clinical trials aimed at defining the maximum tolerated dose, ICIs efficacy results of phase I/II clinical trials had been used for granting accelerated approval in several diseases. In this context, the implementation of biomarkers for predicting immunotherapy efficacy could improve trial results interpretation.
Besides PD‐L1 expression [42], tumor mutational burden [43, 44], and mismatch repair deficiency or microsatellite instability [45], several other potential biomarkers have been investigated or are currently under evaluation [46]. In this regard, the implementation and validation of blood‐derived and serum‐derived biomarkers that can be easily obtained in the daily clinical practice represent an interesting research field [46]. Blood parameters, such as elevated neutrophils, platelets or LDH, and reduced albuminemia, have been historically associated with poor outcomes in several solid tumors [24, 25, 26, 27]. Of note, high pretreatment LDH levels have been recently associated with reduced ICIs efficacy in melanoma and NSCLC [33, 34, 35, 36]. An elevated LDH might be associated with intratumoral acidosis, hypoxia, and glucose depletion, which can dampen T‐cell activity [47]. However, LDH is also associated with tumor burden, which is known to be associated with lower benefit from immunotherapy [48, 49]. Furthermore, some studies have shown how other biomarkers, including NLR and dNLR, may help fine‐tuning risk‐group stratification and contribute to disease‐management strategies for patients with melanoma and NSCLC treated with ICIs [30, 31, 32, 50]. These studies reported that dNLR was greater than 3 in 22% and 35% of patients with melanoma and NSCLC, respectively. Our cohort presented with a lower proportion of cases (20%) with dNLR greater than 3. Such difference can be ascribed to the heterogeneity of our study population that included different tumor types. Furthermore, circulating biomarkers can be also applied for predicting toxicity in patients treated with ICIs [51, 52]. For instance, low baseline levels of NRL and PLR have been found associated with a higher risk for developing immune‐related adverse events from anti–PD‐1/PD‐L1 immunotherapy in NSCLC [53].
Interestingly, the study by Mezquita et al. [31] combined dNLR and LDH to build a prognostic index that has been validated as a predictive tool for ICI‐based immunotherapy in NSCLC. Our results are in line with these findings and suggest that dNLR and LDH are also useful biomarkers for predicting immunotherapy efficacy for other solid tumors and patients enrolled in immunotherapy‐based early phase clinical trials. Conversely, when combined to create two different risk groups, these parameters were only able to predict OS benefit for patient treated with immunotherapy monotherapies, mainly anti–PD1/PD‐L1 agents, but not with immunotherapy combinations. This observation is not fully explainable and could be merely related to the heterogeneity of our patient population. Accordingly, it deserves further investigation in larger cohorts of patients treated with immunotherapy‐based combinatorial treatments.
In contrast to previous work assessing the impact of blood‐based immune‐inflammatory biomarkers in early phase clinical trials [14], our results suggest that dNLR may be more accurate than NLR for patients treated with immunotherapy in the context of phase I trials. Even if these parameters may appear similar at a glance, the dNLR may be more informative than NLR because it includes monocytes as well as other granulocyte subpopulations. Elevated NLR and dNLR can be associated with a systemic inflammatory response, as suggested by a positive linear association between these markers and circulating cytokine levels [54]. Finally, our analysis evidenced that PS ECOG of 1 or higher and more than two prior lines of systemic treatment were associated with a reduced ORR but did not predict for reduced PFS and OS.
We acknowledge that our study presents some limitations. First, it is a retrospective study without an external validation cohort. In spite of this, our results support further studies aimed to prospectively validate these biomarkers. Second, the sample size included in this study comprises a small number of patients and is heterogenous, as it includes several tumor types as well as different drugs. Such limitations do not allow us to draw definitive conclusions on whether there are some subpopulations (tumor type, type of combination, etc.) that cannot be affected by high pretreatment levels of LDH and/or dNLR higher than 3. Third, the absence of a control group, either untreated or treated with other classes of drugs, does not allow us to determine if the evaluated biomarkers are predictive or prognostic because a formal statistical analysis for interaction cannot be performed [55]. Nevertheless, an increasing body of evidence suggests that these parameters are very strong prognostic rather than predictive biomarkers in several tumors [56]. In line with this hypothesis, our study highlighted that LDH and dNLR were related to survival and CBR but not with ORR. Finally, we are aware that our study population, consisted of subjects enrolled in phase I trials with good clinical condition (ECOG PS ≤1, few comorbidities, nonactive brain metastases, etc.), is not truly representative of the real‐world cancer patients’ population. [57]. In this regard, our results cannot be extrapolated for patients treated with immunotherapy combinations in the daily clinical practice, deserving further investigation in real‐world experiences. In contrast, despite these limitations, our study including patients enrolled in prospective clinical trials guaranteed a high quality of data collection.
