Abstract
Background
Little is known regarding risk for co-occurring mental health conditions among pediatric patients with congenital adrenal hyperplasia (CAH). The objective of the current study was to investigate the prevalence of medically managed attention-deficit/hyperactivity disorder (ADHD) in 2 large administrative samples of insured children and adolescents with and without CAH in the United States.
Methods
We assessed the prevalence of CAH and of medically managed ADHD using algorithms defined from diagnosis codes and filled prescriptions data using the IBM MarketScan Commercial and Multi-State Medicaid claims databases. We evaluated subjects who were continuously enrolled for ≥ 12 months with a first claim during October 2015 through December 2017 when they were 5 to 18 years old.
Results
The administrative prevalence of CAH in the Commercial (N = 3 685 127) and Medicaid (N = 3 434 472) samples was 10.1 per 100 000 (n = 372) and 7.2 per 100 000 (n = 247), respectively. The prevalence of medically managed ADHD in the non-CAH population was 8.4% in the Commercial sample and 15.1% in the Medicaid sample. Among children with CAH, there was no increased prevalence of ADHD in the Commercial (9.2%, prevalence ratio [PR] = 1.1; 95% confidence interval [CI], 0.82-1.54; P = 0.48) or Medicaid (13.8%; PR = 0.91; 95% CI, 0.67–1.24; P = 0.55) samples compared with the general population.
Conclusions
Using 2 large samples of insured children and adolescents in the United States, we found similar prevalence of medically managed ADHD among those with CAH and the general population. Future research to assess the validity of our claims algorithm for identifying pediatric CAH cases is warranted.
Keywords: congenital adrenal hyperplasia, attention-deficit/hyperactivity disorder, administrative data, health insurance claims, behavioral health
Congenital adrenal hyperplasia (CAH) is a form of primary adrenal insufficiency characterized by impaired cortisol synthesis and increased adrenal androgen production. CAH is most commonly from 21-hydroxylase deficiency (21OHD). Depending on the degree of enzyme deficiency, CAH is classified as either the classic severe form, which includes salt-wasting and simple-virilizing subtypes, or as nonclassic CAH, which is a milder form and often asymptomatic. Classic CAH, which is detected by newborn screening programs in the United States and in many other countries, is a rare disease that occurs in roughly 1:16 000 in US children; nonclassic CAH resulting from 21OHD is more common, with a population prevalence of perhaps 1:500 [1]. Individuals affected with classic CAH are at risk of acute adrenal crises and salt-wasting crises that if undetected can lead to death. Treatment for CAH involves lifelong glucocorticoid replacement and mineralocorticoid therapy if salt-wasting is present [1]. Most research on health outcomes in classic CAH has focused on physical consequences of 21OHD and hormone therapy. The mental and behavioral health of persons living with classic CAH has been less well studied, and study findings are often inconsistent or inconclusive [2]. Reports of increased frequency of white matter abnormalities and decreased volume on magnetic resonance imaging scans in adult patients with CAH associated with glucocorticoid dosing [3-5] are concerning.
Children and adolescents with classic CAH may be at risk of behavioral or mental health disturbances resulting from hormonal imbalances associated with excess production of adrenal androgens in utero and postnatally. A negative feedback loop in the hypothalamic-pituitary-adrenal (HPA) axis normally maintains cortisol and ACTH in a state of dynamic equilibrium, but hypocortisolemia in CAH leads to ACTH-driven adrenal gland stimulation in which cortisol precursors are shunted to the androgen pathway. Disruption of the HPA axis and fluctuations of cortisol and androgen could potentially affect psychosocial and behavioral outcomes in children and adolescents with CAH through multiple mechanisms.
First, basal HPA axis function, cortisol secretion, and stress reactivity are intimately intertwined and associated with mental health during development. For example, both basal dysregulation of the HPA axis associated with elevated cortisol levels and impaired inhibitory feedback and hypercortisolemia as part of abnormal stress responsivity have been associated with mood and anxiety disorders in children exposed to adverse life events [6, 7]. Higher cortisol reactivity to stress has also been associated with increased cumulative cortisol exposure and behavioral problems among preschool-aged children [8], whereas hypocortisolemia among older children and adolescents has been associated with behavioral problems [9]. Attention problems may have a sex-specific relationship with cortisol, with 1 study finding a relationship between higher basal cortisol levels and attention problems in preadolescent boys versus lower basal cortisol levels in preadolescent girls [10]. The interplay between cortisol dysfunction and risk for mental health symptoms may evolve over time and differ based on stage of development [11, 12], especially with respect to puberty, when interactions with sex steroid hormone levels have also been reported [13].
Second, early and long-term androgen exposure may increase susceptibility to behavioral problems, especially among girls. In the general population, males with disruptive behavioral problems have been found to have higher androgen levels [14]. Furthermore, the effects of androgens on behavior have been shown to significantly interact with cortisol levels [15], with 1 study showing a positive relationship between androgen level and aggression among adolescents with lower cortisol levels, but not those with higher levels [16]. Interestingly, girls with CAH have been shown to have more masculinized behaviors, higher activity levels, and more aggressive behaviors than their nonaffected counterparts, which has been hypothesized to result from early androgen exposure [17-19].
Finally, because of the pharmacokinetic properties of exogenous glucocorticoids, current medical therapy is unable to replicate the circadian and ultradian cortisol secretion rhythms associated with normal adrenal function [20]. In children, hydrocortisone given 3 times per day is used as cortisol replacement to avoid the adverse impact on growth from long-acting steroids [1]. Fluctuating cortisol levels of short peaks and long troughs occur because hydrocortisone has a short median elimination half-life in children with CAH of 58 minutes (range, 41-105 minutes) allowing most of the hydrocortisone dose to be eliminated from the body within 4 to 5 hours [21]. Corticosteroids have been shown to affect brain structure and function in mice, both developmentally and in response to stress [22]. Chronic corticosteroid administration has been linked with deficits in short-term memory and acute use to disturbances in executive function and memory [23].
A heterogenous body of literature has examined executive functioning, behavior, and psychosocial outcomes in the pediatric CAH population [17, 18, 24-30]. Studies have reported diverse and often contradictory findings, which may in part be due to the use of diverse measurement techniques ranging from screening scales to semistructured interviews. Importantly, all of these studies have included small numbers of CAH cases, typically less than 100, with most having subjects recruited from a single institute or center. Thus, although it is plausible that children and adolescents with CAH may be at increased risk for childhood mental health conditions, there is currently a dearth of conclusive studies on this topic.
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common mental health disorders diagnosed in children [31, 32]. Children with ADHD often have high activity levels and difficulty focusing and controlling their behavior. In the current study, we aimed to adapt a health insurance claims-based algorithm for CAH to identify a large sample of pediatric CAH cases and to investigate the prevalence of medically managed ADHD in 2 large administrative samples of insured children and adolescents with and without CAH in the United States.
