Abstract
This experiment evaluated the impacts of administering a bovine appeasing substance (BAS) at feedlot entry to receiving cattle. Angus-influenced steers (n = 342) from 16 sources were purchased from an auction yard on day –1, and transported (12 hr; 4 trucks) to the feedlot. Upon arrival on day 0, shrunk body weight (BW; 240 ± 1 kg) was recorded and steers were ranked by load, shrunk BW, and source and assigned to receive BAS (IRSEA Group, Quartier Salignan, France; n = 171) or placebo (diethylene glycol monoethyl ether; CON; n = 171). The BAS is a mixture of fatty acids that replicate the composition of the bovine appeasing pheromone. Treatments (5 mL) were topically applied to each individual steer on their nuchal skin area. Steers were allocated to 1 of 24 drylot pens (12 pens/treatment) and received a free-choice diet until day 46. Steers were assessed daily for bovine respiratory disease (BRD) signs, and feed intake was recorded from each pen daily. Steer unshrunk BW was recorded on days 7, 17, 31, 45, and 46. Shrunk BW on day 0 was added an 8% shrink to represent initial BW, and final BW was calculated by averaging BW from days 45 and 46. Blood samples were collected from 5 steers/pen on days 0, 7, 11, 31, and 45. Pen was considered the experimental unit. Steer BW gain was greater (P = 0.04) in BAS vs. CON (1.01 vs. 0.86 kg/d, SEM = 0.05). Feed intake did not differ (P = 0.95) between treatments, resulting in greater (P = 0.05) feed efficiency in BAS vs. CON (171 vs. 142 g/kg, SEM = 10). Plasma cortisol concentration was greater (P = 0.05) and plasma glucose concentration was less in CON vs. BAS on day 7 (treatment × day; P = 0.07 and <0.01, respectively). Mean plasma β-hydroxybutyrate concentration was greater (P < 0.01) in BAS vs. CON (3.23 and 2.75 mg/mL; SEM = 0.12). Incidence of BRD was greater (P ≤ 0.05) in BAS vs. CON from days 6 to 10 and days 19 to 23 (treatment × day; P < 0.01), although overall BRD incidence did not differ (P = 0.20) between treatments (82.4% vs. 76.6%, respectively; SEM = 3.2). A greater proportion (P = 0.04) of BAS steers diagnosed with BRD required one antimicrobial treatment to regain health compared with CON (59.3% vs. 47.6%, SEM = 4.2). Hence, BAS administration to steers upon feedlot arrival improved BW gain during a 45-d receiving period by enhancing feed efficiency. Moreover, results suggest that BAS improved steer performance by facilitating early detection of BRD signs, lessening the disease recurrence upon first antimicrobial treatment.
Keywords: appeasing substance, beef cattle, feedlot receiving, health, performance, stress
Introduction
Feedlot receiving is one of the most critical phases within the beef production cycle, when cattle are exposed to several stress and health challenges that impact their welfare and productivity (Duff and Galyean, 2007). These stressors include recent weaning, road transport, exposure to novel diets and environments, and commingling with different animals (Cooke, 2017), which elicit adrenocortical and acute-phase protein responses known to impair cattle immunocompetence and growth (Carroll and Forsberg, 2007). Accordingly, incidence of bovine respiratory diseases (BRD) is extremely elevated during feedlot receiving, despite efforts to minimize stress and vaccination protocols against BRD pathogens (Wilson et al., 2017).
With increased restrictions regarding the use of feed-grade antimicrobials in livestock systems (US Food and Drug Administration, 2015), management strategies to minimize stress and enhance performance and immunity of receiving cattle are warranted. One example includes the use of the bovine appeasing substance (BAS); a mixture of fatty acids that replicate the composition of the original bovine appeasing pheromone (Pageat, 2001; Cooke et al., 2020a). Recent research from our group reported that BAS administration to beef calves at weaning alleviated the resultant acute-phase protein response, enhanced humoral immunity against BRD pathogens, and improved body weight (BW) gain during a 6-wk postweaning period (Cappellozza et al., 2020; Cooke et al., 2020a; Schubach et al., 2020). These studies were novel and suggest the use of BAS to improve health and productive responses of cattle exposed to stressful conditions, including the challenges associated with feedlot receiving. Based on this rationale, we hypothesized that administration of BAS will alleviate stress-induced inflammatory responses, improve immunocompetence, and enhance performance of receiving cattle. To test this hypothesis, this experiment evaluated the impacts of BAS administration at feedlot entry on BRD incidence, physiological responses, feed intake, and efficiency, and BW gain during a 45-d feedlot receiving period.
Materials and Methods
This experiment was conducted at the New Mexico State University, Clayton Livestock Research Center (Clayton, NM). All animals were cared for in accordance with acceptable practices and experimental protocols reviewed and approved by the New Mexico State University, Institutional Animal Care and Use Committee (#2019-025).
Animals and treatments
Three hundred and forty-two recently weaned Angus-influenced steers were purchased from a commercial auction facility (Cattlemen’s Livestock Commission Company, Dalhart, TX) and used in this experiment. Steers were originated from 16 cow–calf operations, and no previous health or management history was available. On the day of purchase (day –1; 1800 hours), steers were loaded into 4 commercial livestock trailers (Legend 50’ cattle liner; Barrett LLC, Purcell, OK) at the auction facility and transported for 800 km (12 hr) to stimulate the stress of a long-haul (Cooke et al., 2013). On day 0 of the experiment (0600 hours), steers were unloaded and immediately weighed [initial shrunk BW = 240 ± 1 kg]. Steers were ranked according to truck load, source, and shrunk BW and assigned to receive BAS (IRSEA Group, Quartier Salignan, France; n = 171) or placebo (diethylene glycol monoethyl ether; CON, n = 171) in a manner that treatments had equivalent initial shrunk BW and proportion of load and sources. Steers were segregated by treatment (2 groups) and immediately processed again for treatment administration, with CON steers processed first to avoid cross-contamination during treatment application (Schubach et al., 2020). Treatments (5 mL) were applied topically to the nuchal skin area of each individual steer, according to Cooke et al. (2020a) for dose and route of administration. The placebo used herein is also known as transcutol (Sigma-Aldrich, St. Louis, MO) and used as excipient for the BAS active ingredients. The BAS active ingredient is based on a proprietary mixture of fatty acids including palmitic, oleic, and linoleic acids, added at 1% of the excipient and estimated to remain in treated animals for 15 d according to the manufacturer (Schubach et al., 2020). Treatment groups had no physical contact upon segregation and were maintained in 2 separate nonadjacent paddocks (100 × 12 m) for a 16 hr rest with ad libitum access to water and a complete starter feed (RAMP; Cargill Corn Milling, Blair, NE; Schneider et al., 2017).