A conceptualization of the comprehensive view of immunotherapy in cancer treatment has already been proposed and included in the framework of the “cancer immunogram” [47]. The abovementioned biomarkers should not be interpreted as interchangeable but as complementary, becauseeach one describes a feature of the complex cancer–immune interplay. Therefore, they could help enriching study populations, thus providing the rationale and the tools to design precision immunotherapy trials.
Conclusions
Our study indicates the potential role of dNLR and LDH as prognostic biomarkers in patients treated with immunotherapy across several solid tumors. If independently validated, these parameters might provide simple and broadly available biomarkers, which could help interpreting the immunotherapy activity in early clinical trials and be applied as stratification factor in randomized trials of immunotherapy agents and combinations. In this regard, a prospective validation of these biomarkers in immunotherapy‐based clinical trials is warranted.
Author Contributions
Conception/design: Carmen Criscitiello, Antonio Marra, Giuseppe Curigliano
Provision of study material or patients: Carmen Criscitiello, Antonio Marra, Stefania Morganti, Paola Zagami, Giulia Viale, Angela Esposito, Giuseppe Curigliano
Collection and/or assembly of data: Carmen Criscitiello, Antonio Marra, Stefania Morganti, Paola Zagami, Giulia Viale, Angela Esposito, Giuseppe Curigliano
Data analysis and interpretation: Carmen Criscitiello, Antonio Marra, Stefania Morganti, Paola Zagami, Giulia Viale, Angela Esposito, Giuseppe Curigliano
Manuscript writing: Carmen Criscitiello, Antonio Marra, Giuseppe Curigliano
Final approval of manuscript: Carmen Criscitiello, Antonio Marra, Stefania Morganti, Paola Zagami, Giulia Viale, Angela Esposito, Giuseppe Curigliano
Disclosures
Carmen Criscitiello: Eli Lilly & Co, Roche Novartis, Pfizer (Speakers’ bureau, C/A); Giuseppe Curigliano: Roche, AZ, Daichii Sankyo, Novartis, Eli Lilly & Co, Pfizer (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Figures.
Appendix S2. Tables.
Acknowledgments
This work was partially supported by the Italian Ministry of Health with Ricerca Corrente and 5x1000 funds.
The study protocol was approved by the Internal Review Board and the Local Ethics Committee and was conducted in accordance with the Declaration of Helsinki. All patients signed an informed consent for the use of their data for research purposes.
All data generated or analyzed during this study are included in this published article (and its additional files) and are available from the corresponding author on reasonable request.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Eisenhauer EA, O'Dwyer PJ, Christian M et al. Phase I clinical trial design in cancer drug development. J Clin Oncol 2000;18:684–692. [DOI] [PubMed] [Google Scholar]
- 2. Adashek JJ, LoRusso PM, Hong DS et al. Phase I trials as valid therapeutic options for patients with cancer. Nat Rev Clin Oncol 2019;16:773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manji A, Brana I, Amir E et al. Evolution of clinical trial design in early drug development: Systematic review of expansion cohort use in single‐agent phase I cancer trials. J Clin Oncol 2013;31:4260–4267. [DOI] [PubMed] [Google Scholar]
- 4. Horstmann E, McCabe MS, Grochow L et al. Risks and benefits of phase 1 oncology trials, 1991 through 2002. N Engl J Med 2005;352:895–904. [DOI] [PubMed] [Google Scholar]
- 5. Schwaederle M, Zhao M, Lee JJ et al. Association of biomarker‐based treatment strategies with response rates and progression‐free survival in refractory malignant neoplasms: A meta‐analysis. JAMA Oncol 2016;2:1452–1459. [DOI] [PubMed] [Google Scholar]
- 6. Chakiba C, Grellety T, Bellera C et al. Encouraging trends in modern phase 1 oncology trials. N Engl J Med 2018;378:2242–2243. [DOI] [PubMed] [Google Scholar]
- 7. Bachelot T, Ray‐Coquard I, Catimel G et al. Multivariable analysis of prognostic factors for toxicity and survival for patients enrolled in phase I clinical trials. Ann Oncol 2000;11:151–156. [DOI] [PubMed] [Google Scholar]
- 8. Arkenau HT, Olmos D, Ang JE et al. Clinical outcome and prognostic factors for patients treated within the context of a phase I study: The Royal Marsden Hospital experience. Br J Cancer 2008;98:1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wheler J, Tsimberidou AM, Hong D et al. Survival of patients in a phase 1 clinic: The M. D. Anderson Cancer Center experience. Cancer 2009;115:1091–1099. [DOI] [PubMed] [Google Scholar]
- 10. Arkenau HT, Barriuso J, Olmos D et al. Prospective validation of a prognostic score to improve patient selection for oncology phase I trials. J Clin Oncol 2009;27:2692–2696. [DOI] [PubMed] [Google Scholar]