Materials and Methods
To identify a large sample of children and adolescents with CAH, we used the IBM MarketScan Commercial and Multi-State Medicaid research databases, which contain medical and drug insurance claims data on outpatient and inpatient services and admissions and outpatient pharmacy claims. The Commercial data relate to employees and their dependents enrolled in participating employer-sponsored health insurance plans throughout the United States. The Medicaid database includes data for children and adolescents enrolled in Medicaid or Children’s Health Insurance programs from participating states. Data from both databases were accessed and tabulated using IBM MarketScan Treatment Pathways, an online analytic platform.
We used a study period of October 1, 2015, through December 31, 2017, for a first claim of CAH or ADHD and through December 31, 2018, for follow-up claims. We limited subject enrollment to individuals with plans with complete outpatient pharmacy claims data, at least 1 outpatient claim during the initial 2015 to 2017 study period and continuous enrollment for at least 12 months from the date of the first claim within the initial 2015 to 2017 study period. Age in years was assigned at the date of the first outpatient claim during the initial study period; records were analyzed for all individuals who ages 5 to 18 years at the time of their first encounter.
We restricted the Commercial sample to plans with complete data on mental health encounters. We were unable to similarly restrict the Medicaid sample because of insufficient reporting of explicit mental health coverage.
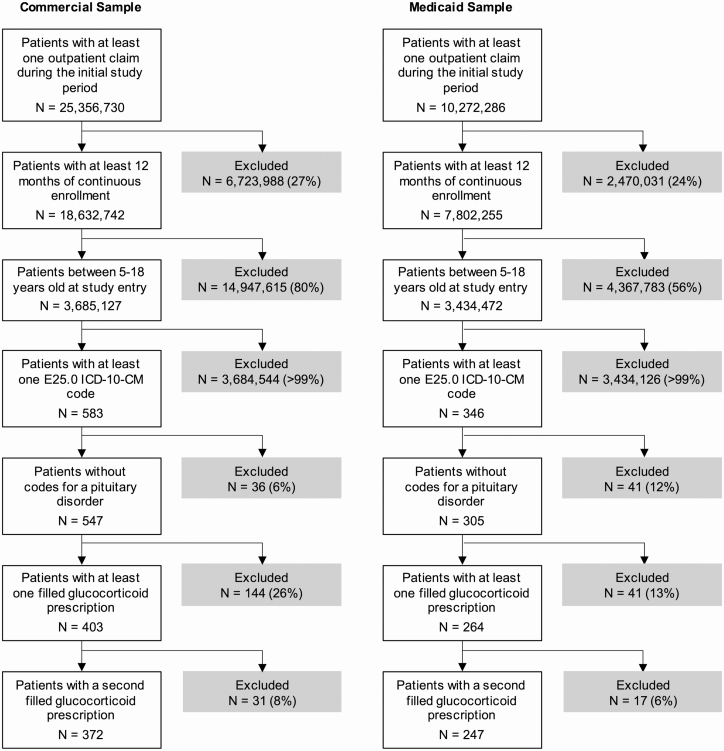
We created an algorithm for defining pediatric CAH cases requiring glucocorticoid treatment using diagnostic codes from the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Clinical Modification (ICD-10-CM) diagnostic codes as well as filled prescription drug claims (Fig. 1). We classified individuals as having CAH if they had at least 1 claim with the ICD-10-CM code E25.0 (“Congenital adrenogenital disorders associated with enzyme deficiency”) in any setting, no ICD-9-CM or ICD-10 claims with a diagnosis code for a pituitary disorder (ICD-9: 253.2, 253.4, 253.7, 253.8, 253.9; ICD-10: E228.x, E229.x, E236.x, E237.x), and at least 2 filled prescriptions for a glucocorticoid, with the second fill at least 28 days apart and within 365 days of the first fill. The prevalence of CAH was defined as the percentage of children and adolescents meeting these criteria. We restricted our CAH algorithm to the ICD-10-CM E25.0 code, as the previously used ICD-9-CM code, 255.2 (“Adrenogenital disorders”), is less specific to CAH. We adapted our approach from Stewart and colleagues [33] and Jenkins-Jones and colleagues [34], who used national administrative data sets in the United States and United Kingdom, respectively, to identify patients with CAH.
Figure 1.
Attrition diagram for CAH cases in the Commercial and Medicaid samples. International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Clinical Modification (ICD-10-CM). Percentages reflect the percentage of patients excluded from the prior total. CAH, congenital adrenal hyperplasia.
We assessed the presence of medically managed ADHD using an algorithm previously defined using data from the MarketScan Commercial and Medicaid MAX databases [35]. Individuals were classified as receiving clinical care for ADHD if they had either: (1) 2 or more outpatient claims with a diagnosis code for ADHD (ICD-9-CM: 314.XX; ICD-10-CM: F90.0X) 7 or more days apart; or (2) 1 outpatient claim with an ADHD code and at least 2 prescription drug claims at least 14 days apart for a Food and Drug Administration-approved ADHD medication: amphetamine and mixed amphetamine salts, atomoxetine, clonidine, dextroamphetamine, dexmethylphenidate, guanfacine, lisdexamfetamine, or methylphenidate. The prevalence of medically managed ADHD was defined as the percentage of children and adolescents ages 5 to 18 years at study entry who met these criteria within the study period, October 2015 through December 2018.
All statistical analyses were calculated using MATLAB (MathWorks, Natick, MA) and SAS 9.4 (SAS Institute, Cary, NC). All analyses were calculated separately by payer type (Commercial sample and Medicaid sample). Demographic groups were summarized on the basis of sex (boys vs girls) and age (5-11 years vs 12-18 years). CAH prevalence was calculated for each sample overall and by age and sex. To compare the prevalence of CAH across demographic groups, prevalence ratios (PR), 95% confidence intervals (CI), and χ 2 tests were calculated. ADHD prevalence was calculated for each sample overall and by age and sex. To compare the prevalence of ADHD across demographic groups within the non-CAH and CAH groups separately, χ 2 tests, PRs, and 95% CIs were calculated. To compare the prevalence of ADHD between the non-CAH and CAH groups, analyses were conducted separately by sex and with stratification by age using χ 2 tests, PRs, and 95% CIs. For the main effect of CAH in each subsample, Mantel-Haenszel adjusted prevalence ratios (aPR) are presented after stratification by age. The PRs presented for both strata (5-11 years and 12-18 years) are unadjusted. All statistical tests are 2-sided and statistical significance was defined as P < 0.05.