After the rest period (day 1), steers within each group were ranked again by truck load, source, and shrunk BW, and allocated to 1 of 24 drylot pens (35 × 12 m; 14 or 15 steers/pen; n = 12/treatment) in a manner that pens had equivalent initial shrunk BW and load proportion, with steers from multiple sources to stimulate the stress of commingling (Step et al., 2008). Pens were arranged in 4 rows of 6 pens/row, and rows were alternately assigned to BAS and CON pens to preserve distance between pens from different treatments (Schubach et al., 2020). Steers were vaccinated against Clostridium (Covexin 8; Merck Animal Health, Madison, NJ), Mannheimia haemolytica, bovine respiratory syncytial virus, bovine herpesvirus-1, bovine viral diarrhea virus 1 and 2, and parainfluenza-3 virus (Vista Once SQ; Merck Animal Health), administered an anthelmintic (Safe-Guard, Merck Animal Health), and received a growth-promoting implant (Revalor-IS; Merck Animal Health) on day 1. Steers had free-choice access to water and the aforementioned starter feed (RAMP; Cargill Corn Milling) from days 1 to 46, which was fed once daily (0800 hours) in a manner to yield 10% residual orts (Colombo et al., 2019).
Sampling
Samples of starter feed were collected weekly, pooled across weeks, and analyzed for nutrient content (Dairy One Forage Laboratory, Ithaca, NY). Steer unshrunk BW was recorded on days 7, 17, 31, 45, and 46 before the feeding of the day. Shrunk BW collected on day 0 was added an 8% shrink (Cooke et al., 2013) to represent initial BW, whereas final BW was calculated by averaging unshrunk BW recorded on days 45 and 46. Steer BW gain during the experiment was calculated by modeling linear regression of BW against sampling days. Feed intake (dry matter basis) was evaluated from days 1 to 45 from each pen by collecting and weighing offered and nonconsumed feed daily. Samples of offered and nonconsumed feed were dried for 96 hr at 50 °C in forced-air ovens for dry matter calculation. Feed intake of each pen was divided by the number of steers within each pen, and expressed as kilogram per steer/day. Feed efficiency was calculated using total BW gain and total feed intake of each pen during the experiment.
Cattle were observed daily for BRD signs according to the DART system (Zoetis, Florham Park, NJ) as described by Sousa et al. (2019), and received antimicrobial treatment as in the study by Lopez et al. (2018). More specifically, steers diagnosed with BRD signs received florfenicol antibiotic with flunixin meglumine (Resflor Gold, Merck Animal Health) at 1 mL/7.6 kg of BW subcutaneously as the first antimicrobial administered, followed by a 5-d moratorium. Steers diagnosed with BRD signs after first antimicrobial treatment were administered ceftiofur crystalline free acid (Excede; Zoetis) at 1 mL/30.3 kg of BW, followed by another 5-d moratorium. Steers diagnosed with BRD signs after the second antimicrobial treatment were administered oxytetracycline (Bio-Mycin 200; Boehringer Ingelheim, Ridgefield, CT) at 1 mL/10 kg of BW and removed from the experiment. Mortality was observed daily, whereas steers deceased after removal from the experiment were not included into the mortality calculation. Immediately prior to processing for treatment administration (day 0), 120 steers that represented average initial BW, truck loads, and sources were selected for blood and hair collection during the experimental period (60 steers/treatment). These steers were then allocated to pens as previously described, in a manner that each pen had 5 steers selected for sampling. Blood was collected on day 0 (prior to treatment administration), 7, 17, 31, and 45 into commercial blood collection tubes (Vacutainer, 10 mL; Becton Dickinson, Franklin Lakes, NJ) containing either no additive or freeze-dried sodium heparin for serum and plasma collection, respectively. Hair samples were collected from the tail switch on days 0 (prior to treatment administration), 17, 31, and 45 as in the study by Schubach et al. (2017).
Laboratorial analyses
Feed samples were analyzed by wet chemistry procedures for concentrations of crude protein (method 984.13; AOAC, 2006), acid detergent fiber (method 973.18 modified for use in an Ankom 200 fiber analyzer, Ankom Technology Corp., Fairport, NY; AOAC, 2006), and neutral detergent fiber using α-amylase and sodium sulfite (Van Soest et al., 1991; modified for use in an Ankom 200 fiber analyzer, Ankom Technology Corp.). Net energy for maintenance and gain were calculated using the equations proposed by NRC (2000). Nutrient profile of the starter feed was (dry matter basis) 22.1% crude protein, 38.3% neutral detergent fiber, 19.1% acid detergent fiber, 1.83 Mcal/kg of net energy for maintenance, and 1.20 Mcal/kg of net energy for gain.
After collection, all blood samples were placed immediately on ice, centrifuged (2,500 × g for 30 min; 4 °C) for plasma or serum harvest, and stored at –80 °C on the same day of collection. All plasma samples were analyzed for concentrations of cortisol (radioimmunoassay kit #07221106, MP Biomedicals, Santa Ana, CA; Colombo et al., 2019) and haptoglobin (Cooke and Arthington, 2013), as well as nonesterified fatty acids (NEFA), β-hydroxybutyrate (BHBA), and glucose (Carysta High Volume Chemistry Analyzer; Zoetis). Serum samples collected on days 0, 17, 31, and 45 were analyzed for antibodies against bovine respiratory syncytial virus (#P00651-2; IDEXX Switzerland AG, Liebefeld-Bern, Switerland), bovine herpesvirus-1 (#99-41459; IDEXX), parainfluenza-3 virus (#P0652-2; IDEXX), bovine viral diarrhea viruses types I and II (#99-44000; IDEXX), and Mannheimia haemolytica (BIOK139 Monoscreen AbELISA; Bio-X Diagnostics S.A., Rochefort, Belgium). However, only samples from steers not diagnosed with BRD signs were analyzed for antibodies against BRD pathogens to ensure that this response was associated with vaccine efficacy rather than pathogenic infection (Callan, 2001). The intra- and interassay CV were, respectively, 4.5% and 7.2% for haptoglobin, 2.9% and 6.1% for cortisol, 4.3% and 4.7% for NEFA, 1.1% and 1.5% for glucose, 2.7% and 3.0% for BHBA, 2.9% and 4.4% for bovine respiratory syncytial virus, 4.1% and 3.6% for bovine respiratory syncytial virus, 5.1% and 5.0% for bovine herpesvirus-1, 1.9% and 1.8% for bovine viral diarrhea viruses, and 4.1% and 4.8% for M. haemolytica, Hair samples were analyzed for cortisol concentrations as described by Schubach et al. (2017), with an intra- and interassay CV of 5.1% and 3.9%, respectively.