- 11. Füssenich LM, Desar IM, Peters ME et al. A new, simple and objective prognostic score for phase I cancer patients. Eur J Cancer 2011;47:1152–1160. [DOI] [PubMed] [Google Scholar]
- 12. Olmos D, A'Hern R P, Marsoni S et al. Patient selection for oncology phase I trials: A multi‐institutional study of prognostic factors. J Clin Oncol 2012;30:996–1004. [DOI] [PubMed] [Google Scholar]
- 13. Wheler J, Tsimberidou AM, Hong D et al. Survival of 1,181 patients in a phase I clinic: The MD Anderson Clinical Center for targeted therapy experience. Clin Cancer Res 2012;18:2922–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bigot F, Castanon E, Baldini C et al. Prospective validation of a prognostic score for patients in immunotherapy phase I trials: The Gustave Roussy Immune Score (GRIm‐score). Eur J Cancer 2017;84:212–218. [DOI] [PubMed] [Google Scholar]
- 15. Sen S, Hess K, Hong DS et al. Development of a prognostic scoring system for patients with advanced cancer enrolled in immune checkpoint inhibitor phase 1 clinical trials. Br J Cancer 2018;118:763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Day D, Guo C, Kanjanapan Y et al. Survival in early phase immuno‐oncology trials: Development and validation of a prognostic index. JNCI Cancer Spectr 2019;3:pkz071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robert C, Long GV, Brady B et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–330. [DOI] [PubMed] [Google Scholar]
- 18. Larkin J, Chiarion‐Sileni V, Gonzalez R et al. Five‐year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019;381:1535–1546. [DOI] [PubMed] [Google Scholar]
- 19. Robert C, Ribas A, Schachter J et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE‐006): Post‐hoc 5‐year results from an open‐label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 2019;20:1239–1251. [DOI] [PubMed] [Google Scholar]
- 20. Brahmer JR, Govindan R, Anders RA et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of non‐small cell lung cancer (NSCLC). J Immunother Cancer 2018;6:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rini BI, Battle D, Figlin RA et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of advanced renal cell carcinoma (RCC). J Immunother Cancer 2019;7:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rodriguez‐Vida A, Perez‐Gracia JL, Bellmunt J. Immunotherapy combinations and sequences in urothelial cancer: Facts and hopes. Clin Cancer Res 2018;24:6115–6124. [DOI] [PubMed] [Google Scholar]
- 23. Cohen EEW, Bell RB, Bifulco CB et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer 2019;7:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McMillan DC. The systemic inflammation‐based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat Rev 2013;39:534–540. [DOI] [PubMed] [Google Scholar]
- 25. Petrelli F, Cabiddu M, Coinu A et al. Prognostic role of lactate dehydrogenase in solid tumors: A systematic review and meta‐analysis of 76 studies. Acta Oncol 2015;54:961–970. [DOI] [PubMed] [Google Scholar]
- 26. Paramanathan A, Saxena A, Morris DL. A systematic review and meta‐analysis on the impact of pre‐operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol 2014;23:31–39. [DOI] [PubMed] [Google Scholar]
- 27. Templeton AJ, Ace O, McNamara MG et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: A systematic review and meta‐analysis. Cancer Epidemiol Biomarkers Prev 2014;23:1204–1212. [DOI] [PubMed] [Google Scholar]
- 28. Laird BJ, Fallon M, Hjermstad MJ et al. Quality of life in patients with advanced cancer: Differential association with performance status and systemic inflammatory response. J Clin Oncol 2016;34:2769–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Templeton AJ, McNamara MG, Šeruga B et al. Prognostic role of neutrophil‐to‐lymphocyte ratio in solid tumors: A systematic review and meta‐analysis. J Natl Cancer Inst 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- 30. Ferrucci PF, Ascierto PA, Pigozzo J et al. Baseline neutrophils and derived neutrophil‐to‐lymphocyte ratio: Prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol 2016;27:732–738. [DOI] [PubMed] [Google Scholar]
- 31. Mezquita L, Auclin E, Ferrara R et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non‐small cell lung cancer. JAMA Oncol 2018;4:351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Capone M, Giannarelli D, Mallardo D et al. Baseline neutrophil‐to‐lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer 2018;6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weide B, Martens A, Hassel JC, et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res 2016;22:5487–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Petrelli F, Ardito R, Merelli B et al. Prognostic and predictive role of elevated lactate dehydrogenase in patients with melanoma treated with immunotherapy and BRAF inhibitors: A systematic review and meta‐analysis. Melanoma Res 2019;29:1–12. [DOI] [PubMed] [Google Scholar]