Results
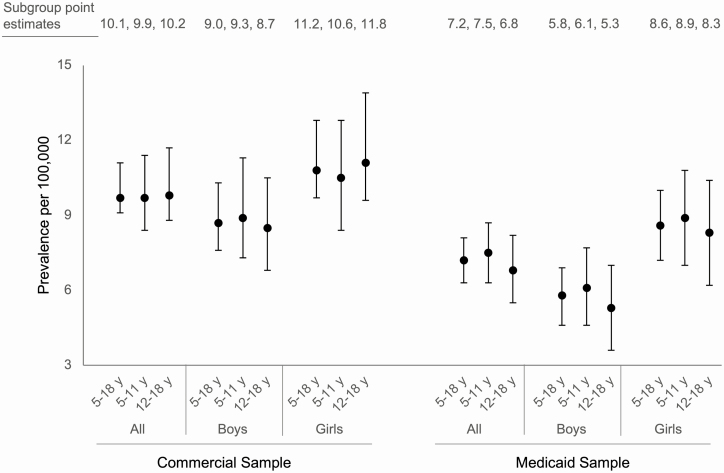
Using our claims-based algorithm, we identified a combined total of 619 CAH cases of approximately 7.1 million individual subjects across the Commercial (N = 3 685 127) and Medicaid (N = 3 434 472) samples (Fig. 1). Descriptive data for each sample are presented in Table 1. The prevalence of CAH in the Commercial sample was 1 in 9906 (10.1/100 000; Fig. 2, left). In the Medicaid sample, the prevalence of CAH was 1 in 13 905 (7.2/100 000; Fig. 2, right). For both the Commercial and Medicaid samples, prevalence of CAH did not vary significantly by age (Fig. 2; Table 2). In both samples, the prevalence of CAH was higher among girls compared with boys (Fig. 2). In the Commercial sample, the prevalence of CAH was 26% higher among girls (11.2 vs 9.0 per 100 000; PR = 1.26; 95% CI, 1.02-1.54, P = 0.029; Table 2). In the Medicaid sample, the prevalence of CAH was approximately 50% higher among girls (8.6 vs 5.8 per 100 000; PR = 1.49; 95% CI, 1.16-1.92; P = 0.002; Table 2).
Table 1.
Descriptive Statistics for the Commercial and Medicaid Samples
| Non-CAH Group | CAH Group | |||||
|---|---|---|---|---|---|---|
| All, n (%) | Boys, n (%) | Girls, n (%) | All, n (%) | Boys, n (%) | Girls, n (%) | |
| Commercial sample | ||||||
| All | 3 684 755 | 1 853 374 (50)a | 1 831 381 (50)a | 372 | 166 (45)a | 206 (55)a |
| 5-11 y | 1 731 153 (47)b | 885 800 (48)b | 845 353 (46)b | 172 (46)b | 82 (49)b | 90 (44)b |
| 12-18 y | 1 953 602 (53)b | 967 574 (52)b | 986 028 (54)b | 200 (54)b | 84 (51)b | 116 (56)b |
| Medicaid sample | ||||||
| All | 3 434 225 | 1 730 160 (50)a | 1 704 065 (50)a | 247 | 100 (40)a | 147 (60)a |
| 5-11 y | 1 968 112 (57)b | 1 010 302 (58)b | 957 810 (56)b | 147 (60)b | 62 (62)b | 85 (58)b |
| 12-18 y | 1 466 113 (43)b | 719 858 (42)b | 719 858 (44)b | 100 (40)b | 38 (38)b | 62 (42)b |
aPercentages add to 100 across each row within each study group (non-CAH or CAH) and sample (Commercial or Medicaid).
bPercentages add to 100 down each column within each study group and sample for sex-specific subgroups (all, goys, or girls).
Abbreviation: CAH, congenital adrenal hyperplasia.
Figure 2.
Comparison of CAH prevalence across age and sex for the Commercial and Medicaid samples. Years (y). Error bars represent 95% confidence intervals for each prevalence estimate. Data labels show prevalence estimates for each subgroup. CAH, congenital adrenal hyperplasia.
Table 2.
Prevalence Ratios for CAH by Age and Sex for the Commercial and Medicaid Samples
| Met Criteria for CAH | ||||
|---|---|---|---|---|
| Prevalence | PR | 95% CI | P Value | |
| Commercial sample | ||||
| All | 1:9906 | |||
| Age | ||||
| 5-11 y (reference) | 1:10 066 | 1 | ||
| 12-18 y | 1:9769 | 1.03 | (0.84-1.26) | 0.773 |
| Sex | ||||
| Boys (reference) | 1:11 166 | 1 | ||
| Girls | 1:8891 | 1.26 | (1.02-1.54) | 0.029 |
| Medicaid sample | ||||
| All | 1:13 905 | |||
| Age | ||||
| 5-11 y (reference) | 1:13 390 | 1 | ||
| 12-18 y | 1:14 662 | 0.91 | (0.71-1.18) | 0.484 |
| Sex | ||||
| Boys (reference) | 1:17 303 | 1 | ||
| Girls | 1:11 593 | 1.49 | (1.16-1.92) | 0.002 |
Prevalence ratio (PR), confidence interval (CI), years (y), reference category (Ref). P-values represent chi-square tests.
Abbreviations: CAH, congenital adrenal hyperplasia; PR, prevalence ratio.
For the Commercial sample, the prevalence of ADHD in the non-CAH group was 8.4%. Within the non-CAH group, the prevalence of ADHD was lower among girls compared with boys (PR = 0.48; 95% CI, 0.48-0.49; P < 0.001; Table 3). There was no clinically meaningful difference in prevalence of ADHD across age groups (8.4% vs 8.4%; PR = 0.99; 95% CI, 0.98-1.0; P = 0.003; Table 3). Within the CAH group, the prevalence of ADHD was 9.4%. In contrast with the non-CAH group, the prevalence of ADHD in the CAH group was not significantly lower in girls than boys (PR = 0.96; 95% CI, 0.51-1.80; P = 0.892; Table 3). The prevalence of ADHD was slightly higher among older than younger children with CAH (PR = 1.29; 95% CI, 0.68-2.46; P = 0.438; Table 3), but this difference did not reach statistical significance.
Table 3.
ADHD Prevalence for the Non-CAH Pediatric Population and Those Meeting Criteria for CAH Within the Commercial and Medicaid Samples
| Met Criteria for ADHD | ||||||||
|---|---|---|---|---|---|---|---|---|
| Non-CAH Group | CAH Group | |||||||
| n (%) | PR | 95% CI | P Value | n (%) | PR | 95% CI | P Value | |
| Commercial sample | ||||||||
| All | 309 302 (8.4) | -- | -- | -- | 35 (9.4) | -- | -- | -- |
| Age | ||||||||
| 5-11 y (reference) | 146 107 (8.4) | 1 | 14 (8.1) | 1 | ||||
| 12-18 y | 163 195 (8.4) | 0.99 | (0.98-1.00) | 0.003 | 21 (10.5) | 1.29 | (0.68-2.46) | 0.438 |
| Sex | ||||||||
| Boys (reference) | 209 426 (11.3) | 1 | 16 (9.6) | 1 | ||||
| Girls | 99 876 (5.5) | 0.48 | (0.48-0.49) | <0.001 | 19 (9.2) | 0.96 | (0.51-1.80) | 0.892 |
| Medicaid sample | ||||||||
| All | 519 920 (15.1) | -- | -- | -- | 34 (13.8) | -- | -- | -- |
| Age | ||||||||
| 5-11 y (reference) | 325 755 (16.6) | 1 | 24 (16.3) | 1 | ||||
| 12-18 y | 194 165 (13.2) | 0.80 | (0.80-0.80) | <0.001 | 10 (10.0) | 0.61 | (0.31-1.22) | 0.158 |
| Sex | ||||||||
| Boys (reference) | 355 166 (20.5) | 1 | 18 (18.0) | 1 | ||||
| Girls | 164 754 (9.7) | 0.47 | (0.47-0.47) | <0.001 | 16 (10.9) | 0.60 | (0.32-1.13) | 0.112 |
P values are from χ 2 tests comparing ratio of subjects meeting criteria for ADHD by age and gender within the non-CAH group and the CAH group.