Statistical analysis
All data were analyzed using pen as the experimental unit, and Satterthwaite approximation to determine the denominator degrees of freedom for tests of fixed effects. Quantitative data were analyzed using the MIXED procedure of SAS (SAS Inst. Inc., Cary, NC), whereas binary data were analyzed using the GLIMMIX procedure of SAS (SAS Inst. Inc.) with a binomial distribution and logit link function. All models included pen(treatment) and steer(pen) as random variables, but for feed intake and efficiency that used pen(treatment) as random variable. Model statements for BW parameters, feed efficiency, and morbidity-related results contained the effects of treatment. Model statements for feed intake, cumulative BRD incidence, and blood variables contained the effects of treatment, day, and the resultant interaction. Plasma, serum, and hair variables were analyzed using results from day 0 as independent covariate. Steers were selected for sampling prior to any BRD incidence occurred; hence, number of BRD treatments received was also included as an independent covariate for plasma and hair variables (if mortality, value of 4 was used). The specified term for all repeated statements was day, with pen(treatment) as subject for feed intake and efficiency, and steer(pen) as subject for all other analyses. The covariance structure used was first-order autoregressive, which provided the smallest Akaike information criterion and hence the best fit for all variables analyzed. All results are reported as least square means, or covariately adjusted least square means for blood and hair variables. Significance was set at P ≤ 0.05 and tendencies were determined if P > 0.05 and ≤ 0.10. Repeated measures are reported according to main treatment effect if the treatment × day interaction was P > 0.10.
Results
As designed, initial BW (day 0) was similar (P = 0.96) between treatments (Table 1). Steer BW gain during the experiment was greater (P = 0.04) in BAS vs. CON steers, although intermediate and final BW did not differ (P ≥ 0.15) between treatments (Table 1). No treatment effects were detected for feed intake (P = 0.95), resulting in greater (P = 0.05) feed efficiency in BAS vs. CON steers during the experiment (Table 1).
Table 1.
Performance parameters during a 45-d feedlot receiving period of beef steers administered (BAS; n = 12) or not (CON; n = 12) a BAS at feedlot entry (day 0)1
| Item | CON | BAS | SEM | P-value |
|---|---|---|---|---|
| BW,2 kg | ||||
| Day 0 (initial) | 261.5 | 261.3 | 2.7 | 0.96 |
| Day 7 | 243.5 | 243.5 | 2.7 | 0.99 |
| Day 17 | 254.1 | 257.1 | 2.7 | 0.42 |
| Day 31 | 267.4 | 272.9 | 2.7 | 0.15 |
| Day 45 (final) | 291.1 | 294.7 | 2.7 | 0.35 |
| BW gain, kg/d | 0.857 | 1.013 | 0.050 | 0.04 |
| Feed intake,3 kg/d | 4.95 | 4.98 | 0.21 | 0.95 |
| Feed efficiency,4 g/kg | 142 | 171 | 10 | 0.05 |
1Steers individually received 5 mL of a BAS (IRSEA Group, Quartier Salignan, France) or placebo (CON; diethylene glycol monoethyl ether) upon feedlot arrival on day 0. Treatments (5 mL) were applied topically to the nuchal skin area of each animal.
2Steer initial BW was calculated based on arrival shrunk BW and added an 8% shrink (Cooke et al., 2013). Steer final BW was the average of 2 BW recorded on days 45 and 46. The BW gain (kg/d) of each steer was modeled by linear regression of BW against sampling days, and each regression coefficient was used as individual response.
3Steers received a complete starter feed (RAMP; Cargill Corn Milling, Blair, NE) for ad libitum consumption from days 0 to 46. Feed intake was recorded daily from days 1 to 45 by measuring offer and refusals from each pen, divided by the number of steers within each pen, and expressed as kilogram per steer per day.
4Feed efficiency was calculated using total BW gain (in grams), and total feed intake (kg of dry matter) of each pen during the experimental period.
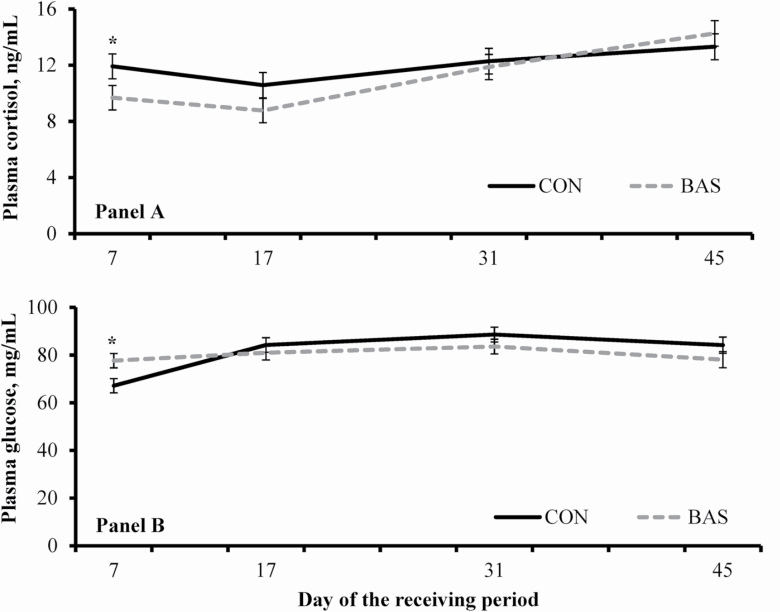
No treatment effects were detected (P ≥ 0.48) for concentrations of plasma haptoglobin, plasma NEFA, and hair cortisol (Table 2). Mean plasma BHBA concentration was greater (P < 0.01) in BAS vs. CON steers (Table 2). A treatment × day interaction was detected (P < 0.01) for plasma glucose concentration, which was greater (P = 0.01) in BAS vs. CON steers on day 7 (Figure 1). A tendency for a similar interaction was noted (P = 0.07) for plasma cortisol concentration, which was greater (P = 0.05) in CON vs. BAS steers on day 7 (Figure 1). Day effects were detected (P < 0.01) for all hormones and metabolites variables reported herein (Table 3). No treatment effects were detected (P ≥ 0.27) for serum antibodies against BRD pathogens (Table 2), which increased (day effects; P < 0.01) across treatments with the advance of the experiment (Table 3).