- 35. Diem S, Kasenda B, Spain L et al. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti‐PD‐1 therapy in metastatic melanoma. Br J Cancer 2016;114:256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Z, Li Y, Yan X et al. Pretreatment lactate dehydrogenase may predict outcome of advanced non small‐cell lung cancer patients treated with immune checkpoint inhibitors: A meta‐analysis. Cancer Med 2019;8:1467–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tang J, Shalabi A, Hubbard‐Lucey VM. Comprehensive analysis of the clinical immuno‐oncology landscape. Ann Oncol 2018;29:84–91. [DOI] [PubMed] [Google Scholar]
- 38. Schemper M, Smith TL. A note on quantifying follow‐up in studies of failure time. Control Clin Trials 1996;17:343–346. [DOI] [PubMed] [Google Scholar]
- 39. Schmid P, Rugo HS, Adams S et al. Atezolizumab plus nab‐paclitaxel as first‐line treatment for unresectable, locally advanced or metastatic triple‐negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol 2020;21:44–59. [DOI] [PubMed] [Google Scholar]
- 40. Marra A, Viale G, Curigliano G. Recent advances in triple negative breast cancer: The immunotherapy era. BMC Med 2019;17:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ciardiello D, Vitiello PP, Cardone C et al. Immunotherapy of colorectal cancer: Challenges for therapeutic efficacy. Cancer Treat Rev 2019;76:22–32. [DOI] [PubMed] [Google Scholar]
- 42. Patel SP, Kurzrock R. Pd‐L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 2015;14:847–856. [DOI] [PubMed] [Google Scholar]
- 43. Hellmann MD, Ciuleanu TE, Pluzanski A et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018;378:2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Samstein RM, Lee CH, Shoushtari AN et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019;51:202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Le DT, Uram JN, Wang H et al. PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 2019;19:133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Blank CU, Haanen JB, Ribas A et al. Cancer immunology. The "cancer immunogram". Science 2016;352:658–660. [DOI] [PubMed] [Google Scholar]
- 48. Joseph RW, Elassaiss‐Schaap J, Kefford R et al. Baseline tumor size is an independent prognostic factor for overall survival in patients with melanoma treated with pembrolizumab. Clin Cancer Res 2018;24:4960–4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Katsurada M, Nagano T, Tachihara M et al. Baseline tumor size as a predictive and prognostic factor of immune checkpoint inhibitor therapy for non‐small cell lung cancer. Anticancer Res 2019;39:815–825. [DOI] [PubMed] [Google Scholar]
- 50. Kazandjian D, Gong Y, Keegan P et al. Prognostic value of the lung immune prognostic index for patients treated for metastatic non‐small cell lung cancer. JAMA Oncol 2019;5:1481–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Valpione S, Pasquali S, Campana LG et al. Sex and interleukin‐6 are prognostic factors for autoimmune toxicity following treatment with anti‐CTLA4 blockade. J Transl Med 2018;16:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gowen MF, Giles KM, Simpson D et al. Baseline antibody profiles predict toxicity in melanoma patients treated with immune checkpoint inhibitors. J Transl Med 2018;16:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pavan A, Calvetti L, Dal Maso A et al. Peripheral blood markers identify risk of immune‐related toxicity in advanced non‐small cell lung cancer treated with immune‐checkpoint inhibitors. The Oncologist 2019;24:1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guthrie GJ, Charles KA, Roxburgh CS et al. The systemic inflammation‐based neutrophil‐lymphocyte ratio: Experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218–230. [DOI] [PubMed] [Google Scholar]
- 55. Ballman KV. Biomarker: Predictive or prognostic? J Clin Oncol 2015;33:3968–3971. [DOI] [PubMed] [Google Scholar]
- 56. Dolan RD, Laird BJA, Horgan PG et al. The prognostic value of the systemic inflammatory response in randomised clinical trials in cancer: A systematic review. Crit Rev Oncol Hematol 2018;132:130–137. [DOI] [PubMed] [Google Scholar]
- 57. Kim ES, Bruinooge SS, Roberts S et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J Clin Oncol 2017;35:3737–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Figures.
Appendix S2. Tables.