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; CAH, congenital adrenal hyperplasia; CI, confidence interval; PR, prevalence ratio.
ADHD prevalence was similar between boys with CAH and those without in the Commercial sample (aPR = 0.94; 95% CI, 0.59-1.49; P = 0.496; Table 4). Although the prevalence was substantially lower among younger boys with CAH than those without (6.1% vs 11.7%), numbers were small and this difference did not reach statistical significance (PR = 0.52; 95% CI, 0.22-1.22; P = 0.116; Table 4). The prevalence of ADHD was similar between older boys with CAH and those without (PR = 1.20; 95% CI, 0.69-2.07; P = 0.530; Table 4). The prevalence of ADHD among girls with CAH was 9.2% compared with 5.5% among girls without CAH (aPR = 1.71; 95% CI, 1.11-2.62; P = 0.018; Table 4). This difference was driven mainly by an increased prevalence among younger girls (PR = 1.98; 95% CI, 1.07-3.68; P = 0.032; Table 4). There was also an increase in prevalence among older girls with CAH compared with those without (PR = 1.49; 95% CI, 0.82-2.69; P = 0.194; Table 4); however, this difference did not reach statistical significance.
Table 4.
Prevalence Ratios for ADHD Between Those Meeting Criteria for CAH and the non-CAH Group in the Commercial and Medicaid Calculated Separately by Sex and Stratified Across Age
| Strata | Met Criteria for ADHD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Boys | Girls | |||||||||
| n (%) | PRa | 95% CI | P Value | n (%) | PRa | 95% CI | P Value | |||
| Commercial sample | ||||||||||
| Non-CAH group (reference) | All | 209 426 (11.3) | 1 | 99 876 (5.5) | 1 | |||||
| CAH group | 16 (9.6) | 0.94 | (0.59-1.49) | 0.496 | 19 (9.2) | 1.71 | (1.11-2.62) | 0.018 | ||
| Non-CAH group (reference) | 5-11 y | 103 412 (11.7) | 1 | 42 695 (5.1) | 1 | |||||
| CAH group | 5 (6.1) | 0.52 | (0.22-1.22) | 0.116 | 9 (10.0) | 1.98 | (1.07-3.7) | 0.032 | ||
| Non-CAH group (reference) | 12-18 y | 106 014 (11.0) | 1 | 57 181 (5.8) | 1 | |||||
| CAH group | 11 (13.1) | 1.20 | (0.69-2.07) | 0.530 | 10 (8.6) | 1.49 | (0.82-2.69) | 0.194 | ||
| Medicaid sample | ||||||||||
| Non-CAH group (reference) | All | 355 166 (20.5) | 1 | 164 754 (9.7) | 1 | |||||
| CAH group | 18 (18.0) | 0.87 | (0.57-1.32) | 0.507 | 16 (10.9) | 1.12 | (0.70-1.78) | 0.626 | ||
| Non-CAH group (reference) | 5-11 y | 225 972 (22.4) | 1 | 99 783 (10.4) | 1 | |||||
| CAH group | 13 (21.0) | 0.94 | (0.58-1.52) | 0.442 | 11 (12.9) | 1.24 | (0.72-2.16) | 0.446 | ||
| Non-CAH group (reference) | 12-18 y | 129 194 (17.9) | 1 | 64 971 (8.7) | 1 | |||||
| CAH group | 5 (13.2) | 0.73 | (0.32-1.66) | 0.463 | 5 (8.1) | 0.93 | (0.40-2.15) | 0.858 | ||
P values represent χ 2 tests.
aPRs presented for the main effect of CAH in each subsample have been adjusted after stratification by age. PRs for each stratum (5-11 years and 12-18 years) are unadjusted.
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; CI, confidence interval; PR, prevalence ratio.
For the Medicaid sample, prevalence of ADHD was 15.1% in the non-CAH group. In the non-CAH group, prevalence was lower among girls (PR = 0.47; 95% CI, 0.47-0.47; P < 0.001; Table 3), and among older children (PR = 0.80; 95% CI, 0.80-0.80; P < 0.001; Table 3). There were similar patterns within the CAH group, with 13.8% meeting criteria for ADHD. The prevalence was lower among girls (PR = 0.60; 95% CI, 0.32-1.13; P = 0.112; Table 3) and older children (PR = 0.61; 95% CI, 0.31-1.22; P = 0.158; Table 3). However, these differences did not reach statistical significance.
When comparing ADHD prevalence between the CAH and non-CAH groups in the Medicaid sample, there was no significant difference in prevalence for either boys (aPR = 0.88; 95% CI, 0.58-1.33; P = 0.507; Table 4) or girls (aPR = 1.14; 95% CI, 0.72-1.80; P = 0.626; Table 4), either overall or after stratifying by age (Table 4).
Discussion
In this retrospective cohort study, we used 2 large health insurance claims samples of children and adolescents in the United States to identify CAH cases and investigate the prevalence of medically managed ADHD. We found no significant difference in the receipt of clinical care for ADHD overall for either privately or publicly insured children and adolescents with CAH as compared with their peers without CAH. However, the prevalence of medically managed ADHD was higher among privately insured girls with CAH compared with their peers without CAH.
In our study, the prevalence of medically managed CAH in both the Commercial and Medicaid samples (1 in 9906 and 1 in 13 905, respectively) was somewhat higher than the reported birth prevalence of 21OHD CAH in the United States over the past several decades of approximately 1 in 16 000 to 1 in 18 000 [36, 37]. The higher prevalence in our samples may be attributed in part to the inclusion of nonclassic CAH cases, the prevalence of which is as high as 0.1% to 0.5% in populations of European ancestry [38]. Racial disparities may also help explain why our prevalence estimates in the Commercial sample were higher than in the Medicaid sample. The prevalence of CAH is reported to vary by race/ethnicity, with non-Hispanic Black infants having a particularly low frequency [36, 37]. In the United States, disadvantaged racial and ethnic minorities are less likely to be insured [39], and among those that are insured, more likely to be insured under public programs [40].