Table 2.
Physiological responses during a 45-d feedlot receiving period of beef steers administered (BAS; n = 12) or not (CON; n = 12) a BAS at feedlot entry (day 0)1,2
| Item | CON | BAS | SEM | P-value |
|---|---|---|---|---|
| Hormones and metabolites2 | ||||
| Plasma BHBA, mg/mL | 2.75 | 3.23 | 0.12 | <0.01 |
| Plasma haptoglobin, mg/mL | 0.769 | 0.724 | 0.052 | 0.56 |
| Plasma NEFA, μEq/L | 0.273 | 0.291 | 0.018 | 0.48 |
| Hair cortisol, pg/mg of hair | 4.17 | 4.19 | 0.18 | 0.92 |
| Serum antibodies against respiratory viruses3 | ||||
| Parainfluenza-3 virus | 68.2 | 79.1 | 6.5 | 0.27 |
| Bovine respiratory syncytial virus | 98.8 | 111 | 10.5 | 0.43 |
| Bovine viral diarrhea viruses type I and II | 1.09 | 0.978 | 0.095 | 0.42 |
| Bovine herpesvirus-1 | 205.5 | 205.1 | 16.5 | 0.98 |
| Mannhemia haemolytica | 102 | 100 | 8 | 0.91 |
1Steers individually received 5 mL of a BAS (IRSEA Group, Quartier Salignan, France) or placebo (CON; diethylene glycol monoethyl ether) upon feedlot arrival on day 0. Treatments (5 mL) were applied topically to the nuchal skin area of each animal.
2Blood samples were collected on days 0, 7, 17, 31, and 45. Hair samples were collected on days 0, 17, 31, and 45 as in Schubach et al. (2017). Results from day 0 were used as covariate in each respective analysis.
3Steers received vaccination against respiratory pathogens on day 1 (Vista Once SQ; Merck Animal Health, Madison, NJ). Samples collected on days 0, 17, 31, and 45 were analyzed and results expressed as sample:positive control ratio as in the study by Cooke et al. (2020c). Results from day 0 were used as covariate in each respective analysis.
Figure 1.
Plasma concentrations of cortisol (Panel A) and glucose (Panel B) in beef steers administered (BAS; n = 12) or not (CON; n = 12) a BAS at feedlot entry (day 0). Steers individually received 5-mL of a BAS (IRSEA Group, Quartier Salignan, France) or placebo (CON; diethylene glycol monoethyl ether) topically to their nuchal skin area. Values obtained on day 0 were used as independent covariate. A tendency for treatment × day interaction was detected (P = 0.07) for plasma cortisol, whereas a treatment × day interaction was detected for glucose (P < 0.01). Within days, * P ≤ 0.05.
Table 3.
Serum concentrations of antibodies against parainfluenza-3 virus (PI3), bovine respiratory syncytial virus (BRSV), bovine viral diarrhea viruses types I and II (BVD-1), bovine herpesvirus-1 (BHV), Mannhemia haemolytica (MH), plasma concentrations of cortisol (ng/mL), BHBA (mmol/L), haptoglobin (mg/dL), NEFA (μEq/L), and concentrations of cortisol in tail-switch hair (HC, pg/mg of hair) from beef steers during a 45-d feedlot receiving period1
| Serum antibodies against respiratory pathogens | Hormones and metabolites | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | PI3 | BRSV | BVDV | BHV | MH | Cortisol | Haptoglobin | Glucose | BHBA | NEFA | HC |
| 0 | 36.4c | 45.3b | 0.657c | 137a | 71.3c | 13.1ab | 0.885b | 93.0a | 2.02d | 0.400b | 3.28c |
| 7 | — | — | — | — | — | 10.8c | 1.20a | 72.1d | 2.48c | 0.528a | — |
| 17 | 59.9b | 107a | 0.853b | 201a | 108a | 9.70c | 0.995b | 82.5bc | 3.38a | 0.244c | 4.12b |
| 31 | 83.0a | 103a | 1.07a | 204a | 103ab | 12.0b | 0.52c | 86.0b | 3.44a | 0.127d | 3.80b |
| 45 | 79.4a | 104a | 1.13a | 196a | 96b | 13.6a | 0.40c | 80.8c | 3.00b | 0.111d | 4.90a |
| SEM | 6.2 | 8.7 | 0.101 | 16 | 9 | 0.65 | 0.086 | 2.2 | 0.14 | 0.022 | 0.22 |
| P-value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
1Within columns, values with different superscripts differ (P ≤ 0.05). Serum antibodies reported as in the study by Cooke et al. (2020c). Steers received vaccination against respiratory pathogens on day 1 (Vista Once SQ; Merck Animal Health, Madison, NJ).
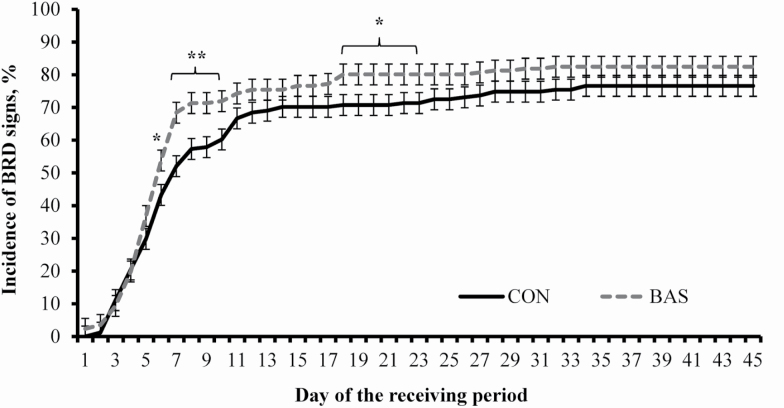
A treatment × day interaction was detected (P < 0.01) for incidence of BRD signs, being greater (P ≤ 0.05) in BAS vs. CON steers on days 6 to 10 and days 19 to 23 (Figure 2). However, the overall incidence of BRD signs during the experiment (Table 4) did not differ (P = 0.20) between treatments. A greater proportion (P = 0.04) of BAS steers diagnosed with BRD signs required one antimicrobial treatment to regain health compared with CON cohorts (Table 4). Hence, the proportion of steers diagnosed with BRD signs that required a second antimicrobial treatment was greater (P = 0.05) in CON vs. BAS steers (Table 4). No treatment differences were observed (P ≥ 0.84) for mortality rate and proportion of steers that required 3 antimicrobial treatments against BRD and were removed from the experiment (Table 4).