Our prevalence estimates for medically managed ADHD in the Commercial and Medicaid samples (8.4% and 15.1%, respectively) are consistent with those reported previously for the MarketScan databases [41]. They are also in line with national estimates of 8.4% to 8.7% for the pediatric point prevalence of ADHD in the United States over the past 2 decades [42, 43]. Importantly, the prevalence of ADHD is known to be higher among those with public insurance [43]. Among other factors, ADHD can be a qualifying condition for disability benefits under the Supplemental Security Income program, which in turn makes individuals eligible for Medicaid [44].
There is limited prior research evaluating ADHD symptoms or diagnoses among patients with CAH. Four studies have used small cohorts of pediatric patients with CAH identified in clinical settings [26, 30, 45, 46]. One study of 54 pediatric patients in the United States, who were recruited as part of natural history study of patients with excess androgen at the National Institutes of Health Clinical Center, used a semistructured clinical interview to characterize mental disorders [46]. The authors hypothesized that prolonged exposure to androgens might predispose children to risk for mental disorders. Relative to the national prevalence of ADHD at a similar timepoint, 8.4% [47], they reported a higher rate of ADHD among boys with CAH (18.2%) but not among girls with CAH (4.9%) [46]. A second center-based study of 49 patients in Malaysia, a country without newborn screening for CAH, used the Child Behavior Checklist (CBCL) to characterize behavioral outcomes in their cohort [45]. They found that children with CAH, compared with a control group made up of nonaffected siblings, scored significantly higher on the attention problem syndrome subscale of the CBCL. Furthermore, 14% to 15% of boys and girls with CAH had clinical range scores on the attention subscale compared with 0% of the sibling controls. A third study of 57 pediatric patients in England and Italy diagnosed with classic CAH through newborn screening recruited as part of a longitudinal study on pre- and postnatal treatment of CAH, also used the CBCL to assess behavioral outcomes [30]. They reported that children in the CAH group did not score higher on the attention subscale compared with a mixed control group of unaffected relatives and individuals enlisted through a population registry [30]. Finally, a fourth study of 82 younger patients (ages 4-11 years) with classic 21OHD in the United Kingdom used the Strengths and Difficulties Questionnaire to assess subjects’ emotional and behavioral adjustment [26]. They found that girls with CAH, but not boys, had higher scores on the Hyperactivity/Inattention subscale compared with the general pediatric population, but not compared with unaffected sisters and female first cousins. Notably, each of these studies included a small number of CAH cases, and their assessments of ADHD were based on clinical screening scales or clinical interviews conducted within the context of the research study.
Additionally, 2 recent studies used national patient registries in Sweden to assess mental health diagnoses in a mixed sample of children and adults with CAH [48, 49]. For the male CAH cohort, they reported no statistically significant difference in risk for ADHD compared with age- and sex-matched controls drawn from the total population registry [49]. Notably, though, there was only 1 ADHD case in the CAH population (0.4%, N = 253), and only 1.1% of the control population had an ADHD diagnosis (n = 268 of 25 300 controls). Similarly, for the female CAH cohort, there was no statistically significant difference in risk for ADHD (0.6%, n = 2 of 335) compared with either age-matched female controls (0.5%, n = 177 of 33 500) or age-matched male controls (1.1%, n = 366 of 33 500) [48]. After stratifying their analyses, the authors found that adult women with the most severe CAH genotype, most of whom were born before the introduction of newborn screening, were at higher risk for ADHD. Although these studies are strengthened by their use of national registries and ICD codes to determine outcomes, the limited number of ADHD cases present in their study populations makes it difficult to directly compare their findings with our findings from the general pediatric population in the United States, which has a much higher baseline rate of ADHD [43].
Although previous research has suggested that dysfunction in the HPA axis and the hypothalamic-pituitary-gonadal axis is tied to mental disorder symptoms in children and adolescents [6, 7, 50], the biological underpinnings of a possible increased risk for ADHD are not entirely clear. A genetic family-based study found an association between functional polymorphisms of the gene encoding the glucocorticoid receptor and childhood ADHD-related behaviors, which, given the pivotal role of glucocorticoid receptor on the HPA axis, suggests a possible involvement of the HPA axis in ADHD [51]. Many studies have focused on differences in cortisol level, particularly in stress responsivity [52, 53], although there has been significant heterogeneity in approach and findings have been inconclusive, particularly with respect to differences in ADHD subtype, presence of co-occurring mental health conditions, and sex. Studies on the relationship between androgen levels and attention and conduct problems in children and adolescents have been similarly divergent [14, 54, 55], likely because of the diversity of specific hormones being investigated, consideration of prenatal versus postnatal exposures, and the complicating role of sex. Thus, further understanding of how dysfunction in the HPA and hypothalamic-pituitary-gonadal axes may predispose children and adolescents to the development of ADHD may help to identify risk among pediatric patients with CAH.
It has been suggested, on the basis of studies in both humans and animal models, that early exposure to androgens may increase stress vulnerability and lead to a downstream effect on mood and anxiety-related behaviors [56]. Our findings suggest that in childhood, the risk for ADHD, which is characterized by inattentiveness, hyperactivity, and reduced impulse control, is not substantially elevated among all pediatric patients with CAH. However, given the nature of our study design, we were not able to control in our analysis for differences in CAH disease severity or a patient’s history of hormonal control. Consideration of these factors as potential moderators in future studies may help to further elucidate the relationship between androgen and cortisol levels and mental health in children and adolescents with CAH.
An alternative explanation for our findings, given that we relied on ADHD diagnosis and pharmacy billing codes rather than measures of symptoms, is that families with children with CAH experiencing ADHD symptoms may be less likely to pursue evaluation or treatment. Such a disparity may be due to incorrectly attributing symptoms of ADHD to CAH. It could also reflect lack of time or resources to pursue additional medical treatment given the high burden of care associated with CAH in pediatric patients. Future studies may wish to consider whether high health care utilization for treatment of CAH presents a barrier to pursuing care for other mental or physical health problems. Investigations of the rate at which children with CAH are identified and referred for mental disorder symptoms to other care providers by their pediatric endocrinologist, who in many cases may be a more frequent source of follow-up than their primary care provider, may also be fruitful.
One of the main strengths of our study is our identification of a large number of children and adolescents with CAH through the use of health insurance claims data. To our knowledge, our study is the first to use such an approach to assess the prevalence of CAH in the pediatric population in the United States and to evaluate a co-occurring mental health condition. Rare diseases can be challenging to investigate with sufficient statistical power [57], and CAH is no exception to this challenge. Thus, an important finding from our study is the refinement of a claims data algorithm for identifying pediatric CAH cases. We do note that given the relatively small number of subjects identified with both CAH and ADHD, post hoc tests on the effects of age and sex may not be adequately powered in our analysis.
Our findings in the non-CAH groups are consistent with the increased risk for medically managed ADHD among boys in the general US pediatric population. In contrast, our finding of a similar prevalence of medically managed ADHD between privately insured boys and girls with CAH is intriguing. In particular, we found an increased prevalence of ADHD among privately insured girls with CAH as compared with their non-CAH peers, especially among young girls. ADHD symptoms that are often more pronounced in girls (eg, inattentiveness, poor school performance, depressive affect) may be less likely to lead to clinical diagnosis of ADHD in comparison with disruptive behaviors commonly observed in boys with ADHD [58]. It is possible that girls with CAH with elevated adrenal androgen levels who are also affected by ADHD may have increased disruptive behaviors that are more likely to be recognized by teachers and parents, leading to a greater likelihood of diagnosis and treatment in this group as compared to their non-CAH peers. Alternatively, several studies have reported increased aggressive behaviors and activity levels among girls with CAH [17, 18, 59], both of which are stereotypically more masculine behaviors. Thus, it may also be possible that the presence of these behaviors among girls with CAH in excess of perceived cultural norms may lead to the misdiagnosis of ADHD. We do note, however, that these differences have not been reported uniformly across the literature. Finally, our nonsignificant findings are also suggestive of a decreased risk for medically managed ADHD among privately insured younger boys with CAH. These hypotheses are deserving of further study if a larger sample could be assembled.
Using claims data to measure the prevalence of chronic conditions and medical comorbidities is a well-established practice [60, 61], but it is subject to misclassification. Use of claims data requires careful methodological consideration to produce accurate estimates, especially when shorter time frames are used. Thus, our findings should be interpreted cautiously in the context of a relatively short, 3-year study period. We did not use data before October 1, 2015, because during this time ICD-9-CM codes were in use, which included a less specific code, 255.2 “Adrenogenital disorders.” By limiting our study period to after the adoption of ICD-10-CM codes, we were able to select for the code E25.0 “Congenital adrenogenital disorders associated with enzyme deficiency,” which is more specific for CAH [62]. However, we were unable to further distinguish individuals on the basis of specific enzyme deficiency, disease severity (ie, salt-wasting, simple-virilizing, or nonclassic CAH), or degree of androgen excess. Although the majority of nonclassic cases are asymptomatic and may go undetected, a subset of patients may require glucocorticoid treatment. Thus, our study is limited in that our cohort captures all CAH cases with filled glucocorticoid prescriptions, which likely includes a minority of patients with nonclassic 21OHD or enzyme deficiencies other than 21OHD.
Importantly, our prevalence estimates for CAH were higher among girls in both the Commercial and Medicaid samples. This suggests a sex-based difference in the recording of CAH cases in billing claims, particularly among adolescents, where the prevalence estimates were lowest in males. In general, adolescent females have more health care contacts than adolescent males [63]. In addition, it is also possible that the more visible virilizing effects on girls with CAH may prompt more frequent medical follow-up, or, alternatively, the less visible effects among boys may cause more nonclassic cases to go undetected during childhood.
Our study also has several other limitations. First, the Commercial sample does not include children and adolescents from families with individual health insurance plans or those without prescription or mental health coverage. Second, our Medicaid sample is from a small number of unidentified states. Finally, and perhaps most critically, the accuracy of our claims algorithm for identifying CAH cases has not been validated through comparison with an external source such as medical records. It is likely that some of our CAH cases are false positives, as coding errors and the use of rule out diagnoses can decrease the accuracy of using claims data for prevalence estimates, especially in outpatient settings [64, 65]. In our approach, we augmented our CAH claims algorithm from previously published work [34, 48, 49] to include a requirement of at least 2 filled glucocorticoid prescriptions. The combination of diagnostic codes with disease-specific medication claims can improve the performance of claims algorithms for chronic conditions in some cases [66, 67]. Future research to assess the sensitivity and specificity of our claims algorithm for identifying pediatric CAH cases relative to clinically validated diagnoses will be helpful in advancing our approach.
In summary, using a claims-based approach, we found that the prevalence of medically managed ADHD among children and adolescents with CAH in the United States was not elevated above the baseline prevalence within the privately and publicly insured pediatric populations. There is some evidence suggesting that younger, privately insured girls with CAH may have a higher prevalence of medically managed ADHD than girls without CAH, whereas younger, privately insured boys may have a lower prevalence. Further refinement of claims-based methodologies and multicenter longitudinal studies that examine the role of sex, newborn screening, and hormonal control may help to elucidate the development of co-occurring mental health conditions among the CAH population.
Acknowledgments
Financial Support: This research was supported by the National Institutes of Health grants F30 CA223591 to L.A.H. and T32 GM008244 to the University of Minnesota Medical Scientist Training Program. This research was also supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, grant UL1TR002494.
MarketScan Research Databases were analyzed at the Centers for Disease Control and Prevention under license from IBM for public health purposes. MarketScan is a registered trademark of IBM Corporation in the United States, other countries or both. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Glossary
Abbreviations
- 21OHD
21-hydroxylase deficiency
- ADHD
attention-deficit/hyperactivity disorder
- aPR
adjusted prevalence ratio
- CAH
congenital adrenal hyperplasia
- CBCL
Child Behavior Checklist
- CI
confidence interval
- HPA
hypothalamic-pituitary-adrenal
- ICD-9-CM
International Statistical Classification of Diseases and Related Health Problems, Ninth Revision, Clinical Modification
- ICD-10-CM
International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Clinical Modification
- PR
prevalence ratio
Additional Information
Disclosure Summary: K.S. receives research support from the National Institutes of Health National Cancer Institute (R01 CA181024); Office of Orphan Products Development of the Food and Drug Administration (R01FDR0006100); Alexion, Inc.; Spruce Biosciences; and Neurocrine Biosciences. L.A.H. and S.D.G. have no financial disclosures to report.