Figure 2.
Cumulative incidence of BRD symptoms during a 45-d feedlot receiving period of beef steers administered (BAS; n = 12) or not (CON; n = 12) a BAS at feedlot entry (day 0). Steers individually received 5 mL of a BAS (IRSEA Group, Quartier Salignan, France) or placebo (CON; diethylene glycol monoethyl ether) topically to their nuchal skin area. Steers were observed daily for BRD signs according to the DART system (Zoetis, Florham Park, NJ) and received antimicrobial treatment as in the study by Lopez et al. (2018). A treatment × day interaction was detected (P < 0.01). Within days: * P ≤ 0.05; ** P < 0.01.
Table 4.
Morbidity parameters during a 45-d feedlot receiving period of beef steers administered (BAS; n = 12) or not (CON; n = 12) a BAS at feedlot entry (day 0)1,2
| Item | CON | BAS | SEM | P-value |
|---|---|---|---|---|
| Steers treated for respiratory disease, % | 76.6 | 82.4 | 3.2 | 0.20 |
| 1 treatment required | 47.6 | 59.3 | 4.2 | 0.04 |
| 2 treatments required | 42.3 | 31.4 | 4.1 | 0.05 |
| 3 treatments required | 10.1 | 9.34 | 3.1 | 0.84 |
| Mortality, % | 7.60 | 7.00 | 1.99 | 0.83 |
1Steers individually received 5 mL of a BAS (IRSEA Group, Quartier Salignan, France) or placebo (CON; diethylene glycol monoethyl ether) upon feedlot arrival on day 0. Treatments (5 mL) were applied topically to the nuchal skin area of each animal.
2Steers were observed daily for BRD signs according to the DART system (Zoetis, Florham Park, NJ), and received antimicrobial treatment as in the study by Lopez et al. (2018). Steers were removed from the experiment if a third medical treatment was warranted.
Discussion
The steers used in this experiment were considered high-risk as their previous management and health history were not fully known (Wilson et al., 2017; Sousa et al., 2019). All steers also experienced the stress of transport, commingling with cattle from different sources, vaccination against BRD pathogens, and exposure to a new environment within a 72-h period. The combination of these stressors impacts physiological and immune responses in cattle (Duff and Galyean, 2007; Cooke, 2017). Day effects noted for cortisol (plasma and hair), and haptoglobin concentrations indicate that steers experienced adrenocortical and acute-phase protein responses elicited by road transport and feedlot entry (Cooke et al., 2013; Lippolis et al., 2017; Sousa et al., 2019). Day effects observed for plasma glucose, BHBA, and NEFA denote the alterations in steer nutritional and metabolic status upon feedlot arrival (Hersom et al., 2004; Lippolis et al., 2017; Sousa et al., 2019). All of these stress-induced metabolic and inflammatory challenges predispose cattle to the BRD complex, corroborating the substantial incidence of BRD signs observed in this experiment (Berry et al., 2004; Cooke, 2017). As designed, this experimental model represented the metabolic and health challenges that high-risk feeder cattle typically experience during feedlot receiving (Duff and Galyean, 2007).
Administering BAS upon feedlot entry increased steer BW gain, in accordance with research reporting improved growth in calves administered BAS at weaning (Cappellozza et al., 2020; Cooke et al., 2020a; Schubach et al., 2020). Cooke et al. (2020a) also evaluated BAS administration to bulls at feedlot entry, and reported similar BW gain during the subsequent 45 d. This latter study, however, used older bulls that did not experience the stress challenges that high-risk feeder steers are exposed to during feedlot receiving. In turn, Schubach et al. (2020) reported increased growth rate during the initial 28 d after weaning, which was associated with greater feed intake in calves receiving BAS. Feed intake was not impacted by treatments in this experiment, and BAS steers had improved receiving BW gain due to increased feed efficiency. Nonetheless, BAS steers had greater plasma glucose concentration on day 7, and greater mean plasma BHBA concentration during the experiment compared with CON. Plasma glucose, NEFA, and BHBA are considered metabolic indicators of nutrient intake in beef cattle (Hess et al., 2005). Glucose concentrations are positively linked with feed intake, nutrient availability, and BW gain in beef cattle (Vizcarra et al., 1998; Hersom et al., 2004; Cappellozza et al., 2014), suggesting improved nutritional status of BAS steers on day 7. Concentrations of NEFA and BHBA are typically associated with mobilization of body fat reserves due to insufficient nutrient intake (Hess et al., 2005), with BHBA being mostly investigated in transition dairy cows (Vazquez-Anon et al., 1994). Although circulating BHBA is produced upon hepatic ketogenesis of NEFA, the metabolism of butyrate and other volatile fatty acids (VFA) by the rumen wall also result in BHBA synthesis (Baird, 1981; Herdt, 1988). Hence, plasma BHBA concentrations herein were likely reflective of heightened VFA synthesis rather than lipolysis, as steer BW and plasma BHBA concentrations increased across treatments with the advance of the experiment. Collectively, treatment differences noted for plasma glucose and BHBA concentrations suggest that BAS administration improved utilization of dietary nutrients, or perhaps increased feed intake that was not perceived by our experimental design, resulting in greater BW gain during the 45-d receiving period.