Data Availability
Restrictions apply to some or all the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References and Notes
- 1. Speiser PW, Arlt W, Auchus RJ, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018;103(11):4043-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berenbaum SA. Cognitive function in congenital adrenal hyperplasia. Endocrinol Metab Clin North Am. 2001;30(1):173-192. [DOI] [PubMed] [Google Scholar]
- 3. Webb EA, Elliott L, Carlin D, et al. Quantitative brain MRI in congenital adrenal hyperplasia: in vivo assessment of the cognitive and structural impact of steroid hormones. J Clin Endocrinol Metab. 2018;103(4):1330-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Merke DP, Fields JD, Keil MF, Vaituzis AC, Chrousos GP, Giedd JN. Children with classic congenital adrenal hyperplasia have decreased amygdala volume: potential prenatal and postnatal hormonal effects. J Clin Endocrinol Metab. 2003;88(4):1760-1765. [DOI] [PubMed] [Google Scholar]
- 5. Herting MM, Azad A, Kim R, Tyszka JM, Geffner ME, Kim MS. Brain differences in the prefrontal cortex, amygdala, and hippocampus in youth with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2020;105(4):1098-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Juruena MF, Eror F, Cleare AJ, Young AH. The role of early life stress in HPA axis and anxiety. Adv Exp Med Biol. 2020;1191:141-153. [DOI] [PubMed] [Google Scholar]
- 7. Guerry JD, Hastings PD. In search of HPA axis dysregulation in child and adolescent depression. Clin Child Fam Psychol Rev. 2011;14(2):135-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kao K, Doan SN, St John AM, Meyer JS, Tarullo AR. Salivary cortisol reactivity in preschoolers is associated with hair cortisol and behavioral problems. Stress. 2018;21(1):28-35. [DOI] [PubMed] [Google Scholar]
- 9. Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Dev Psychopathol. 2005;17(1):167-184. [DOI] [PubMed] [Google Scholar]
- 10. Sondeijker FE, Ferdinand RF, Oldehinkel AJ, et al. Disruptive behaviors and HPA-axis activity in young adolescent boys and girls from the general population. J Psychiatr Res. 2007;41(7):570-578. [DOI] [PubMed] [Google Scholar]
- 11. Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Dev Psychobiol. 2008;50(7):690-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruttle PL, Shirtcliff EA, Serbin LA, Fisher DB, Stack DM, Schwartzman AE. Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: longitudinal and concurrent associations with cortisol. Horm Behav. 2011;59(1):123-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Susman EJ, Peckins MK, Bowes JL, Dorn LD. Longitudinal synergies between cortisol reactivity and diurnal testosterone and antisocial behavior in young adolescents. Dev Psychopathol. 2017;29(4):1353-1369. [DOI] [PubMed] [Google Scholar]
- 14. van Goozen SH, van den Ban E, Matthys W, Cohen-Kettenis PT, Thijssen JH, van Engeland H. Increased adrenal androgen functioning in children with oppositional defiant disorder: a comparison with psychiatric and normal controls. J Am Acad Child Adolesc Psychiatry. 2000;39(11):1446-1451. [DOI] [PubMed] [Google Scholar]
- 15. van Goozen SH, Fairchild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychol Bull. 2007;133(1):149-182. [DOI] [PubMed] [Google Scholar]
- 16. Grotzinger AD, Mann FD, Patterson MW, Tackett JL, Tucker-Drob EM, Harden KP. Hair and salivary testosterone, hair cortisol, and externalizing behaviors in adolescents. Psychol Sci. 2018;29(5):688-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pasterski V, Hindmarsh P, Geffner M, Brook C, Brain C, Hines M. Increased aggression and activity level in 3- to 11-year-old girls with congenital adrenal hyperplasia (CAH). Horm Behav. 2007;52(3):368-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spencer D, Pasterski V, Neufeld S, et al. Prenatal androgen exposure and children’s aggressive behavior and activity level. Horm Behav. 2017;96:156-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berenbaum SA, Duck SC, Bryk K. Behavioral effects of prenatal versus postnatal androgen excess in children with 21-hydroxylase-deficient congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2000;85(2):727-733. [DOI] [PubMed] [Google Scholar]
- 20. Young EA, Abelson J, Lightman SL. Cortisol pulsatility and its role in stress regulation and health. Front Neuroendocrinol. 2004;25(2):69-76. [DOI] [PubMed] [Google Scholar]
- 21. Sarafoglou K, Zimmerman CL, Gonzalez-Bolanos MT, Willis BA, Brundage R. Interrelationships among cortisol, 17-hydroxyprogesterone, and androstenendione exposures in the management of children with congenital adrenal hyperplasia. J Investig Med. 2015;63(1):35-41. [DOI] [PubMed] [Google Scholar]
- 22. Joëls M. Corticosteroids and the brain. J Endocrinol. 2018;238(3):R121-R130. [DOI] [PubMed] [Google Scholar]
- 23. Prado CE, Crowe SF. Corticosteroids and cognition: a meta-analysis. Neuropsychol Rev. 2019;29(3):288-312. [DOI] [PubMed] [Google Scholar]
- 24. Berenbaum SA, Korman Bryk K, Duck SC, Resnick SM. Psychological adjustment in children and adults with congenital adrenal hyperplasia. J Pediatr. 2004;144(6):741-746. [DOI] [PubMed] [Google Scholar]
- 25. Knickmeyer R, Baron-Cohen S, Fane BA, et al. Androgens and autistic traits: a study of individuals with congenital adrenal hyperplasia. Horm Behav. 2006;50(1):148-153. [DOI] [PubMed] [Google Scholar]
- 26. Kung KTF, Spencer D, Pasterski V, et al. Emotional and behavioral adjustment in 4 to 11-year-old boys and girls with classic congenital adrenal hyperplasia and unaffected siblings. Psychoneuroendocrinology. 2018;97:104-110. [DOI] [PubMed] [Google Scholar]
- 27. Agoston AM, Gonzalez-Bolanos MT, Semrud-Clikeman M, Vanderburg N, Sarafoglou K. Executive functioning in children with congenital adrenal hyperplasia. J Investig Med. 2017;65(1):49-52. [DOI] [PubMed] [Google Scholar]
- 28. Browne WV, Hindmarsh PC, Pasterski V, et al. Working memory performance is reduced in children with congenital adrenal hyperplasia. Horm Behav. 2015;67:83-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Collaer ML, Hindmarsh PC, Pasterski V, Fane BA, Hines M. Reduced short term memory in congenital adrenal hyperplasia (CAH) and its relationship to spatial and quantitative performance. Psychoneuroendocrinology. 2016;64:164-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Messina V, Hirvikoski T, Karlsson L, et al. Good overall behavioural adjustment in children and adolescents with classic congenital adrenal hyperplasia. Endocrine. 2020;68(2):427-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perou R, Bitsko RH, Blumberg SJ, et al. ; Centers for Disease Control and Prevention Mental health surveillance among children--United States, 2005–2011. MMWR Suppl. 2013;62(2):1-35. [PubMed] [Google Scholar]
- 32. Wolraich ML, Hagan JF Jr., Allan C, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2019;144(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stewart PM, Biller BM, Marelli C, Gunnarsson C, Ryan MP, Johannsson G. Exploring inpatient hospitalizations and morbidity in patients with adrenal insufficiency. J Clin Endocrinol Metab. 2016;101(12):4843-4850. [DOI] [PubMed] [Google Scholar]
- 34. Jenkins-Jones S, Parviainen L, Porter J, et al. Poor compliance and increased mortality, depression and healthcare costs in patients with congenital adrenal hyperplasia. Eur J Endocrinol. 2018;178(4):309-320. [DOI] [PubMed] [Google Scholar]