Stress-induced physiological and acute-phase responses impact feed intake and efficiency in cattle by, respectively, reducing appetite and increasing maintenance requirements (Elsasser et al., 1997; Johnson, 1997; Nelson and Cox, 2005). Cooke et al. (2020a) reported that BAS administration reduced plasma haptoglobin concentrations on day 15 after weaning and associated this response with the increased postweaning BW gain, whereas cortisol concentrations in plasma or hair were not impacted by BAS. Schubach et al. (2020) reported that calves administered BAS at weaning had lessened plasma haptoglobin and hair cortisol responses during a 42-d preconditioning, which partially explained the increased feed intake of BAS calves. In this experiment, BAS administration at feedlot entry did not impact plasma haptoglobin and hair cortisol concentrations during the 45-d receiving period. However, plasma cortisol concentrations were lessened by BAS administration on day 7 of the experiment, differing from our previous research efforts (Cooke et al., 2020a; Schubach et al., 2020). These outcomes suggest that BAS administration alleviated the adrenocortical response typical of the receiving period (Cooke, 2017), at least transiently during the period which BAS is expected to be active (Osella et al., 2018; Cooke et al., 2020a; Schubach et al., 2020). Although circulating cortisol has been considered a stress biomarker in cattle (Carroll and Forsberg, 2007), handling for blood sampling stimulates an acute stress response that rapidly increases circulating cortisol (Moya et al., 2013). Hair cortisol concentrations were evaluated here and in previous research, as cortisol gradually accumulates in the emerging hair and represents long-term adrenocortical responses (Schubach et al., 2020). Hence, treatment differences observed for plasma cortisol concentration should be interpreted with caution, but may have contributed to improved feed efficiency and BW gain of BAS steers. Conversely, plasma haptoglobin and hair cortisol results do not corroborate with treatment effects noted for plasma cortisol (Cooke et al., 2012) and failed to further elucidate the biological benefits of BAS administration to stressed cattle (Cooke et al., 2020a; Schubach et al., 2020).
Steers effectively acquired humoral immunity against BRD pathogens upon vaccination, given that serum concentrations of antibodies against these antigens increased across treatments during the 45-d receiving period (Richeson et al., 2008). Schubach et al. (2020) reported that BAS administration improved acquired immunity to BRD viruses in calves vaccinated at weaning, and attributed these outcomes to lessened adrenocortical and acute-phase protein responses in BAS calves (Blecha et al., 1984; Munck et al., 1984; Biolatti et al., 2005). In this experiment, BAS did not improve acquired humoral immunity against BRD pathogens despite the transient differences noted in plasma cortisol concentration. Nonetheless, statistical power for these analyses was limited as serum samples from steers observed with BRD signs during the experiment were not analyzed (~80% of samples not analyzed). Numerical differences noted for bovine respiratory syncytial virus and parainfluenza-3 virus indicate a 15% and 13% improvement in acquired immunity for BAS steers, which are numerically superior to treatment differences reported by Schubach et al. (2020). Additional research with adequate sample size is warranted to evaluate the potential benefits of BAS on humoral immunity against BRD pathogens, including vaccination against these pathogens at weaning, during preconditioning programs, and upon feedlot arrival.
Overall incidence of BRD in this experiment was substantial and similar between treatments. Signs of BRD were detected earlier in BAS steers, which may suggest hastened susceptibility and establishment of the disease. Alternatively, the BRD diagnosis used herein are based on behavioral traits such as decreased activity and abnormal feeding and drinking (Sousa et al., 2019). Cattle are prey species that attempt to mask signs of sickness, especially if these signs make them vulnerable for predation (Weary et al., 2009). Therefore, initial BRD signs may be disguised by cattle as part of their natural defensive behavior (Edwards, 2010; Cooke et al., 2020b), and the appeasing effects of BAS may have facilitated expression and recognition of these early behavioral signs (Schubach et al., 2020). Early detection of BRD enhances the efficiency of the antimicrobial treatment (Ferran et al., 2011), corroborating the greater proportion of BAS steers that regained health after the first and did not required a second antimicrobial treatment. Moreover, BW gain and feed efficiency are often associated negatively with the number of antimicrobial treatments to BRD (Waggoner et al., 2007; Thompson et al., 2012; Blakebrough-Hall et al., 2020). Therefore, BAS administration at feedlot entry likely contributed to improved steer performance during the 45-d receiving period by facilitating early detecting of BRD signs, and lessening the disease recurrence upon first antimicrobial treatment.
In summary, BAS administration high-risk steers at feedlot arrival improved BW gain by enhancing feed efficiency during a 45-d receiving period. These outcomes may be associated with improved utilization of dietary nutrients as denoted by plasma glucose and BHBA results, and a transient decrease in adrenocortical activity and resultant plasma cortisol responses. More importantly, BAS administration appears to have contributed to performance responses by allowing earlier detection of BRD, and alleviating the reappearance of BRD signs. Additional research is warranted to evaluate the potential benefits of BAS to receiving cattle, including multiple BAS administrations, carryover effects throughout growing and finishing periods, and potential benefits to carcass traits. Nonetheless, results from this experiment suggest BAS as a novel management strategy to promote performance and health responses of receiving cattle.
Acknowledgments
Financial support for this research was provided by Nutricorp (Araras, SP, Brazil), which distributes BAS in the United States, Canada, and Brazil. Kelsey M. Schubach is a Tom Slick Graduate Research Fellow at Texas A&M University. Alice P. Brandão is supported by CAPES, Brazil (#88881.128327/2016-01).
Glossary
Abbreviations
- BAS
bovine appeasing substance
- BHBA
β-hydroxybutyrate
- BRD
bovine respiratory disease
- BW
body weight
- NEFA
nonesterified fatty acids
- VFA
volatile fatty acids
Conflict of interest statement
Bruno I. Cappellozza is employed by the funder of this project (Nutricorp; Araras, SP, Brazil) and contributed to research design and data interpretation. However, the principal investigator (Reinaldo F. Cooke) and all other authors declare no real or perceived conflicts of interest.