- 35. Visser SN, Danielson ML, Wolraich ML, et al. Vital signs: national and state-specific patterns of attention deficit/hyperactivity disorder treatment among insured children aged 2–5 years - United States, 2008–2014. MMWR Morb Mortal Wkly Rep. 2016;65(17):443-450. [DOI] [PubMed] [Google Scholar]
- 36. Therrell BL Jr, Berenbaum SA, Manter-Kapanke V, et al. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics. 1998;101(4 Pt 1):583-590. [DOI] [PubMed] [Google Scholar]
- 37. Pearce M, DeMartino L, McMahon R, et al. Newborn screening for congenital adrenal hyperplasia in New York State. Mol Genet Metab Rep. 2016;7:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Speiser PW, Dupont B, Rubinstein P, Piazza A, Kastelan A, New MI. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37(4):650-667. [PMC free article] [PubMed] [Google Scholar]
- 39. Sohn H. Racial and ethnic disparities in health insurance coverage: dynamics of gaining and losing coverage over the life-course. Popul Res Policy Rev. 2017;36(2):181-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shone LP, Dick AW, Brach C, et al. The role of race and ethnicity in the State Children’s Health Insurance Program (SCHIP) in four states: are there baseline disparities, and what do they mean for SCHIP? Pediatrics. 2003;112(6 Pt 2):e521. [PubMed] [Google Scholar]
- 41. Nyarko KA, Grosse SD, Danielson ML, Holbrook JR, Visser SN, Shapira SK. Treated prevalence of attention-deficit/hyperactivity disorder increased from 2009 to 2015 among school-aged children and adolescents in the United States. J Child Adolesc Psychopharmacol. 2017;27(8):731-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161(9):857-864. [DOI] [PubMed] [Google Scholar]
- 43. Danielson ML, Bitsko RH, Ghandour RM, Holbrook JR, Kogan MD, Blumberg SJ. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47(2):199-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perrin JM, Houtrow A, Kelleher K, Hoagwood K, Stein REK, Zima B. Supplemental security income benefits for mental disorders. Pediatrics. 2016;138(1):e20160354. [DOI] [PubMed] [Google Scholar]
- 45. Idris AN, Chandran V, Syed Zakaria SZ, Rasat R. Behavioural outcome in children with congenital adrenal hyperplasia: experience of a single centre. Int J Endocrinol. 2014;2014:483718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mueller SC, Ng P, Sinaii N, et al. Psychiatric characterization of children with genetic causes of hyperandrogenism. Eur J Endocrinol. 2010;163(5):801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pastor PN, Reuben CA. Diagnosed attention deficit hyperactivity disorder and learning disability: United States, 2004–2006. Vital Health Stat 10. 2008(237):1-14. [PubMed] [Google Scholar]
- 48. Engberg H, Butwicka A, Nordenström A, et al. Congenital adrenal hyperplasia and risk for psychiatric disorders in girls and women born between 1915 and 2010: a total population study. Psychoneuroendocrinology. 2015;60:195-205. [DOI] [PubMed] [Google Scholar]
- 49. Falhammar H, Butwicka A, Landén M, et al. Increased psychiatric morbidity in men with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2014;99(3):E554-E560. [DOI] [PubMed] [Google Scholar]
- 50. Marceau K, Ruttle PL, Shirtcliff EA, Essex MJ, Susman EJ. Developmental and contextual considerations for adrenal and gonadal hormone functioning during adolescence: implications for adolescent mental health. Dev Psychobiol. 2015;57(6):742-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fortier MÈ, Sengupta SM, Grizenko N, Choudhry Z, Thakur G, Joober R. Genetic evidence for the association of the hypothalamic-pituitary-adrenal (HPA) axis with ADHD and methylphenidate treatment response. Neuromolecular Med. 2013;15(1):122-132. [DOI] [PubMed] [Google Scholar]
- 52. Corominas M, Ramos-Quiroga JA, Ferrer M, et al. Cortisol responses in children and adults with attention deficit hyperactivity disorder (ADHD): a possible marker of inhibition deficits. Atten Defic Hyperact Disord. 2012;4(2):63-75. [DOI] [PubMed] [Google Scholar]
- 53. Kamradt JM, Momany AM, Nikolas MA. A meta-analytic review of the association between cortisol reactivity in response to a stressor and attention-deficit hyperactivity disorder. Atten Defic Hyperact Disord. 2018;10(2):99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang LJ, Lee SY, Chou MC, Lee MJ, Chou WJ. Dehydroepiandrosterone sulfate, free testosterone, and sex hormone-binding globulin on susceptibility to attention-deficit/hyperactivity disorder. Psychoneuroendocrinology. 2019;103:212-218. [DOI] [PubMed] [Google Scholar]
- 55. Roberts BA, Martel MM. Prenatal testosterone and preschool disruptive behavior disorders. Pers Individ Dif. 2013;55(8):962-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mueller SC, Grissom EM, Dohanich GP. Assessing gonadal hormone contributions to affective psychopathologies across humans and animal models. Psychoneuroendocrinology. 2014;46:114-128. [DOI] [PubMed] [Google Scholar]
- 57. Mitani AA, Haneuse S. Small data challenges of studying rare diseases. JAMA Netw Open. 2020;3(3):e201965. [DOI] [PubMed] [Google Scholar]
- 58. Quinn P, Wigal S. Perceptions of girls and ADHD: results from a national survey. Medgenmed. 2004;6(2):2. [PMC free article] [PubMed] [Google Scholar]
- 59. Berenbaum SA, Resnick SM. Early androgen effects on aggression in children and adults with congenital adrenal hyperplasia. Psychoneuroendocrinology. 1997;22(7):505-515. [DOI] [PubMed] [Google Scholar]
- 60. Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care. 2002;40(8 Suppl):IV-26. [DOI] [PubMed] [Google Scholar]
- 61. Rassen JA, Bartels DB, Schneeweiss S, Patrick AR, Murk W. Measuring prevalence and incidence of chronic conditions in claims and electronic health record databases. Clin Epidemiol. 2019;11:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. World Health O. International statistical classification of diseases and related health problems. 10th revision, 5th ed. Geneva: World Health Organization; 2016. [Google Scholar]
- 63. Rand CM, Shone LP, Albertin C, Auinger P, Klein JD, Szilagyi PG. National health care visit patterns of adolescents: implications for delivery of new adolescent vaccines. Arch Pediatr Adolesc Med. 2007;161(3):252-259. [DOI] [PubMed] [Google Scholar]
- 64. Metcalfe A, Sibbald B, Lowry RB, Tough S, Bernier FP. Validation of congenital anomaly coding in Canada’s administrative databases compared with a congenital anomaly registry. Birth Defects Res A Clin Mol Teratol. 2014;100(2):59-66. [DOI] [PubMed] [Google Scholar]
- 65. Andrade SE, Scott PE, Davis RL, et al. Validity of health plan and birth certificate data for pregnancy research. Pharmacoepidemiol Drug Saf. 2013;22(1):7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rector TS, Wickstrom SL, Shah M, et al. Specificity and sensitivity of claims-based algorithms for identifying members of Medicare+Choice health plans that have chronic medical conditions. Health Serv Res. 2004;39(6 Pt 1):1839-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kolodner K, Lipton RB, Lafata JE, et al. Pharmacy and medical claims data identified migraine sufferers with high specificity but modest sensitivity. J Clin Epidemiol. 2004;57(9):962-972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to some or all the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.