Literature Cited
- AOAC 2006. Official methods of analysis. 18th ed. Arlington VA: Association of Official Analytical Chemists. [Google Scholar]
- Baird G D. 1981. Ruminant ketosis. Biochem. Soc. Trans. 9:348–349. doi: 10.1042/bst0090348. [DOI] [PubMed] [Google Scholar]
- Berry B A, Confer A W, Krehbiel C R, Gill D R, Smith R A, and Montelongo M. . 2004. Effects of dietary energy and starch concentrations for newly received feedlot calves: II. Acute-phase protein response. J. Anim. Sci. 82:845–850. doi: 10.2527/2004.823845x. [DOI] [PubMed] [Google Scholar]
- Biolatti B, Bollo E, Cannizzo F T, Zancanaro G, Tarantola M, Dacasto M, Cantiello M, Carletti M, Biolatti P G, and Barbarino G. . 2005. Effects of low-dose dexamethasone on thymus morphology and immunological parameters in veal calves. J. Vet. Med. A Physiol. Pathol. Clin. Med. 52:202–208. doi: 10.1111/j.1439-0442.2005.00714.x. [DOI] [PubMed] [Google Scholar]
- Blakebrough-Hall C, McMeniman J P, and González L A. . 2020. An evaluation of the economic effects of bovine respiratory disease on animal performance, carcass traits, and economic outcomes in feedlot cattle defined using four BRD diagnosis methods. J. Anim. Sci. 98:skaa005. doi: 10.1093/jas/skaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blecha F, Boyles S L, and Riley J G. . 1984. Shipping suppresses lymphocyte blastogenic responses in Angus and Brahman X Angus feeder calves. J. Anim. Sci. 59:576–583. doi: 10.2527/jas1984.593576x. [DOI] [PubMed] [Google Scholar]
- Callan R J. 2001. Fundamental considerations in developing vaccination protocols. Bovine Pract. 34:14–22. [Google Scholar]
- Cappellozza B I, Bastos J P, and Cooke. R F. 2020. Administration of an appeasing substance to Bos indicus-influenced beef cattle improves performance after weaning and carcass pH. Livest. Sci. 238:104067. doi: 10.1016/j.livsci.2020.104067. [DOI] [Google Scholar]
- Cappellozza B I, Cooke R F, Reis M M, Moriel P, Keisler D H, and Bohnert D W. . 2014. Supplementation based on protein or energy ingredients to beef cattle consuming low-quality cool-season forages: II. Performance, reproductive, and metabolic responses of replacement heifers. J. Anim. Sci. 92:2725–2734. doi: 10.2527/jas.2013-7442. [DOI] [PubMed] [Google Scholar]
- Carroll J A, and Forsberg N E. . 2007. Influence of stress and nutrition on cattle immunity. Vet. Clin. North Am. Food Anim. Pract. 23:105–149. doi: 10.1016/j.cvfa.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Colombo E A, Cooke R F, Millican A A, Schubach K M, Scatolin G N, Rett B, and Brandão A P. . 2019. Supplementing an immunomodulatory feed ingredient to improve thermoregulation and performance of finishing beef cattle under heat stress conditions. J. Anim. Sci. 97:4085–4092. doi: 10.1093/jas/skz266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R F. 2017. Invited paper: nutritional and management considerations for beef cattle experiencing stress-induced inflammation. Prof. Anim. Sci. 33(1)1–11. doi: 10.15232/pas.2016-01573. [DOI] [Google Scholar]
- Cooke R F, and Arthington J D. . 2013. Concentrations of haptoglobin in bovine plasma determined by ELISA or a colorimetric method based on peroxidase activity. J. Anim. Physiol. Anim. Nutr. (Berl.). 97:531–536. doi: 10.1111/j.1439-0396.2012.01298.x. [DOI] [PubMed] [Google Scholar]
- Cooke R F, Carroll J A, Dailey J, Cappellozza B I, and Bohnert D W. . 2012. Bovine acute-phase response after different doses of corticotropin-releasing hormone challenge. J. Anim. Sci. 90:2337–2344. doi: 10.2527/jas.2011-4608. [DOI] [PubMed] [Google Scholar]
- Cooke R F, Daigle C L, Moriel P, Smith S B, Tedeschi L O, and Vendramini J M B. . 2020b. Board Invited Review - Cattle adapted to tropical and subtropical environments (I): social, nutritional, and carcass quality considerations. J. Anim. Sci. 98:skaa015. doi: 10.1093/jas/skaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R F, Guarnieri Filho T A, Cappellozza B I, and Bohnert D W. . 2013. Rest stops during road transport: impacts on performance and acute-phase protein responses of feeder cattle. J. Anim. Sci. 91:5448–5454. doi: 10.2527/jas.2013-6357. [DOI] [PubMed] [Google Scholar]
- Cooke R F, Millican A, Brandão A P, Schumaher T F, de Sousa O A, Castro T, Farias R S, and Cappellozza B I. . 2020a. Short communication: administering an appeasing substance to Bos indicus-influenced beef cattle at weaning and feedlot entry. Animal 14:566–569. doi: 10.1017/S1751731119002490. [DOI] [PubMed] [Google Scholar]
- Cooke R F, Paiva R, and Pohler K G. . 2020c. Using enzyme-linked immunosorbent assays to evaluate humoral responses to vaccination against respiratory viruses in beef cattle. J. Anim. Sci. doi: 10.1093/jas/skaa249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff G C, and Galyean M L. . 2007. Board-invited review: recent advances in management of highly stressed, newly received feedlot cattle. J. Anim. Sci. 85:823–840. doi: 10.2527/jas.2006-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards T A. 2010. Control methods for bovine respiratory disease for feedlot cattle. Vet. Clin. North Am. Food Anim. Pract. 26:273–284. doi: 10.1016/j.cvfa.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Elsasser T H, Kahl S, Steele N C, and Rumsey T S. . 1997. Nutritional modulation of somatotropic axis-cytokine relationships in cattle: a brief review. Comp. Biochem. Physiol. 116A:209–221. doi: 10.1016/S0300-9629(96)00279-4. [DOI] [PubMed] [Google Scholar]
- Ferran A A, Toutain P L, and Bousquet-Mélou A. . 2011. Impact of early versus later fluoroquinolone treatment on the clinical; microbiological and resistance outcomes in a mouse-lung model of Pasteurella multocida infection. Vet. Microbiol. 148:292–297. doi: 10.1016/j.vetmic.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdt T H. 1988. Fuel homeostasis in the ruminant. Vet. Clin. North Am. Food Anim. Pract. 4:213–231. doi: 10.1016/s0749-0720(15)31045-8. [DOI] [PubMed] [Google Scholar]
- Hersom M J, Wettemann R P, Krehbiel C R, Horn G W, and Keisler D H. . 2004. Effect of live weight gain of steers during winter grazing: III. Blood metabolites and hormones during feedlot finishing. J. Anim. Sci. 82:2059–2068. doi: 10.2527/2004.8272059x. [DOI] [PubMed] [Google Scholar]
- Hess B W, Lake S L, Scholljegerdes E J, Weston T R, Nayigihugu V, Molle J D C, and Moss. G E. 2005. Nutritional controls of beef cow reproduction. J. Anim. Sci. 83:E90–E106. doi: 10.2527/2005.8313_supplE90x. [DOI] [Google Scholar]
- Johnson R W. 1997. Inhibition of growth by pro-inflammatory cytokines: an integrated view. J. Anim. Sci. 75:1244–1255. doi: 10.2527/1997.7551244x. [DOI] [PubMed] [Google Scholar]
- Lippolis K D, Cooke R F, Schumaher T, Brandão A P, Silva L G T, Schubach K M, Marques R S, and Bohnert D W. . 2017. Physiologic, health, and performance responses of beef steers supplemented with an immunomodulatory feed ingredient during feedlot receiving. J. Anim. Sci. 95:4945–4957. doi: 10.2527/jas2017.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez F A, Oosthuysen E R, Duff G C, Richeson J T, Samuelson K L, Hubbert M E, and Löest C A. . 2018. Health, performance, and complete blood counts of newly received feedlot heifers in response to an oral drench of water and crude glycerin. Transl. Anim. Sci. 2(Suppl. 1):S74–S78. doi: 10.1093/tas/txy028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya D, Schwartzkopf-Genswein K S, and Veira D M. . 2013. Standardization of a non-invasive methodology to measure cortisol in hair of beef cattle. Livest. Sci. 158:138–144. doi: 10.1016/j.livsci.2013.10.007. [DOI] [Google Scholar]
- Munck A, Guyre P M, and Holbrook N J. . 1984. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr. Rev. 5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Nelson D L, and Cox M M. . 2005. Lehninger principles of biochemistry. 4th ed. New York, NY: W. H. Freeman and Company. [Google Scholar]
- NRC 2000. Nutrient requirement of beef cattle. 7th ed. Washington, DC: National Academies Press. [Google Scholar]
- Osella M C, Cozzi A, Spegis C, Turille G, Barmaz A, Lecuelle C L, Teruel E, Bienboire-Frosini C, Chabaud C, Bougrat L, . et al. 2018. The effects of a synthetic analogue of the bovine appeasing pheromone on milk yield and composition in Valdostana dairy cows during the move from winter housing to confined lowland pastures. J. Dairy Res. 85:174–177. doi: 10.1017/S0022029918000262. [DOI] [PubMed] [Google Scholar]
- Pageat P. 2001. Appeasing pheromones to decrease stress, anxiety and aggressiveness. US Patent 6,169,113 B1. Jan. 2, 2001.
- Richeson J T, Beck P A, Gadberry M S, Gunter S A, Hess T W, Hubbell D S, and Jones C. . 2008. Effects of on-arrival versus delayed modified-live virus vaccination on health, performance, and serum infectious bovine rhinotracheitis titers of newly-received beef calves. J. Anim. Sci. 86:999–1005. doi: 10.2527/jas.2007-0593. [DOI] [PubMed] [Google Scholar]
- Schneider C J, Nuttelman B L, Shreck A L, Burken D B, Griffin W A, Gramkow J L, Stock R A, Klopfenstein T J, and Erickson G E. . 2017. Use of a complete starter feed in grain adaptation programs for feedlot cattle. J. Anim. Sci. 95:3639–3653. doi: 10.2527/jas.2016.1115. [DOI] [PubMed] [Google Scholar]
- Schubach K M, Cooke R F, Brandão A P, Lippolis K D, Silva L G T, Marques R S, and Bohnert D W. . 2017. Impacts of stocking density on development and puberty attainment of replacement beef heifers. Animal 11:2260–2267. doi: 10.1017/S1751731117001070. [DOI] [PubMed] [Google Scholar]
- Schubach K M, Cooke R F, Daigle C L, Brandão A P, Rett B, Ferreira V S M, Scatolin G N, Colombo E A, Pohler K G, and Cappellozza B I. . 2020. Administering an appeasing substance to beef calves at weaning to optimize productive and health responses during a 42-d preconditioning program. J. Anim. Sci. 98. doi: 10.1093/jas/skaa269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa O A, Cooke R F, Brandão A P, Schubach K M, Schumaher T F, Bohnert D W, and Marques R S. . 2019. Productive and physiological responses of feeder cattle supplemented with Yucca schidigera extract during feedlot receiving. J. Anim. Sci. 97:208–219. doi: 10.1093/jas/sky412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Step D L, Krehbiel C R, DePra H A, Cranston J J, Fulton R W, Kirkpatrick J G, Gill D R, Payton M E, Montelongo M A, and Confer A W. . 2008. Effects of commingling beef calves from different sources and weaning protocols during a forty-two-day receiving period on performance and bovine respiratory disease. J. Anim. Sci. 86:3146–3158. doi: 10.2527/jas.2008-0883. [DOI] [PubMed] [Google Scholar]
- Thompson D U, Moore E S, White B J, and Reinhardt C D. . 2012. Case study: effects of undifferentiated bovine respiratory disease on performance and marbling deposition in feedlot steers fed to a common yield grade endpoint. Bov. Pract. 46:52–58. doi: 10.21423/bovine-vol46no1p52-58. [DOI] [Google Scholar]
- US Food and Drug Administration 2015. FACT SHEET: veterinary feed directive final rule and next steps. http://www.fda.gov/AnimalVeterinary/DevelopmentApprovalProcess/ucm449019.htm. – [accessed August 25, 2020].
- Van Soest P J, Robertson J B, and Lewis B A. . 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Vazquez-Añon M, Bertics S, Luck M, Grummer R R, and Pinheiro J. . 1994. Peripartum liver triglyceride and plasma metabolites in dairy cows. J. Dairy Sci. 77:1521–1528. doi: 10.3168/jds.S0022-0302(94)77092-2. [DOI] [PubMed] [Google Scholar]
- Vizcarra J A, Wettemann R P, Spitzer J C, and Morrison D G. . 1998. Body condition at parturition and postpartum weight gain influence luteal activity and concentrations of glucose, insulin, and nonesterified fatty acids in plasma of primiparous beef cows. J. Anim. Sci. 76:927–936. doi: 10.2527/1998.764927x. [DOI] [PubMed] [Google Scholar]
- Waggoner J, Mathis C, Löest C, and Sawyer J. . 2007. Case study: impact of morbidity in finishing beef steers on feedlot average daily gain, carcass characteristics, and carcass value. Prof. Anim. Sci. 23:174–178. doi: 10.15232/S1080-7446(15)30958-X. [DOI] [Google Scholar]
- Weary D M, Huzzey J M, and von Keyserlingk M A. . 2009. Board-invited review: using behavior to predict and identify ill health in animals. J. Anim. Sci. 87:770–777. doi: 10.2527/jas.2008-1297. [DOI] [PubMed] [Google Scholar]
- Wilson B K, Richards C J, Step D L, and Krehbiel C R. . 2017. Best management practices for newly weaned calves for improved health and well-being. J. Anim. Sci. 95:2170–2182. doi: 10.2527/jas.2016.1006. [DOI] [PubMed] [Google Scholar]